Introduction
Ovarian carcinoma therapeutic care remains
inefficient owing to the high level of chemoresistance to
conventional treatment. With approximately 145,000 new cases each
year in developed countries and approximately 100,000 deaths,
ovarian carcinoma is the first leading cause of death from
gynecological malignancies.
We previously showed that apoptosis control defects
are responsible for the strong protection of ovarian cancer cells
against cell death (1,2). In particular, we demonstrated that the
concomitant inhibition of the anti-apoptotic proteins
Bcl-xL and Mcl-1 either by siRNAs (3,4), or by
combining a BH3-mimetic molecule targeting Bcl-xL with a
siRNA or a BH3-mimetic molecule targeting Mcl-1 (5–8)
triggers massive apoptotic cell death in chemoresistant ovarian
cancer cells in vitro, even in the absence of
chemotherapeutic treatment. This effect is due to the ‘primed for
death’ feature of cancer cells (9).
Unlike normal cells, cancer cells contain a high level of the
activated apoptosis inducers Bax and Bak, or activator BH3-only
proteins such as Bim and Puma, which are sequestered by
anti-apoptotic proteins such as Bcl-xL or Mcl-1
(10). Strategies aimed at the
release of Bax/Bak or Bim/Puma from their inhibitors are thus
highly attractive in a clinical perspective. In this context, the
design of innovative BH3-mimetic molecules able to target
Bcl-xL in vivo, such as ABT-263 (Navitoclax),
opens up new perspectives for clinical validation of such
approaches. However, pharmacological inhibition of Mcl-1 in
vivo remains problematic despite recent promising advances
(8,11–13).
The versatility in the choice of siRNA targets as
well as their high efficiency for the downregulation of a specific
gene makes the perspective of clinically available siRNA strategies
highly attractive. Recent advances in chemistry are now allowing a
panel of chemical modifications in siRNAs that avoid unwanted
immunostimulatory and off-target effects, thus overcoming some of
the major issues preventing the safe and efficient use of siRNAs in
clinical practice (14). However,
efficient vectorization and delivery of siRNAs in vivo
remains a major challenge (15),
despite the huge amount of research carried out on this topic.
Among the numerous strategies developed for the
in vivo vectorization of siRNAs (16), atelocollagen constitutes an
interesting option. Atelocollagen is a derivative of collagen that
has been used for the in vivo transfection of nucleic acids
such as DNA, antisense oligonucleotides (ASO) and siRNA (17). It is processed from bovine dermal
collagen after digestion of pepsine-mediated telopeptides to avoid
immunogenicity (18). Atelocollagen
is a highly biocompatible and biodegradable compound used in
numerous medicinal, surgical and cosmetic applications.
A molecular model has been proposed for
atelocollagen/siRNA interactions in which the interaction between
atelocollagen, which is positively charged in physiological
conditions (19), and the siRNA
phosphates promotes the formation of a fibrillar structure
containing 5 atelocollagen molecules for 1 siRNA duplex (20). This structure efficiently protects
the siRNA from nucleases and is compatible with cellular uptake. In
addition, modification of the atelocollagen concentration during
complex formation modifies the viscosity of the final product. Low
concentration formulations lead to a viscosity close to that of
blood and are adapted to intravenous administration. Conversely,
higher atelocollagen concentrations lead to a gel-like formation,
allowing the continuous delivery of siRNA over an extended period
of time after local administration. This plasticity of formulation
enables the use of atelocollagen for various administration routes
(intravenous, intraperitoneal, intramuscular, intratumoral,
peritumoral) (19,21). Atelocollagen has thus been proposed
as an adequate vector for the in vivo local or systemic
siRNA administration in various tumor models (17,21–24).
Several studies exploring the antitumor effects of
atelocollagen-mediated siRNA administration have utilized either
intratumoral (24–29) or peritumoral (18,22,30–32)
administration. The only study describing atelocollagen delivery of
siRNA in a subcutaneous ovarian cancer model used peritumoral
administration (32). Using a
vasohibin 2 siRNA to target angiogenesis, the authors reported a
decrease in tumor growth, tumor dissemination and angiogenesis.
However, intratumoral or peritumoral administration is not suitable
for treating ovarian carcinoma, in which peritoneal carcinomatosis
is sometimes composed of hundreds of disseminated tumor nodes.
The poor overall survival of ovarian carcinoma
patients is mainly due to disease recurrence within the peritoneal
cavity (33). We thus decided to
explore the possibility of siRNA delivery in peritoneal
carcinomatosis. In this context, intraperitoneal (i.p.)
administration is an attractive option, particularly since the
small tumor nodes lack vascularization. Furthermore, local
treatment carries a reduced risk of systemic toxicity and could
retain some of the properties of peritumoral injections, at least
next to the injection site and possibly throughout the peritoneal
compartment. Atelocollagen delivery of siRNA via the i.p. route has
been used in several studies (33,34),
providing an interesting proof-of-concept.
Numerous studies with in vivo administration
of siRNA-vectorized by atelocollagen involve intravenous (i.v.)
administration (24,35,36).
It is more feasible in routine clinical use due to the reduced risk
for patients compared to i.p. administration as the use of
catheters for repeated treatments can lead to serious side-effects
(34). Moreover, i.v.-delivered
particles likely reach ‘in-depth’ larger vascularized tumor nodes
more easily. The opportunity to deliver siRNAs via systemic
administration would make it suitable for several pathologies,
including cancer, provided that the chosen site of delivery is
vascularized. In the context of ovarian carcinoma, this would mean
that peritoneal tumor nodes of only a few millimeters in size would
be amenable to siRNA delivery.
As both i.p. and i.v. routes present significant
advantages in the context of ovarian carcinoma, we decided to
explore the efficiency of atelocollagen-vectorized siRNA delivery
in two independent models: a peritoneal carcinomatosis model
submitted to i.p. siRNA/vector complex injection, and an s.c.
vascularized tumor node model submitted to i.v. siRNA/vector
complex injection. To this end, we first used longitudinal imaging
studies performed on luciferase-expressing SKOV3 tumors to define
optimal administration modalities in both models. Our results
evidenced the interest of i.v. administration in an s.c. model of
tumor development. We next studied these administration modalities
for the siRNA-mediated silencing of Bcl-xL and Mcl-1 in
the same animal models.
Materials and methods
Cell culture and treatment
SKOV3 ovarian carcinoma cell line and its
luciferase-expressing counterpart SKOV3-Luc were obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA). They
were grown in RPMI-1640 medium supplemented with 2 mM Glutamax™, 25
µM HEPES, 10% fetal calf serum and 33 mM sodium bicarbonate (Fisher
Scientific Bioblock, Illkirch, France) and were maintained in a 5%
CO2 humidified atmosphere at 37°C.
In vitro transfection
All siRNAs used in the present study were chemically
synthesized and PAGE-purified by Eurogentec (Liège, Belgium) and
received as annealed oligonucleotides. siRNA guide sequences
targeting the indicated genes were as follows: Mcl-1 siRNA (noted
siMcl1), 5′-uguuuagccacaaaggcac-3′, Bcl-xL siRNA (noted
siBcl-xL), 5′-ugcgauccgacucaccaau-3′, luciferase siRNA (noted
siLuc), 5′-ucgaaguacucagcguaag-3′ and GFP siRNA (noted siGFP,
negative control), 5′-acuuguggccguuuacguc-3′. Exponentially growing
cells were seeded (2.5×105 cells/25 cm2
flask) the day before transfection to reach 30–50% confluency at
the time of transfection. Briefly, the transfecting reagent
INTERFERin™ (Polyplus Transfection, Strasbourg, France) was added
to siRNA diluted in OptiMEM® serum-free medium (Life
Technologies, Saint Aubin, France), and the formation of
siRNA/vector complexes was allowed to proceed for 15 min at RT
before distribution in culture flasks. The next day, cell medium
was changed to remove the remaining transfecting reagent. At the
indicated time, cells were trypsinized and washed with cold
phosphate-buffered saline (PBS). Cell pellets were used directly or
stored at −80°C for later use.
