Introduction
Immunotherapy has shown excellent promise for
various types of cancers (1). The
most promising approach to activate or enhance antitumor
immunotherapy is the blockade of immune checkpoints. Immune
checkpoint therapy was first exploited with the success of
anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) in clinical
treatment (2). Another immune
checkpoint therapy includes antibodies against programmed death 1
(PD-1) or its ligand PD-L1 (3), and
this immunotherapy has been confirmed effective for head and neck
squamous cell carcinoma (HNSCC) treatment (4). However, most patients do not respond
to anti-PD1 therapy or develop immunoresistance. Therefore, there
is an urgent need to identify new modulators that can prevent
immunoresistance or enhance immunotherapy.
miRNAs are a type of 18–24 nucleotide non-coding
RNAs with the ability to regulate messenger RNA (mRNA) expression
via binding to 3′ untranslated regions (UTRs) (5). Previous studies have indicated that
miRNAs play critical roles in tumor proliferation, metastasis and
many other processes (6). However,
the roles and the related mechanisms of miRNAs in HNSCC
immunotherapy have not been well elucidated.
Previous study has shown that interferon-γ (IFN-γ)
is a major CD4+ T cell effector cytokine and is
prerequisite to constitutive PD-L1 expression (7). Thus, inhibition of IFN-γ signaling
downregulates PD-L1 expression and increases tumor lysis (8). IFN-γ secretion from natural killer
(NK) cells activates the JAK2/STAT1 pathway and further increases
PD-L1 expression (8). Blockade of
the JAK pathway could be a potential method to act synergistically
with other immune therapies (9).
Notably, bioinformatics assay indicated that JAK2 is a potential
target of miR-375 which is downregulated in HNSCC. Additionally,
miR-375 inhibits the metastasis of colorectal cancer via targeting
SP1 (10). miR-375 was found to
suppress metastasis by directly targeting SHOX2 in esophageal
squamous cell carcinoma (11).
Although it has been previously reported that JAK2 is a target of
miR-375 in gastric cancer (6,12), the
roles of miR-375/JAK2 signaling in HNSCC immunotherapy remain
unclear.
In the present study, we hypothesized that miR-375
inhibits STAT1-dependent PD-L1 expression induced by IFN-γ exposure
in HNSCC cells, and we assessed the roles of JAK2/STAT1 signaling
in this process. We provide evidence that miR-375-mediated
inhibition of IFN-γ-induced PD-L1 expression in HNSCC cells may
allow T cells to create an antitumor immune environment.
Materials and methods
Cell culture and patient samples
HNSCC cell lines Hp-2 and FaDu were purchased from
the Chinese Academy of Sciences Cell Bank. Jurkat cells, a human T
leukemia cell line, were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA). HNSCC cell lines were
maintained in Dulbecco's minimum essential medium (DMEM), and
Jurkat T leukemia cells were maintained in RPMI-1640 medium,
supplemented with 10% fetal bovine serum (FBS) (all from Gibco,
Grand Island, NY, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin in a humidified atmosphere with 5% CO2 at
37°C. Paired mRNA profiling data were downloaded from the TCGA, The
Cancer Genome Atlas (TCGA) data portal (http://cancergenome.nih.gov). The dataset from the
Tumor Head Neck Squanmous Cell Carcinoma-TCGA-520-rsem-tcgars
(http://hgserver1.amc.nl/cgi-bin/r2/main.cgi), which
includes 520 HNSCC samples, was obtained as a validation set. The
R2 platform was used to analyze the microarray (http://r2.amc.nl).
Reagents
miR-375 mimics/inhibitor and the related negative
control (NC) were synthesized by GenePharma, Inc. (Shanghai,
China). Recombinant human IFN-γ (cat. no. 300-02) was purchased
from PeproTech (Rocky Hill, NJ, USA). Phycoerythrin (PE)-conjugated
anti-human PD-L1 Ab (cat. no. 560795; dilution rate, 1:5,000) and
PE-conjugated anti-human PD-1 Ab (cat. no. 561272; dilution rate,
1:5,000) were purchased from BD Biosciences (San Jose, CA,
USA).
Cell viability assay
Cells with different treatment were seeded into
96-well plates at 3×103 cells/well and incubated at 37°C
for 24, 48 and 72 h, respectively. The cell viability was measured
using a Cell Counting Kit-8 assay (cat. no. C0037; Beyotime,
Nanjing, China) following the manufacturer's protocol.
Flow cytometric assay
Hep-2 and FaDu cells were seeded into 6-well plates
at 2×105 or 4×105 cells/well overnight,
respectively. Cells were added with 10 ng/ml IFN-γ, and then
transfected with miR-375 mimics (50 nM) and the related NC (50 nM)
by Lipofectamine 2000 (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) for 48 h. Then, the cells were washed with ice-cold PBS and
re-suspended in flow cytometry buffer (0.2% NaN3 and 1%
BSA in PBS). Jurkat cells in fully supplemented RPMI-1640 medium
were also maintained as suspension cultures. Furthermore, the cells
were stained with 0.5 µg of the desired fluorophore-conjugated Ab
or isotype control for 30 min in the dark at 4°C, then washed twice
with flow cytometry buffer and fixed in 1% paraformaldehyde.
Fluorescence data were acquired using a FACSCalibur flow cytometer
and BD CellQuest™ software (BD Biosciences). Counts/sample
(2×104) were analyzed for all fluorescence experiments
using FCS Express software (De Novo Software, Glendale, CA,
USA).
T cells and HNSCC cell
co-cultures
After 24 h of transfection with miR-375 mimics (50
nM) using Lipofectamine 2000 and/or IFN-γ (10 ng/ml), HNSCC cells
were collected, washed and re-suspended in fully supplemented DMEM,
and further seeded into 24-well plates at 2×105 or
40×105 cells/well for 6 h to allow attachment. Jurkat T
leukemia cells stained with Oregon Green 488 dye were added to
HNSCC cell monolayers at 5×104 cells/well. The resulting
co-cultures were maintained for another 72 h. Then, the
supernatants were centrifuged and collected for examination of
IL-2. The proliferation of Jurkat T cells was analyzed by flow
cytometry. The number of cell divisions was calculated using the
formula: Mean fluorescence intensity (MFI) control = 2n
× MFIsample, where MFIcontrol is the MFI of
the non-proliferative control and n is the number of cell divisions
(9). Cell divisions were normalized
to the medium control.
