Introduction
Oral squamous cell carcinoma (OSCC) is among the
most prevalent malignant neoplasms worldwide, and frequently occurs
in developing countries, including those of Southeast Asia
(1,2). Despite recent advances in surgical and
radiotherapeutic strategies, a high proportion of patients with
OSCC still have poor prognosis, which has remained relatively
unchanged over the past 20 years (3,4).
Therefore, it is necessary to explore novel molecular targets for
the development of more effective therapeutic strategies for
patients with OSCC.
Mesenchymal-epithelial transition factor (c-Met) is
the only known high-affinity receptor for hepatocyte growth factor
(HGF). The HGF/c-Met signaling pathway can stimulate the growth of
hepatocytes, and may also promote proliferation, migration,
survival, angiogenesis, and invasion in a broad range of human
solid tumors, including ovarian, stomach, lung, breast, liver, and
brain tumors (5–12). Moreover, high expression of c-Met
was found in pancreatic cancer stem cells, and knockdown of c-Met
or treatment with a c-Met inhibitor blocked the formation of tumor
spheres in a population of pancreatic cancer cells with stem cell
like properties (13,14). Furthermore, inhibition of the c-Met
signaling pathway has been reported to increase mitochondrial
release of cytochrome c and the Bax/Bcl-2 ratio (15). These data regarding the aberrant
expression and activity of c-Met revealed that it may play an
important role in the progression of human cancers, and may be an
important target in cancer therapy. However, little is known about
the biological functions of the HGF/c-Met signaling pathway in the
progression of human OSCC.
To elucidate the underlying functions of the
HGF/c-Met signaling pathway in the progression of OSCC, the present
study first examined c-Met expression in 40 human OSCC tissues, 20
human normal oral mucosa and two OSCC cell lines. Subsequently, we
evaluated the effects of a c-Met inhibitor on the viability,
migration, and apoptosis of the OSCC cell lines. Finally, we
preliminarily investigated the potential molecular mechanisms
underlying the activity of the HGF/c-Met signaling pathway in the
progression of OSCC.
Materials and methods
Cell lines and cell culture
conditions
The human HIOEC, HN30 and CAL-27 cell lines were
obtained from the Laboratory of Oral Oncology, Ninth People's
Hospital, School of Medicine, Shanghai Jiaotong University,
Shanghai, China. The three cell lines were cultured in DMEM and
medium supplemented with 10% FBS (Gibco, Grand Island, NY, USA) and
of 1% penicillin/streptomycin (Gibco). Cells were incubated at 37°C
in a humidified incubator containing 5% CO2.
Reagents and antibodies
Human recombinant HGF was purchased from GenScript
(Nanjing, China). The c-Met kinase inhibitors JNJ38877605 (JNJ) was
obtained from Selleckchem (Houston, TX, USA). Antibodies used
included: Met mAb (#8198), phosphor-Met (Tyr1234/1235) mAb (#3077),
AKT mAb (#9272), phosphor-AKT (Ser473) mAb (#4060), p44/42 MAP
kinase mAb (#4696), phosphor-p44/42 MAP kinase (Thr202/Tyr204) mAb
(#4376), NF-κB p65 mAb (#6131), GAPDH mAb (#2118), MMP-9 mAb
(#3852), HRP-linked anti-mouse IgG antibody (#7076), and HRP-linked
anti-rabbit IgG antibody (#7074). The PE-labeled anti-rabbit IgG
antibody was used as a secondary antibody. All antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
VEGF mAb (#19003) was purchased from Proteintech (Chicago, IL,
USA).
Immunohistochemistry (IHC). IHC was performed as
previously described (16). Forty
human OSCC specimens were collected from patients who had undergone
surgery between September 2009 and September 2010 at the Department
of Oral and Maxillofacial Surgery, Ninth People's Hospital, School
of Medicine, Shanghai Jiao Tong University. All experimental
procedures received ethics approval from the independent Ethics
Committee of the Shanghai Ninth People's Hospital Affiliated to
Shanghai Jiaotong University School of Medicine (no. 200926). The
pathological characterization of the OSCC patients included in this
study is summarized in Table I. For
immunohistochemical examination, tissues were fixed with 4%
paraformaldehyde and embedded with paraffin. Sections of the
samples were blocked with 10% goat serum in PBS and incubated
overnight at 4°C with anti-c-Met, anti-VEGF-A or anti-MMP-9
antibodies. After 3 washes with PBS, the sections were incubated
with the peroxidase-conjugated goat anti-rabbit antibody for 1 h,
following by incubation with 3,3′-diaminobenzidine (DAB) substrate
for 3 min. Counter-staining was performed with hematoxylin, and
dehydration was then performed with ethanol and dimethyl benzene.
The IHC results in tissues were scored by two independent
investigators based on the level of staining intensity as follows:
none (−), 0% of stained cells; weak (+), 1–25% of stained cells;
moderate (++), 26–50% of stained cells; strong (+++), >50% of
stained cells.
 | Table I.The correlation between
clinicopathological features and expression of c-Met. |
Table I.
The correlation between
clinicopathological features and expression of c-Met.
|
Characteristics | Case no. | c-Met positive
grade | Non-parametric test
value | P-value |
|---|
| Tobacco |
|
| Z= −0.588 | 0.565 |
|
Yes | 17 | 1.25±1.09 |
|
|
| No | 23 | 1.04±1.12 |
|
|
| Alcohol |
|
| Z= −0.271 | 0.798 |
|
Yes | 18 | 1.17±1.04 |
|
|
| No | 22 | 1.09±1.15 |
|
|
| Sex |
|
| Z= −0.062 | 0.965 |
|
Male | 28 | 1.12±1.03 |
|
|
|
Female | 12 | 1.17±1.13 |
|
|
| Tumor site |
|
|
χ2=0.318, d.f.=3 | 0.957 |
| Oral
cavity | 21 | 1.10±1.09 |
|
|
|
Gingiva | 6 | 1.0±1.27 |
|
|
| Mouth
floor | 7 | 1.29±1.11 |
|
|
|
Other | 6 | 1.17±1.17 |
|
|
| Tumor stage |
|
|
χ2=1.698, d.f.=3 | 0.637 |
| T1 | 17 | 1.18±1.13 |
|
|
| T2 | 12 | 0.92±0.99 |
|
|
| T3 | 6 | 1.33±1.21 |
|
|
| T4 | 5 | 1.20±1.30 |
|
|
| Nodal status |
|
| Z= −0.987 | 0.442 |
| N0 | 27 | 1.07±1.12 |
|
|
|
N1–2 | 13 | 1.23±1.09 |
|
|
| Pathological
differentiation grade |
|
|
χ2=0.505, d.f.=2 | 0.777 |
|
Well | 23 | 1.09±1.08 |
|
|
|
Moderately | 13 | 1.08±1.11 |
|
|
|
Poorly | 4 | 1.50±1.29 |
|
|
RNA isolation and RT-PCR
Total RNA was isolated and RT-PCR was performed as
previously described (16). The
primer pairs were as follows: c-Met, forward
5′-TTC-ACC-GCG-GAA-ACA-CCC-ATC-3′, and reverse
5′-GTC-TTC-CAG-CCA-GGC-CCA-3′; GAPDH, forward
5′-CAT-CTC-TGC-CCC-CTC-TGC-TGA-3′, and reverse
5′-GGA-TGA-CCT-TGC-CCA-CAG-CCT-3′.
