Introduction
Hepatocellular carcinoma (HCC) is one of the most
common cancers worldwide and the third most common cause of
cancer-related death (1,2). It is particularly prevalent in Asia
and sub-Saharan Africa countries (3,4). A
progressive increase in HCC-related mortality has been observed in
the US and Western Europe (5–7).
Accumulating evidence has revealed that inflammatory-related
cytokines participate in the carcinogenesis and progression of HCC
(8). Research has revealed that
higher expression of interleukin-32 (IL-32), a novel
pro-inflammatory cytokine, is detected in HCC (9). However, the potential roles of IL-32
in the carcinogenesis and progression of HCC remain unclear.
IL-32, originally called natural killer (NK) cell
transcript 4, is a recently described cytokine that is mainly
produced by T, NK and epithelial cells after stimulation (10,11).
Six splice variants have been reported in the IL-32 family,
including IL-32α, IL-32β, IL-32δ, IL-32γ, IL- 32ε and IL-32ζ
(12). Besides its pluripotent
pro-inflammatory properties, it has been unambiguously shown that
IL-32α enhances the migration and invasion of cancers, such as
breast cancer, gastric cancer and lung cancer (13–15).
However, the function and role of IL-32α in HCC progression remain
unknown.
The present study explored the expression of IL-32α
in HCC and its role in vascular invasion and tumor progression.
Mechanistic investigation was conducted to show the potential
downstream factor in the IL-32 signaling pathway. The results
suggested a specific mechanism of IL-32 in HCC and present a
potential therapeutic target for HCC treatment and drug
development.
Materials and methods
Patients and tissue specimens
Tumor tissues and paired non-cancerous hepatic
parenchyma were collected from 100 patients with primary HCC who
received surgical resection from May 2010 to June 2011 at the
Department of Hepatobiliary Surgery, Shandong Provincial Hospital
Affiliated to Shandong University. Serum specimens were collected
from the patients and 30 control patients without HCC. None of the
patients had received preoperative chemotherapy or other treatment
before surgery. Patient written informed consent was obtained, and
the study protocol was approved by the Health Service Ethics
Committee of Shandong Provincial Hospital Affiliated to Shandong
University. HCC was histologically diagnosed by two pathologists
independently and the clinical characteristics of each patient were
recorded as shown in Table I.
 | Table I.Correlations between serum IL-32
expression and clinicopathological parameters in 100 patients with
HCC. |
Table I.
Correlations between serum IL-32
expression and clinicopathological parameters in 100 patients with
HCC.
| Clinicopathological
parameters | Cases | T/Na (mean ± SE) | P-value |
|---|
| Age (years) |
|
| 0.312b |
|
<60 | 72 |
16.49±9.10 |
|
|
≥60 | 28 |
13.50±3.03 |
|
| Sex |
|
| 0.184b |
|
Male | 80 |
15.78±4.88 |
|
|
Female | 20 |
13.56±3.20 |
|
| Virus |
|
|
|
|
HBV | 54 |
14.48±3.56 | 0.271c |
|
HCV | 6 |
14.31±4.96 |
|
|
None | 40 |
16.64±5.72 |
|
| AFP (ng/ml) |
|
| 0.157b |
|
<20 | 30 |
13.89±3.33 |
|
|
≥20 | 70 |
15.96±5.02 |
|
| Tumor
multiplicity |
|
| 0.249b |
|
Single | 90 |
15.07±4.70 |
|
|
Multiple | 10 |
17.69±3.72 |
|
| Tumor size
(cm) |
|
| 0.460b |
|
<3.5 | 46 |
14.80±4.03 |
|
|
≥3.5 | 54 |
15.79±5.15 |
|
|
Differentiation |
|
| 0.798c |
|
Well | 16 |
14.42±3.45 |
|
|
Moderate | 60 |
15.35±5.10 |
|
|
Poor | 24 |
15.91±4.35 |
|
| Liver
cirrhosis |
|
| 0.811b |
|
Yes | 40 |
15.14±3.95 |
|
| No | 60 |
15.47±5.12 |
|
| Vascular
invasion |
|
| 0.007 |
|
Yes | 18 |
19.05±5.99b |
|
| No | 82 |
14.52±3.94 |
|
| Metastasis |
|
| 0.011b |
|
Yes | 24 |
18.26±5.85 |
|
| No | 76 |
14.41±3.84 |
|
Ethical approval
The present study was performed in accordance with
the Declaration of Helsinki and approved by the local Ethics
Committee. All patients provided their informed consent.
Quantitative real-time RT-PCR
Fresh HCC tissues were treated with TRIzol reagent
for total RNA extraction (Invitrogen Carlsbad, CA, USA) and
purified by phenol/CHCl3 according to the manufacturer's
instructions. Total RNA (5 µg) was reversely transcribed to cDNA
using the MBI Fermantas reverse transcription kit (MBI Fermentas,
Vilnius, Lithuania). The Quantitative SYBR-Green PCR kit and ABI
Prism 7000 Sequence Detection System (both from ABI, USA) were
applied to test the expression level of IL-32α under the following
conditions: 30 cycles: 1 cycle at 95°C for 5 min, then 30 cycles at
94°C for 30 sec and 60°C for 45 sec; quantitative RT-PCR was
repeated at least 3 times. β-actin expression was used for
normalization. Primer sequences are listed as follows: IL-32α F,
5′-ACAGTGGCGGCTTATTATGAGGA-3′ and R, 5′-GTTGCCTCGGCACCGTAATC-3′;
β-actin F, 5′-AATGCTTCTAGGCGGACTATGA-3′ and R,
5′-CAAGAAAGGGTGTAACGCAACT-3′.