Cytokine measurement
IL-2 level was measured in cell supernatants from T
cells and HNSCC cell co-cultures using IL-2-specific ELISA kit from
Boster (EK0397; Wuhan, China) according to the manufacturer's
instructions.
RNA immune co-precipitation (RIP)
assay
The procedure for RIP assay was performed as
previously described (13). RNA in
the IP materials was measured by qRT-PCR.
Plasmid construction and
transfection
JAK2 coding sequences were inserted into the
pcDNA3.1 (+) plasmid, named as JAK2-CDS. Cells were seeded into
6-well plates at a density of 2.5×105 cells/well. Fifty
nanomoles of miRNA mimics or NC, and JAK2-CDS were transfected into
the cells using Lipofectamine 2000 reagent according to the
manufacturer's instructions.
Quantitative real-time PCR
(qRT-PCR)
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions. cDNA was reverse transcribed with
M-MLV transcriptase (Promega, Madison, WI, USA). qRT-PCR was
performed on the ABI StepOnePlus Real-Time PCR system [Applied
Biosystems (ABI,) Foster City, CA, USA USA] using SYBR-Green PCR
kit (Takara, Japan). U6 and GAPDH were used as the internal
controls for miRNA and mRNA expression, respectively. The primers
of JAK2 for qRT-PCR are listed below: forward,
5′-CTGCAGGAAGGAGAGAGGAAGAGGA-3′ and reverse,
5′-GAATGTTATTGGCAGTCAG-3′. Relative quantification was calculated
using the 2−ΔΔCt method.
Western blotting
Cells were lysed with radioimmunoprecipitation assay
(RIPA) lysis buffer containing protease and phosphatase inhibitors.
The concentration of protein was quantified by BCA (bicinchoninic
acid) protein assay kit (Beyotime). Equal amount of protein was
loaded onto 10% SDS-PAGE, followed by wet transferring to
polyvinylidene fluoride (PVDF) membranes. The membranes were
blocked with 5% non-fat milk at room temperature for 1 h, and then
incubated with primary antibodies at 4°C overnight. The membranes
were subsequently blotted with HRP-conjugated secondary antibodies
