Introduction
Compared with normal cells, the majority of cancer
cells generally rely on aerobic glycolysis, known as the Warburg
effect, to produce the energy required for cellular processes
rather than mitochondrial oxidative phosphorylation, which is more
efficient, even in the availability of oxygen (1). The reprogramming of glycometabolism
not only allows the high energy demand of cancer cells to be met
under hypoxic conditions, but also provides substrates for rapid
synthesis of nucleotides and lipids, thereby conferring a growth
advantage to cancer cells (2).
Recently, the Warburg effect, or cellular energy metabolism
reprogramming, was considered a new cancer biomarker (3).
MicroRNAs (miRNAs) have a crucial role in aerobic
glycolysis (4). miRNAs are
naturally occurring, 22-nucleotide small non-coding RNA (ncRNA)
molecules (5). Recently, it was
demonstrated that miR-143 has an important role in regulating
glycolysis in cancer and in cancer cell proliferation; further
study showed that, in lung cancer, miR-143 reduced hexokinase 2
(HK2) protein expression by targeting the mammalian target of
rapamycin (mTOR) pathway (6).
Another study indicated that inhibition of miR-199a, miR-138,
miR-150 and miR-532-5p expression in renal cell carcinoma increased
the expression of glucose transporter isoform 1 (GLUT1);
conversely, increasing miR-130b, miR-19a, miR-19b and miR-301a
expression downregulated GLUT1 (7).
These findings demonstrate that ncRNAs may be essential regulators
of aerobic glycolysis in cancer. Another indispensable ncRNA class,
long ncRNAs (lncRNA) are composed of more than 200 nucleotides. To
date, more than 10,000 lncRNAs have been found in the human genome
(8). Several studies have found
that lncRNAs can be carcinogenic or cancer-suppressor genes
(9). The expression of genes
involved in tumors by the comparison of tumor and normal cells has
revealed abnormal lncRNA expression in different types of tumors.
In addition to studies on phenotype, some in-depth studies have
reported on the mechanism of lncRNAs in tumors (10,11).
For example, lncRNAs correlate with tumor cell apoptosis,
metastasis, drug resistance and autophagy (12). Furthermore, lncRNAs have been
detected in clinical routine examination of patients with cancer,
suggesting that they can be used as a prognostic factor for
diagnosing tumors. It is expected that lncRNAs may be new tumor
markers and targets of tumor therapy (13). However, their role in cancer
metabolism, particularly the Warburg effect, remains unknown. HOX
transcript antisense RNA (HOTAIR) is the first lncRNA found to have
inverse transcription, and is found in the HOXC gene on
chromosome 12 (14). HOTAIR is
expressed in various tumors, such as liver cancer and breast
cancer, and high HOTAIR expression and poor prognosis are closely
related (15,16). HOTAIR expression levels were
significantly positively correlated with hepatocellular carcinoma
(HCC) recurrence and metastasis and with the overall survival time
of patients with HCC (17).
Silencing of HOTAIR expression was found to activate tumor necrosis
factor (TNF)-α-induced apoptosis, significantly decrease hepatoma
cell proliferation and distant invasion ability, and also improve
cancer cell sensitivity to doxorubicin and cisplatin (18). These findings suggest that HOTAIR
upregulation is closely related to HCC development, metastasis,
recurrence and drug resistance. Anaerobic glycolysis provides
indispensable material and energy in HCC development and
metastasis. Altogether, this suggests that HOTAIR may be a key role
player in HCC glucose metabolism. However, the specific
relationship between HOTAIR and glucose metabolism is unclear.
In the present study, we found that HOTAIR promotes
HCC cell consumption of glucose and production of lactate. HOTAIR
is involved in glucose metabolic processes that are essential to
our understanding of tumor progression. However, the mechanism by
which HOTAIR directly regulates glycolysis has not been proven.
Therefore, we researched the potential mechanism of HOTAIR-mediated
metabolic transformation in HCC cells.
Materials and methods
Clinical samples
From July 2012 to November 2014, 84 HCC and paired
adjacent normal tissues were obtained from patients undergoing
surgical hepatectomy at the Shengjing Hospital of China Medical
University and who were pathologically diagnosed with HCC. None of
the patients received chemotherapy or radiation therapy,
preoperatively. The Shengjing Hospital Ethics Committee approved
the present study; we obtained informed consent from all
patients.
Cell lines and culture
HepG2, SMMC-7721, Hep3B, Huh7, and Bel-7402 cells,
and the normal liver Chang cell line were purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). All
cells were cultured with Dulbecco's modified Eagle's medium that
contained 2 mM L-glutamine, 0.1 mM non-essential amino acids, 1.0
mM sodium pyruvate, and 10% fetal bovine serum in 5% CO2
and 95% O2 at 37°C.
Lactate and glucose level
measurement
Lactate and glucose levels were detected using
Lactate Assay Kit II and High Sensitivity Glucose Assay kit,
respectively. The two kits are purchased from Sigma-Aldrich,
Shanghai, China.
Cell Counting Kit-8 (CCK-8)
CCK-8 (Beyotime) was used to detect cell
proliferation. Cells (5×103 cells/ml) were seeded in
96-well plates, incubated in 5% CO2 overnight at 37°C,
and then incubated for 1 h with 10 µl CCK-8 reagent. We measured
the CCK-8 absorption values (A) at 450 nm using a microplate reader
to determine the rate of cell growth.
