Introduction
Photodynamic therapy (PDT) is an advancing medical
technology that uses photosensitizing drugs. When the
photosensitizing agent is exposed to an appropriate wavelength of
light, reactive oxygen species (ROS) are produced which may kill
nearby tumor cells (1–5). Unlike conventional therapeutics, PDT
can selectively destroy tumor tissues, as well as control and
reduce the degree of damage to normal tissues. Photosensitizers
play a key role in PDT. Currently, only a few porphyrin
photosensitizers (e.g. 5-aminolevulinic acid, temoporfin) are
approved for the treatment of cancer in humans. Although these
photosensitizers have demonstrated a wide spectrum of antitumor
effects, they have various deficiencies. Thus, the development of
better photosensitizing candidate drugs is required.
Phthalocyanines, which have desirable electronic absorption and
photophysical properties, are some of the most promising potential
photosensitizer candidates. Phthalocyanines have a
hydrophilic/lipophilic structure. The hydrophilic group enhances
the transport of the drug in the body and the lipophilic group
enhances the uptake of the drug by the cancer cells. Recently,
accumulating evidence revealed that PDT using
hydrophilic/lipophilic phthalocyanines achieved highly selective
antitumor effects (6–9).
Mitochondria play a pivotal role in the initiation
and amplification of most apoptotic pathways. When mitochondria are
stimulated by apoptotic signals, the mitochondrial
membrane-permeability is increased, leading to the release of
cytochrome c (Cyto c) and the apoptosis-inducing
factor (AIF) into the cytoplasm resulting in mitochondria-mediated
apoptosis (10–16). Additionally, mitochondria are
regulated by the Bcl-2 family, the caspase family and the
mitogen-activated protein kinase (MAPK) proteins (17–22).
The Bcl-2 family of proteins are a group of evolutionarily related
apoptosis-regulation proteins that regulate mitochondrial
membrane-permeabilization; Bax and Bid are pro-apoptotic proteins
and Bcl-2 is an anti-apoptotic protein. Recent studies demonstrated
that the mitochondrial transmembrane potential of cancer cells is
altered by phthalocyanine-PDT (23,24).
Caspase-9, a member of the caspase family of
proteases, is the main executor of apoptosis signaling. Once
activated, initiator caspase-9 cleaves effector caspase-3, which
subsequently, induces the morphological and functional changes
associated with apoptosis. Accumulating evidence has demonstrated
that caspase-9 and caspase-3 play crucial regulatory roles in the
phthalocyanine-PDT-induced apoptosis of cancer cells (23,24).
In addition, accumulating evidence has revealed that caspase-9 is
involved in the regulation of cell autophagy and is an important
determinant of the balance between cell apoptosis and autophagy
(25,26).
MAPKs, a family of protein serine/threonine kinases,
include extracellular signal-regulated kinase (ERK), c-Jun
NH2-terminal protein kinase (JNK) and p38 kinase. MAPKs are
activated in response to cellular stimuli and are involved in the
regulation of cellular processes such as proliferation, apoptosis
and autophagy (27,28). Among the MAPKs subfamily, p38
MAPK-activation is mainly implicated in stress stimuli (oxidative
stress, ultraviolet irradiation, chemotherapeutics,
hyperthermia)-induced apoptosis (29,30).
Accumulating evidence has indicated that p38 MAPK activation plays
a crucial regulatory role in phthalocyanine-PDT-induced apoptosis
of cancer cells (8,31,32).
Our previous study demonstrated that p38 MAPK and
caspase-8 regulated the apoptosis of Bel-7402 human hepatocellular
carcinoma cells induced by hydrophilic/lipophilic
tetra-α-(4-carboxyphenoxy) phthalocyanine zinc (TαPcZn)-PDT
(6,8). However, the manner through which p38
MAPK or caspase-9 regulated mitochondria-mediated apoptosis in LoVo
human colon carcinoma cells treated with TαPcZn-PDT is not clear.
In the present study, a siRNA targeting p38 MAPK mRNA
(siRNA-p38 MAPK) and the caspase-9-specific inhibitor
z-LEHD-fmk were used to investigate the crosstalk between p38 MAPK
and caspase-9 as part of apoptosis in the TαPcZn-PDT-treated LoVo
cells. The findings indicated that p38 MAPK in a crosstalk between
p38 MAPK and caspase-9 may play an important regulatory role and
that direct crosstalk between p38 MAPK and caspase-9 may regulate
mitochondria-mediated apoptosis in the TαPcZn-PDT-treated LoVo
cells.
Materials and methods
Antibodies and reagents
Dulbecco's modified Eagle's medium (DMEM), opti-MEM,
Silencer Select siRNA-p38 MAPK, negative control siRNA,
GAPDH positive control siRNA and lipofectamine RNAiMAX were all
purchased from Thermo Fisher Scientific (Carlsbad, CA, USA). Fetal
bovine serum (FBS) was purchased from PAA Laboratories (Cölbe,
Germany). Diethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich
(St. Louis, MO, USA). Anti-p38 MAPK, anti-phosphorylated (p)-p38
MAPK, anti-Bcl-2, anti-Bax, anti-AIF, anti-Cyto c, anti-Bid,
anti-caspase-9 and anti-caspase-3 antibodies were all purchased
from Cell Signaling Technology (Danvers, MA, USA). The anti-β-actin
antibody was obtained from Santa Cruz Biotechnology (Santa Cruz,
CA, USA). Z-LEHD-fmk was obtained from Merck Chemicals GmbH
(Darmstadt, Germany). The Annexin V-FLUOS staining kit was
purchased from Roche (Basel, Switzerland). The reverse
transcription-polymerase chain reaction (RT-PCR) kit was obtained
from Takara (Dalian, China). The
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine
iodide (JC-1) staining kit was purchased from Genmed Scientifics
(Wilmington, DE, USA). Catch and Release v2.0 kit was purchased
from Millipore (Billerica, MA, USA). TαPcZn was synthesized as
described in our previous study (33). The TαPcZn stock solution was
prepared in DMSO and stored in the dark at 4°C. When used, the
stock solution was appropriately diluted to obtain the desired
concentration with a final DMSO concentration of 0.1%. All other
chemicals and reagents were of analytical grade.
Cell culture and treatment
The LoVo cells were obtained from Harbin Medical
University (Harbin, China) and cultured in DMEM supplemented with
10% FBS, 100 U/ml penicillin and 100 mg/l streptomycin in a
humidified atmosphere of 5% CO2 at 37°C. The LoVo cells
were incubated for 48 h. After treatment with the TαPcZn stock
solution for 2.5 h at 37°C in the presence or absence of
siRNA-p38 MAPK or z-LEHD-fmk, the cells were irradiated with
an SS-B instrument (Wuxi Holyglow Physiotherapy Instrument Co.,
Ltd., Jiangsu, China) that emitted red light within a wavelength of
600–700 nm. The light dose was ~53.7 J/cm2. The cells
were harvested after 3 h. The control cells were exposed to red
light irradiation (53.7 J/cm2).
siRNA transfection
The cells were transfected with siRNAs using
lipofectamine RNAiMAX reagent according to the manufacturer's
instructions and assayed 48 h after transfection. Control
non-targeting siRNA, GAPDH positive control siRNA and siRNA against
p38 MAPK (12.5 nM) were all obtained from Thermo Fisher
Scientific. The target sequence of the p38 MAPK siRNA was
5′-GAAGCUCUCCAGACCAUUUTT-3′. The silencing efficiency was validated
by RT-PCR and immunoblot analysis.
Semi-quantitative RT-PCR
Total cellular RNA was extracted and the cDNA was
synthesized using standard protocols. PCR primers specific for p38
MAPK (forward, 5′-GACAATCTGGGAGGTGCC-3′ and reverse,
5′-GACCCAGTCCAAAATCCA-3′) and GAPDH (forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′)
were applied. RT was performed on 1 µg total RNA with a reaction
mixture containing 10 µl denatured RNA in a 96-well thermal cycler
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), 1 µl 10X RT
buffer, 2 µl (12.5 mM) MgCl2, 1 µl dNTP mix, 0.5 µl AMV
reverse transcriptase, 0.5 µl Oligo dT-adaptor primer, 0.25 µl
RNase inhibitor and 3.75 µl distilled water. cDNA was synthesized
by incubation at 30°C for 10 min and then 42°C for 30 min, 99°C for
5 min and 5°C for 5 min. The PCR was performed on 5 µl cDNA
product, which was added to a 20-µl PCR mixture comprised of 5 µl
PCR Buffer, 0.125 µl Takara Ex Taq HS, 0.5 µl forward and reverse
primers and 14.375 µl distilled water. The PCR reaction was carried
out using one cycle at 94°C for 2 min, followed by 35 cycles at
94°C for 30 sec, annealing at 59°C for 30 sec, polymerization at
72°C for 1 min and a final extension at 72°C for 10 min. The RT-PCR
products were separated by electrophoresis in 1.5% agarose gels and
bands were visualized and quantified on a Molecular
Imager® Gel Doc™XR system with Image Lab™ software v.4.1
(Bio-Rad Laboratories). The samples exhibiting 220 and 460 bp bands
were considered positive. GAPDH was used as an internal control.