In vitro bioluminescence
measurement
Firefly luciferase activity was assayed 72 h after
siLuc transfection with the Dual-Luciferase Reporter Assay System
(Promega) and measured with a luminometer Centro LB 960 (Berthold,
Thoiry, France). Each assay contained 3 technical replicates.
Animal experiments
Female nude mice (Janvier Laboratories, Saint
Berthevin, France) 6-weeks of age were injected with human ovarian
carcinoma cell lines (SKOV3-Luc or SKOV3) i.p. (20×106
cells in 200 µl PBS) or s.c. bilaterally on the flanks
(5×106 cells in 200 µl PBS). The animals were kept under
pathogen-free conditions and fed and watered ad libitum, in
cages of 4 to 5 animals (in compliance with recommended area
surface/animal), in a dedicated room with a 12h/12h light/dark
cycle at a constant temperature of 22°C. A period of 4 weeks was
allowed for tumor development before the beginning of the
experiments. In vivo experiments were performed in the
animal core facilities of the Comprehensive Cancer Centre F.
Baclesse in Caen and of the Institute of Advanced Biosciences in
Grenoble, both of which are certified by the French Ministry of
Higher Education and Research and regularly controlled by the
Departmental Management of Population Protection (certification
nos. B14 118 003 and C38 516 10001, respectively). Animal
experiments were performed according to the current regulations in
the respect of animal experiment ethics. Animals were euthanatized
at the end points of the experiments, i.e. after a total of 5 weeks
of tumoral development. At this stage, s.c. tumors were ~4 mm in
size, 33.5 mm3 (V = L × l2 × π/6), whereas for
micro-carcinomatosis, nodules were just becoming visible on the
peritoneum with the presence of a few nodules (usually 1–5) ~10
mm3 in size in the peritoneal cavity. At this stage no
ascites was noted. For euthanasia, animals were anesthetized with a
gaseous mix of isoflurane/oxygen (5/95) and thereafter euthanatized
with a gaseous mix of oxygen/carbon dioxide (20/80).
In vivo treatments
For local administration, 100 µg of siRNA complexed
with atelocollagen at a final 0.5% concentration was injected i.p.
in 400 µl. For systemic administration, 125 µg of siRNA complexed
with atelocollagen at a final concentration of 0.05% was injected
i.v. in 200 µl. NaCl injections for control animals were performed
with the same volumes. Local (0.5% Atelocollagen) or systemic
(0.05% Atelocollagen) administration kits were purchased from
Cosmobio (Koken Co., Ltd., Tokyo, Japan). siRNA/vector complexes
were prepared according to the manufacturer's protocol for local
use kit, and with a modified protocol, i.e. increase in siRNA
concentration in the formulation for the systemic kit, according to
the protocol published by Mu et al (24). In experiments involving siMcl1 and
siBcl-xL, animals were distributed into 3 subgroups
prior to treatment according to the size of the s.c. tumor, i.e.
large, medium and small. We thus avoided any possible bias due to
unequal average tumor size between groups. Mice from these
subgroups were then equally distributed into 4 experimental groups
of 5 mice each for a total of 20 mice: vehicle (NaCl), control
siRNA (siGFP), Mcl-1 siRNA (siMcl1) and Bcl-xL siRNA
(siBcl-xL).
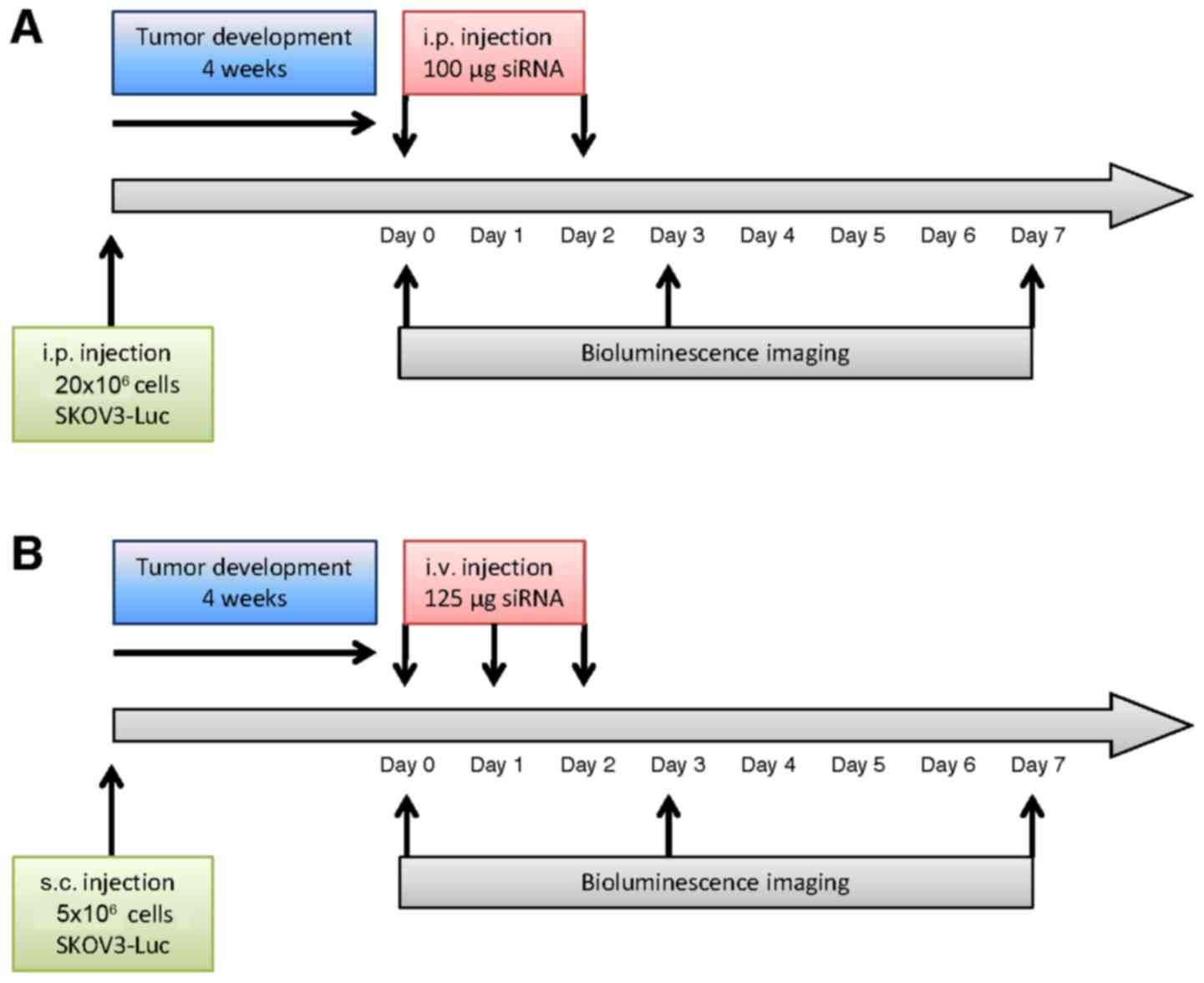
Bioluminescence imaging
In vivo imaging was conducted at Optimal
(Grenoble, France), a core facility for small animal optical
imaging. After 4 weeks of tumor development, mice received an i.p.
injection of 150 µg/g luciferin (Promega, Charbonnières-les-Bains,
France) for non-invasive bioluminescence imaging (IVIS Kinetic;
PerkinElmer, Waltham, MA, USA). Semi-quantitative data of
luciferase-positive tumor cell signals were obtained using the
manufacturer's software (Living Image; PerkinElmer). Results were
expressed as photons/second (photons/s). For animal randomization
prior to treatment, mice were separated into 3 subgroups with high,
medium and low levels of bioluminescent signals. Mice from each of
these subgroups were then distributed into 3 experimental groups
for a total of 20 mice: vehicle (NaCl, 4 and 3 mice bearing i.p. or
s.c. tumors respectively), control siRNA (siGFP, 3 and 3 mice
bearing i.p. or s.c. tumors, respectively) and luciferase siRNA
(siLuc, 4 and 3 mice bearing i.p. or s.c. tumors, respectively)
treatment. Bioluminescence imaging was performed at day 0, 3 and 7.
