Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer worldwide which represents more than 90% of primary
liver cancers and is a major global health problem in excess of one
million cases every year (1,2).
Despite the fact that surgical operation has made great progress
during the past decades, patients with HCC still suffer a high
incidence of postoperative recurrence and metastasis. Therefore, it
is necessary to investigate the molecular pathogenesis of HCC to
develop novel treatment strategies.
Increasing evidence suggested that metastasis is
initiated by epithelial-mesenchymal transition (EMT) at the
invasive front of primary carcinoma (3,4). EMT
is recognized as an important step in invasion and metastasis which
could be induced by cytokines (5,6),
transcription factors (7,8) and other factors (9,10).
Paired-related homeobox 1 (PRRX1) was recently identified as a new
EMT inducer (11). Furthermore,
aberrant expression of PRRX1 is significantly associated with poor
prognosis in various solid tumors including breast (11), colorectal (12), gastric cancer (13) and HCC (14). High PRRX1 expression levels were
significantly associated with reduced metastasis and good prognosis
in breast cancer (11), but the
opposite relationship was observed in colorectal cancer and gastric
cancer (12,13). Downregulation of PRRX1 expression
contributed to the poor prognosis of patients with breast cancer
and HCC through acquisition of CSC-like properties (11,14).
However, the direct mechanisms through which PRRX1 regulates HCC
cells is still unclear.
The tumor suppressor p53 is one of the most
frequently mutated genes in human cancers that regulates the
expression of stress response genes and mediates a variety of
anti-proliferative processes (15,16).
Previous studies have shown that deletions or mutations of p53 are
frequently found in cancers (16,17)
and that p53 is involved in tumor metastasis as well as tumor
progression (18–20). In the present study, we investigated
the expression of PRRX1 and p53 in HCC cells and clinical samples.
We also found that aberrant expression of PRRX1 affect biological
behavior of HCC cells by regulating p53. Finally, decreased
expression of PRRX1 and p53 in HCC tissues is associated with poor
prognosis.
Materials and methods
Patients and tissue specimens
Samples for the laboratory investigations were
collected from April 2006 until February 2008. Formalin-fixed
paraffin-embedded tumor tissues and matched adjacent non-tumorous
hepatic tissues were collected from 116 HCC patients who underwent
hepatectomy as an initial treatment at Eastern Hepatobiliary
Surgery Hospital. For each patient, the diagnosis of HCC was
confirmed on the basis of postoperative pathology (Fig. 1, representative pathohistological
image). Preoperatively, no neoadjuvant radio- or chemotherapy was
applied, and no invasive interventions, such as percutaneous
ablation or chemo-embolization were performed. Each patient was
followed up until March 2015. The Hospital Research Ethics
Committee approved the research protocol. Written informed consents
and voluntary participation in the study were obtained from every
patient before the surgery. The clinical baseline characteristics
of the HCC patients are presented in Table I.
 | Table I.Relationship between PRRX1 and p53
expression and clinicopathological features (n = 116). |
Table I.
Relationship between PRRX1 and p53
expression and clinicopathological features (n = 116).
|
| PRRX1 expression |
| p53 expression |
|
|---|
|
|
|
|
|
|
|---|
| Variables | Low (n=77) | High (n=39) | P-valuea | Low (n=45) | High (n=71) | P-valuea |
|---|
| Sex |
|
| 0.553 |
|
| 0.290 |
| Male | 47 | 26 |
| 31 | 42 |
|
|
Female | 30 | 13 |
| 14 | 29 |
|
| Age (years) |
|
| 0.213 |
|
| 0.934 |
| ≤50 | 40 | 25 |
| 25 | 40 |
|
|
>50 | 37 | 14 |
| 20 | 31 |
|
| Tumor size (cm) |
|
| 0.91 |
|
| 0.582 |
| ≤5 | 58 | 29 |
| 35 | 52 |
|
|
>5 | 19 | 10 |
| 10 | 19 |
|
| Serum AFP
(ng/ml) |
|
| 0.209 |
|
| 0.582 |
| ≤20 | 45 | 18 |
| 23 | 40 |
|
|
>20 | 32 | 21 |
| 22 | 31 |
|
| HBsAg |
|
| 0.724 |
|
| 0.661 |
|
Positive | 67 | 33 |
| 38 | 62 |
|
|
Negative | 10 | 6 |
| 7 | 9 |
|
| Anti-HCV |
|
| 0.988 |
|
| 0.778 |
|
Positive | 4 | 2 |
| 2 | 4 |
|
|
Negative | 73 | 37 |
| 43 | 67 |
|
| Liver
cirrhosis |
|
| 0.507 |
|
| 0.903 |
|
Yes | 65 | 31 |
| 37 | 59 |
|
| No | 12 | 8 |
| 8 | 12 |
|
| Vascular
invasion |
|
| 0.001 |
|
| 0.001 |
|
Yes | 53 | 11 |
| 34 | 30 |
|
| No | 24 | 28 |
| 11 | 41 |
|
| Intrahepatic
metastasis |
|
| 0.002 |
|
| 0.001 |
|
Yes | 56 | 17 |
| 20 | 53 |
|
| No | 21 | 22 |
| 25 | 18 |
|
| Distant
metastasis |
|
| 0.001 |
|
| 0.004 |
|
Yes | 49 | 4 |
| 28 | 25 |
|
| No | 28 | 35 |
| 17 | 46 |
|
| TNM stage |
|
| 0.036 |
|
| 0.005 |
|
I–II | 51 | 33 |
| 22 | 62 |
|
|
III–IV | 26 | 6 |
| 23 | 9 |
|
| BCLC stage |
|
| 0.013 |
|
| 0.001 |
|
0-A | 62 | 23 |
| 41 | 44 |
|
|
B-C | 15 | 16 |
| 4 | 27 |
|
Cell culture
The normal liver cell line LO2 and human HCC cell
lines Hep3B, Huh7, HepG2, SMMC7721 (purchased from the Cell Bank of
the Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences, Shanghai, China) were cultured in Dulbecco's modified
Eagle's medium (DMEM), supplemented with 10% fetal bovine serum
(FBS; Gibco, Carlsbad, CA, USA), in humidified 5% CO2,
95% air at 37°C.