Western blotting
Five fresh HCC and paired non-cancerous tissues were
lysed by cold RIPA buffer containing protease inhibitor on ice for
30 min, and centrifuged at 12,000 × g at 4°C for 20 min. The
protein concentration was determined using a BCA protein assay kit
(Biocolor Biotech, Shanghai, China). Proteins suspended in loading
buffer were denatured and separated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
electrophoretically transferred onto a polyvinylidene difluoride
(PVDF) membrane (Millipore, Bedford, MA, USA) in transfer buffer at
40 V for 105 min. The membrane was blocked using 5% skimmed milk in
Tris-buffered saline with Tween-20 (TBST) for 2 h, washed with TBST
and incubated with mouse anti-IL-32 antibody (R&D Systems,
Minneapolis, MN, USA) overnight at 4°C. The membrane was incubated
with an anti-mouse horseradish peroxidase-conjugated secondary
antibody (Dako, Glostrup, Denmark) at a dilution of 1:200 at room
temperature for 1 h. Protein bands were visualized by SuperSignal
West Pico Chemiluminescent Substrate kit (Pierce, Rockford, IL,
USA) and exposed using Kodak X-ray film (Kodak, Rochester, NY,
USA). Proteins were re-blotted with anti-GAPDH (Zymed, South San
Francisco, CA, USA) as an internal control.
Immunohistochemical staining
For immunohistochemical analysis, 4-µm tissue
sections were cut from paraffin blocks and baked at 60°C for 2 h
before staining with mouse anti-IL-32α antibody (dilution 1:100;
R&D Systems). Endogenous peroxidase activity was blocked with
3% H2O2 for 30 min. Then tissue sections were
pre-treated in citrate buffer using a water bath for 15 min for
antigen retrieval. Goat serum (1%) was applied to prevent a
non-specific reaction. The primary antibody was incubated overnight
at 4°C. An anti-mouse antibody kit (Jing Mei Biotech, Shanghai,
China) was applied and DAB reaction was performed following the
protocol. Control IgG antibody was used as a negative control.
Histomorphometric analysis was performed by Image-Pro Plus image
analysis system (Media Cybernetics, Inc., Rockville, MD, USA).
Enzyme-linked immununosorbent assay
(ELISA)
A sandwich ELISA was designed for the quantification
of IL-32α in human serum. A 96-well microtiter plate was coated
overnight at 4°C with goat antibody (PAb; R&D Systems) to
IL-32α (1 µg/ml in PBS, 100 µl/well) and rinsed with PBST. The
wells were then coated with 1% BSA solution in PBS. IL-32α standard
samples were prepared using a serial dilution of a recombinant
human IL-32α solution. Samples were grouped into control and HCC.
IL-32α ELISA was carried out according to the manufacturer's
instructions as follows: assay diluent (80 µl) was added in
duplicate to all wells. Each prepared standard dilution (20 µl) was
added to samples and incubated at room temperature.
Biotin-conjugate (100 µl) was added to all wells and incubated at
room temperature. Diluted streptavidin-HRP (100 µl) was added to
all wells and incubated at room temperature. The enzyme reaction
was stopped by quickly pipetting 100 µl of stop solution into each
well. Absorbance of the reaction product was measured at 490 nm on
an ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
Cell culture and siRNA
transfection
HCC cell lines Hu7 and HepG2 were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 100
U/ml penicillin, 100 µg/ml streptomycin, 25 ng/ml amphotericin B,
and 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) at
37°C in a humidified incubator with 5% CO2. For the RNA
interference assay, an siRNA for IL-32α was designed to silence
IL-32α expression in HCC cell lines, Hu7 and HepG2 (Santa Cruz
Biotechnology, Santa Cruz, CA, USA). The cells were transfected
with 40 nM of siRNA using Lipofectamine™ LTX (Invitrogen).
Silencing efficiency was verified by western blot analysis.
Exogenous IL-32α at a similar concentration (500 pg/ml) was added
to the culture medium for the rescue assay.
Detection of invasion and migration by
scratch and Transwell assays
Transfected and control cells were subjected to cell
scratch and Transwell invasion assays. For the scratch assay cells
were seeded into 6-well plates and cultured until reaching
confluence. A wound was created with a sterile pipette tip. The
distance was measured by a Nikon DS-5M Camera System mounted on a
phase-contrast Leitz microscope. Images of the wound were captured
under a phase-contrast microscope at 0, 24 and 48 h. For each
experiment, 5 visual fields and 2 repeated wells were measured with
3 replications.
For the Transwell assay a 24-well Transwell chamber
(8-mm; Millipore) coated with 30 µl Matrigel was used for the
invasion assay. A 100-µl cell suspension was loaded into the upper
Matrigel-coated chamber. DMEM (600 ml) with 10% FBS was added to
the bottom chamber. Cells were then allowed to migrate or invade
for 48 h at 37°C. The cells in the bottom chamber were fixed in
paraformaldehyde and permeabilized in methanol, and then stained
with crystal violet dye. Cell images were obtained under a light
microscope (Leica DM4000 B; Leica Microsystems, Wetzlar,
Germany.
