Introduction
Angiogenesis, the process of new blood vessel
formation from pre-existing ones, is essential in physiological
processes, including revascularization, wound healing, reproduction
and pregnancy (1). In contrast,
abnormal angiogenesis results in many pathological diseases, such
as tumors, rheumatoid arthritis, psoriasis and vasoproliferative
retinopathy (2). Particularly,
tumorigenesis depends on angiogenesis for metabolic supplies such
as essential nutrients, various growth factors and molecular oxygen
(3). Therefore, suppression of
angiogenesis presents a promising strategy for cancer treatment and
other angiogenesis-related human diseases.
Endothelial cell proliferation, metastasis and
tubulogenesis are the major hallmarks of angiogenesis, which are
induced by the crucial regulator, vascular endothelial growth
factor (VEGF) (3,4). VEGF acts on endothelial cells via the
vascular endothelial growth factor receptor (VEGFR) family, among
which VEGFR2 is a key mediator of proangiogenic activities induced
by VEGF (5). During the process of
angiogenesis, VEGFR2 signaling activates many downstream mediators,
including signal transducer and activator of transcription (STAT)3,
extracellular signal-regulated kinase (ERK) 1/2 and AKT, which
promote the proliferation, migration and differentiation of
endothelial cells (3–5). In addition, in VEGFR2-mediated
cascades, matrix metalloproteinases (MMPs) play a key role in
extracellular matrix (ECM) degradation and cyclin D1 is required
for progression through the G1 phase of the cell cycle, which are
deeply involved in the regulation of endothelial cell survival,
migration and invasion (6,7). Thus, inhibition of VEGFR2 signal
transduction is considered as a therapeutic strategy for
antiangiogenesis.
Hypoxia areas arise as a result of the rapid growth
of solid tumors (8). During the
process of hypoxia-induced malignant progression, tumors may
undergo increased invasive growth, tumor cell spreading and
metastasis (9). Cancer cells
exposed to hypoxic conditions exhibit altered protein levels, such
as hypoxia-inducible factor (HIF)-1α accumulation. HIF-1α is a
crucial transcription factor involved in the angiogenesis in tumor
cells, which controls transcription of hypoxia-regulated genes such
as VEGF (10,11). Therefore, inhibition of HIF-1α may
be regarded as an antiangiogenic therapy.
Natural products are rich source of
angiogenesis-regulating compounds (12). Some angiogenesis inhibitors have
been isolated from natural products, such as paclitaxel (Taxus
brevifolia), camptothecin (Camptotheca acuminate),
combretastatin (Combretum caffrum) and farnesiferol C
(Ferula assafoetida) (13–16).
To date, many studies have demonstrated that a variety of natural
products have antiangiogenic activities (17). Hovenia dulcis Thunb.,
Japanese raisin tree or Oriental raisin tree, is a hardy tree found
in Asia, Eastern China and Korea, and its roots, seeds, branches,
leaves and fruits have been known to possess various biological
activities, including antifatigue, antidiabetic, neuroprotective
and hepatoprotective activity (18–22).
Recently, several studies have revealed that Hovenia dulcis
Thunb. extract contains a variety of pharmacological compounds,
such as alkaloids, flavonoids and triterpenoids (23). Ampelopsin, also known as
dihydromyricetin, is a flavanonol of Hovenia dulcis Thunb.
extract and possesses antiinflammatory, antioxidative and
anticancer activity (24–26). However, whether Hovenia
dulcis Thunb. extract and its active compound ampelopsin
inhibit angiogenesis and the underlying mechanisms in endothelial
cells are still unknown. In the present study, we investigated the
in vitro and in vivo antiangiogenic effects and the
molecular mechanisms of Hovenia dulcis Thunb. extract and
ampelopsin. Our results demonstrated that Hovenia dulcis
Thunb. extract and ampelopsin efficiently suppressed angiogenesis
by downregulating both VEGFR2 signaling and HIF-1α expression.
Materials and methods
Materials
Ampelopsin was purchased from Sigma-Aldrich (St.
Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO) at a
concentration of 100 mM as a stock solution. Endothelial growth
medium-2 (EGM-2) was obtained from Lonza (Walkersville, MD, USA).
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
(FBS) were purchased from Invitrogen (Grand Island, NY, USA).
Recombinant human vascular endothelial growth factor (VEGF),
Matrigel and Transwell chamber systems were obtained from Koma
Biotech (Seoul, Korea), BD Biosciences (San Jose, CA, USA) and
Corning Costar (Acton, MA, USA), respectively. Anti-hypoxia
inducible factor-1α (HIF-1α) antibody was purchased from BD
Biosciences. Anti-phospho-VEGFR2, anti-VEGFR2, anti-phospho-STAT3,
anti-STAT3, anti-phospho-AKT, anti-AKT, anti-phospho-ERK1/2,
anti-ERK1/2, cyclin D1, MMP-2, MMP-9 and anti-β-actin antibodies
were purchased from Cell Signaling Technology (Beverly, MA,
USA).
Preparation of an ethanol extract of
Hovenia dulcis Thunb
The fresh branches of Hovenia dulcis Thunb.
(Jangheung, Korea) were fragmented and stored in a closed
container. Ethanol extract was obtained by dissolving 150 g of raw
material in 1 liter of 100% (vol/vol) ethanol and shaken for 24 h
at room temperature. The mixture was filtered and the clear
filtrate was concentrated, vacuum dried and kept at −20°C.
Cell culture and hypoxic
condition
Human umbilical vein endothelial cells (HUVECs) and
HepG2 (human hepatocarcinoma) cells were obtained from the Korean
Cell Line Bank (KCLB) and grown in EGM-2 and DMEM supplemented with
10% FBS, respectively. All cells were maintained at 37°C in a
humidified 5% CO2 incubator. For hypoxic condition,
cells were incubated in a hypoxic chamber (Forma Scientific,
Marietta, OH, USA) under 5% CO2 and 1% O2
balanced with N2.
Cell proliferation assay
Serum-starved HUVECs (3×103 cells/well)
seeded in a 96-well culture plate were stimulated by VEGF (30
ng/ml) with or without various concentrations of Hovenia
dulcis Thunb. extract and ampelopsin for 72 h. Cell
proliferation was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay. Briefly, 50 µl of MTT solution (2 mg/ml;
Sigma-Aldrich) was added to each well followed by incubation for 3
h at 37°C. To dissolve formazan crystals, the culture medium was
removed and an equal volume of DMSO was added to each well. The
absorbance of each well was determined at a wavelength of 540 nm
using a microplate reader (Thermo Scientific, Vantaa, Finland).