Immunohistochemistry (IHC)
The paraffin-embedded tissue specimens were cut into
4-µm serial sections and placed on polylysine-coated slides. After
deparaffinization in xylene, sections were rehydrated using a
series of graded alcohols and microwave antigen retrievals. Slides
were incubated in monoclonal antibodies against goat polyclonal
anti-PRRX1 (NBP1-06067, 1:50 dilutions; Novus Biologicals LLC,
Littleton, CO, USA), rabbit monoclonal anti-p53 (ab179477, 1:100
dilutions; Abcam, Cambridge, UK) at 4°C overnight, followed by
incubation in the corresponding secondary antibodies at 37°C for 30
min. Staining was performed with DAB and counterstaining with
Mayer's hematoxylin. Negative controls were performed by omitting
the primary antibodies.
To evaluate the expression of PRRX1 and p53, tissue
sections were examined under a microscope at a magnification of
×200. Ten fields were randomly selected to count tumor cells and to
calculate the percentage of tumor cells with a stronger PRRX1 and
p53 expression. In order to quantify the gene expression level, we
created a score based on two criteria: i) the intensity of PRRX1
and p53 staining classified according to the following scale:
negative, 0; weak, 1; and strong, 2. ii)The percentage of
immunoreactive tumor cells was calculated and classified on a
5-point scale (0, 0%, 1, 1–25%, 2, 26–50%, 3, 51–75%, and 4,
76–100%). For statistical analysis, a final score of 0–1 indicates
low gene expression; a score of 2–4 indicates high expression of
PRRX1 and p53.
Western blot analysis
Proteins from clinical specimens and HCC cell lines
were extracted with lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). Tissues and cell lysates were
subjected to 10% PAGE and transferred to nitrocellulose filter
membranes. The membranes were blocked for 1 h in 5% non-fat dry
milk diluted with TBST (10 mM Tris-HCl and 0.05% Tween-20). The
membranes were then incubated with primary antibodies at 4°C
overnight, followed by incubation with appropriate secondary
antibodies at room temperature for 2 h. The primary antibodies were
goat polyclonal anti-PRRX1 (NBP1-06067, 1:500 dilutions; Novus
Biologicals), rabbit monoclonal anti-p53 (ab179477, 1:10,000
dilutions; Abcam), mouse monoclonal anti-caspase-3 (ab2171, 1:500
dilutions; Abcam), rabbit polyclonal anti-Bax (ab7977, 1:1,000
dilutions; Abcam), mouse monoclonal anti-Bcl2 (ab117115, 1:1,000
dilutions; Abcam), and mouse monoclonal anti-GAPDH (sc-365062,
1:5,000 dilutions; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
The membranes were washed three times with phosphate-buffered
saline (PBS), and the immunoreactive bands were visualized using an
ECL Plus kit, according to the manufacturers instructions. GAPDH
was used as a gel loading control.
Small interfering RNA (siRNA) and
transient transfection
PRRX1 siRNA was purchased from Santa Cruz
Biotechnology (sc-106455). A non-functional siRNA (scrambled
sequence) was used as control. p53 siRNA was purchased from Santa
Cruz Biotechnology (sc-29435). The siRNA transfection was optimized
using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according
to the manufacturers instructions; 24–48 h after the transfection,
cells were analyzed using the assays described below.
Detection of cell migration and
invasion ability
SMMC7721 and HepG2 were cultured and transfected
with PRRX1 siRNA. The scrambled siRNA was used as control group,
the parental cells were cultured at the same time as a blank
control. Cells were added to the top chamber of Transwell plate
(3×105 cells/200 µl). Normal medium (500 µl) containing
10% FBS was added to the bottom chamber. When we detected cell
invasion ability, Matrigel was plated to the top chamber. After
culture for 48 h, cells in the top chamber were removed and stained
with 0.1% crytal violet for 15 min. Ten fields were randomly imaged
using the light microscope for counting. The experiment was
repeated three times.
Wound healing assays and Transwell
assays
For wound healing assays, cells were seeded in
6-well plates to a confluency of ~60–70%. Wounds were created in
the cell monolayer with a 200-µl pipette tip and the indicated
plasmids were transfected into cells. Dead cells were eliminated
with PBS wash. Wound closure was monitored at 0 and 24 h. Cell
invasion assays were evaluated using Transwell chamber assay
(Millipore, Billerica, MA, USA) according to the manufacturers
instruction. Matrigel (BD Biosciences, San Jose, CA USA) was left
to polymerize at the base of the top chamber of a 24-well Transwell
plate (8 µm; Corning Costar Corp., Corning, NY, USA) for 45 min at
37°C. Cells (5×104 cells/well) were exposed to
starvation by eliminating serum and growth factor for 24 h and then
added to the top chambers. The bottom chambers were filled with
serum-containing medium. Cultures were maintained for 48 h. Cells
adherent to the upper surface of the filter were removed using a
cotton applicator, and then stained with crystal violet. Cells were
counted in 10 random fields at ×100 magification and the mean ± SD
was calculated. To assure a representative conduct of the assays,
they were performed in triplicate wells and repeated twice.
