Introduction
Although the rates of death due to cancer have been
continuously declining for the past 2 decades in developed nations,
cancer remains a major public health threat in many parts of the
world (1). The incidence of cancer
in the developing world is currently increasing. Specifically, 55%
of new cases arise in developing nations, a figure that could reach
60% by 2020 and 70% by 2050. Worldwide, cancer also causes a
substantial burden of economic cost and human suffering; the cost
associated with cancer cases worldwide was approximately US$1.16
trillion in 2010, the equivalent of >2% of the total global
gross domestic product. Nevertheless, this high figure is a lower
bound and does not include the substantial longer-term costs to
families and caregivers (2).
The current gold standard of care for cancer is a
combination of surgery, radiation therapy, and chemotherapy
(3–5). However, traditional methods are
associated with drawbacks, such as a lack of screening tests for
early diagnosis and a lack of tumor-specific drug delivery systems.
Moreover, most classical anticancer drugs cannot differentiate
between cancerous and normal cells, thus leading to systemic
toxicity and adverse side effects. Selective and more efficient new
drugs are urgently needed to address this problem. In this context,
bioactive peptides are increasingly being considered as good drug
candidates for cancer therapeutic applications. A growing body of
peptides from natural animal sources has been demonstrated to
possess physiological functions, such as immunomodulatory (6), antimicrobial (7), antihypertensive (8), antithrombotic (9), anticancer (10), anti-oxidative (11), and cholesterol-lowering activities
(12). However, this review focuses
on bioactive peptides from animal sources that may specifically
target cancer cells and could consequently serve as anticancer
agents that are less toxic to normal tissues. In addition, as shown
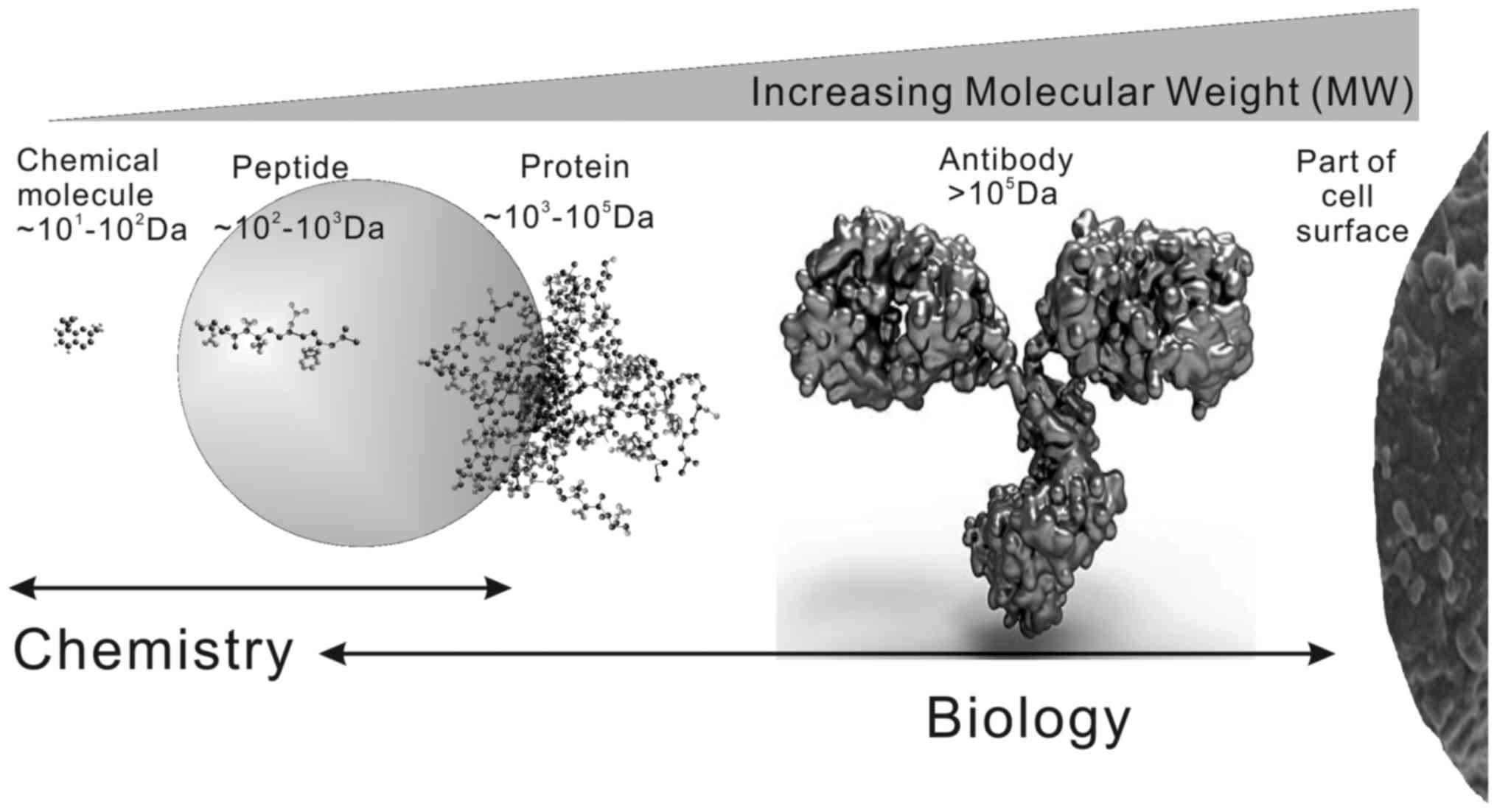
in Fig. 1, bioactive peptides
usually consist of 2–50 amino acid residues
(~102-103 Da). Thus, they easily traverse or
disrupt the cell membrane and result in apoptosis or necrosis.
Therefore, the study and modification of bioactive peptides with
anticancer activity will offer new opportunities for cancer
prevention and treatment.
The objectives of this study are to 1) review the
current understanding of anticancer bioactive peptides derived from
different animal sources and 2) summarize the mechanisms of action
by which bioactive peptides affect cancer cells. In addition, this
review highlights the potential applications of natural animal
source-derived peptides as pharmaceutical candidates in the
auxiliary therapy of cancer.
Animal sources of bioactive peptides with
anticancer activity
Terrestrial mammals and
by-products
Although bioactive peptides with anticancer activity
from terrestrial mammals are not well documented, one report has
described four bovine meat-derived peptides that inhibit
angiotensin-converting enzyme (ACE) and also exhibit
anti-proliferative activity (13).
Specifically, this study has demonstrated the cytotoxic effect of
four peptides: GFHI, DFHING, FHG, and GLSDGEWQ. GFHI has been found
to exhibit the most potent cytotoxic effect on the human breast
cancer cell line (MCF-7) and to decrease the viability of a stomach
adenocarcinoma cell line (AGS) in a dose-dependent manner, whereas
GLSDGEWQ significantly inhibits the proliferation of AGS cells.
The group of Su identified the novel anticancer
bioactive peptide-3 (ACPB-3) (14,15),
which was isolated from goat spleens or livers. This peptide has
been found to exhibit anticancer activity against a human gastric
cancer cell line (BGC-823) and gastric cancer stem cells (GCSCs)
in vitro and in vivo. Moreover, it significantly
inhibits the growth of BGC-823 and CD44+ cells in a
dose-dependent manner, suppressing the proliferation of spheroid
cell colonies and inhibiting their clone-forming capacity. In
vivo, ACBP-3 alone or in combination with cisplatin suppresses
xenograft tumor growth, and this peptide enhances the chemotherapy
tolerance of mice by reducing the toxicity of the treatment during
long-term experiments (15,16). The Su group also investigated the
anticancer activity of ACBPs in a human colorectal tumor cell line
(HCT116) in vitro and in vivo (17). Specifically, treatment with ACBPs
significantly inhibits HCT116 cell growth, enhances UV-induced
apoptosis, increases the expression levels of PARP and p53, and
decreases the expression of Mcl-1. Moreover, ACBPs markedly inhibit
human colorectal tumor growth in a xenograft nude mouse model and
induce changes in the expression levels of PARP, P53, and Mcl-1,
consistently with the changes observed in vitro, without
producing apparent changes in body weight. These studies indicate
that ACBPs inhibit human colorectal tumor cell growth and induce
apoptosis by modulating the PARP-p53-Mcl-1 signaling pathway.
Milk and dairy products contain numerous components
that exhibit a wide variety of physiological and functional
activities. Moreover, bioactive peptides have been considered to be
the important bioactive components of milk and dairy products, and
they have been identified within the amino acid sequences of milk
proteins. The intrinsic bioactivities of peptides encrypted in
major milk proteins are latent until they are released and
activated in three ways: 1) digestive enzyme-mediated hydrolysis,
2) hydrolysis by proteins from proteolytic microorganisms, and 3)
digestion by proteolytic enzymes derived from microorganisms or
plants (18).
A number of studies have reported the anticancer
effects of milk protein-derived peptides on various cancer cells.
Roy et al (19) found that
bovine skim milk digested with cell-free extract from the yeast
Saccharomyces cerevisiae inhibits the proliferation of a
human leukemia cell line (HL-60). Meisel and FitzGerald reported
the anticancer activity of casein fraction-derived
caseinophosphopeptides (CPPs) (20), which inhibit cancer cell growth and
stimulate the activity of immunocompetent cells and neonatal
intestinal cells. Moreover, the bacterial hydrolysis of casein by
commercial yogurt starter cultures yields bioactive peptides that
influence colon cell kinetics in vitro (21), and a yogurt fraction obtained by
membrane dialysis has been found to have an anti-proliferative
effect on Coca-2 and IEC-6 mammalian intestinal cells (22).
Lactoferrin is an 80-kDa iron-binding glycoprotein
that belongs to the transferrin family and has a variety of
biological functions, including antibacterial, antiviral,
anticancer, anti-inflammatory, and immunomodulatory activities
(23). Moreover, lactoferricin is a
cationic peptide generated by the acid-pepsin hydrolysis of
lactoferrin and exhibits a range of biological activities,
including cytotoxic activity against various microorganisms
(24,25) and cancer cells (26–28).
The major anticancer mechanisms of lactoferrin and lactoferricin
include cell cycle arrest, apoptosis, anti-angiogenesis effects,
anti-metastasis effects, immune modulation and necrosis (28). Thus, the aforementioned studies
suggest that milk proteins are not only a nutritious part of a
normal daily diet but also have potential for the prevention and/or
management of cancer.
Marine animals
Bioactive peptide compounds from marine animals have
been reported to have a broad range of bioactive properties
(29–31). An increasing number of recent
studies have focused on bioactive peptides with potential
anticancer activity isolated from various marine animals, such as
sponges, tunicates, ascidians, mollusks, fish, and other marine
organisms (32–35).
Fish
Fish is a popular seafood item worldwide and have
been identified as a source of bioactive peptides with potential
anticancer activities. Selected fish-derived bioactive peptides
with potential anticancer activity are listed in Table I.
 | Table I.Fish sources of selected bioactive
peptides with potential anticancer activity. |
Table I.
Fish sources of selected bioactive
peptides with potential anticancer activity.
| Fish | Peptide name | Anticancer
activity | References |
|---|
| Anchovy |
| Induce apoptosis in
a U937 cells | (37,38) |
| Blue whiting |
| Antiproliferative
activity on MCF-7/6 and MDA-MB-231 | (39) |
| Cod | TFD100 | Inhibited adhesion
of PC3 to endothelial cells, angiogenesis, and gal3-induced T-cell
apoptosis | (33) |
| Cod |
| Antiproliferative
activity on MCF-7/6 and MDA-MB-231 | (39) |
| Grouper | Epinecidin-1 | Inhibited the
proliferation of U937 cells | (42) |
| Plaice |
| Antiproliferative
activity on MCF-7/6 and MDA-MB-231 | (39) |
| Red sea bream | Chrysophsin-1 | Antitumor
activities and modulates the inflammatory response in RAW264.7
cells | (43) |
| Red sea
flatfish | Pardaxin | Antitumor activity
toward MN-11 cells in vitro and in vivo | (44) |
| Salmon |
| Antiproliferative
activity on MCF-7/6 and MDA-MB-231 | (39) |
| Tilapia | TH1-5, TH2-2, and
TH2-3 | TH1-5 and TH2-3
exhibited anticancer activity against HeLa cells and HT-1080 cells,
respectively | (40,41) |
| Tuna |
| Antiproliferative
on MCF-7 cells | (36) |
The potential anti-proliferative activity of
hydrolysate of a by-product from dark tuna muscle has been examined
in MCF-7 cells (36). Specifically,
peptide fractions with molecular weights ranging from 400–1400 Da
exhibit the strongest anti-proliferative activity. Two
anti-proliferative peptides, LPHVLTPEAGAT from papain hydrolysate
and PTAEGVYMVT from Protease XXIII, have been identified in those
fractions. Guha and others (33)
have reported a 100-kDa Thomsen-Friedenreich disaccharide
(TFD)-containing glycopeptide, named TFD-100, purified from the
Pacific cod. TFD-100 binds to galactin-3 (β-galactoside-binding
lectin) and inhibits the adhesion of androgen-independent prostate
cancer cells (PC3) to endothelial cells, angiogenesis, and
gal3-induced T-cell apoptosis.
Lee and colleagues (37,38)
have reported that peptides isolated from anchovy sauce induce
apoptosis in a human lymphoma cell line (U937) by increasing the
activities of caspase-3 and caspase-8 activity, and the authors
have purified a 440.9-Da hydrophobic peptide. Moreover, the
anti-proliferative activities of protein hydrolysates from
different fish species, including blue whiting, cod, plaice and
salmon, have been investigated in vitro in two human breast
cancer cell lines (MCF-7/6 and MDA-MB-231) (39). Hepcidin consists of three
hepcidin-like antimicrobial peptides (named TH1-5, TH2-2, and
TH2-3) and has been isolated from tilapia. Of these peptides, TH1-5
and TH2-3 exhibit anticancer activity against epithelial carcinoma
cells (HeLa) and human fibrosarcoma cells (HT-1080), respectively
(40,41).