Chemoinvasion assay
The invasiveness of HUVECs was investigated using a
Transwell chamber system with polycarbonate filter inserts with a
pore size of 8.0 µm. Briefly, the lower side of the filter was
coated with 10 µl gelatin (1 mg/ml) and the upper side was coated
with 10 µl Matrigel (3 mg/ml). Serum-starved HUVECs
(8×104 cells) were placed in the upper chamber of the
filter and Hovenia dulcis Thunb. extract and ampelopsin were
added to the lower chamber in the presence of VEGF (30 ng/ml). The
chamber was incubated at 37°C for 18 h, and then the cells were
fixed with methanol and then stained with hematoxylin and eosin
(H&E). The total number of cells that invaded the lower chamber
of the filter was counted using an optical microscope (Olympus,
Center Valley, PA, USA) at a magnification of ×100.
Capillary tube formation assay
Serum-starved HUVECs (4×104 cells) were
inoculated on a surface containing Matrigel (10 mg/ml) and were
incubated with Hovenia dulcis Thunb. extract and ampelopsin
for 6 h in the presence of VEGF (30 ng/ml). Morphological changes
in the cells and tube formation were visualized under a microscope
and photographed at a magnification of ×100 (Olympus). Tube
formation was quantified by counting the total number of branched
tubes in randomly selected fields at a magnification of ×100.
Cell migration assay
The confluent monolayer HUVECs were scratched using
a tip and each well was washed with PBS to remove non-adherent
cells. The cells were treated with Hovenia dulcis Thunb.
extract and ampelopsin in the presence of VEGF (30 ng/ml) and then
incubated for up to 48 h. The perimeter of the area with a central
cell-free gap was confirmed at the time intervals 0, 24, and 48 h
under an optical microscope (Olympus).
Chorioallantoic membrane (CAM)
assay
Fertilized chick eggs were maintained in a
humidified incubator at 37°C for 3 days. Approximately 5–6 ml egg
albumin was removed with a hypodermic needle, allowing the CAM and
yolk sac to drop away from the shell membrane. After 2 days, the
shell was punched out and peeled away. Thermanox coverslips (Nalge
Nunc International, Rochester, NY, USA) with or without Hovenia
dulcis Thunb extract and ampelopsin were air-dried and applied
to the CAM surface, respectively. Two days later, 1 ml of 10%
intralipid (Sigma-Aldrich) was injected into the chorioallantois
and the CAM was observed under a microscope.
Western blot analysis
Cell lysates were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the
separated proteins were transferred to polyvinylidene difluoride
(PVDF) membranes (Millipore, Billerica, MA, USA) using standard
electroblotting procedures. The blots were blocked and
immunolabeled with primary antibodies against phospho-VEGFR2,
VEGFR2, phospho-STAT3, STAT3, phospho-AKT, AKT, phospho-ERK1/2,
ERK1/2, cyclin D1, MMP-2, MMP-9, HIF-1α and β-actin overnight at
4°C. Immunolabeling was detected with an enhanced chemiluminescence
(ECL) kit (Bio-Rad Laboratories, Hercules, CA, USA), according to
the manufacturer's instructions.
Reactive oxygen species (ROS)
measurement
ROS levels were detected with
2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular
Probes, Eugene, OR, USA). For the assay, serum-starved HUVECs
seeded at a density of 1×105 cells/well in 96-black well
culture plates were pretreated with Hovenia dulcis Thunb.
