Introduction
Bladder cancer (BC) is one of the most common
malignancies worldwide, with a global age-standardized incidence
rate of 9.0 for men and 2.2 for women (per 100,000 person-years)
(1,2). Approximately 25% of newly diagnosed
cases of BC present with muscle invasion and require radical
surgery or radiotherapy, although these patients often have poor
outcomes despite systemic therapy (3). BC affects the urinary lining of the
bladder through a complicated, multifactorial etiology that
involves both genetic and environmental factors (4). Novel prognostic markers and effective
treatment strategies are therefore essential to improve outcomes
for patients with BC.
MicroRNAs (miRNAs) are small non-coding RNAs that
are ~22 nucleotides in length. They play pivotal regulatory roles
in post-transcriptional suppression (5) and have been reported to be involved in
many cellular processes, such as differentiation, morphogenesis and
tumorigenesis (6–8). Aberrant miRNA expression is found in a
variety of cancers, including BC, suggesting that they may have
roles as oncogenes or tumor-suppressor genes (9,10). For
example, miR-497 was significantly downregulated in bladder
transitional cell carcinoma cells and tissues. This led to the
upregulation of the transcription factor E2F3 and suppression of
cell proliferation and invasion (11). Similarly, miR-106a had an inhibitory
effect on the proliferation of BC cells through the modulation of
MAPK signaling (12). Previous
studies have also shown that miR-154, located in the human
imprinted 14q32 domain, acts as a tumor suppressor in various types
of human cancer, including prostate (13), colorectal (14) and lung cancer (15). Moreover, low miR-154 levels in
glioma (16) and colorectal cancer
(17) were significantly associated
with poor prognosis. Nevertheless, the levels of miR-154 and their
clinical significance in human BC have not yet been evaluated.
In our analysis, we first investigated the levels of
miR-154 in multiple BC tissues. We found that miR-154 was
downregulated in BC tissues and lower miR-154 expression levels
were strongly associated with worse overall survival (OS) rates in
patients with BC. Further study suggested that this was due to
decreased inhibition of BC cell proliferation, migration and
invasion mediated by miR-154. Subsequent bioinformatics analysis
revealed that both runt-related transcription factor 2
(RUNX2) and remodeling and spacing factor 1 (RSF1)
were targets of miR-154 and were involved in miR-154-induced
inhibition of the tumorigenic properties of BC cells.
Materials and methods
Patients and tissue samples
In total, 86 fresh BC tissue specimens, along with
adjacent normal tissue specimens, were collected from 2010 to 2013
at the Department of Urology, Peking Union Medical College Hospital
(Beijing, China). None of the patients had received any treatment
prior to surgery. The pathological type of BC was observed and
microscopically validated by an independent pathologist. Tumor
grading and staging were carried out according to the 6th AJCC TNM
staging system and graded according to Fuhrman's nuclear grading
system. The exclusion criteria included incomplete clinical or
follow-up information, recurrent tumors, other chronic system
diseases, and an unwillingness to participate in the present study.
Clinical characteristics and follow-up information for individual
patients were collected from their medical records. OS was defined
as the time from inclusion to death of the patient, for any reason.
The study was approved by the Research Ethics Committee of the
Peking Union Medical College Hospital and written informed consent
was provided by all patients.
Real-time quantitative PCR
(RT-qPCR)
Total RNA was isolated from tissues and cells using
TRIzol reagent, according to the manufacturer's protocol
(Invitrogen, Carlsbad, CA, USA). For the detection of miR-154,
complementary DNAs were synthesized using a TaqMan MicroRNA Reverse
Transcription kit and a stem-loop RT primer. Amplification was
performed using a TaqMan miRNA assay (Applied Biosystems, Foster
City, CA, USA) on an ABI PRISM 7500 Real-Time PCR System with the
following conditions: 95°C for 2 min, followed by 40 cycles at 94°C
(15 sec), 60°C (60 sec) and 72°C (30 sec). For mRNA detection,
complementary DNAs were synthesized via a PrimeScript RT-PCR kit
(Takara, Otsu, Japan) and quantification was performed using RT
Real-Time SYBR-Green assays (Bio-Rad Laboratories, Berkeley, CA,
USA). Each sample was examined in triplicate and the relative miRNA
and mRNA expression levels were determined using the
2−ΔΔCt method and normalized to the housekeeping genes
U6 and GAPDH. Primers used for qPCR are shown in
Table I.
 | Table I.The forward and reverse primers for
real-time PCR. |
Table I.
The forward and reverse primers for
real-time PCR.
| Name |
| Sequence |
|---|
| has-miR-154 | RT |
GTCGTATCCAGTGCGTGTCG |
|
| primer |
TGGAGTCGGCAATTGCACTGGATACGACCGAAGG |
|
| Forward |
GTGGTACTTGAAGATAGGTT |
|
| Reverse |
TTGGTACTGAAAAATAGGTC |
| RSF1 | Forward |
GAGGAGGATGCCGATACTATGC |
|
| Reverse |
TGCTTTCAGGAGTGCAAGAGTC |
| RUNX2 | Forward |
AAGTGAGGTTAGGGCGAAATG |
|
| Reverse |
AAGGTAGTTGATTGCCAACGAA |
| U6 | Forward |
GAGCGGTAGCACCATTTGAA |
|
| Reverse |
GTGCAGGGTCCGAGG |
| GAPDH | Forward |
GAAGGTGAAGGTCGGAGTC |
|
| Reverse |
GAAGATGGTGATGGGATTTC |
Cell culture and transfection
The T24 human BC cell line was purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA) and
cultured in Dulbeccos modified Eagles medium (DMEM) (Mediatech,
Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS),
and 1% U/ml penicillin and streptomycin (both from Invitrogen). All
cells were maintained at 37°C in a humidified atmosphere of air
containing 5% CO2. Cells were transfected with miR-154
mimics (sense, 5′UAGGUUAUCCGUGUUGCCUUCG3′ and antisense,
5′AAGGCAACACGAUAACCUAUU3′) and negative controls (scrambled oligos,
miR-NC) (Shanghai GenePharma, Shanghai, China) using Lipofectamine
2000 (Invitrogen) at a final concentration of 100 nM, following the
manufacturer's instructions. Overexpression of RSF1 and RUNX2 was
achieved using RSF1 and RUNX2 ORF expression clones
(Vector Gene Technology of Beijing, Beijing, China) and a pcDNA3.1
empty vector was used as a negative control.
