Introduction
An increasing body of evidence suggests that the
tumor microenvironment has a profound impact on cancer progression
and therapeutic responses (1–4).
Extracellular matrix (ECM), a major component of the
microenvironment, is composed of a combination of macromolecules
(e.g. collagens, laminin, elastin, fibronectin, proteoglycans and
polysaccharides). Cellular attachment to ECM components triggers
intracellular signaling pathways that regulate numerous cellular
functions, including cell proliferation, differentiation, cell
shape, survival, migration and invasion (1,5).
Cell-ECM interactions can influence patterns of tissue-specific
gene expression, leading to different biological phenotypes. For
instance, exposure of mammary epithelial cells to a laminin-rich
basement membrane has been revealed to induce milk protein
(beta-casein) expression (6,7).
Factors in the tissue microenvironment have been suggested to
influence the expression of ECM-degrading enzymes, thus modifying
the ability of human colon carcinoma cells to metastasize at two
different implantation sites (8). A
previous study demonstrated the role of the microenvironment in the
expression of urokinase-type plasminogen activator (uPA), and thus
in the metastatic potential of KG-2 human renal carcinoma cells
(9).
Metastasis, the spread of cancer to distant
locations in the body, is a primary cause of cancer-related deaths.
It has previously been reported that disseminated tumor cells can
lie dormant in secondary organs for prolonged periods of time
(10,11). These quiescent dormant cells remain
undetectable to screening methodologies and evade conventional
therapies that target actively proliferating cells (12). Recent studies have demonstrated that
the dormant cells can switch to proliferative metastatic growth
when conditions are favorable, and thus may be responsible for
disease recurrence (13). Tumor
dormancy, chemotherapy resistance and cancer recurrence are major
problems associated with cancer treatment. Components of the
microenvironment have been reported to play a central role in
regulating tumor dormancy and chemoresistance (1,14,15).
Elucidating the mechanisms underlying ECM-mediated tumor dormancy
and the switch to metastatic growth should provide useful targets
for the development of therapeutic approaches to eliminate these
inactive tumor cells. This would reduce the risk of dormant cells
becoming proliferate after long periods.
The microenvironment of cells cultured in
vitro on traditional plastic substrata differs considerably
from that of cells in vivo. Cells require not only nutrients
and growth factors, but also appropriate interaction with ECM
components in order to trigger intracellular signal transduction.
Critical signals are lost when cells are cultured on plastic
surfaces. Recent experiments revealed that most cell lines can
readily proliferate as a monolayer on plastic plates, but do not
develop tumors in vivo (11). Moreover, standard cell culture on
plastic surfaces fails to predict realistic responses to drug
candidates. However, most research has been performed with cancer
cells grown on plastic substrata. Thus, the development of a cell
culture system that recreates an appropriate environment for cells
should provide a better model for studies of cellular activity.
Matrigel is a biological ECM extracted from mouse
Englebreth-Holm-Swarm (EHS) sarcoma, a tumor that is rich in
basement membrane and ECM proteins. Matrigel is composed of
laminin, collagen IV, entactin, heparan sulfate proteoglycans,
nidogens and a small quantity of protease, as well as growth
factors (16). Matrigel polymerizes
at room temperature to form a matrix material resembling the
basement membrane found in many tissues. It is commonly used as a
substrate for cell culture (17).
In the present study, human non-small cell lung cancer (NSCLC) A549
cells were embedded in a semi-solid Matrigel matrix to enable close
interaction with ECM proteins, a method that better imitates the
in vivo microenvironment compared with standard cell culture
on plastic plates. Cancer cell behaviors, including cell migration,
invasion, chemosensitivity and proliferation, were assessed. In
addition, the signaling pathways mediating the effect of Matrigel
were studied to assess the molecular mechanisms involved in tumor
dormancy and drug resistance.
Materials and methods
Cell culture
Human A549 NSCLC cells were purchased from the
American Type Culture Collection (Rockville, MD, USA). Cells were
maintained in RPMI-1640 supplemented with 10% fetal bovine serum
and 1% antibiotic-antimycotic solution (Gibco; Thermo Fisher
Scientific, Inc., Grand Island, NY, USA). For semi-solid
Matrigel-embedded cell culture, a cell suspension (3×105
cells in 350 µl of complete medium) was gently mixed with 150 µl of
Matrigel (containing growth factors) (Corning Life Sciences,
Bedford, MA, USA), with a final concentration of 30% v/v Matrigel.
This was plated into a 24-well plate and incubated at 37°C for 30
min. Then, 500 µl of complete media was gently added to the well
and the culture was maintained for 3 days. This diluted Matrigel
polymerizes at 37°C to form a semi-solid gel that is soft enough to
allow cells to spread as a monolayer beneath the gel. Cells were
harvested from the semi-solid culture environment, for further
analysis of cell behavior, by tapping the plate, aspirating the
collapsed gel and washing with PBS. This was followed by
conventional trypsinization. Cell morphology was examined by
inverted phase-contrast microscopy. In all experiments, the cells
were maintained at 37°C in a humidified atmosphere containing 5%
CO2.
Cell proliferation assay
Cell proliferation was determined using the
Quant-iT™ PicoGreen® dsDNA Assay kit (Invitrogen; Thermo
Fisher Scientific, Inc., San Diego, CA, USA) to quantify DNA,
according to the manufacturer's protocol. After harvesting cells
from standard cell culture or semi-solid Matrigel-embedded cell
culture, the cells were seeded into 96-well plates at a density of
1×104 cells/well in 100 µl of culture medium. Cell
proliferation was assessed on days 0, 2, 4, 7 and 9 after plating.
Fluorescence-based dsDNA measurements were obtained using a
SpectraMax fluorescence microplate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA), with an excitation wavelength of 485 nm and an
emission wavelength of 520 nm.