Densitometric quantifications of the objective RNA levels were made
relative to GAPDH. Quantitative data were presented as the mean ±
standard deviation (SD)from three independent experiments and were
analyzed using an analysis of variance (ANOVA).
Annexin V-FLUOS/Propidium iodide (PI)
double-staining analysis of apoptosis
Cell apoptosis was assayed using the Annexin
V-FLUOS/PI apoptosis detection kit. The harvested LoVo cells were
washed with ice-cold PBS and suspended in 500 µl Annexin V binding
buffer A, after which 100 µl aliquots were collected. Subsequently,
2.0 µl Annexin V-FLUOS and 2.0 µl PI were added and the mixture was
incubated for 5 min in the dark at room temperature. After 5 min,
400 µl binding buffer was added to the cells and 1×104
cells were analyzed on a FACScan flow cytometer (Becton Dickinson,
Franklin Lakes, NJ, USA) using CellQuest software. The results are
shown as dotplots. In each graph, the percentage of apoptotic cells
is indicated in the right upper and lower quadrant; the y-axis
corresponds to the relative PI staining and the x-axis corresponds
to the log of the FLUOS signal.
JC-1 assay of the mitochondria
membrane potential (ΔΨm)
The ΔΨm was assayed by JC-1 assay using fluorescence
microscopy and flow cytometry. For the fluorescence microscopic
analysis, after being treated with TαPcZn-PDT in the absence or
presence of siRNA-p38 MAPK or z-LEHD-fmk, the cells were
incubated in fresh culture medium containing 2.5 µg/ml JC-1 for 20
min in the dark at 37°C and washed twice with PBS and then, assayed
using an inverted fluorescence microscope (Chongqing Photoelectric
Instruments Co. Ltd., Chongqing, China). For the flow cytometric
analysis, after being treated with TαPcZn-PDT in the absence or
presence of siRNA-p38 MAPK or z-LEHD-fmk, the cells were
trypsinized and washed twice with PBS and then incubated in PBS
containing 2.5 µg/ml JC-1 for 20 min in the dark at 37°C. About
1×104 cells were analyzed on a FACScan flow cytometer
(Becton Dickinson) using CellQuest software. The results observed
by flow cytometry are demonstrated as dotplots. In each graph, the
shift of fluorescence from red to green indicated the collapse of
the ΔΨm.
Immunoblot assay
All immunoblots were performed using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to
the manufacturer's instructions. To obtain total cellular protein,
the LoVo cells were lysed in buffer containing 25 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.5), 0.3 M
NaCl, 1.5 mM MgCl2, 0.2 mM ethylenediaminetetraacetic
acid, 0.1% Triton X-100, 20 mM β-glycerophosphate, 0.5 mM
dithiothreitol, 1.0 mM sodium orthovanadate, 0.1 mM okadaic acid
and 1.0 mM phenylmethylsulfonyl fluoride. Equal amounts of protein
lysate were run on 10% SDS-PAGE and electrophoretically transferred
to polyvinylidene fluoride membranes. After blocking, the blots
were incubated with specific primary antibodies (anti-p38 MAPK,
anti-phos-p38 MAPK, anti-caspase-3, anti-caspase-9, anti-Bcl-2,
anti-Bax and anti-Bid antibodies) overnight at 4°C and further
incubated for 1 h with horseradish peroxidase-conjugated secondary
antibodies. Bound antibodies were detected using an enhanced
chemiluminescence (ECL) kit with Lumino Image analyzer (Founder
Group, Beijing, China). The mitochondria and cytosolic fractions
isolated from the cells were collected for immunoblot assay of Cyto
c and AIF, as previously described (34). The Cyto c and AIF proteins
were assayed using anti-Cyto c and anti-AIF antibodies. All
densitometric quantifications of the protein levels were made
relative to β-actin and expressed in arbitrary units.
Immunoprecipitation/immunoblot
assay
The cell lysates and the affinity ligands were
incubated with the p38 MAPK antibody or caspase-9 antibody in a
spin-column at 4°C overnight. After washing the column three times
with 1X washing buffer, the protein was eluted from the column in
native form. The immunoprecipitates were separated by 10% SDS-PAGE.
After being transferred, the membranes were immunoblotted with the
p38 MAPK antibody or caspase-9 antibody, washed with Tween-PBS and
developed using the ECL system.
Localized surface plasmon resonance
(LSPR) signal detection
A COOH sensor chip was loaded into the OpenSPR
instrument (Nicoya Lifesciences, Kitchener, ON, Canada), running
buffer (1X PBS, pH 7.4) was pumped and 200 µl
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (0.04
mg/ml)/N-hydroxysuccinimide (0.06 mg/ml) mixture in the activation
buffer was injected into the instrument. Subsequently, after 5 min,
200 µl anti-p38 MAPK antibody in PBS solution (8.55 µg/ml) was
injected into the instrument. After 5 min, a 200 µl blocking
solution was injected and then, after a further 5 min, 200 µl of
treated-proteins in PBS (80 µg/ml) and 200 µl anti-caspase-9
antibody PBS solution (11.56 µg/ml) were injected into the
instrument to assess the interactions between p38 MAPK, caspase-9
and total protein. The interactions were assayed by the resonance
wavelength that was obtained by fitting the LSPR signals to the
Lorentzian equation.
Statistical analysis
All data are presented as the mean ± standard
deviation (SD) and were analyzed using an analysis of variance
(ANOVA). P<0.05 was considered to indicate a statistically
significant difference and P<0.01 was considered to indicate a
highly statistically significant difference in all cases.
Statistical analyses were performed using SPSS version 18.0 (SPSS,
Inc., Chicago, IL, USA).
Results
siRNA silencing of p38 MAPK
To analyze the effect of p38 MAPK silencing in the
TαPcZn-PDT-treated LoVo cells, the cells were transfected with
siRNA-p38 MAPK. The siRNA transfection rate was ~90%
(Fig. 1A and B). After the
siRNA-p38 MAPK transfection, the expression levels of p38
MAPK mRNA and p38 MAPK protein in the LoVo cells decreased
significantly (Fig. 1C-F),
indicating that siRNA silenced the expression of p38
MAPK.
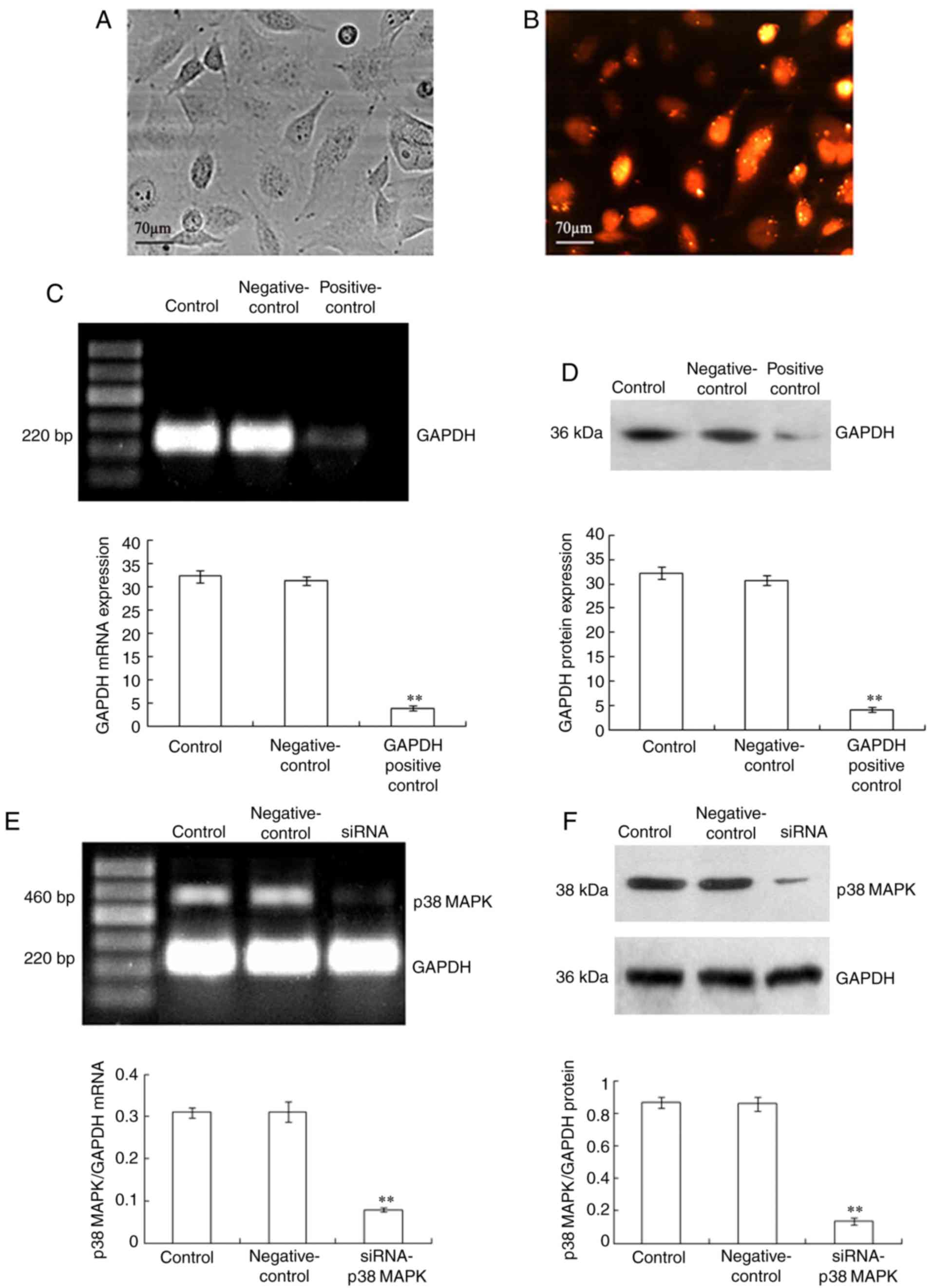 | Figure 1.Silencing of p38 MAPK in LoVo cells.