Bioinformatics analysis and luciferase
reporter assay
The downstream targets of miR-154 were predicted
using miRBase, miTarget and TargetScan. Putative complementary
sequences for miR-154 were identified in the 3′-untranslated
regions (3′-UTRs) of RSF1 and RUNX2 mRNA. The 3′-UTRs
of RSF1 and RUNX2, containing the miR-154 target
sequence, were synthesized and inserted into the XbaI and
FseI sites of a pGL3 control vector (Promega, Madison, WI,
USA) to generate a overall survival an RSF1-wild-type and
RUNX2-wild-type luciferase reporter. Mutation of the binding site
was performed using site-directed mutagenesis (Promega) to create
the pGL3-RSF1- and pGL3-RUNX2-mutant type plasmids. For detection,
T24 cells were cultured in 96-well plates and co-transfected with
miR-1545 mimics (or miR-NC) and reporter plasmids using
Lipofectamine 2000. After 48 h, the cells were assayed with a
Dual-Luciferase Reporter Assay System (Promega). Firefly luciferase
activity for each sample was normalized to Renilla
luciferase activity.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto,
Japan) assays were used for analysis of cell proliferation. T24
cells were seeded into 96-well plates at 1×104
cells/well and incubated in 10% CCK-8 solution at 37°C until color
conversion had occurred. The absorbance was assessed at 450 nm
using a microplate reader (Multiskan; Devices, Menlo Park, CA, USA)
24, 48, 72 and 96 h after transfection.
Wound-healing and Transwell invasion
assays
T24 cells were seeded in 24-well plates. When cells
were 80–90% confluent, a sterile pipette tip was used to manually
scratch the monolayer. After three washes with phosphate-buffered
saline (PBS), cell migration was observed using an inverted
microscope. The cells were then incubated at 37°C for a further 48
h before cell migration was assessed again. For the invasion
assays, transfected cells were transferred to the top of
Matrigel-coated invasion chambers (24-well plates, 8 µm pore size;
Becton-Dickinson, San Jose, CA, USA) with serum-free medium. Medium
containing 10% FBS was used as the chemoattractant in the lower
chambers. After 24 h of incubation, the media were removed and the
wells were washed twice with PBS. Invasive cells that were attached
to the lower surface were fixed with 4% paraformaldehyde, stained
with 0.1% crystal violet, and transferred to a microscope slide.
Microphotographs were collected in six representative fields using
an inverted microscope at a magnification of ×200 (Olympus BX53;
Olympus, Tokyo, Japan).
Western blot analyses
T24 cells and human BC specimens were lysed in 1%
RIPA lysis buffer (Beyotime, Jiangsu, China), and supplemented with
protease inhibitors at 4°C for 1 h. Protein quantification was
performed using the BCA method (Beyotime). Equal amounts of protein
were ran on 10% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE) gels, and transferred to polyvinylidene
fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After
blocking with 1% bovine serum albumin (BSA) for 30 min, the
membranes were incubated with primary antibodies against either
RSF1 (ab109002), RUNX2 (ab23981) or GAPDH (ab9485) (all from Abcam,
Cambridge, UK) at 4°C overnight. The membranes were then washed
three times with Tris-buffered saline with Tween-20 (TBST),
followed by incubation with horseradish peroxidase-conjugated (HRP)
goat anti-rabbit secondary antibody (Sigma, St. Louis, MO, USA) for
1 h at room temperature. The blots were incubated with an enhanced
chemiluminescence substrate (ECL; Plus) and visualized on a G:BOX
Chemi XR5 imaging system (Syngene, Cambridge, UK).
Statistical analysis
All values are expressed as the median and range, or
mean ± standard deviation (SD), where appropriate. Data analysis
was performed using GraphPad Prism software 6.0 (GraphPad Software,
Inc., La Jolla, CA, USA). Comparisons between two groups were
performed using unpaired two-tailed Student's t-tests or
χ2 test and comparisons among >2 groups were carried
out with one-way ANOVA plus post hoc Bonferroni or post hoc Dunnett
tests. Assessment of any association between miR-154 expression and
various clinicopathological parameters were made using Pearson's
χ2 tests and SPSS version 19.0 (SPSS, Inc., Chicago, IL,
USA). Spearman's correlation analysis was performed to compare
mRNAs and miR-154 levels. Analysis of OS was performed according to
the Kaplan-Meier method and comparisons were carried out using
log-rank tests. P<0.05 was considered statistically
significant.