extract and ampelopsin for 3 h. After incubation with H2DCFDA (10
µM) for 5 min, the cells were stimulated with VEGF (30 ng/ml) for 5
min. The fluorescence intensity of DCF was detected using an
Optinity KI-2000F fluorescence microscope (Korea Lab Tech) or a
multimode microplate reader (Thermo Scientific, Vantaa, Finland) at
the excitation and emission wavelengths of 495 and 529 nm,
respectively.
Measurement of VEGF by enzyme-linked
immunosorbent assay (ELISA)
VEGF concentration in the media from Hovenia
dulcis Thunb. extract- or ampelopsin-treated cells was
determined using a VEGF immunoassay kit (R&D Systems,
Minneapolis, MN, USA) according to the manufacturer's instructions.
The results were expressed as the concentration of VEGF relative to
the total amount of protein from each well.
Statistical analysis
The results are expressed as the mean ± standard
error (SE). Student's t-test was used to determine statistical
significance between the control and the test groups. A P-value of
<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of Hovenia dulcis Thunb.
extract on in vitro angiogenesis
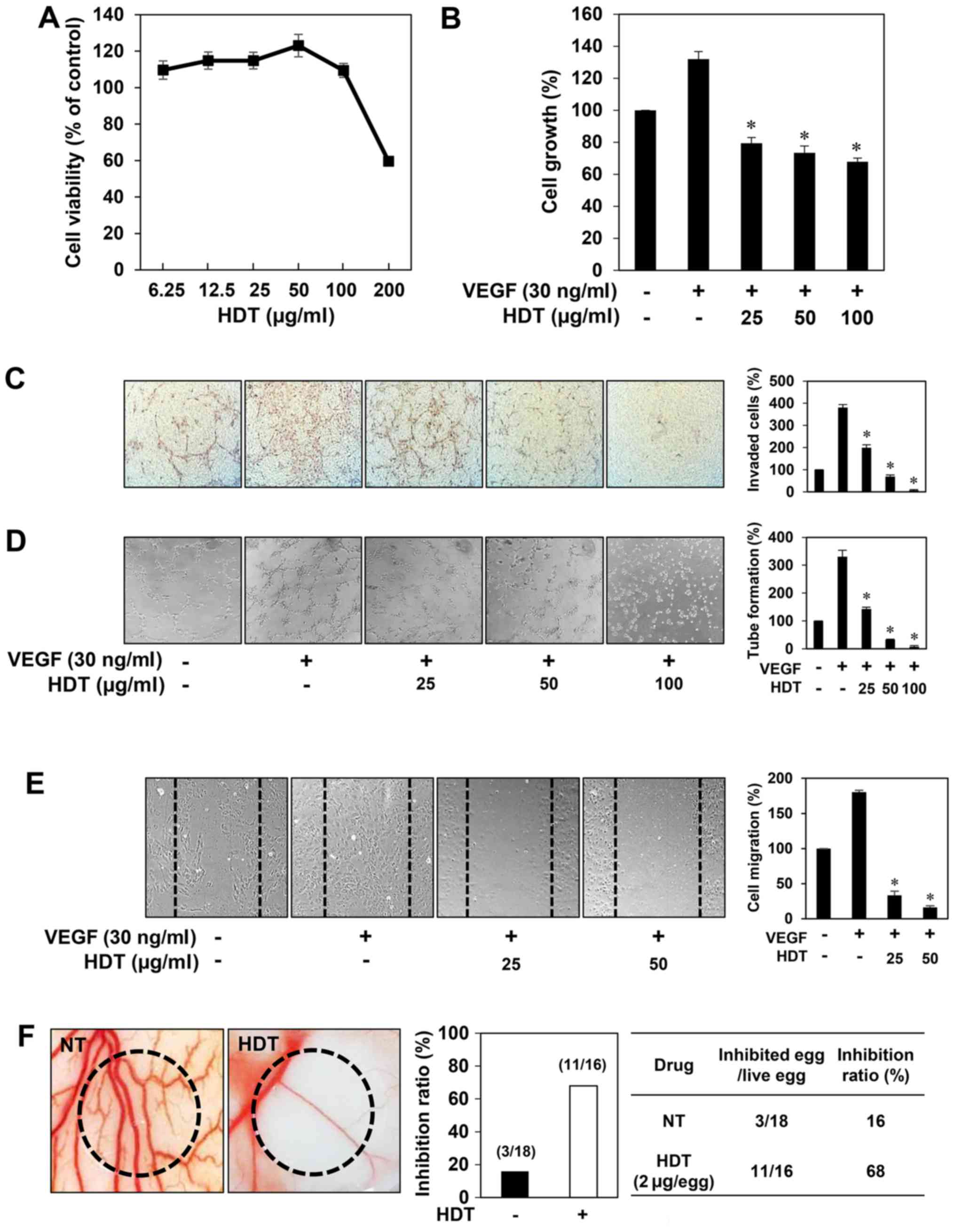
First, to determine the optimum dose of 100% ethanol
extract of Hovenia dulcis Thunb. (HDT) with no cytotoxicity
for angiogenesis assays, various concentrations of HDT were applied
to human umbilical vein endothelial cells (HUVECs) for 72 h. The
viability of the HUVECs was not influenced up to 100 µg/ml of HDT
treatment (Fig. 1A). Based on this
result, in vitro angiogenesis assays were performed in a
concentration range (0–100 µg/ml) of HDT at which no cytotoxicity
was observed. To assess the antiangiogenic activity of HDT, we
investigated the effect of HDT on the proliferation of HUVECs
induced by VEGF. Serum-starved HUVECs were stimulated by VEGF with
or without HDT and then cell growth was measured using the MTT
colorimetric assay. As shown in Fig.
1B, HDT inhibited the VEGF-induced proliferation of HUVECs. We
next examined the effect of HDT on key angiogenic phenotypes of
endothelial cells such as cell invasion, tube formation and
migration. HDT dose-dependently decreased the VEGF-induced
invasiveness, tube forming ability and migration of HUVECs at
subtoxic doses (Fig. 1C-E). These
results suggest that Hovenia dulcis Thunb. extract
significantly inhibits VEGF-induced angiogenesis of HUVECs in
vitro.
Effect of Hovenia dulcis Thunb.
extract on in vivo angiogenesis
The antiangiogenic activity of HDT was also
validated in vivo using a chorioallantoic membrane (CAM)
assay. Coverslips containing HDT were placed on the CAM surface,
and angiogenesis zones were observed under a microscope. As shown
in Fig. 1F, the inhibition of
neovascularization on control coverslips was 16% (n=18), whereas
HDT much more potently inhibited the angiogenesis of the CAM (68%
at 2 µg/egg, n=16) without toxicity against pre-existing vessels.
Taken together, these results demonstrated that Hovenia
dulcis Thunb. extract efficiently inhibits angiogenesis without
affecting endothelial cell viability both in vitro and in
vivo.
Effect of Hovenia dulcis Thunb.
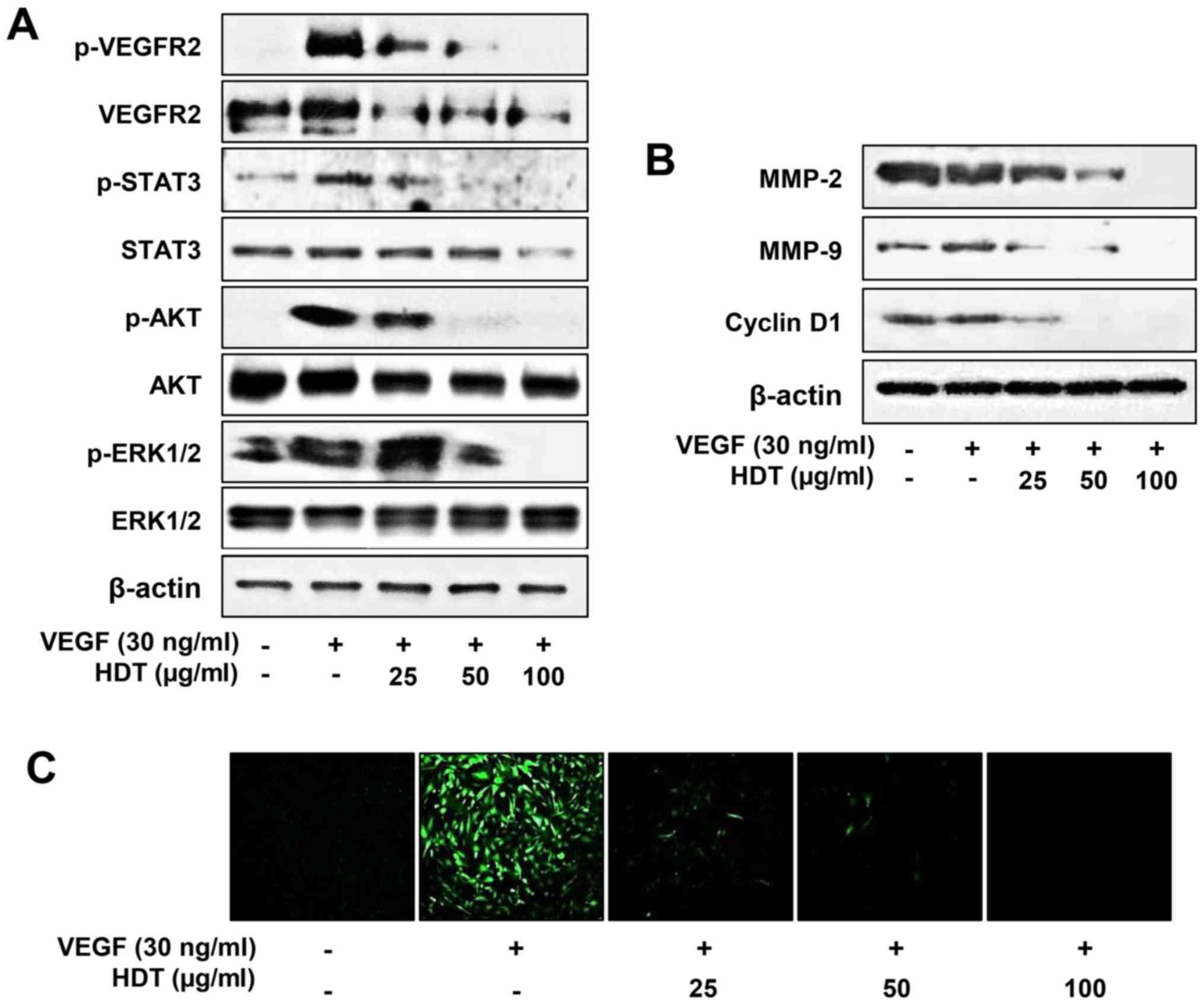
extract on VEGFR2 signaling
VEGFR2 is considered the major mediator of
VEGF-induced downstream signaling in endothelial cells, including
survival, mitogenesis and especially angiogenesis (27–29).