LoVo cells were transfected with siRNA-p38 MAPK (12.5
nmol/l), the negative control siRNA and the GAPDH positive control
siRNA using Lipofectamine RNAiMAX. In the presence of Block-iT
Alexa Fluor Red Fluorescent Control for 48 h, the LoVo cells were
exposed to red light irradiation (53.7 J/cm2), incubated
for 3 h and then, the transfected cells were observed using (A) an
inverted microscope and (B) a fluorescence microscope. Following
transfection, the expression levels of p38 MAPK mRNA and p38
MAPK protein were analyzed using (C and E) reverse
transcription-PCR and (D and F) immunoblot assay, respectively. The
LoVo cells were treated with Lipofectamine RNAiMAX only as the
control treatment. GAPDH was used as an internal control. The
values presented are representative of three independent
experiments (mean ± standard deviation; **P<0.01, compared to
the control treatment). Scale bar, 70 µm. siRNA, small interfering
RNA; MAPK, mitogen-activated protein kinase. |
Effect of TαPcZn-PDT on the apoptosis
of LoVo cells
The effect of TαPcZn-PDT on the apoptosis of LoVo
cells was detected in several ways. Firstly, the morphological
changes of the cells were observed using an inverted microscope.
Compared with the control group, the TαPcZn-PDT group had a
significantly greater proportion of cells exhibiting morphological
characteristics of apoptosis, such as cell shrinkage and an
increased number of vacuoles in the cytoplasm (Fig. 2A). In addition, cell apoptosis was
quantitated by flow cytometric analysis of Annexin V-FLUOS/PI
double-stained cells. Compared with the control treatment,
TαPcZn-PDT significantly induced apoptosis of LoVo cells (Fig. 2B).
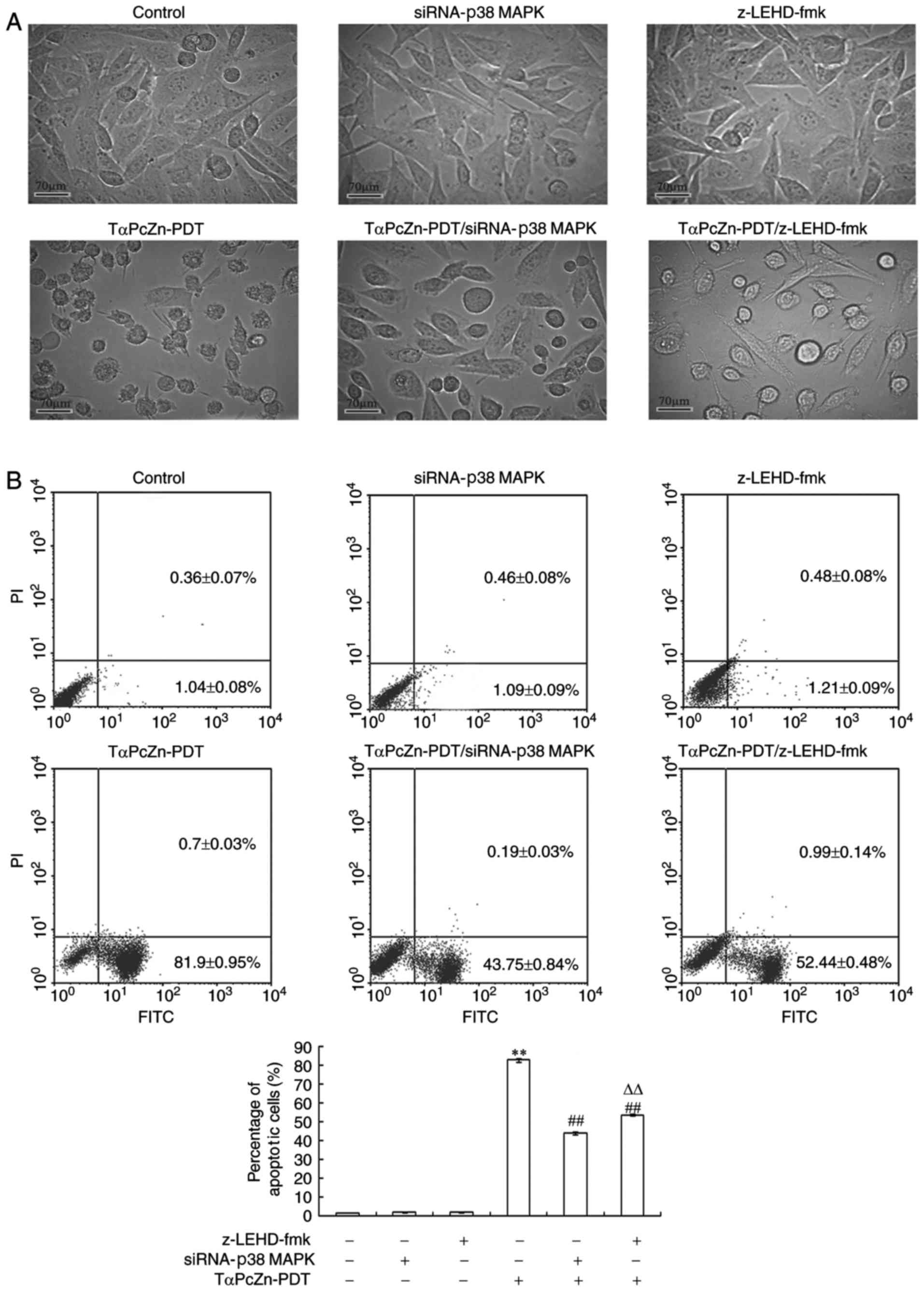 | Figure 2.Effect of siRNA-p38 MAPK or
z-LEHD-fmk on the TαPcZn-PDT-induced apoptosis of LoVo cells. In
the absence or presence of siRNA-p38 MAPK (12.5 nmol/l) for
48 h or z-LEHD-fmk (10 µmol/l) for 2.5 h, LoVo cells were treated
with TαPcZn (54 µmol/l), exposed to red light irradiation (53.7
J/cm2), and then incubated for 3 h. The morphology of
the apoptotic cells was observed by (A) microscopy and the
percentage of apoptotic cells was assayed by (B) flow cytometric
analysis of Annexin V-FLUOS/PI double-stained cells. Scale bar, 70
µm. Values presented are representative of three independent
experiments (mean ± standard deviation; **P<0.01, compared with
the control treatment; ##P<0.01, compared with the
TαPcZn-PDT treatment; ΔΔP<0.01, compared with the
TαPcZn-PDT/siRNA-p38 MAPK treatment). siRNA, small
interfering RNA; MAPK, mitogen-activated protein kinase; TαPcZn,
tetra-α-(4-carboxyphenoxy) phthalocyanine zinc; PDT, photodynamic
therapy. |
Crosstalk between p38 MAPK and
caspase-9 regulates the TαPcZn-PDT-induced apoptosis of LoVo
cells
To evaluate whether p38 MAPK and caspase-9 are
involved in the TαPcZn-PDT-induced apoptosis of LoVo cells, an
immunoblot assay was used to investigate the effect of TαPcZn-PDT
on p38 MAPK, p-p38 MAPK and caspase-9 with or without siRNA-p38
MAPK or z-LEHD-fmk. Compared with the control treatment,
TαPcZn-PDT increased p38 MAPK phosphorylation and active caspase-9,
but had less influence on p38 MAPK (Fig. 3). However, compared with TαPcZn-PDT
treatment only, siRNA-p38 MAPK decreased the level of p38
MAPK and p-p38 MAPK and z-LEHD-fmk decreased the level of activated
caspase-9 (Fig. 3).