Results
miR-154 is specifically downregulated
in BC tissues and is a predictor of poor outcome
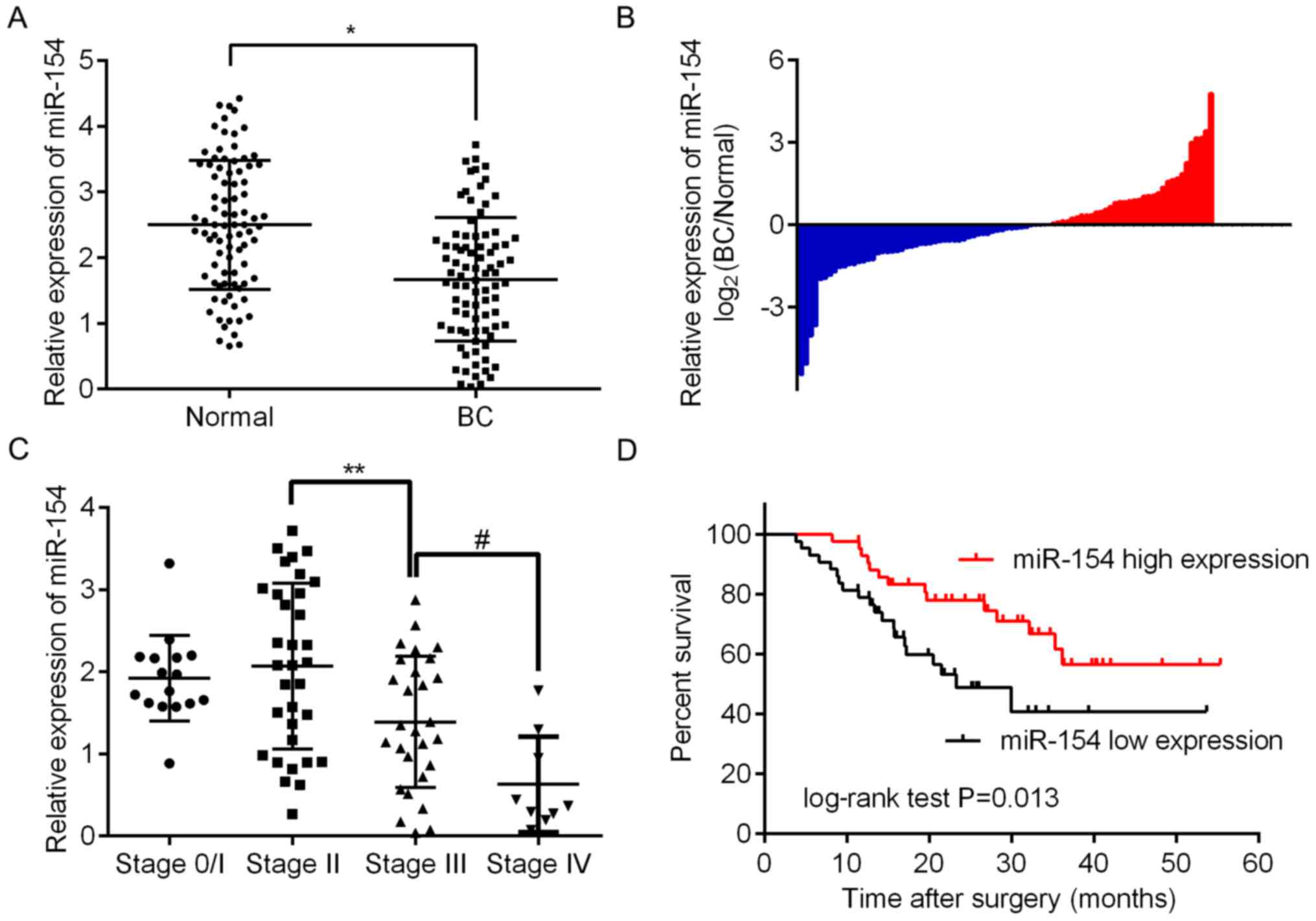
To assess the expression of miR-154 in BC tissue, we
analyzed 86 paired BC/normal tissue samples by RT-qPCR. The results
revealed that there was significantly less miR-154 in BC tissues
relative to adjacent normal tissue (Fig. 1A and B; P<0.05). To assess any
correlation between miR-154 and the clinical or pathological
features of BC, patients were assigned to two groups, one with high
expression relative to the median and the other with low
expression. As shown in Table II,
it was determined that low miR-154 expression was significantly
associated with more advanced tumor staging (P=0.020), higher
histologic grades (P=0.041) and lymph node metastasis (P=0.024).
However, there were no significant associations between miR-154
expression and age, sex or tumor size. These findings revealed that
miR-154 may play an important role in the progression of BC. This
supports results shown in Fig. 1C
demonstrating that there is a trend for miR-154 to be lower in more
advanced tumor, node and metastasis (TNM) stages, with the lowest
expression found in stage 4. Furthermore, Kaplan-Meier curves
revealed that low levels of miR-154 had a statistically significant
association with shorter OS in patients when compared to those with
higher expression levels (P<0.01; Fig. 1D).
 | Table II.Correlation between miR-154
expression and clinicopathological parameters of baldder
cancer. |
Table II.
Correlation between miR-154
expression and clinicopathological parameters of baldder
cancer.
|
| miR-154
expression |
|
|---|
|
|
|
|
|---|
| Clinical
characteristics | Low | High |
P-valuea |
|---|
| Age (years) |
|
| 0.914 |
|
<60 | 19 | 17 |
|
|
≥60 | 27 | 23 |
|
| Sex |
|
| 0.279 |
|
Male | 20 | 23 |
|
|
Female | 26 | 17 |
|
| Tumor size
(cm) |
|
| 0.363 |
|
<3 | 28 | 29 |
|
| ≥3 | 18 | 11 |
|
| T stage |
|
| 0.020 |
|
Ta/T1 | 8 | 8 |
|
| T2 | 12 | 21 |
|
| T3 | 18 | 10 |
|
| T4 | 8 | 1 |
|
| Histologic
grade |
|
| 0.041 |
| G1 | 12 | 20 |
|
| G2 | 17 | 13 |
|
| G3 | 17 | 7 |
|
| Lymph node
metastasis |
|
| 0.024 |
|
N0/1 | 19 | 28 |
|
| N2 | 20 | 10 |
|
| N3 | 7 | 2 |
|
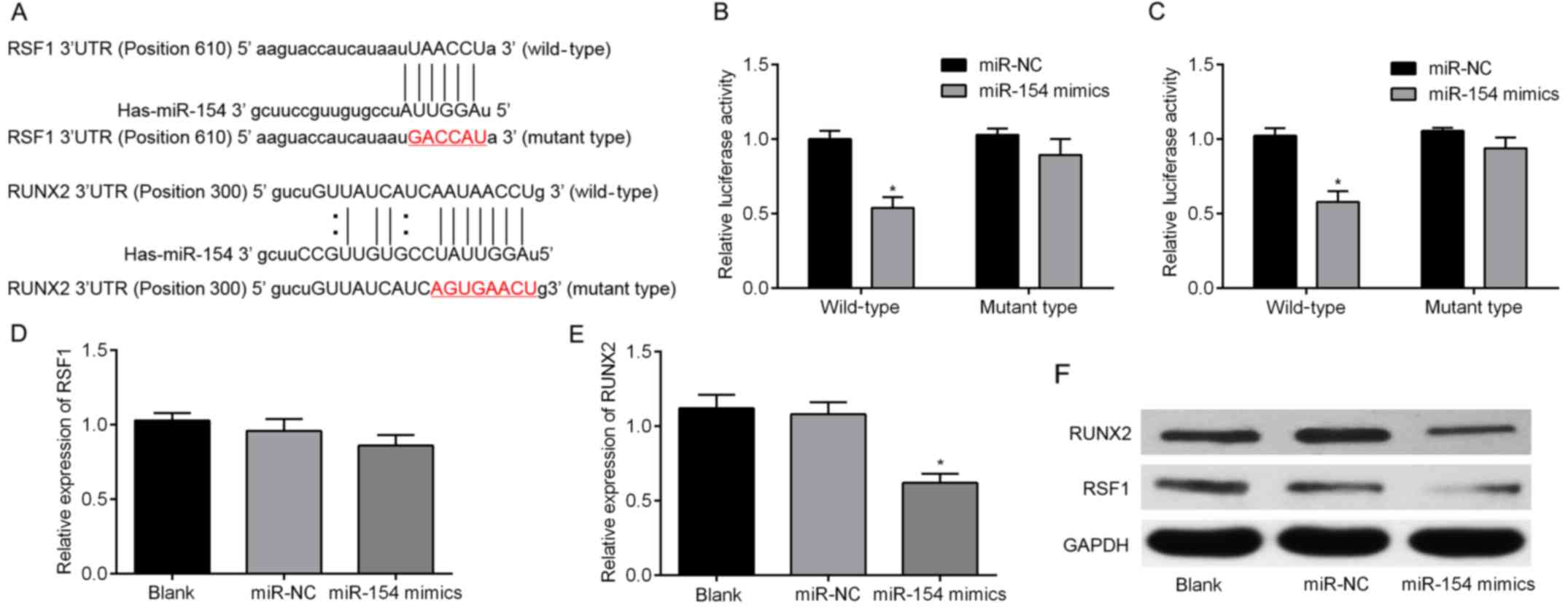