In addition, several studies have shown that
peptides from different fish sources exert clear anticancer
activity against various carcinoma cell lines, such as human
hepatocellular liver carcinoma cells (HepG2), U937 cells, HeLa
cells and murine fibrosarcoma cells (MN-11) (42–44).
These data suggest the potential of fish as a valuable source of
anticancer peptides for incorporation into functional foods.
Shrimp
Wilson-Sanchez et al (45) have demonstrated the anti-mutagenic
and anti-proliferative activities of lipidic extracts from white
shrimp. Specifically, the lipid fraction of white shrimp contains
compounds that have been found to reduce the mutagenicity of
aflatoxin B1 and the proliferation of a B-cell lymphoma cell line.
Moreover, shrimp anti-lipopolysaccharide factor (SALF), an
antimicrobial peptide from black tiger shrimp (46,47),
enhances the anticancer activity of cisplatin in vitro and
inhibits HeLa cell growth in nude mice. These peptides also exhibit
significant anticancer activity in human colon and liver cancer
cell lines, even when they are isolated from shrimp waste (48).
Ascidians
Bioactive peptides with anticancer activity have
also been identified in tunicates and ascidians. Didemnins are a
family of cytotoxic peptides isolated from tunicates (49), and acyclic depsipeptide, Didemnin B,
has been widely studied because of its high anticancer potential
(50,51). Didemnin B inhibits proliferation by
inhibiting the synthesis of RNA, DNA and protein (51,52).
Aplidine, a cyclic depsipeptide isolated from the Mediterranean
tunicate Aplidium albicans, has anti-angiogenic activity
both in vitro and in vivo (53), and aplidine was first identified on
the basis of its enhanced cytotoxicity against different tumor cell
lines, such as breast, melanoma, lung and ovarian cancer cell
lines, and its lower myelotoxicity relative to Didemnin B (53–57).
Tamandarins A and B are also cytotoxic depsipeptides from a marine
ascidian of the family Didemnidae, and the effects of these
peptides have been evaluated in various human cancer cell lines
(58,59). Mollamide and Trunkamide A obtained
from ascidians both are cytotoxic to different human tumor cell
lines (58).
A novel polypeptide (CS5931) with anticancer
activity has been identified by the group of Lin (60,61),
who have demonstrated that CS5931 extracted from Ciona
savignyi induces apoptosis via a mitochondria-mediated pathway
in human colorectal carcinoma cells (HCT-8) in a dose- and
time-dependent manner. CS5931 is strongly anti-angiogenic in
vitro and in vivo, and this effect may be mediated by
vascular endothelial growth factor (VEGF) and matrix
metalloproteinases (MMPs). These studies indicate that CS5931 has
the potential to be developed as a novel angiogenesis inhibitor for
the treatment of cancer.
Sponges
Marine sponges are an abundant source of bioactive
peptides with anticancer potential. In recent years, most
researchers have focused on bioactive cyclic peptides and
depsipeptides with highly unique structures that contain a wide
variety of unusual amino acids and other building blocks (62–64).
Seven cytotoxic cyclic peptides (Callyaerins A-F and
H) isolated from the Indonesian sponge (Callyspongia
aerizusa) are cytotoxic to murine lymphoma cells (L5178Y), HeLa
cells, and pheochromocytoma tumor cells (PC12) (63). Furthermore, reniochalistatins A-E,
five cyclic peptides (including four heptapeptides and one
octapeptide) from the marine sponge Reniochalina
stalagmitis, and the cyclic octapeptide reniochalistatin E are
cytotoxic to different tumor cell lines (65). Rolloamides A and B are cytotoxic
cyclic heptapeptides isolated from the Caribbean sponge (Eurypon
laughlini), androlloamide A has been found to significantly
suppress the growth of a panel of histologically diverse cancer
cells (66). A new peptide,
gombamide A, isolated from the Korean sponge Clathria
gombawuiensis is weakly cytotoxic to human lung carcinoma
(A549) and myelogenous leukemia (K562) cell lines and moderately
inhibits Na+/K+-ATPase.
Jaspamide is a cyclic depsipeptide isolated from
sponges of the genus Jaspis and Hemiastrella
(32) and induces apoptosis in
HL-60 (67,68) and Jurkat T cells (69). Two jaspamide derivatives, jaspamide
2 and 3, have been isolated from an Indonesian sponge (Jaspis
splendens), and low concentrations of these peptides inhibit
the growth of L5178Y cells in vitro (70). Nine cyclodepsipeptides from the
sponge Homophymia sp., homophymines B-E and A1-E1, have
exhibited very potent cytotoxic activity with IC50
values in the nM range against a panel of human cancer cell lines
(71). Recently, two cyclic
depsipeptides isolated from a Madagascan sponge (Homophymia
lamellosa), pipecolidepsins A and B (64), have been found to exhibit cytotoxic
activity against human lung, colon, and breast cancer cells
(64,72).
Geodiamolide H, a depsipeptide isolated from a
Brazilian sponge (Geodia corticostylifera), inhibits the
migration and invasion of breast cancer cells by modifying the
actin cytoskeleton (73).
Three lipodepsipeptides (Lipodiscamides A-C)
isolated from the marine sponge Discodermia kiiensis are
moderately cytotoxic to murine leukemia cells (P-388) and HeLa
cells (74). Moreover, taumycin A,
another lipodepsipeptide from a Madagascan sponge
(Fascaplysinopsis sp.), has been found to inhibit the growth
of a human leukemic cell line (75).
Callyptide A, a newly identified cytotoxic peptide
from the Red Sea marine sponge (Callyspongia), has been
found to inhibit the growth of different cancer cell lines,
including MDA-MB-231 cells, A549 cells and human colorectal
adenocarcinoma cells (HT-29), with GI50 values of 29,
18.5 and 30 µM, respectively (31).
Smenamides A and B are two isomerichybrid peptide/polyketide
compounds isolated from a Caribbean sponge (Smenospongia
aurea) that contain a dolapyrrolidinone unit and show potent
cytotoxic activity at nanomolar levels against lung cancer Calu-1
cells (76).
Mollusks
Several studies have reported that mollusks, such as
shellfish, sea slugs, and sea hares, are rich sources of bioactive
peptides that exhibit anticancer activity. Wang et al have
isolated oligopeptide-enriched hydrolysates from oysters by using
protease (77) and have shown that
these hydrolysates markedly and dose-dependently inhibits
sarcoma-S180 tumor cell growth in BALB/c mice. Furthermore, Cheong
et al (78) and Kim et
al (79) have reported two
novel anticancer peptides isolated from oysters and mussels,
respectively. The sequences of these two anticancer peptides
differ, but both exhibit clearly superior cytotoxic activity and
effectively induce cell death in prostate, breast and lung cancer
cells.
Keyhole limpet hemocyanin (KLH) is a
high-molecular-weight copper-containing protein found in the
hemolymph of the marine mollusk Megathura crenulata (80). This extracellular respiratory
protein has many bioactive properties (81–83),
including immunostimulatory, antitumor, and antimicrobial activity.
Riggs et al and McFadden et al (84,85)
have shown that KLH from the giant keyhole limpet significantly
inhibits the growth of different cancer cells in vitro,
including estrogen-dependent breast cancer cells (MCF-7),
estrogen-independent breast cancer cells (ZR75-1), pancreatic
cancer cells (PANC-1), prostate cancer cells (DU145), and Barrett's
esophageal adenocarcinoma cells (SEG-1 and BIC-1). Moreover, a
cytokine analysis has revealed that KLH directly affects the
production of cellular inflammatory and pro-apoptotic mediators.
Furthermore, KLH increases early and late apoptotic activity in
MCF-7 cells, whereas it reduces late apoptotic activity in the
ZR75-1 cells. In contrast, KLH does not affect the early or late
apoptotic activity of PANC-1 cells. These results suggest that KLH
directly inhibits the growth of human breast and pancreatic cancer
in vitro by modulating apoptotic and non-apoptotic
mechanisms (86).
Dolastatins are a family of cytotoxic peptides
isolated from the mollusk Dolabella auricularia. In this
family, the linear pentapeptide Dolastatin 10 and the depsipeptide
Dolastatin 15 have been reported to exhibit promising
anti-proliferative activity (87,88).
In recent years, synthetic dolastatin 10 analogs have been widely
used in anticarcinogen drug development (89–91).
These studies have provided strong evidence showing that Dolastatin
10 analogs effectively inhibit cell growth by dampening microtubule
dynamics, inducing apoptotic cell death, and inhibiting tumor
growth.
Aurilide is a small cyclodepsipeptide isolated from
Dolabella auricularia that induces apoptosis in human cancer
cells at low concentrations (92).
Specifically, aurilide selectively binds to prohibitin 1 (PHB1) in
the mitochondria, activating the proteolytic processing of
dynamin-like GTPase optic atrophy 1 (OPA1) and resulting in
mitochondria-induced apoptosis. The mechanism of aurilide
cytotoxicity suggests that PHB1 is an apoptosis-regulating protein
amenable to modulation by small molecules. Thus, aurilide may serve
as a small-molecule tool for studies of mitochondria-induced
apoptosis (93,94).
Kahalalides are a family of peptides isolated from
Elysia rufescens. Among them, Kahalalide F is regarded as an
important anticancer candidate for tumor therapeutics, owing to its
high cytotoxicity (95–97). However, the mechanism of action of
Kahalalide F is not well understood, and Kahalalide F has been
observed to disturb lysosomal function and to potentially result in
intracellular acidification and cell death. Thus, this peptide may
effectively combat cancer cells exhibiting high lysosomal activity,
such as prostate and cervical cancer cells (98). Moreover, Janmaat and others have
demonstrated that ErbB3 and the downstream phosphatidylinositol
3-kinase (PI3K)-Akt signaling pathway are important determinants of
the cytotoxic activity of Kahalalide F in vitro (99).
Finally, Keenamide A is a cytotoxic cyclic
hexapeptide isolated from the notaspidean mollusk Pleurobranchus
forskalii that significantly inhibits the proliferation of
P-388, A-549, and HT-29 cells (100).
Amphibians
Frog and toad skin secretions
Skin secretions from amphibians (e.g., frogs and
toads) contain a wide range of compounds with biological activity
and have garnered attention because of their potential for drug
development (101). In addition,
the Chinese have traditionally administered secretions from frog
skin and toad parotid glands for medicinal purposes since ancient
times (102). Hundreds of such
peptides have been identified since the discovery of the first
antimicrobial peptide from this source (103), and some naturally occurring
amphibian skin peptides and analogs are selectively cytotoxic to
tumor cells and are promising anticancer agents (101). The primary structures of selected
bioactive peptides with anticancer properties isolated from frog
skin secretions are listed in Table
II.
 | Table II.Primary structures of selected
bioactive peptides with anticancer properties from frog skin
secretions. |
Table II.
Primary structures of selected
bioactive peptides with anticancer properties from frog skin
secretions.
| Species | Family | Peptide name | Primary
structure | Activity | References |
|---|
| Midwife toad
(Alytes obstetricans) | Alytidae | Alyteserin-2 |
ILGKLLSTAAGLLSNL | Cytotoxicity on
A549 cells | (105) |
| Tailed frog
(Ascaphus truei) | Ascaphidae | Ascaphin-8 |
GFKDLLKGAAKALVKTVLF | Cytotoxicity on
HepG2 cells | (106) |
| Green and golden
bell frog (Litoria aureus) | Hylidae | Aurein 1.2 | GLFDIIKKIAESF | Anticancer
activity | (107) |
| Green and golden
bell frog (Litoria aureus) | Hylidae | Aurein 3.1 |
GLFDIVKKIAGHIAGSI | Anticancer
activity | (107) |
| Giant monkey frog
(Phyllomedusa bicolor) | Hylidae | Dermaseptin B2 |
GLWSKIKEVGKEAAKAAAKAAGKAALGAVSEAV | Inhibited the
proliferation of PC-3 cells | (108) |
| Giant monkey frog
(Phyllomedusa bicolor) | Hylidae | Dermaseptin B3 |
ALWKNMLKGIGKLAGQAALGAVKTLVGAE | Inhibited the
proliferation of PC-3 cells | (108) |
| Phyllomedusine leaf
frog (Pachymedusa dacnicolor) | Hylidae |
Dermaseptin-PD-1 | GMWSKIKETAMAAAK
EAAKAAGKTISDMIKQ | Inhibited growth of
PC-3 cells, H157 cells, U251MG cells | (109) |
| Phyllomedusine leaf
frog (Pachymedusa dacnicolor) | Hylidae |
Dermaseptin-PD-2 |
GMWSKIKNAGKAAAKAAAKAAGKAALDAVSEAI | Inhibited growth of
PC-3 cells, H157 cells, U251MG cells | (109) |
| Lemur leaf frog
(Agalychnis lemur) | Hylidae | Dermaseptin L1 |
GLWSKIKEAAKAAGKAALNAVTGLVNQGDQPS | Cytotoxic activity
against HepG2 cells | (110) |
| Lemur leaf frog
(Agalychnis lemur) | Hylidae | Phylloseptin
L1 |
LLGMIPLAISAISALSKL | Cytotoxic activity
against HepG2 cells | (110) |
| Peruvian
purple-sided leaf frog (Phyllomedusa baltea) | Hylidae |
Phylloseptin-PBa |
MAFLKKSLFLVLF(F/L)GLVSLSIC | Anti-proliferative
activity against H460 cells, PC3 cells and tU251MG cells | (111) |
| Pepper frog
(Leptodactylus labyrinthicus) |
Leptodactylidae | Pentadactylin |
GLLDTLKGAAKNVVGSLASKVMEKL | Cytotoxic activity
on B16F10 cells without high specificity | (118) |
| Congo dwarf clawed
frog (Hymenochirus boettgeri) | Pipidae |
Hymenochirin-1B |
KLSPETKDNLKKVLKGAIKGAIVAKMV | Cytotoxic activity
against A549 cells, MDA-MB-231 cells, HT-29 cells, and HepG2
cells | (119) |
| South African
clawed frog (Xenopus laevis) | Pipidae | Magainin-2 |
GIGKFLHSAKKFGKAFVGEIMNS | Tumoricidal
activity against human small cell lung cancer cell lines and
bladder cancer cell lines | (112,113) |
| South African
clawed frog (Xenopus laevis) | Pipidae | XLAsp-P1 | DEDDD | Inhibition activity
against breast cancer cell | (117) |
| Tropical clawed
frog (Silurana tropicalis) | Pipidae | Peptide XT-7 |
GLLGPLLKIAAKVGSNLL | Cytotoxicity on
HepG2 cells | (106) |
| Chiricahua leopard
frog (Lithobates chiricahuensis) | Ranidae |
Esculentin-2CHa |
GFSSIFRGVAKFASKGLGKDLAKLGVDLVACKISKQC | Cytotoxic activity
against A549 cells | (120) |
Alyteserin-2a, obtained from the midwife toad
(Alytes obstetricans), exhibits relatively weak
antimicrobial and cytotoxic activities (104). However, analogs of alyteserin-2a
are potently cytotoxic to A549 cells, human hepatocarcinoma cells
(HepG2), MDA-MB-231 cells, and HT-29 cells (105).