Cell migration (wound-healing)
assay
Cell migration was assessed using a wound-healing
assay. Wounds were generated by scratching confluent cultures of
cells on plastic plates, or cells in semi-solid Matrigel (after
removing the gel and washing with PBS), with a sterile 20-µl
pipette tip. These wounds were then washed twice with PBS to remove
cell debris, covered with complete medium or 30% v/v Matrigel, and
incubated for a further 24 h. Wounds were marked and digitally
photographed in the same field at 0 and 24 h after scratching. The
wound area was assessed with ImageJ software, and the extent of
healing was calculated as follows:
% Wound closure = (wound area at 0 h - wound area at
24 h)/wound area at 0 h × 100%
Invasion assay
The invasiveness of cancer cells was evaluated using
Matrigel-coated Transwell chambers (8 µm pore size). The upper
chamber was seeded with 1×105 cells in a 200-µl
suspension, while 500 µl of conditioned medium prepared from human
lung fibroblasts (MRC-5) was added to the lower chamber. After
incubation for 24 h at 37°C, non-invaded cells on the upper surface
of the insert membrane were removed with a cotton swab. Invaded
cells on the lower surface of the membrane were fixed with 25%
methanol, stained with crystal violet, and acid-extracted with 0.1
N HCl in methanol. The absorbance at 550 nm was measured.
Chemosensitivity assay
The chemosensitivity of the cells was determined
using an MTT assay for assessing metabolically active cells, as
previously described (18).
Briefly, the cell suspension was seeded into 96-well culture plates
at a density of 1×104 cells/100 µl/well. Then, 100 µl of
vehicle (cell culture medium) or various concentrations of
cytotoxic agents were added, and the plates were incubated for 48
h. Cell culture medium containing MTT (Sigma-Aldrich, St. Louis,
MO, USA) was added to each well and incubated for 2 h. The number
of viable cells was assessed by determining the absorbance at 550
nm and subtracting the absorbance at 650 nm (reference wavelength).
The IC50 value of each drug, defined as the
concentration of the drug that results in a 50% decrease in cell
viability, was extrapolated from a concentration-dependent curve of
the drug. Assays were performed in quadruplicate, and data were
expressed as the percentage of viability relative to the
control.
Cell cycle assay
Cell cycle distribution was analyzed using a Muse™
Cell Analyzer (Merck Millipore, Hayward, CA, USA). The Muse™ Cell
Cycle Assay kit (Merck Millipore) was used according to the
manufacturer's protocol. Briefly, cell pellets from each condition
were washed three times with PBS and fixed in ice-cold 70% ethanol
overnight at −20°C prior to staining. Cells were washed again with
PBS, stained with 200 µl of Muse™ Cell Cycle reagent, incubated in
the dark for 30 min at room temperature and then processed for cell
cycle analysis. Results were expressed as a percentage of the cells
in the G0/G1, S and G2/M phases based on differential DNA content.
Experiments were performed in triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Gene expression levels were determined by RT-qPCR.
Cells were harvested and total RNA isolation was performed using
the RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA), following
the manufacturer's instructions. Approximately 2 µg of total RNA
was reverse transcribed to cDNA with SuperScript® III
Reverse Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.).
RT-qPCR was performed using KAPA SYBR® FAST qPCR kits
(Kapa Biosystems, Inc., Woburn, MA, USA) and a StepOnePlus™
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc., Foster City, CA, USA) with the following steps: 95°C for 10
min, followed by 40 cycles at 95°C for 15 sec, 60°C for 30 sec and
72°C for 30 sec. All primers used to amplify genes are listed in
Table I. The level of gene
expression relative to an internal control, RPS13 (ribosomal
protein S13), was determined using the 2−ΔΔCq method
(19).
 | Table I.Primers used for quantitative
real-time RT-PCR analysis. |
Table I.
Primers used for quantitative
real-time RT-PCR analysis.
| Gene name | Forward sequence
5′-3′ | Reverse sequence
5′-3′ |
|---|
| uPA |
TCGTCTGTTCCCTCCAAGGC |
TGCGGATCCAGGGTAAGAAG |
| uPAR |
TAAGACCAACGGGGATTGCC |
TCTCCTTCTTCCCACAAGCG |
| MMP2 |
CAAGGACCGGTTCATTTGGC |
GGCCTCGTATACCGCATCAA |
| MMP7 |
AGTGGTCACCTACAGGATCG |
GGGATCTCTTTGCCCCACAT |
| MMP9 |
AAGGATGGGAAGTACTGGCG |
GCTCCTCAAAGACCGAGTCC |
| MMP11 |
ACCTTTACTGAGGTGCACGAG |
CAAATTCATGGGCTGCCACC |
| MMP13 |
CTTAGAGGTGACTGGCAAAC |
GCCCATCAAATGGGTAGAAG |
| HGF |
CGACAGTGTTTCCCTTCTCG |
ATTGAGAACCTGTTTGCGTTTCT |
| CXCR4 |
ACTTCAGTTTGTTGGCTGCGGC |
ACCGCTGGTTCTCCAGATGCG |
| MDR-1 |
GTCTTTGGTGCCATGGCCGT |
ATGTCCGGTCGGGTGGGATA |
| MRP-3 |
GGGACCCTGCGCATGAACCTG |
TAGGCAAGTCCAGCATCTCTGG |
| RPS13 |
CGAAAGCATCTTGAGAGGAACA |
TCGAGCCAAACGGTGAATC |
Western blot analysis
The expression levels of the signaling proteins were
determined by western blot analysis. Cells cultured on plastic
plates or embedded in semi-solid Matrigel were harvested, and then
lysed in cell signaling lysis buffer (Merck Millipore) containing
protease inhibitors and phosphatase inhibitors (1 mM
Na3VO4, 10 mM NaF and 20 mM
β-glycerophosphate). Cell extracts were resolved by 10% SDS-PAGE
and transferred to Immobilon-P Transfer Membranes (EMD Millipore,
Bedford, MA, USA). After blocking with 3% bovine serum albumin in
Tris-buffered saline containing 0.1% Tween-20 at room temperature
for 1 h, the membranes were probed with the following primary
antibodies overnight at 4°C: Akt (1:1,000; rabbit, polyclonal; cat.
no 9272), phospho-Akt (1:500; rabbit, polyclonal; cat. no. 9271),
ERK (1:4,000; rabbit, polyclonal; cat. no. 9102), phospho-ERK
(1:10,000; rabbit, polyclonal; cat. no. 9101), FAK (1:2,000;
rabbit, polyclonal; cat. no. 3285), phospho-FAK (1:1,000; rabbit,
monoclonal; cat. no. 8556), STAT3 (1:3,000; rabbit, polyclonal;
cat. no. 9132), phospho-STAT3 (1:500; rabbit, monolonal; cat. no.