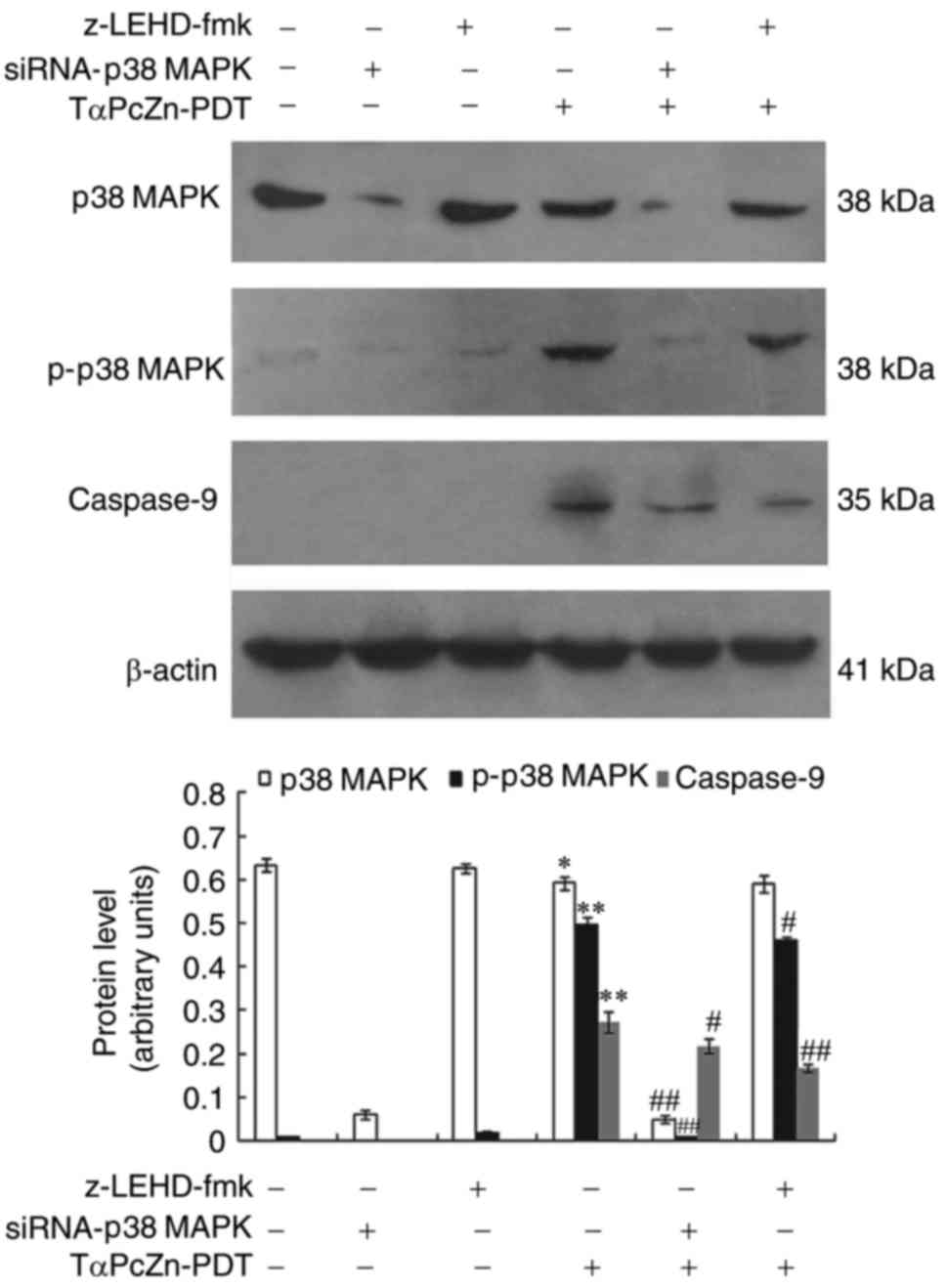 | Figure 3.Changes in p38 MAPK, p-p38 MAPK and
caspsase-9 levels in response to treatment with TαPcZn-PDT, with or
without siRNA-p38 MAPK or z-LEHD-fmk, in LoVo cells.
Following treatment with siRNA-p38 MAPK (12.5 nmol/l) for 48
h or z-LEHD-fmk (10 µmol/l) for 2.5 h, the LoVo cells were treated
with TαPcZn (54 µmol/l), exposed to red light irradiation (53.7
J/cm2) and then incubated for 3 h. The expression of p38
MAPK, p-P38MAPK and cleaved caspase-9 was analyzed by an immunoblot
assay. The values presented are representative of three independent
experiments (mean ± standard deviation; *P<0.05, **P<0.01,
compared to the control treatment; #P<0.05,
##P<0.01, compared with the TαPcZn-PDT treatment).
siRNA, small interfering RNA; MAPK, mitogen-activated protein
kinase; TαPcZn, tetra-α-(4-carboxyphenoxy) phthalocyanine zinc;
PDT, photodynamic therapy. |
siRNA-p38 MAPK and z-LEHD-fmk were used to
assess whether p38 MAPK and caspase-9 regulated apoptosis in the
TαPcZn-PDT-treated LoVo cells. Compared with the TαPcZn-PDT
treatment only, siRNA-p38 MAPK and z-LEHD-fmk inhibited the
TαPcZn-PDT-induced apoptosis of LoVo cells (Fig. 2A and B). Additionally, the results
revealed that siRNA-p38 MAPK had a more significant
inhibitory effect on TαPcZn-PDT-induced apoptosis compared with
that of z-LEHD-fmk (Fig. 2B).
To evaluate whether p38 MAPK regulated caspase-9 and
caspase-9 in turn regulated p38 MAPK, immunoblotting was performed
to investigate the effect of siRNA-p38 MAPK on caspase-9 and
the effect of z-LEHD-fmk on p38 MAPK during the TαPcZn-PDT-induced
apoptosis in LoVo cells. Compared with the TαPcZn-PDT treatment
alone, siRNA-p38 MAPK downregulated the expression of
activated caspase-9 and z-LEHD-fmk downregulated the expression of
p-p38 MAPK (Fig. 3).