Results were then expressed as values relative to day 0.
Statistical analysis
In vivo luciferase assays were compared using
the non-parametrical Wilcoxon rank-sum test, and p-values <0.05
were considered as significant. Statistical analyses were
calculated using the 32-bit R Console software V3.1.0 (R Foundation
for Statistical Computing, Vienna, Austria).
Immunohistochemical analysis
Automated immunohistochemistry using a Ventana
Discovery XT autostainer was performed on 4 µm-thick paraffin
sections. Slides were deparaffinized with EZPrep buffer and
epitopes were unmasked by 15 min of high-temperature treatment in
CC1 EDTA buffer. Sections were incubated for 40 min at 37°C with an
anti Mcl-1 (ab32087; Abcam, Cambridge, MA, USA) or
Bcl-xL antibody (556361; BD Pharmingen, Franklin Lakes,
NJ, USA). Secondary antibody (Omnimap Rabbit; Ventana Medical
System Inc., Tucson, AZ, USA) was incubated for 16 min at room
temperature. Immunodetection performed without the primary antibody
was used as the control. After washes, the staining was performed
with DAB and sections were counterstained with hematoxylin using
Ventana reagents according to the manufacturer's protocol. Stained
slides were then digitized using an Aperio ScanScope slide scanner
(Aperio Technologies, Vista, CA, USA).
RNA isolation and quantitative reverse
transcriptase-PCR (RT-PCR)
Total RNA was isolated from the SKOV3 cell line
using TRIzol (Qiagen, Courtaboeuf, France) and 1 µg was
reverse-transcribed using Omniscript reverse transcriptase (Qiagen)
with random hexamers according to the manufacturer's instructions.
cDNA was combined with 10 µmol/l of each forward and reverse
primer, 50 µmol/l of the TaqMan® probe and 2X
TaqMan® Fast Universal PCR Master Mix (Applied
Biosystems, Foster City, CA, USA) in a 20 µl final reaction volume.
The following probes were used: for Mcl-1 (Hs00172036_m1), for
Bcl-xL (forward, 5′-TGCGTGGAAAGCGTAGACAA-3′ and reverse,
5′-AGGTAAGTGGCCATCCAAGCT-3′; probe, 5′-AGATGCAGGTATTGGTG-3′) and
GAPDH (Hs99999905_m1). All PCR amplification reactions were carried
out in triplicate on an Applied ABI Prism 7500 Fast PCR system
(Applied Biosystems). The Mcl-1 and Bcl-xL transcripts
were quantified relative to GAPDH endogenous reference by the
comparative 2−ΔΔCt method and results are expressed as a
percentage of untransfected tumors from NaCl-injected mice.
Results
Nude mice were xenografted with SKOV3 ovarian cancer
cells constitutively expressing luciferase (SKOV3-Luc) by i.p. or
bilateral s.c. injection to induce the development of peritoneal
carcinomatosis or s.c. tumors, respectively. The use of in
vivo bioluminescence imaging allowed individual longitudinal
follow-up of tumor development for each animal. Therefore, by
measuring the evolution of bioluminescence signals over time, each
tumor constitutes its own control, thus limiting the heterogeneity
of response due to inter-animal variation in the rate of tumor
development.
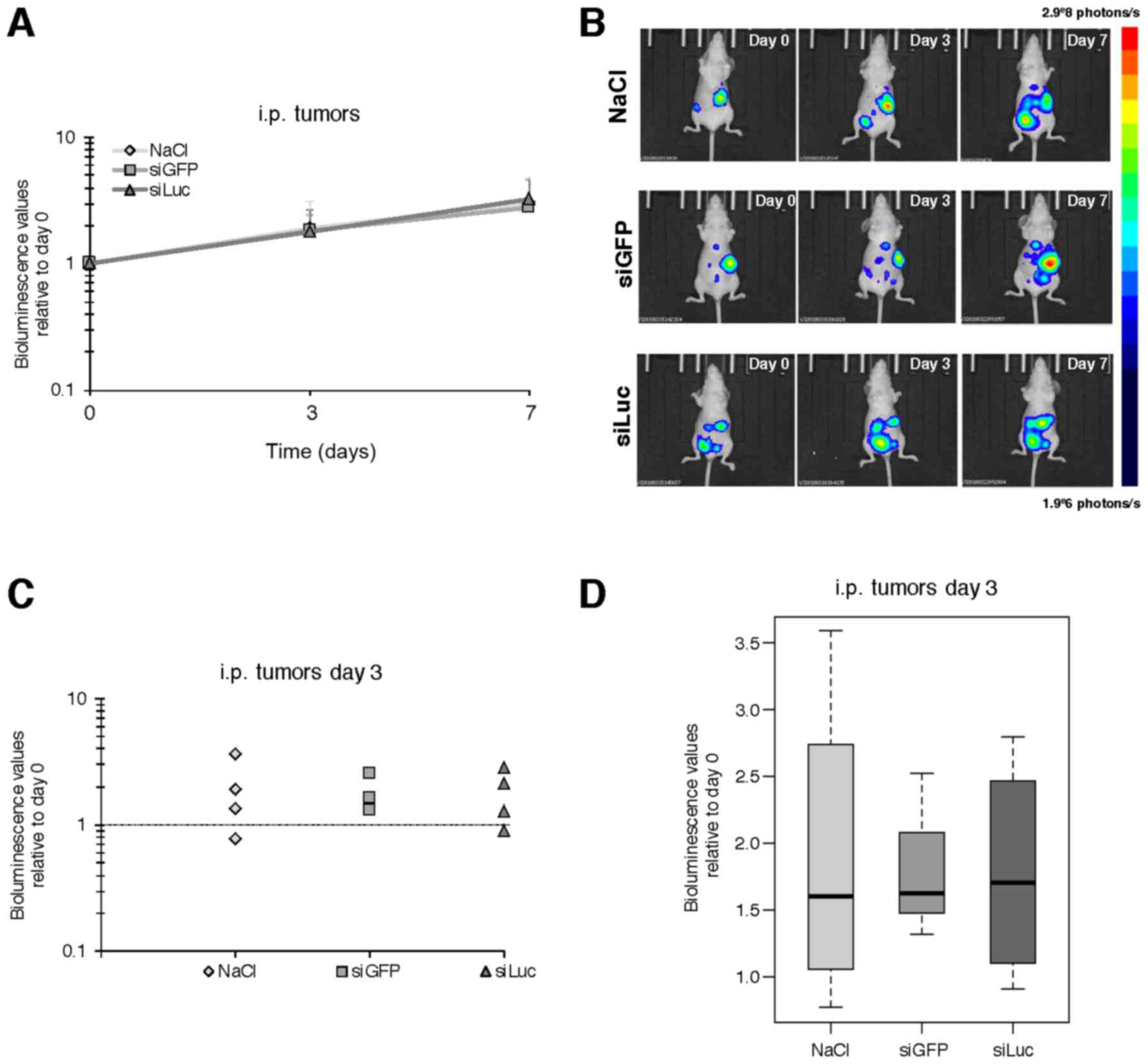
Atelocollagen-vectorized siRNA
targeting luciferase injected by i.p. route does not decrease
bioluminescence in carcinomatosis tumor nodes
In the peritoneal carcinomatosis model,
atelocollagen was complexed with siRNA to a ‘high’ final
concentration of atelocollagen (0.5%) to obtain a gel-like
compound, according to the manufacturer's protocol. This gel-like
formulation was used to allow the continuous release of siRNA in
the peritoneal cavity. siRNA/vector complexes or vehicle were
injected i.p. at day 0 and day 2. Bioluminescence was measured at
day 0 (before treatment), day 3 and day 7. Treatment and the
imaging schedules are represented in Fig. 1A.
