Introduction
The morbidity of prostate cancer in the world is
25.3/100,000, which ranks in the second place among all malignant
tumors in male (1). In addition,
its morbidity is associated with distinct geographical and racial
differences, with countries such as America, Canada, Australia/New
Zealand, Northern Europe and Western Europe having higher morbidity
(1). The morbidity of prostate
cancer in America has surpassed that of lung cancer, making it the
top tumor threatening male health. It is estimated by the American
Cancer Society that approximately 217,730 new prostate cancer cases
occurred in America in 2010, and 32,050 of them die of such disease
(2). Prostate cancer is the most
common cancer in male in European Union, which accounts for 18.1%
of all new cases. Approximately 2.6 million out of the new prostate
cancer cases are confirmed every year. Prostate cancer takes up 11%
of all male cancers and accounts for 9% of all male cancer deaths
(3).
Autophagy is an evolutionally conserved process in
eukaryotic cell that is regulated by genes. During such dynamic
process, regulating degradation of intracellular proteins and
organelles contributes to the formation of intracellular
autophagosome with double-layer membrane structure, so that
cytoplasm, proteins and organelles can be partly degraded for
recycling (4). The formation of
autophagosome is a multi-step process involving multiple
autophagosome-related proteins and 2 ubiquitin-like covalence
systems (5). Such process can
induce fusion of autophagosome with lysosome to form the autophagic
lysosome; and lysosomal hydrolase will degrade the contents that it
swallows (5). Autophagy is
effective in treating prostate cancer, because it can enhance
sensitivity of tumor cells to various therapies, including DNA
damaging agent, anti-hormone therapy (such as aniline) and
radiotherapy (6).
Endoplasmic reticulum (ER) is a kind of
membrane-bound organelle in eukaryotic cells, which is mainly
responsible for the correct folding and post-translational
modification of membrane proteins and secretory proteins (7). In addition, ER also plays an important
role in biosynthesis of lipids, energy metabolism, intracellular
Ca2+ homeostasis and redox equilibrium. Protein folding
function in ER is extremely sensitive to extracellular and
intracellular stimulations, including ischemia reperfusion,
inflammation, glycosylation and Ca2+ disequilibrium
(8). Aggregation of misfolded or
unfolded proteins in ER lumen will induce endoplasmic reticulum
stress (ER stress), thus, activating unfolded protein response
(9).
Death-associated protein kinase-3 (DAPk3) is a kind
of Ca2+/CaM-regulating serine/threonine protein kinase
discovered by Israel scientist Adi Kimchi by means of gene knockout
in 1995. At first, DAPk3 was discovered as a canonical tumor
suppressor gene (10). In recent
years, increasing studies have verified that DAPk3 is involved in
multiple cellular functions. In addition, it participates in
multiple signal pathways to regulate apoptosis, autophagy,
caspase-dependent cell death, adhesion and migration (11). Therefore, it not only plays a
certain role in inflammatory response, but also exerts antitumor
functions inhibiting metastasis (11).
PI3K/Akt pathway is one of the important
intracellular signal transduction pathways, which exerts extremely
important biological effects in multiple physiological functions,
such as cell growth, proliferation, apoptosis, angiogenesis and
autophagy, as well as development and protection of the nervous
system (12). Disorder of such
pathway will result in multiple diseases, such as cancer genesis
and progression, nervous system disease, autoimmune disease and
hematopoietic system disease (13).
Traditional Chinese herbal medicines are verified to
be extremely effective through thousands of years of practice, but
compositions and mechanisms of action of numerous Chinese herbal
medicines remain unclear (14).
Therefore, Chinese herbal medicine monomer with safe and wide
source as well as definite action is the first choice in drug
screening (15). Bark of plants of
Anacardiaceae is frequently used in treating gastric ulcer,
gastritis and gastric cancer in Mexico (16). Anacardic acid is an important
antitumor bioactive substance purified from Amphipterygium
adstringens, the bark of which is used traditionally to treat
gastric ulcer, gastritis and gastric cancer (17). Recent research has indicated that
anacardic acid possesses the effects of inhibiting proliferation of
multiple tumor cells (such as lung cancer, liver cancer, multiple
myeloma and prostate cancer) and inducing apoptosis (18). In the present study, we investigated
the anticancer effects of anacardic acid on cell apoptosis of
prostatic cancer and molecular mechanisms of this phenomenon.
Materials and methods
Cell lines and cell culture
Human prostate carcinoma cell line LNCaP cells were
obtained from the American Type Cell Culture (ATCC; Manassas, VA,
USA) and cultured in Dulbeccos modified Eagles medium (DMEM;
HyClone Laboratories, Inc., Logan, UT, USA), 10% fetal bovine serum
(FBS; HyClone Laboratories) at 37°C and 5% CO2.
Proliferation assay
LNCaP cells were treated with 12.5, 25 and 50 µM of
anacardic acid for 24, 48 and 72 h.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bro mide
(MTT; Sigma-Aldrich, St. Louis, MO, USA) was added into cells and
incubated for 4 h at 37°C. Old medium was removed and dimethyl
sulfoxide (DMSO) was added for solution at 37°C for 20 min. The
intensity was measured using an ELISA reader (Molecular Devices,
Sunnyvale, CA, USA) at 490 nm.
Apoptosis assay
LNCaP cells were treated with 12.5, 25 and 50 µM of
anacardic acid for 48 h. LNCaP cells were stained with Annexin
V/FITC (Becton-Dickinson, San Jose, CA, USA) and prodium iodide
(PI; Becton-Dickinson) at darkness for 30 min. Apoptosis was
quantitatively estimated on a FACScan flow cytometry
(Becton-Dickinson).
Western blot analysis
Cells were washed with phosphate-buffered saline
(PBS) and split using RIPA assay (Pierce, Rockford, IL, USA). The
protein was quantified by a BCA assay (Pierce). Denatured proteins
were separated by 8–12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and then transferred to nitrocellulose
(Amersham, Bensheim, Germany). Nitrocellulose was blocked with
5%-BSA in TBST and incubated with Bax, BiP, CHOP, p-eIF2α, LC3,
Beclin-1, Atg 7, DAPK3, p-Akt, p-mTOR and GAPDH (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) at 4°C overnight.
Nitrocellulose was washed with TBST, incubated with horseradish
peroxidase (HRP)-conjugated appropriate secondary antibodies and
reacted with ECL detection reagents (Amersham Bioscience).
Statistical analysis
All data are shown as mean ± SD. Comparison of
multiple groups was made with the one-way ANOVA followed by the
Tukeys test or the Newman-Keuls test. Differences with P<0.05
were considered statistically significant.
Results
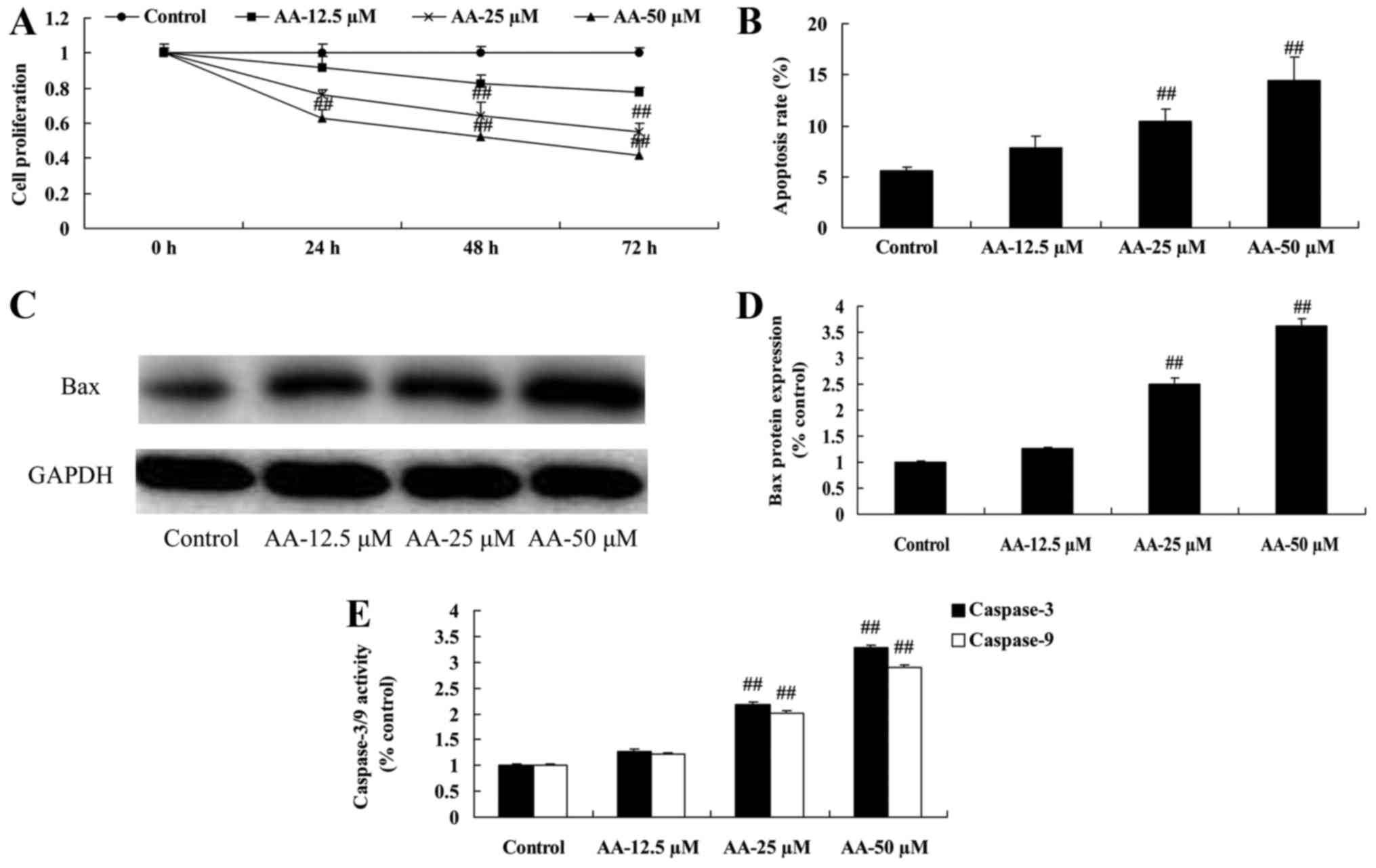
The effects of anacardic acid on cell
proliferation and apoptosis of prostatic cancer cells
In our initial study, cell proliferation and
apoptosis of prostatic cancer cell were determined. As shown in
Fig. 1A and B, anacardic acid
effectively inhibited cell proliferation and induced apoptosis of
prostatic cancer cells. Moreover, anacardic acid significantly
induced Bax promoted and caspase-3/9 activities of prostatic cancer
cell (Fig. 1C-E).