Statistical analysis
Statistical analyses were performed using SPSS 18.0
software. Chi-square tests and Fishers exact tests were used to
compare the clinicopathological data. Kaplan-Meier analysis was
used to estimate survival rates and the two-sided log-rank test was
used to compare differences. Univariate and multivariate analyses
were based on a Cox proportional hazard regression model. In
vitro data were analyzed using one-way ANOVA method. A
P<0.05 was considered statistically significant.
Results
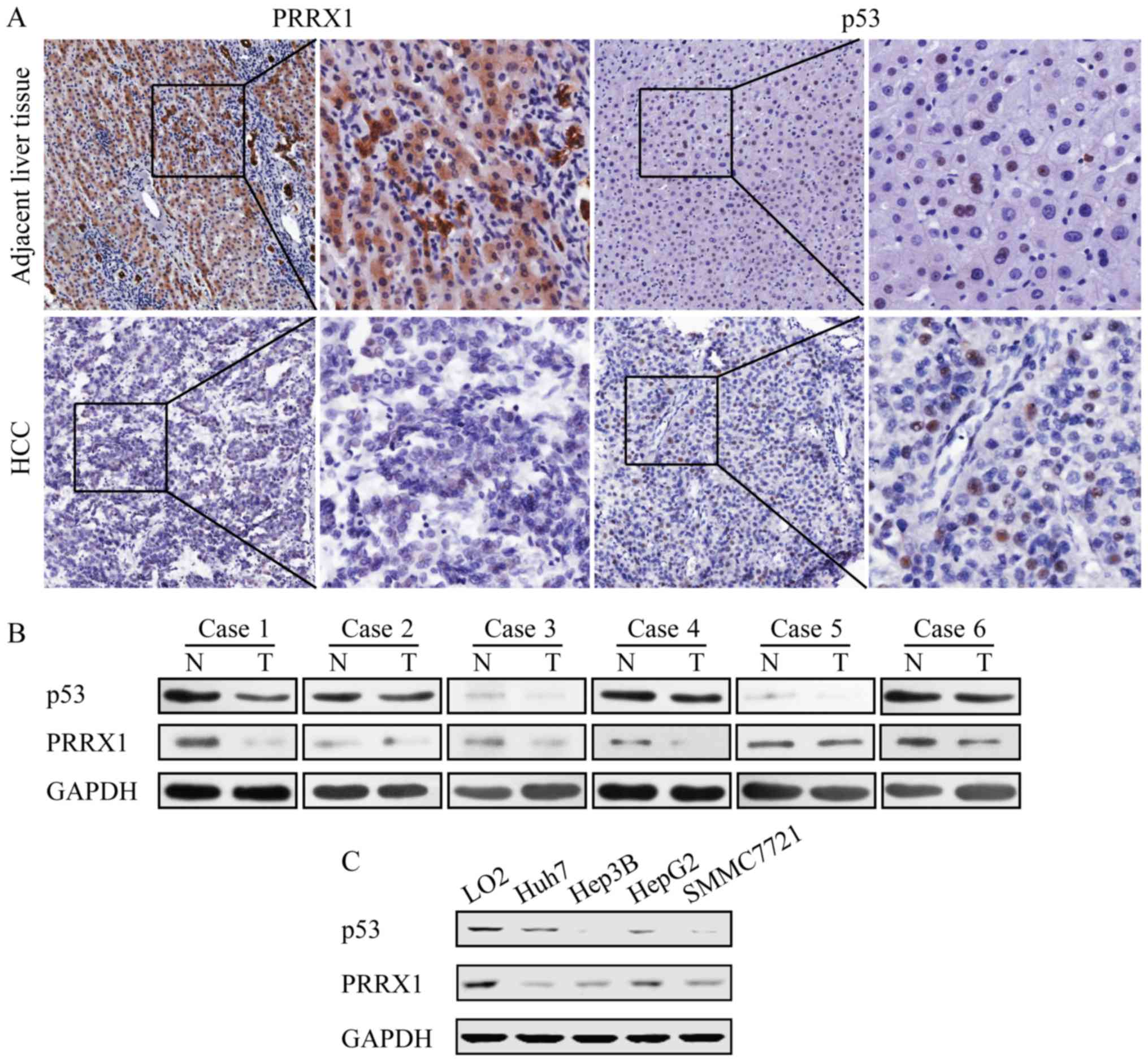
PRRX1 and p53 gene expression profiles
in HCC
The expression of PRRX1 and p53 were measured in
paraffin-embedded serial sections from 116 HCC patients who had
undergone hepatectomy. Results showed that the expression of PRRX1
and p53 is downregulated in HCC tissues compared to adjacent liver
tissues (Fig. 1A). Furthermore, the
expression level of PRRX1 and p53 protein were lower in tumors than
that in the corresponding non-malignant liver tissues (Fig. 1B). These results were confirmed by
western blot assay with HCC cell lines including Hep3B (p53 null),
Huh7 (p53 mutation), SMMC7721 (p53 wild-type), HepG2 (p53
wild-type) and normal liver cell line LO2 for comparison. The
expression of PRRX1 and p53 decreased in all HCC cell lines
compared to normal liver cells (Fig.