Statistical analysis
The Mann-Whitney U test or Kruskal-Wallis was used
for between-group comparisons, where appropriate, and the
correlation between the results obtained with the two different
analyses was analyzed with the Spearman's test. A paired Student's
t-test was used to compare the differences of IL-32-α mRNA and
protein expression in tumor tissues and non-cancerous tissues. The
correlations of mRNA expression levels were analyzed with Pearson
test. P<0.05 was considered statistically significant. All data
were analyzed with SPSS 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
IL-32α overexpression in HCC
correlates with vascular invasion
In order to investigate the expression pattern of
IL-32α in HCC tissue and its prognostic role in HCC patients, we
examined the IL-32α expression levels in 100 HCC samples. IL-32α
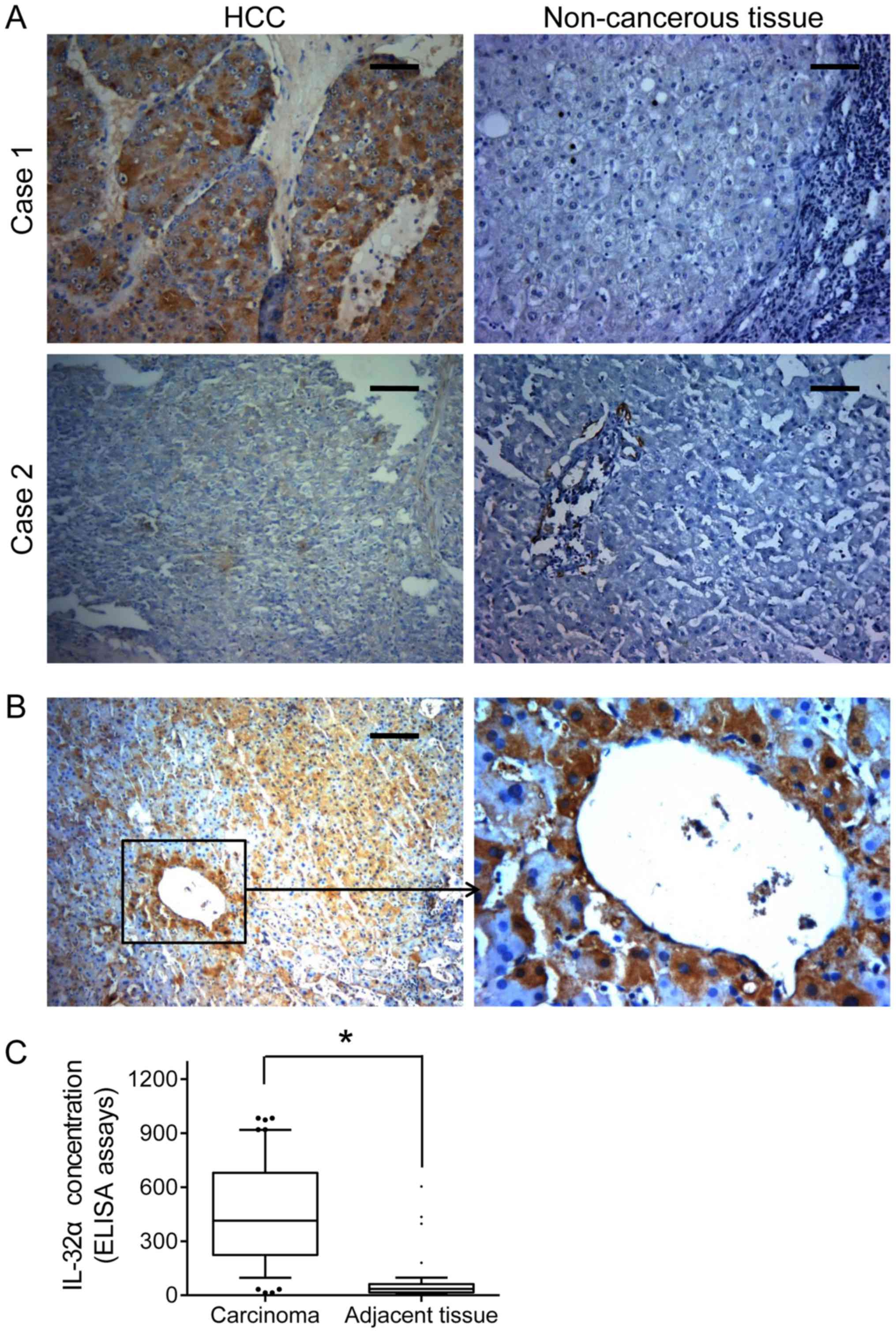
expression was found widely elevated in the HCC tissues, as
compared with that in the paired non-cancerous tissues (Fig. 1A). Moreover, we analyzed the
correlation between IL-32α serum levels of HCC patients and
clinicopathological parameters, including tumor size, virus
infection, liver cirrhosis, vascular invasion and metastasis.
Statistical results revealed that IL-32α was much higher in the
serum samples of patients with distant metastasis than those
without distant metastasis and in patients with vascular invasion
(Table I; P=0.01). Similarly, IHC
staining revealed that high IL-32α expression was often observed in
vessel invasion foci (Fig. 1B).
Importantly, HCC patients showed a higher IL-32α serum
concentration than the controls (571.45±102.28 vs. 144.60±51.172
pg/ml, P=0.007, Fig. 1C). Taken
together, these findings suggest that IL-32α overexpression can
serve as a predictive indicator for distant metastasis and vascular
invasion of HCC patients.