9145), p38 (1:1,000; rabbit, polyclonal; cat. no. 9212),
phospho-p38 (1:500; rabbit, polyclonal; cat. no. 9211), p65
(1:1,000; rabbit, polyclonal; cat. no. 3034), phospho-p65 (1:500;
rabbit, polyclonal; cat. no. 3031), cyclin D1 (1:1,000; rabbit,
polyclonal; cat. no. 2922), p21 (1:2,000; rabbit, monoclonal; cat.
no. 2947), Bcl-xL (1:10,000; rabbit, monoclonal; cat. no. 2764),
Bax (1:5,000; rabbit, polyclonal; cat. no. 2772), IκB (1:1,000;
rabbit, monoclonal; cat. no. 4812) (all from Cell Signaling
Technology, Danvers, CA, USA), uPAR (1:500; rabbit, polyclonal;
cat. no. sc-10815; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), MYLK (1:500; mouse, monoclonal; cat. no. sc-365352; Santa
Cruz Biotechnology, Inc.) and GAPDH (1:50,000; rabbit, monoclonal;
cat. no. ab190480; Abcam, Cambridge, MA, USA). Following incubation
with the appropriate horseradish peroxidase-conjugated secondary
antibody (1:5,000; goat, monoclonal; cat. no. 7074; Cell Signaling
Technology, Danvers, CA, USA; or 1:5,000; rabbit, polyclonal; cat.
no. P0260; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA),
the protein bands were visualized using enhanced chemiluminescence
reagents.
Statistical analysis
All experiments were performed in triplicate, and
the data are presented as the mean ± standard deviation.
Statistical analysis was performed using the Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
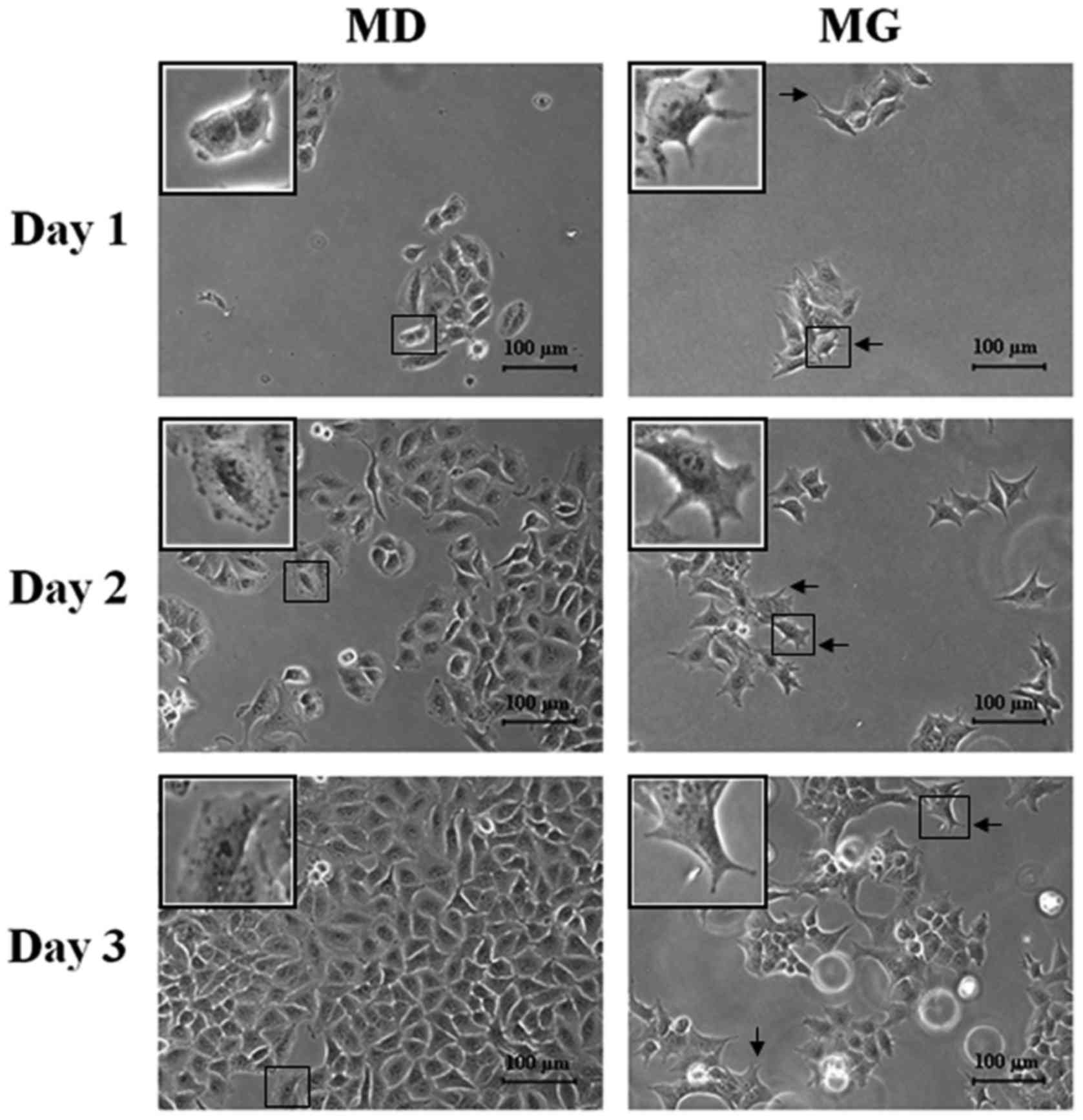
Morphology of A549 cells grown on
plastic plates vs. in semi-solid Matrigel
When A549 cells plated on plastic dishes or embedded
in semi-solid Matrigel were examined by inverted phase-contrast
microscopy, the cells grown within the Matrigel matrix had
undergone morphological changes by 24 h after plating (Fig. 1), transforming from their typical
shape to branched cells. This altered morphology could be reversed
by replating the semi-solid Matrigel embedded cells back into
plastic plates (data not shown).