Western blotting
Western blot analysis was performed as previously
described (16). The cells were
lysed with M-PER® mammalian protein extraction (Pierce,
Rockford, IL, USA). Proteins were quantified by the BCA Protein
Assay kit (Pierce) according to the manufacturer's instructions.
Samples containing a total of 50 µg protein were incubated at 100°C
for 5 min, separated by SDS-polyacrylamide gel electrophoresis, and
subsequently electrotransferred onto a polyvinylidene difluoride
membrane. Essential component detection in the cells was performed
using an antibody with overnight incubation at 4°C, and then an
HRP-conjugated secondary antibody (1:5,000 dilution) was added for
1 h at room temperature, followed by the development of reactions
in a chemiluminescent detection system.
Viability and apoptosis assays
Cells were seeded in a 96-well plate at
1×104 cells/well and were grown in the presence of JNJ
for 2 h. Then, they were treated with HGF. After 3 days, 20 µl MTS
(Sango, Shanghai, China) was added to each sample and incubated for
4 h. The absorbance of the solution was recorded at 490 nm with a
Thermo microplate reader. The results of the MTS assay reflected
the cell viability.
Apoptotic cells were assessed by flow cytometry as
follows: cells were harvested and washed with PBS, resuspended in
pre-diluted binding buffer, and stained with Annexin V-FITC (BD
Biosciences, San Diego, CA, USA) for 30 min at room temperature.
After being washed and resuspended in PI binding buffer, the cells
were immediately subjected to apoptosis analyses by flow cytometry
using Cell Quest Software.
In vitro scratch wound healing
migration assays
HN30 or CAL-27 cells were plated in a 6-well plate.
After overnight incubation, a sterile 10 µl pipette tip was used to
make a wound across a cell culture monolayer. Cells were incubated
in DMEM-0% FBS in the presence of JNJ for 2 h, and then were
treated with HGF (100 ng/ml). Multiple images of the wound were
taken immediately after wounding at 0 and 24 h under a
phase-contrast microscope. The efficiency of the wound healing
process was determined by calculating the area of the cell gap at
the indicated time-points (0 and 24 h), using ImageJ software.
Three images were used for each wound at each experimental point.
The results are expressed as percentage of healing at 24 h with
respect to time-point zero.
Immunofluorescence analysis
Tumor cells deposited on glass slides were washed
twice with PBS and fixed in 4% paraformaldehyde in PBS for 20 min.
The cells were further permeabilized with 0.1% Triton X in PBS for
8 min, washed and blocked with 5% bovine serum albumin in PBS for
30 min, and then treated with a monoclonal mouse anti-p65 (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) antibody overnight.
PE-labeled (1:100) anti-rabbit IgG served as the secondary
antibody. The sections were then mounted in a medium containing
DAPI for 5 min to visualize cell nuclei. The slides were evaluated
with fluorescence microscope TCS SP2 (Leica, Wetzlar, Germany).
Tumor xenograft study
Approximately 5- to 6-week-old male athymic nude
mice (approximately 20 g) were obtained from SLAC Laboratory Animal
Co., Ltd. (Shanghai, China) and were kept in a specific
pathogen-free (SPF) facility. CAL-27 cells (5×106) were
subcutaneously injected into the right flank region of mice. After
6 days, allowing the tumors to grow to approximately 50
mm3, the mice were randomized into control and treatment
groups. For the CAL-27 xenograft, the mice were treated as follows:
JNJ (10 mg/kg) oral administration drugs once a day for 4 weeks.