RSF1 and RUNX2 are directly targeted
by miR-154 in BC cells
Three algorithms, miRBase, TargetScan and miRanda,
were used to predict the putative targets of miR-154. Two genes,
RSF1 and RUNX2, were then selected for further
validation since they both occurred in all three algorithms. As
shown in Fig. 2A, the 3′-UTRs of
RSF1 and RUNX2 were inserted into a reporter plasmid
downstream of luciferase. Matching mutants were also created, with
changes underlined in the presented sequences. T24 cells were then
transiently co-transfected with miR-154 mimics (or miR-NC) and
individually constructed plasmids. Luciferase reporter assays were
performed to detect luciferase expression. These revealed that
overexpression of miR-154 resulted in downregulation of luciferase
when fused to the RSF1 and RUNX2 3′-UTRs in T24 cells
(Fig. 2B and C). To determine
whether miR-154 suppressed endogenous RSF1 and RUNX2,
miR-154 was overexpressed using mimic transfection into T24 cells.
Notably, upregulation of miR-154 only downregulated RUNX2
mRNA, but failed to suppress endogenous expression of RSF1
mRNA (Fig. 2D and E). For further
validation, cells were collected after transfection and analyzed by
western blotting. The results revealed that both RSF1 and RUNX2
protein were decreased by miR-154 overexpression (Fig. 2F). Collectively, our results
revealed that RSF1 and RUNX2 are both target genes of
miR-154.
RSF1 and RUNX2 are highly expressed in
BC tissues and are negatively correlated with miR-154 levels
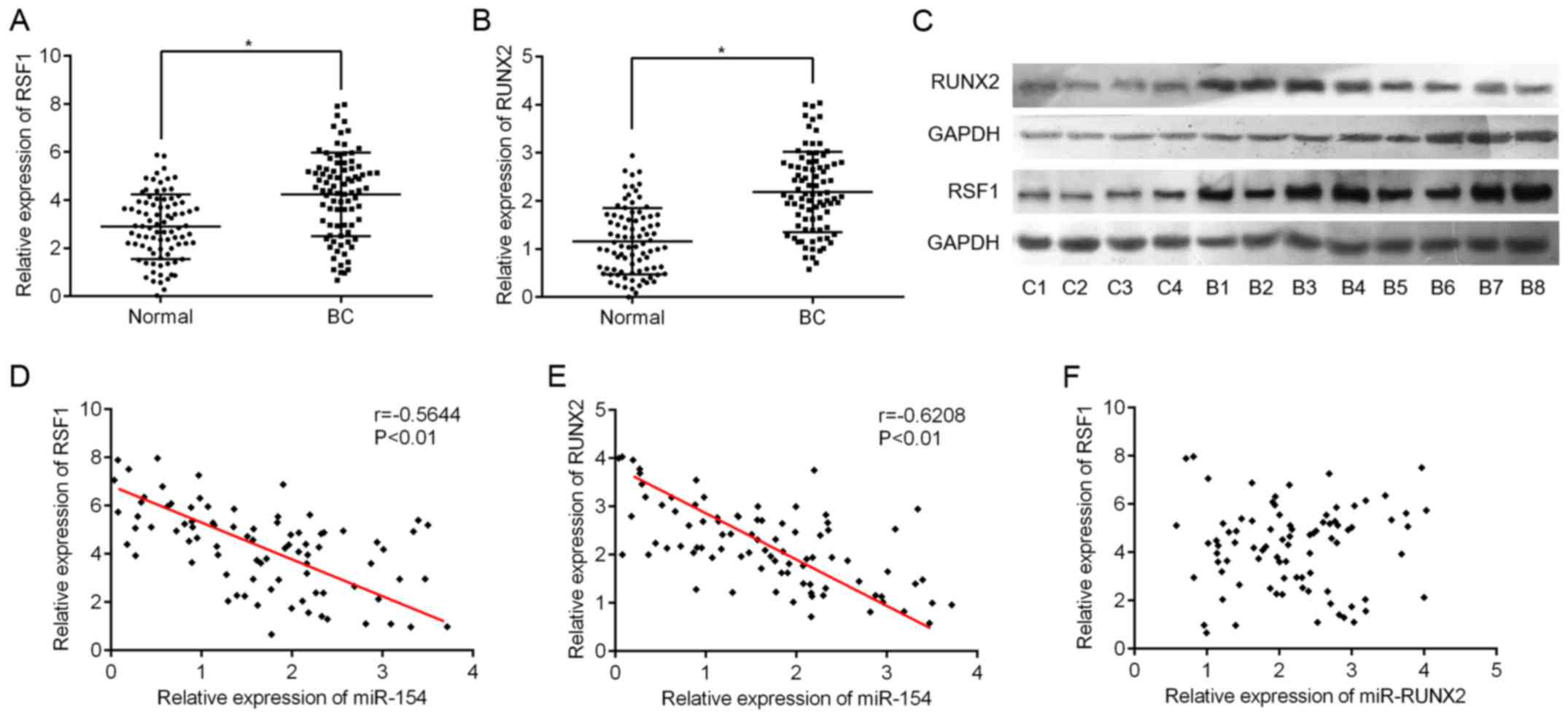
Next, we used RT-qPCR to assess the expression
levels of RSF1 and RUNX2 in BC tissues. As expected,
RSF1 and RUNX2 mRNA expression was significantly
higher in BC tissues compared to adjacent normal tissues (Fig. 3A and B). Supporting the RT-qPCR
data, the levels of RSF1 and RUNX2 protein also exhibited the same
pattern in eight BC and four normal samples (Fig. 3C). Furthermore, we found that
RSF1 gene expression was negatively correlated with miR-154
levels (Pearson correlation r=-0.5644, P<0.001; Fig. 3D). A similar correlation was also
observed between RUNX2 and miR-154 (r=-0.6208, P<0.001;
Fig. 3E). However, no linear
relationship was found between RSF1 and RUNX2
expression in BC tissues (r=0.016, P=0.893; Fig. 3F).