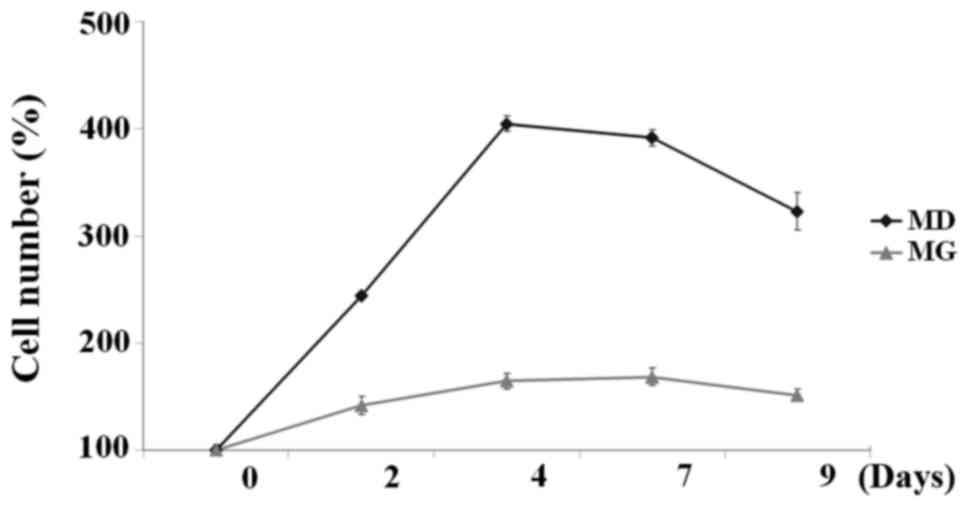
Proliferation of A549 cells grown on
plastic plates vs. in semi-solid Matrigel
Proliferation of A549 cells cultured on plastic
plates or embedded in semi-solid Matrigel was examined on days 0,
2, 4, 7 and 9 via DNA quantification using a PicoGreen DNA assay.
The data revealed that the growth dynamics differed between the two
cell populations; proliferative and quiescent growth
characteristics were observed. Proliferative growth characteristics
were observed in cells cultured on plastic plates, while quiescent
growth was observed in cells cultured in semi-solid Matrigel
(Fig. 2). A549 cells grown within
the Matrigel matrix exhibited a small increase in the number of
cells during the 9 days of culture, while the number of the cells
grown on plastic plates had increased 4-fold by day 4.
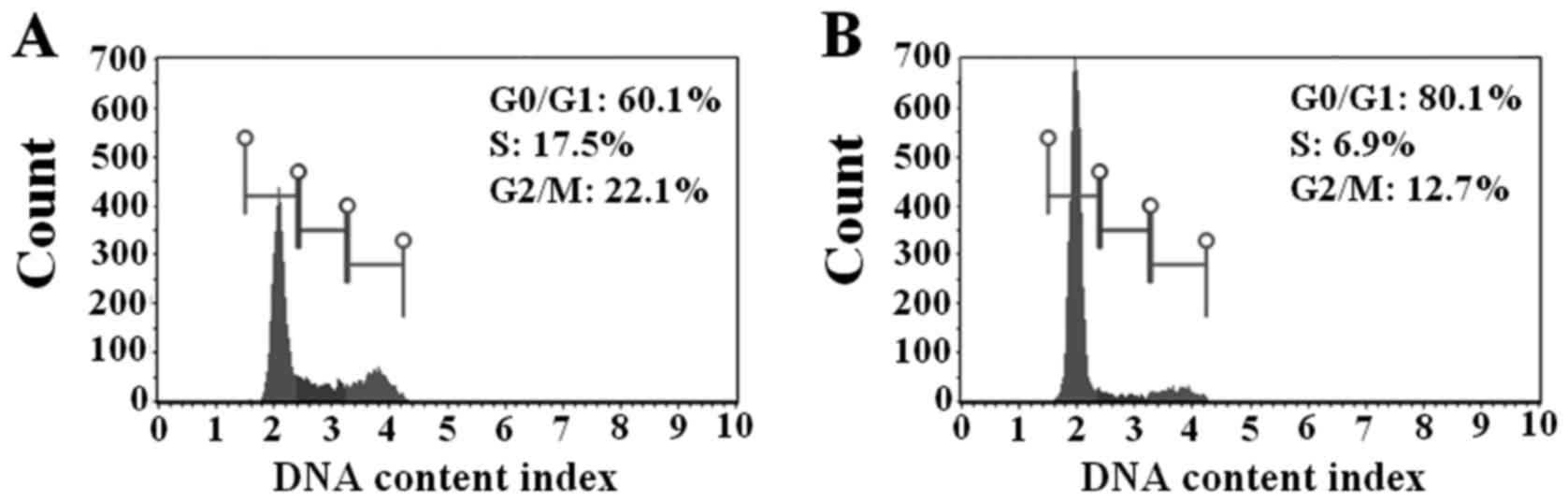
Semi-solid Matrigel embedding induces
G0/G1 cell cycle arrest in A549 cells
To assess possible mechanisms underlying
Matrigel-mediated cell growth inhibition, the cell cycle
distribution of A549 cells cultured on plastic plates and in
semi-solid Matrigel was investigated. The results demonstrated that
cells embedded in semi-solid Matrigel exhibited a higher G0/G1
population compared with cells cultured on plastic plates (80.1±2.2
vs. 60.1±1.5%; Fig. 3A and B).
Consistent with this result, Matrigel embedding caused a decrease
in the proportion of cells in the S phase (6.9±0.8%) and G2/M phase
(12.7±1.7%) of the cell cycle compared with cells grown on plastic
plates (17.5±0.4 and 22.1±1.0%, respectively). These data revealed
that Matrigel embedding induced cell cycle arrest (cellular
dormancy) at the G0/G1 phase, resulting in growth inhibition of
A549 cells.