Each group consisted of 5–6 mice. Concomitantly, the body weight
and tumor size were assessed using an electronic balance and a
vernier caliper. The tumor volume was calculated using the formula:
volume = (length × width2) × 1/2. Following the endpoint
(28 days after the implantation), mice were euthanized when
moribund for the collection of tumors. This study was performed via
protocols approved by the Institutional Animal Care and Use
Committee of Fudan University (Shanghai, China).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS Inc., Chicago, IL, USA). Data are presented as the
mean ± standard deviation (SD) of at least three separate
experiments. One-way ANOVA was performed with post hoc SNK for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of c-Met in human OSCC
tissues and cell lines
To investigate whether c-Met was expressed in human
OSCC tissues, 40 human OSCC specimens and 20 normal oral tissue
samples adjacent to the tumor were assessed by
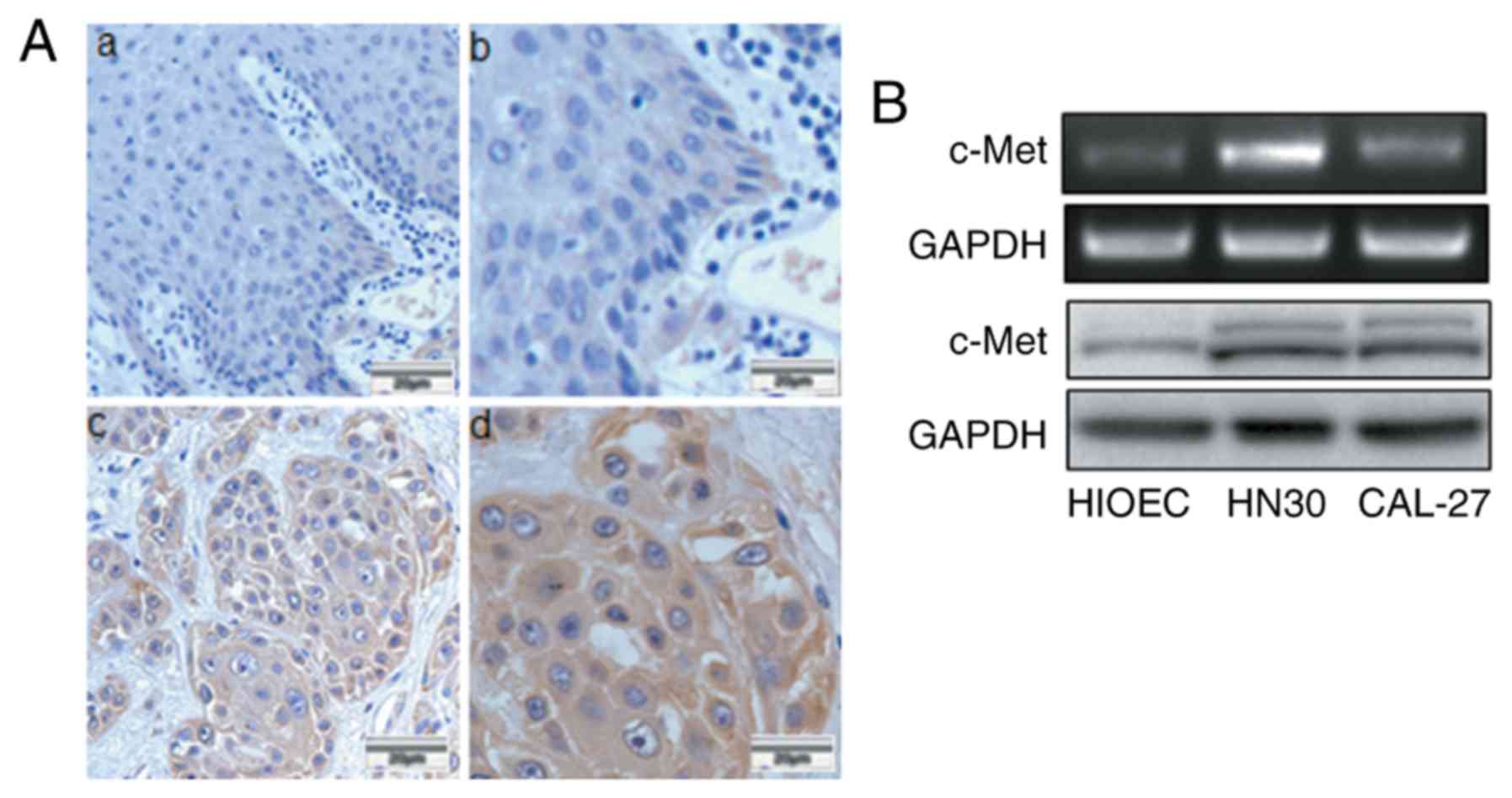
immunohistochemistry. A high level of c-Met expression was detected
in 60% (24/40) of the carcinoma samples, while only in 25% (5/20)
of the normal oral epithelial tissues. In the tumor samples, c-Met
was found to be localized in the cell membrane and cytoplasm
(Fig. 1A). The expression of c-Met
in situ was not correlated with patient clinicopathological
characteristics, including tobacco use, alcohol consumption, sex,
tumor site, tumor stage, nodal status or pathological
differentiation grade (Table I).
Additionally, we detected upregulated c-Met in HIOEC and the OSCC
cell lines (HN30 and CAL-27) by RT-PCR and western blot analysis,
as shown in Fig. 1B. Thus, the
increased expression of c-Met in OSCC tissues and cell lines
revealed that c-Met may be functionally important in the
progression of human OSCC.
JNJ inhibits the effects of HGF on cancer cell
viability, migration, and anti-apoptosis. Since the HGF/c-Met
signaling pathway can mediate tumor growth, migration, invasion,
and survival (6,7), we first sought to assess the effect of
JNJ on tumor cell viability. JNJ is a small-molecule
ATP-competitive inhibitor of the catalytic activity of c-Met, which