and developed with the ECL detection system (Thermo Fisher
Scientific, Inc.).
Luciferase reporter analysis
For the luciferase assay, the full length of JAK2
3′UTR containing the wild-type or the mutant binding sites for
miRNA-375 was cloned into the pMIR-Report vector downstream of the
luciferase reporter gene, which was denoted as Luc-JAK2-3′UTR-WT
and Luc-JAK2-3′UTR-MUT, respectively. These constructs were
co-transfected with miR-375 mimics or NC in HNSCC cells using
Lipofectamine 2000. Luminescent signals were measured 48 h later by
luminometer (Thermo Fisher Scientific, Inc.). β-gal was used to
normalize the luciferase activity.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). The differences between the groups were analyzed using ANOVA
with the Tukey-Kramer post test, and P<0.05 was considered to
indicate a statistically significant result.
Results
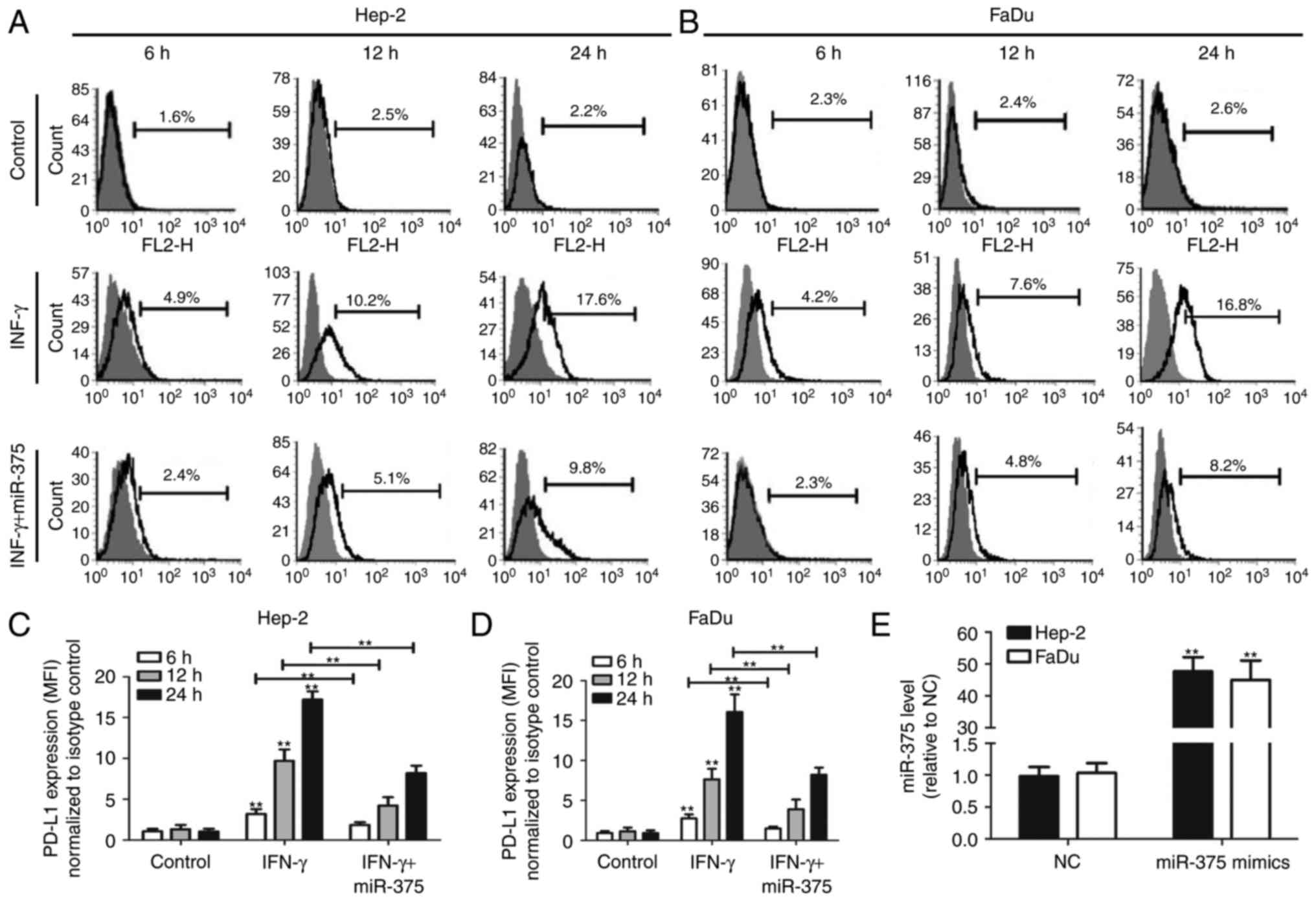
miR-375 inhibits IFN-γ-induced PD-L1
expression in HNSCC cells
Consistent with previous studies (7,8), we
found that IFN-γ-treated Hep-2 and FaDu cells showed a
time-dependent increase in PD-L1 expression (Fig. 1A-D). However, this effect was
attenuated following co-transfection with miR-375. The transfection
efficiency of miR-375 mimics was confirmed by qRT-PCR, and the
result indicated that transfection with miR-375 mimics
significantly upregulated miR-375 level in Hep-2 and FaDu cells
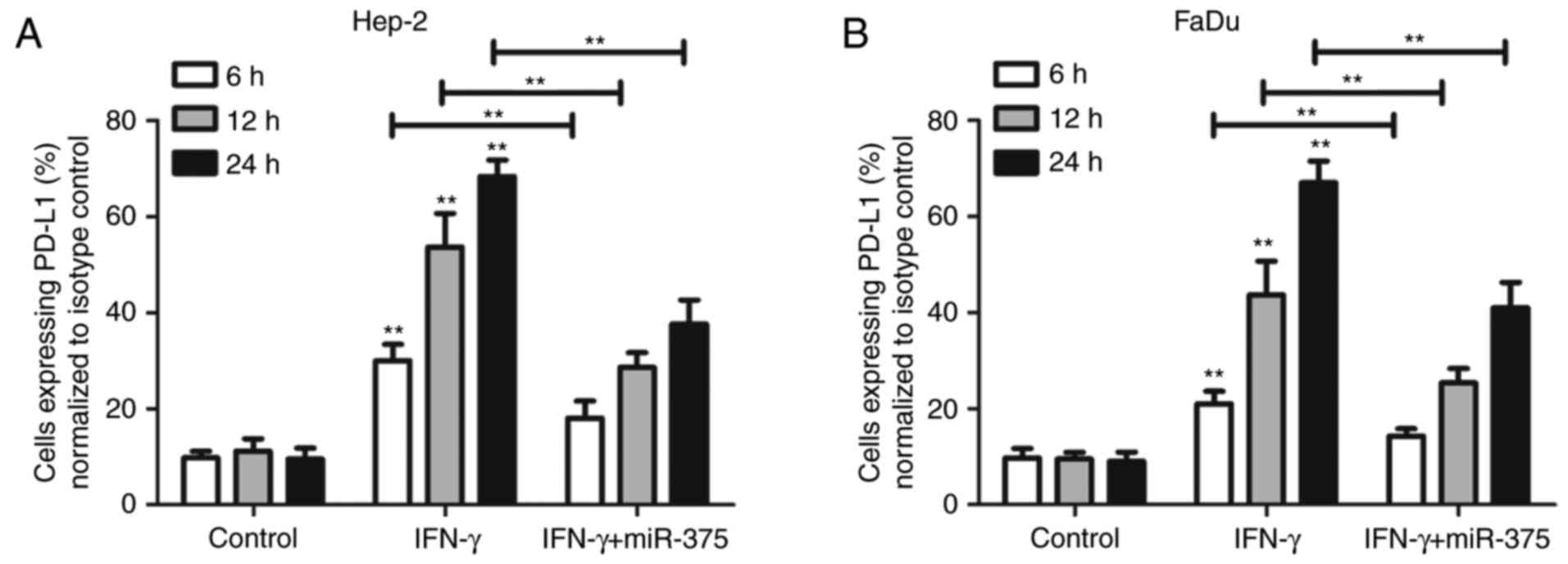
(Fig. 1E). Notably, miR-375 reduced
both the percentage of PD-L1-expressing HNSCC cells and the amount
of PD-L1 expressed in individual HNSCC cells (Fig. 2A and B).
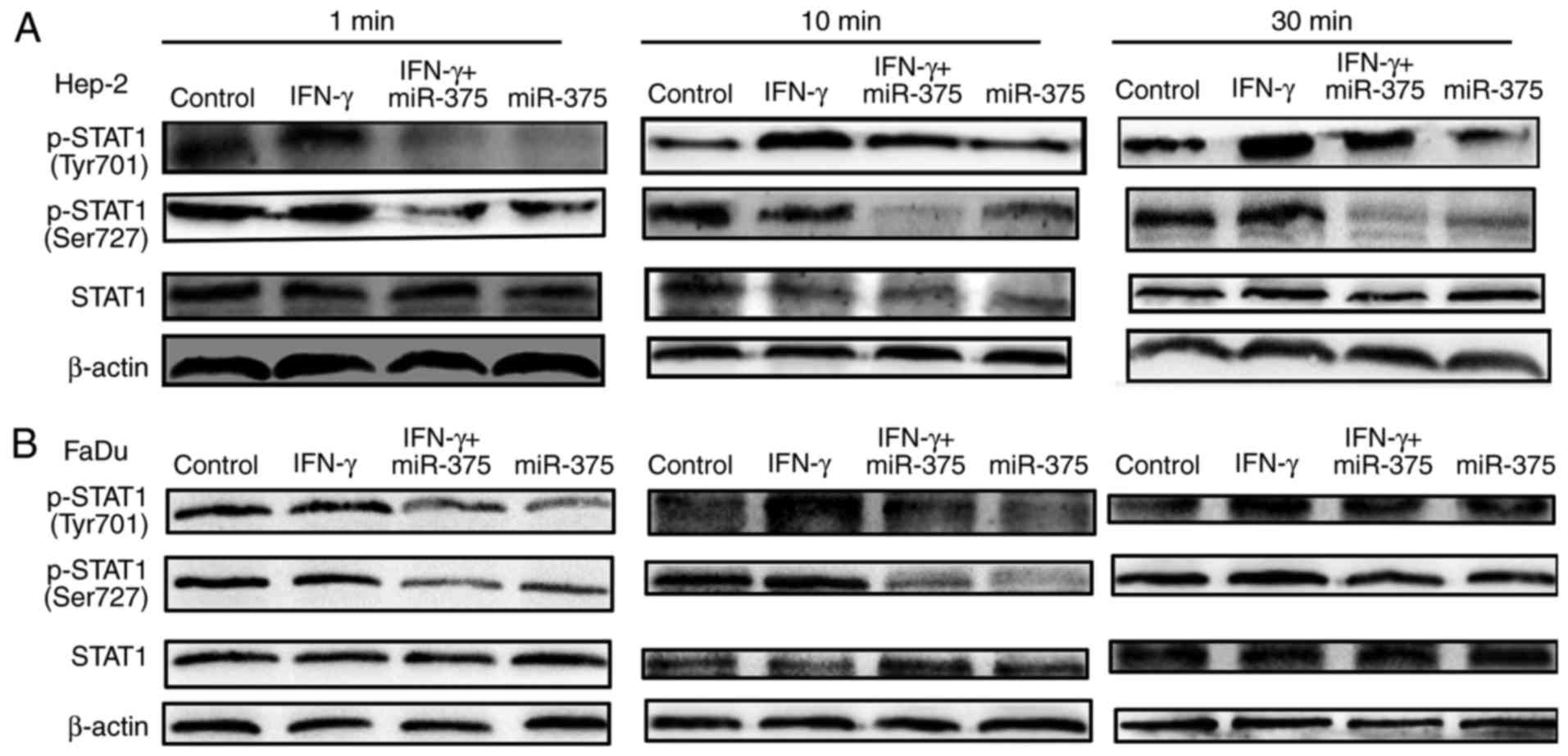
miR-375 inhibits IFN-γ-induced STAT1
activation
Previous research has shown that IFN-γ induction
activates JAK1 and JAK2, further leading to the phosphorylation of
STAT1 (8). We found this to be the
case in HNSCC cells. Treatment of HNSCC cells with 10 ng/ml IFN-γ
caused STAT1 phosphorylation (Fig.