Effect of semi-solid Matrigel
embedding on the chemosensitivity of A549 cells
Drug sensitivities of A549 cells cultured on plastic
plates and embedded in semi-solid Matrigel were explored. Multiple
anticancer drugs commonly used to treat a variety of cancers were
used in this study. The harvested cells from both culture
conditions were treated with various concentrations of the drugs
for 48 h, then MTT assays were performed to determine the survival
rates. The drug concentration causing a 50% decrease in the number
of cells (IC50) was extrapolated. As presented in
Table II, the results demonstrated
that cell culture in semi-solid Matrigel caused a marked increase
in IC50 for anticancer drugs that target actively
dividing cells [etoposide (10.6-fold), paclitaxel (5.7-fold),
vinblastine (4.4-fold), doxorubicin (4.1-fold) and
2-deoxy-D-glucose (3.9-fold)], as compared with cells cultured on
plastic plates. This indicated a higher degree of chemoresistance
in cells cultured in semi-solid Matrigel. However, the responses of
the two cell populations to cytotoxic agents that are not related
to cell proliferation rate were similar, such as curcumin (1.5-fold
increase in IC50 in cells cultured in Matrigel) and Bay
11–7085 (0.9-fold increase; Table
II). These results indicated that the chemoresistance of
semi-solid Matrigel-embedded cells may be the result of a decreased
rate of cell proliferation.
 | Table II.Chemosensitivity of A549 cells
cultured on plastic plates (MD) or in semi-solid Matrigel (MG)
against various anticancer agents. |
Table II.
Chemosensitivity of A549 cells
cultured on plastic plates (MD) or in semi-solid Matrigel (MG)
against various anticancer agents.
|
| IC50
values |
|
|---|
|
|
|
|
|---|
| Anticancer
agents | MD | MG | Fold change |
|---|
| Etoposide (µM) |
35.3±3.3 |
371.7±15.5 |
10.6±0.8 |
| Paclitaxel
(nM) |
79.8±17.7 |
430.0±21.6 |
5.7±1.6 |
| Vinblastine
(nM) |
18.3±1.9 |
79.8±5.3 |
4.4±0.4 |
| Doxorubicin
(µM) |
1±0.2 |
3.8±0.2 |
4.1±0.7 |
| 2-Deoxy-D-glucose
(mM) |
3.2±0.6 |
12.2±1.6 |
3.9±0.3 |
| Curcumin (µM) |
29.9±5.4 |
44.5±5.9 |
1.5±0.1 |
| Bay 11–7085
(µM) |
16.7±1.0 |
15.2±2.7 |
0.9±0.2 |
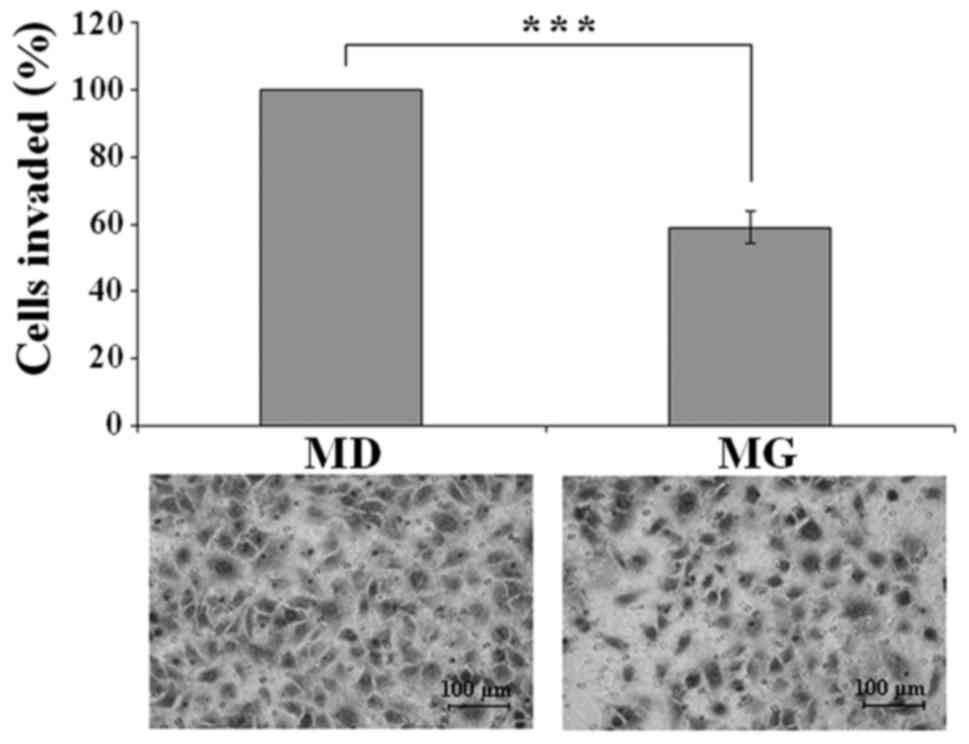
Effect of semi-solid Matrigel
embedding on invasion
A Transwell invasion assay was performed to assess
the effect of semi-solid Matrigel embedding on cell invasiveness.
A549 cells were cultured on plastic plates or in semi-solid
Matrigel for 72 h, then the cells were harvested and subjected to
an invasion assay. Photography and quantitative analysis of the
results revealed a significant decrease in the invasion rate of
cells cultured in semi-solid Matrigel (Fig. 4). The number of invading cells was
41% lower among cells cultured in semi-solid Matrigel, as compared
with those grown on plastic plates (P<0.001).