Western blotting
Western blot assays were performed as previously
described (12). Anti-GLUT1 and
anti-phosphorylated (p)-mTOR rabbit monoclonal antibody and
anti-immunoglobulin (Ig)G rabbit monoclonal antibody were purchased
from ProteinTech Group (Chicago, IL, USA). We purchased horseradish
peroxidase-linked secondary antibodies from Cell Signaling
Technology (Shanghai, China). Anti-β-actin was purchased from
TransGen Biotech (Beijing, China).
Real-time PCR
Isolation of total RNA, reverse transcription of
RNA, and quantitative real-time PCR were performed as previously
described (12) using the following
forward and reverse primers: HOTAIR,
5′-CAGTGGGGAACTCTGACTCG-3′ and 5′-GTGCCTGGTGCTCTCTTACC-3′;
GLUT1, 5′-TTATTGCCCAGGTGTTCGGC-3′ and
5′-GTAGCAGGGCTGGGATGAAG-3′; 18S, 5′-GGAGCGAGATCCCTCCAAAAT-3′
and 5′-GGCTGTTGTCATACTTCTCATGG-3′.
RNA binding protein
immunoprecipitation (RIP)
A RIP kit was purchased from Millipore (Bedford, MA,
USA); the RIP assays were performed according to the manufacturer's
protocol. The negative control and positive control were normal
rabbit IgG and GLUT1, respectively. The co-precipitated RNAs were
determined by quantitative reverse transcription-PCR (qRT-PCR).
HOTAIR small interfering RNA (siRNA)
vector construction and transfection
We purchased plasmids that contained HOTAIR
complementary DNA (cDNA), HOTAIR siRNA (si-HOTAIR) and control
siRNA from GeneChem (Shanghai, China), and transfected them into
cells using Lipofectamine 2000 according to the manufacturer's
instructions.
Statistical analysis
We analyzed the data using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). Data are presented as the mean ± standard
deviation (SD). The Students t-test or analysis of variance was
used for the statistical analyses. P<0.05 was considered
statistically significant.
Results
HOTAIR expression is high in HCC cells
and tissues
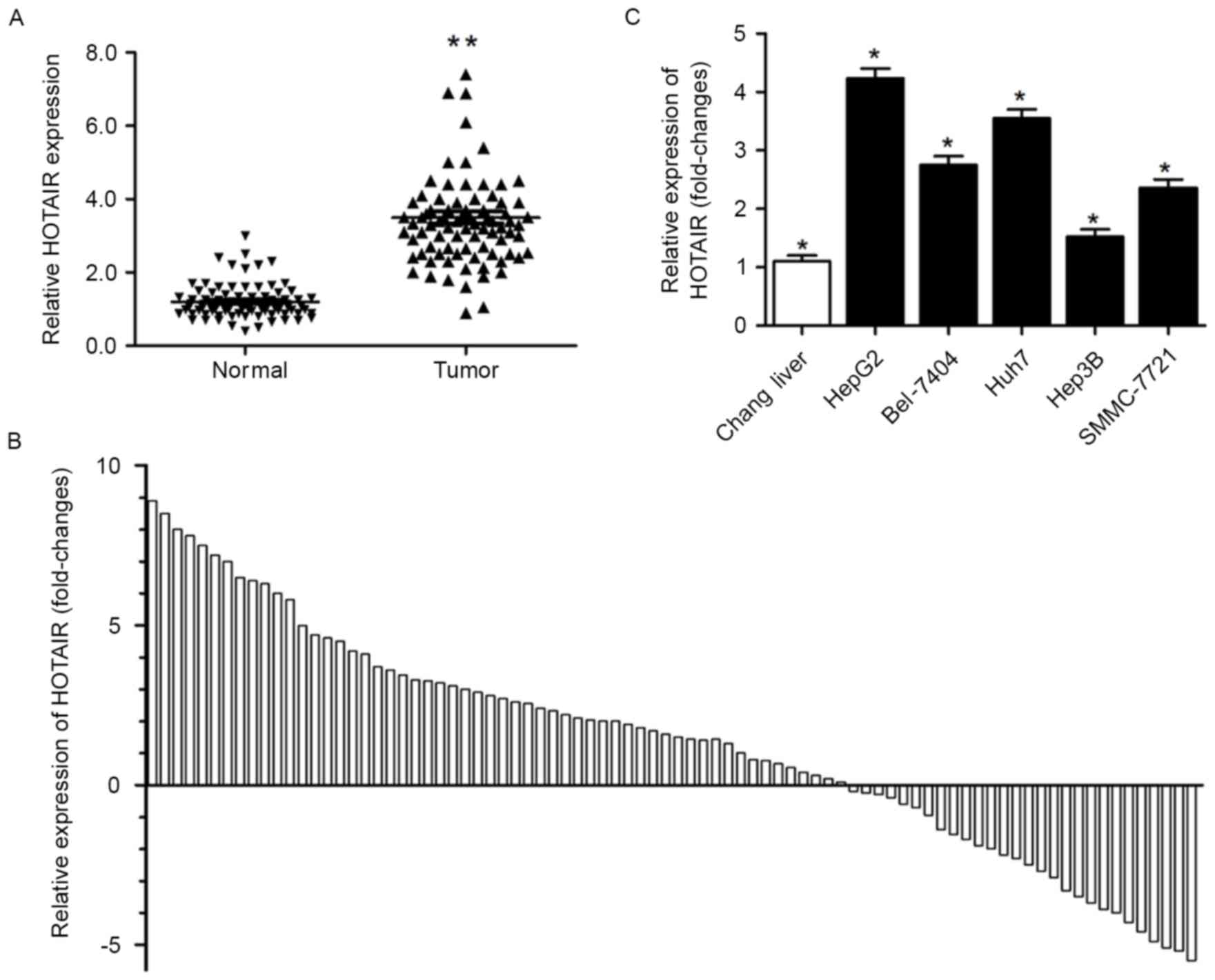
Real-time PCR was used to investigate HOTAIR
expression levels in HCC tissues: HOTAIR expression in the tumor
samples was significantly upregulated as compared to the normal
tissue samples (P<0.01; Fig.