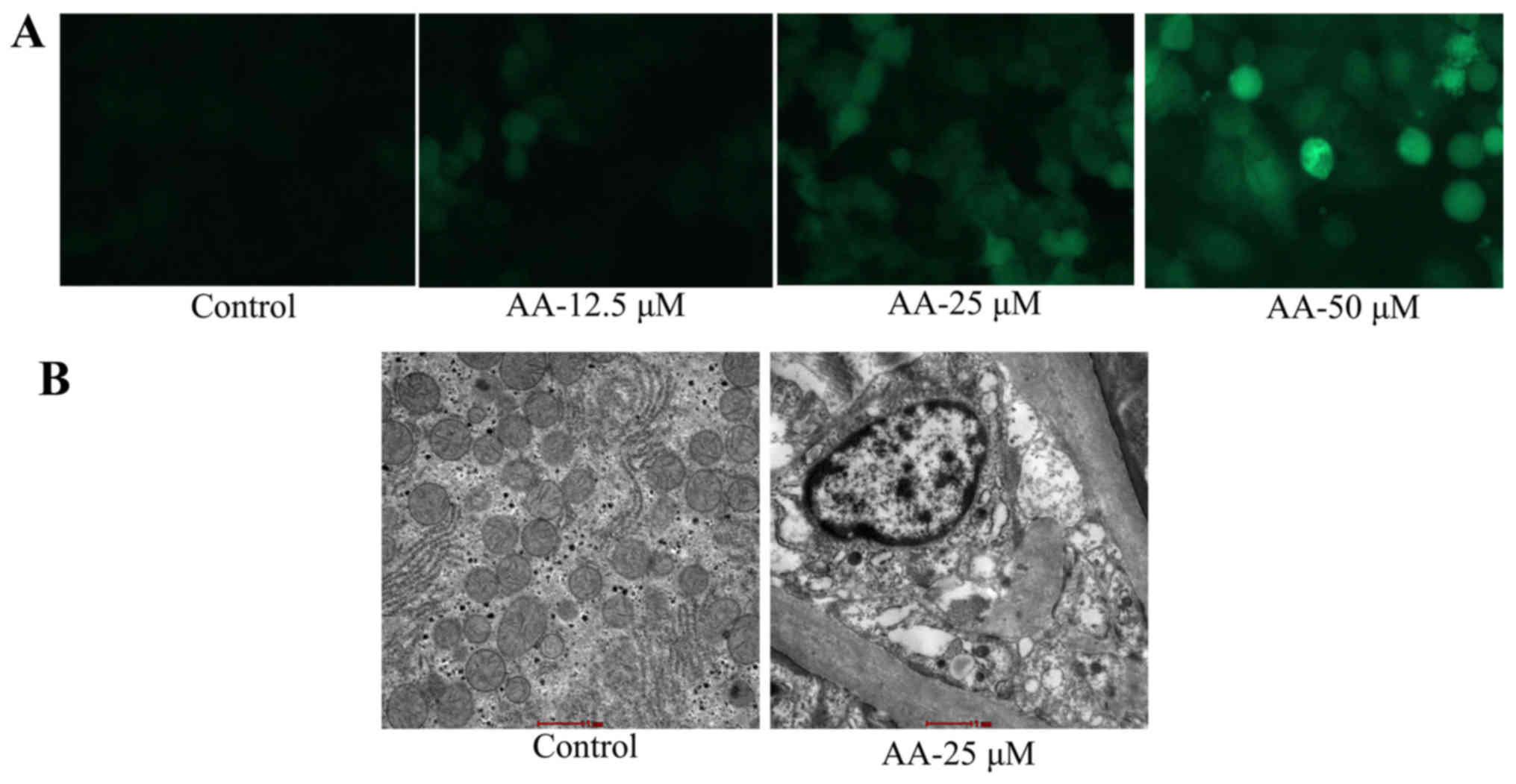
The effects of anacardic acid on
autophagy of prostatic cancer cells
We evaluated the anticancer effects of anacardic
acid on autophagy of prostatic cancer cells. As shown in Fig. 2A, anacardic acid caused autophagy
and LC3 protein expression of prostatic cancer cells in a
dose-dependent manner. Anacardic acid significantly produced
autophagy vesicles of prostatic cancer cells (Fig. 2B).
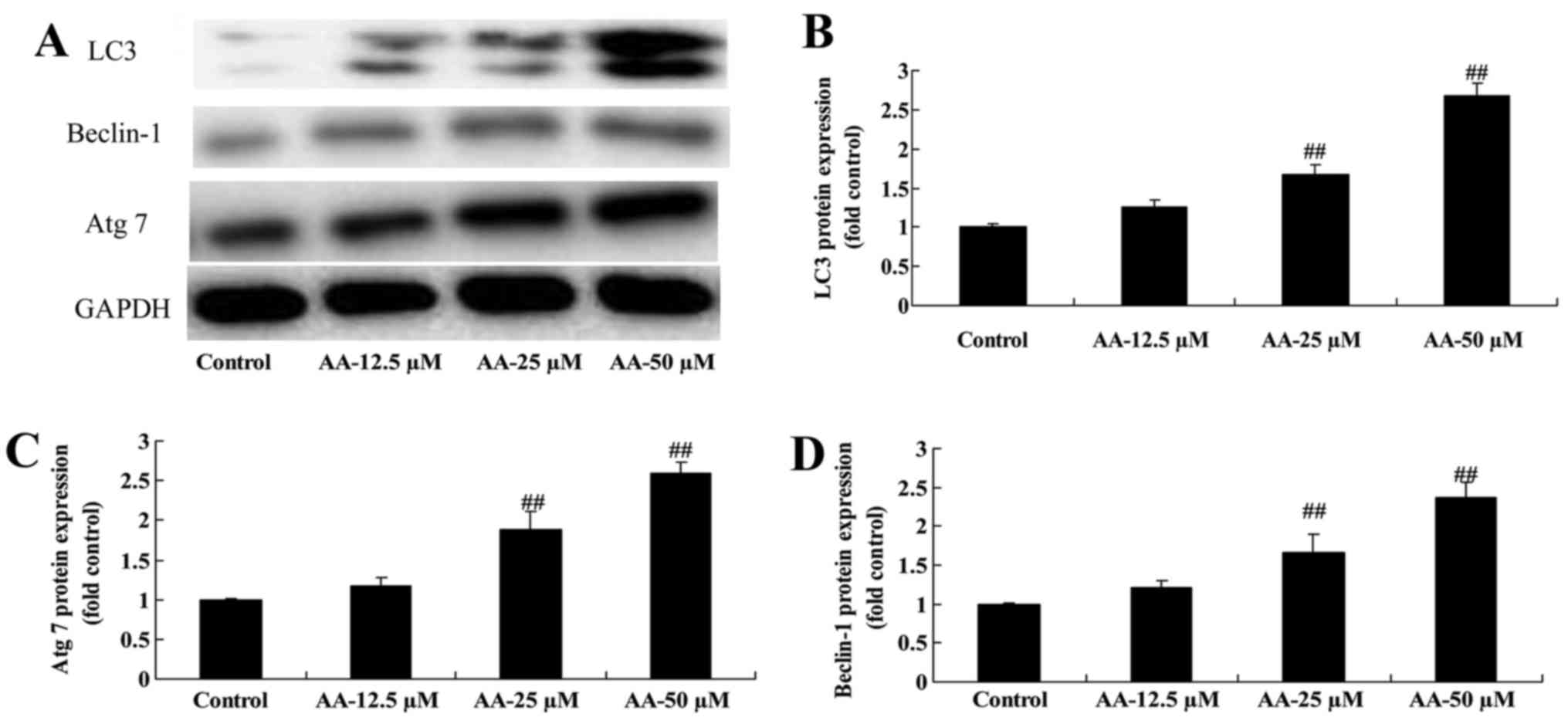
The effects of anacardic acid on
autophagy associated protein of prostatic cancer cells
We determined whether the effects of anacardic acid
on autophagy associated with protein of prostatic cancer cells. The
results in Fig. 3 revealed that
anacardic acid significantly induced LC3, Beclin-1 and Atg 7
protein expression of prostatic cancer cells in a dose-dependent
manner.