To examine the underlying molecular mechanism of HDT on
angiogenesis, we evaluated the effect of HDT on VEGF-mediated
VEGFR2 signaling pathways in HUVECs. As shown in Fig. 2A, HDT decreased VEGF-induced VEGFR2,
STAT3, AKT and ERK1/2 phosphorylation in a dose-dependent manner.
In addition, HDT reduced the total protein levels of VEGFR2,
indicating that the inhibitory effect on VEGFR2 expression affected
VEGF-induced VEGFR2 phosphorylation. VEGFR2 signaling induces the
expression of endothelial cell-derived matrix metalloproteases
(MMPs), including MMP-2 and MMP-9, which degrade the matrix to
allow for endothelial sprouting. The cell cycle regulatory protein,
cyclin D1, also plays a key role in endothelial cell proliferation
during angiogenic process (6,7). As
shown in Fig. 2B, HDT suppressed
the protein expression of MMP-2, MMP-9 and cyclin D1 in a
dose-dependent manner. Furthermore, HDT remarkably reduced the
VEGF-stimulated ROS generation in HUVECs (Fig. 2C). Thus, these data suggest that HDT
exhibits the antiangiogenic activity by inhibiting VEGFR2-mediated
downstream signaling cascades in HUVECs.
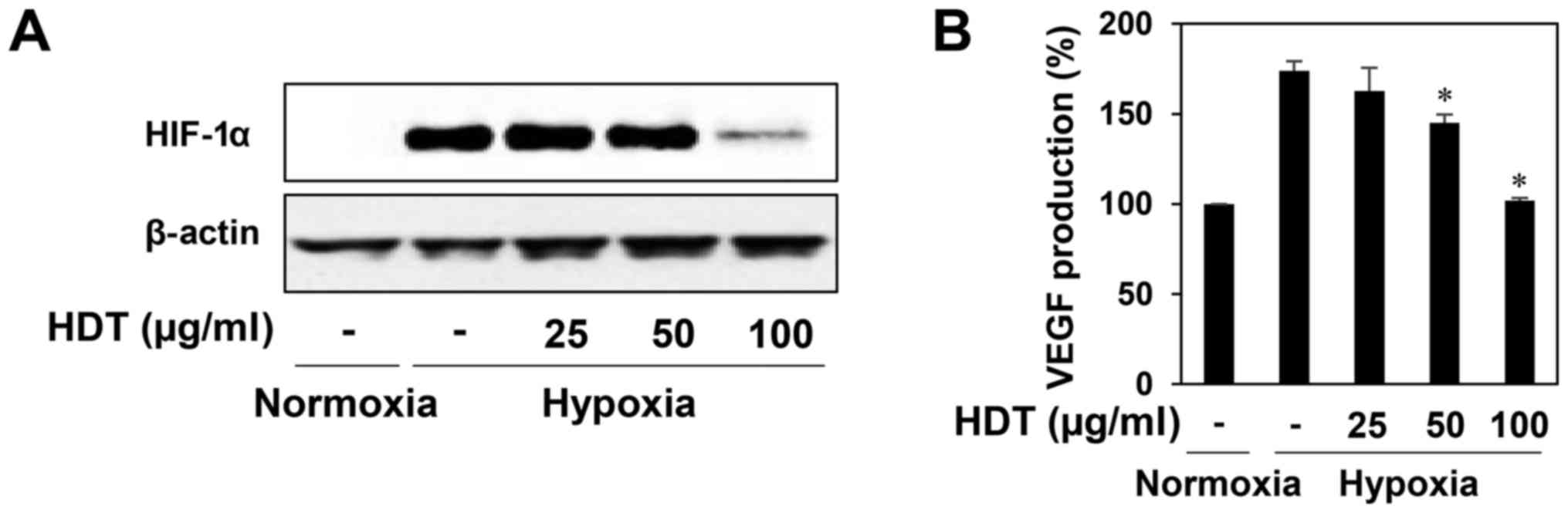
Effect of Hovenia dulcis Thunb.
extract on hypoxia-induced accumulation of HIF-1α protein
HIF-1α is a crucial transcription factor of the
angiogenesis in tumor cells, which controls transcription of
hypoxia-regulated genes such as VEGF (10,11).
Thus, we evaluated the HIF-1α inhibitory activity of HDT in the
human hepatoma cell line HepG2, a hypervascularized tumor.
HDT-treated HepG2 cells attentively reduced the hypoxia-induced
accumulation of HIF-1α protein at 100 µg/ml (Fig. 3A). We further assessed the effect of
HDT on the expression of VEGF during hypoxia. As shown in Fig. 3B, treatment with HDT under hypoxia
decreased the VEGF production in HepG2 cells, indicating that HDT
inhibits tumor angiogenesis by downregulating HIF-1α and VEGF
expression.