1A), and HOTAIR expression was higher in 57 of the 84 HCC
tissues (Fig. 1B). We also detected
HOTAIR expression levels in HepG2, Bel-7402, Huh7, Hep3B and
SMMC-7721 cell lines and in Chang cells. Real-time PCR confirmed
HOTAIR upregulation in all HCC cell lines except Hep3B cells
(Fig. 1C). Accordingly, we used
Hep3B and HepG2 cells in the present study.
HOTAIR overexpression promotes in
vitro proliferation of HCC cells
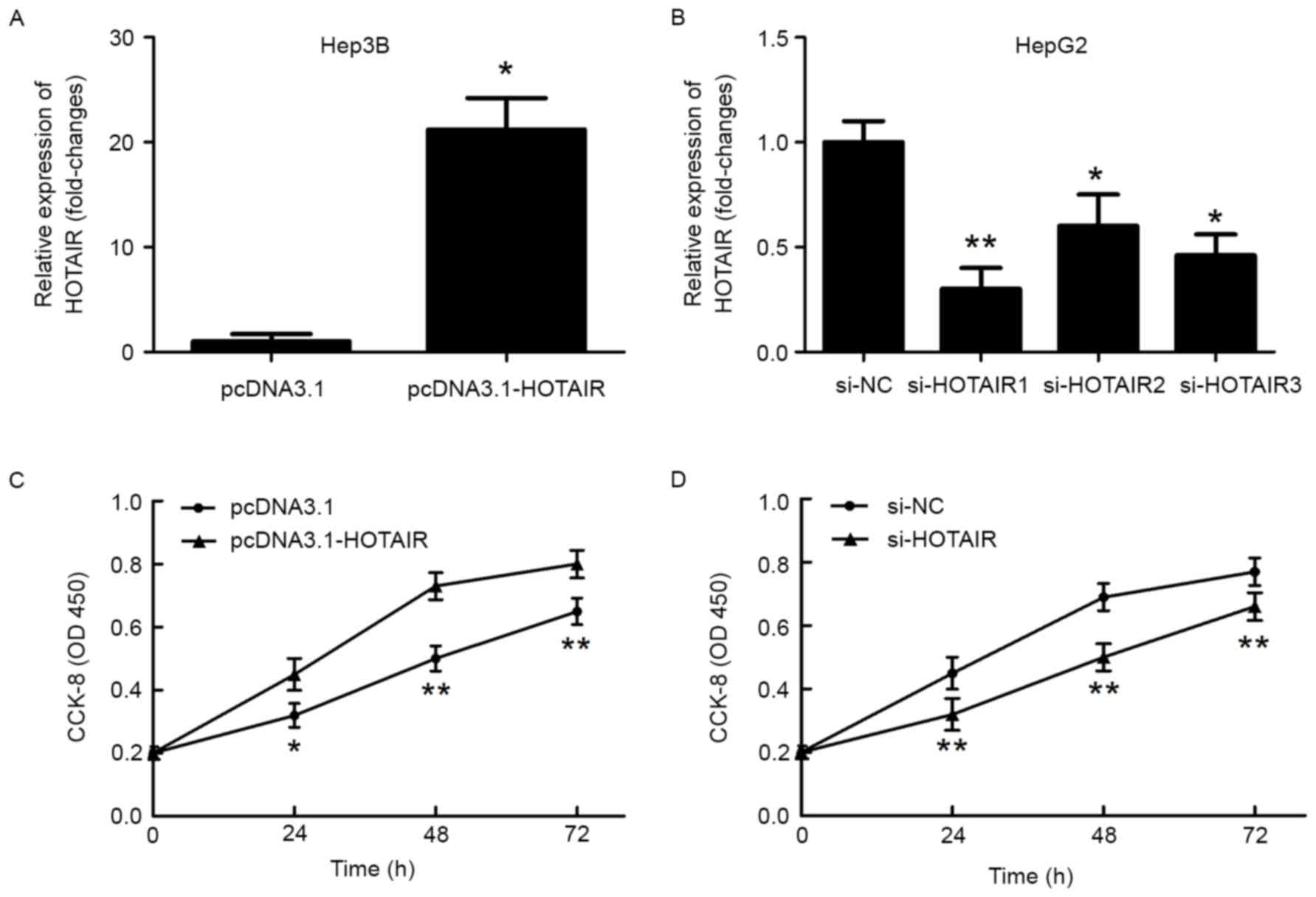
To evaluate the role of HOTAIR in HCC cell
proliferation, we first transfected si-HOTAIR into HepG2 cells to
decrease HOTAIR levels, and transfected Hep3B cells with
pcDNA3.1-HOTAIR to upregulate HOTAIR levels. After a 48-h
transfection, HOTAIR levels were measured using real-time PCR to
evaluate transfection efficiency (Fig.