3) at different time points. Additionally, transfection with
miR-375 mimics before IFN-γ treatment decreased the STAT1
phosphorylation level (Fig. 3),
indicating that ablation of STAT1 phosphorylation is necessary for
the inhibitory effects of miR-375 on IFN-γ-induced PD-L1
expression.
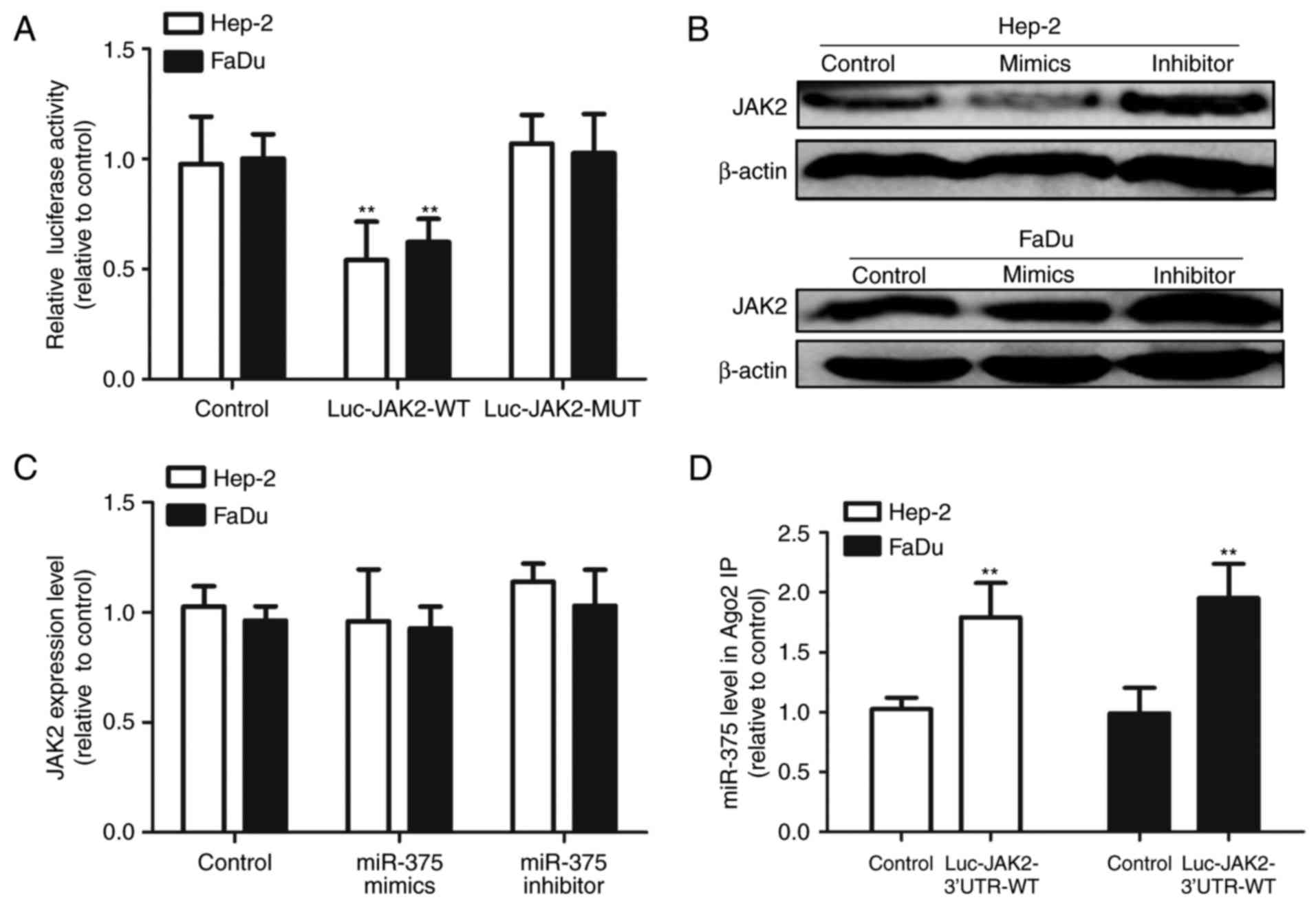
JAK2 is a bona fide target of miR-375
in HNSCC cells
Since JAK2 has been identified as a potential target
of miR-375 in gastric tumor and myeloid-derived suppressor cells
(6,12,13),
we speculated that miR-375/JAK2 signaling also exists in HNSCC
cells. As shown in Fig. 4A,
co-transfection with miR-375 mimics and the Luc-JAK2-3′UTR-WT
construct significantly reduced the luciferase activity; whereas
the luciferase activity of Luc-JAK2-3′UTR-MUT was unaffected by
miR-375 transfection. Additionally, western blot results showed
that upregulation of miR-375 markedly decreased JAK2 protein
expression in Hep-2 and FaDu cells, while transfection with miR-375
inhibitor increased JAK2 protein expression (Fig. 4B). However, no significant
difference was observed at the JAK2 mRNA level (Fig. 4C), indicating that miR-375 regulates
JAK2 expression at the post-transcriptional level. To further
confirm the direct binding between miR-375 and JAK2 3′UTR in HNSCC
cells, JAK2 3′UTR was introduced into HNSCC cells by
Luc-JAK2-3′UTR-WT transfection, and RIP assay was performed to
detect the bound miRNAs in the Ago2-binding complex. As shown in
Fig. 4D, the miR-375 level which
bound to Ago2 was increased in the Luc-JAK2-3′UTR-WT-introduced
cells. Consequently, we demonstrated that JAK2 is a direct target
of miR-375 in HNSCC cells.