Conlon et al (106) have reported two bioactive
peptides, ascaphin-8 and peptide XT-7, isolated from the skin
secretions of Ascaphus truei and Silurana tropicalis.
These peptides are highly cytotoxic to HepG2 cells. Moreover, the
analogs of these peptides are more cytotoxic to HepG2 cells than
the natural bioactive peptides.
Several aurein peptides exhibiting anticancer
activity have been reported by Rozek et al (107), who extracted Aureins 1, 2 and 3.1
from the green and golden bell frog (Litoria aureus) and the
southern bell frog (Litoria raniformis).
van Zoggel et al (108) have reported that two bioactive
peptides of the dermaseptin family (dermaseptin B2 and B3) isolated
from skin secretions of the South American tree frog
(Phyllomedusa bicolor) exhibit antitumor and angiostatic
properties. Specifically, the authors demonstrated that these two
peptides inhibit both the proliferation of a human prostatic
adenocarcinoma cell line (PC-3) by >90% in vitro and the
differentiation of bovine aortic endothelial cells. Most recently,
Shi et al (109) have
identified two novel members of the dermaseptin antimicrobial
peptide family, dermaseptin-PD-1 and dermaseptin-PD-2, in the
skins/skin secretions of the phyllomedusine leaf frog
(Pachymedusa dacnicolor). These two peptides have been found
to modulate the growth of PC-3 cells, a human non-small cell lung
cancer cell line (H157), and a human neuronal glioblastoma cell
line (U251MG) with low hemolytic activity. Moreover, both
dermaseptins are less cytotoxic to normal human cell lines
(109). Dermaseptin L1 and
phylloseptin L1, isolated from the skin secretions of the lemur
leaf frog (Agalychnis lemur), are both cytotoxic to HepG2
cells (110). Dermaseptin L1 is
cytolytic to HepG2 cells but not human erythrocytes, whereas
phylloseptin L1 is approximately equipotent against both HepG2
cells and erythrocytes. In addition, the novel phylloseptin-PBa,
isolated from the skin secretion of the purple-sided leaf frog
(Phyllomedusa baltea), has been found to inhibit the
proliferation of several human cancer cell lines: lung cancer cells
(H460), PC-3 cells and a neurospongioma cell line (U251MG).
However, it is less active in a normal human micro-vessel
endothelial cell line (HMEC-1) (111).
Magainin-2, isolated from Xenopus laevis, and
its analog magainin G, exhibits tumoricidal activity against human
small cell lung cancer cell lines (112) and bladder cancer cell lines
(113). Another modified
magainin-2 peptide, MSI-238, is markedly more potent than the
parent peptide, displaying a significant cytotoxic effect on A549
cells in vitro and P-388 cells, ascites (S180), and a
spontaneous ovarian tumor in vivo (114). In addition, several studies have
reported other magainin-2 analogs that are cytotoxic to U937
(115) and HeLa cells (116). Li et al (117) have isolated a small antibacterial
peptide, Xenopus laevis antibacterial peptide-P1 (XLAsp-P1),
from the skin of Xenopus laevis by using reverse-phase
high-performance liquid chromatography, and this peptide strongly
and dose-dependently inhibits breast cancer cells.
Moreover, pentadactylin from Leptodactylus
labyrinthicus reduces the viability of murine melanoma (B16F10)
cells in a dose-dependent manner without significantly affecting
normal human fibroblast cells (118). Specifically, pentadactylin alters
cell morphology, disrupts the membrane, fragments DNA, arrests
cells in the S phase of the cell cycle, and alters mitochondrial
membrane potential, thus suggesting that this peptide affects
B16F10 cells via an apoptosis pathway.
Attoub and colleagues have extracted the
frog-derived peptide Hymenochirin-1B, which is highly cytotoxic to
A549 cells, MDA-MB-231 cells, HT-29 cells, and HepG2 cells
(119). Moreover, the (D9K) analog
is most potent against all four cell lines (up to 6-fold increase
in cytotoxicity), but its hemolytic activity is also increased. In
contrast, the (D9k) and (E6k, D9k) analogs retain relatively high
cytotoxicity against tumor cells but are less hemolytic than the
parent peptide (119). Moreover,
the same group has identified another frog-derived peptide,
Esculentin-2Cha, which is highly cytotoxic to A549 cells (120). In this study, the authors found
that two analogs both remain cytotoxic to A549 cells but have
completely contrary effects on hemolytic activity.
Crocodile and turtle
Crocodilians are minimally affected by infections or
death from microorganisms, and cancer has not been observed in
crocodiles to date, thus suggesting that these animals have a
strong innate immune system that protects against undesirable
cells. These characteristics make crocodilians a good choice for
the study of anticancer agents. Previous studies have indicated
that alligator serum, including leukocyte extract, has a broad
spectrum of activity against bacteria, viruses and amoeba via the
complement system (121–124). Pata et al have reported
four novel antibacterial peptides isolated from the white blood
cell extract of the Siamese crocodile, Leucrocin I–IV, which
exhibit strong antibacterial activity against Staphylococcus
epidermidis, Salmonella typhi and Vibrio cholera
(125). On the basis of this work,
Yaraksa et al designed and synthesized the novel
antibacterial peptides L-and D-NY15 by using the peptide Leucrocin
I as a sequence template, and these peptides exhibit potent
antibacterial activity without any toxicity to mammalian cells at
their bacteriolytic concentrations (126).
Additionally, Patathananone and colleagues (127) have investigated the anticancer
activity of crocodile leukocyte extracts. Specifically, they have
shown that the percentage of viable HeLa cells significantly
decreases in a dose- and time-dependent manner after treatment with
white blood cell extracts. They have further demonstrated that the
anticancer compounds from crocodile leukocyte extracts induces
apoptosis in HeLa cells via both caspase-dependent and
caspase-independent pathways (127).
Recently, Theansungnoen et al (128) have indicated that the cationic
antimicrobial peptides KT2, RT2 and RP9 from Crocodylus
siamensis leukocyte extract exhibit anticancer activities
against human cervical cancer cells but do not affect non-cancer
cells.
He et al (129) have reported antitumor peptides
derived from the enzymatic hydrolysates of the Chinese
three-striped box turtle (Cuora trifasciata). Two fractions,
T1 and T2, inhibit HepG2 and MCF-7 cancer cells, and three peptides
have been identified in these fractions: RGVKGPR (T1-1), KLGPKGPR
(T1-2), and SSPGPPVH (T2-1). T2-1 was found to be a novel peptide
that had not been listed in any database and exhibits the most
potent inhibition toward MCF-7 cancer cells.
Animal venoms
Animal venoms and toxins consist of a complex
cocktail of proteins and peptides and are enriched in approximately
100–1,000 biologically active peptides. Thus, they have been used
as a therapeutic resource in folk and traditional medicine for
centuries, and they remain largely unexplored resource for the
discovery of novel bioactive peptides.
Scorpion venom
Among venomous animals, scorpions, the oldest
arthropods on Earth, possess a venom apparatus connected to the
telson, which is used to inject the venom. Scorpions can be
phylogenetically divided into 18 distinct families consisting of
>1,500 species (130), and
scorpion venom has been used in traditional medicine for many
centuries (131). However,
possibly <1% of all venoms from known scorpion species have been
studied in detail (132).
Scorpion venom is a source of peptidyl neurotoxins,
which are used as tools to study different ion channels, such as
the Na+, K+, Ca+, and
Cl− ion channels. Chlorotoxin (CTX) is a small
neurotoxin of 36 amino acids that was isolated in 1993 from the
venom of the Israeli scorpion Leiurus quinquestriatus
(133). Initially, CTX was used as
a pharmacological tool to characterize chloride channels. However,
CTX cannot kill cancer cells on its own, despite its ability to
inhibit tumor invasion. CTX can target cancer cells, including
glioma, melanoma, small cell lung carcinoma, neuroblastoma and
medulloblastoma cells. These properties make CTX a very attractive
peptide for targeted cancer therapy or imaging (134). Moreover, CTX has been demonstrated
to deeply diffuse into tumors, unlike other targeting agents, such
as antibodies (135,136). Therefore, CTX should limit changes
in cell shape in the setting of glioma, thereby hampering the
ability of the tumor to invade tissue. This mechanism corroborates
the reported anti-invasive effects of CTX on glioma cells and the
inhibition of metastasis (137–140). Recently, Guo et al
identified two linear α-helical peptides in the venom of the
Brazilian yellow scorpion, TsAP-1 and TsAP-2 (Tityus
serrulatus antimicrobial peptide) and demonstrated their
anti-proliferative effects on human cancer cells, namely a human
squamous carcinoma cell line (NCI-H157) and a human lung
adenocarcinoma cell line (NCI-H838). Moreover, TsAP-2 is three
times more active than TsAP-1 against an androgen-independent
prostate adenocarcinoma cell line (PC-3), MCF-7 cells, and a human
glioblastoma cell line (U251) (141). Ali et al have isolated a
new chlorotoxin-like peptide (Bs-Tx7) from the venom of the common
yellow scorpion (Buthus sindicus). This peptide inhibits
thechlorotoxin (ClTx) and CFTR channels (GaTx1) by 66% and 82%,
respectively, and an amino acid sequence analysis of Bs-Tx7 has
identified a scissile peptide bond (i.e., Gly-Ile) for human MMP2,
whose activity is increased in malignant tumors. This finding
suggests that Bs-Tx7 inhibits tumor proliferation by decreasing
MMP2 activity (142).
Spider venom
Spider venom contains versatile proteins and
peptides including enzymes (such as proteases, hyaluronidases, and
phospholipases), neurotoxins (most have disulfide-rich peptides
affecting ion channels), and cytolytic peptides (143). Latarcin 2a (Ltc2a), a short
cationic linear α-helical peptide isolated from the venom of a
spider (Lachesana tarabaevi), is cytotoxic against human
erythroleukemia K562 cells. This cytotoxicity is primarily related
to plasma membrane destabilization; Ltc2a induces the formation of
small (approximately 2.0 nm) membrane pores on the plasma membrane
of K562 cells and subsequent blebbing, swelling and eventual cell
death (144). Spider venom-derived
peptide lycosin-1 strongly inhibits cancer cell growth in
vitro and effectively suppresses tumor growth in vivo by
interfering with cell signaling pathways via the attenuation of the
activities of key proteins (145).
Bee and wasp venom
Venom from bees and wasps is now being studied to
design and develop new therapeutic drugs from the proteins and
peptides in venom (146). Melittin
(MEL), an amphiphilic peptide (26 amino acid residues) isolated
from the honey bee Apis mellifera, is the most studied and
well-known bee venom-derived peptide (147). MEL inhibits different cancer cells
in vitro, including astrocytoma, leukemic, lung tumor,
ovarian carcinoma, squamous carcinoma, glioma, hepatocellular
carcinoma, osteosarcoma, prostate cancer and renal cancer cells
(148–152). Although it is cytotoxic to a broad
spectrum of tumor cells, this peptide is also toxic to normal
cells. Thus, MEL must be accurately delivered to a targeted area to
optimize results (153,154).
Similarly to MEL, mastoparan is a well-studied
14-amino acid amphipathic and cationic peptide obtained from
Vespula lewisii venom that has shown antitumor activity in
vitro (146). It also needs to
be precisely delivered to avoid side effects, as described by
Yamada and colleagues (155).
Moreover, several structural modifications may improve the
pharmacodynamic parameters of chimeric mitoparan in vivo
(146,156).
Snake venom
The therapeutic use of snake venoms is frequently
studied by scientists. Most venoms are a complex mixture of several
proteins, peptides, enzymes, toxins and non-protein components.
Bioactive peptides from snake venoms have significantly contributed
to the treatment of many medical conditions, and some peptides and
enzymes from snake venom may specifically target cancer cell
membranes, affecting the migration and proliferation of these cells
(157,158).
Crotamine, a polypeptide of 42 amino acids first
isolated from South American rattle snake venom, was the first
venom-derived peptide classified as a natural cell-penetrating and
antimicrobial peptide with pronounced antifungal activity (158). Pereira et al have
investigated the toxicity of this peptide toward cancer cells in
vitro and in vivo in a mouse model of melanoma; they
have tested the viability of B16-F10 (murine melanoma cells),
SK-Mel-28 (human melanoma cells), and Mia PaCa-2 (human pancreatic
carcinoma cells) at crotamine concentrations of 1–5 µg/ml.
Noteworthy, a final crotamine concentration of 5 µg/ml is lethal to
B16-F10, MiaPaCa-2, and SK-Mel-28 cells but not to normal cells
(159,160).