To determine the manner in which p38 MAPK and
caspase-9 regulated each other via crosstalk during the
TαPcZn-PDT-induced apoptosis in LoVo cells,
immunoprecipitation/immunoblot analysis was used to investigate the
interaction between p38 MAPK and caspase-9 with or without
siRNA-p38 MAPK or z-LEHD-fmk. Compared with the control
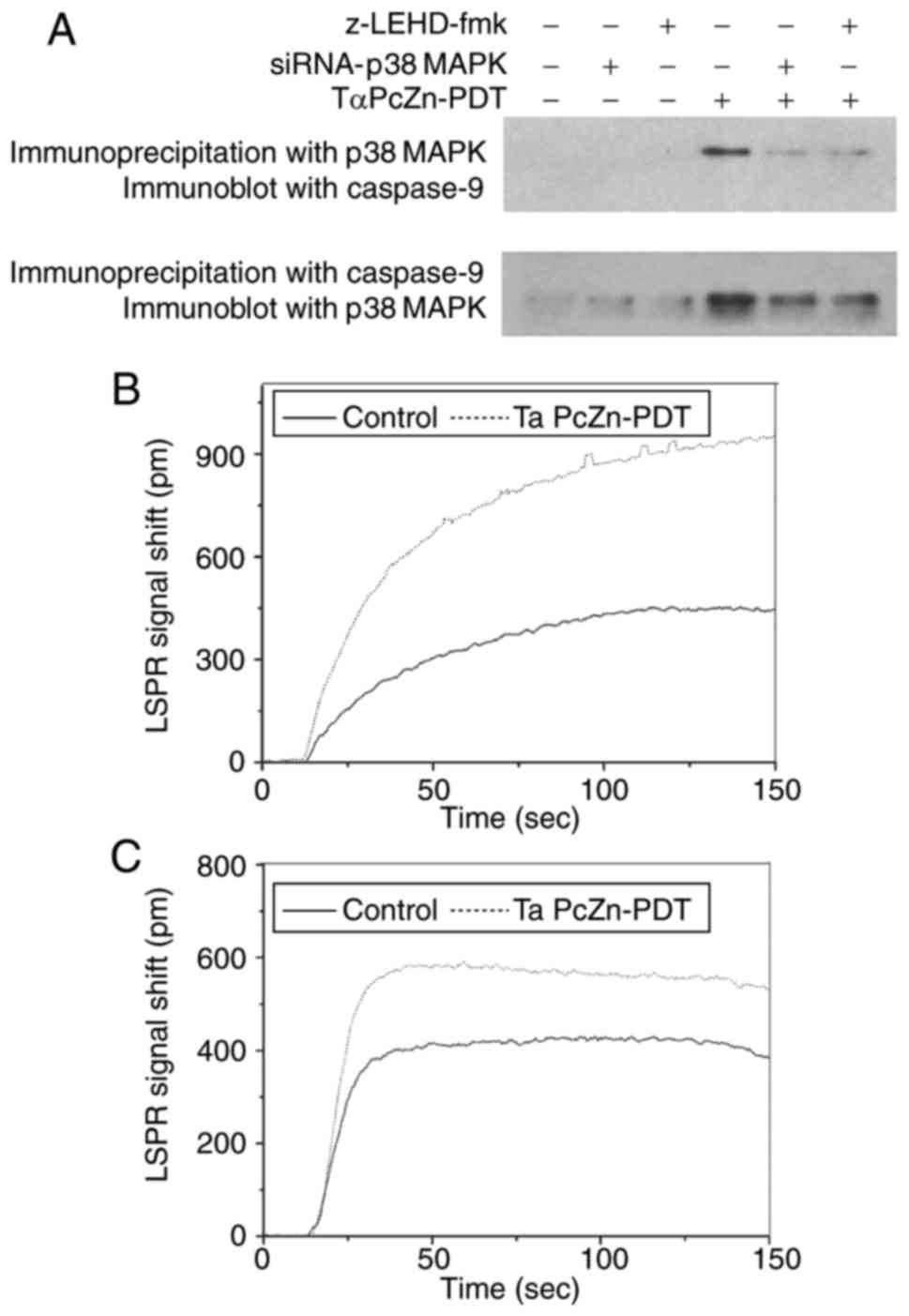
treatment, TαPcZn-PDT upregulated p38 MAPK and caspase-9 (Fig. 4A), indicating that TαPcZn-PDT
induced the formation of p38 MAPK/caspase-9 complexes. However,
compared with the TαPcZn-PDT treatment alone, siRNA-p38 MAPK
or z-LEHD-fmk downregulated p38 MAPK or caspase-9, respectively
(Fig. 4A). Furthermore, the
interaction between p38 MAPK and caspase-9 in the
TαPcZn-PDT-induced apoptosis of LoVo cells was detected by an LSPR
assay. Firstly, the LSPR signal shift was used to assess the
interaction between p38 MAPK and the total cellular protein.
Compared with the control treatment, TαPcZn-PDT markedly increased
the LSPR signal shift (Fig. 4B),
indicating that p38 MAPK bound to total protein. Secondly, the LSPR
signal shift was used to detect the interaction between caspase-9
and the total protein/p38 MAPK complexes. Compared with the control
treatment, TαPcZn-PDT obviously increased the LSPR signal shift
(Fig. 4C), indicating that
caspase-9 bound to the total protein/p38 MAPK complexes and to p38
MAPK.
Crosstalk between p38 MAPK and
caspase-9 regulates mitochondria in TαPcZn-PDT-induced apoptosis of
LoVo cells
To assess whether the crosstalk between p38 MAPK and
caspase-9 regulates mitochondria during the TαPcZn-PDT-induced
apoptosis of LoVo cells, a JC-I assay was used to detect the
influence of TαPcZn-PDT on ΔΨm with or without siRNA-p38
MAPK or z-LEHD-fmk. Compared with the control treatment,
TαPcZn-PDT increased the number of green fluorescent apoptotic
cells (Fig. 5A) and decreased the
ratio of red/green fluorescence intensity (Fig. 5B). However, compared with TαPcZn-PDT
treatment only, siRNA-p38 MAPK or z-LEHD-fmk decreased the
number of green fluorescent apoptotic cells (Fig. 5A) and increased the ratio of
red/green fluorescence intensity (Fig.
5B).
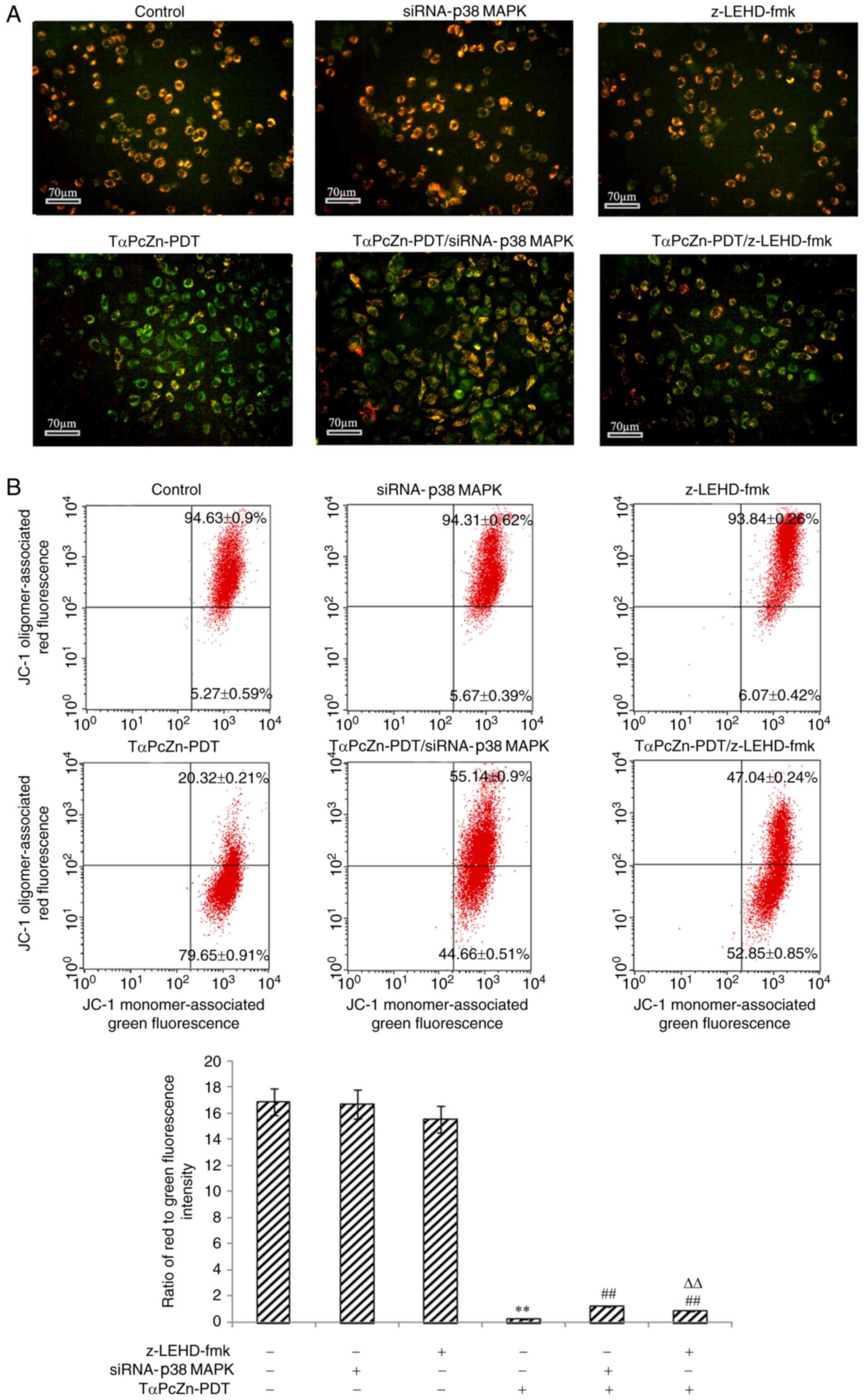 | Figure 5.Effect of TαPcZn-PDT treatment, with
or without siRNA-p38 MAPK or z-LEHD-fmk, on ΔΨm in the LoVo
cells analyzed by JC-1 staining assay. In the absence or presence
of siRNA-P38MAPK (12.5 nmol/l) for 48 h or z-LEHD-fmk (10
µmol/l) for 2.5 h, the LoVo cells were treated with TαPcZn (54
µmol/l), exposed to red light irradiation (53.7 J/cm2)
and then incubated for 3 h. (A) The red/green fluorescence
intensity and (B) the ratio of red to green fluorescence intensity
in LoVo cells were detected by JC-1 assay using fluorescence
microscopy and flow cytometry, respectively. Scale bar, 70 µm.