Effect of ampelopsin on in vitro and
in vivo angiogenesis
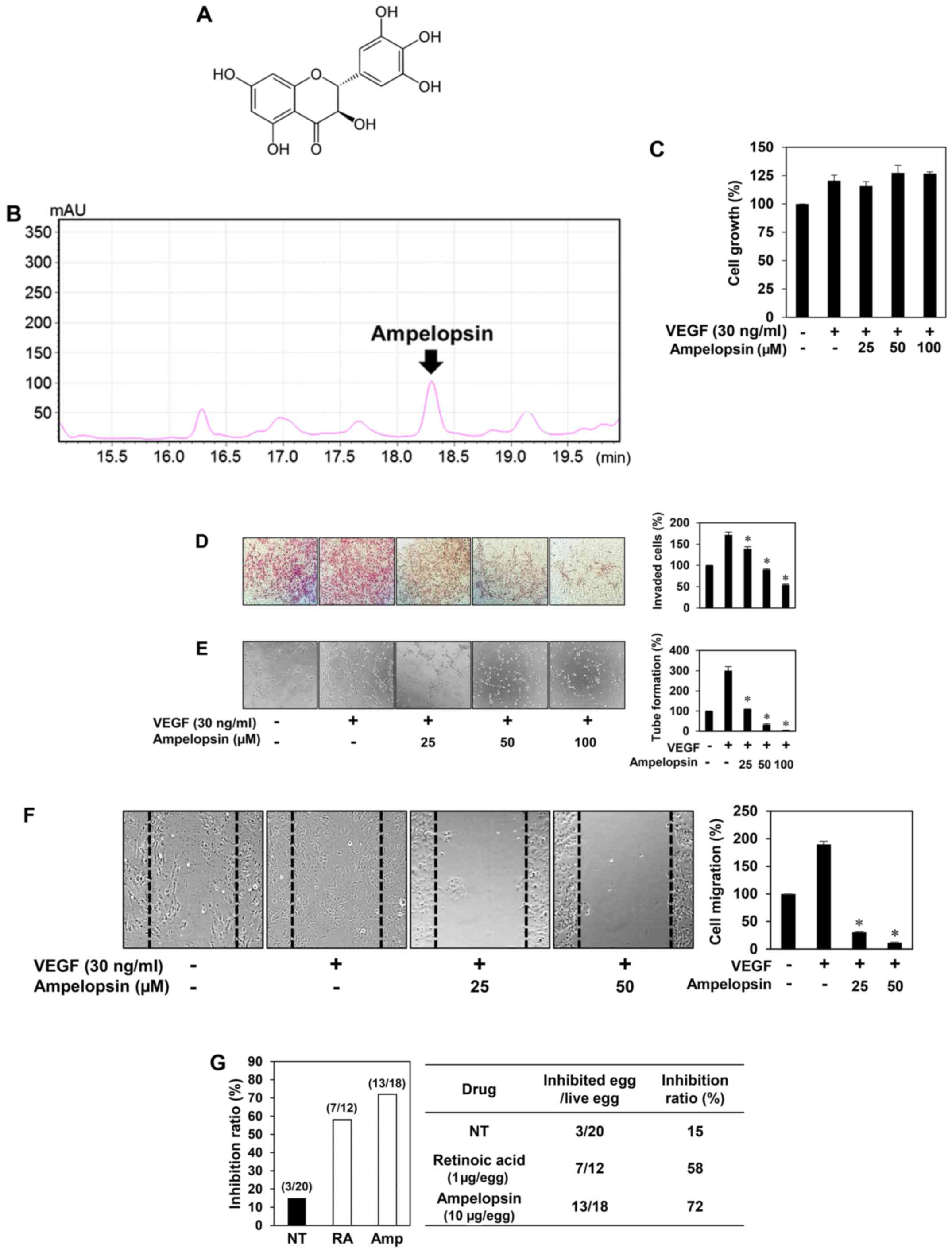
Ampelopsin is a major active ingredient of
Hovenia dulcis Thunb. extract (23) (Fig.
4A). The high-performance liquid chromatography (HPLC) analysis
also revealed that the ethanol extract of Hovenia dulcis
Thunb. branches used in the present study contained ampelopsin
(Fig. 4B). Thus, we assessed
whether ampelopsin is one of the possible pharmaceutically active
compounds contributing to the antiangiogenic activity of Hovenia
dulcis Thunb. extract. Unlike HDT, ampelopsin did not affect
the VEGF-induced proliferation of HUVECs (Fig. 4C). However, ampelopsin significantly
inhibited the VEGF-induced invasiveness, tube forming ability and
migration of HUVECs (Fig. 4D-F).
Furthermore, ampelopsin effectively inhibited the angiogenesis of
the CAM (72% at 10 µg/egg, n=18) without showing any rupture of or
toxicity against pre-existing vessels, when compared to the control
(15%, n=20) (Fig. 4G). These
results demonstrated that ampelopsin suppresses both in
vitro and in vivo angiogenesis without affecting
endothelial cell proliferation.
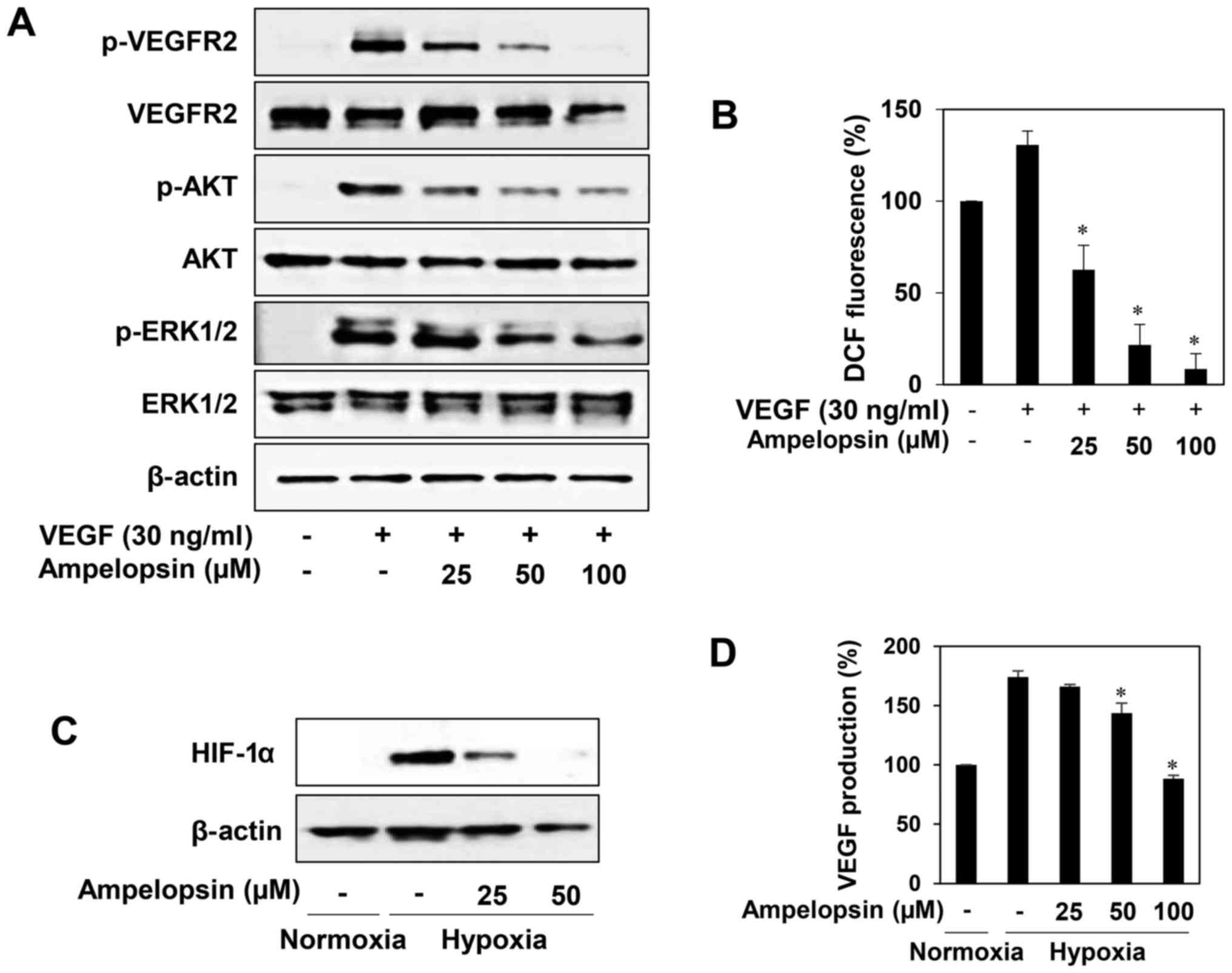
Downregulation of VEGFR2 signal
transduction and HIF-1α expression by ampelopsin
To elucidate whether ampelopsin affects VEGFR2
signal transduction, we investigated the effect of ampelopsin on
VEGF-induced VEGFR2 signaling pathways in HUVECs. As shown in
Fig. 5A, ampelopsin effectively
reduced the phosphorylation of VEGFR2, AKT and ERK1/2 induced by
VEGF without affecting the total protein levels. In addition, we
found that ampelopsin dose-dependently reduced the generation of
ROS induced by VEGF in HUVECs, indicating that the blockade of
VEGFR2 signaling by ampelopsin may be associated with the
downregulation of ROS generation (Fig.