exhibited ~600-fold selectivity for c-Met compared with a panel of
~250 diverse tyrosine and serine-threonine kinases. It was also
found to potently inhibit HGF-stimulated and constitutively
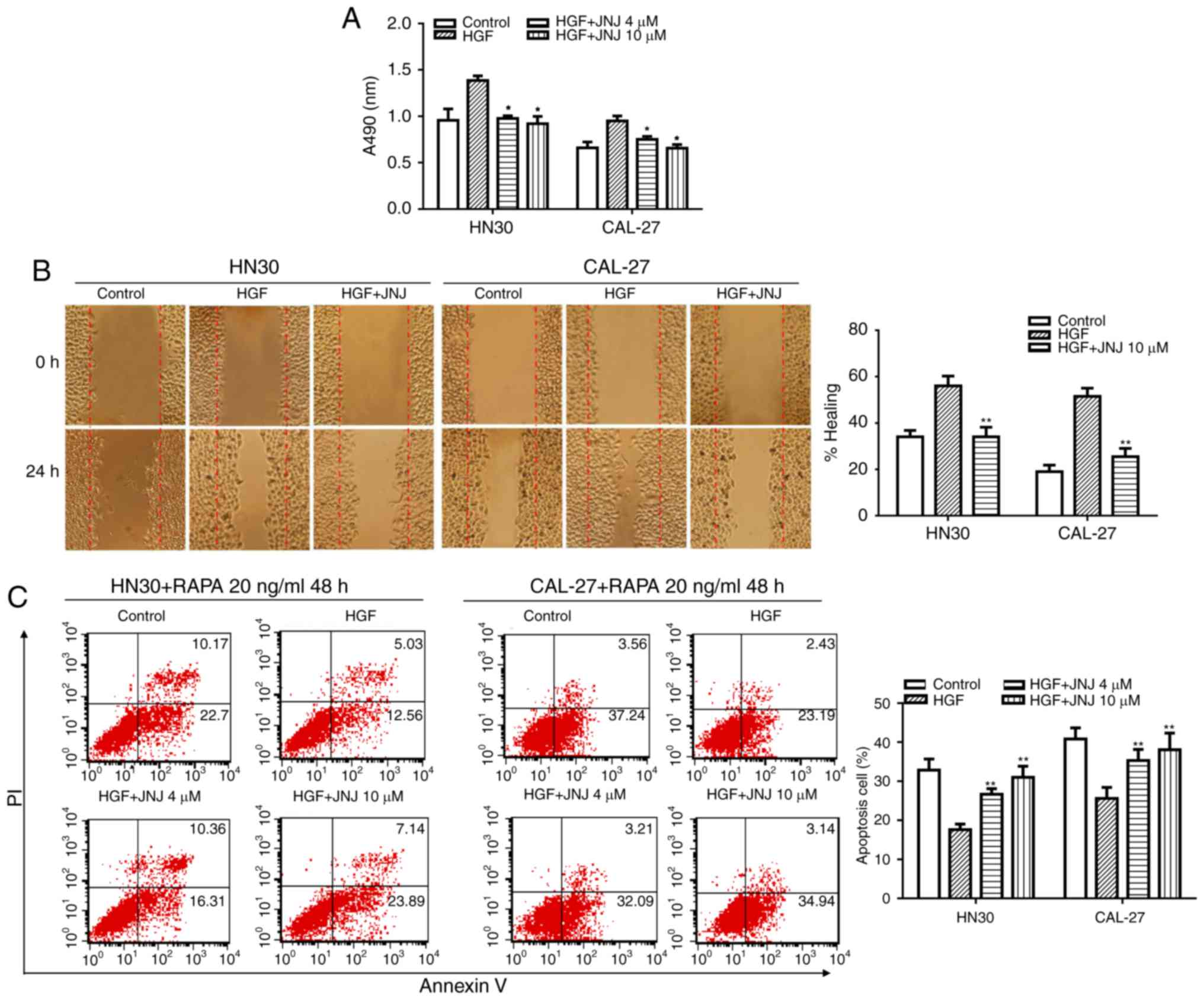
activated c-Met phosphorylation (17). HN30 and CAL-27 cells were treated
with or without JNJ for 2 h prior to treatment with HGF, and an MTS
cell viability assay was performed subsequently. The results
revealed that JNJ could significantly reverse the stimulatory
effect of HGF on cancer cell viability (Fig. 2A). Next, the effect of JNJ on the
migration of HN30 and CAL-27 cells was characterized. In a wound
healing assay, it was observed that JNJ (10 µM) could significantly
suppress HGF-induced migration of the tumor cells (Fig. 2B).
We also evaluated the effect of JNJ on c-Met
signaling in the regulation of cancer cell death. HN30 and CAL-27
cells were treated with JNJ for 2 h, and then with combinations of
HGF and RAPA. After treatment for 48 h, the cells were analyzed by
flow cytometry to evaluate the apoptotic index. The results
revealed that HGF could significantly rescue RAPA-induced HN30 cell
apoptosis when compared with the control group. Notably, the
percentage of RAPA-induced apoptotic cells was decreased from 32.87
to 17.59% following HGF stimulation of the cancer cells. In
addition, pretreatment with JNJ could markedly supress the effect
of HGF. Thus, following JNJ pre-treatment, the percentage of
apoptotic cells increased from 17.59 to 26.91% for the RAPA-induced
+ HGF-treated HN30 cells (Fig. 2C).
The same phenomenon was observed in CAL-27 cells. Altogether, our
results indicated that JNJ may play an important role in inhibiting
the pro-proliferation, pro-migration, and anti-apoptotic effects of
HGF in human OSCC cell lines.
JNJ inhibits tumor development in
vivo
JNJ displayed excellent oral bioavailability
approaching 100% in all examined species and JNJ in a single dose
was observed to inhibit Met phosphorylation in tumor xenografts for
up to 16 h (18). Finally, the
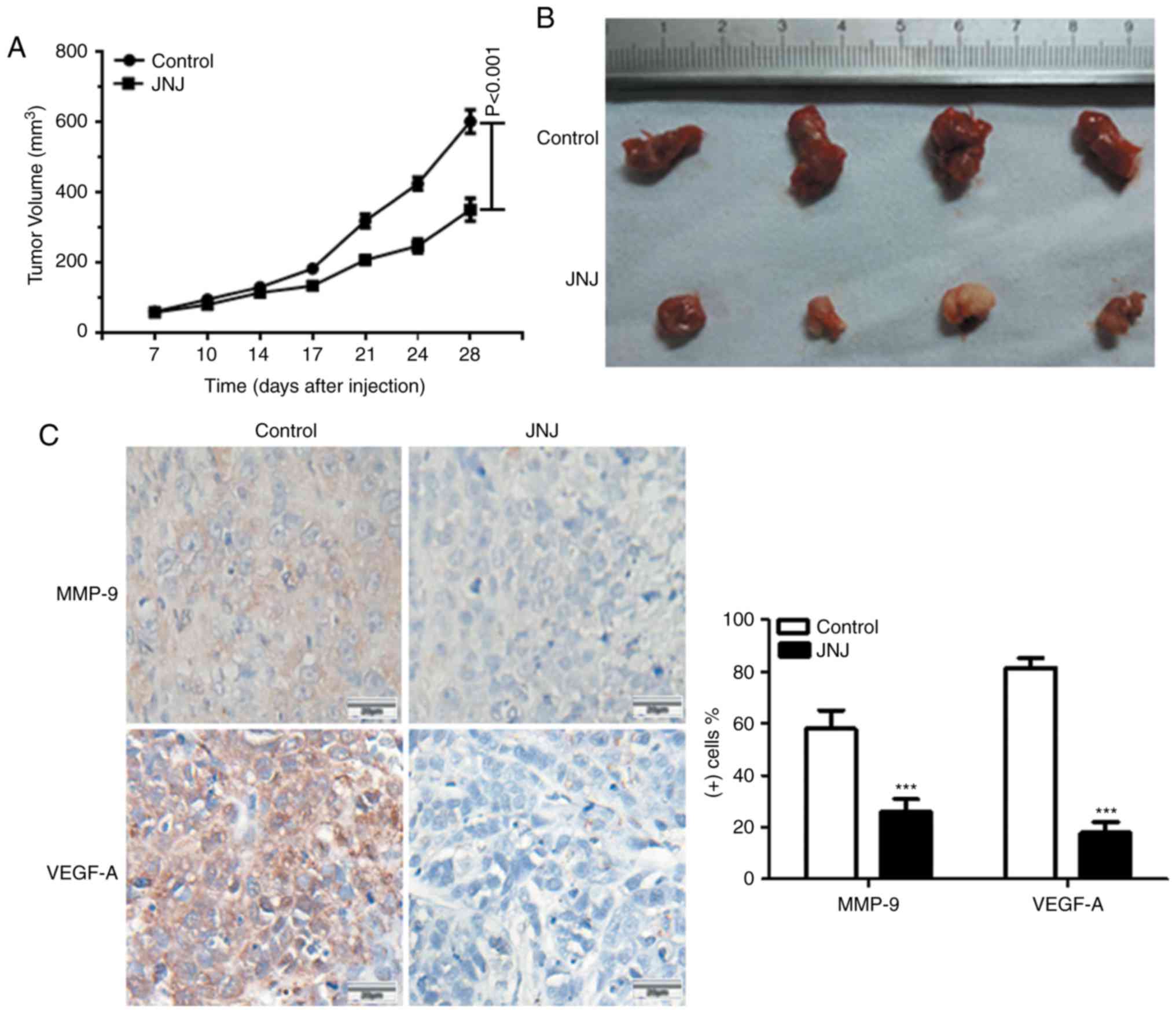
therapeutic efficacy of JNJ was explored in mice in vivo.