Cathelicidin-BF (BF-30) is a cathelicidin-like
polypeptide consisting of 30 amino acids and a natural
antibacterial peptide extracted from the venom of the snake
Bungarus fasciatus. BF-30 inhibits B16F10 cell proliferation
in vitro in a dose- and time-dependent manner. Moreover,
BF-30 significantly suppresses melanoma growth in B16F10
tumor-bearing mice without inducing losses in body weight (161). Naumann et al have isolated
and purified L-amino acid oxidases (LAAOs) from Bothrops
leucurus (Bl-LAAO) and have reported the biochemical features
of Bl-LAAO associated with its effect on platelet function and
cytotoxicity. Bl-LAAO is cytotoxic to the stomach cancer cell line
MKN-45, the adenocarcinoma cell line HUTU, the colorectal cancer
cell line RKO and the human fibroblast cell line LL-24.
Specifically, this enzyme releases sufficient amounts of
H2O2 into the culture medium to induce
apoptosis in cells in a dose- and time-dependent manner (162).
Mechanisms of action of bioactive peptides
underlying their anticancer effects
Since anticancer peptides non-specifically destroy
the plasma membrane, they show therapeutic potential for tumors
that are not responsive to conventional pharmaceutical therapy.
Although some major mechanisms of action have already been
outlined, the exact mechanism by which bioactive peptides kill
cancerous cells remains controversial. In general, the anticancer
effect of bioactive peptides may be mediated either by
membranolytic or by non-membranolytic mechanisms (163)
Membrane-related mechanisms
The plasma membrane of cells is a very effective
selectively permeable barrier. Although this phospholipid bilayer
is essential for cell survival and function, many studies have
indicated that natural antimicrobial peptides kill cancer cells by
disrupting the cellular membrane (128,164,165). Specifically, peptides target
negatively charged membrane components in the membrane, such as
phosphatidylserine (PS), sialic acid or heparan sulfate. In fact,
the exposure of the negatively charged lipid PS on the outer
leaflet of the cancer cell membrane is a key difference between
cancerous and non-cancerous cells, which are overall neutrally
charged, owing to zwitterionic phosphatidylcholine and
sphingomyelin (166,167).
Papo et al have found that a short host
defense-like peptide selectively targets cancer cells, primarily by
binding to PS exposed on the surfaces of cells, thus resulting in
cytoplasmic membrane depolarization and cell death. Consequently,
peptide-lipid interaction is a critical step for the effective
disruption of the cell membrane (168). Latarcins 2a (Ltc2a), a peptide
extracted from the venom of the spider Lachesana tarabaevi,
is cytotoxic to human erythroleukemia K562 cells. Specifically, the
peptide affects the plasma membrane of cells and induces membrane
blebbing, swelling and eventual cell death, as observed with
fluorescently labeled Ltc2a. Moreover, the peptide binds to the
outer membrane leaflet of K562 cells, consequently triggering PS
externalization. Cytotoxicity is due to the formation of membrane
pores (approximately 3.7 nm), which are more permeable to anionic
than cationic molecules, and the redistribution of PS toward the
outer leaflet of the membrane has been detected in the cells. Of
note, the peptide does not activate apoptosis (144). Pore formation is accompanied by
self-assisted Ltc2a internalization and accumulation in
mitochondria, mitochondrial inactivation and apoptosis-independent
phosphatidylserine externalization (169).
The mechanism underlying the membranolytic activity
of each peptide depends on the characteristics of the bioactive
peptide and those of the target membrane, which in turn modulate
peptide selectivity and toxicity. Bioactive peptide-induced
membrane disruption can occur via different modes: pore formation
in the lipid (barrel-stave and toroidal pore models), the thinning
of the membrane bilayer, membrane dissolution (carpet model), or
lipid-peptide domain formation (166,170).
The barrel-stave model describes the lateral
insertion and diffusion of peptides through the lipid bilayer,
where they arrange into helices and create barrel/stave-like
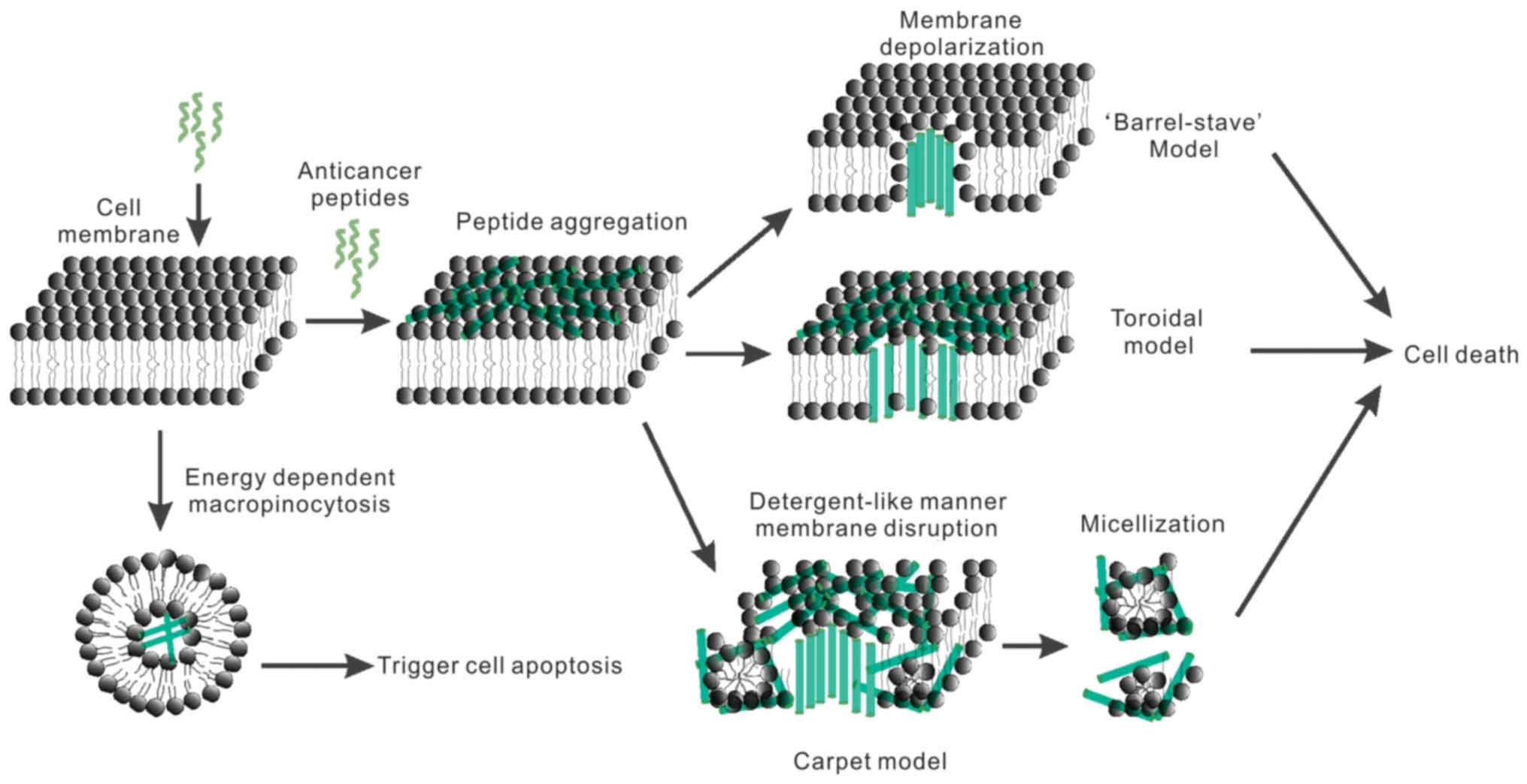
channels that span the membrane (171). As shown in Fig. 2, cecropins (from moths) (172), pardaxin (from the Red Sea sole)
(173), magainins (from frogs)
(174) and melittin (from the
European honey bee) (175,176) induce cell lysis via pore formation
and follow this model.
According to the toroidal model, peptide molecules
maintain a predominantly parallel orientation to the membrane, and
a water core forms the center of the pore, with the bioactive
peptides and lipid head groups forming the wall of the pore
(177). As shown in Fig. 2, Magainins (from frogs) (174), melittin (from bee venom) (175,176), and protegrins (from porcine
leukocytes) (178) all follow this
mode of action.
Another classical mechanism of action is described
by the carpet model. In this model, peptides do not form pores but
bind parallel to the membrane surface, forming a ‘carpet’ in
association with other peptide monomers. The bilayers are disrupted
and form micelles, destroying the membrane structure in a
detergent-like manner at a certain peptide concentration showed in
Fig. 2 (179,180).
Since peptides and lipids are highly dynamic,
Bechinger (181) has proposed the
‘Soft Membranes Adapt and Respond, also Transiently’ (SMART) model,
which describes the interaction between peptides and the membrane
from a global dynamic viewpoint: peptides and lipids change and
mutually adapt their conformations, and membrane penetration and
morphology are described in detail on a local and a global level.
As a result, peptides and lipids can form a wide variety of
supramolecular assemblies. In contrast, charged amphipathic
sequences tend to remain intercalated at the membrane interface,
where they cause pronounced disruptions of phospholipid fatty acyl
packing. With increasing local or global concentrations, the
peptides result in transient membrane opening, rupture and
ultimately lysis. Therefore, the same peptide sequence can result
in a variety of these responses, depending on the peptide-to-lipid
ratio, lipid composition and environmental factors (temperature,
buffer composition and ionic strength).
Mitochondrial membrane disruption and
mitochondrial-dependent apoptosis
In addition to inducing cell death by disrupting
the plasma membrane, some anticancer peptides induce apoptosis via
the mitochondrial pathway (182),
and mitochondrial membrane disruption-induced apoptosis plays a
crucial role in both carcinogenesis and cancer therapy as showed in
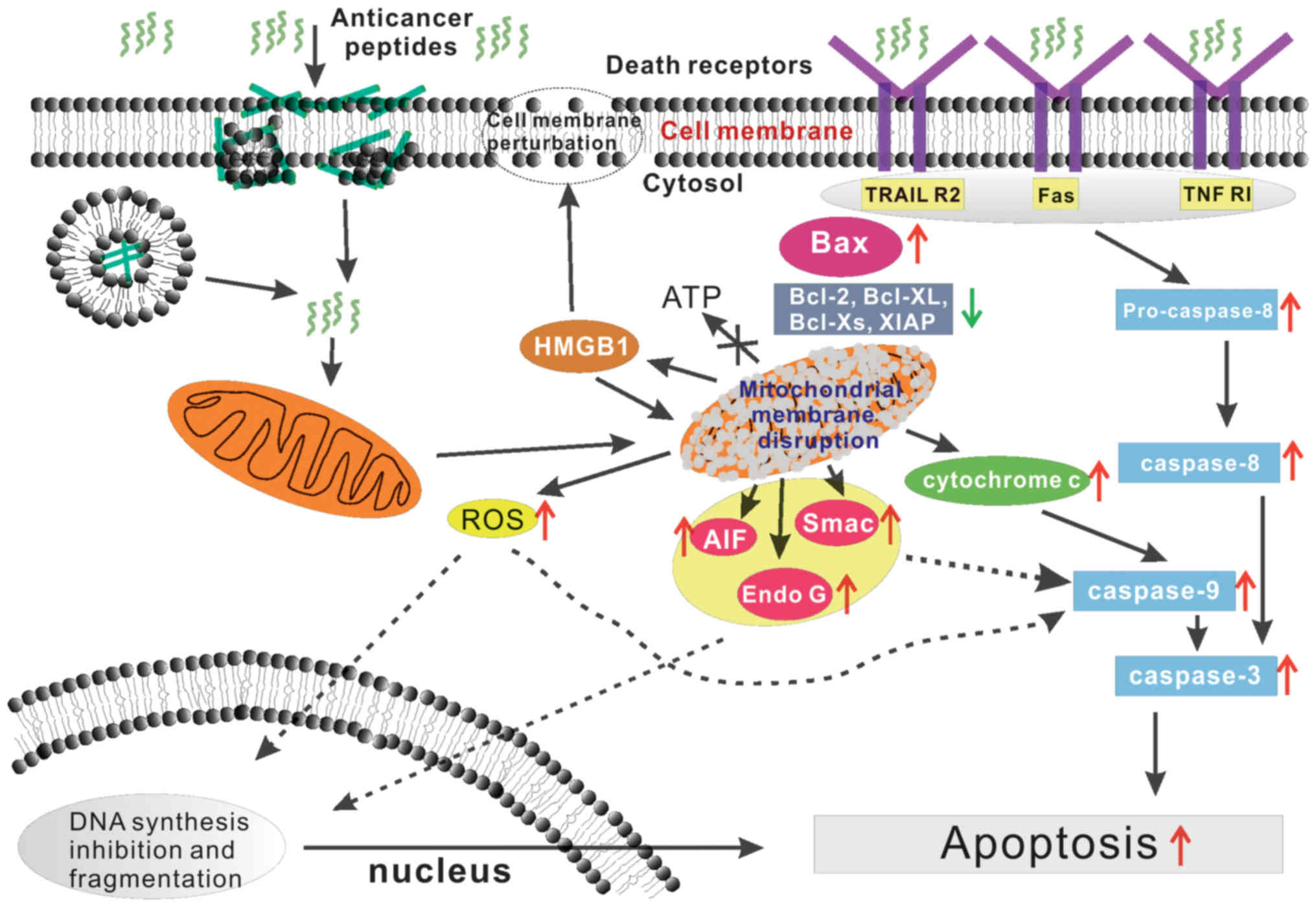
Fig. 3 (183). Hence, understanding this pathway
is very important for bioactive peptide applications. The early
opening of the mitochondrial permeability transition pore (mPTP) in
the inner mitochondrial membrane (IMM) is a key event in primary
necrosis. These events interrupt ATP synthesis and result in the
influx of large amounts of water and small solutes to the matrix
along their electrochemical gradients, which results in severe
osmotic swelling of mitochondria and ultimately in necrotic cell
death. Furthermore, mitochondrial outer membrane permeabilization
(MOMP), allows the release of pro-apoptotic factors, including
cytochrome c (Cyt c), which activate caspases, apoptosis-inducing
factor (AIF), second mitochondria-derived activator of caspase
(Smac) and endonucleases showed in Fig.