5B). We next evaluated the HIF-1α inhibitory activity of
ampelopsin in HepG2 cells. Ampelopsin prominently inhibited the
expression of HIF-1α and its transcriptional target, VEGF, during
hypoxia (Fig. 5C and D). Taken
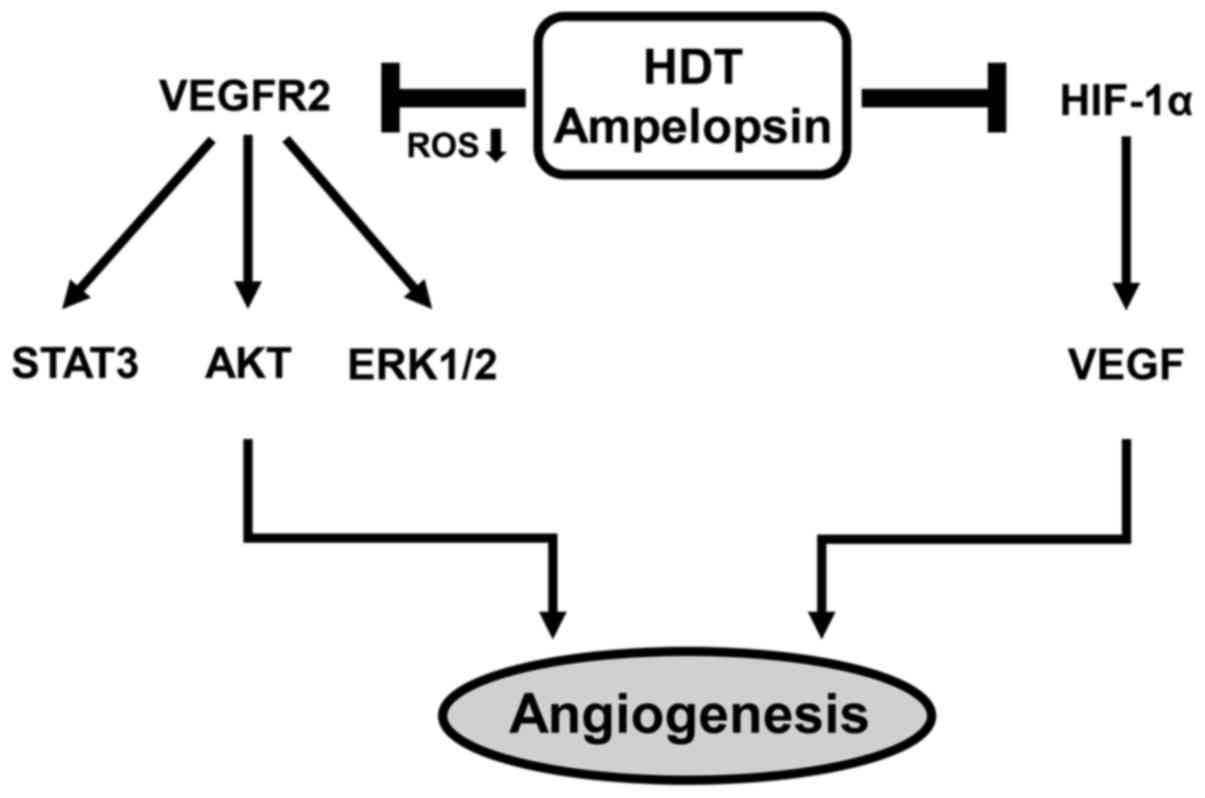
together, these results suggest that ampelopsin shows
antiangiogenic activity through the blockade of both VEGFR2
signaling and HIF-1α expression and, therefore, the antiangiogenic
effect of Hovenia dulcis Thunb. extract is at least in part
due to ampelopsin (Fig. 6).
Discussion
To date, the discovery of angiogenesis inhibitors
for the treatment of angiogenesis-related diseases including cancer
has generated drug-related toxicity issues in the coagulation
cascade, immune system and blood flow regulation (30). For this reason, we expect that the
development of non-toxic and more potent antiangiogenic agents will
lead to an improved therapeutic effect in patients as well as
provide further insight into the biological process of
angiogenesis. Accumulating evidence has shown that natural products
play a positive role in the discovery of new bioactive compounds
including antiangiogenic agents (31).
Previous studies have revealed that Hovenia
dulcis Thunb. possesses various biological activities such as
antifatigue, antidiabetic, neuroprotective and hepatoprotective
effects (18–22). In addition, the molecular mechanisms
underlying its biological effects were partially identified.
Hovenia dulcis Thunb. extract reduced lipid accumulation in
oleic acid-induced steatosis of HepG2 cells via activation of AMPK
and PPARα/CPT-1 pathway (32).
Hovenia dulcis Thunb. extract also suppressed
lipopolysaccharide (LPS)-stimulated inflammatory responses through
nuclear factor-κB (NF-κB) pathway in Raw 264.7 cells (33). However, the antiangiogenic activity
of Hovenia dulcis Thunb. extract has not yet been
reported.