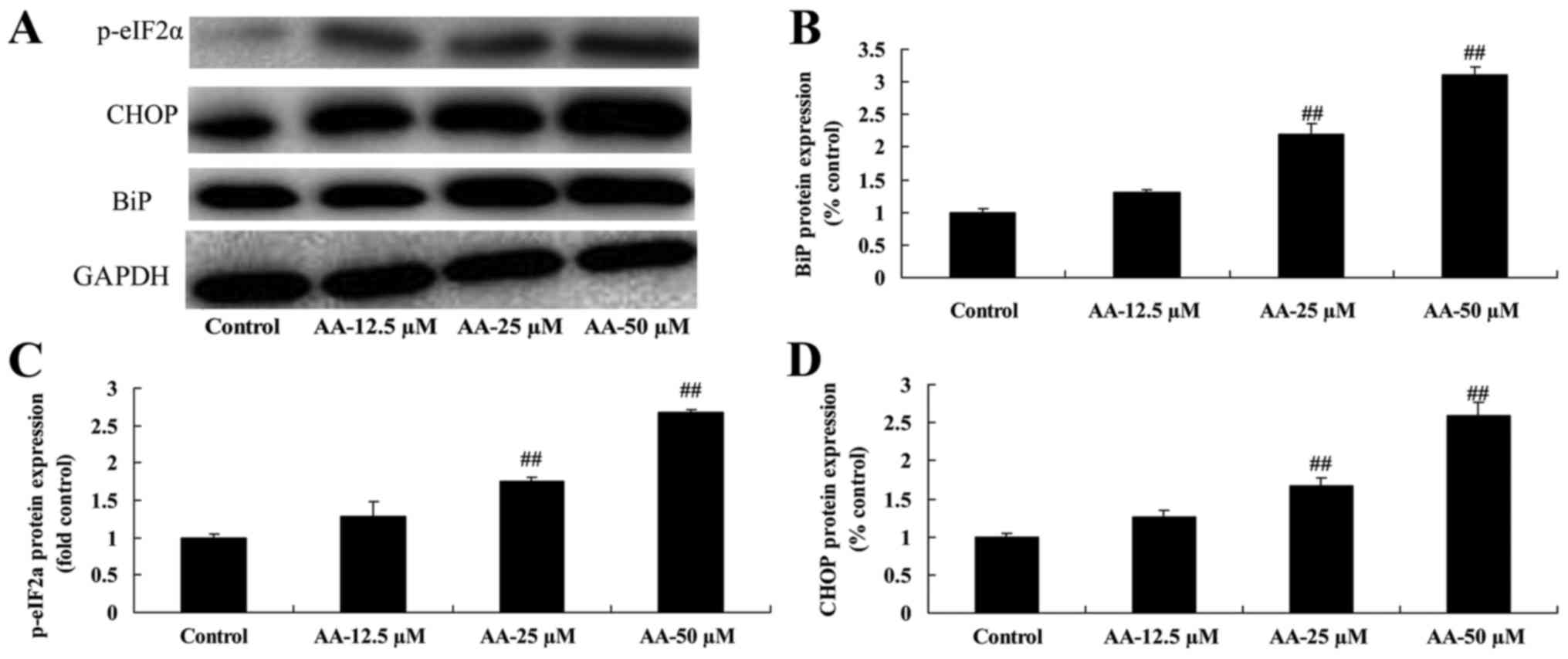
The effects of anacardic acid on ER
stress of prostatic cancer cells
To determine whether ER stress participates in the
anticancer effects of anacardic acid on prostatic cancer cell
growth, BiP, CHOP and p-eIF2α protein expression was measured using
western blot analysis. The results in Fig. 4 showed that anacardic acid
significantly increased ER stress, and induced BiP, CHOP and
p-eIF2α protein expression of prostatic cancer cells in a
dose-dependent manner.
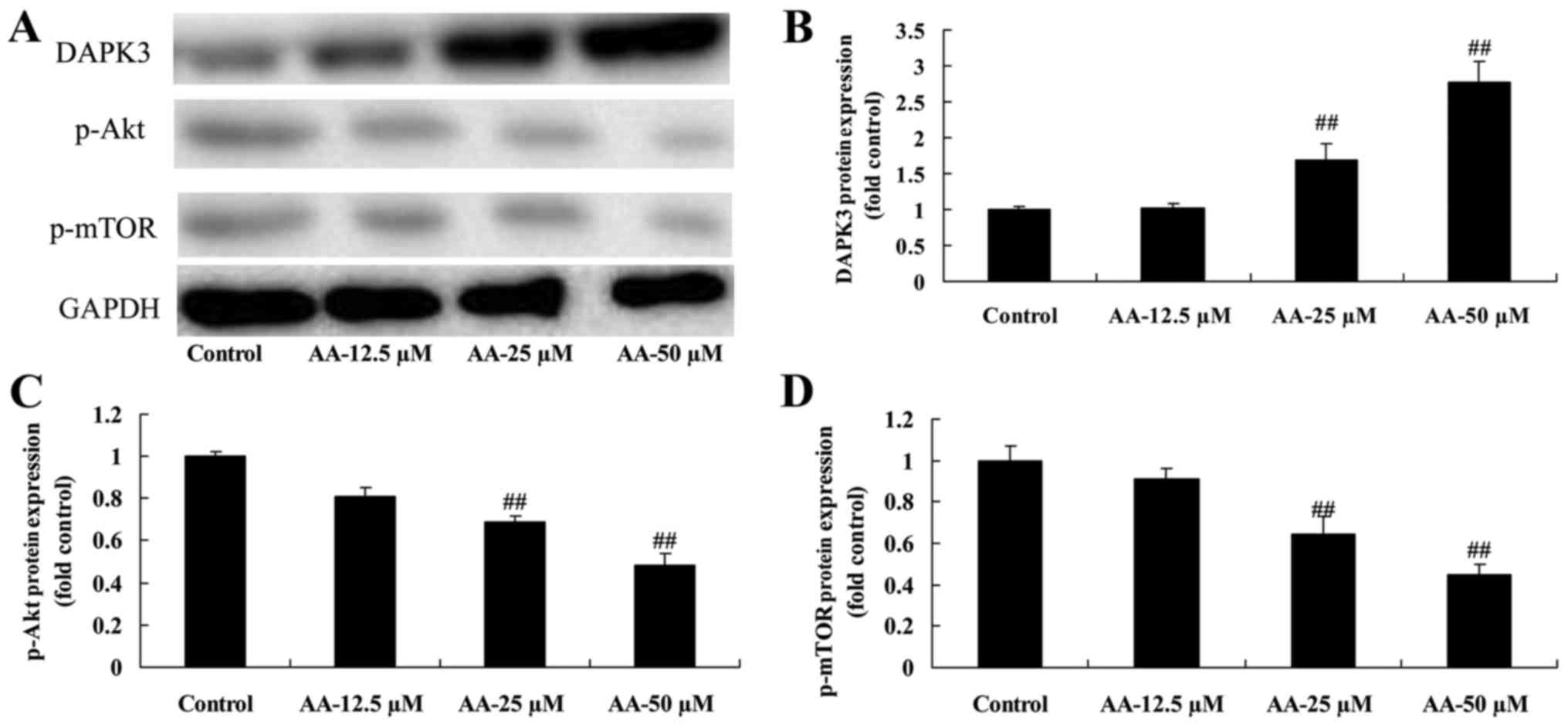
The effects of anacardic acid on
DAPK3/Akt signaling of prostatic cancer cells
In order to further prove the autophagy induced by
anacardic acid in prostatic cancer cells, DAPK3/Akt signaling was
measured using western blot analysis. Treatment with anacardic acid
significantly induced DAPK3 and suppressed p-Akt and p-Mtor protein
expression in prostatic cancer cells in a dose-dependent manner
(Fig. 5).
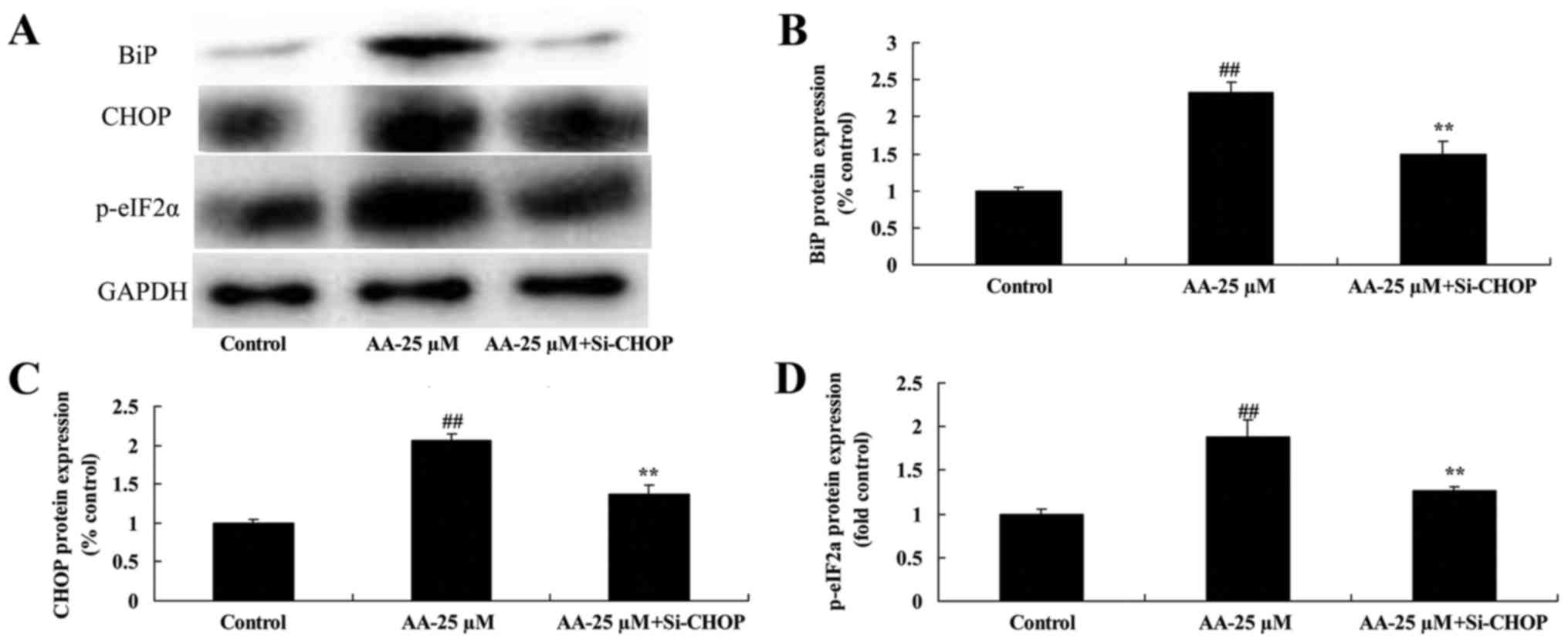
The inhibition of ER stress in
prostatic cancer cell treated by anacardic acid
To investigate whether ER stress participated in
apoptosis of prostatic cancer cells treated by anacardic acid, we
performed si-CHOP transiently. The induction of BiP, CHOP and
p-eIF2α protein expression by anacardic acid was significantly
suppressed in prostatic cancer cells (Fig. 6).