IL-32α promotes migration and invasion
of HCC cells
In order to verify the functions of IL-32α in HCC
in vitro, an siRNA of IL-32α was designed to silence the
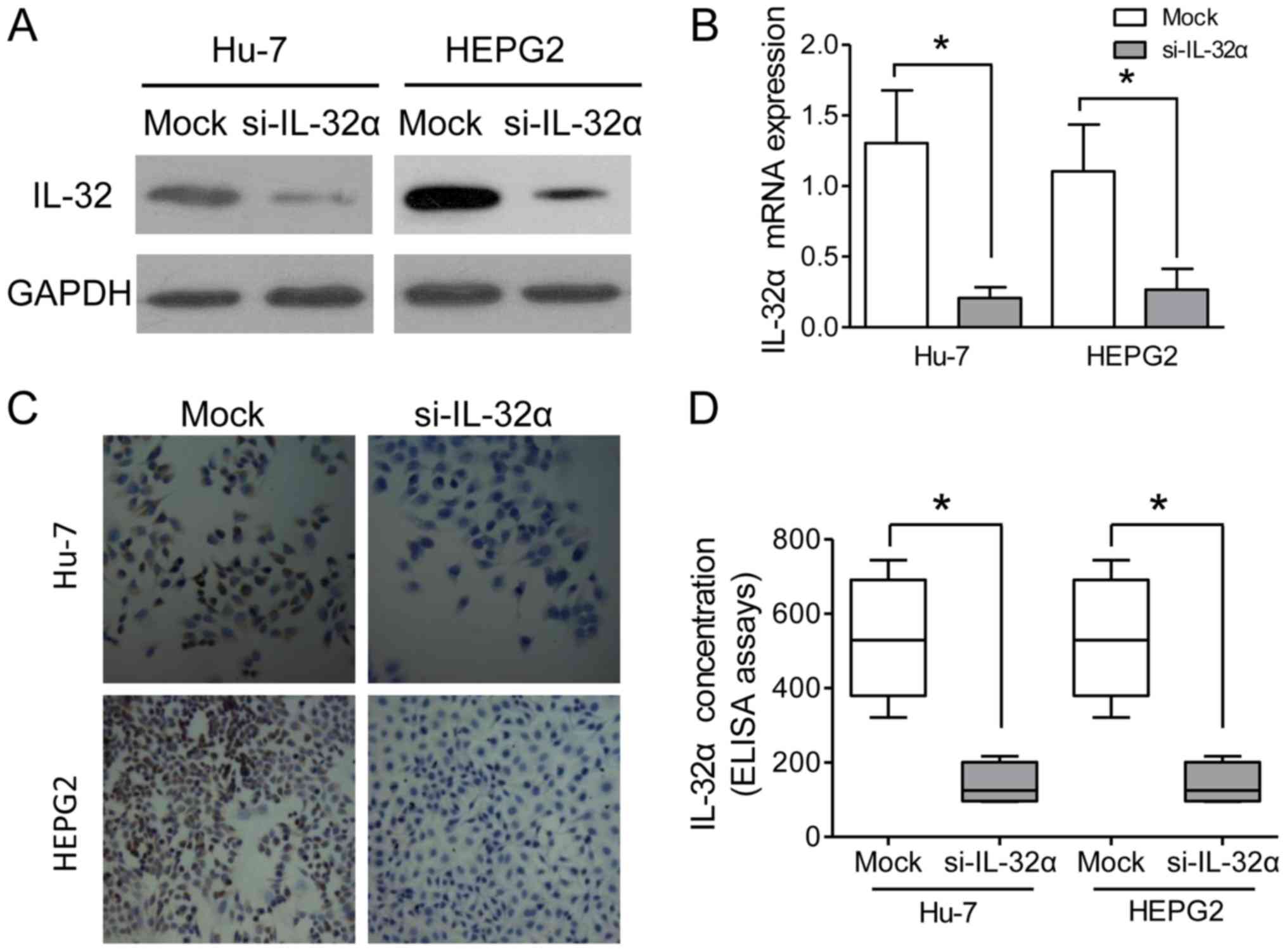
IL-32α expression in HCC cell lines, Hu7 and HepG2. The results
indicated that IL-32α was significantly downregulated in the
si-IL-32α-treated Hu7 and HepG2 cells, as compared with the mock
group (Fig. 2A and B). IHC staining
assays also confirmed the knockdown of IL-32α in the HepG2 Hu7
cells (Fig. 2C). As a secreted
factor, IL-32α was also detected in the culture medium, and its
concentration in the supernatant was decreased after siRNA
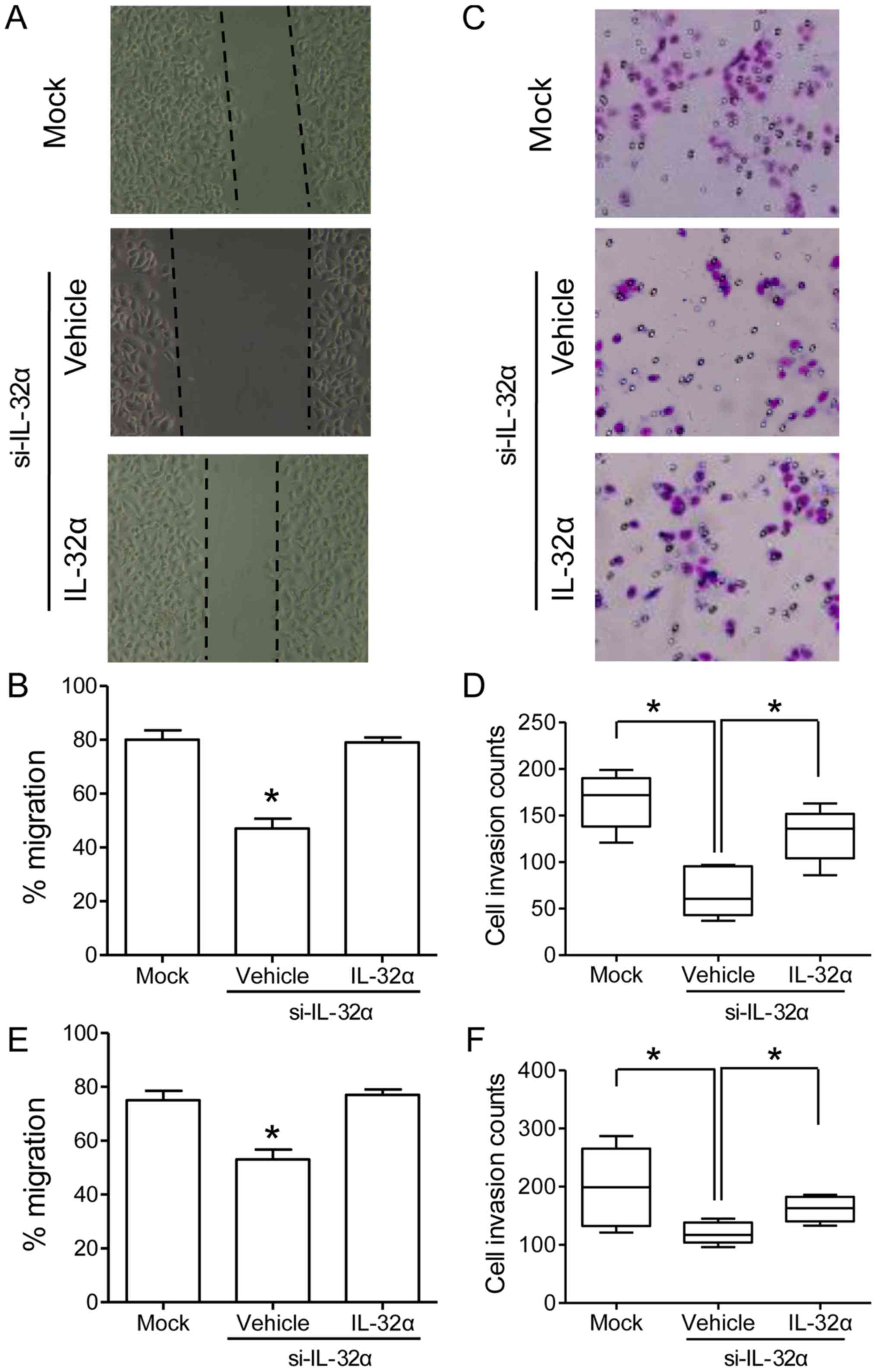
knockdown (Fig. 2D). Cell scratch
and Transwell invasion assays were carried out in order to examine
the cell migration and invasion abilities, respectively. Cell
scratch assays revealed that Hu7-si-IL-32α cells showed sharply
reduced migration ability as compared with that of mock cells
(Fig. 3A and B; P<0.05). For
Transwell invasion assays, Hu7-si-IL-32α cells showed decreased