Following CAL-27 cell xenografts, the mice were treated with or
without JNJ (10 mg/kg). As shown in Fig. 3A and B, JNJ treatment significantly
reduced the tumor size when compared with the control group
(P<0.001). We also found that JNJ could reduce the expression of
VEGF-A and MMP-9, two key proteins related to angiogenesis and
migration, which indicated its ability to inhibit cancer cell
angiogenesis, migration, and invasion in vivo (Fig. 3C). These data collectively indicated
the potency of JNJ in inhibiting tumor growth, angiogenesis, and
migration in vivo.
JNJ inhibits HGF-mediated upregulation
of c-Met downstream molecules
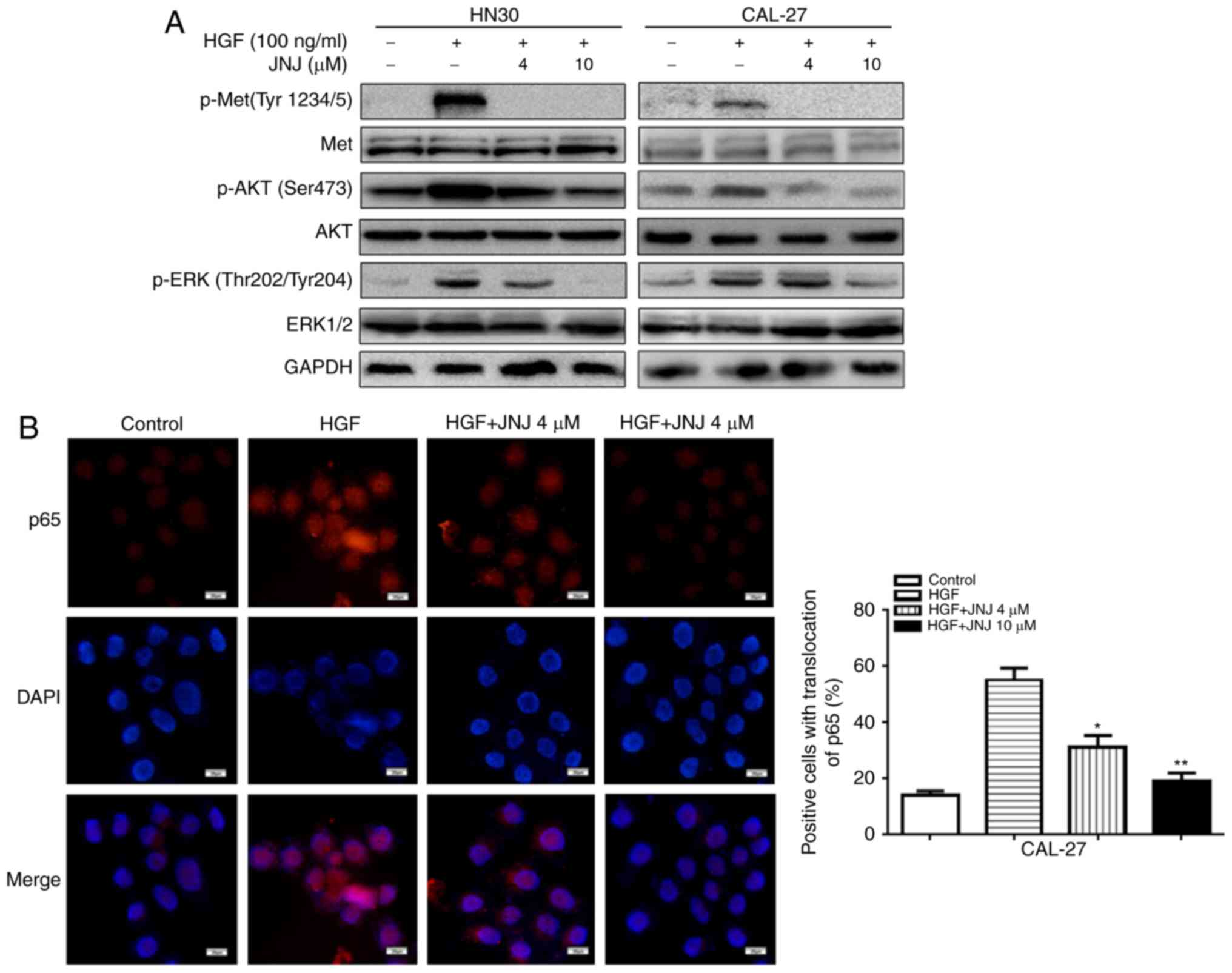
The PI3K/AKT and ERK signaling pathways act
downstream of c-Met signaling, and thus we examined the effects of
JNJ on these downstream pathways in HN30 and CAL-27 cells. Western
blot analysis revealed that the HGF-mediated upregulation of
p-c-Met in the cancer cells was abolished by JNJ treatment
(Fig. 4A). In addition,
upregulation of p-AKT was also observed in the cancer cells in
response to HGF stimulation, which was also inhibited by treatment
with JNJ. Similarly, HGF-mediated upregulation of p-ERK1/2 was
inhibited by JNJ. Meanwhile, in CAL-27 cells immunofluorescence
staining indicated that there was a significant increase in the
nuclear translocation of the NF-κB subunit p65 after HGF
stimulation (Fig. 4B), which could
also be significantly inhibited by JNJ treatment (4 and 10 µM).