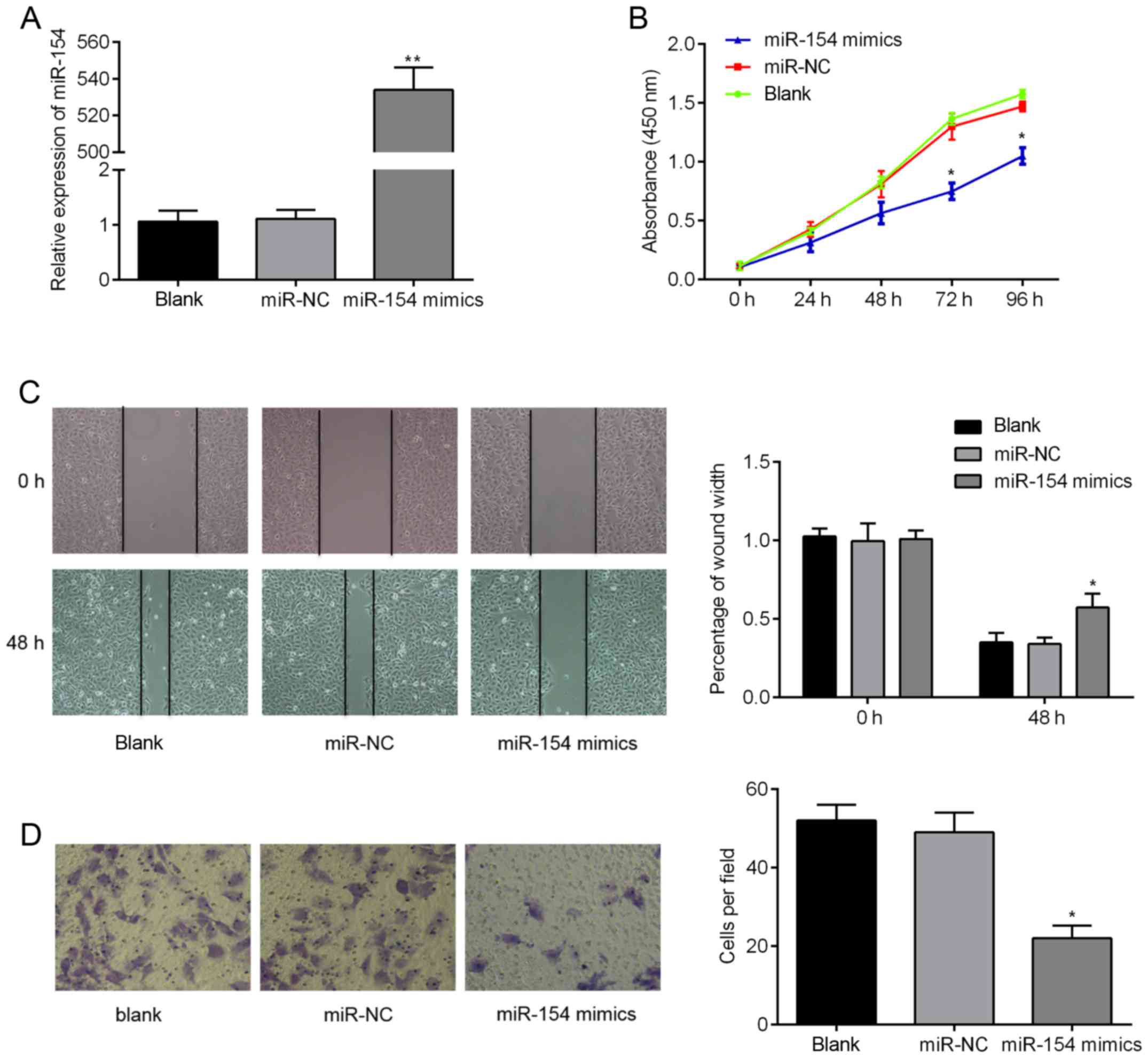
miR-154 suppresses proliferation,
migration and invasion of BC cells
We next assessed the in vitro biological
functions of miR-154 in BC cells in more detail. RT-qPCR analysis
confirmed that miR-154 mimics were successfully transfected into
T24 cells and could be used for subsequent analysis. As shown in
Fig. 4A, miR-154 expression levels
were >500-fold higher in the miR-154 mimic group compared to
controls (P<0.01). We then examined the effects that miR-154 had
on cell proliferation, migration and invasion. We found using CCK-8
assays that ectopic miR-154 expression suppressed proliferation of
T24 cells. Additionally, the migratory capacity of cells
transfected with miR-154 mimics was lower 48 h after wound
creation, when compared to the control cells (Fig. 4C; P<0.05). Finally, Transwell
migration assays over 24 h revealed that there were twice as many
migratory cells transfected with miR-NC relative to cells
transfected with miR-154 mimics (Fig.