2A and B). CCK-8 assay showed that HOTAIR overexpression
significantly promoted Hep3B cell growth as compared with the
vector control (Fig. 2C). HCC cell
proliferation was decreased following HOTAIR knockdown (Fig. 2D).
HOTAIR promotes glycolysis in HCC
cells
Cancer cells usually utilize energy metabolism, such
as glycolysis, to survive and proliferate. Therefore, we aimed to
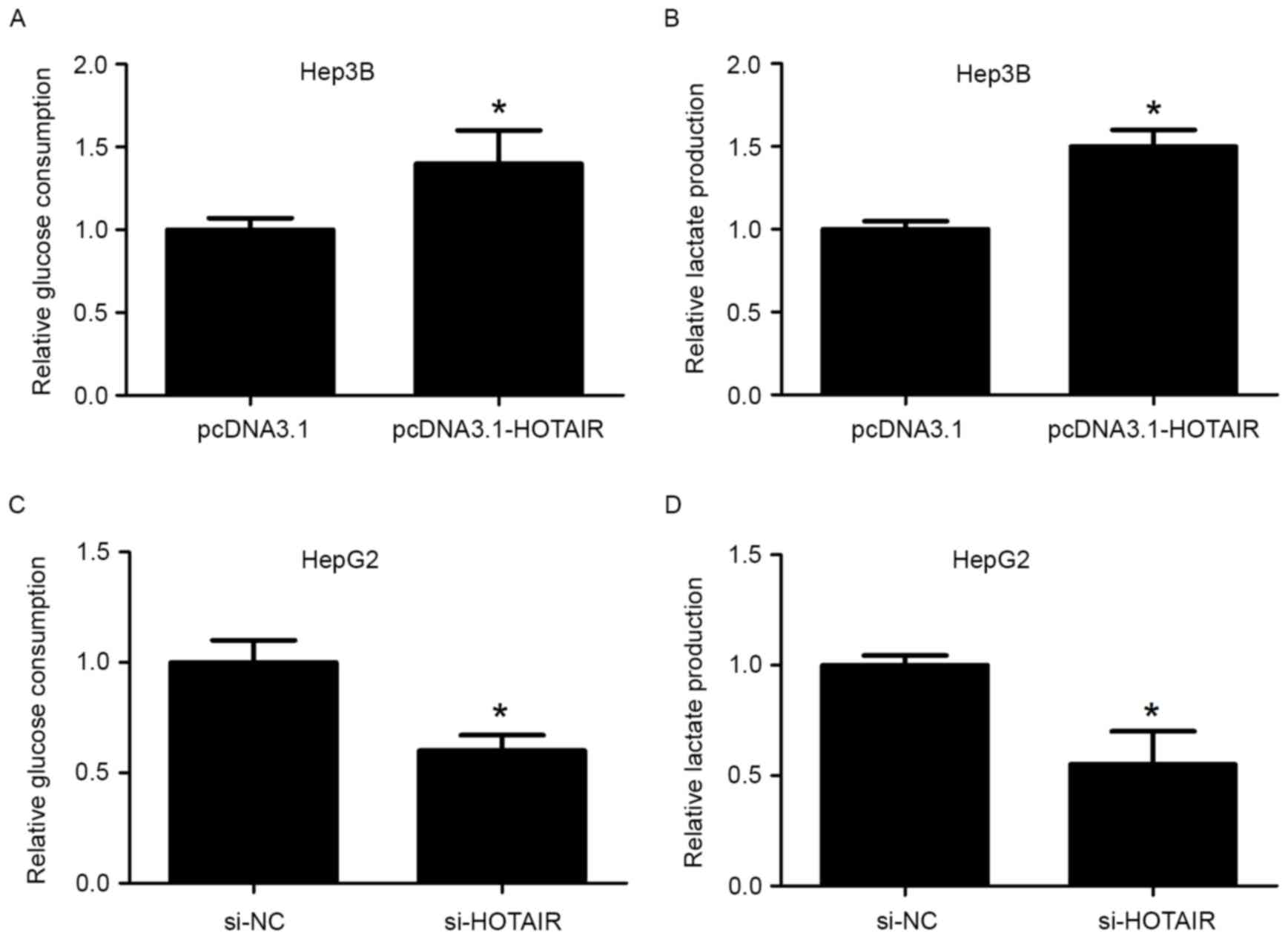
ascertain whether HOTAIR promotes cancer cell glycolysis. We first
examined the effect of HOTAIR on HCC cell glucose metabolism.
HOTAIR overexpression significantly increased Hep3B cell glucose
consumption and lactate production rates (Fig. 3A and B), while HOTAIR knockdown in
HepG2 cells significantly decreased glucose consumption and lactate
production (Fig. 3C and D). The
findings indicate that HOTAIR promotes glycolysis in HCC cells.