invasive potential than that of Hu7-mock cells (Fig. 3C and D; P<0.05). Similar results
were also observed in HepG2 cells both in cell scratching and
Transwell invasion assays (Fig. 3E and
F). To further confirm the role of IL-32α in regulating cell
migration and invasion, exogenous IL-32α at a similar concentration
(500 pg/ml) was added to the culture medium. Cell scratch assays
showed that Hu7-si-IL-32α cells restored the migration ability with
IL-32α treatment (Fig. 3A and B;
P<0.05). For Transwell invasion assays, Hu7-si-IL-32α cells also
displayed elevated invasive potential after IL-32α treatment
(Fig. 3C and D; P<0.05). To sum
up, these results indicated that IL-32α could positively regulate
the migration and invasion ability of HCC cells.
IL-32α regulates VEGF in HCC
cells
It has been reported that IL-32α regulates VEGF
levels in breast cancer, which is linked to angiogenesis and tumor
invasion (14,16). In order to test whether IL-32α also
modulates VEGF in HCC cells, we examined the level of VEGF after
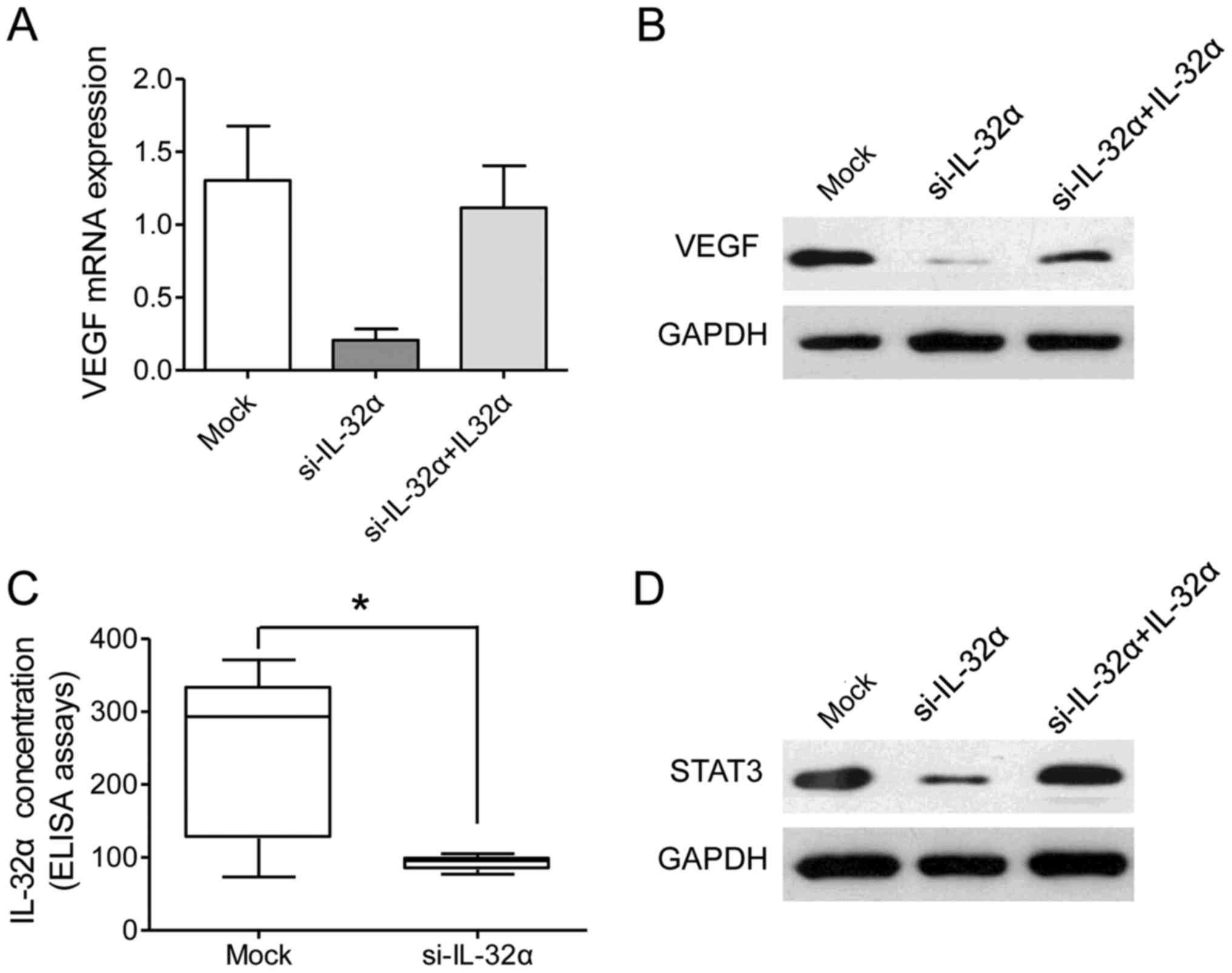
IL-32α was transiently silenced in the Hu7 and HepG2 cell lines.