1C).
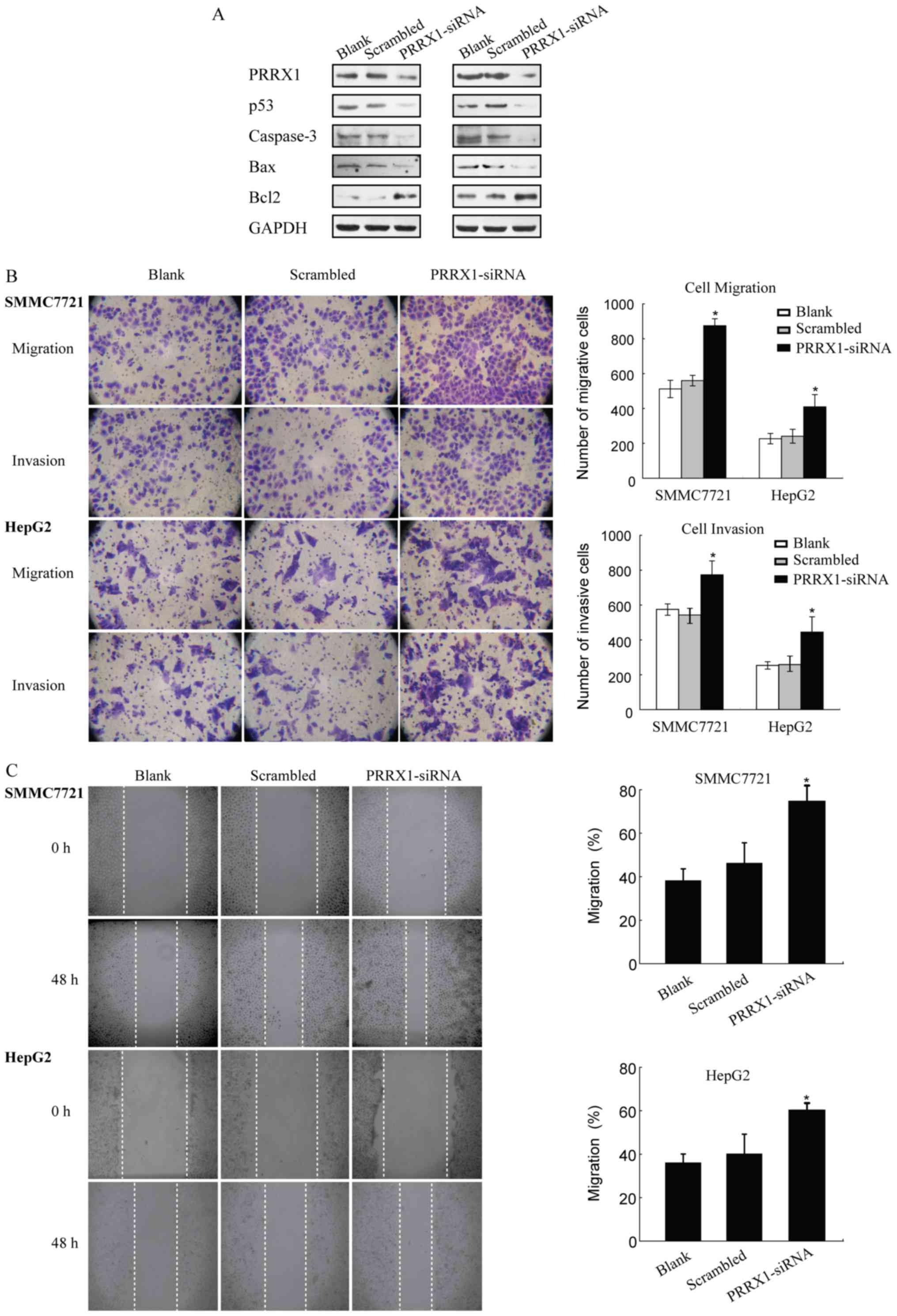
The loss of PRRX1 promotes HCC cell
mobility in vitro
Western blot assays were used to evaluate the effect
of PRRX1 silencing on the expression of p53 in HCC cells (SMMC7721
and HepG2). Our results showed that siRNA silencing of PRRX1
significantly decreased the expression of p53 compared to controls
and scrambled groups (Fig. 2A). The
decrease of PRRX1 was correlated with downregulation of p53
expression in HCC cells. Transwell and wound healing results showed
that PRRX1 siRNA had a stronger promotive effect on cell migration
and invasion ability of SMMC7721 and HepG2 cells compared to blank
and scrambled group (Fig. 2B and
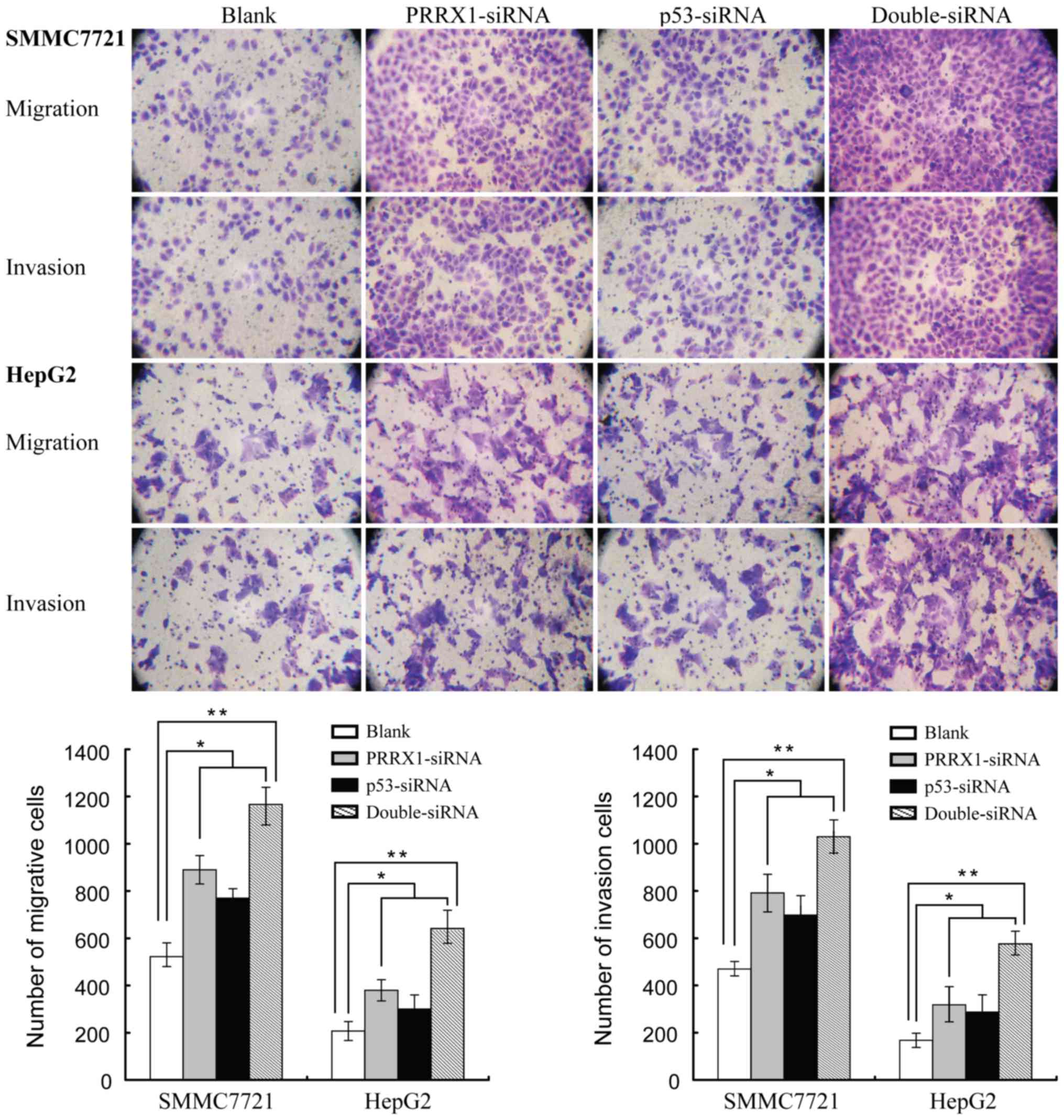
C). Furthermore, HCC cells presented strongest migration and
invasion ability when PRRX1 and p53 were both downregulated
(Fig. 3). These findings indicated
that decreased expression of PRRX1 and/or p53 induced both the
migration and the invasion features of HCC cells.
The loss of PRRX1 inhibits HCC cells
apoptosis via regulating p53 expression
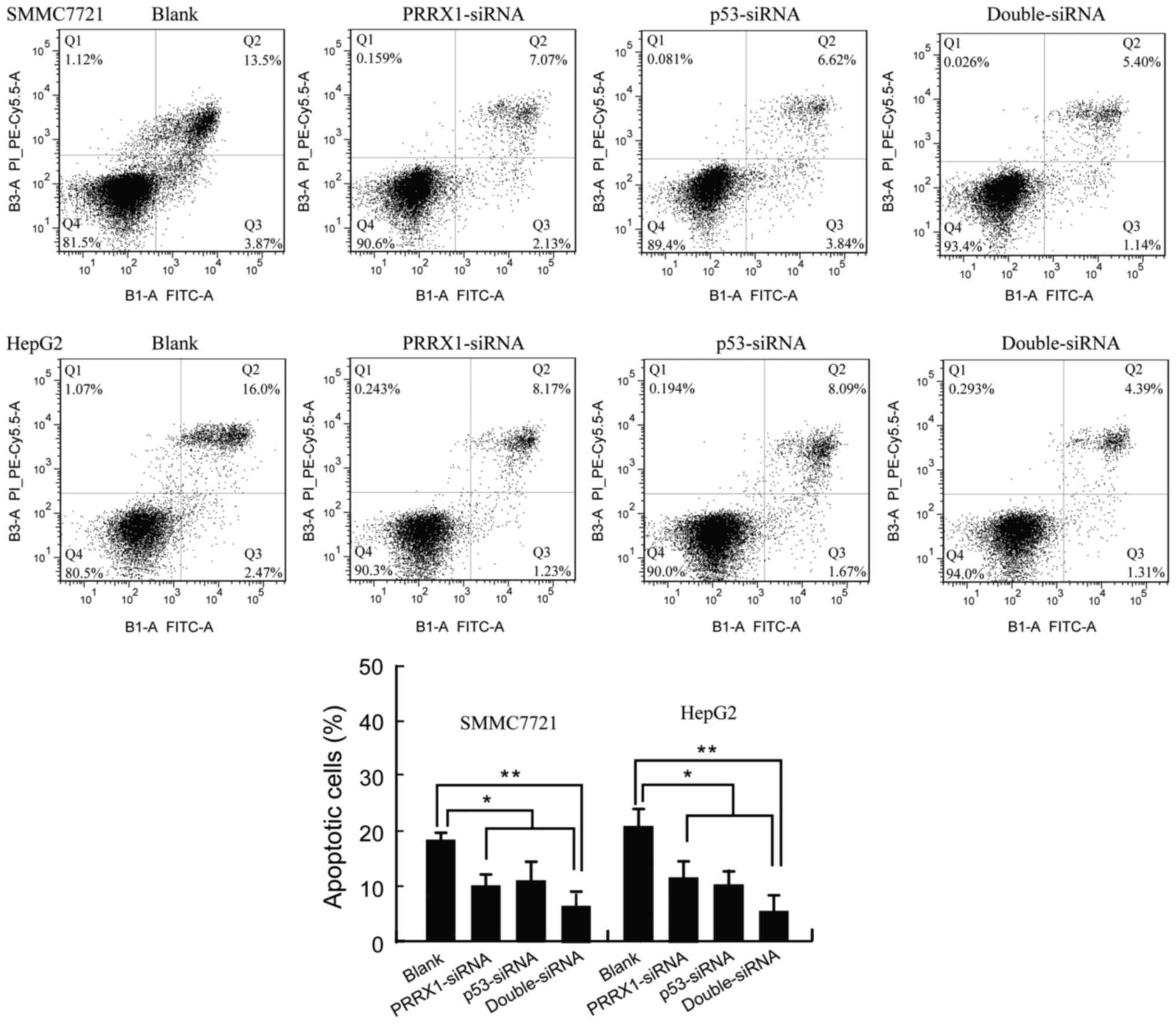
The Annexin V/PI apoptosis kit was used to quantify
the percentage of cells undergoing apoptosis. As shown in Fig. 4, the apoptosis rate of SMMC7721 and
HepG2 was 9.18±2.36 and 9.40±3.28% in response to transfection with
PRRX1 siRNA, respectively. The apoptosis of SMMC7721 and HepG2 was
10.65±3.74 and 9.24±2.32% in response to transfection with p53
siRNA, respectively. Apoptosis of SMMC7721 and HepG2 was 6.65±2.74
and 5.24±3.02% in response to transfection with both PRRX1 siRNA
and p53 siRNA, respectively (Fig.
4). Therefore, silencing PRRX1 and/or p53 exhibited a strong
effect on inhibition of apoptosis of HCC cells. In accordance with
the observed apoptotic effect induced by PRRX1 siRNA, an