4D; P<0.05). Combined, these findings suggest that
overexpression of miR-154 suppresses the tumorigenic behavior of BC
cells in vitro.
miR-154 inhibits proliferation,
migration and invasion in BC cells by regulating RSF1 and
RUNX2
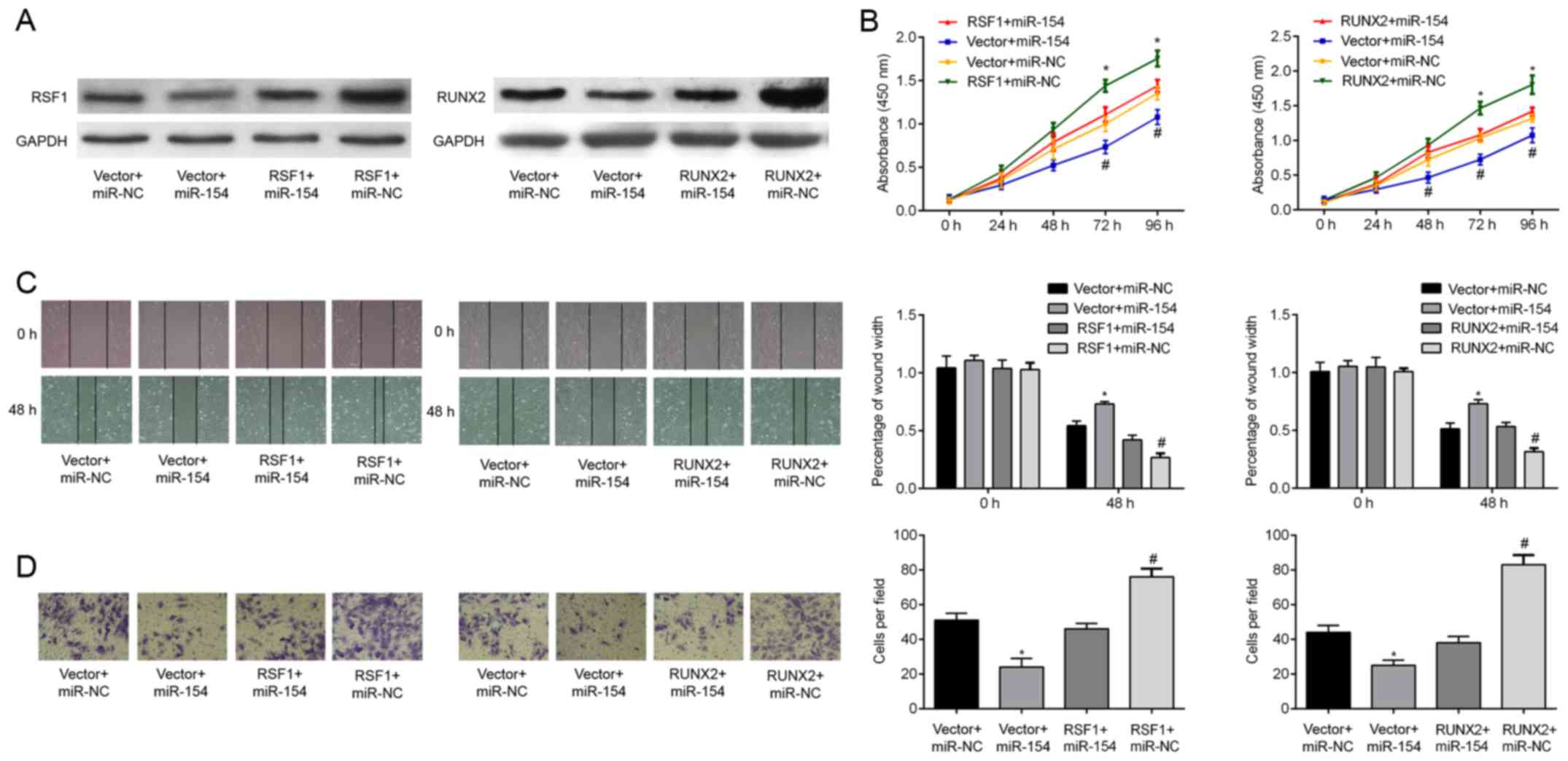
We finally determined whether overexpression of RSF1
or RUNX2 could counteract the inhibitory effects of miR-154 on T24
cells. To achieve this, cells were transfected with either
RSF1 or RUNX2 inserted into a pcDNA3.1 overexpression
plasmid. Successful transfection was ascertained by western
blotting, demonstrating that the relative expression of RSF1 and
RUNX2 protein was increased relative to the controls.
Significantly, increasing RSF1 and RUNX2 protein expression in BC
cells reversed the cell proliferation induced by miR-154
overexpression (Fig. 5B). Wound
healing assays revealed that the decrease in T24 cell migration
caused by miR-154 mimics was attenuated by co-transfection with
RSF1 and RUNX2 (Fig.
5C; P<0.05). Similarly, upregulation of RSF1 and RUNX2
resulted in a greater cell invasive capacity and rescued
miR-154-induced suppression of cell invasion (Fig. 5D; all P<0.05). Collectively,
these data support the hypothesis that miR-154 inhibits T24 cell
proliferation, migration, and invasion by negatively targeting the
RSF1 and RUNX2 genes.
Discussion
A growing body of evidence has suggested that miRNAs
play a fundamental role in the proliferation, invasion and
metastasis of malignant human carcinomas (18). Therefore, targeting miRNAs may be an
effective approach for the treatment of advanced cancer. Recently,
aberrant miRNAs involved in tumorigenesis and cancer progression
were identified in bladder cancer (BC) (19,20).
In the present study, we demonstrated that miR-154 was
downregulated in BC tissues compared to adjacent normal tissues. We
also found that miR-154 levels were strongly associated with the
aggressive clinicopathological features of BC, such as progression
to advanced TNM stages, high histologic grades and lymph node
status. In addition, survival analysis revealed that patients with
low miR-154 expression had shorter OS relative to those with high
miR-154 expression. Finally, we found that miR-154 could suppress
BC cell proliferation, migration and invasion in vitro. To
the best of our knowledge, this is the first study confirming the
clinical significance and functional attributes of miR-154 in
BC.
Previous studies have identified miR-154 as a
tumor-suppressor involved in several types of cancer. For example
Xu et al (2016) revealed that miR-154 is frequently
downregulated in breast cancer tissue and cell lines where it is
involved in the proliferation, migration and invasion of breast
cancer cells by directly targeting E2F transcription factor 5
(E2F5) (21). In addition,
miR-154 was identified as a suppressor of epithelial mesenchymal
transition by downregulating ZEB2 (22) and HMGA2 (23). A recent study revealed by Lin et
al (2016) also revealed that ZEB2 overexpression can reverse
miR-154-induced inhibition of migration and invasion in lung cancer
(15). Building on this research,
the present study demonstrated that overexpression of miR-154
decreased RSF1 and RUNX2 protein expression levels in T24 cells.
This is likely through miR-154 binding the 3′-UTR of RUNX2
mRNA, promoting its degradation. There was however no significant
effect on RSF1 mRNA levels, which may be due to incomplete
complementation with the 3′-UTR.