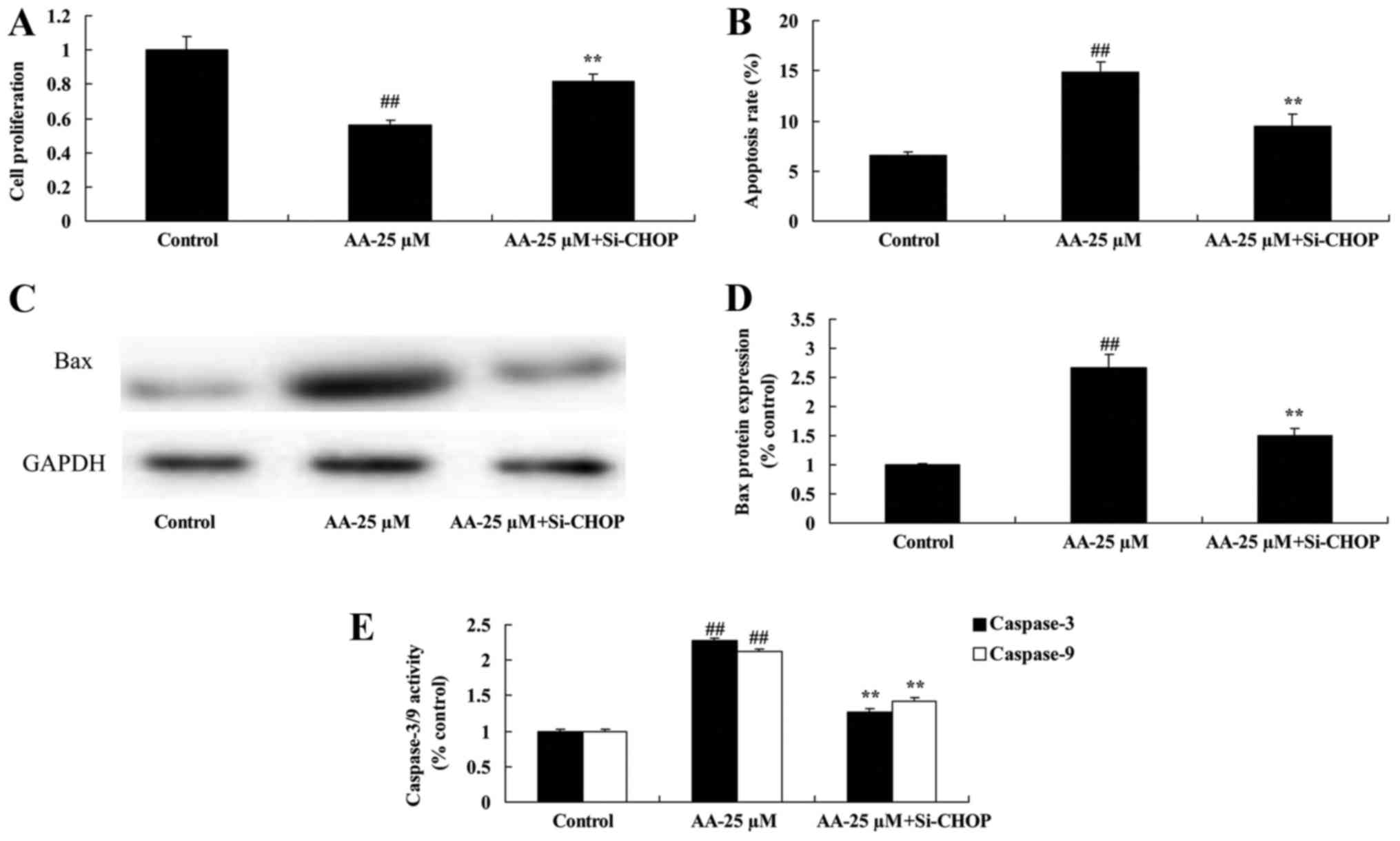
The inhibition of ER stress on cell
proliferation and apoptosis of prostatic cancer cells treated by
anacardic acid
We tested the inhibition of ER stress on cell
proliferation and apoptosis of prostatic cancer cells treated by
anacardic acid. The result indicated that the inhibition of ER
stress significantly reversed the anticancer effects of anacardic
acid on the cell proliferation inhibition and induction of
apoptosis, Bax and caspase-3/9 activities of prostatic cancer cells
(Fig. 7).
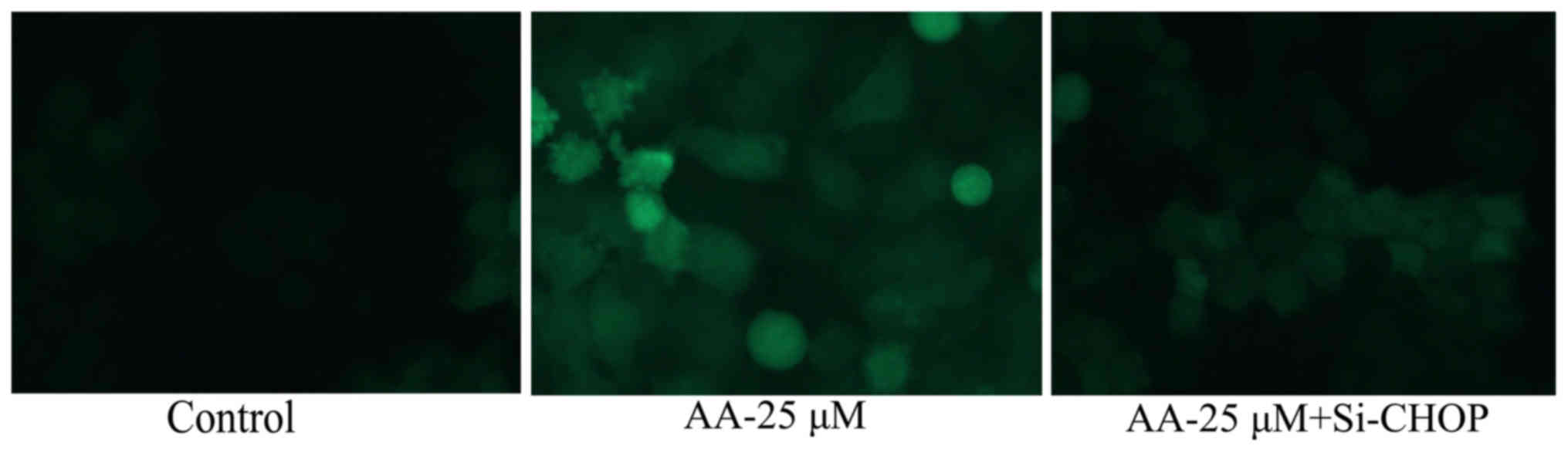
Inhibition of ER stress on autophagy
of prostatic cancer cells treated by anacardic acid
Fig. 8 shows that
the inhibition of ER stress significantly inhibited autophagy and
LC3 protein expression of prostatic cancer cells.