anti-apoptotic expression profile was upregulated accompanied by
downregulated expression of p53 (Fig.
3A).
Decreased expression of PRRX1 and p53
in HCC is associated with poor prognosis
We first observed a lower PRRX1 and p53 expression
in 116 HCC samples (as compared to matched adjacent non-tumor liver
tissues (Fig. 1A and B). We
additionally found a correlation between the expression level and
tumor features. The decreased expression of PRRX1 was found to be
significant in HCC patients with vascular invasion (P<0.001),
TNM stage (P=0.036), BCLC stage (P=0.013), intrahepatic (P=0.002)
and distant metastasis (P<0.001; Table I). The decreased expression of p53
was correlated with vascular invasion (P<0.001), TNM stage
(P=0.005), BCLC stage (P=0.001), intrahepatic (P=0.001) and distant
metastasis (P=0.004; Table I).
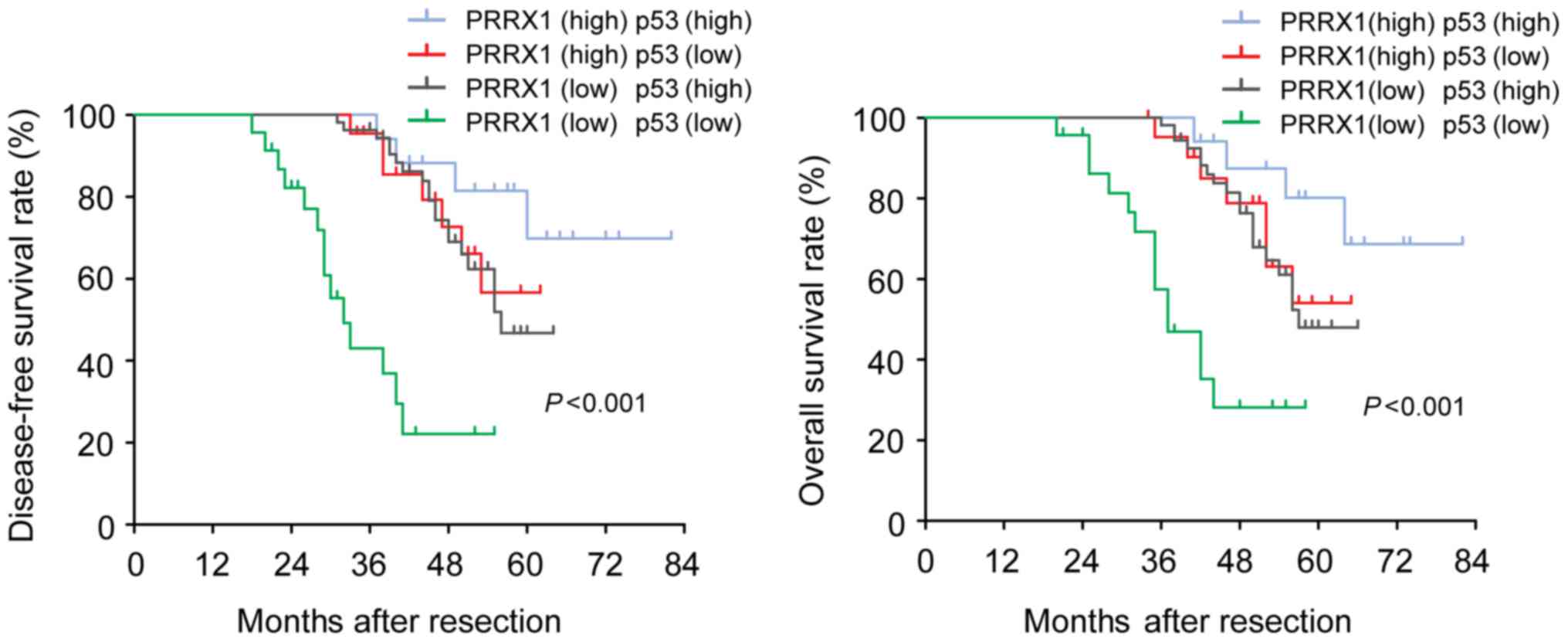
Based on these results, we divided 116 HCC patients into 4 groups:
both high-expression of PRRX1 (n=17), both low-expression of PRRX1
(n=23), high-expression of PRRX1 and low-expression of p53 (n=22),
high-expression of p53 and low-expression of PRRX1 (n=54). HCC
patients with low-expression of both PRRX1 and p53 had a
significantly shorter overall and disease-free survival than
patients with only PRRX1- or only p53 high expression (Fig. 5). Their correlations are detailed in
Table II, and PRRX1 is positively
correlated with p53 expression (r=0.257, P=0.006). These
observations are suggestive that PRRX1 and p53 expression levels
could be valuable predictive factors for recurrence and survival in
patients with HCC. Co-downregulation of both PRRX1 and p53 was
confirmed to be an independent negative factor for overall and
diseased-free survival.
 | Table II.Correlation of PRRX1 with p53 in 116
HCC patients. |
Table II.
Correlation of PRRX1 with p53 in 116
HCC patients.
|
| PRRX1 |
|