HOTAIR upregulates GLUT1 to promote
glycolysis
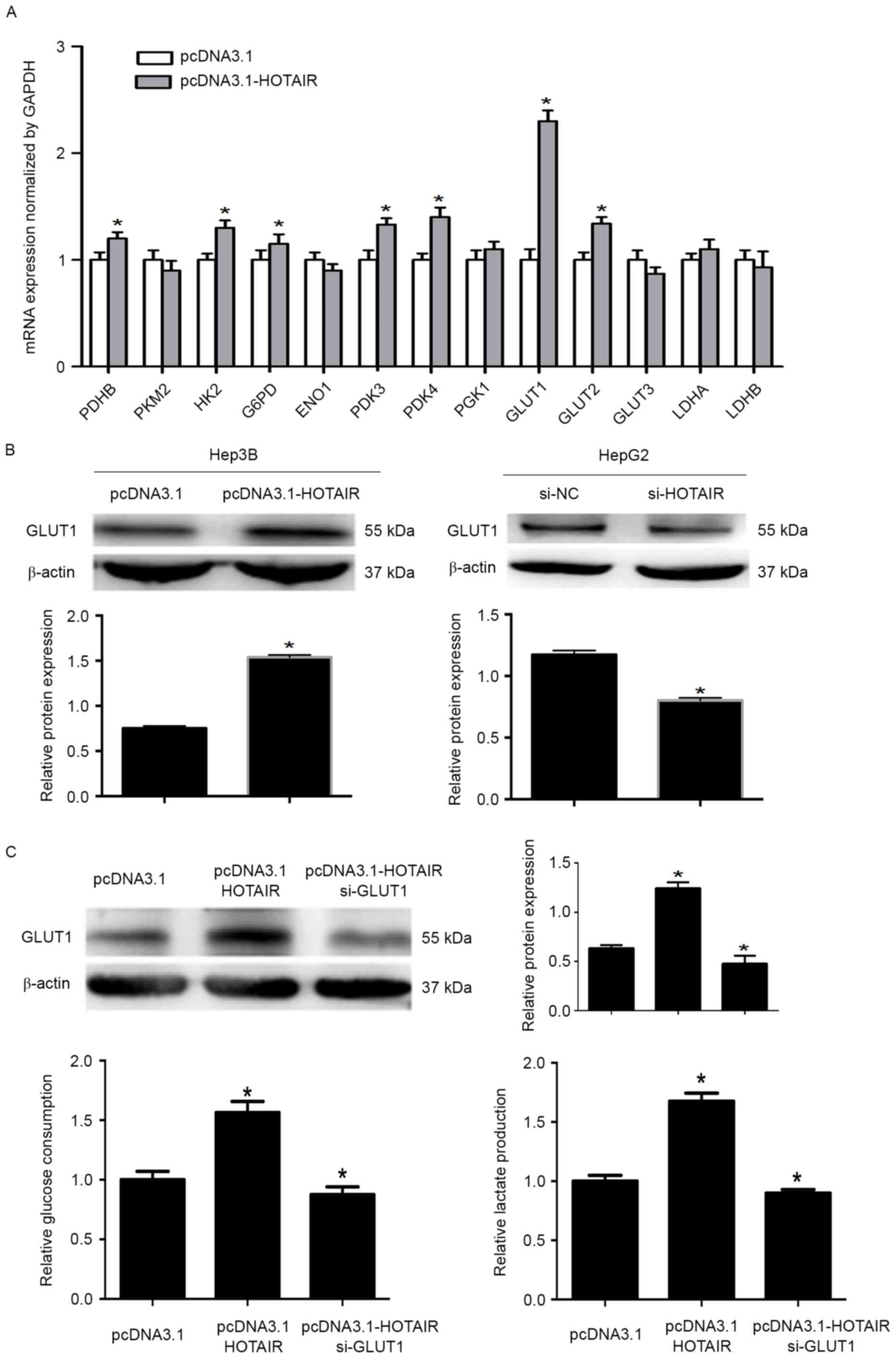
Aerobic glycolysis involves numerous enzymes. To
elucidate the potential HOTAIR regulatory mechanism of HCC cell
glycolysis, we investigated the expression levels of key enzymes
following HOTAIR upregulation. Real-time PCR showed that HOTAIR
significantly upregulated GLUT1 mRNA levels (Fig. 4A). Western blotting showed that
HOTAIR enhanced GLUT1 protein expression, while HOTAIR knockdown
significantly reduced GLUT1 protein levels (Fig. 4B). In addition, GLUT1 knockdown
significantly decreased the effect of HOTAIR on the consumption of
glucose and production of lactate (Fig.
4C). These results indicate that HOTAIR regulates glycolysis
via GLUT1.