miR-375 inhibits IFN-γ-induced STAT1
activation in a JAK2-dependent manner
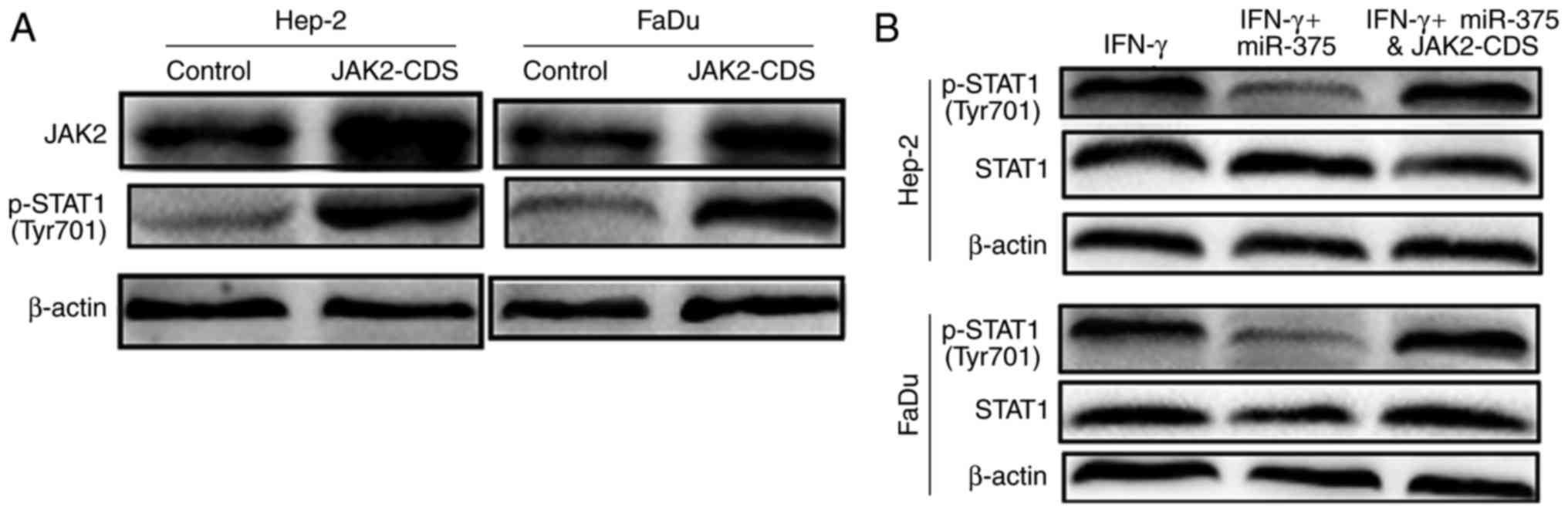
To further confirm the essential role of JAK2 in
miR-375-mediated inhibition on IFN-γ-induced STAT1 activation. JAK2
was overexpressed in Hep-2 and FaDu cells by transfection with
JAK2-CDS. The transfection efficiency of JAK-CDS was confirmed by
western blot analysis (Fig. 5A).
Overexpression of JAK2 significantly upregulated the
phosphorylation level of STAT1. Additionally, co-transfection with
JAK2-CDS and miR-375 reversed the inhibitory effects of miR-375 on
IFN-γ-induced STAT1 activation (Fig.
5B) and PD-L1 expression (Fig.