RSF1, also known as hepatitis B X-antigen-associated
protein (HBXAP), is a member of the ATP-dependent chromatin
remodeling factor family (24).
RSF1 interacts with its partner, sucrose non-fermenting protein 2
homologous (hSNF2H), to form the RSF-1/hSNF2H complex. This complex
responds to a variety of growth signals and environmental cues to
remodel chromatin and is essential for transcriptional
activation/suppression and cell cycle progression (25–27).
There is also evidence to suggest that expression of the
RSF1 gene is increased in various cancers and its
upregulation is critical to tumor development (28). Ren et al (2013) revealed that
high-expression of RSF1 was associated with larger tumors and more
advanced TNM staging in primary breast cancer (29). A study by Liang et al (2012)
also found that increased levels of RSF1 were associated with poor
outcomes for patients with BC, suggesting that the protein is
involved in cell survival and growth (30). In the present study, we found that
RSF1 was directly targeted by miR-154. Furthermore, it
played an important role in controlling the malignant behavior of
BC cells, explaining the clinical significance of the RSF1.
However, the exact molecular mechanisms associated with the effects
of RSF1 protein, such as NF-κB signaling (31), in BC still remain undefined in the
present study.
In addition, we also identified another target gene
of miR-154, RUNX2. This is a member of the mammalian RUNX family of
transcription factors that have been well-established to be
involved in development and cancer (32). For example, RUNX2, a key regulator
of osteoblast differentiation, has been implicated in the evolution
of breast and prostate cancer metastasis (33, 34).
Consistent with our findings, Abdelzaher et al (2016)
reported that RUNX2 is frequently upregulated in urothelial
carcinomas and is likely involved in both carcinogenesis and tumor
aggressiveness (35). There are
also numerous studies examining the oncogenic role of RUNX2 through
its regulatory effects on various tumorigenesis-associating genes
and pathways. For example, Lim et al (2010) demonstrated
that RUNX2 regulated survivin (BIRC5) expression in prostate cancer
and protected cancer cells against apoptosis (36). Another study concluded that RUNX2
could act as a negative regulator for p53-dependent apoptosis and
may therefore be an attractive therapeutic target for cancer
(37). Our own study revealed an
association between upregulation of RUNX2 in malignant epithelial
BC carcinoma tissues, although we demonstrated that this was
regulated by miR-154. We therefore suggest that RUNX2 promotes cell
proliferation, migration, and invasion in BC and that these factors
may be controlled by miR-154. However, it is still unclear whether
RUNX2 affects cell apoptosis in BC cells and more studies are
required to better understand the molecular mechanisms and
biological pathways mediated by RUNX2. Since miRNAs can regulate
various genes simultaneously, targeting miRNAs could influence
multiple critical molecules involved in tumorigenesis (38). iTRAQ proteomics and bioinformatics
(39) could be further used to
identify more miR-154 targets.
In summary, the present study found that miR-154
downregulation in BC was correlated with a poor survival rate.
Additionally, miR-154 inhibited BC cell proliferation, migration,
and invasion by regulating RSF1 and RUNX2 expression. Our data
revealed that the miR-154-RSF1/RUNX2 interaction in BC may be an
attractive target for future cancer treatment.
Glossary
Abbreviations
Abbreviations:
|
BC
|
bladder cancer
|
|
miR-154
|
microRNA-154
|
|
RUNX2
|
runt-related transcription factor
2
|
|
RSF1
|
remodeling and spacing factor 1
|
References
|
1
|
Babjuk M, Böhle A, Burger M, Capoun O,
Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M,
et al: EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma
of the Bladder: Update 2016. Eur Urol. 71:447–461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S, et al: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stenzl A, Cowan NC, De Santis M, Kuczyk
MA, Merseburger AS, Ribal MJ, Sherif A and Witjes JA: European
Association of Urology (EAU): Treatment of muscle-invasive and
metastatic bladder cancer: Update of the EAU guidelines. Eur Urol.
59:1009–1018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye F, Wang L, Castillo-Martin M, McBride
R, Galsky MD, Zhu J, Boffetta P, Zhang DY and Cordon-Cardo C:
Biomarkers for bladder cancer management: Present and future. Am J
Clin Exp Urol. 2:1–14. 2014.PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang SJ, Weng SL, Hsieh JY, Wang TY,
Chang MD and Wang HW: MicroRNA-34a modulates genes involved in
cellular motility and oxidative phosphorylation in neural
precursors derived from human umbilical cord mesenchymal stem
cells. BMC Med Genomics. 4:652011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui X, Kong C, Zhu Y, Zeng Y, Zhang Z, Liu
X, Zhan B, Piao C and Jiang Z: miR-130b, an onco-miRNA in bladder
cancer, is directly regulated by NF-κB and sustains NF-κB
activation by decreasing Cylindromatosis expression. Oncotarget.
7:48547–48561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song T, Zhang X, Yang G, Song Y and Cai W:
Decrement of miR-199a-5p contributes to the tumorigenesis of
bladder urothelial carcinoma by regulating MLK3/NF-κB pathway. Am J
Transl Res. 7:2786–2794. 2015.PubMed/NCBI
|
|
9
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JY, Ryu DS, Kim WJ and Kim SJ:
Aberrantly expressed microRNAs in the context of bladder
tumorigenesis. Investig Clin Urol. 57 Suppl 1:S52–S59. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Zhang Z, Li Z, Gong D, Zhan B,
Man X and Kong C: MicroRNA-497 inhibits the proliferation,
migration and invasion of human bladder transitional cell carcinoma
cells by targeting E2F3. Oncol Rep. 36:1293–1300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shin SS, Park SS, Hwang B, Kim WT, Choi
YH, Kim WJ and Moon SK: MicroRNA-106a suppresses proliferation,
migration, and invasion of bladder cancer cells by modulating MAPK
signaling, cell cycle regulators, and Ets-1-mediated MMP-2
expression. Oncol Rep. 36:2421–2429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu C, Shao P, Bao M, Li P, Zhou H, Cai H,
Cao Q, Tao L, Meng X, Ju X, et al: miR-154 inhibits prostate cancer
cell proliferation by targeting CCND2. Urol Oncol. 32:31.e9–31.e16.