HOTAIR promotes HCC cell glycolysis
partly by inducing GLUT1 expression by upregulating mTOR and by
binding GLUT1 directly. The role of mTOR in the regulation of cell
metabolism is crucial
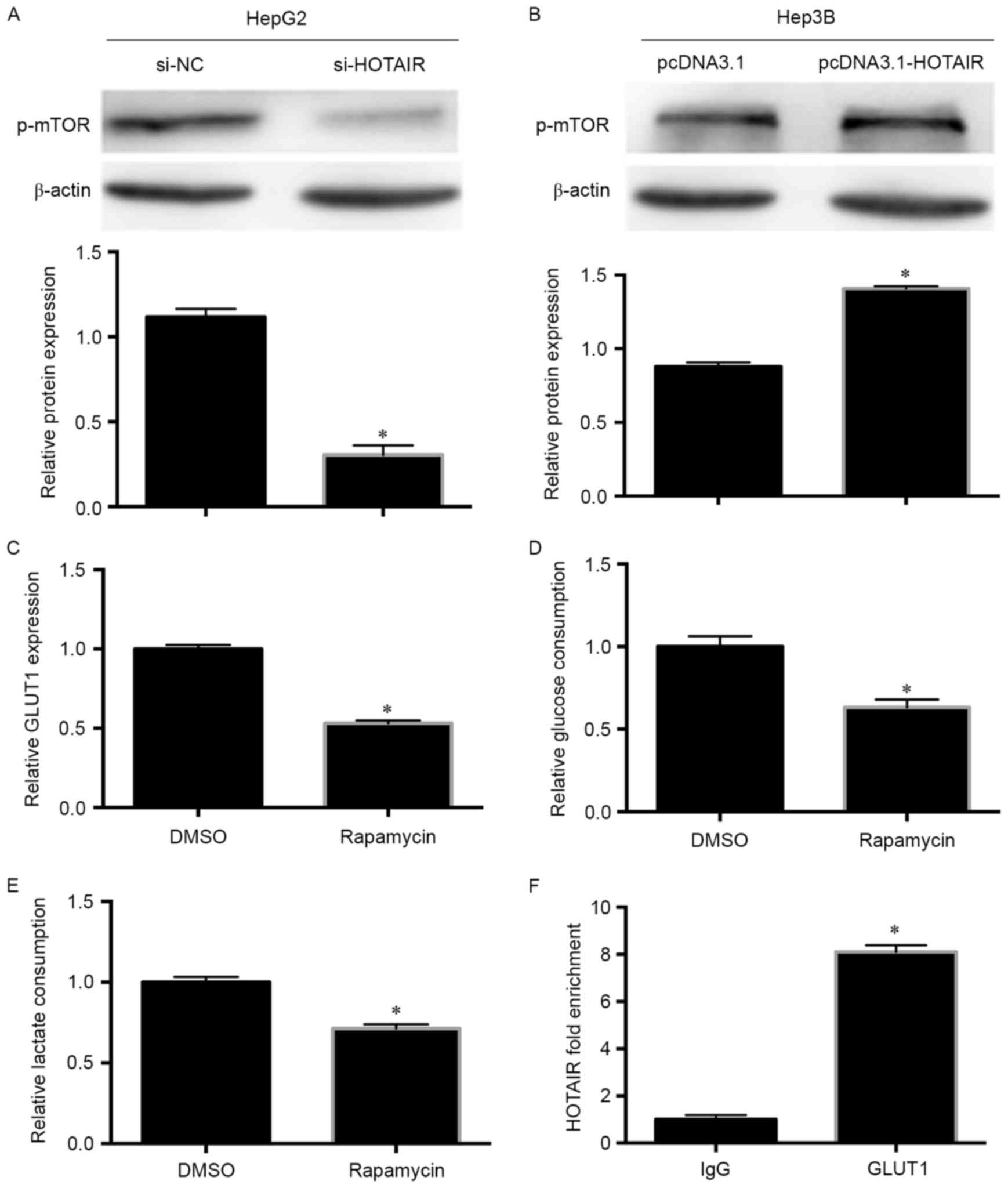
Accordingly, we detected mTOR protein expression
using western blotting. There was a positive relation between mTOR
phosphorylation and HOTAIR in HCC cells (Fig. 5A and B), and rapamycin reduced
GLUT1 mRNA levels (Fig. 5C).
We also found that rapamycin significantly decreased the glucose
consumption and lactate production rates (Fig. 5D and E). Furthermore, lncRNA can
also bind proteins directly, thus we used RIP to detect the
interaction between HOTAIR and GLUT1, and HOTAIR bound with GLUT1
directly (Fig. 5F). Taken together,
the results suggest that HOTAIR promotes HCC cell glycolysis partly
by inducing GLUT1 expression by upregulating mTOR, and by binding
with GLUT1 directly.
Discussion
Previously, ncRNAs were considered transcriptional
noise since they did not encode proteins. However, completion of
the Human Genome Project has changed this viewpoint (19). An increasing number of studies have
confirmed that ncRNAs play an important regulatory role in many
cellular activities and human disease, including cancer (20). In recent years, it has been reported
that many lncRNAs influence the malignant biological behavior in
tumor development, such as proliferation, apoptosis, invasion and
metastasis (21–23). More attention has been focused on
the role of lncRNAs in cancer cell development; however, the
relationship between lncRNAs and cancer cell glucose metabolism
remains unclear. An increasing number of studies suggest that
altered metabolism is important in supporting cancer cell
proliferation (24). In the present
study, we demonstrated that HOTAIR is essential for accelerating
glycolysis in HCC cells, which supplements the function of HOTAIR
in tumor cell development.
Rinn et al first described HOTAIR as a
spliced, polyadenylated ncRNA containing 2,158 nucleotides and 6
exons (25). Transcription of the
HOXC gene antisense strand, situated between HOXC11
and HOXC12 on chromosome 12q13.13, yields HOTAIR. HOTAIR
interacts with polycomb repressive complex 2 (PRC2), which is
necessary for PRC2 occupancy and histone H3 lysine 27
trimethylation (26). As an
oncogenic factor, HOTAIR has been detected with deregulation in
many cancers, such as breast and gastric cancer (27,28).