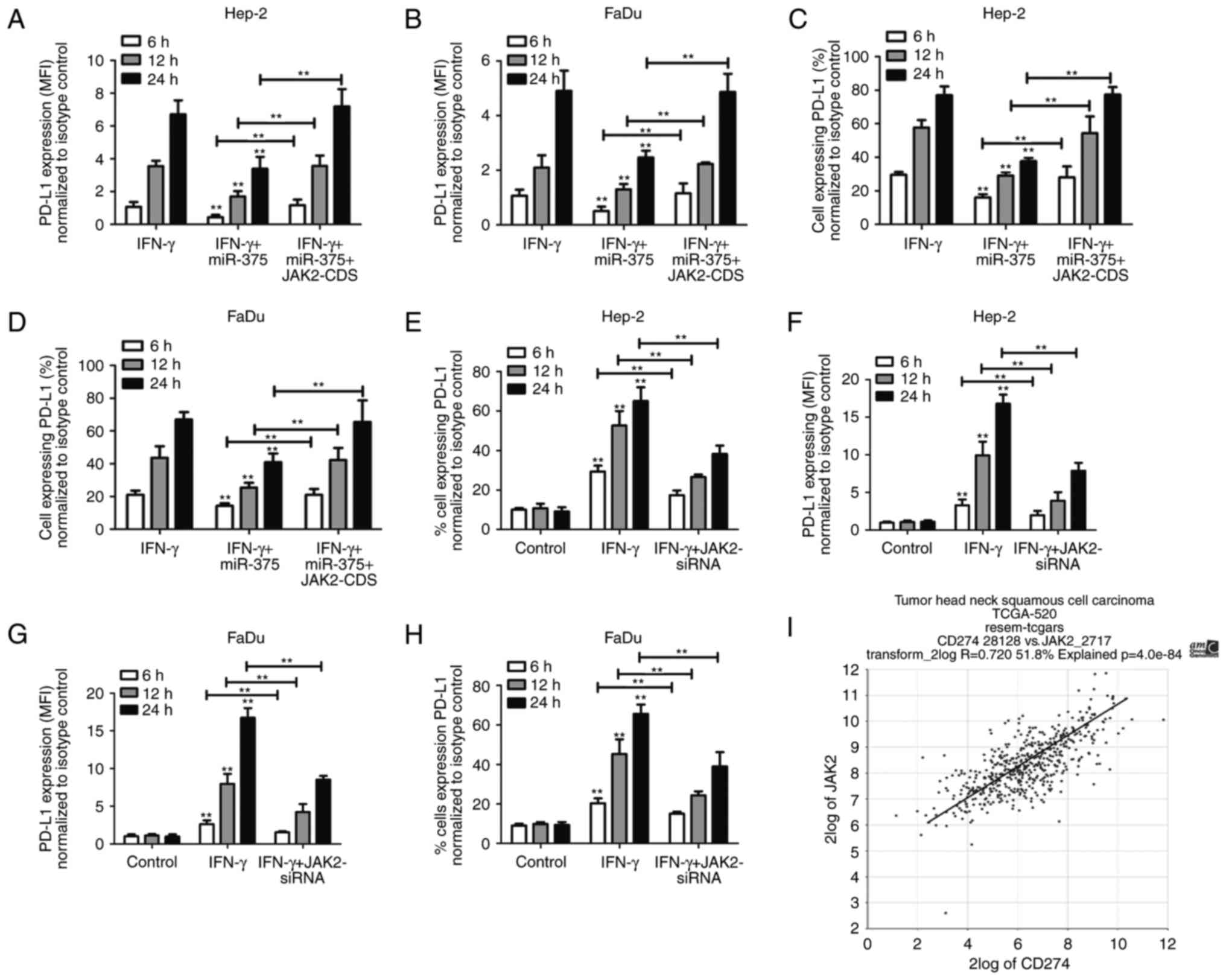
6A-D). Moreover, knockdown of JAK2 also reduced IFN-γ-induced
PD-L1 expression (Fig. 6E-H).
Importantly, by analyzing the mRNA expression in the mRNA
microarrays from TCGA, we confirmed that the levels of JAK2 and
PD-L1 were positively correlated (P=4.0e-84) in HNSCC patients
(Fig. 6I). All together, these
results indicate that JAK2/STAT1 signaling by miR-375 participates
in IFN-γ-induced STAT1 activation and thus suppresses PD-L1
expression.
Upregulation of miR-375 contributes to
increased T cell proliferation in HNSCC co-cultures
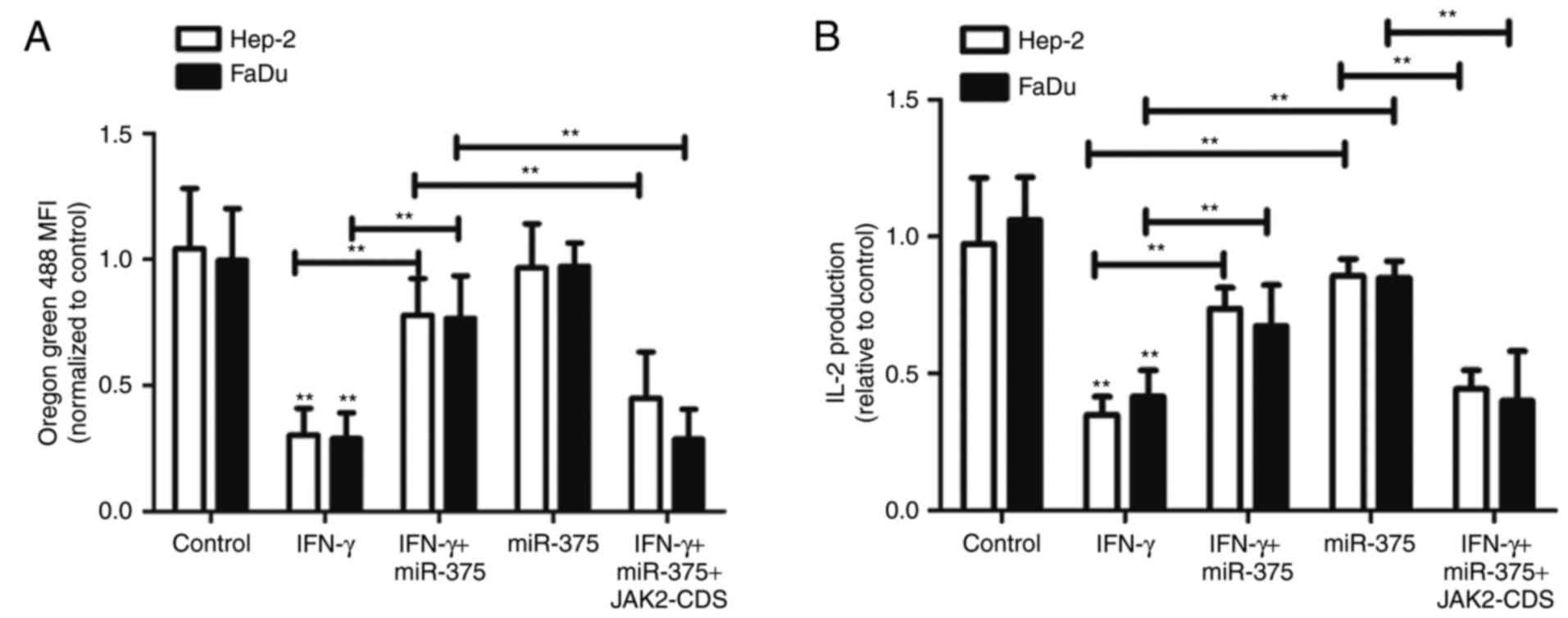
Having shown that miR-375 inhibited IFN-γ-induced
STAT1 activation and thus decreased PD-L1 expression, we sought to
investigate the potential relevance of this phenotype with T cell
proliferation. We co-cultured Oregon Green 488-stained Jurkat T
cells with Hep-2 and FaDu cells that were previously treated with
10 ng/ml IFN-γ in the absence or presence of miR-375
overexpression. After 72 h of co-culture, T cell proliferation was
tested by flow cytometry. Fig. 7A
showed that T cell proliferation, characterized as decreased MFI,
was reduced in the presence of IFN-γ-treated Hep-2 and FaDu cells,
and this effect was presumably caused by the inhibition of
PD-1/PD-L1 interaction. Most importantly, T cell proliferation was
reversed to the control level in co-cultures of Hep-2 and FaDu
cells with miR-375 introduction after IFN-γ treatment. In addition,
introduction of JAK2-CDS in HNSCC cells reversed the
miR-375-mediated effects. Furthermore, IL-2 concentration secreted
by Jurkat T cells was upregulated in the co-cultures when HNSCC
cells were treated with miR-375 plus IFN-γ compared with the
co-cultures of HNSCC cells with IFN-γ treatment only (Fig. 7B); this result was reversed by JAK2
overexpression. Collectively, our findings demonstrate that the
inhibitory effects of miR-375 on IFN-γ-induced expression of PD-L1
in HNSCC cells is associated with increased T cell responses in
co-cultures and dependent on JAK2 expression.