Effect of semi-solid Matrigel
embedding on cell migration
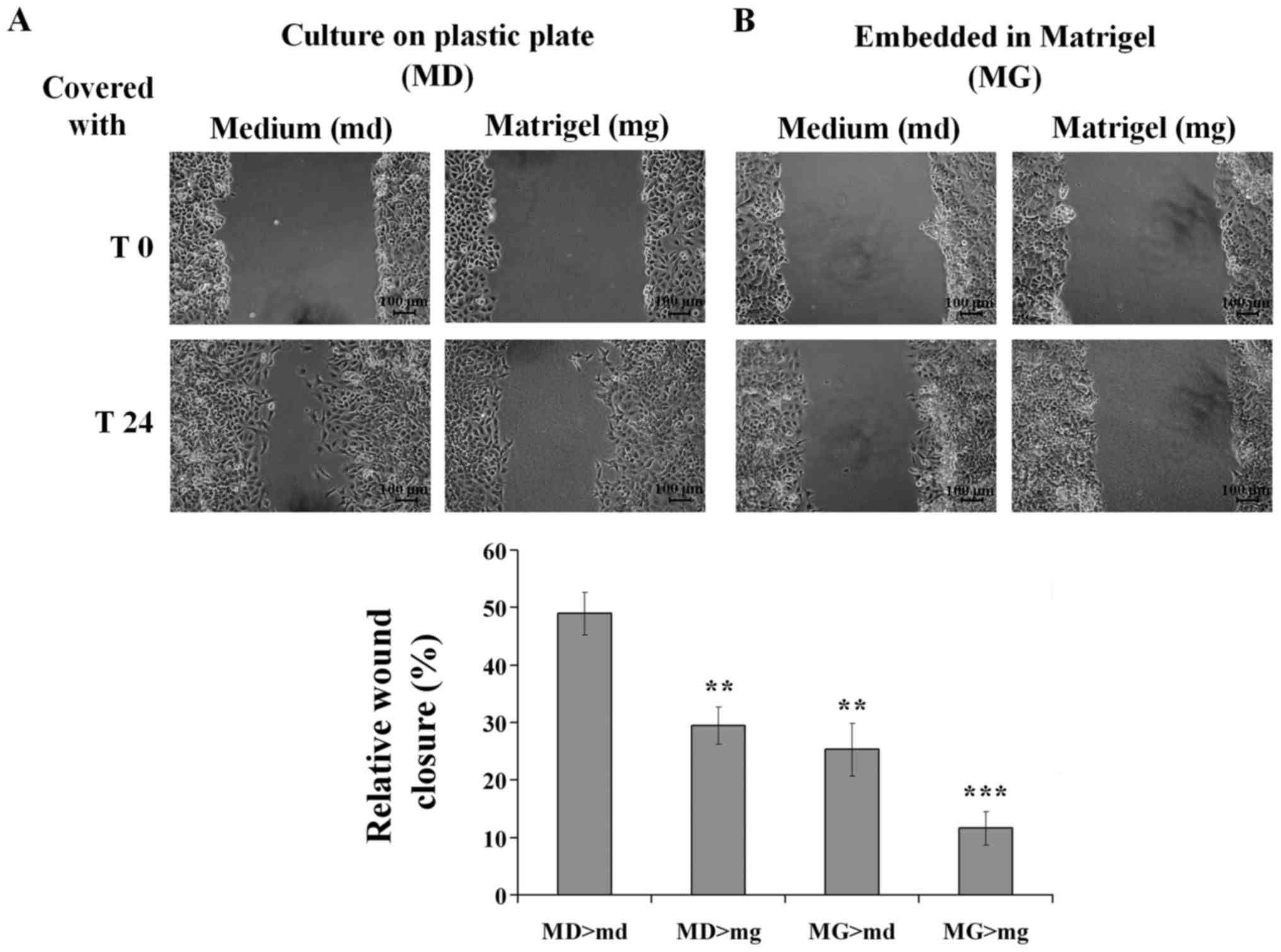
A wound-healing assay was performed to investigate
the effect of semi-solid Matrigel embedding on cell motility. Cell
migration was expressed as a percentage of wound closure. The
results demonstrated that, at 24 h, ~50% of the gap had closed for
A549 cells grown on plastic plates and covered with culture medium,
while only 30% of the initial gap had closed for cells grown on
plastic plates and covered with semi-solid Matrigel (Fig. 5A). For A549 cells grown within
semi-solid Matrigel, only 25 and 10% of the gap had closed when
covered with culture medium and semi-solid Matrigel, respectively
(Fig. 5B). These results revealed
that A549 cells cultured on plastic plates exhibited higher
motility compared with cells grown within semi-solid Matrigel in
both conditions. In addition, cell motility was decreased in the
presence of Matrigel.
Effect of semi-solid Matrigel
embedding on gene expression
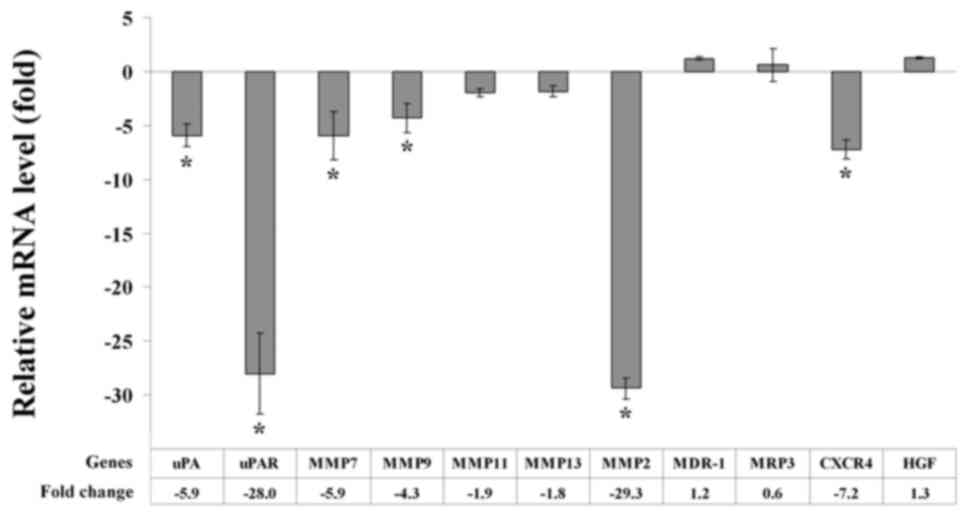
It has previously been reported that gene expression
is altered upon cell contact with ECM proteins (4). Therefore, we investigated the
consequences of semi-solid Matrigel embedding on the expression
patterns of genes associated with invasion (MMP2, MMP7, MMP9,
MMP11, MMP13, HGF and CXCR4) and drug resistance (MDR-1 and MRP3).