|
|---|
|
|
|
|
|
|---|
|
| Low | High | r | P-value |
|---|
| p53 |
|
|
|
|
|
Low | 23 | 22 | 0.257 | 0.006 |
|
High | 54 | 17 |
|
|
Discussion
Hepatocellular carcinoma (HCC) is a common
malignancy worldwide, especially occurring in Asia and South
Africa. The incidence of HCC in China is still high. Molecular
mechanisms leading to malignant transformation of normal liver
cells have not yet been fully elucidated. PRRX1 is a transcription
co-activator with the function of enhancing the DNA-binding
activity of serum response factor. It also regulates muscle
creatine kinase, indicating a role in the establishment of diverse
mesodermal muscle types. Several recent studies demonstrate that
PRRX1 can regulate differentiation of mesenchymal precursors. Ocaña
et al (11) showed that
PRRX1 is an EMT inducer conferring migratory and invasive
properties. Hirata et al (14) found that downregulation of PRRX1
expression contributes to poor prognosis of patients with HCC
through acquisition of CSC-like properties. The loss of PRRX1 is
required for breast cancer cells and HCC cells to metastasize in
vivo. In contrast to studies of breast cancer, overexpression
of PRRX1 was significantly associated with metastasis and poor
prognosis in CRC (12). It
indicates that heterogeneity exists in different tumors. The
present study demonstrated that PRRX1 expression is lower in HCC
tissues than adjacent normal liver tissues and is significantly
correlated with the survival and metastasis of HCC cells in
vitro. The mechanism underlying PRRX1 expression and HCC
remains unclear.
The tumor suppressor p53 is a transcription factor
that responds to various types of cellular stress, such as oncogene
activation and genotoxic drug-induced DNA damage (21). p53 regulates a variety of cellular
behaviors, such as cell growth, DNA repair, cell cycle arrest and