Values presented are representative of three independent
experiments (mean ± standard deviation; **P<0.01, compared with
control treatment; ##P<0.01, compared with TαPcZn-PDT
treatment; ΔΔP<0.01, compared with the
TαPcZn-PDT/siRNA-p38 MAPK treatment). siRNA, small interfering RNA;
MAPK, mitogen-activated protein kinase; ΔΨm, mitochondrial membrane
potential; TαPcZn, tetra-α-(4-carboxyphenoxy) phthalocyanine zinc;
PDT, photodynamic therapy. |
To further assess whether the crosstalk between p38
MAPK and caspase-9 regulates mitochondria during the
TαPcZn-PDT-induced apoptosis of the LoVo cells, immunoblotting was
performed to examine the levels of factors and proteins associated
with mitochondria, including Bcl-2, Bax, AIF, Cyto c, Bid
and caspase-3, with or without siRNA-p38 MAPK or z-LEHD-fmk
treatment. Compared with the control treatment, TαPcZn-PDT
significantly downregulated the expression of Bcl-2, upregulated
the expression of Bax, promoted the release of AIF and Cyto
c and activated Bid and caspase-3 (Fig. 6A-C). However, compared with the
TαPcZn-PDT treatment alone, siRNA-p38 MAPK or z-LEHD-fmk
attenuated the effects of TαPcZn-PDT on Bcl-2, Bax, AIF, Cyto
c, Bid and caspase-3 (Fig.
6A-C). In addition, the results revealed that the inhibitory
effect of siRNA-p38 MAPK on ΔΨm, Bcl-2, Bax, AIF, Cyto
c, Bid and caspase-3 was more significant than the effects
of z-LEHD-fmk on these factors in the TαPcZn-PDT-induced apoptosis
of LoVo cells (Figs. 5B and
6A-C).
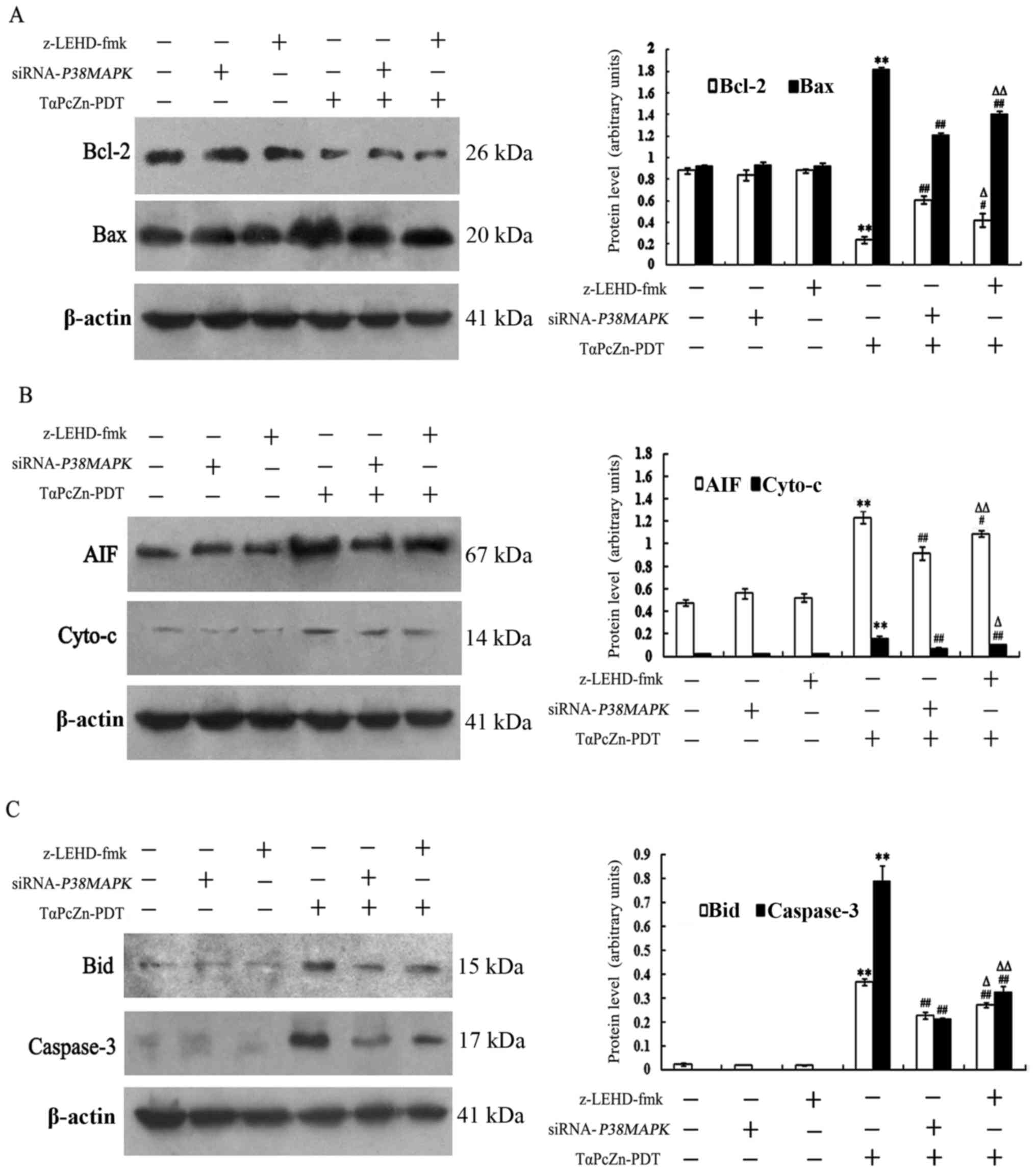 | Figure 6.Effect of TαPcZn-PDT treatment, with
or without siRNA-p38 MAPK or z-LEHD-fmk, on Bcl-2, Bax, AIF, Cyto
c, Bid and caspase-3 levels in LoVo cells analyzed by
immunoblot assay. In the absence or presence of siRNA-p38
MAPK (12.5 nmol/l) for 48 h or z-LEHD-fmk (10 µmol/l) for 2.5
h, LoVo cells were treated with TαPcZn (54 µmol/l), exposed to red
light irradiation (53.7 J/cm2) and then incubated for 3
h. The expression of (A) Bcl-2 and Bax, (B) AIF and Cyto c
and (C) Bid and caspase-3 was analyzed by immunoblot assay. Values
presented are representative of three independent experiments (mean
± standard deviation; **P<0.01, compared with the control
treatment; #P<0.05, ##P<0.01, compared
with the TαPcZn-PDT treatment; ΔP<0.05,
ΔΔP<0.01, compared with the TαPcZn-PDT/siRNA-p38
MAPK treatment). siRNA, small interfering RNA; MAPK,
mitogen-activated protein kinase; AIF, apoptosis-inducing factor;
Cyto c, cytochrome c; TαPcZn,
tetra-α-(4-carboxyphenoxy) phthalocyanine zinc; PDT, photodynamic
therapy. |
Discussion
Accumulating evidence has revealed that
phthalocyanine-PDT induces apoptosis of tumor cells (11,35).
However, it was unclear whether TαPcZn-PDT induced the apoptosis of
the LoVo cells. Therefore, the present study performed an initial
investigation of this, confirming that TαPcZn-PDT induced apoptosis
of LoVo cells.
p38 MAPK is an important signal in stress responses
and caspase-9 is a key initiator in the mitochondrial apoptotic
pathway (36–40). Recent studies revealed that p38 MAPK
and caspase-9 were involved in the apoptotic process of tumor cells
treated with phthalocyanine-PDT (8,23,24,32,41).
However, the mechanism through which p38 MAPK and caspase-9
regulated the TαPcZn-PDT-induced apoptosis of LoVo cells remains
unclear. Therefore, we examined the effect of TαPcZn-PDT on p38
MAPK, p-p38 MAPK and caspase-9 levels, in combination with
siRNA-p38 MAPK or z-LEHD-fmk. The results indicated that
TαPcZn-PDT activated p38 MAPK and caspase-9, but siRNA-p38
MAPK and z-LEHD-fmk both reduced the level of activated p38
MAPK and caspase-9, indicating that the activation of p38 MAPK and
caspase-9 may be involved in the TαPcZn-PDT-induced apoptosis of
LoVo cells. To assess whether p38 MAPK and caspase-9 regulated
apoptosis in the TαPcZn-PDT-treated LoVo cells, we analyzed the
effect of siRNA-p38 MAPK and z-LEHD-fmk on apoptosis. We
observed that both siRNA-p38 MAPK and z-LEHD-fmk reduced
apoptosis of TαPcZn-PDT-treated LoVo cells, indicating that
activated p38 MAPK and caspase-9 are essential for
TαPcZn-PDT-induced apoptosis of LoVo cells.