In addition, genes that are associated with tumor progression and
metastasis [uPA and uPA receptor (uPAR)] were analyzed. RT-qPCR was
performed to determine the level of gene expression. Gene
expression was normalized against RPS13 expression. Using a
threshold value of 3-fold expression change, the results revealed
that A549 cells cultured in semi-solid Matrigel exhibited decreased
expression levels of uPA, uPAR and multiple genes associated with
invasion (MMP2, MMP7, MMP9 and CXCR4), as compared with cells
cultured on plastic plates. However, no notable differences were
observed in the expression levels of drug resistance genes, MDR-1
and MRP3 (Fig. 6).
Effect of semi-solid Matrigel
embedding on protein expression
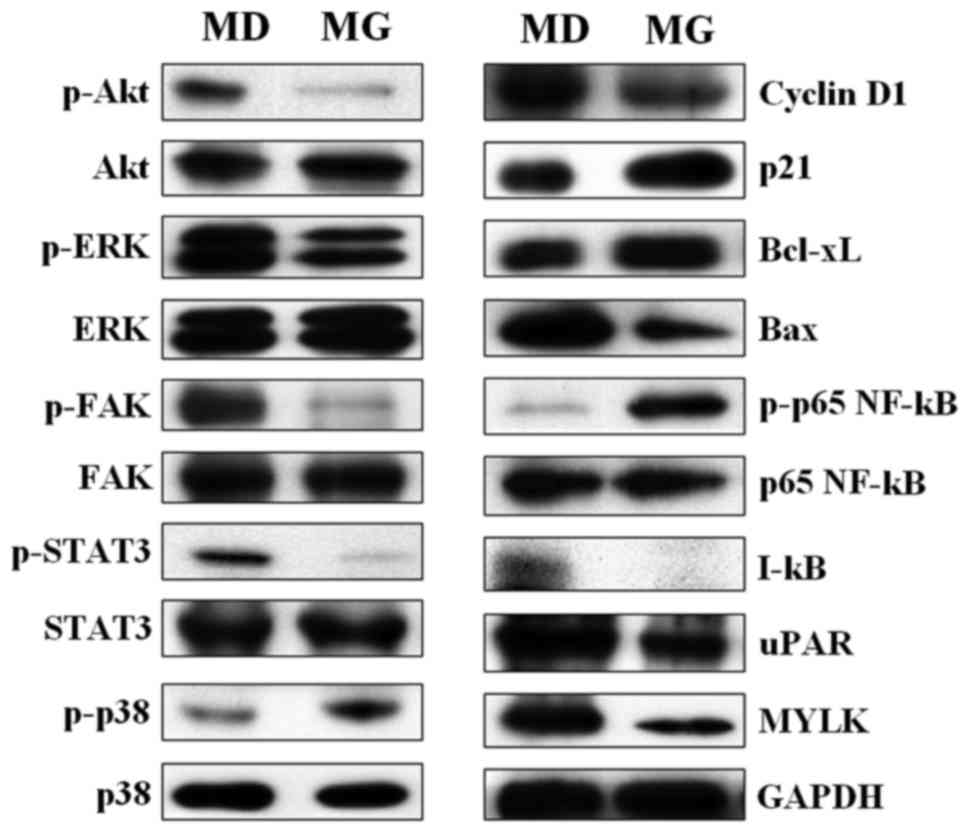
Protein expression levels of signaling molecules
associated with cell proliferation, metastasis, chemoresistance and
apoptosis were assessed. Western blot analysis of proteins
extracted from A549 cells indicated that cells grown within
semi-solid Matrigel had reduced expression of p-ERK, p-Akt and
p-STAT3 (positive regulators of cell growth, proliferation and
survival), compared with cells cultured on plastic plates (Fig. 7). Bcl-xL, an anti-apoptotic protein,
was up-regulated in cells cultured in semi-solid Matrigel, while a
decreased level of the pro-apoptotic protein, Bax, was detected
(Fig. 7). An increased level of
p-p65 (NF-κB) protein and decreased levels of IκB were observed in
cells cultured in semi-solid Matrigel (Fig. 7), suggested that NF-κB signaling was
activated in the Matrigel-embedded cells. Furthermore, the
upregulation of p-p38 and p21 was observed in cells cultured in
semi-solid Matrigel, while the expression of p-FAK, cyclin D1, uPAR
and myosin light-chain kinase (MYLK) was decreased, compared with
cells cultured on plastic plates (Fig.
7). These findings revealed that changes in the expression of
signaling molecules in cells cultured in semi-solid Matrigel
shifted the cells from a highly proliferative to a less
proliferative state, and also enhanced apoptotic resistance.
Inhibition of MAPK and Akt pathways
induces G0/G1 cell cycle arrest and protects against
etoposide-induced cell death
Specific kinase inhibitors of MEK1/2 (U0126), PI3K
(LY-294002) and JAK2 (AG-490) were used to investigate whether
these signaling pathways are associated with Matrigel-mediated
dormancy and chemoresistance. First, the effects of U0126,
LY-294002 and AG-490 on cell viability were evaluated. A549 cells
were treated with various concentrations of U0126, LY-294002 and
AG-490, after which an MTT assay was performed to determine
cytotoxicity following treatment. Inhibitor concentrations that
resulted in >80% cell viability were used. Pretreatment for 24 h
with 30 µM of U0126, 20 µM of LY-294002 and 30 µM of AG-490 did not
significantly affect A549 cell viability (data not shown). Western
blot analysis revealed decreased phosphorylation of ERK1/2, Akt and
STAT3 following treatment with 30 µM of U0126, 20 µM of LY-294002
and 30 µM of AG-490, respectively, for 24 h (data not shown). Cell
cycle analysis revealed that blockade of ERK1/2 and Akt with U0126
and LY-294002 resulted in G0/G1 phase cell cycle arrest, similar to
that observed in cells embedded in semi-solid Matrigel (Table III). Treatment with AG-490 had no
notable effect on the cell cycle distribution of A549 cells
(Table III).
 | Table III.Cell cycle progression of A549
cells. |
Table III.