apoptosis (15). Wild-type p53 gene
mutation and inactivation in liver cells leading by a variety of
environmental factors play an important role in carcinogenesis.
When the cell genome DNA was damaged by exogenous factors, p53 will
build a complex regulatory network with related genes and regulate
cell characteristics by p53-related signaling pathway.
In this study, the expression of PRRX1 and p53 were
found decreased in some HCC cell lines and clinical samples.
Moreover, we found that p53 expression was correlated with PRRX1
expression in HCC. siRNA silencing of PRRX1 significantly decreased
the expression of p53 in HepG2 and SMMC7721. Our results indicated
that downregulation of PRRX1 expression in HCC cells presenting
more aggressive cellular motility. It is reported that p53
participates in inducing apoptosis in HCC cells (22–24).
Our data revealed that silencing PRRX1 exhibited a stronger effect
on inhibiting apoptosis via regulating p53 expression of HCC cells.
Furthermore, we demonstrated that decreased expression of PRRX1 and
p53 was significantly associated with poor prognosis in patients
with HCC.
In summary, we report that PRRX1 regulates p53 by
inhibiting apoptosis in HCC cells. The loss of PRRX1 expression
stimulates invasion and metastasis of HCC cells, contributing to
poor prognosis. Our results concerning the relationship between
PRRX1 expression and p53 expression suggest that HCC patients who
have both low expression of PRRX1 and p53 are more likely to
develop metastases and have the worst prognosis, and this knowledge
can be used to predict patient outcomes. Our finding suggested that
PRRX1 and p53 synergistically inhibit HCC progression and
metastasis by inducing apoptosis. Further experiments are necessary
to determine whether they have a positive effect on HCC
therapy.