As expected, an increase in the bioluminescence
signal was detected over time from day 0 to 3, and day 7 in the
control tumors, corresponding to tumor growth. However,
siLuc-treated animals did not display any decrease in
bioluminescent signal (178 and 325% of the signal at day 3 and day
7, respectively, relative to day 0) compared to siGFP (182 and
278%) or vehicle (189 and 302%) (Fig.
2 and data not shown).
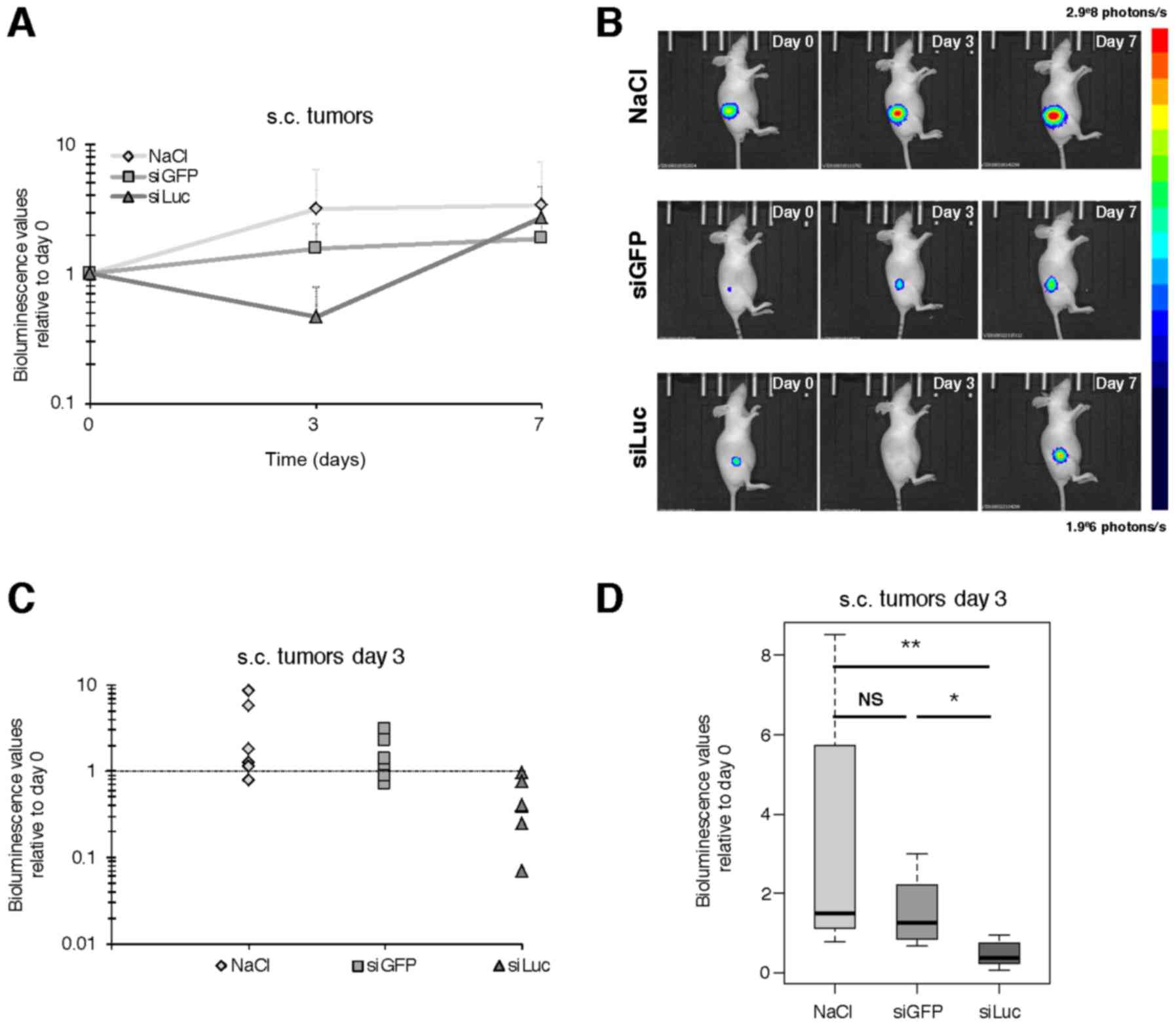
Atelocollagen-vectorized siRNA
targeting luciferase injected i.v. decreases bioluminescence in
s.c. tumors
For the s.c. tumor model, atelocollagen was
complexed with siRNA to a ‘low’ final concentration of
atelocollagen (0.05%), so that the siRNA/vector complexes presented
a viscosity comparable to blood. SiRNAs - or vehicle - were
injected i.v. at day 0, 1 and 2, and bioluminescence was assessed
at day 0 (before treatment), day 3 and 7, as described in Fig. 1B. An increase of bioluminescence
over time was observed for NaCl and siGFP, consistent with the
expected growth of tumors over time (Fig. 3A and B). However, at day 3 the
bioluminescence values were significantly lower in siLuc compared
to day 0 (47% of day 0). This decrease was even stronger compared
to the bioluminescence values of NaCl (319% of day 0) with p=0.004,
and vs. siGFP (155% of day 0) with p=0.015 (Fig. 3C and D), which means a decrease in
bioluminescence signal of 85 and 70% relative to NaCl and siGFP
respectively. In contrast, there was no significant difference at
day 7 between groups, with siLuc bioluminescence signal intensity
catching up with other groups, supporting that the decrease in
bioluminescence intensity observed at day 3 was not the consequence
of impaired tumor growth and/or tumor mass reduction for siLuc
group (Fig. 3 and data not
shown).