3 (184–186).
Penaeidin-2 (Pen-2) is an important antimicrobial
peptide derived from the Pacific white shrimp, and recombinant
pen-2 (rPen-2) has been found to strongly inhibit the growth of
ACHN and A498 kidney cancer cells in a time- and dose-dependent
manner. This effect is less pronounced in renal tubular epithelial
HK-2 cells. Two different phenomena, apoptosis and lysis, have been
observed, thus suggesting that rPen-2 caused membrane disruption
and apoptosis of tumor cells (187). The antimicrobial peptides NRC-03
and NRC-07 from the Atlantic flounder target and damage
mitochondria and consequently induce a loss of transmembrane
potential in breast cancer cells. These peptides also induce the
production of reactive oxygen species (ROS) and cell death via
mitochondrial-dependent apoptosis or the inhibition of DNA
synthesis showed in Fig. 3
(188,189).
Recently, Patathananone et al have reported
that leukocyte extract from the crocodile (C. siamensis) is
cytotoxic to human cervical cancer cells in a protein
concentration-dependent manner. Specifically, the mitochondrial
membrane potential (DWm) of HeLa cells rapidly decreases,
indicating the formation of open mitochondrial pores, which
increase the levels of the pro-apoptotic protein Bax and reduce the
levels of the anti-apoptotic proteins Bcl-2, Bcl-XL, Bcl-Xs, and
XIAP. Simultaneously, the open mitochondrial pore leads to the
release of cytochrome c and the activation of caspase-9 and
caspase-3. Mitochondrial membrane disruption also results in the
release of the apoptosis-inducing factor endonuclease G (Endo G)
via induction of the caspase-independent apoptotic pathway by
mitochondria. Noteworthy, Endo G has not been found to translocate
into the nuclei. Overall, these results suggest that anticancer
agents in leukocyte extract induce apoptosis in HeLa cells via both
caspase-dependent and caspase-independent pathways (127). Furthermore, Theansungnoen and
colleagues have shown that the peptides KT2 and RT2, derived from
crocodile leukocyte extract, act as death ligands and could
upregulate death receptors including TRAIL R2, Fas and TNF RI.
Fas-associated death domain is activated by peptide-receptor
binding, and pro-caspase-8 is subsequently cleaved, thus generating
caspase-8; high expression levels of pro-caspase-3 in HeLa cells
and activation of the caspase-8 and caspase-3 apoptosis pathway
have also been observed (Fig. 3)
(128).
Aurilide, isolated from the Japanese sea hare,
selectively binds to prohibitin 1 (PHB1) in mitochondria. PHB1
localizes in the inner membrane of mitochondria and may activate
the proteolytic processing of optic atrophy1 (OPA1) and result in
mitochondria-induced apoptosis. In detail, aurilide induces
prolonged mitochondrial fragmentation by enhancing OPA1 processing,
which results in a loss of membrane potential and induces apoptosis
(93).
The nonamer peptide LTX-315, derived from bovine
lactoferricin, exhibits oncolytic properties. Eike et al
have further investigated the oncolytic activity of LTX-315 in
human melanoma cells (A375) and have shown that LTX-315 treatment
depolarizes the mitochondrial membrane and significantly alters
mitochondrial morphology at the ultrastructural level.
Simultaneously, death-associated molecular patterns (DAMPs), such
as cytochrome-c, ATP, and HMGB1, are released and consequently
damage cellular integrity in several ways. Specifically, the
release of DAMPs perturb both the cell membrane and mitochondria as
shown in Fig. 3 (190). Burns et al have reported a
pH-selective peptide (KL AKLAK)2 analog that inhibits
breast cancer cell growth in a dose- and pH-dependent manner. In
addition, they have identified pHLIP-KLAKLAK as a better modifier
because of its low cytotoxicity at physiological pH levels,
chemical stability, high anti-proliferation potency and specific
induction of apoptosis at lower pH levels via mitochondrial
membrane disruption (191).
The group of Su has reported that ACBP extracted
from goat spleens induces apoptosis and blocks the cell cycle by
decreasing the gene expression levels of cyclin D1, c-myc, and
bcl-2 as well as the protein expression of PCNA. It also increases
p16Ink4, p21Waf1, p27Kip1 and bax expression (14). Furthermore, in vitro and
in vivo findings suggest that PARP, p53, and Mcl-1 mediate
ACBP-induced apoptosis. These studies suggest that ACBPs inhibit
human colorectal tumor cell growth and induce apoptosis by
modulating the PARP-p53-Mcl-1 signaling pathway. Further studies
are needed to elucidate the role of mitochondrial membrane
disruption in this apoptosis cascade (17).
Summary and perspective
The use of anticancer peptides has become more
prevalent for the clinical treatment of cancer. However, in
addition to their many advantages these peptides also have
drawbacks, such as their lack of oral bioavailability and low
stability under physiological conditions; gastric acids and complex
enzymes in the gastrointestinal environment make anticancer
peptides vulnerable to degradation (192,193). Strategies to develop a selective
delivery system have been described (194,195), and these strategies result in
highly efficacious treatment. Some cancer-targeting peptides have
been designed on the basis of the pH difference between tumor
tissue and normal tissues (196);
the peptide selectively kills tumor cells at acidic pH levels but
is nontoxic against normal cells. Furthermore, owing to their
unique optical, electronic, magnetic, photoresponsive, and
structural properties, nanotechnology and nanomaterials have
provided tremendous potential for application of anticancer
peptides in tumor-targeted therapy, bio-imaging, and diagnosis
(197,198). Moreover, a new methodology based
on dynamic multiple complex views is needed to study the mechanism
of action of anticancer bioactive peptides (181). Anticancer peptide-related
pharmaceutical research and development are likely to garner
significant attention and investment over the next several decades,
to integrate their characteristics and fully exploit their
potential to benefit thousands of patients who are suffering from
cancer.
Glossary
Abbreviations
Abbreviations:
|
ACE
|
angiotensin-converting enzyme
|
|
ACPB-3
|
anticancer bioactive peptide-3
|
|
SALF
|
shrimp anti-lipopolysaccharide
factor
|
|
KLH
|
keyhole limpet hemocyanin
|
|
CTX
|
chlorotoxin
|
|
LAAOs
|
L-amino acid oxidases
|
|
Bl-LAAO
|
Bothrops leucurus
|
|
Ltc2a
|
latarcins 2a
|
|
Pen-2
|
penaeidin-2
|
|
mPTP
|
mitochondrial permeability transition
pore
|
|
MOMP
|
mitochondrial outer membrane
permeabilization
|
|
OPA1
|
optic atrophy1
|
|
PHB1
|
prohibitin 1
|
|
DAMPs
|
death-associated molecular
patterns
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGuire S: World Cancer Report 2014.
Geneva, Switzerland: World Health Organization, International
Agency for Research on Cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peer D, Karp JM, Hong S, Farokhzad OC,
Margalit R and Langer R: Nanocarriers as an emerging platform for
cancer therapy. Nat Nanotechnol. 2:751–760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amit D and Hochberg A: Development of
targeted therapy for bladder cancer mediated by a double promoter
plasmid expressing diphtheria toxin under the control of H19 and
IGF2-P4 regulatory sequences. J Transl Med. 8:1342010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang TH, Mao CP, He L, Tsai YC, Liu K, La
V, Wu TC and Hung CF: Tumor-targeted delivery of IL-2 by NKG2D
leads to accumulation of antigen-specific CD8+ T cells
in the tumor loci and enhanced anti-tumor effects. PLoS One.
7:e351412012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blaurock N, Schmerler D, Hünniger K,
Kurzai O, Ludewig K, Baier M, Brunkhorst FM, Imhof D and Kiehntopf
M: C-terminal alpha-1 antitrypsin peptide: A new sepsis biomarker
with immunomodulatory function. Mediators Inflamm.
2016:61294372016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Porta A, Petrone AM, Morello S, Granata I,
Rizzo F, Memoli D, Weisz A and Maresca B: Design and expression of
peptides with antimicrobial activity against Salmonella
typhimurium. Cell Microbiol. 19:e126452017.doi: 10.1111/cmi.12645.
View Article : Google Scholar
|
|
8
|
Dabarera MC, Athiththan LV and Perera RP:
Antihypertensive peptides from curd. Ayu. 36:214–219. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shiratsuchi E, Ura M, Nakaba M, Maeda I
and Okamoto K: Elastin peptides prepared from piscine and mammalian
elastic tissues inhibit collagen-induced platelet aggregation and
stimulate migration and proliferation of human skin fibroblasts. J
Pept Sci. 16:652–658. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qin Y, Zhou J, Zhang W, Yang X, Wang J,
Wei C, Gu F and Lei T: Construction of an anticancer fusion peptide
(ACFP) derived from milk proteins and an assay of anti-ovarian
cancer cells in vitro. Anticancer Agents Med Chem. Jun
26–2016.(Epub ahead of print).
|
|
11
|
Kongcharoen A, Poolex W, Wichai T and
Boonsombat R: Production of an antioxidative peptide from hairy
basil seed waste by a recombinant Escherichia coli.
Biotechnol Lett. 38:1195–1201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iwaniak A, Darewicz M, Minkiewicz P,
Protasiewicz M and Borawska J: (Biologically active peptides
derived from food proteins as the food components with
cardioprotective properties). Pol Merkur Lekarski. 36:403–406.
2014.(In Polish). PubMed/NCBI
|
|
13
|
Jang A, Jo C, Kang K-S and Lee M:
Antimicrobial and human cancer cell cytotoxic effect of synthetic
angiotensin-converting enzyme (ACE) inhibitory peptides. Food Chem.
107:327–336. 2008. View Article : Google Scholar
|
|
14
|
Su L, Xu G, Shen J, Tuo Y, Zhang X, Jia S,
Chen Z and Su X: Anticancer bioactive peptide suppresses human
gastric cancer growth through modulation of apoptosis and the cell
cycle. Oncol Rep. 23:3–9. 2010.PubMed/NCBI
|
|
15
|
Yu L, Yang L, An W and Su X: Anticancer
bioactive peptide-3 inhibits human gastric cancer growth by
suppressing gastric cancer stem cells. J Cell Biochem. 115:697–711.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su X, Dong C, Zhang J, Su L, Wang X, Cui H
and Chen Z: Combination therapy of anti-cancer bioactive peptide
with Cisplatin decreases chemotherapy dosing and toxicity to
improve the quality of life in xenograft nude mice bearing human
gastric cancer. Cell Biosci. 4:72014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su LY, Shi YX, Yan MR, Xi Y and Su XL:
Anticancer bioactive peptides suppress human colorectal tumor cell
growth and induce apoptosis via modulating the PARP-p53-Mcl-1
signaling pathway. Acta Pharmacol Sin. 36:1514–1519. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park YW and Nam MS: Bioactive peptides in
milk and dairy products: A review. Korean J Food Sci Anim Resour.
35:831–840. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roy MK, Watanabe Y and Tamai Y: Induction
of apoptosis in HL-60 cells by skimmed milk digested with a
proteolytic enzyme from the yeast Saccharomyces cerevisiae.
J Biosci Bioeng. 88:426–432. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meisel H and FitzGerald RJ: Biofunctional
peptides from milk proteins: Mineral binding and cytomodulatory
effects. Curr Pharm Des. 9:1289–1295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
MacDonald RS, Thornton WH Jr and Marshall
RT: A cell culture model to identify biologically active peptides
generated by bacterial hydrolysis of casein. J Dairy Sci.
77:1167–1175. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ganjam LS, Thornton WH Jr, Marshall RT and
MacDonald RS: Antiproliferative effects of yogurt fractions
obtained by membrane dialysis on cultured mammalian intestinal
cells. J Dairy Sci. 80:2325–2329. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Legrand D, Pierce A, Elass E, Carpentier
M, Mariller C and Mazurier J: Lactoferrin structure and functions.
Adv Exp Med Biol. 606:163–194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sánchez-Gómez S, Ferrer-Espada R, Stewart
PS, Pitts B, Lohner K and de Martínez Tejada G: Antimicrobial
activity of synthetic cationic peptides and lipopeptides derived
from human lactoferricin against Pseudomonas aeruginosa
planktonic cultures and biofilms. BMC Microbiol. 15:1372015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin C, Wong JH and Ng TB: Recent studies
on the antimicrobial peptides lactoferricin and lactoferrampin.
Curr Mol Med. 14:1139–1154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mader JS, Salsman J, Conrad DM and Hoskin
DW: Bovine lactoferricin selectively induces apoptosis in human
leukemia and carcinoma cell lines. Mol Cancer Ther. 4:612–624.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eliassen LT, Berge G, Leknessund A, Wikman
M, Lindin I, Løkke C, Ponthan F, Johnsen JI, Sveinbjørnsson B,
Kogner P, et al: The antimicrobial peptide, lactoferricin B, is
cytotoxic to neuroblastoma cells in vitro and inhibits xenograft
growth in vivo. Int J Cancer. 119:493–500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin CM, Wong JH, Xia J and Ng TB: Studies
on anticancer activities of lactoferrin and lactoferricin. Curr
Protein Pept Sci. 14:492–503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Harnedy PA and FitzGerald RJ: Bioactive
peptides from marine processing waste and shellfish: A review. J
Funct Foods. 4:6–24. 2012. View Article : Google Scholar
|
|
30
|
Zhou QJ, Wang J, Liu M, Qiao Y, Hong WS,
Su YQ, Han KH, Ke QZ and Zheng WQ: Identification, expression and
antibacterial activities of an antimicrobial peptide NK-lysin from
a marine fish Larimichthys crocea. Fish Shellfish Immunol.