Ampelopsin, a flavanonol of Hovenia dulcis
Thunb. extract, possesses antiinflammatory, antioxidative and
anticancer effects (24–26). The molecular mechanisms underlying
the biological effects of ampelopsin were also partly demonstrated.
Ampelopsin protected endothelial cells from hyperglycemia-induced
oxidative damage by inducing autophagy via the AMPK signaling
pathway (34). Ampelopsin also
induced apoptosis by regulating multiple c-Myc/S-phase
kinase-associated protein 2/F-box and WD repeat-containing protein
7/histone deacetylase 2 pathways in human lung adenocarcinoma cells
(35). Moreover, ampelopsin
attenuated LPS-induced inflammatory response through the inhibition
of NF-κB and JAK2/STAT3 signaling pathways in microglia (24). However, whether ampelopsin inhibits
angiogenesis and its underlying mechanism in endothelial cells are
still unknown.
Angiogenesis, the formation of new blood vessels,
proceeds by a multistep process, including endothelial cell
proliferation, migration, tube formation and invasion. Inhibition
at any step may result in suppression of angiogenesis (36). Our results importantly showed that
Hovenia dulcis Thunb. extract dose-dependently inhibited
VEGF-induced endothelial cell proliferation, whereas ampelopsin had
no affect. Thus, the antiproliferative effect of Hovenia
dulcis Thunb. extract may be associated with the action of
another bioactive component in the extract other than ampelopsin.
Nevertheless, our results demonstrated that Hovenia dulcis
Thunb. extract and ampelopsin exhibited potent inhibitory effects
on VEGF-induced invasion, tube formation and migration of HUVECs.
Furthermore, our data showed that Hovenia dulcis Thunb.
extract and ampelopsin inhibited in vivo neovascularization
of the CAM without toxicity.
VEGFR2 signaling is a main pathway involved in
angiogenesis. Therefore, suppression of the VEGFR2 signal
transduction cascade is the major strategy in antiangiogenic
therapy. In addition, targeting the transcription regulators of
proangiogenic factors such as HIF-1α in cancer cells can be a
powerful strategy to effectively inhibit tumor angiogenesis through
prevention of VEGF expression (37,38).
In this study, we demonstrated that Hovenia dulcis Thunb.
extract and ampelopsin downregulated both VEGFR2 signaling of
endothelial cells and the HIF-1α expression of tumor cells.
Notably, unlike ampelopsin, Hovenia dulcis Thunb. extract
also reduced the total protein levels of VEGFR2, indicating that
other ingredients of the extract may result in the inhibitory
effect on VEGFR2 expression. Meanwhile, Hovenia dulcis
Thunb. extract and ampelopsin suppressed the VEGF-induced
generation of ROS in HUVECs. In recent studies, VEGF stimulated ROS
production and in turn promoted VEGFR2 autophosphorylation by
reversibly oxidizing and inactivating protein tyrosine phosphatases
(PTPs) (39,40). Therefore, they may block the VEGFR2
signaling via the downregulation of ROS generation. In addition,
ampelopsin more potently inhibited the expression of HIF-1α in
HepG2 hepatoma cells than Hovenia dulcis Thunb. extract.
Thus, ampelopsin could be a promising antiangiogenic agent for
cancer treatment.
In conclusion, our results demonstrated that the
antiangiogenic effects of Hovenia dulcis Thunb. extract and
its active compound ampelopsin on HUVECs were exerted through the
inhibition of VEGFR2 signal transduction, leading to decreased cell
proliferation, migration, invasion and tube formation. Hovenia
dulcis Thunb. extract and ampelopsin may also exert inhibitory
activity on tumor angiogenesis by downregulating the expression of
HIF-1α. In addition, we found that Hovenia dulcis Thunb.
extract and ampelopsin could suppress basic fibroblast growth
factor (bFGF)-stimulated invasion and tube formation of HUVECs
in vitro, suggesting that they may also affect other growth
factor-related angiogenesis (data not shown). These findings
provide support for the potential use of Hovenia dulcis
Thunb. extract and ampelopsin for antiangiogenic therapy. However,
further investigation of their therapeutic effects in
angiogenesis-related disease models and action mechanisms would be
required for their development as natural pharmaceutical
agents.
Acknowledgements
This study was carried out with the support of the
‘Cooperative Research Program for Agriculture Science and
Technology Development (project no. PJ01188001)’ Rural Development
Administration, Republic of Korea, the Basic Science Research
Program through the National Research Foundation of Korea (NRF)
funded by the Ministry of Education (NRF-2016R1D1A1B03932956), and
the Brain Korea 21 Plus Project, Republic of Korea.
References
|
1
|
Folkman J: Seminars in Medicine of the
Beth Israel Hospital, Boston. Clinical applications of research on
angiogenesis. N Engl J Med. 333:1757–1763. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karamysheva AF: Mechanisms of
angiogenesis. Biochemistry (Mosc). 73:751–762. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holash J, Wiegand SJ and Yancopoulos GD:
New model of tumor angiogenesis: Dynamic balance between vessel
regression and growth mediated by angiopoietins and VEGF. Oncogene.
18:5356–5362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holmes K, Roberts OL, Thomas AM and Cross
MJ: Vascular endothelial growth factor receptor-2: Structure,
function, intracellular signalling and therapeutic inhibition. Cell
Signal. 19:2003–2012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stetler-Stevenson WG: Matrix
metalloproteinases in angiogenesis: A moving target for therapeutic
intervention. J Clin Invest. 103:1237–1241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Semenza GL: Regulation of mammalian
O2 homeostasis by hypoxia-inducible factor 1. Annu Rev
Cell Dev Biol. 15:551–578. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Greer SN, Metcalf JL, Wang Y and Ohh M:
The updated biology of hypoxia-inducible factor. EMBO J.
31:2448–2460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forsythe JA, Jiang BH, Iyer NV, Agani F,
Leung SW, Koos RD and Semenza GL: Activation of vascular
endothelial growth factor gene transcription by hypoxia-inducible
factor 1. Mol Cell Biol. 16:4604–4613. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan TP, Yeh JC, Leung KW, Yue PY and Wong
RN: Angiogenesis: From plants to blood vessels. Trends Pharmacol
Sci. 27:297–309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Avramis IA, Kwock R and Avramis VI:
Taxotere and vincristine inhibit the secretion of the angiogenesis
inducing vascular endothelial growth factor (VEGF) by wild-type and
drug-resistant human leukemia T-cell lines. Anticancer Res.