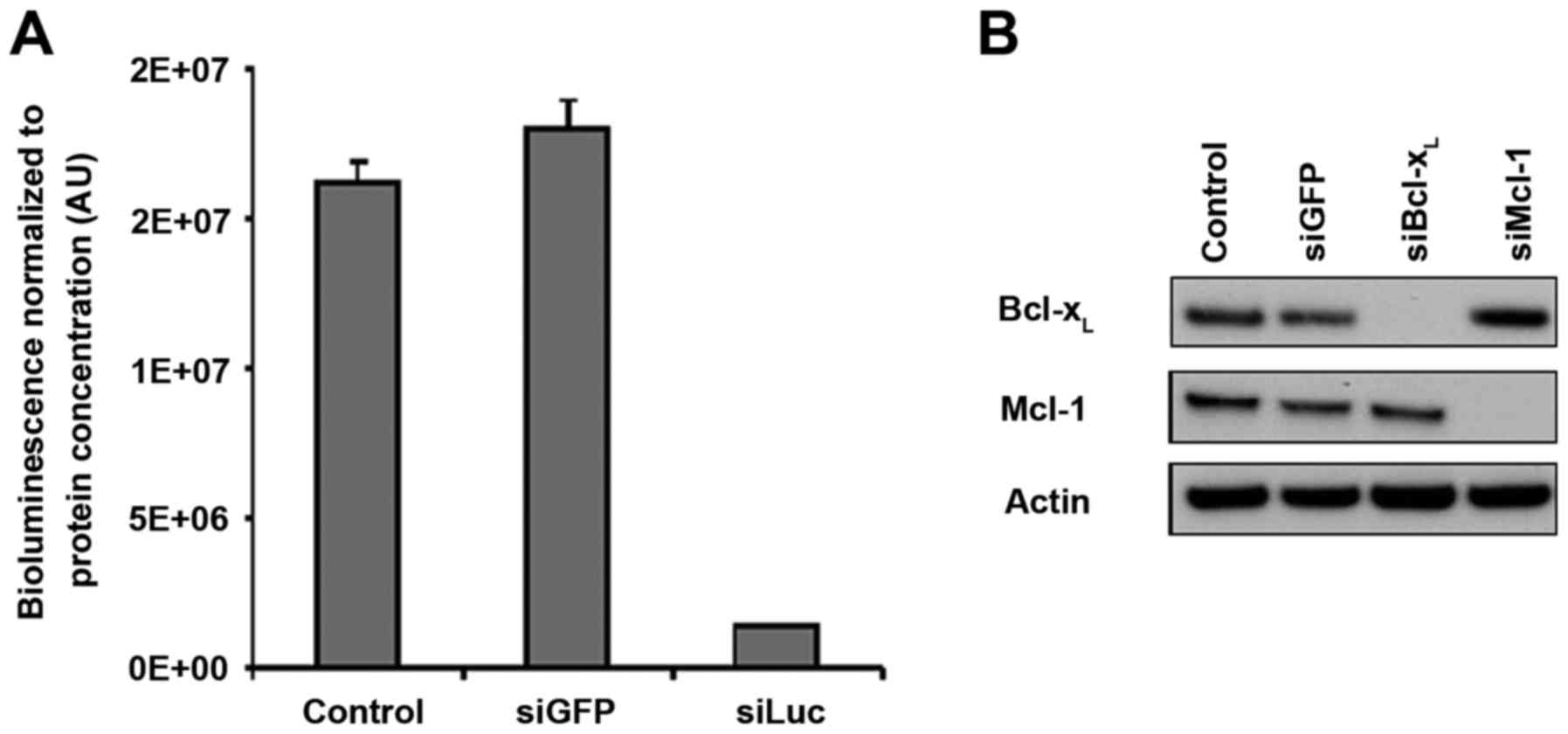
Unlike luciferase-directed siRNA,
Mcl-1- and Bcl-xL-directed siRNAs vectorized with
atelocollagen do not induce target downregulation in vivo
After defining the successful conditions for in
vivo siRNA vectorization with atelocollagen, we sought to
downregulate the cancer-related targets of interest, Mcl-1 and
Bcl-xL. We used siRNA sequences which had been validated
by our group in previous studies for their ability to downregulate
their targets specifically with high efficiency (3,4,6). In
addition, the batches of siMcl1 and siBcl-xL that we
used in vivo were checked for their ability to downregulate
their respective targets in vitro in SKOV3 cells. The
complete disappearance of the protein-specific band was observed in
western blotting (Fig. 4A),
demonstrating that these siRNAs enable full silencing of their
respective targets. These results were comparable to what was
obtained upon siLuc transfection in SKOV3-Luc cells, which
triggered 89% luminescence inhibition compared to the control
conditions (Fig. 4B).
SKOV3 cells were injected into nude mice to develop
bilateral s.c. tumors and the above-described protocol and
treatment schedule (Fig. 1B) were
repeated, with 3 exceptions; i) siLuc condition was removed and
replaced with siMcl1 or siBcl-xL; ii) we discarded the
day 7 analysis time point as it did not lead to a decrease in
bioluminescence in the above-described experiment; and iii) we used
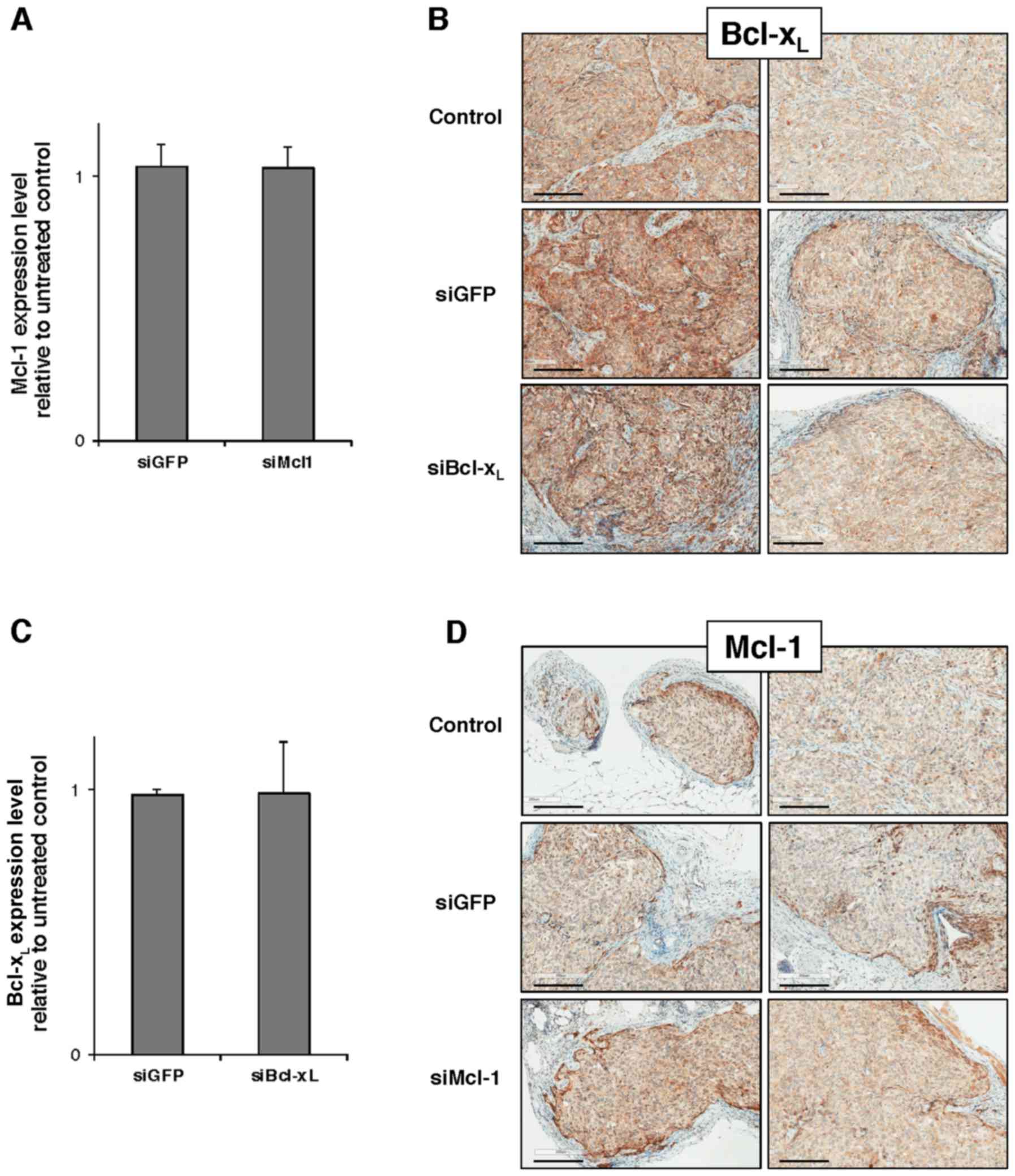
5 mice/group with a total of 20 mice. Unfortunately, neither
RT-qPCR analysis performed on tumor lysates nor IHC performed in
situ on tumor slices revealed any Mcl-1 or Bcl-xL
downregulation at day 3 after the beginning of the protocol
(Fig. 5).
Discussion
Our group has previously shown that the concomitant
inhibition of Bcl-xL and Mcl-1 is sufficient to trigger
apoptosis in ovarian carcinoma cell lines (3), as well as in cell lines from other
malignancies (37–39). The use of siRNAs is an attractive
option to downregulate Bcl-2 anti-apoptotic family members in
ovarian tumors, particularly Mcl-1 for which no pharmacological
inhibitor is yet available for clinical use.
The development of safe and efficient tools for
siRNA vectorization is a very intensive research topic. More than
50 clinical trials involving siRNAs have been reported (40) but no siRNA-based drug has yet
obtained FDA approval and none of the vectorization systems
utilized have achieved a consensus (40,41).
Furthermore, a large number of different vectorization approaches
have been considered for in vivo preclinical studies
(40,42,43).