Discussion
Low-level expression of miR-375 has been shown to be
correlated with poor outcome and metastasis while altering the
invasive properties of head and neck squamous cell carcinomas
(14). Additionally, miRNA-375
suppresses extracellular matrix degradation and invadopodial
activity via regulating oncogene AEG-1/MTDH in HNSCC (15,16).
However, the immune regulatory roles and the related mechanisms of
miR-375 in HNSCC have not been defined, and the mechanisms that
underlie IFN-γ-induced expression of PD-L1 have not been clearly
elucidated.
miR-375 has been extensively explored for its
inhibitory effects on tumor development (6,10,11).
However, to the best of our knowledge, the present study is the
first to provide evidence of a potential role of miR-375 in
improving a T cell-mediated antitumor immune response against HNSCC
cells by modulating IFN-γ-induced expression of PD-L1, which is
validated as a promising target for immunotherapy in HNSCC
treatment (4,17). We indicated here that miR-375
inhibited IFN-γ-induced PD-L1 expression via targeting JAK2 and
thus inactivating JAK2/STAT1 signaling This means that miR-375
inhibits PD-1/PD-L1-mediated immune escape in HNSCC patients whose
tumor-associated PD-L1 expression is elevated due to IFN-γ
inductive effects or JAK2/STAT1-mediated pro-inflammatory
cytokines, such as IL-2 in the tumor microenvironment.
Specifically, although JAK2 phosphorylated STAT1 at
Tyr701 and Ser727 residues, phosphorylation of STAT1 at the Tyr701
residue is independent of Ser727 phosphorylation (18). In addition, the rapid
phosphorylation of STAT1 (pY701), but not activation of other STATs
is the main mediator of IFN-γ induction and suppresses tumor cell
susceptibility to NK cells through upregulation of PD-L1 (8). Our results showed that treating HNSCC
cells with miR-375 caused a significant and early decrease in
IFN-γ-induced phosphorylation of STAT1 at Tyr701. However, miR-375
still could decrease the phosphorylation level of STAT1 (pS727),
and this could be due to miR-375-mediated inactivation of JAK2
which could phosphorylate STAT1 at Tyr701 and Ser727 residues. More
importantly, the inhibitory effect of miR-375 on STAT1
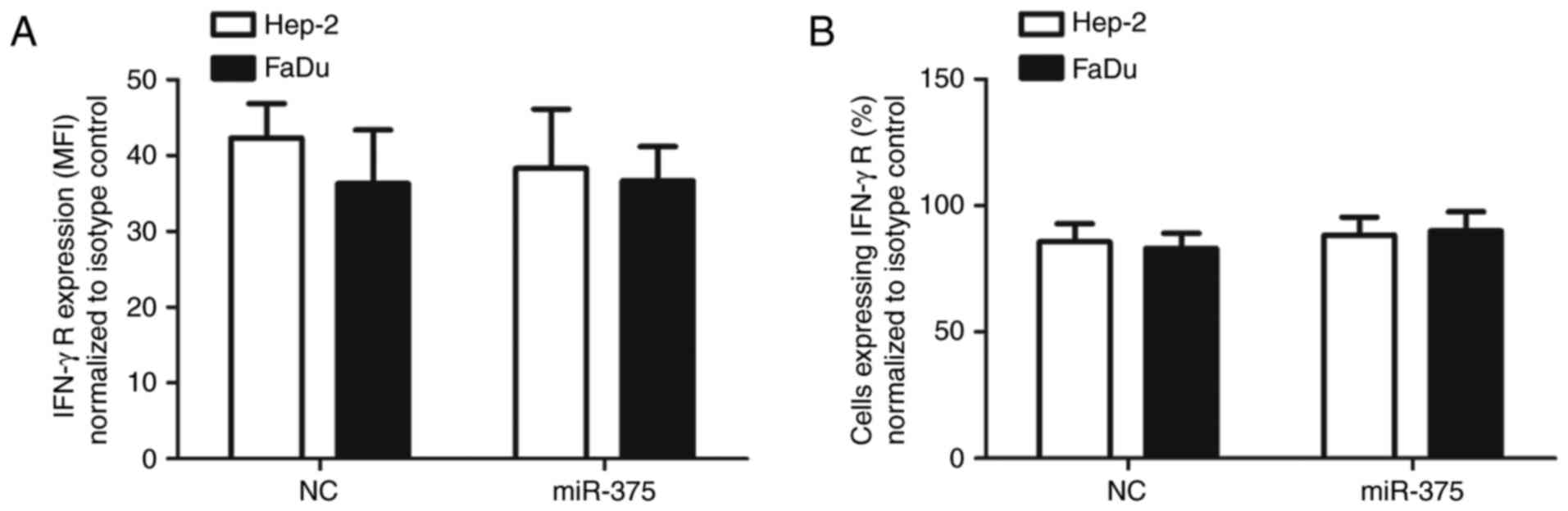
phosphorylation was not due to the decreased expression of the
IFN-γ receptor on HNSCC cells (Fig. 8A
and B). Rather, since our results indicate that miR-375
directly targets JAK2, it follows that miR-375 can affect
JAK2-dependent PD-L1 expression induced by IFN-γ treatment of HNSCC
cells. As PD-L1 upregulation is a common immune escape of tumor
cells (19), and higher PD-L1
expression in tumor patients predicates a poor prognosis in various
cancers, including HNSCC (20).