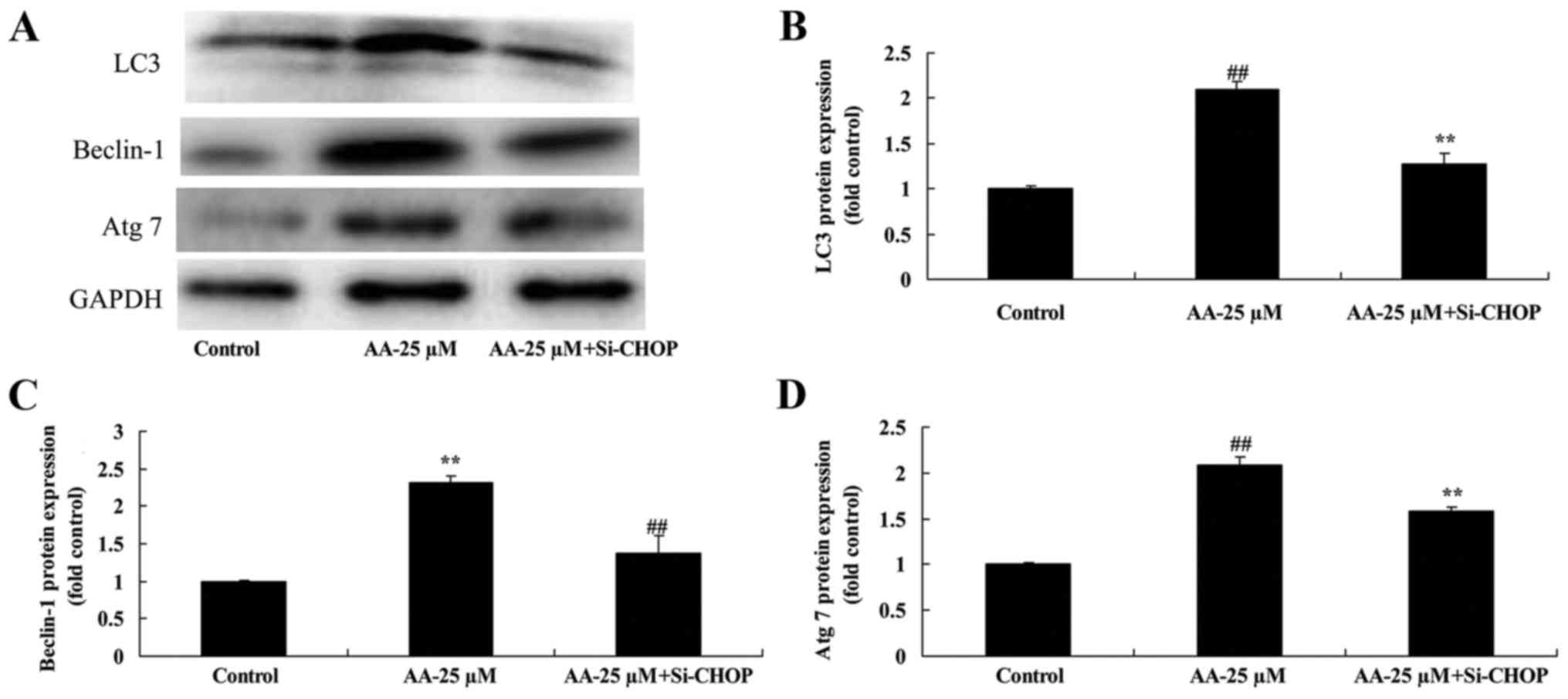
Inhibition of ER stress on autophagy
associated protein of prostatic cancer cells treated by anacardic
acid
Next, to investigate crosstalk between ER stress and
autophagy in prostatic cancer cells treated by anacardic acid, we
examined LC3, Beclin-1 and Atg 7 protein expression. Notably, the
inhibition of ER stress significantly suppressed the increase of
LC3, Beclin-1 and Atg 7 protein expression of prostatic cancer
cells treated by anacardic acid (Fig.
9).
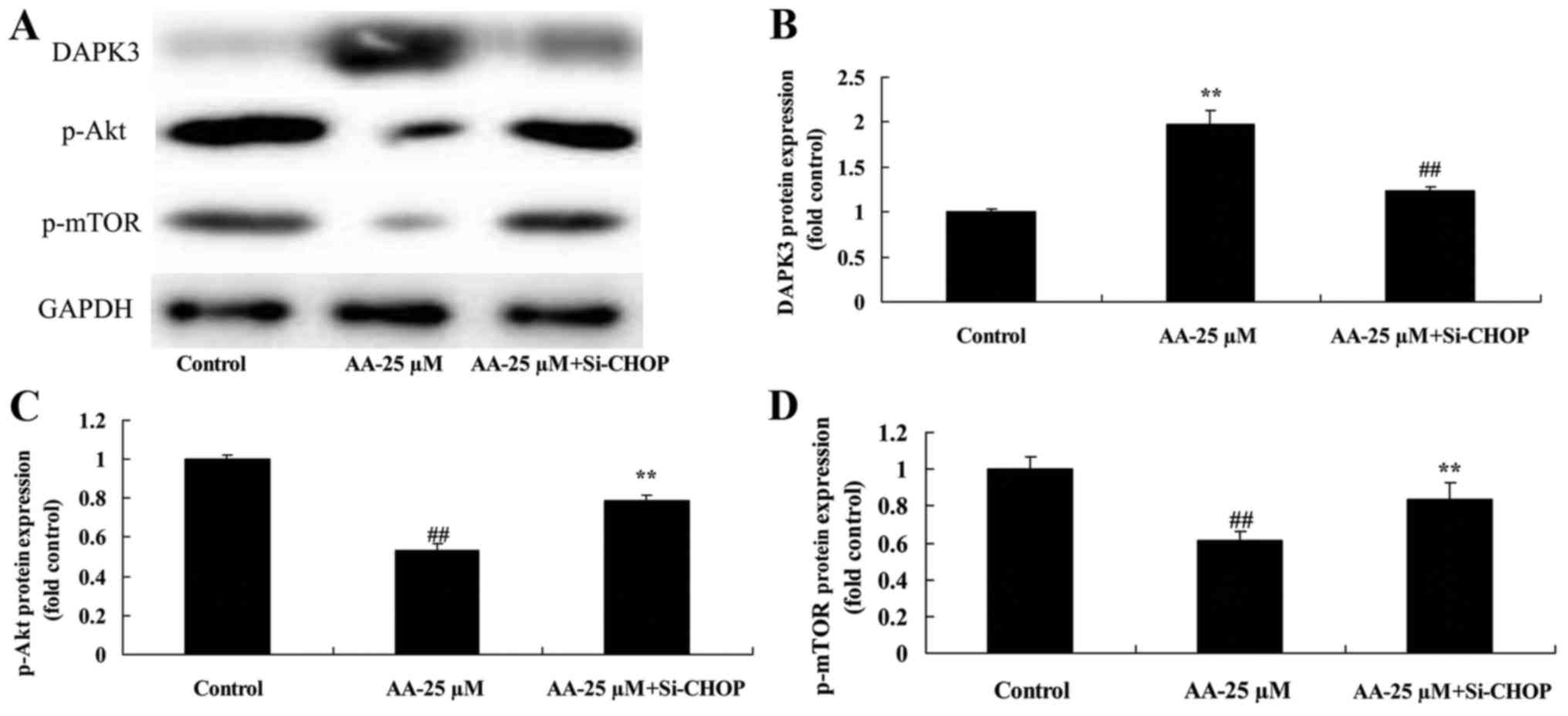
The inhibition of ER stress on
DAPK3/Akt signaling of prostatic cancer cell treated by anacardic
acid
We further confirmed that the inhibition of ER
stress affects on DAPK3/Akt signaling of prostatic cancer cell
treated by anacardic acid. As shown in Fig. 10, the inhibition of ER stress
significantly suppressed DAPK3 protein expression, and induced
p-Akt and p-mTOR protein expression of prostatic cancer cells
treated by anacardic acid.
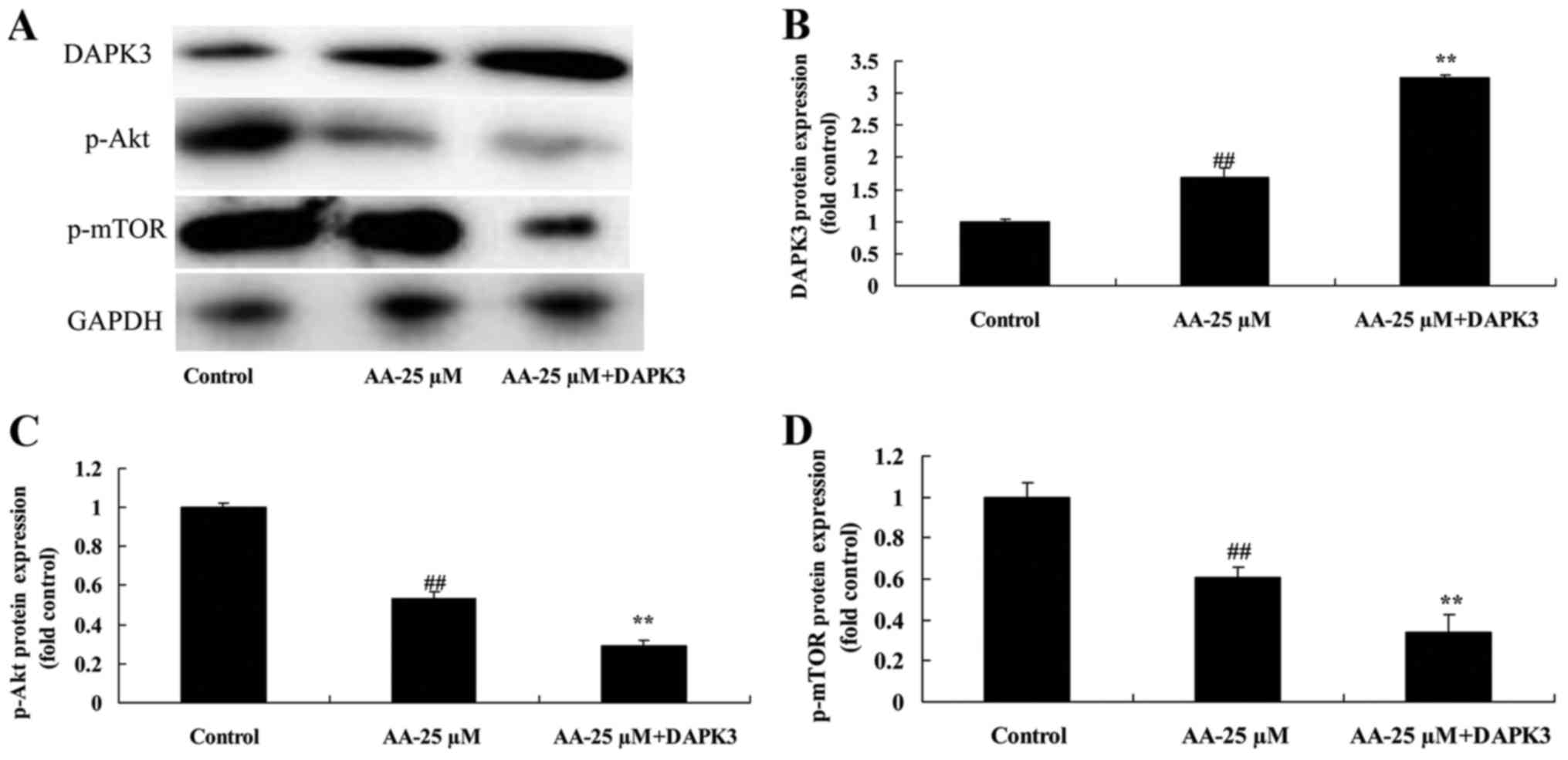
The promotion of DAPK3 on DAPK3/Akt
signaling of prostatic cancer cell treated by anacardic acid
We next analyzed the role of DAPK3 in DAPK3/Akt
signaling of prostatic cancer cell treated by anacardic acid. As
shown in Fig. 11, DAPK3 protein
increased DAPK3 protein expression, and suppressed p-Akt and p-mTOR
protein expression of prostatic cancer cells treated by anacardic
acid.