In the present study, 57 tumor samples (57/84, 68%) had higher
HOTAIR expression than the matched adjacent normal tissue, which is
consistent with other results on HOTAIR gene expression in
HCC (18). In addition, numerous
studies have focused on the effect of HOTAIR on HCC development, a
complex process associated with malignant biology. Gao et al
found that HOTAIR promotes cell proliferation and invasion partly
by regulating the Wnt/β-catenin signaling pathway (29). Ding et al demonstrated that
HOTAIR increases HCC cell aggression and invasion by suppressing
RBM38 expression, which plays a role in regulating cell motility
(30). Recently, emerging evidence
has demonstrated that HOTAIR mediates hepatocarcinogenesis by
decreasing miR-128 expression, and then activating P14 and P16
signaling, functioning as a competitive endogenous RNA (ceRNA)
(31). Previously, our research
indicated that HOTAIR promotes autophagy activation in HCC cells by
upregulating the expression of ATG3 and ATG7,
autophagy-related genes, highlighting the novel role of HOTAIR in
HCC cell progression (12).
However, an increasing number of studies have focused on the
mechanisms of HOTAIR regulation in cancer cells, but it is still
limited to research on the relationship between metabolism
reprogramming in cancer cells and HOTAIR.
It was recently suggested that the lncRNA CRNDE,
controlled by insulin/insulin-like growth factor (IGF) signaling,
promotes cellular metabolism in a pattern that suggests the Warburg
effect (32). The lncRNA UCA1
promotes glycolysis by upregulating HK2 via the mTOR-signal
transducer and activator of transcription (STAT)3/miR-143 pathway
(33). However, whether HOTAIR
regulates HCC cell glucose metabolism remains unknown. In the
present study, we confirmed that HOTAIR promotes glycolysis
significantly in HCC cells, indicating that HOTAIR is involved in
the metabolic regulatory process in cancer. As lncRNAs mainly
function as key regulators of gene expression, we used qRT-PCR to
screen a series of genes related to glucose metabolism in cells
overexpressing HOTAIR as compared with negative control cells.
HOTAIR upregulated GLUT1 most obviously at both the mRNA and
protein levels. GLUT1 is an important rate-limiting factor in
glucose transport and metabolism in cancer cells (34). Amann et al found
significantly higher GLUT1 expression in HCC tissues which
functionally affected HCC cell proliferation and invasiveness
(35). Mechanistically, we showed
that HOTAIR increased GLUT1 expression by upregulating mTOR and by
binding GLUT1 directly. lncRNAs, such as H19 and Linc00152, are
gene enhancers by direct binding with proteins (36,37).
Therefore, the present study reveals a novel HOTAIR/mTOR/GLUT1 axis
linking HOTAIR and glucose metabolism in HCC cells.
To summarize, HOTAIR is increased in HCC tissues and
promotes cell proliferation by regulating glucose metabolism via
the activation of mTOR signaling. The identification of
GLUT1 as a HOTAIR-regulated gene provides a potential link
between HOTAIR and cancer metabolism. However, additional
mechanisms underlying the regulation between HOTAIR and metabolism
warrant further exploration. The present study identifies a
potential target for developing HCC therapeutic strategies.
References
|
1
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeBerardinis RJ and Thompson CB: Cellular
metabolism and disease: What do metabolic outliers teach us? Cell.
148:1132–1144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Munkley J and Elliott DJ: Hallmarks of
glycosylation in cancer. Oncotarget. 7:35478–35489. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bensinger SJ and Christofk HR: New aspects
of the Warburg effect in cancer cell biology. Semin Cell Dev Biol.
23:352–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yin S, Fan Y, Zhang H, Zhao Z, Hao Y, Li
J, Sun C, Yang J, Yang Z, Yang X, et al: Differential TGFβ pathway
targeting by miR-122 in humans and mice affects liver cancer
metastasis. Nat Commun. 7:110122016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fang R, Xiao T, Fang Z, Sun Y, Li F, Gao
Y, Feng Y, Li L, Wang Y, Liu X, et al: MicroRNA-143 (miR-143)
regulates cancer glycolysis via targeting hexokinase 2 gene. J Biol
Chem. 287:23227–23235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koh HJ, Toyoda T, Fujii N, Jung MM, Rathod
A, Middelbeek RJ, Lessard SJ, Treebak JT, Tsuchihara K, Esumi H, et
al: Sucrose nonfermenting AMPK-related kinase (SNARK) mediates
contraction-stimulated glucose transport in mouse skeletal muscle.
Proc Natl Acad Sci USA. 107:15541–15546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao XY and Lin JD: Long Non-coding RNAs:
A new regulatory code in metabolic control. Trends Biochem Sci.
40:586–596. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmitt AM and Chang HY: Long non-coding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long non-coding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martens-Uzunova ES, Böttcher R, Croce CM,
Jenster G, Visakorpi T and Calin GA: Long non-coding RNA in
prostate, bladder, and kidney cancer. Eur Urol. 65:1140–1151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang L, Zhang X, Li H and Liu J: The long
non-coding RNA HOTAIR activates autophagy by upregulating ATG3 and
ATG7 in hepatocellular carcinoma. Mol Biosyst. 12:2605–2612. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma Y, Huang D, Yang F, Tian M, Wang Y,
Shen D, Wang Q, Chen Q and Zhang L: Long non-coding RNA highly
upregulated in liver cancer regulates the tumor necrosis
factor-α-induced apoptosis in human vascular endothelial cells. DNA
Cell Biol. 35:296–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee M, Kim HJ, Kim SW, Park SA, Chun KH,
Cho NH, Song YS and Kim YT: The long non-coding RNA HOTAIR
increases tumour growth and invasion in cervical cancer by
targeting the Notch pathway. Oncotarget. 7:44558–44571. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Serghiou S, Kyriakopoulou A and Ioannidis
JP: Long non-coding RNAs as novel predictors of survival in human
cancer: A systematic review and meta-analysis. Mol Cancer.