2014. View Article : Google Scholar
|
|
14
|
Xin C, Zhang H and Liu Z: miR-154
suppresses colorectal cancer cell growth and motility by targeting
TLR2. Mol Cell Biochem. 387:271–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin X, Yang Z, Zhang P, Liu Y and Shao G:
miR-154 inhibits migration and invasion of human non-small cell
lung cancer by targeting ZEB2. Oncol Lett. 12:301–306.
2016.PubMed/NCBI
|
|
16
|
Wang L, Wu L and Wu J: Downregulation of
miR-154 in human glioma and its clinicopathological and prognostic
significance. J Int Med Res. 44:994–1001. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kai Y, Qiang C, Xinxin P, Miaomiao Z and
Kuailu L: Decreased miR-154 expression and its clinical
significance in human colorectal cancer. World J Surg Oncol.
13:1952015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baranwal S and Alahari SK: miRNA control
of tumor cell invasion and metastasis. Int J Cancer. 126:1283–1290.
2010.PubMed/NCBI
|
|
19
|
Xiong F, Liu K, Zhang F, Sha K, Wang X,
Guo X and Huang N: MiR-204 inhibits the proliferation and invasion
of renal cell carcinoma by inhibiting RAB22A expression. Oncol Rep.
35:3000–3008. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou J, Dai W and Song J: miR-1182
inhibits growth and mediates the chemosensitivity of bladder cancer
by targeting hTERT. Biochem Biophys Res Commun. 470:445–452. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu H, Fei D, Zong S and Fan Z:
MicroRNA-154 inhibits growth and invasion of breast cancer cells
through targeting E2F5. Am J Transl Res. 8:2620–2630.
2016.PubMed/NCBI
|
|
22
|
Pang X, Huang K, Zhang Q, Zhang Y and Niu
J: miR-154 targeting ZEB2 in hepatocellular carcinoma functions as
a potential tumor suppressor. Oncol Rep. 34:3272–3279. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu C, Li J, Cheng G, Zhou H, Tao L, Cai
H, Li P, Cao Q, Ju X, Meng X, et al: miR-154 inhibits EMT by
targeting HMGA2 in prostate cancer cells. Mol Cell Biochem.
379:69–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shih IeM, Sheu JJ, Santillan A, Nakayama
K, Yen MJ, Bristow RE, Vang R, Parmigiani G, Kurman RJ, Trope CG,
et al: Amplification of a chromatin remodeling gene, Rsf-1/HBXAP,
in ovarian carcinoma. Proc Natl Acad Sci USA. 102:pp. 14004–14009.
2005; View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cosma MP, Tanaka T and Nasmyth K: Ordered
recruitment of transcription and chromatin remodeling factors to a
cell cycle- and developmentally regulated promoter. Cell.
97:299–311. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shamay M, Barak O, Doitsh G, Ben-Dor I and
Shaul Y: Hepatitis B virus pX interacts with HBXAP, a PHD finger
protein to coactivate transcription. J Biol Chem. 277:9982–9988.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maeda D, Chen X, Guan B, Nakagawa S, Yano
T, Taketani Y, Fukayama M, Wang TL and Shih IeM: Rsf-1 (HBXAP)
expression is associated with advanced stage and lymph node
metastasis in ovarian clear cell carcinoma. Int J Gynecol Pathol.
30:30–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sheu JJ, Choi JH, Guan B, Tsai FJ, Hua CH,
Lai MT, Wang TL and Shih IeM: Rsf-1, a chromatin remodelling
protein, interacts with cyclin E1 and promotes tumour development.
J Pathol. 229:559–568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ren J, Chen QC, Jin F, Wu HZ, He M, Zhao
L, Yu ZJ, Yao WF, Mi XY, Wang EH, et al: Overexpression of Rsf-1
correlates with pathological type, p53 status and survival in
primary breast cancer. Int J Clin Exp Pathol. 7:5595–5608.
2014.PubMed/NCBI
|
|
30
|
Liang PI, Wu LC, Sheu JJ, Wu TF, Shen KH,
Wang YH, Wu WR, Shiue YL, Huang HY, Hsu HP, et al: Rsf-1/HBXAP
overexpression is independent of gene amplification and is
associated with poor outcome in patients with urinary bladder
urothelial carcinoma. J Clin Pathol. 65:802–807. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Li G, Liu C, Tang Y and Zhang S:
RSF1 regulates the proliferation and paclitaxel resistance via
modulating NF-κB signaling pathway in nasopharyngeal carcinoma. J
Cancer. 8:354–362. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ito Y: RUNX genes in development and
cancer: Regulation of viral gene expression and the discovery of
RUNX family genes. Adv Cancer Res. 99:33–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akech J, Wixted JJ, Bedard K, van der Deen
M, Hussain S, Guise TA, van Wijnen AJ, Stein JL, Languino LR,
Altieri DC, et al: Runx2 association with progression of prostate
cancer in patients: Mechanisms mediating bone osteolysis and
osteoblastic metastatic lesions. Oncogene. 29:811–821. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pratap J, Wixted JJ, Gaur T, Zaidi SK,
Dobson J, Gokul KD, Hussain S, van Wijnen AJ, Stein JL, Stein GS,
et al: Runx2 transcriptional activation of Indian Hedgehog and a
downstream bone metastatic pathway in breast cancer cells. Cancer
Res. 68:7795–7802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abdelzaher E and Kotb AF: High
Coexpression of Runt-related transcription factor 2 (RUNX2) and p53
independently predicts early tumor recurrence in bladder urothelial
carcinoma patients. Appl Immunohistochem Mol Morphol. 24:345–354.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lim M, Zhong C, Yang S, Bell AM, Cohen MB
and Roy-Burman P: Runx2 regulates survivin expression in prostate
cancer cells. Lab Invest. 90:222–233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ozaki T, Wu D, Sugimoto H, Nagase H and
Nakagawara A: Runt-related transcription factor 2 (RUNX2) inhibits
p53-dependent apoptosis through the collaboration with HDAC6 in
response to DNA damage. Cell Death Dis. 4:e6102013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Gao DY and Huang L: In vivo
delivery of miRNAs for cancer therapy: Challenges and strategies.
Adv Drug Deliv Rev. 81:128–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang Z, Qiu H, Luo L, Liu N, Zhong J, Kang
K and Gou D: miR-34b modulates skeletal muscle cell proliferation
and differentiation. J Cell Biochem. Apr 19–2017.(Epub ahead of
print). doi: 10.1002/jcb.26079. View Article : Google Scholar
|