Certain studies have reported that p38 MAPK
regulates caspase-9 during drug-induced apoptosis of tumor cells
(42,43). However, whether p38 MAPK and
caspase-9 regulate each other has not been ascertained in the
TαPcZn-PDT-induced apoptosis of LoVo cells. Therefore, we examined
the effect of siRNA-p38 MAPK on caspase-9 and the effect of
z-LEHD-fmk on p38 MAPK during TαPcZn-PDT-induced apoptosis of LoVo
cells. The results revealed that siRNA-p38 MAPK decreased
the activation of caspase-9 and that z-LEHD-fmk decreased the
activation of p38 MAPK, indicating that the crosstalk regulation
between p38 MAPK and caspase-9 is involved in the
TαPcZn-PDT-induced apoptosis of LoVo cells. In addition, these
findings indicated that the inhibitory effect of siRNA-p38
MAPK on the TαPcZn-PDT-induced apoptosis was more significant
than the effect of z-LEHD-fmk on apoptosis, revealing that p38 MAPK
may have an important regulatory role in the crosstalk between p38
MAPK and caspase-9 during apoptosis of these treated cells. To
determine the manner in which p38 MAPK and caspase-9 regulate each
other during the TαPcZn-PDT-induced apoptosis of LoVo cells, we
investigated the interaction between p38 MAPK and caspase-9 with or
without siRNA-p38 MAPK or z-LEHD-fmk. The results revealed
that TαPcZn-PDT induced the formation of p38 MAPK/caspase-9
complexes, but siRNA-p38 MAPK or z-LEHD-fmk both decreased
the formation of p38 MAPK/caspase-9 complexes, indicating that p38
MAPK may bind to caspase-9 and that p38 MAPK or caspase-9 can
regulate their interaction. All of the aforementioned results
indicated that p38 MAPK may play an important regulatory role in
the crosstalk between p38 MAPK and caspase-9 and that direct
interaction between p38 MAPK and caspase-9 may regulate apoptosis
in the TαPcZn-PDT-treated LoVo cells.
Previous studies revealed that mitochondria play a
crucial regulatory role in phthalocyanine-PDT-induced apoptosis
(23,24) and that p38 MAPK and caspase-9
regulated mitochondria during cell apoptosis (44–48).
However, it was not clear whether p38 MAPK and caspase-9 regulated
mitochondria in the TαPcZn-PDT-induced apoptosis of LoVo cells.
Therefore, the effect of the TαPcZn-PDT treatment in combination
with siRNA-p38 MAPK or z-LEHD-fmk on ΔΨm and
mitochondria-related factors and proteins (Bcl-2, Bax, AIF, Cyto
c, Bid and caspase-3) was investigated in the present study.
The findings revealed that TαPcZn-PDT resulted in the reduction of
ΔΨm, the downregulation of Bcl-2, the upregulation of Bax
expression, the release of AIF and Cyto c and the activation
of Bid and caspase-3, indicating the involvement of mitochondria in
the process of TαPcZn-PDT-induced apoptosis. However, siRNA-p38
MAPK and z-LEHD-fmk both attenuated the effects of TαPcZn-PDT
on ΔΨm, Bcl-2, Bax, AIF, Cyto c, Bid and caspase-3,
indicating that the crosstalk between p38 MAPK and caspase-9 may
regulate mitochondria during TαPcZn-PDT-induced apoptosis of LoVo
cells. In addition, the results revealed that siRNA-p38 MAPK
had a more significant inhibitory effect on ΔΨm, Bcl-2, Bax, AIF,
Cyto c, Bid and caspase-3 than the effect of z-LEHD-fmk,
indicating that p38 MAPK has a major regulatory role in the
crosstalk between p38 MAPK and caspase-9 during the
TαPcZn-PDT-induced apoptosis in this cell type. As caspase-9 is the
downstream of mitochondria within the apoptosis pathway, caspase-9
may regulate the mitochondria upstream by directly influencing p38
MAPK during the crosstalk between p38 MAPK and caspase-9, which may
explain the reason why p38 MAPK had a more significant influence on
mitochondria than caspase-9 in the crosstalk between p38 MAPK and
caspase-9.
In conclusion, our findings indicated that p38 MAPK
in the crosstalk between p38 MAPK and caspase-9 may play a major
regulatory role and direct crosstalk between p38 MAPK and caspase-9
may regulate mitochondria-mediated apoptosis in the
TαPcZn-PDT-treated LoVo cells.
Acknowledgements
The project was supported by the Natural Science
Foundation of Heilongjiang Province (no. ZD201318).
References
|
1
|
Lucena SR, Salazar N, Gracia-Cazaña T,
Zamarrón A, González S, Juarranz Á and Gilaberte Y: Combined
treatments with photodynamic therapy for non-melanoma skin cancer.
Int J Mol Sci. 16:25912–25933. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Acedo P, Stockert JC, Cañete M and
Villanueva A: Two combined photosensitizers: A goal for more
effective photodynamic therapy of cancer. Cell Death Dis.
5:e11222014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng Y, Zhang Y, Chen D, Chen H, Lin L,
Zheng C and Guo Y: Monascus pigment rubropunctatin: A potential
dual agent for cancer chemotherapy and phototherapy. J Agric Food
Chem. 64:2541–2548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kessel D: Death pathways associated with
photodynamic therapy. Med Laser Appl. 21:219–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yslas EI, Durantini EN and Rivarola VA:
Zinc-(II) 2,9,16,23-tetrakis (methoxy) phthalocyanine: Potential
photosensitizer for use in photodynamic therapy in vitro. Bioorg
Med Chem. 15:4651–4660. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xia C, Wang Y, Chen W, Yu W, Wang B and Li
T: New hydrophilic/lipophilic tetra-α-(4-carboxyphenoxy)
phthalocyanine zinc-mediated photodynamic therapy inhibits the
proliferation of human hepatocellular carcinoma Bel-7402 cells by
triggering apoptosis and arresting cell cycle. Molecules.
16:1389–1401. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Z, Chan PS, Li H, Wong KL, Wong RN,
Mak NK, Zhang J, Tam HL, Wong WY, Kwong DW, et al: Highly selective
mitochondria-targeting amphiphilic silicon(IV) phthalocyanines with
axially ligated rhodamine B for photodynamic therapy. Inorg Chem.
51:812–821. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Xia C, Chen W, Chen Y, Wang Y and
Li T: Autoregulatory feedback mechanism of P38MAPK/Caspase-8 in
Photodynamic Therapy-Hydrophilic/Lipophilic
Tetra-α-(4-carboxyphenoxy) phthalocyanine zinc-induced apoptosis of
human hepatocellular carcinoma Bel-7402 Cells. Int J Photoenergy
2014. 1638132014.doi: org/10.1155/2014/163813.
|
|
9
|
Cakir D, Göksel M, Çakır V, Durmuş M,
Biyiklioglu Z and Kantekin H: Amphiphilic zinc phthalocyanine
photosensitizers: synthesis, photophysicochemical properties and in
vitro studies for photodynamic therapy. Dalton Trans. 44:9646–9658.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seervi M and Xue D: Mitochondrial cell
death pathways in caenorhabiditis elegans. Curr Top Dev Biol.
114:43–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mfouo-Tynga I and Abrahamse H: Cell death
pathways and phthalocyanine as an efficient agent for photodynamic
cancer therapy. Int J Mol Sci. 16:10228–10241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sevrioukova IF: Apoptosis-inducing factor:
Structure, function, and redox regulation. Antioxid Redox Signal.
14:2545–2579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Doti N, Reuther C, Scognamiglio PL, Dolga
AM, Plesnila N, Ruvo M and Culmsee C: Inhibition of the AIF/CypA
complex protects against intrinsic death pathways induced by
oxidative stress. Cell Death Dis. 5:e9932014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karch J and Molkentin JD: Regulated
necrotic cell death: The passive aggressive side of Bax and Bak.
Circ Res. 116:1800–1809. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gillies LA and Kuwana T: Apoptosis
regulation at the mitochondrial outer membrane. J Cell Biochem.