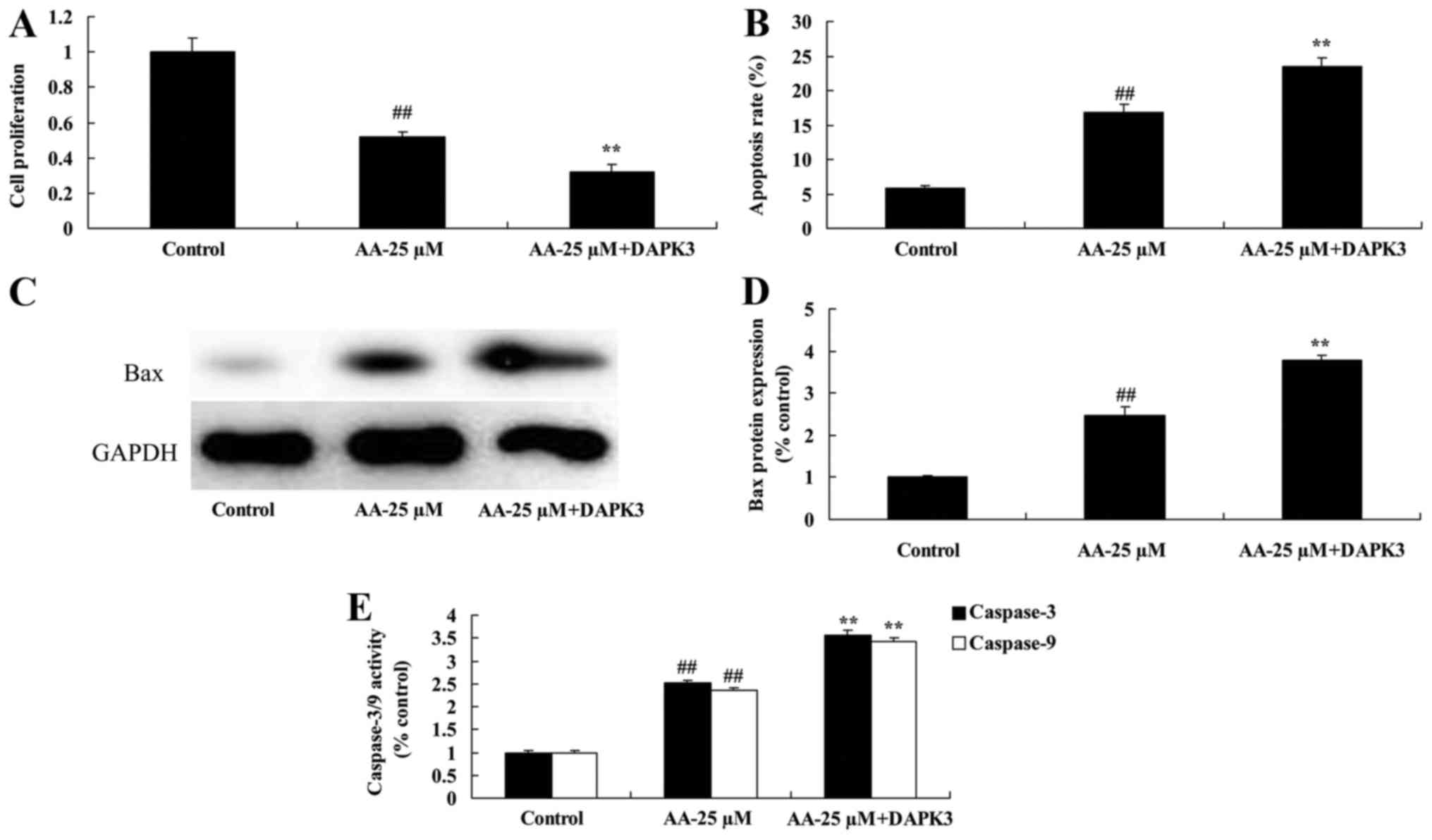
The promotion of DAPK3 on cell
proliferation and apoptosis of prostatic cancer cell treated by
anacardic acid
We confirmed whether the promotion of DAPK3 on cell
proliferation and apoptosis of prostatic cancer cell treated by
anacardic acid. The inhibition of cell proliferation and promotion
of apoptosis of prostatic cancer cell treated by anacardic acid
were effectively enhanced by the promotion of DAPK3 (Fig. 12A and B). There were significant
increases of Bax protein and caspase-3/9 activities in prostatic
cancer cell treated by anacardic acid (Fig. 12C-E).
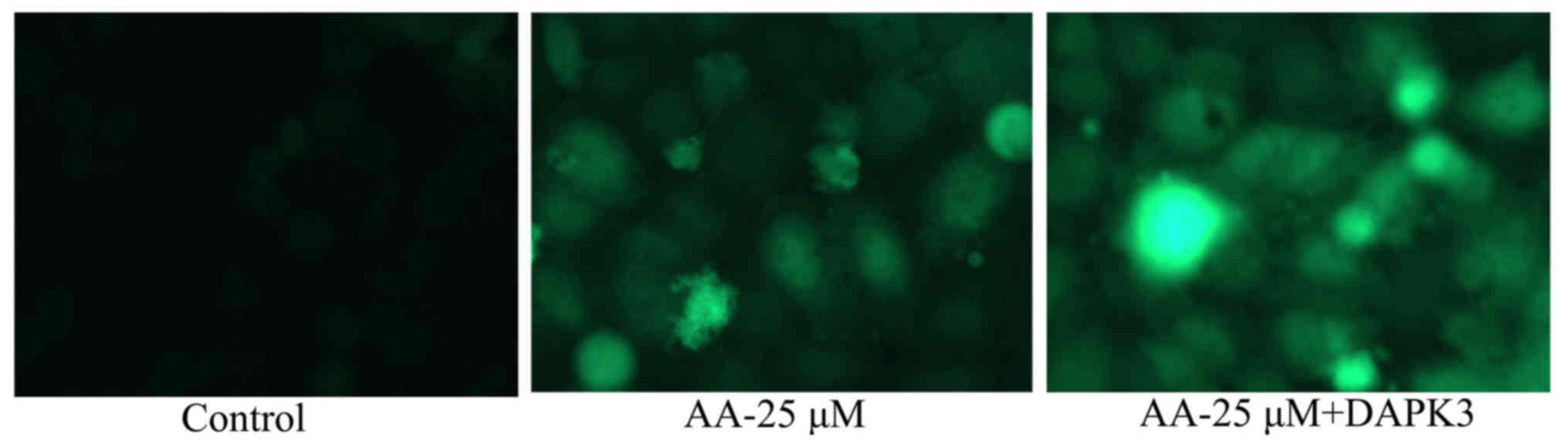
The promotion of DAPK3 on autophagy of
prostatic cancer cell treated by anacardic acid
To confirm the pro-apoptotic function of DAPK3 on
autophagy of prostatic cancer cell treated by anacardic acid, we
observed autophagy of prostatic cancer cell. The autophagy and LC3
protein expression in prostatic cancer cells treated by anacardic
acid were significantly increased by the promotion of DAPK3
(Fig. 13).
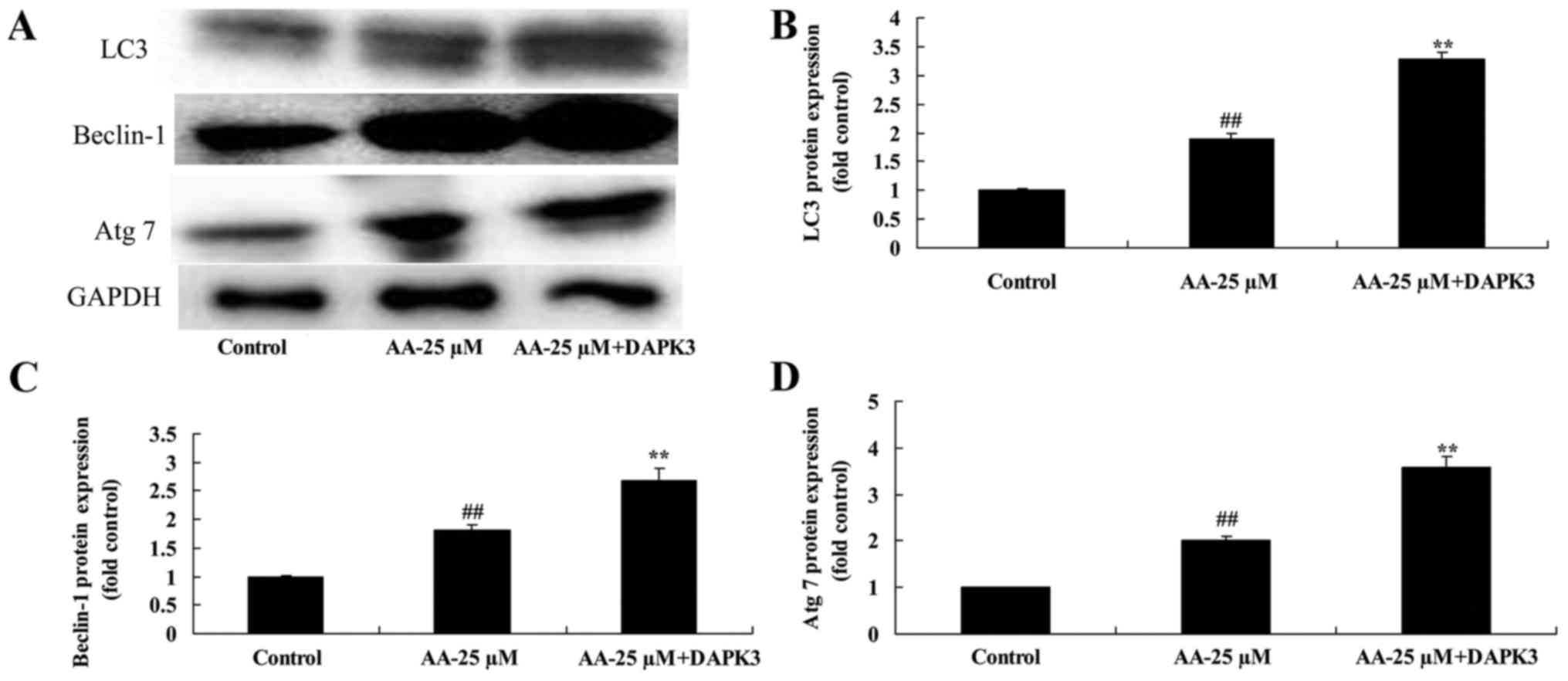
The promotion of DAPK3 on autophagy
associated protein of prostatic cancer cells treated by anacardic
acid
Next, we analyzed autophagy associated protein of
prostatic cancer cells treated by anacardic acid + the promotion of
DAPK3. DAPK3 overexpressing cells exhibited induction of LC3,
Beclin-1 and Atg 7 protein expression of prostatic cancer cells
treated by anacardic acid (Fig.