15:502016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Malek E, Jagannathan S and Driscoll JJ:
Correlation of long non-coding RNA expression with metastasis, drug
resistance and clinical outcome in cancer. Oncotarget. 5:8027–8038.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Zhang P, Wang L, Piao HL and Ma
L: Long non-coding RNA HOTAIR in carcinogenesis and metastasis.
Acta Biochim Biophys Sin. 46:1–5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Z, Zhou L, Wu LM, Lai MC, Xie HY,
Zhang F and Zheng SS: Overexpression of long non-coding RNA HOTAIR
predicts tumor recurrence in hepatocellular carcinoma patients
following liver transplantation. Ann Surg Oncol. 18:1243–1250.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Green ED, Watson JD and Collins FS: Human
Genome Project: Twenty-five years of big biology. Nature.
526:29–31. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Evans JR, Feng FY and Chinnaiyan AM: The
bright side of dark matter: lncRNAs in cancer. J Clin Invest.
126:2775–2782. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Huang H, Li Y, Li L, Hou W and You
Z: Decreased expression of long non-coding RNA GAS5 promotes cell
proliferation, migration and invasion, and indicates a poor
prognosis in ovarian cancer. Oncol Rep. 36:3241–3250.
2016.PubMed/NCBI
|
|
22
|
Jiao C, Song Z, Chen J, Zhong J, Cai W,
Tian S, Chen S, Yi Y and Xiao Y: lncRNA-UCA1 enhances cell
proliferation through functioning as a ceRNA of Sox4 in esophageal
cancer. Oncol Rep. 36:2960–2966. 2016.PubMed/NCBI
|
|
23
|
Xu S, Yi XM, Tang CP, Ge JP, Zhang ZY and
Zhou WQ: Long non-coding RNA ATB promotes growth and
epithelial-mesenchymal transition and predicts poor prognosis in
human prostate carcinoma. Oncol Rep. 36:10–22. 2016.PubMed/NCBI
|
|
24
|
Lee N and Kim D: Cancer metabolism:
Fueling more than just growth. Mol Cells. 39:847–854. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, et
al: Functional demarcation of active and silent chromatin domains
in human HOX loci by non-coding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hajjari M and Salavaty A: HOTAIR:
An oncogenic long non-coding RNA in different cancers. Cancer Biol
Med. 12:1–9. 2015.PubMed/NCBI
|
|
27
|
Soudyab M, Iranpour M and Ghafouri-Fard S:
The role of long non-coding RNAs in breast cancer. Arch Iran Med.
19:508–517. 2016.PubMed/NCBI
|
|
28
|
Zhao W, Dong S, Duan B, Chen P, Shi L, Gao
H and Qi H: HOTAIR is a predictive and prognostic biomarker for
patients with advanced gastric adenocarcinoma receiving
fluorouracil and platinum combination chemotherapy. Am J Transl
Res. 7:1295–1302. 2015.PubMed/NCBI
|
|
29
|
Gao JZ, Li J, Du JL and Li XL: Long
non-coding RNA HOTAIR is a marker for hepatocellular carcinoma
progression and tumor recurrence. Oncol Lett. 11:1791–1798.
2016.PubMed/NCBI
|
|
30
|
Ding C, Cheng S, Yang Z, Lv Z, Xiao H, Du
C, Peng C, Xie H, Zhou L, Wu J, et al: Long non-coding RNA
HOTAIR promotes cell migration and invasion via
down-regulation of RNA binding motif protein 38 in hepatocellular
carcinoma cells. Int J Mol Sci. 15:4060–4076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fu WM, Zhu X, Wang WM, Lu YF, Hu BG, Wang
H, Liang WC, Wang SS, Ko CH, Waye MM, et al: Hotair mediates
hepatocarcinogenesis through suppressing miRNA-218 expression and
activating P14 and P16 signaling. J Hepatol. 63:886–895. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ellis BC, Graham LD and Molloy PL: CRNDE,
a long non-coding RNA responsive to insulin/IGF signaling,
regulates genes involved in central metabolism. Biochim Biophys
Acta. 1843:372–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Z, Li X, Wu S, Xue M and Chen W: Long
non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase
2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci.
105:951–955. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun HW, Yu XJ, Wu WC, Chen J, Shi M, Zheng
L and Xu J: GLUT1 and ASCT2 as predictors for prognosis of
hepatocellular carcinoma. PLoS One. 11:e01689072016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amann T, Maegdefrau U, Hartmann A, Agaimy
A, Marienhagen J, Weiss TS, Stoeltzing O, Warnecke C, Schölmerich
J, Oefner PJ, et al: GLUT1 expression is increased in
hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol.
174:1544–1552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou J, Zhi X, Wang L, Wang W, Li Z, Tang
J, Wang J, Zhang Q and Xu Z: Erratum to: Linc00152 promotes
proliferation in gastric cancer through the EGFR-dependent pathway.
J Exp Clin Cancer Res. 35:302016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li H, Yu B, Li J, Su L, Yan M, Zhu Z and
Liu B: Overexpression of lncRNA H19 enhances carcinogenesis and
metastasis of gastric cancer. Oncotarget. 5:2318–2329. 2014.
View Article : Google Scholar : PubMed/NCBI
|