These results revealed that the AKT, ERK1/2, and NF-κB p65 pathways
play essential roles in the growth, migration, and apoptosis of
OSCC cells, possibly mediating the effects of JNJ on the inhibition
of OSCC progression after targeting c-Met.
Cigarette smoking is considered to be among the
major risk factors for OSCC (19).
Additionally, cigarette smoking has been reported to induce
overexpression of c-Met and HGF (20,21).
Collectively, with our results, these data revealed that c-Met
expression may mediate smoking-induced OSCC progression. Therefore,
inhibition of c-Met may be an effective strategy for preventing the
progression of OSCC.
Discussion
It is well established that cigarette smoking is
among the major factors that induce oral squamous cell carcinoma
(OSCC) (19), and cigarette smokers
have been reported to have a 2–5 times greater risk of developing
oral cancer than non-smokers. Furthermore, the risk of oral cancer
increases with the number of cigarettes smoked and the duration of
smoking (20). In cigarette
smokers, overexpression of c-Met has been found to be induced in
microvessels of oral lichen planus, and the majority of smoker
samples exhibit c-Met-positive expression (21). Additionally, it was reported that
nicotine can induce HGF overexpression in lung cancer tissues, as
well as in type II normal pneumocytes, while overexpression of
c-Met was frequently detected in adenocarcinoma cells, and nicotine
can enhance the pro-migratory effect of HGF on lung cancer cells,
thus possibly contributing to lung cancer progression (22,23).
A previous study on head and neck squamous cell
carcinoma (HNSCC) demonstrated that c-Met-positive cells had cancer
stem cell properties and could resist the effect of cisplatin
(24). c-Met was upregulated and
functional in 90% of HNSCC cell lines and 84% of patient samples
(25,26). It has also been suggested that the
HGF/c-Met signaling pathway is associated with the progression and
invasive behavior of OSCC (27–29).
The length of survival was significantly reduced in oral tongue
carcinoma patients with c-Met expression compared to those without
c-Met expression (30). Activation
of HGF/c-Met was critical for enhanced proliferation, invasion, and
metastasis in HNSCC, which was correlated with decreased survival,
increased recurrence rates and poor patient prognosis (31–33).
c-Met knockdown in OSCC cell lines reduced cervical lymph node
spread and increased survival of mice in an orthotopic animal model
(34). However, the relationship
between the HGF/c-Met signaling pathway and the progression of OSCC
is not fully understood. It is essential to understand the
molecular mechanism so as to aid clinical intervention.
In the present study, we examined the degree of
c-Met expression in OSCC tissues and its potential correlation with
clinicopathological parameters. We found that 60.0% (24/40) of the
OSCC tissues exhibited a high level of c-Met expression, whereas
only 25% (5/20) of the normal oral epithelial tissues expressed
c-Met. This result revealed that the c-Met protein was produced in
the majority of OSCC cases. However, we found that there were no
significant correlations between c-Met expression and
clinicopathological variables, such as tobacco use, alcohol
consumption, sex, tumor site, tumor stage, nodal status or
pathological differentiation grade (Table I). Nevertheless, the aberrant
expression of c-Met in OSCC samples compared with normal tissues
revealed that it may play an important role in the progression of
human OSCC. Cigarette smoking can induce overexpression of c-Met,
which may in turn result in OSCC. However, there was no significant
correlation between c-Met expression and cigarette smoking. This
may be a limitation of the present study, as only 40 specimens of
OSCC were investigated, and thus a larger number of specimens
should be investigated in future studies.
Overexpression of c-Met has been reported in many
types of cancer, and potentially leads to aberrant signaling
associated with cancer development and progression (35,36).
Activation of the HGF/c-Met signaling pathway promotes tumor cell
proliferation, migration, and invasion and tumor angiogenesis, and
was associated with poor prognosis (37–39).
HGF can promoted tumor angiogenesis and reverse suspension-induced
apoptosis (anoikis), which in turn, can increase not only tumor
growth, but also tumor invasion and metastasis (40–44).
To further investigate the role of the HGF/c-Met signaling pathway
in the progression of OSCC, in vitro experiments were
carried out in the present study. We found that c-Met was
overexpressed in two OSCC cell lines (Fig. 1B), indicating that OSCC cell lines
may obtain a growth advantage by upregulating c-Met. Exposure to a
selective c-Met inhibitor, JNJ, had substantial effects on cell
viability, migration, and apoptosis following HGF stimulation of
the OSCC cell lines (Fig. 2A-C).
HGF provides anoikis resistance to HNSCC cells, and anoikis
resistance plays an important role in tumor progression and
metastasis (41). Our results
revealed that HGF can prolong cancer cell survival and promote
metastasis by inhibiting apoptosis. Our findings demonstrated that
OSCC cell lines can be stimulated to grow by HGF stimulation, and
thus c-Met may be aberrantly activated in the progression of
OSCC.
HGF can induce c-Met phosphorylation, which in turn
activates multiple downstream pathways, including the PI3K/AKT and
MAPK/ERK signaling pathways (6,40).
c-Met inhibitors have demonstrated antitumor efficacy in
preclinical studies and are currently being evaluated in human
cancer clinical trials (45,46).
In the present study, the selective c-Met inhibitor JNJ exerted
antitumor effects on OSCC cell growth potentially by blocking
activation of AKT, ERK, and NF-κB p65 (Fig. 4A and B). These data indicated that
activation of the HGF/c-Met system may stimulate cancer cell
survival and growth through the ERK, PI3K/AKT, and NF-κB signaling
pathways.