14).
Discussion
Prostate cancer is a malignant tumor with the
highest morbidity in male in America, which is only second to lung
cancer and ranks in the second place in malignant tumor-related
mortality, and it takes the third place in Europe (19). In China, the morbidity of prostate
cancer is increasing year by year with the universally improved
living standard, as well as diagnosis and treatment levels
(20). Generally speaking, prostate
cancer grows slowly and may be restricted in prostate for years,
and many old people are discovered with prostate cancer in autopsy
(2). In the present study, we found
that anacardic acid inhibited cell proliferation, induced
apoptosis, and caspase-3/9 activities and Bax protein expression of
prostatic cancer. Yao et al (21) reported that anacardic acid
sensitizes radiation therapy-prostate cancer cells by regulating
H2AX expression (21).
Autophagy is a genetic programming and evolutionally
conserved process, which degrades the excess, harmful or aging
proteins and organelles, controls over important cellular
components, and plays an important role in normal organism
(22). It has been discovered that
autophagy is closely related to tumors, which plays an important
role in tumor genesis and development (23). In prostate cancer cells, autophagy
plays an important role in promoting progression and metastasis of
prostate cancer. Besides, it can protect prostate cancer cells from
external environment changes, and develops resistance to agents in
treatment of prostate cancer (24).
Our data showed that anacardic acid induced ER stress prostatic
cancer. Moreover, anacardic acid induced autophagy and ER stress of
prostatic cancer. Seong et al (18) showed that anacardic acid induces ER
stress and autophagy of human lung carcinoma A549 cells.
At the early stage of ER stress, protein synthesis
within ER will be reduced, and related genes regulating protein
translation and correct folding will be activated, which
contributes to maintaining the normal physiological functions of
cells, thus, promoting cell survival (25). However, a large amount of misfolded
or unfolded proteins will be accumulated in ER in the case of
excessive ER duration or UPR function impairment, at this moment,
pro-apoptosis signal will be activated (26). At present, it is indicated in
research that ER dysfunction of myocardial cells and pancreatic
cells may be the major pathogenesis leading to cardio-cerebral
tissue ischemic blocking and diabetes (9). Therefore, ER stress can either serve
as a survival means to maintain cell survival, or an important
mechanism inducing cell apoptosis (27). In this study, we found that the
inhibition of ER stress inhibited the anticancer effects of
anacardic acid on apoptosis and autophagy of prostatic cancer.
PI3K/Akt signal pathway transmits multiple growth
factor and cytokine signals into cells, influences cell
proliferation, survival and apoptosis, and promotes malignant
transformation of cells (28).
Furthermore, it is related to numerous links such as tumor cell
migration, adhesion, tumor angiogenesis and extracellular matrix
degradation (13). Abnormally
elevated protein expression and kinase activity of PI3K/Akt signal
pathway can be seen in multiple human malignant tumors, such as
ovarian, prostate, multiple myeloma, breast, pancreatic, lung,
endometrial, follicular thyroid cancer and melanoma (13). In addition, the signals that it
transmits are remarkably amplified. PI3K/Akt signal pathway is in a
central position among numerous signal transduction pathways, and
plays an important role in tumor genesis and development (29). However, in the present study,
anacardic acid suppressed Akt signaling pathway in prostatic
cancer. Xiu et al (30)
demonstrated that anacardic acid enhances the proliferation of
human ovarian cancer cells through PI3K, VEGF and caspase-3
pathways.
DAPK3 is extensively, but conservatively distributed
in multiple species (31). Human
DAPK3 locates in chromosome 19, which locates in the cell nucleus
as it has nuclear localization signal. DAPK3 forms a dimer through
C-terminal domain, thus activating its kinase activity; besides, it
induces apoptosis through the phosphorylation of substrates
(10). Plenty of data have
demonstrated that DAPK3 can exert its apoptosis-inducing function
through phosphorylating multiple substrates. In addition,
endonuclear DAPK3 can interact with PML body, thus, inducing
apoptosis through activating PAR-4 protein by phosphorylation
(32). We also detected that the
promotion of DAPK3 inhibited the anticancer effects of anacardic
acid on autophagy of prostatic cancer.
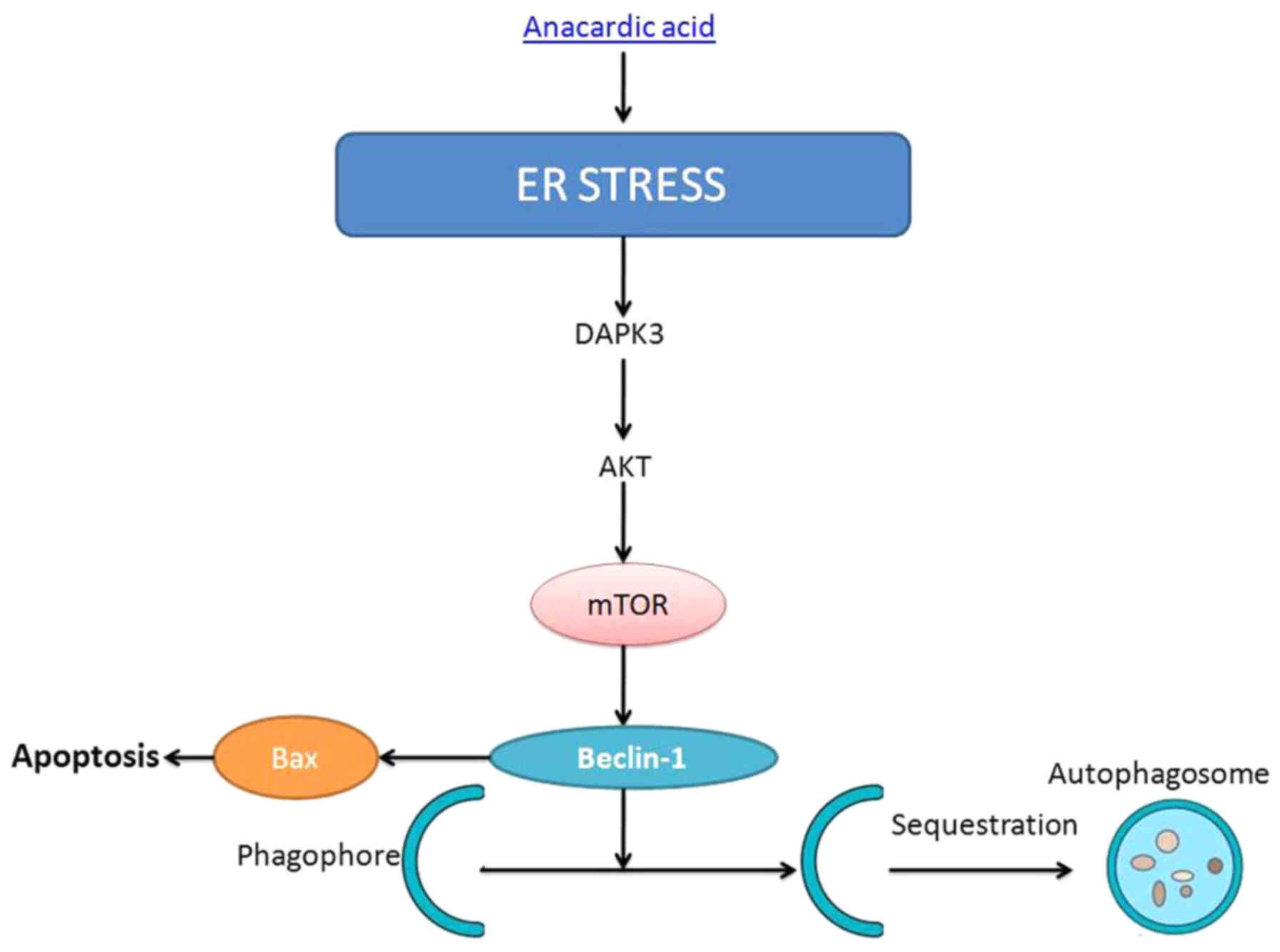
In summary, we demonstrated that the anacardic acid
induces cell apoptosis of prostatic cancer through autophagy by ER
stress/DAPK3/Akt signaling pathway (Fig. 15). Therefore, we suggest that
anacardic acid shows potential as a possible new drug for therapy
of prostatic cancer.