55:195–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shaala LA, Youssef DT, Ibrahim SR and
Mohamed GA: Callyptide A, a new cytotoxic peptide from the Red Sea
marine sponge Callyspongia species. Nat Prod Res. Mar
7–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suarez-Jimenez GM, Burgos-Hernandez A and
Ezquerra-Brauer JM: Bioactive peptides and depsipeptides with
anticancer potential: Sources from marine animals. Mar Drugs.
10:963–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guha P, Kaptan E, Bandyopadhyaya G,
Kaczanowska S, Davila E, Thompson K, Martin SS, Kalvakolanu DV,
Vasta GR and Ahmed H: Cod glycopeptide with picomolar affinity to
galectin-3 suppresses T-cell apoptosis and prostate cancer
metastasis. Proc Natl Acad Sci USA. 110:5052–5057. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jumeri and Kim SM: Antioxidant and
anticancer activities of enzymatic hydrolysates of solitary
tunicate (Styela clava). Food Sci Biotechnol. 20:10752011.
View Article : Google Scholar
|
|
35
|
Kurt O, Ozdal-Kurt F, Tuğlu MI and Akçora
CM: The cytotoxic, neurotoxic, apoptotic and antiproliferative
activities of extracts of some marine algae on the MCF-7 cell line.
Biotech Histochem. 89:568–576. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hsu K-C, Li-Chan ECY and Jao C-L:
Antiproliferative activity of peptides prepared from enzymatic
hydrolysates of tuna dark muscle on human breast cancer cell line
MCF-7. Food Chem. 126:617–622. 2011. View Article : Google Scholar
|
|
37
|
Lee YG, Kim JY, Lee KW, Kim KH and Lee HJ:
Peptides from anchovy sauce induce apoptosis in a human lymphoma
cell (U937) through the increase of caspase-3 and −8 activities.
Ann NY Acad Sci. 1010:399–404. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee YG, Lee KW, Kim JY, Kim KH and Lee HJ:
Induction of apoptosis in a human lymphoma cell line by hydrophobic
peptide fraction separated from anchovy sauce. Biofactors.
21:63–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Picot L, Bordenave S, Didelot S,
Fruitier-Arnaudin I, Sannier F, Thorkelsson G, Bergé JP, Guérard F,
Chabeaud A and Piot JM: Antiproliferative activity of fish protein
hydrolysates on human breast cancer cell lines. Process Biochem.
41:1217–1222. 2006. View Article : Google Scholar
|
|
40
|
Chen JY, Lin WJ and Lin TL: A fish
antimicrobial peptide, tilapia hepcidin TH2-3, shows potent
antitumor activity against human fibrosarcoma cells. Peptides.
30:1636–1642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chang WT, Pan CY, Rajanbabu V, Cheng CW
and Chen JY: Tilapia (Oreochromis mossambicus) antimicrobial
peptide, hepcidin 1–5, shows antitumor activity in cancer cells.
Peptides. 32:342–352. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen JY, Lin WJ, Wu JL, Her GM and Hui CF:
Epinecidin-1 peptide induces apoptosis which enhances antitumor
effects in human leukemia U937 cells. Peptides. 30:2365–2373. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hsu JC, Lin LC, Tzen JT and Chen JY:
Characteristics of the antitumor activities in tumor cells and
modulation of the inflammatory response in RAW264.7 cells of a
novel antimicrobial peptide, chrysophsin-1, from the red sea bream
(Chrysophrys major). Peptides. 32:900–910. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu SP, Huang TC, Lin CC, Hui CF, Lin CH
and Chen JY: Pardaxin, a fish antimicrobial peptide, exhibits
antitumor activity toward murine fibrosarcoma in vitro and in vivo.
Mar Drugs. 10:1852–1872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wilson-Sanchez G, Moreno-Félix C,
Velazquez C, Plascencia-Jatomea M, Acosta A, Machi-Lara L,
Aldana-Madrid ML, Ezquerra-Brauer JM, Robles-Zepeda R and
Burgos-Hernandez A: Antimutagenicity and antiproliferative studies
of lipidic extracts from white shrimp (Litopenaeus
vannamei). Mar Drugs. 8:2795–2809. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lin MC, Lin SB, Chen JC, Hui CF and Chen
JY: Shrimp anti-lipopolysaccharide factor peptide enhances the
antitumor activity of cisplatin in vitro and inhibits HeLa cells
growth in nude mice. Peptides. 31:1019–1025. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Somboonwiwat K, Marcos M, Tassanakajon A,
Klinbunga S, Aumelas A, Romestand B, Gueguen Y, Boze H, Moulin G
and Bachère E: Recombinant expression and anti-microbial activity
of anti-lipopolysaccharide factor (ALF) from the black tiger shrimp
Penaeus monodon. Dev Comp Immunol. 29:841–851. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kannan A, Hettiarachchy NS, Marshall M,
Raghavan S and Kristinsson H: Shrimp shell peptide hydrolysates
inhibit human cancer cell proliferation. J Sci Food Agric.
91:1920–1924. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Aneiros A and Garateix A: Bioactive
peptides from marine sources: Pharmacological properties and
isolation procedures. J Chromatogr B Analyt Technol Biomed Life
Sci. 803:41–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Baker MA, Grubb DR and Lawen A: Didemnin B
induces apoptosis in proliferating but not resting peripheral blood
mononuclear cells. Apoptosis. 7:407–412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ahuja D, Vera MD, SirDeshpande BV,
Morimoto H, Williams PG, Joullié MM and Toogood PL: Inhibition of
protein synthesis by didemnin B: How EF-1alpha mediates inhibition
of translocation. Biochemistry. 39:4339–4346. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Vera MD and Joullié MM: Natural products
as probes of cell biology: 20 years of didemnin research. Med Res
Rev. 22:102–145. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Taraboletti G, Poli M, Dossi R, Manenti L,
Borsotti P, Faircloth GT, Broggini M, D'Incalci M, Ribatti D and
Giavazzi R: Antiangiogenic activity of aplidine, a new agent of
marine origin. Br J Cancer. 90:2418–2424. 2004.PubMed/NCBI
|
|
54
|
Andavan GS and Lemmens-Gruber R:
Cyclodepsipeptides from marine sponges: Natural agents for drug
research. Mar Drugs. 8:810–834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Faivre S, Chièze S, Delbaldo C, Ady-Vago
N, Guzman C, Lopez-Lazaro L, Lozahic S, Jimeno J, Pico F, Armand
JP, et al: Phase I and pharmacokinetic study of aplidine, a new
marine cyclodepsipeptide in patients with advanced malignancies. J
Clin Oncol. 23:7871–7880. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Geldof AA, Mastbergen SC, Henrar RE and
Faircloth GT: Cytotoxicity and neurocytotoxicity of new marine
anticancer agents evaluated using in vitro assays. Cancer Chemother
Pharmacol. 44:312–318. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Albella B, Faircloth G, López-Lázaro L,
Guzmán C, Jimeno J and Bueren JA: In vitro toxicity of ET-743 and
aplidine, two marine-derived antineoplastics, on human bone marrow
haematopoietic progenitors. comparison with the clinical results.
Eur J Cancer. 38:1395–1404. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hamada Y and Shioiri T: Recent progress of
the synthetic studies of biologically active marine cyclic peptides
and depsipeptides. Chem Rev. 105:4441–4482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Vervoort H, Fenical W and Epifanio RA:
Tamandarins A and B: New cytotoxic depsipeptides from a Brazilian
ascidian of the family Didemnidae. J Org Chem. 65:782–792. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cheng L, Wang C, Liu H, Wang F, Zheng L,
Zhao J, Chu E and Lin X: A novel polypeptide extracted from
Ciona savignyi induces apoptosis through a
mitochondrial-mediated pathway in human colorectal carcinoma cells.
Clin Colorectal Cancer. 11:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu G, Liu M, Wei J, Huang H, Zhang Y,
Zhao J, Xiao L, Wu N, Zheng L and Lin X: CS5931, a novel
polypeptide in Ciona savignyi, represses angiogenesis via
inhibiting vascular endothelial growth factor (VEGF) and matrix
metalloproteinases (MMPs). Mar Drugs. 12:1530–1544. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Blunt JW, Copp BR, Hu WP, Munro MH,
Northcote PT and Prinsep MR: Marine natural products. Nat Prod Rep.
26:170–244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ibrahim SR, Min CC, Teuscher F, Ebel R,
Kakoschke C, Lin W, Wray V, Edrada-Ebel R and Proksch P:
Callyaerins A-F and H, new cytotoxic cyclic peptides from the
Indonesian marine sponge Callyspongia aerizusa. Bioorg Med
Chem. 18:4947–4956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Coello L, Reyes F, Martín MJ, Cuevas C and
Fernández R: Isolation and structures of pipecolidepsins A and B,
cytotoxic cyclic depsipeptides from the Madagascan sponge
Homophymia lamellosa. J Nat Prod. 77:298–303. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhan KX, Jiao WH, Yang F, Li J, Wang SP,
Li YS, Han BN and Lin HW: Reniochalistatins A-E, cyclic peptides
from the marine sponge Reniochalina stalagmitis. J Nat Prod.
77:2678–2684. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Williams DE, Yu K, Behrisch HW, Van Soest
R and Andersen RJ: Rolloamides A and B, cytotoxic cyclic
heptapeptides isolated from the Caribbean marine sponge Eurypon
laughlini. J Nat Prod. 72:1253–1257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nakazawa H, Kitano K, Cioca D, Ishikawa M,
Ueno M, Ishida F and Kiyosawa K: Induction of polyploidization by
jaspamide in HL-60 cells. Acta Haematol. 104:65–71. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cioca DP and Kitano K: Induction of
apoptosis and CD10/neutral endopeptidase expression by jaspamide in
HL-60 line cells. Cell Mol Life Sci. 59:1377–1387. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Odaka C, Sanders ML and Crews P:
Jasplakinolide induces apoptosis in various transformed cell lines
by a caspase-3-like protease-dependent pathway. Clin Diagn Lab
Immunol. 7:947–952. 2000.PubMed/NCBI
|
|
70
|
Ebada SS, Wray V, de Voogd NJ, Deng Z, Lin
W and Proksch P: Two new jaspamide derivatives from the marine
sponge Jaspis splendens. Mar Drugs. 7:434–444. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zampella A, Sepe V, Bellotta F, Luciano P,
D'Auria MV, Cresteil T, Debitus C, Petek S, Poupat C and Ahond A:
Homophymines B-E and A1-E1, a family of bioactive
cyclodepsipeptides from the sponge Homophymia sp. Org Biomol
Chem. 7:4037–4044. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pelay-Gimeno M, García-Ramos Y, Martin
Jesús M, Spengler J, Molina-Guijarro JM, Munt S, Francesch AM,
Cuevas C, Tulla-Puche J and Albericio F: The first total synthesis
of the cyclodepsipeptide pipecolidepsin A. Nat Commun. 4:23522013.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Freitas VM, Rangel M, Bisson LF, Jaeger RG
and Machado-Santelli GM: The geodiamolide H, derived from Brazilian
sponge Geodia corticostylifera, regulates actin
cytoskeleton, migration and invasion of breast cancer cells
cultured in three-dimensional environment. J Cell Physiol.
216:583–594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tan KC, Wakimoto T and Abe I:
Lipodiscamides A-C, new cytotoxic lipopeptides from Discodermia
kiiensis. Org Lett. 16:3256–3259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bishara A, Rudi A, Aknin M, Neumann D,
Ben-Califa N and Kashman Y: Taumycins A and B, two bioactive
lipodepsipeptides from the Madagascar sponge
Fascaplysinopsis sp. Org Lett. 10:4307–4309. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Teta R, Irollo E, Della Sala G, Pirozzi G,
Mangoni A and Costantino V: Smenamides A and B, chlorinated
peptide/polyketide hybrids containing a dolapyrrolidinone unit from
the Caribbean sponge Smenospongia aurea. Evaluation of their
role as leads in antitumor drug research. Mar Drugs. 11:4451–4463.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang YK, He HL, Wang GF, Wu H, Zhou BC,
Chen XL and Zhang YZ: Oyster (Crassostrea gigas)
hydrolysates produced on a plant scale have antitumor activity and
immunostimulating effects in BALB/c mice. Mar Drugs. 8:255–268.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Cheong SH, Kim EK, Hwang JW, Kim YS, Lee
JS, Moon SH, Jeon BT and Park PJ: Purification of a novel peptide
derived from a shellfish, Crassostrea gigas, and evaluation
of its anticancer property. J Agric Food Chem. 61:11442–11446.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kim EK, Joung HJ, Kim YS, Hwang JW, Ahn
CB, Jeon YJ, Moon SH and Park PJ: Purification of a novel
anticancer peptide from enzymatic hydrolysate of Mytilus coruscus.
J Microbiol Biotechnol. 22:1381–1387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Harris JR and Markl J: Keyhole limpet
hemocyanin: Molecular structure of a potent marine immunoactivator.