115:632–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Juric V, Chen CC and Lau LF: TNFα-induced
apoptosis enabled by CCN1/CYR61: Pathways of reactive oxygen
species generation and cytochrome c release. PLoS One.
7:e313032012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ameeramja J, Panneerselvam L, Govindarajan
V, Jeyachandran S, Baskaralingam V and Perumal E: Tamarind seed
coat ameliorates fluoride induced cytotoxicity, oxidative stress,
mitochondrial dysfunction and apoptosis in A549 cells. J Hazard
Mater. 301:554–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park HS, Hwang HJ, Kim G-Y, Cha H-J, Kim
W-J, Kim ND, Yoo YH and Choi YH: Induction of apoptosis by fucoidan
in human leukemia U937 cells through activation of p38 MAPK and
modulation of Bcl-2 family. Mar Drugs. 11:2347–2364. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kong D, Zheng T, Zhang M, Wang D, Du S, Li
X, Fang J and Cao X: Static mechanical stress induces apoptosis in
rat endplate chondrocytes through MAPK and mitochondria-dependent
caspase activation signaling pathways. PLoS One. 8:e694032013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tait SWG and Green DR: Mitochondrial
regulation of cell death. Cold Spring Harb Perspect Biol.
5:a0087062013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsiao P-C, Chou YE, Tan P, Lee WJ, Yang
SF, Chow JM, Che HY, Lin CH, Lee LM and Chien MH: Pterostilbene
simultaneously induced G0/G1-phase arrest and MAPK-mediated
mitochondrial-derived apoptosis in human acute myeloid leukemia
cell lines. PLoS One. 9:e1053422014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tor YS, Yazan LS, Foo JB, Wibowo A, Ismail
N, Cheah YK, Abdullah R, Ismail M, Ismail IS and Yeap SK: Induction
of apoptosis in MCF-7 cells via oxidative stress generation,
mitochondria-dependent and caspase-independent pathway by ethyl
acetate extract of dillenia suffruticosa and its chemical profile.
PLoS One. 10:e01274412015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shao J, Dai Y, Zhao W, Xie J, Xue J, Ye J
and Jia L: Intracellular distribution and mechanisms of actions of
photosensitizer Zinc(II)-phthalocyanine solubilized in Cremophor EL
against human hepatocellular carcinoma HepG2 cells. Cancer Lett.
330:49–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marino J, García Vior MC, Furmento VA,
Blank VC, Awruch J and Roguin LP: Lysosomal and mitochondrial
permeabilization mediates zinc(II) cationic phthalocyanine
phototoxicity. Int J Biochem Cell Biol. 45:2553–2562. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeong HS, Choi HY, Lee ER, Kim JH, Jeon K,
Lee HJ and Cho SG: Involvement of caspase-9 in autophagy-mediated
cell survival pathway. Biochim Biophys Acta. 1813:80–90. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han J, Hou W, Goldstein LA, Stolz DB,
Watkins SC and Rabinowich H: A Complex between Atg7 and Caspase-9:
A novel mechanism of cross-regulation between autophagy and
apoptosis. J Biol Chem. 289:6485–6497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bu HQ, Liu DL, Wei WT, Chen L, Huang H, Li
Y and Cui JH: Oridonin induces apoptosis in SW1990 pancreatic
cancer cells via p53- and caspase-dependent induction of p38 MAPK.
Oncol Rep. 31:975–982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lewis TS, Shapiro PS and Ahn NG: Signal
transduction through MAP kinase cascades. Adv Cancer Res.
74:49–139. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xue L, He J and Oleinick NL: Promotion of
photodynamic therapy-induced apoptosis by stress kinases. Cell
Death Differ. 6:855–864. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Whitacre CMFD, Feyes DK, Satoh T,
Grossmann J, Mulvihill JW, Mukhtar H and Oleinick NL: Photodynamic
therapy with the phthalocyanine photosensitizer Pc 4 of SW480 human
colon cancer xenografts in athymic mice. Clin Cancer Res.
6:2021–2027. 2000.PubMed/NCBI
|
|
33
|
Wu HY, Chen W, Li T, Wang Y, Xia CH and Li
XL: Study on synthesis and antineoplastic activity of
α-tetra-(4-carboxyphenoxy)phthalocyanine zinc. J Liaoning Normal
Univ (Natural Science Edition). 1:94–97. 2009.(In Chinese).
|
|
34
|
Bi MC, Rosen R, Zha RY, McCormick SA, Song
E and Hu DN: Zeaxanthin induces apoptosis in human uveal melanoma
cells through Bcl-2 family proteins and intrinsic apoptosis
pathway. Evid Based Complement Alternat Med. 2013:2050822013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kuzyniak W, Ermilov EA, Atilla D, Gürek
AG, Nitzsche B, Derkow K, Hoffmann B, Steinemann G, Ahsen V and
Höpfner M: Tetra-triethyleneoxysulfonyl substituted zinc
phthalocyanine for photodynamic cancer therapy. Photodiagn Photodyn
Ther. 13:148–157. 2016. View Article : Google Scholar
|
|
36
|
Kim JY, Yu SJ, Oh HJ, Lee JY, Kim Y and
Sohn J: Panaxydol induces apoptosis through an increased
intracellular calcium level, activation of JNK and p38 MAPK and
NADPH oxidase-dependent generation of reactive oxygen species.
Apoptosis. 16:347–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Festa M, Capasso A, D'Acunto CW, Masullo
M, Rossi AG, Pizza C and Piacente S: Xanthohumol induces apoptosis
in human malignant glioblastoma cells by increasing reactive oxygen
species and activating MAPK pathways. J Nat Prod. 74:2505–2513.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Zhang S, Su D, Liu J, Cheng Y, Zou
L, Li W and Jiang Y: Inhibiting (pro)renin receptor-mediated p38
MAPK signaling decreases hypoxia/reoxygenation-induced apoptosis in
H9c2 cells. Mol Cell Biochem. 403:267–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hui K, Yang Y, Shi K, Luo H, Duan J, An J,
Wu P, Ci Y, Shi L and Xu C: The p38 MAPK-regulated PKD1/CREB/Bcl-2
pathway contributes to selenite-induced colorectal cancer cell
apoptosis in vitro and in vivo. Cancer Lett. 354:189–199. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao W, Yang Y, Zhang YX, Zhou C, Li HM,
Tang YL, Liang XH, Chen T and Tang YJ: Fluoride-containing
podophyllum derivatives exhibit antitumor activities through
enhancing mitochondrial apoptosis pathway by increasing the
expression of caspase-9 in HeLa cells. Sci Rep. 5:171752015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wright DB and Loftus EF: Measuring
dissociation: Comparison of alternative forms of the dissociative
experiences scale. Am J Psychol. 112:497–519. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sreekanth GP, Chuncharunee A,
Sirimontaporn A, Panaampon J, Noisakran S, Yenchitsomanus PT and
Limjindaporn T: SB203580 modulates p38 MAPK signaling and dengue
virus-induced liver injury by reducing MAPKAPK2, HSP27, and ATF2
phosphorylation. PLoS One. 11:e01494862016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zou S, Wang C, Cui Z, Guo P, Meng Q, Shi
X, Gao Y, Yang G and Han Z: β-Elemene induces apoptosis of human
rheumatoid arthritis fibroblast-like synoviocytes via reactive
oxygen species-dependent activation of p38 mitogen-activated
protein kinase. Pharmacol Rep. 68:7–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park GB, Kim YS, Lee HK, Song H, Kim S,
Cho DH and Hur DY: Reactive oxygen species and p38 MAPK regulate
Bax translocation and calcium redistribution in salubrinal-induced
apoptosis of EBV-transformed B cells. Cancer Lett. 313:235–248.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang JB, Khan M, He YY, Yao M, Li YM, Gao
HW and Ma TH: Tubeimoside-1 induces oxidative stress-mediated
apoptosis and G0/G1 phase arrest in human prostate carcinoma cells
in vitro. Acta Pharmacol Sin. 37:950–962. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guerrero AD, Schmitz I, Chen M and Wang J:
Promotion of caspase activation by caspase-9-mediated feedback
amplification of mitochondrial damage. J Clin Cell Immunol.
3:10001262012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen M, Guerrero AD, Huang L, Shabier Z,
Pan M, Tan TH and Wang J: Caspase-9-induced mitochondrial
disruption through cleavage of anti-apoptotic BCL-2 family members.
J Biol Chem. 282:33888–33895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nie C, Luo Y, Zhao X, Luo N, Tong A, Liu
X, Yuan Z, Wang C and Wei Y: Caspase-9 mediates Puma activation in
UCN-01-induced apoptosis. Cell Death Dis. 5:e14952014. View Article : Google Scholar : PubMed/NCBI
|