In summary, our results ascetaoned that JNJ can
inhibit HGF-stimulated cell viability and migration, and further
confirmed the potent opposing activity of JNJ against the
anti-apoptotic effect of HGF. These findings indicated the
potential role of c-Met in the progression of OSCC. The HGF/c-Met
system functions as a potent pro-growth signal that exacerbates the
malignant progression of OSCC. Thus, c-Met inhibition is considered
to exert a potent pro-apoptotic effect as part of its direct impact
on OSCC. Accordingly, our data revealed that JNJ inhibited
HGF-induced survival of the OSCC cell lines in vitro. In
particular, JNJ markedly inhibited the pro-proliferative,
pro-migration, and anti-apoptotic effects of HGF by inhibiting the
c-Met pathway. JNJ also inhibited tumor growth, angiogenesis, and
migration in OSCC xenografts (Fig.
3A-C). The expression of Ki-67 related to tumor proliferation
was also analyzed. However, for some reason, there were no
differences between the control and JNJ-treated group. In addition,
immunohistochemical staining of human OSCC tissues revealed high
expression of c-Met. Therefore, we have not only demonstrated the
pro-proliferative, pro-promigratory, and anti-apoptotic activity of
HGF/c-Met signaling in OSCC cells, but also provided solid data on
the possible molecular mechanisms mediating such activity, which
may serve important roles in the progression of OSCC. Therefore,
c-Met may be an important target for the development of new
therapeutic approaches in the treatment of OSCC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81001205, 81202132, 81472179,
81500371) and the Three-year Planning for Strengthening the
Construction of Public Health System in Shanghai (2015–2017)
(15GWZK0301).
References
|
1
|
Kademani D: Oral cancer. Mayo Clin Proc.
82:pp. 878–887. 2007; View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petersen PE: The World Oral Health Report
2003: Continuous improvement of oral health in the 21st century -
the approach of the WHO Global Oral Health Programme. Community
Dent Oral Epidemiol. 31 Suppl 1:3–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lothaire P, de Azambuja E, Dequanter D,
Lalami Y, Sotiriou C, Andry G, Castro G Jr and Awada A: Molecular
markers of head and neck squamous cell carcinoma: Promising signs
in need of prospective evaluation. Head Neck. 28:256–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cecchi F, Rabe DC and Bottaro DP:
Targeting the HGF/Met signalling pathway in cancer. Eur J Cancer.
46:1260–1270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Birchmeier C, Birchmeier W, Gherardi E and
Woude GF Vande: Met, metastasis, motility and more. Nat Rev Mol
Cell Biol. 4:915–925. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trusolino L and Comoglio PM:
Scatter-factor and semaphorin receptors: Cell signalling for
invasive growth. Nat Rev Cancer. 2:289–300. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maulik G, Shrikhande A, Kijima T, Ma PC,
Morrison PT and Salgia R: Role of the hepatocyte growth factor
receptor, c-Met, in oncogenesis and potential for therapeutic
inhibition. Cytokine Growth Factor Rev. 13:41–59. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laterra J, Nam M, Rosen E, Rao JS, Lamszus
K, Goldberg ID and Johnston P: Scatter factor/hepatocyte growth
factor gene transfer enhances glioma growth and angiogenesis in
vivo. Lab Invest. 76:565–577. 1997.PubMed/NCBI
|
|
10
|
Mhawech-Fauceglia P, Afkhami M and Pejovic
T: MET/HGF signaling pathway in ovarian carcinoma: Clinical
implications and future direction. Pathol Res Int. 2012:9603272012.
View Article : Google Scholar
|
|
11
|
Chu JS, Ge FJ, Zhang B, Wang Y, Silvestris
N, Liu LJ, Zhao CH, Lin L, Brunetti AE, Fu YL, et al: Expression
and prognostic value of VEGFR-2, PDGFR-β, and c-Met in advanced
hepatocellular carcinoma. J Exp Clin Cancer Res. 32:16–23. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Landi L, Minuti G, D'Incecco A and
Cappuzzo F: Targeting c-MET in the battle against advanced
nonsmall-cell lung cancer. Curr Opin Oncol. 25:130–136. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li C, Wu JJ, Hynes M, Dosch J, Sarkar B,
Welling TH, di Magliano M Pasca and Simeone DM: c-Met is a marker
of pancreatic cancer stem cells and therapeutic target.
Gastroenterology. 141:2218–2227.e5. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herreros-Villanueva M, Zubia-Olascoaga A
and Bujanda L: c-Met in pancreatic cancer stem cells: Therapeutic
implications. World J Gastroenterol. 18:5321–5323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Liu JH, Chai K, Tashiro S, Onodera
S and Ikejima T: Inhibition of c-Met promoted apoptosis, autophagy
and loss of the mitochondrial transmembrane potential in
oridonin-induced A549 lung cancer cells. J Pharm Pharmacol.
65:1622–1642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Z, Hu S, Luo Q, Ye D, Hu D and Chen F:
Overexpression of SENP3 in oral squamous cell carcinoma and its
association with differentiation. Oncol Rep. 29:1701–1706. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Perera T, Lavrijssen T, Janssens B, Geerts
T, King P, Mevellec L, Cummings M, Lu T, Johnson D and Page M:
JNJ-38877605: A selective Met kinase inhibitor inducing regression
of Met-driven tumor models. Presented at the 99th AACR Annual
Meeting. Apr 12–16, 2008; San Diego, CA, USA. pp. 4837
|
|
18
|
Torti D, Sassi F, Galimi F, Gastaldi S,
Perera T, Comoglio PM, Trusolino L and Bertotti A: A preclinical
algorithm of soluble surrogate biomarkers that correlate with
therapeutic inhibition of the MET oncogene in gastric tumors. Int J
Cancer. 130:1357–1366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weiner D, Khankin EV, Levy Y and Reznick
AZ: Effects of cigarette smoke borne reactive nitrogen species on
salivary alpha-amylase activity and protein modifications. J
Physiol Pharmacol. 60 Suppl 5:127–132. 2009.PubMed/NCBI
|
|
20
|
Wald NJ and Hackshaw AK: Cigarette
smoking: An epidemiological overview. Br Med Bull. 52:3–11. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kłosek SK, Sporny S, Stasikowska-Kanicka O
and Kurnatowska AJ: Cigarette smoking induces overexpression of
c-Met receptor in microvessels of oral lichen planus. Arch Med Sci.
7:706–712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen JT, Lin TS, Chow KC, Huang HH, Chiou
SH, Chiang SF, Chen HC, Chuang TL, Lin TY and Chen CY: Cigarette
smoking induces overexpression of hepatocyte growth factor in type
II pneumocytes and lung cancer cells. Am J Respir Cell Mol Biol.