Previous study has shown that PD-L1/PD-1 interaction contributes to
the functional suppression of T-cell responses characterized as
impaired IL-2 production by Jurkat T cells (21). Indeed, our results showed that the
inhibitory effect of miR-375 on IFN-γ-induced PD-L1 expression by
HNSCC cells was associated with increased proliferation and IL-2
synthesis by Jurkat T cells that were co-cultured with HNSCC cells,
which is consistent with an attenuation of PD-1/PD-L1 interactions.
Future studies could be performed to confirm this immune-modulating
effects of miR-375 in vivo using immune-competent mice.
Additionally, since miR-375 could target JAK2 in gastric tumor, the
effects of miR-375 on IFN-γ-induced PD-L1 expression should also be
explored. Furthermore, previous research has shown that miR-375
suppresses the invasive properties of HNSCC (14). miR-375 therefore has the potential
to modulate HNSCC growth in an indirect or direct manner,
indicating an important potential therapeutic target and providing
a strong rationale for the development of miRNA-based therapeutic
strategies for HNSCC.
References
|
1
|
Khalil DN, Smith EL, Brentjens RJ and
Wolchok JD: The future of cancer treatment: Immunomodulation, CARs
and combination immunotherapy. Nat Rev Clin Oncol. 13:3942016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma P, Wagner K, Wolchok JD and Allison
JP: Novel cancer immunotherapy agents with survival benefit: Recent
successes and next steps. Nat Rev Cancer. 11:805–812. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ansell SM, Lesokhin AM, Borrello I,
Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry
D, Freeman GJ, et al: PD-1 blockade with nivolumab in relapsed or
refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saâda-Bouzid E, Defaucheux C, Karabajakian
A, Coloma VP, Servois V, Paoletti X, Even C, Fayette J, Guigay J,
Loirat D, et al: Hyperprogression during anti-PD-1/PD-L1 therapy in
patients with recurrent and/or metastatic head and neck squamous
cell carcinoma. Ann Oncol. 28:1605–1611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Zheng L, Zhang F, Hu J, Chou J, Liu
Y, Xing Y and Xi T: STARD13-correlated ceRNA network inhibits EMT
and metastasis of breast cancer. Oncotarget. 7:23197–23211.
2016.PubMed/NCBI
|
|
6
|
Miao L, Liu K, Xie M, Xing Y and Xi T:
miR-375 inhibits Helicobacter pylori-induced gastric carcinogenesis
by blocking JAK2-STAT3 signaling. Cancer Immunology Immunother.
63:699–711. 2014. View Article : Google Scholar
|
|
7
|
Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI,
Park YM, Oh S, Shin JG, Yao S, Chen L and Choi IH: Interferon
regulatory factor-1 is prerequisite to the constitutive expression
and IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett.
580:755–762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bellucci R, Martin A, Bommarito D, Wang K,
Hansen SH, Freeman GJ and Ritz J: Interferon-γ-induced activation
of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells
through upregulation of PD-L1 expression. Oncoimmunology.
4:e10088242015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coombs MR, Harrison ME and Hoskin DW:
Apigenin inhibits the inducible expression of programmed death
ligand 1 by human and mouse mammary carcinoma cells. Cancer Lett.
380:424–433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui F, Wang S, Lao I, Zhou C, Kong H,
Bayaxi N, Li J, Chen Q, Zhu T and Zhu H: miR-375 inhibits the
invasion and metastasis of colorectal cancer via targeting SP1 and
regulating EMT-associated genes. Oncol Rep. 36:487–93. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yi J, Jin L, Chen J, Feng B, He Z, Chen L
and Song H: MiR-375 suppresses invasion and metastasis by direct
targeting of SHOX2 in esophageal squamous cell carcinoma. Acta
Biochim Biophys Sin. 49:159–169. 2017.PubMed/NCBI
|
|
12
|
Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y,
Yao H, Liu X, Ke Y, Si J and Zhou T: MiR-375 frequently
downregulated in gastric cancer inhibits cell proliferation by
targeting JAK2. Cell Res. 20:784–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sheng B, Zhao L, Zang X, Zhen J and Chen
W: miR-375 ameliorates sepsis by downregulating miR-21 level via
inhibiting JAK2-STAT3 signaling. Biomed Pharmacother. 86:254–261.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harris T, Jimenez L, Kawachi N, Fan JB,
Chen J, Belbin T, Ramnauth A, Loudig O, Keller CE, Smith R, et al:
Low-level expression of miR-375 correlates with poor outcome and
metastasis while altering the invasive properties of head and neck
squamous cell carcinomas. Am J Pathol. 180:917–928. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nohata N, Hanazawa T, Kikkawa N, Mutallip
M, Sakurai D, Fujimura L, Kawakami K, Chiyomaru T, Yoshino H,
Enokida H, et al: Tumor suppressive microRNA-375 regulates oncogene
AEG-1/MTDH in head and neck squamous cell carcinoma (HNSCC). J Hum
Genet. 56:595–601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jimenez L, Sharma VP, Condeelis J, Harris
T, Ow TJ, Prystowsky MB, Childs G and Segall JE: MicroRNA-375
suppresses extracellular matrix degradation and invadopodial
activity in head and neck squamous cell carcinoma. Arch Pathol Lab
Med. 139:1349–1361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ock CY, Kim S, Keam B, Kim M, Kim TM, Kim
JH, Jeon YK, Lee JS, Kwon SK, Hah JH, et al: PD-L1 expression is
associated with epithelial-mesenchymal transition in head and neck
squamous cell carcinoma. Oncotarget. 7:15901–15914. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu X, Wen Z, Xu LZ and Darnell JE Jr:
Stat1 serine phosphorylation occurs independently of tyrosine
phosphorylation and requires an activated Jak2 kinase. Mol Cell
Biol. 17:6618–6623. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mann JE, Hoesli R, Michmerhuizen NL,
Devenport SN, Ludwig ML, Vandenberg TR, Matovina C, Jawad N,
Mierzwa M, Shuman AG, et al: Surveilling the potential for
precision medicine-driven PD-1/PD-L1-targeted therapy in HNSCC. J
Cancer. 8:332–344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang W, Chen PW, Li H, Alizadeh H and
Niederkorn JY: PD-L1: PD-1 interaction contributes to the
functional suppression of T-cell responses to human uveal melanoma
cells in vitro. Invest Ophthalmol Vis Sci. 49:2518–2525. 2008.
View Article : Google Scholar : PubMed/NCBI
|