The present study found that VEGF was significantly reduced after
IL-32α knockdown at both the mRNA level and protein level (Fig. 4A and B). Furthermore, a decreased
VEGF level in the culture medium was observed after IL-32α
knockdown (Fig. 4C; P<0.05).
Collectively, these data showed that VEGF was a downstream response
factor of IL-32α in HCC cells.
Previous reports have revealed that VEGF-STAT3
signaling is important for vascular invasion in a series of tumors
(17,18). In the present study, we provided
further proofs for the correlation between IL-32α and VEGF-STAT3
signaling. STAT3 was significantly reduced in the Hu7-si-IL-32α
cells than that noted in the mock cells (Fig. 4D). However, exogenous IL-32α
treatment increased the level of STAT3 in the Hu7-si-IL-32α cells
(Fig. 4D). Taken together, our data
revealed that the IL-32α/VEGF/STAT3 signaling pathway plays an
essential role in the vascular invasion in HCC.
IL-32α is positively correlated with
VEGF in both HCC tissues and serum
To further confirm the correlation between IL-32α
and VEGF in HCC, VEGF staining was performed on the HCC tissues and
corresponding non-cancerous liver tissues. Our result verified that
VEGF expression levels were in accordance with IL-32α in the HCC
tissues, whereas their expression was low in paired non-cancerous
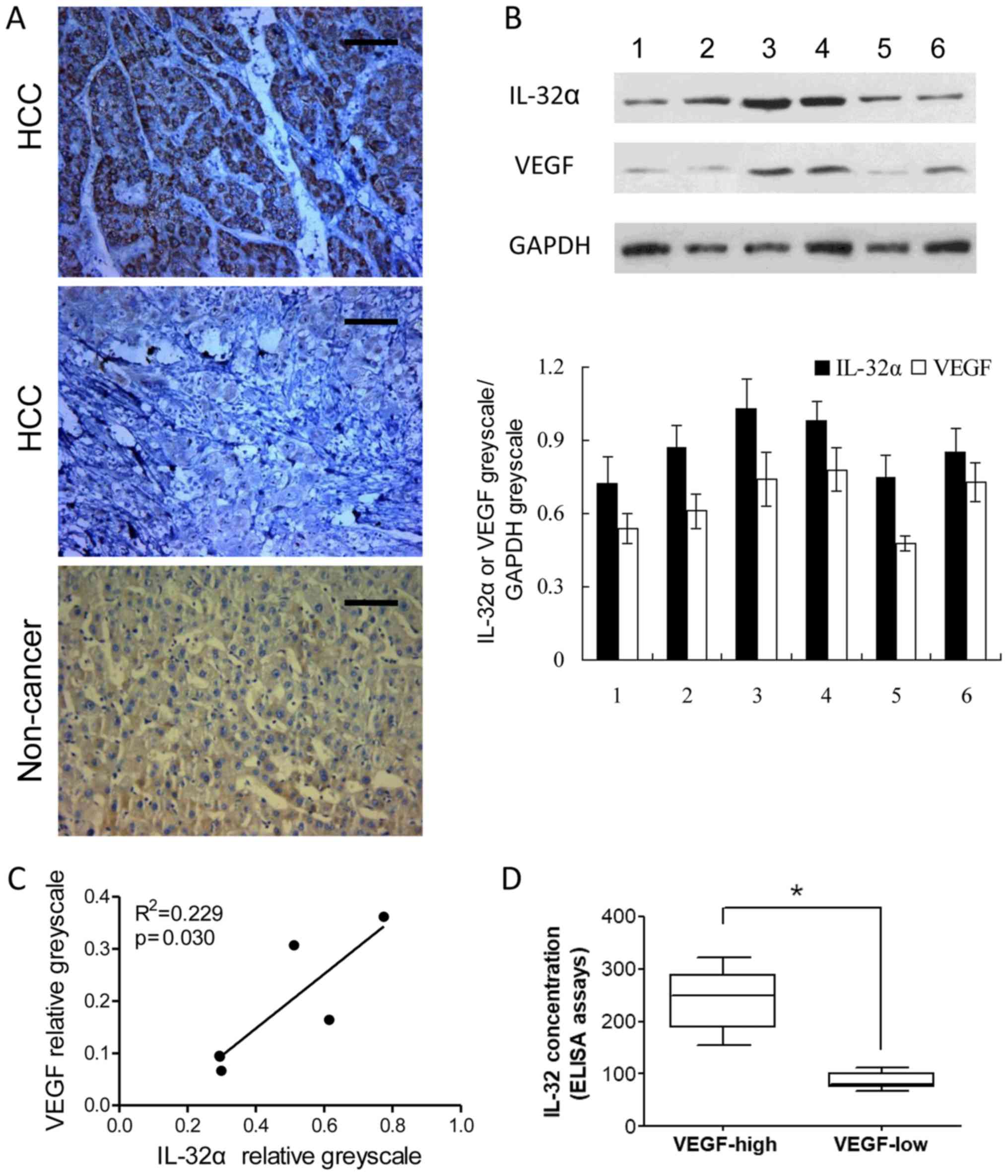
tissues (Fig. 5A). Moreover,
western blotting of IL-32α and VEGF were also conducted in 6 cases
of HCC and corresponding non-cancerous liver tissues. A significant
correlation was also detected between VEGF and IL-32α at the
relative protein level (VEGF greyscale/GAPDH greyscale and IL-32α
greyscale/GAPDH greyscale) (Fig.