34:264–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoneyama R, Aoshiba K, Furukawa K, Saito
M, Kataba H, Nakamura H and Ikeda N: Nicotine enhances hepatocyte
growth factor-mediated lung cancer cell migration by activating the
α7 nicotine acetylcholine receptor and phosphoinositide
kinase-3-dependent pathway. Oncol Lett. 11:673–677. 2016.PubMed/NCBI
|
|
24
|
Sun S and Wang Z: Head neck squamous cell
carcinoma c-Met+ cells display cancer stem cell properties and are
responsible for cisplatin-resistance and metastasis. Int J Cancer.
129:2337–2348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seiwert TY, Jagadeeswaran R, Faoro L,
Janamanchi V, Nallasura V, El Dinali M, Yala S, Kanteti R, Cohen
EE, Lingen MW, et al: The MET receptor tyrosine kinase is a
potential novel therapeutic target for head and neck squamous cell
carcinoma. Cancer Res. 69:3021–3031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Herdt MJ and de Jong RJ Baatenburg: HGF
and c-MET as potential orchestrators of invasive growth in head and
neck squamous cell carcinoma. Front Biosci. 13:2516–2526. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Freudlsperger C, Alexander D, Reinert S
and Hoffmann J: Prognostic value of c-Met expression in oral
squamous cell carcinoma. Exp Ther Med. 1:69–72. 2010.PubMed/NCBI
|
|
28
|
Hanzawa M, Shindoh M, Higashino F, Yasuda
M, Inoue N, Hida K, Ono M, Kohgo T, Nakamura M, Notani K, et al:
Hepatocyte growth factor upregulates E1AF that induces oral
squamous cell carcinoma cell invasion by activating matrix
metalloproteinase genes. Carcinogenesis. 21:1079–1085. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murai M, Shen X, Huang L, Carpenter WM,
Lin CS, Silverman S, Regezi J and Kramer RH: Overexpression of
c-met in oral SCC promotes hepatocyte growth factor-induced
disruption of cadherin junctions and invasion. Int J Oncol.
25:831–840. 2004.PubMed/NCBI
|
|
30
|
Kim CH, Koh YW, Han JH, Kim JW, Lee JS,
Baek SJ, Hwang HS and Choi EC: c-Met expression as an indicator of
survival outcome in patients with oral tongue carcinoma. Head Neck.
32:1655–1664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lim YC, Kang HJ and Moon JH: C-Met pathway
promotes self-renewal and tumorigenecity of head and neck squamous
cell carcinoma stem-like cell. Oral Oncol. 50:633–639. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hartmann S, Bhola NE and Grandis JR:
HGF/Met signaling in head and neck cancer: Impact on the tumor
microenvironment. Clin Cancer Res. 22:4005–4013. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lim YC, Han JH, Kang HJ, Kim YS, Lee BH,
Choi EC and Kim CH: Overexpression of c-Met promotes invasion and
metastasis of small oral tongue carcinoma. Oral Oncol.
48:1114–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tao X, Hill KS, Gaziova I, Sastry SK, Qui
S, Szaniszlo P, Fennewald S, Resto VA and Elferink LA: Silencing
Met receptor tyrosine kinase signaling decreased oral tumor growth
and increased survival of nude mice. Oral Oncol. 50:104–112. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yao HP, Zhou YQ, Zhang R and Wang MH:
MSP-RON signalling in cancer: Pathogenesis and therapeutic
potential. Nat Rev Cancer. 13:466–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma PC, Tretiakova MS, Nallasura V,
Jagadeeswaran R, Husain AN and Salgia R: Downstream signalling and
specific inhibition of c-MET/HGF pathway in small cell lung cancer:
Implications for tumour invasion. Br J Cancer. 97:368–377. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu X, Newton RC and Scherle PA:
Developing c-MET pathway inhibitors for cancer therapy: Progress
and challenges. Trends Mol Med. 16:37–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matsumoto K and Nakamura T: Hepatocyte
growth factor and the Met system as a mediator of tumor-stromal
interactions. Int J Cancer. 119:477–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
You WK and McDonald DM: The hepatocyte
growth factor/c-Met signaling pathway as a therapeutic target to
inhibit angiogenesis. BMB Rep. 41:833–839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zeng Q, Chen S, You Z, Yang F, Carey TE,
Saims D and Wang CY: Hepatocyte growth factor inhibits anoikis in
head and neck squamous cell carcinoma cells by activation of ERK
and Akt signaling independent of NFkappa B. J Biol Chem.
277:25203–25208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zeng Q, McCauley LK and Wang CY:
Hepatocyte growth factor inhibits anoikis by induction of activator
protein 1-dependent cyclooxygenase-2. Implication in head and neck
squamous cell carcinoma progression. J Biol Chem. 277:50137–50142.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rosen EM and Goldberg ID: Scatter factor
and angiogenesis. Adv Cancer Res. 67:257–279. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dong G, Chen Z, Li ZY, Yeh NT, Bancroft CC
and Van Waes C: Hepatocyte growth factor/scatter factor-induced
activation of MEK and PI3K signal pathways contributes to
expression of proangiogenic cytokines interleukin-8 and vascular
endothelial growth factor in head and neck squamous cell carcinoma.
Cancer Res. 61:5911–5918. 2001.PubMed/NCBI
|
|
44
|
Michi Y, Morita I, Amagasa T and Murota S:
Human oral squamous cell carcinoma cell lines promote angiogenesis
via expression of vascular endothelial growth factor and
upregulation of KDR/flk-1 expression in endothelial cells. Oral
Oncol. 36:81–88. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sequist LV, von Pawel J, Garmey EG,
Akerley WL, Brugger W, Ferrari D, Chen Y, Costa DB, Gerber DE,
Orlov S, et al: Randomized phase II study of erlotinib plus
tivantinib versus erlotinib plus placebo in previously treated
non-small-cell lung cancer. J Clin Oncol. 29:3307–3315. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Smith DC, Smith MR, Sweeney C, Elfiky AA,
Logothetis C, Corn PG, Vogelzang NJ, Small EJ, Harzstark AL, Gordon
MS, et al: Cabozantinib in patients with advanced prostate cancer:
Results of a phase II randomized discontinuation trial. J Clin
Oncol. 31:412–419. 2013. View Article : Google Scholar : PubMed/NCBI
|