Acknowledgements
This study was funded by the Inherited Project of
Xiangya Famous Doctor of the Third Xiangya Hospital of Central
South University.
References
|
1
|
Aggarwal RR, Beer TM, Weinberg VK, Higano
C, Taplin ME, Ryan CJ, Lin AM, Alumkal J, Graff JN, Nordquist LT,
et al: Intermittent chemotherapy as a platform for testing novel
agents in patients with metastatic castration-resistant prostate
cancer: A Department of Defense Prostate cancer clinical trials
consortium randomized phase II trial of intermittent docetaxel with
prednisone with or without maintenance GM-CSF. Clin Genitourin
Cancer. 13:e191–e198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Figg WD, Chau CH, Madan RA, Gulley JL, Gao
R, Sissung TM, Spencer S, Beatson M, Aragon-Ching J, Steinberg SM,
et al: Phase II study of satraplatin and prednisone in patients
with metastatic castration-resistant prostate cancer: A
pharmacogenetic assessment of outcome and toxicity. Clin Genitourin
Cancer. 11:229–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scheltema MJ, van den Bos W, de Bruin DM,
Wijkstra H, Laguna MP, de Reijke TM and de la Rosette JJ: Focal vs
extended ablation in localized prostate cancer with irreversible
electroporation; a multi-center randomized controlled trial. BMC
Cancer. 16:2992016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo J, Huang X, Wang H and Yang H:
Celastrol induces autophagy by targeting AR/miR-101 in prostate
cancer cells. PLoS One. 10:e01407452015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo J, Mei Y, Li K, Huang X and Yang H:
Downregulation of miR-17-92a cluster promotes autophagy induction
in response to celastrol treatment in prostate cancer cells.
Biochem Biophys Res Commun. 478:804–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liao H, Xiao Y, Hu Y, Xiao Y, Yin Z and
Liu L: microRNA-32 induces radioresistance by targeting DAB2IP and
regulating autophagy in prostate cancer cells. Oncol Lett.
10:2055–2062. 2015.PubMed/NCBI
|
|
7
|
Rah B, ur Rasool R, Nayak D, Yousuf SK,
Mukherjee D, Kumar LD and Goswami A: PAWR-mediated suppression of
BCL2 promotes switching of 3-azido withaferin A (3-AWA)-induced
autophagy to apoptosis in prostate cancer cells. Autophagy.
11:314–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mathur A, Elmageed ZY Abd, Liu X,
Kostochka ML, Zhang H, Abdel-Mageed AB and Mondal D: Subverting
ER-stress towards apoptosis by nelfinavir and curcumin coexposure
augments docetaxel efficacy in castration resistant prostate cancer
cells. PLoS One. 9:e1031092014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang J, Wei J, Wu Y, Wang Z, Guo Y, Lee P
and Li X: Metformin induces ER stress-dependent apoptosis through
miR-708-5p/NNAT pathway in prostate cancer. Oncogenesis.
4:e1582015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujiwara N, Usui T, Ohama T and Sato K:
Regulation of Beclin 1 protein phosphorylation and autophagy by
protein phosphatase 2A (PP2A) and death-associated protein kinase 3
(DAPK3). J Biol Chem. 291:10858–10866. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Das TP, Suman S, John AM Papu, Pal D,
Edwards A, Alatassi H, Ankem MK and Damodaran C: Activation of AKT
negatively regulates the pro-apoptotic function of death-associated
protein kinase 3 (DAPK3) in prostate cancer. Cancer Lett.
377:134–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Utermark T, Schmit F, Lee SH, Gao X,
Schaffhausen BS and Roberts TM: The phosphatidylinositol 3-kinase
(PI3K) isoform dependence of tumor formation is determined by the
genetic mode of PI3K pathway activation rather than by tissue type.
J Virol. 88:10673–10679. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Quan Y, Wang N, Chen Q, Xu J, Cheng W, Di
M, Xia W and Gao WQ: SIRT3 inhibits prostate cancer by
destabilizing oncoprotein c-MYC through regulation of the PI3K/Akt
pathway. Oncotarget. 6:26494–26507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nambiar J, Bose C, Venugopal M, Banerji A,
Patel TB, Kumar GB and Nair BG: Anacardic acid inhibits gelatinases
through the regulation of Spry2, MMP-14, EMMPRIN and RECK. Exp Cell
Res. 349:139–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Philip JY, Da Cruz Francisco J, Dey ES,
Buchweishaija J, Mkayula LL and Ye L: Isolation of anacardic acid
from natural cashew nut shell liquid (CNSL) using supercritical
carbon dioxide. J Agric Food Chem. 56:9350–9354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng C, Zhu J, Sun HC, Huang XP, Zhao WA,
Zheng M, Liu LJ and Tian J: Inhibition of histone H3K9 acetylation
by anacardic acid can correct the over-expression of Gata4 in the
hearts of fetal mice exposed to alcohol during pregnancy. PLoS One.
9:e1041352014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alam-Escamilla D, Estrada-Muñiz E,
Solís-Villegas E, Elizondo G and Vega L: Genotoxic and cytostatic
effects of 6-pentadecyl salicylic anacardic acid in transformed
cell lines and peripheral blood mononuclear cells. Mutat Res Genet
Toxicol Environ Mutagen. 777:43–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seong YA, Shin PG, Yoon JS, Yadunandam AK
and Kim GD: Induction of the endoplasmic reticulum stress and
autophagy in human lung carcinoma A549 cells by anacardic acid.
Cell Biochem Biophys. 68:369–377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gladwish A, Loblaw A, Cheung P, Morton G,
Chung H, Deabreu A, Pang G and Mamedov A: Accelerated
hypofractioned postoperative radiotherapy for prostate cancer: A
prospective phase I/II study. Clin Oncol (R Coll Radiol).
27:145–152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong P, Zhang T, He D and Hsieh JT:
MicroRNA-145 modulates tumor sensitivity to radiation in prostate
cancer. Radiat Res. 184:630–638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao K, Jiang X, He L, Tang Y, Yin G, Zeng
Q, Jiang Z and Tan J: Anacardic acid sensitizes prostate cancer
cells to radiation therapy by regulating H2AX expression. Int J
Clin Exp Pathol. 8:15926–15932. 2015.PubMed/NCBI
|
|
22
|
Morell C, Bort A, Vara-Ciruelos D,
Ramos-Torres Á, Altamirano-Dimas M, Díaz-Laviada I and
Rodríguez-Henche N: Up-regulated expression of LAMP2 and autophagy
activity during neuroendocrine differentiation of prostate cancer
LNCaP cells. PLoS One. 11:e01629772016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramos-Torres Á, Bort A, Morell C,
Rodríguez-Henche N and Díaz-Laviada I: The peppers natural
ingredient capsaicin induces autophagy blockage in prostate cancer
cells. Oncotarget. 7:1569–1583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tai S, Xu L, Xu M, Zhang L, Zhang Y, Zhang
K, Zhang L and Liang C: Combination of Arsenic trioxide and
Everolimus (Rad001) synergistically induces both autophagy and
apoptosis in prostate cancer cells. Oncotarget. 8:11206–11218.
2017.PubMed/NCBI
|
|
25
|
Wang L, Fu P, Zhao Y, Wang G, Yu R, Wang
X, Tang Z, Imperato-McGinley J and Zhu YS: Dissociation of
NSC606985 induces atypical ER-stress and cell death in prostate
cancer cells. Int J Oncol. 49:529–538. 2016.PubMed/NCBI
|
|
26
|
Bruchmann A, Roller C, Walther TV, Schäfer
G, Lehmusvaara S, Visakorpi T, Klocker H, Cato AC and Maddalo D:
Bcl-2 associated athanogene 5 (Bag5) is overexpressed in prostate
cancer and inhibits ER-stress induced apoptosis. BMC Cancer.
13:962013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
QiNan W, XiaGuang G, XiaoTian L, WuQuan D,
Ling Z and Bing C: Par-4/NF-kappaB mediates the apoptosis of islet
beta cells induced by glucolipotoxicity. J Diabetes Res.
4692478:20162016.
|
|
28
|
Qi W, Morales C, Cooke LS, Johnson B,
Somer B and Mahadevan D: Reciprocal feedback inhibition of the
androgen receptor and PI3K as a novel therapy for
castrate-sensitive and -resistant prostate cancer. Oncotarget.
6:41976–41987. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Casar B, Rimann I, Kato H, Shattil SJ,
Quigley JP and Deryugina EI: In vivo cleaved CDCP1 promotes early
tumor dissemination via complexing with activated β1 integrin and
induction of FAK/PI3K/Akt motility signaling. Oncogene. 33:255–268.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiu YL, Zhao Y, Gou WF, Chen S, Takano Y
and Zheng HC: Anacardic acid enhances the proliferation of human
ovarian cancer cells. PLoS One. 9:e993612014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kwon T, Youn H, Son B, Kim D, Seong KM,
Park S, Kim W and Youn B: DANGER is involved in high
glucose-induced radioresistance through inhibiting DAPK-mediated
anoikis in non-small cell lung cancer. Oncotarget. 7:7193–7206.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dai L, Ma C, Zhang Z, Zeng S, Liu A, Tang
S, Ren Q, Sun Y and Xu C: DAPK promoter methylation and bladder
cancer risk: A systematic review and meta-analysis. PLoS One.
11:e01672282016. View Article : Google Scholar : PubMed/NCBI
|