The choice of a suitable system to vectorize siRNAs in vivo
is still greatly dependent on the experimental model.
Ovarian carcinoma development can be classified into
4 different tumoral tissue types: primary tumor, peritoneal tumor
including micro- and macro-carcinomatosis, distant tumor nodes, and
ascites. The primary tumor is usually removed surgically during
first-line treatment, and ascites in vivo in mice is usually
present at a very late stage of tumor development so it cannot be
studied for ethical reasons. Micro-carcinomatosis and secondary
tumor nodes, which are easily developed in mice, are a good model
of the clinically challenging residual disease and recurrence so we
targeted them in our siRNA experiments.
The aim of the present study was to evaluate the
possibility to vectorize siRNAs in two in vivo models of
tumoral development in mice mimicking the setting of ovarian
carcinoma: the development of carcinomatosis matching the
peritoneal dissemination mainly displaying small-sized tumor nodes
with low vascularization, and subcutaneous tumors matching distant
metastasis with larger tumor nodes displaying more developed
vascularization. For the carcinomatosis model with its small islets
of tumor cells lacking vascularization, local i.p. injection is
attractive. For the distant and/or vascularized metastasis model
with i.v. injection, s.c. tumor development is more relevant owing
to the unique presence of larger vascularized tumor nodes. We chose
to use atelocollagen as a vector since its flexibility (two
possible formulations adapted to i.v. and i.p. administration,
respectively) enabled us to use it in both models. In addition, the
possibility to use each tumor as its own control, thereby avoiding
inter-animal and inter-nodular variability, led us to use a
bioluminescent tumoral model to establish a suitable administration
regimen.
In the peritoneal carcinomatosis model, we could not
evidence any effect of atelocollagen-vectorized siRNAs on the
bioluminescence signal in comparison to the control groups. To the
best of our knowledge, this formulation with high viscosity has
been used only for siRNA delivery with peritumoral injection
(18,21,22,30,31),
with two exceptions. One study reported atelocollagen-vectorized
siRNAs with i.p. injection for intraperitoneal NSCLC tumors
(33), but the authors did not give
any information either on the atelocollagen concentration or on the
amount of siRNA used. Another study demonstrated efficient siRNA
delivery and target downregulation on peritoneal tumor nodes in a
gastric cancer model after injection of siRNAs-vectorized with
atelocollagen at 0.5% together with DharmaFECT 1 transfection
reagent (34). Notably, the use of
atelocollagen alone in their model did not allow target
downregulation, in accordance with our observations. However, the
authors did not report the effects of DharmaFECT 1 transfection
reagent in the absence of atelocollagen, so the contribution of
atelocollagen to the successful vectorization of siRNAs and
silencing of their target could not be estimated.
One of the reasons for the lack of efficacy in this
model could be that, unlike with peritumoral or intratumoral
injections, the complexes released in the peritoneal cavity are not
maintained in contact with the majority of tumor cells, as they
cannot cover the entirety of the peritoneal cavity. However, i.p.
injection of atelocollagen complexed with ASO was used for delivery
to s.c. tumors (44), indicating
that small oligonucleotides are at least released efficiently in
the peritoneal cavity even with an atelocollagen concentration of
1.8%, which is higher than the one used in the present study, i.e.
0.5%, the concentration recommended in the commercially available
kit. A possibility is that once released in the peritoneal cavity,
siRNAs, which are more prone to degradation than
phosphorothioate-modified ASO, are rapidly excreted and/or degraded
without the help of a complementary vector system, before they can
reach tumor cells present in this compartment. In order to monitor
biodistribution, direct labeling of siRNAs has been used in several
instances in vivo. However, the peritoneal compartment is
not an organ per se, and such an approach would be difficult
to use in our case to monitor the intra-peritoneal release of
siRNAs.
Regarding the s.c. tumor model, we first evidenced
satisfactory efficiency on the inhibition of luciferase expression
as revealed by in vivo bioluminescence (70% decrease in
signal vs. control siRNA), which is in line with previous studies
in which siRNAs-vectorized with atelocollagen were used (17,23,24,35) on
tumors of various origins. This downregulation effect was present
at day 3 after 3 daily consecutive injections and was absent at day
7, showing that the effect is transient over time suggesting a loss
of the RNA inhibition effect soon after the end of the injections.
In several studies describing a long-lasting effect on target
inhibition, the protocols included multiple repeated injections
(34,45), suggesting that without this, no
long-lasting target inhibition can be obtained, which is again in
line with our own observations. In addition, when targeting genes
involved in cancer progression, successful inhibition by siRNAs
prevents further tumoral development and enables tumoral
regression. In our luciferase model, however, target inhibition is
not supposed to, and indeed does not, impair tumor development.
Therefore, the inhibitory effects of transfected siRNAs are likely
to be diluted over cell divisions, which is another plausible
explanation for a transient decrease in bioluminescence.
Unfortunately, we could not observe the inhibition
of target gene expression in s.c. tumors using Mcl-1- or
Bcl-xL-directed siRNAs. The histology of tumors derived
from SKOV3 cells (used during Mcl-1 and Bcl-xL
experiments) or SKOV3-Luc cells (used during the bioluminescence
experiments) are similar, as confirmed by a certified pathologist
(data not shown). Therefore, the use of SKOV3 instead of SKOV3-Luc
cells to generate tumors does not likely explain the differences
observed between our in vivo experiments.
It has been shown that target mRNA half-life
influences RNAi efficiency; mRNAs with shorter half-life being more
difficult to downregulate (46).
Luciferase, Mcl-1 and Bcl-xL mRNA half-lives are 1.5,
2.5 and 2–3 h, respectively (47,48).
These small differences regarding mRNA half-lives are thus quite
unlikely to induce any difference in the timing of mRNA
downregulation following siRNA injection and do not constitute an
explanation for the absence of observed Bcl-xL or Mcl-1
downregulation. With regard to protein half-lives, it should be
noted that luciferase and Mcl-1 proteins have a 6-fold difference
in their respective half-lives [3 h vs. 30 min, respectively
(49)]. In addition, the half-life
of Bcl-xL protein has been reported to be ~20 h (50). Due to these differences, the effects
of siRNA downregulation on Mcl-1 protein should have been
measurable earlier than luciferase, whereas Bcl-xL
protein downregulation should have lasted longer. Overall, the time
window in which we should have been able to observe any protein
downregulation was larger with Bcl-xL and Mcl-1.
Therefore any difference in the timing of the action of
Bcl-xL or Mcl-1 siRNAs compared to luciferase would have
not compromised our ability to observe the effects of siRNA on
protein, at least on one of our targets. In fact, these differences
in the half-lives of our proteins of interest do not likely explain
the lack of observed siRNA activity.
We did consider an increase in dose and/or number of
administrations to obtain a more stable and reproducible target
downregulation, as well as the use of a panel of alternative siRNA
sequences for Mcl-1 and Bcl-xL. However, in most of the
animals we observed a seizure-like behavior shortly after i.v.
injection of atelocollagen in complex with each of the 4 siRNAs we
used. The mice recovered quickly with no visible sign of
physiological or behavioral consequence. It is unlikely that this
effect was triggered by contamination of our siRNAs as seizures
were observable with 4 different PAGE-purified siRNAs. To the best
of our knowledge, this effect with the use of atelocollagen has not
been reported elsewhere. Considering previous studies in the
literature and the manufacturer's protocol, we could not increase
the siRNA concentration in the siRNA/vector complexes. Therefore,
as we ruled out increasing the volume or frequency of injections
for ethical reasons, we did not push our investigations further
with atelocollagen for in vivo siRNA delivery.