References
|
1
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127:(Suppl 1). S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuen MF, Tanaka Y, Fong DY, Fung J, Wong
DK, Yuen JC, But DY, Chan AO, Wong BC, Mizokami M, et al:
Independent risk factors and predictive score for the development
of hepatocellular carcinoma in chronic hepatitis B. J Hepatol.
50:80–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsai JH, Donaher JL, Murphy DA, Chau S and
Yang J: Spatiotemporal regulation of epithelial-mesenchymal
transition is essential for squamous cell carcinoma metastasis.
Cancer Cell. 22:725–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bae YK, Choi JE, Kang SH and Lee SJ:
Epithelial-mesenchymal transition phenotype is associated with
clinicopathological factors that indicate aggressive biological
behavior and poor clinical outcomes in invasive breast cancer. J
Breast Cancer. 18:256–263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taneyhill LA, Coles EG and Bronner-Fraser
M: Snail2 directly represses cadherin 6B during
epithelial-to-mesenchymal transitions of the neural crest.
Development. 134:1481–1490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deep G, Jain AK, Ramteke A, Ting H,
Vijendra KC, Gangar SC, Agarwal C and Agarwal R: SNAI1 is critical
for the aggressiveness of prostate cancer cells with low
E-cadherin. Mol Cancer. 13:372014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Campbell K, Whissell G, Franch-Marro X,
Batlle E and Casanova J: Specific GATA factors act as conserved
inducers of an endodermal-EMT. Dev Cell. 21:1051–1061. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song K, Li Q, Jiang ZZ, Guo CW and Li P:
Heparan sulfate D-glucosaminyl 3-O-sulfotransferase-3B1, a novel
epithelial-mesenchymal transition inducer in pancreatic cancer.
Cancer Biol Ther. 12:388–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ocaña OH, Córcoles R, Fabra A,
Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A and
Nieto MA: Metastatic colonization requires the repression of the
epithelial-mesenchymal transition inducer Prrx1. Cancer Cell.
22:709–724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takahashi Y, Sawada G, Kurashige J, Uchi
R, Matsumura T, Ueo H, Takano Y, Akiyoshi S, Eguchi H, Sudo T, et
al: Paired related homoeobox 1, a new EMT inducer, is involved in
metastasis and poor prognosis in colorectal cancer. Br J Cancer.
109:307–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo J, Fu Z, Wei J, Lu W, Feng J and Zhang
S: PRRX1 promotes epithelial-mesenchymal transition through the
Wnt/β-catenin pathway in gastric cancer. Med Oncol. 32:3932015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirata H, Sugimachi K, Takahashi Y, Ueda
M, Sakimura S, Uchi R, Kurashige J, Takano Y, Nanbara S, Komatsu H,
et al: Downregulation of PRRX1 confers cancer stem cell-like
properties and predicts poor prognosis in hepatocellular carcinoma.
Ann Surg Oncol. 22:(Suppl 3). S1402–S1409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chari NS, Pinaire NL, Thorpe L, Medeiros
LJ, Routbort MJ and McDonnell TJ: The p53 tumor suppressor network
in cancer and the therapeutic modulation of cell death. Apoptosis.
14:336–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soussi T: p53 alterations in human cancer:
More questions than answers. Oncogene. 26:2145–2156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lewis BC, Klimstra DS, Socci ND, Xu S,
Koutcher JA and Varmus HE: The absence of p53 promotes metastasis
in a novel somatic mouse model for hepatocellular carcinoma. Mol
Cell Biol. 25:1228–1237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen YW, Klimstra DS, Mongeau ME, Tatem
JL, Boyartchuk V and Lewis BC: Loss of p53 and Ink4a/Arf cooperate
in a cell autonomous fashion to induce metastasis of hepatocellular
carcinoma cells. Cancer Res. 67:7589–7596. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hansen JE, Fischer LK, Chan G, Chang SS,
Baldwin SW, Aragon RJ, Carter JJ, Lilly M, Nishimura RN, Weisbart
RH, et al: Antibody-mediated p53 protein therapy prevents liver
metastasis in vivo. Cancer Res. 67:1769–1774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marte B: Cancer: Super p53. Nature.
420:2792002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yee SB, Choi HJ, Chung SW, Park DH, Sung
B, Chung HY and Kim ND: Growth inhibition of luteolin on HepG2
cells is induced via p53 and Fas/Fas-ligand besides the TGF-β
pathway. Int J Oncol. 47:747–754. 2015.PubMed/NCBI
|
|
23
|
Zhu R, Mok MT, Kang W, Lau SS, Yip WK,
Chen Y, Lai PB, Wong VW, To KF, Sung JJ, et al: Truncated
HBx-dependent silencing of GAS2 promotes hepatocarcinogenesis
through deregulation of cell cycle, senescence and p53-mediated
apoptosis. J Pathol. 237:38–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lou G, Liu Y, Wu S, Xue J, Yang F, Fu H,
Zheng M and Chen Z: The p53/miR-34a/SIRT1 positive feedback loop in
quercetin-induced apoptosis. Cell Physiol Biochem. 35:2192–2202.
2015. View Article : Google Scholar : PubMed/NCBI
|