A review. Eur Urol. 37:(Suppl 3). 24–33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tzianabos AO: Polysaccharide
immunomodulators as therapeutic agents: Structural aspects and
biologic function. Clin Microbiol Rev. 13:523–533. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lamm DL, Dehaven JI and Riggs DR: Keyhole
limpet hemocyanin immunotherapy of bladder cancer: Laboratory and
clinical studies. Eur Urol. 37:(Suppl 3). 41–44. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Murai A, Kitahara K, Okumura S, Kobayashi
M and Horio F: Oral antibiotics enhance antibody responses to
keyhole limpet hemocyanin in orally but not muscularly immunized
chickens. Anim Sci J. 87:257–265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Riggs DR, Jackson B, Vona-Davis L and
McFadden D: In vitro anticancer effects of a novel immunostimulant:
Keyhole limpet hemocyanin. J Surg Res. 108:279–284. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
McFadden DW, Riggs DR, Jackson BJ and
Vona-Davis L: Keyhole limpet hemocyanin, a novel immune stimulant
with promising anticancer activity in Barrett's esophageal
adenocarcinoma. Am J Surg. 186:552–555. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Riggs DR, Jackson BJ, Vona-Davis L, Nigam
A and McFadden DW: In vitro effects of keyhole limpet hemocyanin in
breast and pancreatic cancer in regards to cell growth, cytokine
production, and apoptosis. Am J Surg. 189:680–684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Pettit GR, Srirangam JK, Barkoczy J,
Williams MD, Durkin KP, Boyd MR, Bai R, Hamel E, Schmidt JM and
Chapuis JC: Antineoplastic agents 337. Synthesis of dolastatin 10
structural modifications. Anticancer Drug Des. 10:529–544.
1995.PubMed/NCBI
|
|
88
|
Pettit GR, Flahive EJ, Boyd MR, Bai R,
Hamel E, Pettit RK and Schmidt JM: Antineoplastic agents 360.
Synthesis and cancer cell growth inhibitory studies of dolastatin
15 structural modifications. Anticancer Drug Des. 13:47–66.
1998.PubMed/NCBI
|
|
89
|
Maderna A, Doroski M, Subramanyam C, Porte
A, Leverett CA, Vetelino BC, Chen Z, Risley H, Parris K, Pandit J,
et al: Discovery of cytotoxic dolastatin 10 analogues with
N-terminal modifications. J Med Chem. 57:10527–10543. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Gajula PK, Asthana J, Panda D and
Chakraborty TK: A synthetic dolastatin 10 analogue suppresses
microtubule dynamics, inhibits cell proliferation, and induces
apoptotic cell death. J Med Chem. 56:2235–2245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Pettit GR, Hogan F and Toms S:
Antineoplastic agents. 592. Highly effective cancer cell growth
inhibitory structural modifications of dolastatin 10. J Nat Prod.
74:962–968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Suenaga K, Kajiwara S, Kuribayashi S,
Handa T and Kigoshi H: Synthesis and cytotoxicity of aurilide
analogs. Bioorg Med Chem Lett. 18:3902–3905. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sato S, Murata A, Orihara T, Shirakawa T,
Suenaga K, Kigoshi H and Uesugi M: Marine natural product aurilide
activates the OPA1-mediated apoptosis by binding to prohibitin.
Chem Biol. 18:131–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Semenzato M, Cogliati S and Scorrano L:
Prohibitin(g) cancer: Aurilide and killing by Opa1-dependent
cristae remodeling. Chem Biol. 18:8–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Shilabin AG and Hamann MT: In vitro and in
vivo evaluation of select kahalalide F analogs with antitumor and
antifungal activities. Bioorg Med Chem. 19:6628–6632. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cruz LJ, Luque-Ortega JR, Rivas L and
Albericio F: Kahalalide F, an antitumor depsipeptide in clinical
trials, and its analogues as effective antileishmanial agents. Mol
Pharm. 6:813–824. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hosta L, Pla-Roca M, Arbiol J,
López-Iglesias C, Samitier J, Cruz LJ, Kogan MJ and Albericio F:
Conjugation of Kahalalide F with gold nanoparticles to enhance in
vitro antitumoral activity. Bioconjug Chem. 20:138–146. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
García-Rocha M, Bonay P and Avila J: The
antitumoral compound Kahalalide F acts on cell lysosomes. Cancer
Lett. 99:43–50. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Janmaat ML, Rodriguez JA, Jimeno J, Kruyt
FA and Giaccone G: Kahalalide F induces necrosis-like cell death
that involves depletion of ErbB3 and inhibition of Akt signaling.
Mol Pharmacol. 68:502–510. 2005.PubMed/NCBI
|
|
100
|
Wesson KJ and Hamann MT: Keenamide A, a
bioactive cyclic peptide from the marine mollusk Pleurobranchus
forskalii. J Nat Prod. 59:629–631. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Conlon JM, Mechkarska M, Lukic ML and
Flatt PR: Potential therapeutic applications of multifunctional
host-defense peptides from frog skin as anti-cancer, anti-viral,
immunomodulatory, and anti-diabetic agents. Peptides. 57:67–77.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Angeletti LR, Agrimi U, Curia C, French D
and Mariani-Costantini R: Healing rituals and sacred serpents.
Lancet. 340:223–225. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Oelkrug C, Hartke M and Schubert A: Mode
of action of anticancer peptides (ACPs) from amphibian origin.
Anticancer Res. 35:635–643. 2015.PubMed/NCBI
|
|
104
|
Conlon JM, Demandt A, Nielsen PF, Leprince
J, Vaudry H and Woodhams DC: The alyteserins: Two families of
antimicrobial peptides from the skin secretions of the midwife toad
Alytes obstetricans (Alytidae). Peptides. 30:1069–1073.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Conlon JM, Mechkarska M, Prajeep M, Arafat
K, Zaric M, Lukic ML and Attoub S: Transformation of the naturally
occurring frog skin peptide, alyteserin-2a into a potent, non-toxic
anti-cancer agent. Amino Acids. 44:715–723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Conlon JM, Galadari S, Raza H and
Condamine E: Design of potent, non-toxic antimicrobial agents based
upon the naturally occurring frog skin peptides, ascaphin-8 and
peptide XT-7. Chem Biol Drug Des. 72:58–64. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Rozek T, Wegener KL, Bowie JH, Olver IN,
Carver JA, Wallace JC and Tyler MJ: The antibiotic and anticancer
active aurein peptides from the Australian bell frogs Litoria
aurea and Litoria raniformis the solution structure of
aurein 1.2. Eur J Biochem. 267:5330–5341. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
van Zoggel H, Hamma-Kourbali Y, Galanth C,
Ladram A, Nicolas P, Courty J, Amiche M and Delbé J: Antitumor and
angiostatic peptides from frog skin secretions. Amino Acids.
42:385–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Shi D, Hou X, Wang L, Gao Y, Wu D, Xi X,
Zhou M, Kwok HF, Duan J, Chen T, et al: Two novel dermaseptin-like
antimicrobial peptides with anticancer activities from the skin
secretion of Pachymedusa dacnicolor. Toxins (Basel).
8:82016. View Article : Google Scholar
|
|
110
|
Conlon JM, Woodhams DC, Raza H, Coquet L,
Leprince J, Jouenne T, Vaudry H and Rollins-Smith LA: Peptides with
differential cytolytic activity from skin secretions of the lemur
leaf frog Hylomantis lemur (Hylidae: Phyllomedusinae).
Toxicon. 50:498–506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wan Y, Ma C, Zhou M, Xi X, Li L, Wu D,
Wang L, Lin C, Lopez JC, Chen T, et al: Phylloseptin-PBa - A novel
broad-spectrum antimicrobial peptide from the skin secretion of the
peruvian purple-sided leaf frog (Phyllomedusa baltea) which
exhibits cancer cell cytotoxicity. Toxins (Basel). 7:5182–5193.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ohsaki Y, Gazdar AF, Chen HC and Johnson
BE: Antitumor activity of magainin analogues against human lung
cancer cell lines. Cancer Res. 52:3534–3538. 1992.PubMed/NCBI
|
|
113
|
Lehmann J, Retz M, Sidhu SS, Suttmann H,
Sell M, Paulsen F, Harder J, Unteregger G and Stöckle M: Antitumor
activity of the antimicrobial peptide magainin II against bladder
cancer cell lines. Eur Urol. 50:141–147. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Baker MA, Maloy WL, Zasloff M and Jacob
LS: Anticancer efficacy of Magainin2 and analogue peptides. Cancer
Res. 53:3052–3057. 1993.PubMed/NCBI
|
|
115
|
Koszałka P, Kamysz E, Wejda M, Kamysz W
and Bigda J: Antitumor activity of antimicrobial peptides against
U937 histiocytic cell line. Acta Biochim Pol. 58:111–117.
2011.PubMed/NCBI
|
|
116
|
Miyazaki Y, Aoki M, Yano Y and Matsuzaki
K: Interaction of antimicrobial peptide magainin 2 with
gangliosides as a target for human cell binding. Biochemistry.
51:10229–10235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Li S, Hao L, Bao W, Zhang P, Su D, Cheng
Y, Nie L, Wang G, Hou F and Yang Y: A novel short anionic
antibacterial peptide isolated from the skin of Xenopus
laevis with broad antibacterial activity and inhibitory
activity against breast cancer cell. Arch Microbiol. 198:473–482.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Libério MS, Joanitti GA, Azevedo RB, Cilli
EM, Zanotta LC, Nascimento AC, Sousa MV, Pires Júnior OR, Fontes W
and Castro MS: Anti-proliferative and cytotoxic activity of
pentadactylin isolated from Leptodactylus labyrinthicus on
melanoma cells. Amino Acids. 40:51–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Attoub S, Arafat H, Mechkarska M and
Conlon JM: Anti-tumor activities of the host-defense peptide
hymenochirin-1B. Regul Pept. 187:51–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Attoub S, Mechkarska M, Sonnevend A,
Radosavljevic G, Jovanovic I, Lukic ML and Conlon JM:
Esculentin-2CHa: A host-defense peptide with differential
cytotoxicity against bacteria, erythrocytes and tumor cells.
Peptides. 39:95–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Merchant ME, Roche C, Elsey RM and
Prudhomme J: Antibacterial properties of serum from the American
alligator (Alligator mississippiensis). Comp Biochem Physiol
B Biochem Mol Biol. 136:505–513. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Merchant ME, Pallansch M, Paulman RL,
Wells JB, Nalca A and Ptak R: Antiviral activity of serum from the
American alligator (Alligator mississippiensis). Antiviral
Res. 66:35–38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Merchant ME, Leger N, Jerkins E, Mills K,
Pallansch MB, Paulman RL and Ptak RG: Broad spectrum antimicrobial
activity of leukocyte extracts from the American alligator
(Alligator mississippiensis). Vet Immunol Immunopathol.
110:221–228. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Merchant ME, Roche CM, Thibodeaux D and
Elsey RM: Identification of alternative pathway serum complement
activity in the blood of the American alligator (Alligator
mississippiensis). Comp Biochem Physiol B Biochem Mol Biol.
141:281–288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Pata S, Yaraksa N, Daduang S, Temsiripong
Y, Svasti J, Araki T and Thammasirirak S: Characterization of the
novel antibacterial peptide Leucrocin from crocodile (Crocodylus
siamensis) white blood cell extracts. Dev Comp Immunol.
35:545–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yaraksa N, Anunthawan T, Theansungnoen T,
Daduang S, Araki T, Dhiravisit A and Thammasirirak S: Design and
synthesis of cationic antibacterial peptide based on Leucrocin I
sequence, antibacterial peptide from crocodile (Crocodylus
siamensis) white blood cell extracts. J Antibiot (Tokyo).
67:205–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Patathananone S, Thammasirirak S, Daduang
J, Chung JG, Temsiripong Y and Daduang S: Bioactive compounds from
crocodile (Crocodylus siamensis) white blood cells induced
apoptotic cell death in hela cells. Environ Toxicol. 31:986–997.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Theansungnoen T, Maijaroen S, Jangpromma
N, Yaraksa N, Daduang S, Temsiripong T, Daduang J and
Klaynongsruang S: Cationic antimicrobial peptides derived from
Crocodylus siamensis leukocyte extract, revealing anticancer
activity and apoptotic induction on human cervical cancer cells.
Protein J. 35:202–211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
He S, Mao X, Zhang T, Guo X, Ge Y, Ma C
and Zhang X: Separation and nanoencapsulation of antitumor peptides
from Chinese three-striped box turtle (Cuora trifasciata). J
Microencapsul. 33:344–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Chippaux JP and Goyffon M: Epidemiology of
scorpionism: A global appraisal. Acta Trop. 107:71–79. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Goudet C, Chi CW and Tytgat J: An overview
of toxins and genes from the venom of the Asian scorpion Buthus
martensi Karsch. Toxicon. 40:1239–1258. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Srinivasan KN, Gopalakrishnakone P, Tan
PT, Chew KC, Cheng B, Kini RM, Koh JL, Seah SH and Brusic V:
SCORPION, a molecular database of scorpion toxins. Toxicon.
40:23–31. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
DeBin JA, Maggio JE and Strichartz GR:
Purification and characterization of chlorotoxin, a chloride
channel ligand from the venom of the scorpion. Am J Physiol.
264:C361–C369. 1993.PubMed/NCBI
|
|
134
|
Dardevet L, Rani D, Aziz TA, Bazin I,
Sabatier JM, Fadl M, Brambilla E and De Waard M: Chlorotoxin: A
helpful natural scorpion peptide to diagnose glioma and fight tumor
invasion. Toxins (Basel). 7:1079–1101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Mamelak AN and Jacoby DB: Targeted
delivery of antitumoral therapy to glioma and other malignancies
with synthetic chlorotoxin (TM-601). Expert Opin Drug Deliv.
4:175–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Veiseh O, Gunn JW, Kievit FM, Sun C, Fang
C, Lee JS and Zhang M: Inhibition of tumor-cell invasion with
chlorotoxin-bound superparamagnetic nanoparticles. Small.
5:256–264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Deshane J, Garner CC and Sontheimer H:
Chlorotoxin inhibits glioma cell invasion via matrix
metalloproteinase-2. J Biol Chem. 278:4135–4144. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Soroceanu L, Manning TJ Jr and Sontheimer
H: Modulation of glioma cell migration and invasion using Cl(−) and
K(+) ion channel blockers. J Neurosci. 19:5942–5954.
1999.PubMed/NCBI
|
|
139
|
Sontheimer H: An unexpected role for ion
channels in brain tumor metastasis. Exp Biol Med (Maywood).