In summary, formulation with a high concentration of
atelocollagen alone is not suitable for i.p. siRNA transfection in
peritoneal nodes. In contrast, a low concentration formulation for
i.v. injection is able to deliver siRNAs in s.c. tumors and to
induce a strong but transient silencing of the targeted protein
activity. In addition, the efficiency of target silencing is very
sensitive and depends on the nature of the target. Given that we
used the limiting dose for this vector and that the level and
duration of silencing are clearly insufficient to explore possible
therapeutic effects, we decided not to push our investigations with
this vector further for the delivery of siRNAs to ovarian cancer
cell xenografts in vivo in mice.
Acknowledgements
The authors wish to thank Edwige Lemoisson for
technical assistance, the CMAbio3 platform (SF 4206 ICORE UNICAEN)
for IHC and the OPTIMAL platform (Grenoble) for small animal
bioluminescence imaging. The present study is part of the national
program Cartes d'Identité des Tumeurs (CIT) funded and developed by
the Ligue Nationale Contre le Cancer. It was supported by the
‘Ligue contre le Cancer’ (Calvados and Orne Committee), the
‘Conseil Regional de Basse-Normandie’, and the French Government.
E.B., M.M.F., E.V. and C.L. were recipients of a doctoral
fellowship from the ‘Ligue contre le Cancer’ (Calvados Committee:
E.B., E.V. and C.L.; Eure Committee: M.M.F.) and C.D. was recipient
of a post-doctoral fellowship from the ‘Conseil Regional de
Basse-Normandie’. N.V. was a recipient of a doctoral fellowship
from the French Ministry for Higher Education and Research. The
present study was supported by a grant from l'Ecole de l'Inserm
Liliane Bettencourt (N.V.).
Glossary
Abbreviations
Abbreviations:
|
HES
|
hematoxylin, eosin, safran
|
|
s.c.
|
subcutaneous
|
|
i.p.
|
intra-peritoneal
|
|
i.v.
|
intravenous
|
|
ASO
|
antisense oligonucleotide
|
References
|
1
|
Villedieu M, Louis MH, Dutoit S, Brotin E,
Lincet H, Duigou F, Staedel C, Gauduchon P and Poulain L: Absence
of Bcl-xL down-regulation in response to cisplatin is
associated with chemoresistance in ovarian carcinoma cells. Gynecol
Oncol. 105:31–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomasina J, Malzert-Freon A, Giffard F,
Brotin E, Louis MH, Abeilard E, Rault S, Gauduchon P and Poulain L:
Sensitization of ovarian carcinoma cells to
Bcl-xL-targeting strategies through indirect modulation
of Mcl-1 activity by MR22388, a molecule of the tripentone family.
J Ovarian Res. 6:38–2013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brotin E, Meryet-Figuière M, Simonin K,
Duval RE, Villedieu M, Leroy-Dudal J, Saison-Behmoaras E, Gauduchon
P, Denoyelle C and Poulain L: Bcl-XL and MCL-1
constitute pertinent targets in ovarian carcinoma and their
concomitant inhibition is sufficient to induce apoptosis. Int J
Cancer. 126:885–895. 2010.PubMed/NCBI
|
|
4
|
Varin E, Denoyelle C, Brotin E,
Meryet-Figuière M, Giffard F, Abeilard E, Goux D, Gauduchon P,
Icard P and Poulain L: Downregulation of Bcl-xL and Mcl-1 is
sufficient to induce cell death in mesothelioma cells highly
refractory to conventional chemotherapy. Carcinogenesis.
31:984–993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simonin K, Brotin E, Dufort S, Dutoit S,
Goux D, N'diaye M, Denoyelle C, Gauduchon P and Poulain L: Mcl-1 is
an important determinant of the apoptotic response to the
BH3-mimetic molecule HA14-1 in cisplatin-resistant ovarian
carcinoma cells. Mol Cancer Ther. 8:3162–3170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simonin K, N'Diaye M, Lheureux S,
Loussouarn C, Dutoit S, Briand M, Giffard F, Brotin E,
Blanc-Fournier C and Poulain L: Platinum compounds sensitize
ovarian carcinoma cells to ABT-737 by modulation of the Mcl-1/Noxa
axis. Apoptosis. 18:492–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lheureux S, N'diaye M, Blanc-Fournier C,
Dugue AE, Clarisse B, Dutoit S, Giffard F, Abeilard E, Briand M,
Labiche A, et al: Identification of predictive factors of response
to the BH3-mimetic molecule ABT-737: An ex vivo experiment in human
serous ovarian carcinoma. Int J Cancer. 136:E340–E350. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gloaguen C, Voisin-Chiret AS, Sopkova-de
Oliveira Santos J, Fogha J, Gautier F, De Giorgi M, Burzicki G,
Perato S, Pétigny-Lechartier C, Simonin-Le Jeune K, et al: First
evidence that oligopyridines, α-helix foldamers, inhibit Mcl-1 and
sensitize ovarian carcinoma cells to Bcl-xL-targeting strategies. J
Med Chem. 58:1644–1668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Letai AG: Diagnosing and exploiting
cancer's addiction to blocks in apoptosis. Nat Rev Cancer.
8:121–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Certo M, Del Gaizo Moore V, Nishino M, Wei
G, Korsmeyer S, Armstrong SA and Letai A: Mitochondria primed by
death signals determine cellular addiction to antiapoptotic BCL-2
family members. Cancer Cell. 9:351–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Belmar J and Fesik SW: Small molecule
Mcl-1 inhibitors for the treatment of cancer. Pharmacol Ther.
145:76–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Varadarajan S, Vogler M, Butterworth M,
Dinsdale D, Walensky LD and Cohen GM: Evaluation and critical
assessment of putative MCL-1 inhibitors. Cell Death Differ.
20:1475–1484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leverson JD, Zhang H, Chen J, Tahir SK,
Phillips DC, Xue J, Nimmer P, Jin S, Smith M, Xiao Y, et al: Potent
and selective small-molecule MCL-1 inhibitors demonstrate on-target
cancer cell killing activity as single agents and in combination
with ABT-263 (navitoclax). Cell Death Dis. 6:e15902015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deleavey GF and Damha MJ: Designing
chemically modified oligonucleotides for targeted gene silencing.
Chem Biol. 19:937–954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Colombo S, Zeng X, Ragelle H and Foged C:
Complexity in the therapeutic delivery of RNAi medicines: An
analytical challenge. Expert Opin Drug Deliv. 11:1481–1495. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miller AD: Delivery of RNAi therapeutics:
Work in progress. Expert Rev Med Devices. 10:781–811. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeshita F, Minakuchi Y, Nagahara S,
Honma K, Sasaki H, Hirai K, Teratani T, Namatame N, Yamamoto Y,
Hanai K, et al: Efficient delivery of small interfering RNA to
bone-metastatic tumors by using atelocollagen in vivo. Proc Natl
Acad Sci USA. 102:12177–12182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takei Y, Kadomatsu K, Yuzawa Y, Matsuo S
and Muramatsu T: A small interfering RNA targeting vascular
endothelial growth factor as cancer therapeutics. Cancer Res.
64:3365–3370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujimoto I and Takei Y:
Atelocollagen-mediated siRNA delivery: Future promise for
therapeutic application. Ther Deliv. 5:369–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Svintradze DV and Mrevlishvili GM: Fiber
molecular model of atelocollagen-small interfering RNA (siRNA)
complex. Int J Biol Macromol. 37:283–286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ochiya T, Nagahara S, Sano A, Itoh H and
Terada M: Biomaterials for gene delivery: atelocollagen-mediated
controlled release of molecular medicines. Curr Gene Ther. 1:31–52.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Forootan SS, Bao ZZ, Forootan FS, Kamalian
L, Zhang Y, Bee A, Foster CS and Ke Y: Atelocollagen-delivered
siRNA targeting the FABP5 gene as an experimental therapy
for prostate cancer in mouse xenografts. Int J Oncol. 36:69–76.