5B). Protein level analysis of the western blotting revealed
that IL-32α and VEGF levels were positively correlated (P<0.05;
Fig. 5C). Furthermore, we
investigated IL-32α and VEGF levels in the HCC patient serum
samples. IL-32α and VEGF protein were positively correlated
(Fig. 5D; P<0.05). Taken
together, these data provide further proof that VEGF may serve as a
downstream factor regulated by IL-32α in HCC.
Discussion
In the present study, we investigated the expression
pattern and functions of IL-32α in HCC tissues. We demonstrated
that elevated IL-32α in HCC tissues was correlated with the patient
tumor stage as well as vascular invasion. We also revealed that
silencing of IL-32α in HCC cells impaired the tumor migration and
invasion properties. Importantly, we found that VEGF, an essential
factor for cancer growth, invasion and metastasis, served as a
downstream response of the IL-32α signaling pathway in HCC
cells.
Increasing evidence has confirmed that inflammation
plays a crucial role in liver carcinogenesis. Elevated
inflammatory-related cytokines are commonly observed in the
carcinogenesis and progression of HCC (8,19,20).
IL-32 is known as a pro-inflammatory cytokine since it enhances the
production of IL-1β and TNFα (11,21).
Higher expression of IL-32 in tumor tissues was observed compared
with normal tissue or serum (22).
However, different roles are observed with respect to the tumor
types among the 6 members of the IL-32 family (23,24).
IL-32α exhibits significant effects in human inflammatory disorders
and cancers, and may be involved in the pathogenesis and
progression from inflammation to cancer (25).
IL-32α expression has been observed in a series of
tumor tissues, including gastric (26), breast (16) and esophageal cancer (27). Accumulated evidence indicates that
IL-32α participates in cell proliferation and predicts patient
overall outcome. IL-32α knockdown was found to inhibit cell growth
and induce intrinsic apoptosis by decreasing phospho-p38, MAPK,
NF-κB and Bcl-2, but increasing pro-apoptotic proteins, p53 and
PUMA (19,28). Quite consistent with these studies,
we found that IL-32α was elevated in HCC tissues and associated
with patient metastasis as well as vascular invasion, which was
reported for the first time. In vitro experiments provided
convincing evidence that silencing of IL-32α in HCC cells sharply
reduced the migration and invasion properties of HCC cell lines,
which was correlated with VEGF-STAT3 signaling. Further studies
will be conducted to investigate the functional role of IL-32α in
HCC progression.
For HCC patients, tumor angiogenesis contributes to
a poor therapy response and progression of residual disease
(29). Among the tumor angiogenesis
regulators, VEGF, an essential growth factor for cancer
progression, invasion and metastasis, plays vital roles (30). Previous studies suggest IL-32 as a
critical regulator of endothelial cell functions, which possesses
angiogenic properties (31,32). Secreted VEGF was also found to be
altered along with a change in IL-32α in breast cancer cells
(14,17). Moreover, IL-32α induced VEGF
increased migration and invasion through STAT3 activation, which is
a potential target for HCC therapy (33,34).
We demonstrated that VEGF is a downstream factor for IL-32α
signaling in HCC cells. The detailed mechanism by which IL-32α
regulates VEGF expression requires further investigation.
In conclusion, our findings provide evidence for the
clinical relevance and function of IL-32α in HCC. Elevated IL-32α
in clinical specimens is predictive of tumor metastasis and
vascular invasion in HCC patients, which was correlated with
VEGF/STAT3 signaling, IL-32α is a promising therapeutic target for
HCC treatment or drug development.
Acknowledgements
The authors thank the medical and nursing stuff at
the Department of Hepatobiliary Surgery at Provincial Hospital
Affiliated to Shandong University for providing clinical samples.
The present study was supported by the Key Scientific and Medical
Project of Shandong Province Health Department (2011QZ016), and the
Key Scientific and Medical Project of Taian (2016 NS1076).
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
IHC
|
immuno-histochemistry
|
|
IL-32
|
interleukin-32
|
|
NK
|
natural killer
|
|
VEGF
|
vascular endothelial growth factor
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
ELISA
|
enzyme-linked immununosorbent
assay
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu SJ: A concise review of updated
guidelines regarding the management of hepatocellular carcinoma
around the world: 2010–2016. Clin Mol Hepatol. 22:7–17. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poon D, Anderson BO, Chen LT, Tanaka K,
Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, et al:
Asian Oncology Summit: Management of hepatocellular carcinoma in
Asia: Consensus statement from the Asian Oncology Summit 2009.
Lancet Oncol. 10:1111–1118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bellissimo F, Pinzone MR, Cacopardo B and
Nunnari G: Diagnostic and therapeutic management of hepatocellular
carcinoma. World J Gastroenterol. 21:12003–12021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flores A and Marrero JA: Emerging trends
in hepatocellular carcinoma: Focus on diagnosis and therapeutics.
Clin Med Insights Oncol. 8:71–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petrick JL, Kelly SP, Altekruse SF,
McGlynn KA and Rosenberg PS: Future of hepatocellular carcinoma
incidence in the united states forecast through 2030. J Clin Oncol.
34:1787–1794. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han KQ, He XQ, Ma MY, Guo XD, Zhang XM,
Chen J, Han H, Zhang WW, Zhu QG, Nian H, et al: Inflammatory
microenvironment and expression of chemokines in hepatocellular
carcinoma. World J Gastroenterol. 21:4864–4874. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moschen AR, Fritz T, Clouston AD, Rebhan
I, Bauhofer O, Barrie HD, Powell EE, Kim SH, Dinarello CA,
Bartenschlager R, et al: Interleukin-32: A new proinflammatory
cytokine involved in hepatitis C virus-related liver inflammation
and fibrosis. Hepatology. 53:1819–1829. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Felaco P, Castellani ML, De Lutiis MA,
Felaco M, Pandolfi F, Salini V, De Amicis D, Vecchiet J, Tete S,
Ciampoli C, et al: IL-32: A newly-discovered proinflammatory
cytokine. J Biol Regul Homeost Agents. 23:141–147. 2009.PubMed/NCBI
|
|
11
|
Jeong HJ, Shin SY, Oh HA, Kim MH, Cho JS
and Kim HM: IL-32 up-regulation is associated with inflammatory
cytokine production in allergic rhinitis. J Pathol. 224:553–563.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim S: Interleukin-32 in inflammatory
autoimmune diseases. Immune Netw. 14:123–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khawar MB, Abbasi MH and Sheikh N: IL-32:
A novel pluripotent inflammatory interleukin, towards gastric
inflammation, gastric cancer, and chronic rhino sinusitis.