233:779–791. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Lui VC, Lung SS, Pu JK, Hung KN and Leung
GK: Invasion of human glioma cells is regulated by multiple
chloride channels including ClC-3. Anticancer Res. 30:4515–4524.
2010.PubMed/NCBI
|
|
141
|
Guo X, Ma C, Du Q, Wei R, Wang L, Zhou M,
Chen T and Shaw C: Two peptides, TsAP-1 and TsAP-2, from the venom
of the Brazilian yellow scorpion, Tityus serrulatus:
Evaluation of their antimicrobial and anticancer activities.
Biochimie. 95:1784–1794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ali SA, Alam M, Abbasi A, Undheim EA, Fry
BG, Kalbacher H and Voelter W: Structure-activity relationship of
chlorotoxin-like peptides. Toxins (Basel). 8:362016. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Kuhn-Nentwig L: Antimicrobial and
cytolytic peptides of venomous arthropods. Cell Mol Life Sci.
60:2651–2668. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Vorontsova OV, Egorova NS, Arseniev AS and
Feofanov AV: Haemolytic and cytotoxic action of latarcin Ltc2a.
Biochimie. 93:227–241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Liu Z, Deng M, Xiang J, Ma H, Hu W, Zhao
Y, Li DW and Liang S: A novel spider peptide toxin suppresses tumor
growth through dual signaling pathways. Curr Mol Med. 12:1350–1360.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Moreno M and Giralt E: Three valuable
peptides from bee and wasp venoms for therapeutic and
biotechnological use: Melittin, apamin and mastoparan. Toxins
(Basel). 7:1126–1150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Havas LJ: Effect of bee venom on
colchicine-induced tumours. Nature. 166:567–568. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Jo M, Park MH, Kollipara PS, An BJ, Song
HS, Han SB, Kim JH, Song MJ and Hong JT: Anti-cancer effect of bee
venom toxin and melittin in ovarian cancer cells through induction
of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol
Appl Pharmacol. 258:72–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wang C, Chen T, Zhang N, Yang M, Li B, Lü
X, Cao X and Ling C: Melittin, a major component of bee venom,
sensitizes human hepatocellular carcinoma cells to tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis
by activating CaMKII-TAK1-JNK/p38 and inhibiting IkappaBalpha
kinase-NFkappaB. J Biol Chem. 284:3804–3813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Park MH, Choi MS, Kwak DH, Oh KW, Yoon DY,
Han SB, Song HS, Song MJ and Hong JT: Anti-cancer effect of bee
venom in prostate cancer cells through activation of caspase
pathway via inactivation of NF-κB. Prostate. 71:801–812. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Park JH, Jeong YJ, Park KK, Cho HJ, Chung
IK, Min KS, Kim M, Lee KG, Yeo JH, Park KK, et al: Melittin
suppresses PMA-induced tumor cell invasion by inhibiting NF-kappaB
and AP-1-dependent MMP-9 expression. Mol Cells. 29:209–215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Gajski G and Garaj-Vrhovac V: Melittin: A
lytic peptide with anticancer properties. Environ Toxicol
Pharmacol. 36:697–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Pan H, Soman NR, Schlesinger PH, Lanza GM
and Wickline SA: Cytolytic peptide nanoparticles (‘NanoBees’) for
cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol.
3:318–327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Soman NR, Baldwin SL, Hu G, Marsh JN,
Lanza GM, Heuser JE, Arbeit JM, Wickline SA and Schlesinger PH:
Molecularly targeted nanocarriers deliver the cytolytic peptide
melittin specifically to tumor cells in mice, reducing tumor
growth. J Clin Invest. 119:2830–2842. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Yamada Y, Shinohara Y, Kakudo T, Chaki S,
Futaki S, Kamiya H and Harashima H: Mitochondrial delivery of
mastoparan with transferrin liposomes equipped with a pH-sensitive
fusogenic peptide for selective cancer therapy. Int J Pharm.
303:1–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
de Azevedo RA, Figueiredo CR, Ferreira AK,
Matsuo AL, Massaoka MH, Girola N, Auada AV, Farias CF, Pasqualoto
KF, Rodrigues CP, et al: Mastoparan induces apoptosis in
B16F10-Nex2 melanoma cells via the intrinsic mitochondrial pathway
and displays antitumor activity in vivo. Peptides. 68:113–119.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Vyas VK, Brahmbhatt K, Bhatt H and Parmar
U: Therapeutic potential of snake venom in cancer therapy: Current
perspectives. Asian Pac J Trop Biomed. 3:156–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Kerkis I, Hayashi MA, da Prieto Silva AR,
Pereira A, De Sá, Júnior PL, Zaharenko AJ, Rádis-Baptista G, Kerkis
A and Yamane T: State of the art in the studies on crotamine, a
cell penetrating peptide from South American rattlesnake. Biomed
Res Int. 2014:6759852014. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Pereira A, Kerkis A, Hayashi MA, Pereira
AS, Silva FS, Oliveira EB, da Prieto Silva AR, Yamane T,
Rádis-Baptista G and Kerkis I: Crotamine toxicity and efficacy in
mouse models of melanoma. Expert Opin Investig Drugs. 20:1189–1200.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
León G, Sánchez L, Hernández A, Villalta
M, Herrera M, Segura A, Estrada R and Gutiérrez JM: Immune response
towards snake venoms. Inflamm Allergy Drug Targets. 10:381–398.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Wang H, Ke M, Tian Y, Wang J, Li B, Wang
Y, Dou J and Zhou C: BF-30 selectively inhibits melanoma cell
proliferation via cytoplasmic membrane permeabilization and
DNA-binding in vitro and in B16F10-bearing mice. Eur J Pharmacol.
707:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Naumann GB, Silva LF, Silva L, Faria G,
Richardson M, Evangelista K, Kohlhoff M, Gontijo CM, Navdaev A, de
Rezende FF, et al: Cytotoxicity and inhibition of platelet
aggregation caused by an l-amino acid oxidase from Bothrops
leucurus venom. Biochim Biophys Acta. 1810:683–694. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Harris F, Dennison SR, Singh J and Phoenix
DA: On the selectivity and efficacy of defense peptides with
respect to cancer cells. Med Res Rev. 33:190–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Lohner K and Hilpert K: Antimicrobial
peptides: Cell membrane and microbial surface interactions. Biochim
Biophys Acta. 1858:915–917. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Bailly C: Anticancer properties of
lamellarins. Mar Drugs. 13:1105–1123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Riedl S, Leber R, Rinner B, Schaider H,
Lohner K and Zweytick D: Human lactoferricin derived di-peptides
deploying loop structures induce apoptosis specifically in cancer
cells through targeting membranous phosphatidylserine. Biochim
Biophys Acta. 1848:2918–2931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Riedl S, Rinner B, Asslaber M, Schaider H,
Walzer S, Novak A, Lohner K and Zweytick D: In search of a novel
target - phosphatidylserine exposed by non-apoptotic tumor cells
and metastases of malignancies with poor treatment efficacy.
Biochim Biophys Acta. 1808:2638–2645. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Papo N, Seger D, Makovitzki A, Kalchenko
V, Eshhar Z, Degani H and Shai Y: Inhibition of tumor growth and
elimination of multiple metastases in human prostate and breast
xenografts by systemic inoculation of a host defense-like lytic
peptide. Cancer Res. 66:5371–5378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Won A, Ruscito A and Ianoul A: Imaging the
membrane lytic activity of bioactive peptide latarcin 2a. Biochim
Biophys Acta. 1818:3072–3080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Rashid R, Veleba M and Kline KA: Focal
targeting of the bacterial envelope by antimicrobial peptides.
Front Cell Dev Biol. 4:552016. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Sorochkina AI, Kovalchuk SI, Omarova EO,
Sobko AA, Kotova EA and Antonenko YN: Peptide-induced membrane
leakage by lysine derivatives of gramicidin A in liposomes, planar
bilayers, and erythrocytes. Biochim Biophys Acta. 1828:2428–2435.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Ryan L, Lamarre B, Diu T, Ravi J, Judge
PJ, Temple A, Carr M, Cerasoli E, Su B, Jenkinson HF, et al:
Anti-antimicrobial peptides: Folding-mediated host defense
antagonists. J Biol Chem. 288:20162–20172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Han Y, Cui Z, Li YH, Hsu WH and Lee BH: In
vitro and in vivo anticancer activity of pardaxin against
proliferation and growth of oral squamous cell carcinoma. Mar
Drugs. 14:22015. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Pino-Angeles A, Leveritt JM III and
Lazaridis T: Pore structure and synergy in antimicrobial peptides
of the magainin family. PLOS Comput Biol. 12:e10045702016.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Zhang SK, Song JW, Gong F, Li SB, Chang
HY, Xie HM, Gao HW, Tan YX and Ji SP: Design of an α-helical
antimicrobial peptide with improved cell-selective and potent
anti-biofilm activity. Sci Rep. 6:273942016. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Kashiwada A, Mizuno M and Hashimoto J:
pH-Dependent membrane lysis by using melittin-inspired designed
peptides. Org Biomol Chem. 14:6281–6288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Ros U and García-Sáez AJ: More Than a
Pore: The interplay of pore-forming proteins and lipid membranes. J
Membr Biol. 248:545–561. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Bolintineanu DS, Vivcharuk V and Kaznessis
YN: Multiscale models of the antimicrobial peptide protegrin-1 on
gram-negative bacteria membranes. Int J Mol Sci. 13:11000–11011.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Lohner K: Novel antibiotics based upon the
multiple mechanisms of membrane perturbation by antimicrobial
peptides. Curr Top Med Chem. July;2016.(In press). PubMed/NCBI
|
|
180
|
Wang C, Zolotarskaya OY, Nair SS, Ehrhardt
CJ, Ohman DE, Wynne KJ and Yadavalli VK: Real-time observation of
antimicrobial polycation effects on Escherichia coli:
Adapting the carpet model for membrane disruption to quaternary
copolyoxetanes. Langmuir. 32:2975–2984. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Bechinger B: The SMART model: Soft
membranes adapt and respond, also transiently, in the presence of
antimicrobial peptides. J Pept Sci. 21:346–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Mulder KC, Lima LA, Miranda VJ, Dias SC
and Franco OL: Current scenario of peptide-based drugs: The key
roles of cationic antitumor and antiviral peptides. Front
Microbiol. 4:3212013. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Whelan RS, Konstantinidis K, Wei AC, Chen
Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, et
al: Bax regulates primary necrosis through mitochondrial dynamics.
Proc Natl Acad Sci USA. 109:6566–6571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Linkermann A, Konstantinidis K and Kitsis
RN: Catch me if you can: Targeting the mitochondrial permeability
transition pore in myocardial infarction. Cell Death Differ.
23:1–2. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Karch J, Kwong JQ, Burr AR, Sargent MA,
Elrod JW, Peixoto PM, Martinez-Caballero S, Osinska H, Cheng EH,
Robbins J, et al: Bax and Bak function as the outer membrane
component of the mitochondrial permeability pore in regulating
necrotic cell death in mice. eLife. 2:e007722013. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Farsinejad S, Gheisary Z, Samani Ebrahimi
S and Alizadeh AM: Mitochondrial targeted peptides for cancer
therapy. Tumour Biol. 36:5715–5725. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Meng MX, Ning JF, Yu JY, Chen DD, Meng XL,
Xu JP and Zhang J: Antitumor activity of recombinant antimicrobial
peptide penaeidin-2 against kidney cancer cells. J Huazhong Univ
Sci Technolog Med Sci. 34:529–534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Hilchie AL, Doucette CD, Pinto DM,
Patrzykat A, Douglas S and Hoskin DW: Pleurocidin-family cationic
antimicrobial peptides are cytolytic for breast carcinoma cells and
prevent growth of tumor xenografts. Breast Cancer Res. 13:R1022011.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Hilchie AL, Conrad DM, Coombs MR, Zemlak
T, Doucette CD, Liwski RS and Hoskin DW: Pleurocidin-family
cationic antimicrobial peptides mediate lysis of multiple myeloma
cells and impair the growth of multiple myeloma xenografts. Leuk
Lymphoma. 54:2255–2262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Eike LM, Yang N, Rekdal Ø and
Sveinbjørnsson B: The oncolytic peptide LTX-315 induces cell death
and DAMP release by mitochondria distortion in human melanoma
cells. Oncotarget. 6:34910–34923. 2015.PubMed/NCBI
|
|
191
|
Burns KE, McCleerey TP and Thévenin D:
pH-Selective Cytotoxicity of pHLIP-Antimicrobial Peptide
Conjugates. Sci Rep. 6:284652016. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Mandal SM, Pati BR, Chakraborty R and
Franco OL: New insights into the bioactivity of peptides from
probiotics. Front Biosci (Elite Ed). 8:450–459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Gaspar D, Veiga AS and Castanho MA: From
antimicrobial to anticancer peptides. A review. Front Microbiol.
4:2942013. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Liao W, Zhang R, Dong C, Yu Z and Ren J:
Novel walnut peptide-selenium hybrids with enhanced anticancer
synergism: Facile synthesis and mechanistic investigation of
anticancer activity. Int J Nanomed. 11:1305–1321. 2016.
|
|
195
|
Svensen N, Walton JG and Bradley M:
Peptides for cell-selective drug delivery. Trends Pharmacol Sci.
33:186–192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Wakabayashi N, Yano Y, Kawano K and
Matsuzaki K: A pH-dependent charge reversal peptide for cancer
targeting. Eur Biophys J. 46:121–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Hu X and Liu S: Recent advances towards
the fabrication and biomedical applications of responsive polymeric
assemblies and nanoparticle hybrid superstructures. Dalton Trans.
44:3904–3922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Balducci A, Wen Y, Zhang Y, Helfer BM,
Hitchens TK, Meng WS, Wesa AK and Janjic JM: A novel probe for the
non-invasive detection of tumor-associated inflammation.
OncoImmunology. 2:e230342013. View Article : Google Scholar : PubMed/NCBI
|