21:2281–2286. 2001.PubMed/NCBI
|
|
14
|
Clements MK, Jones CB, Cumming M and Daoud
SS: Antiangiogenic potential of camptothecin and topotecan. Cancer
Chemother Pharmacol. 44:411–416. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vincent L, Kermani P, Young LM, Cheng J,
Zhang F, Shido K, Lam G, Bompais-Vincent H, Zhu Z, Hicklin DJ, et
al: Combretastatin A4 phosphate induces rapid regression of tumor
neovessels and growth through interference with vascular
endothelial-cadherin signaling. J Clin Invest. 115:2992–3006. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JH, Choi S, Lee Y, Lee HJ, Kim KH, Ahn
KS, Bae H, Lee HJ, Lee EO, Ahn KS, et al: Herbal compound
farnesiferol C exerts antiangiogenic and antitumor activity and
targets multiple aspects of VEGFR1 (Flt1) or VEGFR2 (Flk1)
signaling cascades. Mol Cancer Ther. 9:389–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han JM, Kwon HJ and Jung HJ: Tricin,
4′,5,7-trihydroxy-3′,5′-dimethoxyflavone, exhibits potent
antiangiogenic activity in vitro. Int J Oncol. 49:1497–1504.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang J, Wu S and Li C: High efficiency
secondary somatic embryogenesis in Hovenia dulcis Thunb.
through solid and liquid cultures. Sci World J. 2013:7187542013.
View Article : Google Scholar
|
|
19
|
Na CS, Yoon SY, Kim JB, Na DS, Dong MS,
Lee MY and Hong CY: Anti-fatigue activity of Hovenia dulcis
on a swimming mouse model through the inhibition of stress hormone
expression and antioxidation. Am J Chin Med. 41:945–955. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji Y, Chen S, Zhang K and Wang W: Effects
of Hovenia dulcis Thunb on blood sugar and hepatic glycogen
in diabetic mice. Zhong Yao Cai. 25:190–191. 2002.(In Chinese).
PubMed/NCBI
|
|
21
|
Li G, Min BS, Zheng C, Lee J, Oh SR, Ahn
KS and Lee HK: Neuroprotective and free radical scavenging
activities of phenolic compounds from Hovenia dulcis. Arch
Pharm Res. 28:804–809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hase K, Ohsugi M, Xiong Q, Basnet P,
Kadota S and Namba T: Hepatoprotective effect of Hovenia
dulcis THUNB. on experimental liver injuries induced by carbon
tetrachloride or D-galactosamine/lipopolysaccharide. Biol Pharm
Bull. 20:381–385. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JS, Kim IS, Shaheed Ur Rehman, Na CS
and Yoo HH: HPLC determination of bioactive flavonoids in
Hovenia dulcis fruit extracts. J Chromatogr Sci. 54:130–135.
2016.PubMed/NCBI
|
|
24
|
Weng L, Zhang H, Li X, Zhan H, Chen F, Han
L, Xu Y and Cao X: Ampelopsin attenuates lipopolysaccharide-induced
inflammatory response through the inhibition of the NF-κB and
JAK2/STAT3 signaling pathways in microglia. Int Immunopharmacol.
44:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hou X, Zhang J, Ahmad H, Zhang H, Xu Z and
Wang T: Evaluation of antioxidant activities of ampelopsin and its
protective effect in lipopolysaccharide-induced oxidative stress
piglets. PLoS One. 9:e1083142014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y, Liang X, Chang H, Shu F, Wu Y,
Zhang T, Fu Y, Zhang Q, Zhu JD and Mi M: Ampelopsin-induced
autophagy protects breast cancer cells from apoptosis through
Akt-mTOR pathway via endoplasmic reticulum stress. Cancer Sci.
105:1279–1287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parast CV, Mroczkowski B, Pinko C,
Misialek S, Khambatta G and Appelt K: Characterization and kinetic
mechanism of catalytic domain of human vascular endothelial growth
factor receptor-2 tyrosine kinase (VEGFR2 TK), a key enzyme in
angiogenesis. Biochemistry. 37:16788–16801. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Verheul HM and Pinedo HM: Possible
molecular mechanisms involved in the toxicity of angiogenesis
inhibition. Nat Rev Cancer. 7:475–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim B, Woo MJ, Park CS, Lee SH, Kim JS,
Kim B, An S and Kim SH: Hovenia dulcis extract reduces lipid
accumulation in oleic acid-induced steatosis of Hep G2 cells via
activation of AMPK and PPARα/CPT-1 pathway and in acute
hyperlipidemia mouse model. Phytother Res. 31:132–139. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park JY, Moon JY, Park SD, Park WH, Kim H
and Kim JE: Fruits extracts of Hovenia dulcis Thunb.
suppresses lipopolysaccharide-stimulated inflammatory responses
through nuclear factor-kappaB pathway in Raw 264.7 cells. Asian Pac
J Trop Med. 9:357–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang X, Zhang T, Shi L, Kang C, Wan J,
Zhou Y, Zhu J and Mi M: Ampelopsin protects endothelial cells from
hyperglycemia-induced oxidative damage by inducing autophagy via
the AMPK signaling pathway. Biofactors. 41:463–475. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen XM, Xie XB, Zhao Q, Wang F, Bai Y,
Yin JQ, Jiang H, Xie XL, Jia Q and Huang G: Ampelopsin induces
apoptosis by regulating multiple c-Myc/S-phase kinase-associated
protein 2/F-box and WD repeat-containing protein 7/histone
deacetylase 2 pathways in human lung adenocarcinoma cells. Mol Med
Rep. 11:105–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cho SG, Yi Z, Pang X, Yi T, Wang Y, Luo J,
Wu Z, Li D and Liu M: Kisspeptin-10, a KISS1-derived decapeptide,
inhibits tumor angiogenesis by suppressing Sp1-mediated VEGF
expression and FAK/Rho GTPase activation. Cancer Res. 69:7062–7070.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
El-Kenawi AE and El-Remessy AB:
Angiogenesis inhibitors in cancer therapy: Mechanistic perspective
on classification and treatment rationales. Br J Pharmacol.
170:712–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen SH, Murphy DA, Lassoued W, Thurston
G, Feldman MD and Lee WM: Activated STAT3 is a mediator and
biomarker of VEGF endothelial activation. Cancer Biol Ther.
7:1994–2003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jung HJ, Kim Y, Chang J, Kang SW, Kim JH
and Kwon HJ: Mitochondrial UQCRB regulates VEGFR2 signaling in
endothelial cells. J Mol Med (Berl). 91:1117–1128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ushio-Fukai M: VEGF signaling through
NADPH oxidase-derived ROS. Antioxid Redox Signal. 9:731–739. 2007.
View Article : Google Scholar : PubMed/NCBI
|