Mediators Inflamm. 2016:84137682016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park JS, Choi SY, Lee JH, Lee M, Nam ES,
Jeong AL, Lee S, Han S, Lee MS, Lim JS, et al: Interleukin-32β
stimulates migration of MDA-MB-231 and MCF-7 cells via the
VEGF-STAT3 signaling pathway. Cell Oncol. 36:493–503. 2013.
View Article : Google Scholar
|
|
15
|
Zeng Q, Li S, Zhou Y, Ou W, Cai X, Zhang
L, Huang W, Huang L and Wang Q: Interleukin-32 contributes to
invasion and metastasis of primary lung adenocarcinoma via
NF-kappaB induced matrix metalloproteinases 2 and 9 expression.
Cytokine. 65:24–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Chen F and Tang L: IL-32 promotes
breast cancer cell growth and invasiveness. Oncol Lett. 9:305–307.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghaffari A, Hoskin V, Szeto A, Hum M,
Liaghati N, Nakatsu K, LeBrun D, Madarnas Y, Sengupta S and Elliott
BE: A novel role for ezrin in breast cancer
angio/lymphangiogenesis. Breast Cancer Res. 16:4382014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Guo X, Li H, Chen J and Qi X:
Src/STAT3 signaling pathways are involved in KAI1-induced
downregulation of VEGF-C expression in pancreatic cancer. Mol Med
Rep. 13:4774–4778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galun E: Liver inflammation and cancer:
The role of tissue microenvironment in generating the
tumor-promoting niche (TPN) in the development of hepatocellular
carcinoma. Hepatology. 63:354–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Q, Carroll HP and Gadina M: The
newest interleukins: Recent additions to the ever-growing cytokine
family. Vitam Horm. 74:207–228. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim SH, Han SY, Azam T, Yoon DY and
Dinarello CA: Interleukin-32: A cytokine and inducer of TNFalpha.
Immunity. 22:131–142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishida A, Andoh A, Inatomi O and Fujiyama
Y: Interleukin-32 expression in the pancreas. J Biol Chem.
284:17868–17876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heinhuis B, Koenders MI, Van den Berg WB,
Netea MG, Dinarello CA and Joosten LA: Interleukin 32 (IL-32)
contains a typical α-helix bundle structure that resembles focal
adhesion targeting region of focal adhesion kinase-1. J Biol Chem.
287:5733–5743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heinhuis B, Netea MG, Van den Berg WB,
Dinarello CA and Joosten LA: Interleukin-32: A predominantly
intracellular proinflammatory mediator that controls cell
activation and cell death. Cytokine. 60:321–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishigami S, Arigami T, Uchikado Y,
Setoyama T, Kita Y, Sasaki K, Okumura H, Kurahara H, Kijima Y,
Harada A, et al: IL-32 expression is an independent prognostic
marker for gastric cancer. Med Oncol. 30:4722013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai CY, Wang CS, Tsai MM, Chi HC, Cheng
WL, Tseng YH, Chen CY, Lin CD, Wu JI, Wang LH, et al:
Interleukin-32 increases human gastric cancer cell invasion
associated with tumor progression and metastasis. Clin Cancer Res.
20:2276–2288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yousif NG, Al-Amran FG, Hadi N, Lee J and
Adrienne J: Expression of IL-32 modulates NF-κB and p38 MAP kinase
pathways in human esophageal cancer. Cytokine. 61:223–227. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang YH, Park MY, Yoon DY, Han SR, Lee CI,
Ji NY, Myung PK, Lee HG, Kim JW, Yeom YI, et al: Dysregulation of
overexpressed IL-32α in hepatocellular carcinoma suppresses cell
growth and induces apoptosis through inactivation of NF-κB and
Bcl-2. Cancer Lett. 318:226–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu K, Min XL, Peng J, Yang K, Yang L and
Zhang XM: The changes of HIF-1α and VEGF expression after TACE in
patients with hepatocellular carcinoma. J Clin Med Res. 8:297–302.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsuchiya K, Asahina Y, Matsuda S, Muraoka
M, Nakata T, Suzuki Y, Tamaki N, Yasui Y, Suzuki S, Hosokawa T, et
al: Changes in plasma vascular endothelial growth factor at 8 weeks
after sorafenib administration as predictors of survival for
advanced hepatocellular carcinoma. Cancer. 120:229–237. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nold-Petry CA, Nold MF, Zepp JA, Kim SH,
Voelkel NF and Dinarello CA: IL-32-dependent effects of IL-1beta on
endothelial cell functions. Proc Natl Acad Sci USA. 106:pp.
3883–3888. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nold-Petry CA, Rudloff I, Baumer Y, Ruvo
M, Marasco D, Botti P, Farkas L, Cho SX, Zepp JA, Azam T, et al:
IL-32 promotes angiogenesis. J Immunol. 192:589–602. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hoshida Y, Fuchs BC and Tanabe KK:
Prevention of hepatocellular carcinoma: Potential targets,
experimental models, and clinical challenges. Curr Cancer Drug
Targets. 12:1129–1159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Welker MW and Trojan J: Antiangiogenic
treatment in hepatocellular carcinoma: The balance of efficacy and
safety. Cancer Manag Res. 5:337–347. 2013.PubMed/NCBI
|