2010.PubMed/NCBI
|
|
23
|
Kawata E, Ashihara E, Kimura S, Takenaka
K, Sato K, Tanaka R, Yokota A, Kamitsuji Y, Takeuchi M, Kuroda J,
et al: Administration of PLK-1 small interfering RNA with
atelocollagen prevents the growth of liver metastases of lung
cancer. Mol Cancer Ther. 7:2904–2912. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mu P, Nagahara S, Makita N, Tarumi Y,
Kadomatsu K and Takei Y: Systemic delivery of siRNA specific to
tumor mediated by atelocollagen: Combined therapy using siRNA
targeting Bcl-xL and cisplatin against prostate cancer. Int J
Cancer. 125:2978–2990. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Minakuchi Y, Takeshita F, Kosaka N, Sasaki
H, Yamamoto Y, Kouno M, Honma K, Nagahara S, Hanai K, Sano A, et
al: atelocollagen-mediated synthetic small interfering RNA delivery
for effective gene silencing in vitro and in vivo. Nucleic Acids
Res. 32:e109–e2004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nozawa H, Tadakuma T, Ono T, Sato M, Hiroi
S, Masumoto K and Sato Y: Small interfering RNA targeting epidermal
growth factor receptor enhances chemosensitivity to cisplatin,
5-fluorouracil and docetaxel in head and neck squamous cell
carcinoma. Cancer Sci. 97:1115–1124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iwaki K, Shibata K, Ohta M, Endo Y, Uchida
H, Tominaga M, Okunaga R, Kai S and Kitano S: A small interfering
RNA targeting proteinase-activated receptor-2 is effective in
suppression of tumor growth in a Panc1 xenograft model. Int J
Cancer. 122:658–663. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takei Y, Kadomatsu K, Goto T and Muramatsu
T: Combinational antitumor effect of siRNA against midkine and
paclitaxel on growth of human prostate cancer xenografts. Cancer.
107:864–873. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takigami I, Ohno T, Kitade Y, Hara A,
Nagano A, Kawai G, Saitou M, Matsuhashi A, Yamada K and Shimizu K:
Synthetic siRNA targeting the breakpoint of EWS/Fli-1
inhibits growth of Ewing sarcoma xenografts in a mouse model. Int J
Cancer. 128:216–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ashihara E, Kawata E, Nakagawa Y,
Shimazaski C, Kuroda J, Taniguchi K, Uchiyama H, Tanaka R, Yokota
A, Takeuchi M, et al: β-Catenin small interfering RNA successfully
suppressed progression of multiple myeloma in a mouse model. Clin
Cancer Res. 15:2731–2738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sudo H, Tsuji AB, Sugyo A, Kohda M, Sogawa
C, Yoshida C, Harada YN, Hino O and Saga T: Knockdown of
COPA, identified by loss-of-function screen, induces
apoptosis and suppresses tumor growth in mesothelioma mouse model.
Genomics. 95:210–216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koyanagi T, Suzuki Y, Saga Y, Machida S,
Takei Y, Fujiwara H, Suzuki M and Sato Y: In vivo delivery of siRNA
targeting vasohibin-2 decreases tumor angiogenesis and suppresses
tumor growth in ovarian cancer. Cancer Sci. 104:1705–1710. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tasaki M, Shimada K, Kimura H, Tsujikawa K
and Konishi N: ALKBH3, a human AlkB homologue, contributes to cell
survival in human non-small-cell lung cancer. Br J Cancer.
104:700–706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fujita T, Yanagihara K, Takeshita F,
Aoyagi K, Nishimura T, Takigahira M, Chiwaki F, Fukagawa T, Katai
H, Ochiya T, et al: Intraperitoneal delivery of a small interfering
RNA targeting NEDD1 prolongs the survival of scirrhous
gastric cancer model mice. Cancer Sci. 104:214–222. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Azuma K, Nakashiro K, Sasaki T, Goda H,
Onodera J, Tanji N, Yokoyama M and Hamakawa H: Anti-tumor effect of
small interfering RNA targeting the androgen receptor in human
androgen-independent prostate cancer cells. Biochem Biophys Res
Commun. 391:1075–1079. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sasaki T, Nakashiro K, Tanaka H, Azuma K,
Goda H, Hara S, Onodera J, Fujimoto I, Tanji N, Yokoyama M, et al:
Knockdown of Akt isoforms by RNA silencing suppresses the growth of
human prostate cancer cells in vitro and in vivo. Biochem Biophys
Res Commun. 399:79–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Woo SM, Min KJ, Seo BR, Nam JO, Choi KS,
Yoo YH and Kwon TK: Cafestol overcomes ABT-737 resistance in
Mcl-1-overexpressed renal carcinoma Caki cells through
downregulation of Mcl-1 expression and upregulation of Bim
expression. Cell Death Dis. 5:e15142014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sadahira K, Sagawa M, Nakazato T, Uchida
H, Ikeda Y, Okamoto S, Nakajima H and Kizaki M: Gossypol induces
apoptosis in multiple myeloma cells by inhibition of interleukin-6
signaling and Bcl-2/Mcl-1 pathway. Int J Oncol. 45:2278–2286. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cao X, Yap JL, Newell-Rogers MK,
Peddaboina C, Jiang W, Papaconstantinou HT, Jupitor D, Rai A, Jung
KY, Tubin RP, et al: The novel BH3 α-helix mimetic JY-1-106 induces
apoptosis in a subset of cancer cells (lung cancer, colon cancer
and mesothelioma) by disrupting Bcl-xL and Mcl-1 protein-protein
interactions with Bak. Mol Cancer. 12:42–2013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ozcan G, Ozpolat B, Coleman RL, Sood AK
and Lopez-Berestein G: Preclinical and clinical development of
siRNA-based therapeutics. Adv Drug Deliv Rev. 87:108–119. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu SY, Lopez-Berestein G, Calin GA and
Sood AK: RNAi therapies: Drugging the undruggable. Sci Transl Med.
6:240ps72014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo J, Fisher KA, Darcy R, Cryan JF and
O'Driscoll C: Therapeutic targeting in the silent era: Advances in
non-viral siRNA delivery. Mol Biosyst. 6:1143–1161. 2010.PubMed/NCBI
|
|
43
|
Kanasty R, Dorkin JR, Vegas A and Anderson
D: Delivery materials for siRNA therapeutics. Nat Mater.
12:967–977. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakazawa K, Nemoto T, Hata T, Seyama Y,
Nagahara S, Sano A, Itoh H, Nagai Y and Kubota S: Single-injection
ornithine decarboxylase-directed antisense therapy using
atelocollagen to suppress human cancer growth. Cancer.
109:993–1002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bosma GC, Custer RP and Bosma MJ: A severe
combined immunodeficiency mutation in the mouse. Nature.
301:527–530. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Larsson E, Sander C and Marks D: mRNA
turnover rate limits siRNA and microRNA efficacy. Mol Syst Biol.
6:4332010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wilsbacher LD, Yamazaki S, Herzog ED, Song
EJ, Radcliffe LA, Abe M, Block G, Spitznagel E, Menaker M and
Takahashi JS: Photic and circadian expression of luciferase in
mPeriod1-luc transgenic mice invivo. Proc Natl Acad Sci USA.
99:489–494. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Reed JC, Tsujimoto Y, Epstein SF, Cuddy M,
Slabiak T, Nowell PC and Croce CM: Regulation of bcl-2 gene
expression in lymphoid cell lines containing normal #18 or t(14;18)
chromosomes. Oncogene Res. 4:271–282. 1989.PubMed/NCBI
|
|
49
|
Wei G, Margolin AA, Haery L, Brown E,
Cucolo L, Julian B, Shehata S, Kung AL, Beroukhim R and Golub TR:
Chemical genomics identifies small-molecule MCL1 repressors
and BCL-xL as a predictor of MCL1 dependency. Cancer Cell.
21:547–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rooswinkel RW, van de Kooij B, de Vries E,
Paauwe M, Braster R, Verheij M and Borst J: Antiapoptotic potency
of Bcl-2 proteins primarily relies on their stability, not binding
selectivity. Blood. 123:2806–2815. 2014. View Article : Google Scholar : PubMed/NCBI
|