Cell cycle progression of A549
cells.
|
| Cell cycle
distribution (%) |
|---|
|
|
|
|---|
| Treatment | G0/G1 | S | G2/M |
|---|
| Cultured on plastic
plates |
60.1±1.5 |
17.5±0.4 |
22.1±1.0 |
| Embedded in
semi-solid Matrigel |
80.1±2.2 |
6.9±0.8 |
12.7±1.7 |
| U0126 (30 µM) |
77.0±1.5 |
10.8±0.7 |
11.9±1.1 |
| LY-294002 (20
µM) |
78.4±1.3 |
9.5±0.6 |
12.0±0.8 |
| AG-490 (30 µM) |
61.3±2.8 |
16.5±1.4 |
21.8±1.4 |
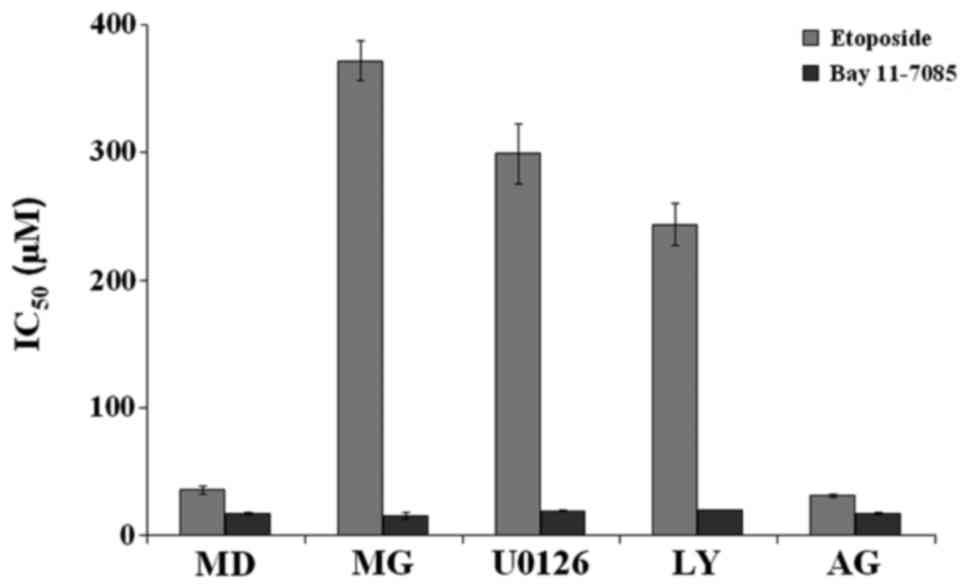
To determine whether the decrease in phosphorylation
of ERK1/2, Akt and STAT3 mediates chemoresistance of semi-solid
Matrigel-embedded cells, chemosensitivity to etoposide and Bay
11–7085 was evaluated in the presence of the specific kinase
inhibitors. Etoposide and Bay 11–7085 were used as representative
examples of drugs that are associated and not associated with,
respectively, the cell proliferation rate. The cells were
pre-incubated with the kinase inhibitors for 24 h prior to and
during the chemosensitivity assay. Comparing the effects on
chemosensitivity, U0126 and LY-294002 were determined to increase
etoposide resistance, similar to that observed in cells cultured in
semi-solid Matrigel (the IC50 value of cells treated
with U0126 and LY-294002 was 299±23.3 µM and 243.3±16.5 µM,
respectively; Fig. 8). However, the
chemoresistance to etoposide was unaffected by STAT3 inhibition
with AG-490 (the IC50 value of cells pre-treated with
AG-490 was 30.8±1.2 µM). For Bay 11–7085, treatment with U0126,
LY-294002 and AG-490 exhibited no effect on chemosensitivity (the
IC50 value of cells treated with U0126, LY-294002 and
AG-490 were 19.0±0.5, 19.4±0.1 and 17.0±1.1 µM, respectively;
Fig. 8). These findings were in
agreement with those of the cell cycle distribution experiment,
suggesting that inhibition of ERK1/2 and Akt with U0126 and
LY-294002 results in cell dormancy (Table III), which leads to enhanced
chemoresistance for the drugs that target actively proliferating
cells. These data revealed that Matrigel-mediated growth inhibition
of A549 cells and protection from chemotherapy-induced apoptosis
occurred predominantly via the MAPK and Akt pathways.
Discussion
Metastasis is the prime cause of cancer-related
deaths. Many cancer patients who have no clinical symptoms after
primary tumor removal suffer from disease relapse years or decades
later (20,21). A likely explanation for cancer
recurrence after a long latent period is that metastatic cells
remain dormant in the body, resistant to current therapies and
begin reactivation in a permissive microenvironment (22). Various factors have been suggested
as possible contributors to cell dormancy, including complex
interactions between metastatic cells and the microenvironment
(23). Interactions between
metastatic cancer cells and the new organ microenvironment have
been demonstrated to influence the proliferative properties of
cancer cells at the metastatic site (24,25).
Recent experiments have indicated that cell-ECM interactions
inhibit the proliferation of metastatic melanoma cells (26,27).
Matrigel is a commercially available matrix
resembling the complex tissue microenvironment. Matrigel contains
various soluble factors, including insulin-like growth factor,
TGF-β, tissue plasminogen activator, EGF, bFGF, and other growth
factors found in EHS tumors. Increasingly, cell-ECM interactions
are being modeled to bridge the gap between in vitro and
in vivo experiments. For instance, it has been reported that
MCF-7 and MDA-MB-231 cells cultured in ECM exhibited growth
characteristics that correspond with their behavior at secondary
sites in vivo (13).
Vascular smooth muscle cells grown on Matrigel were morphologically
similar to those found in in vivo studies of contractile
vascular smooth muscle (28). This
was consistent with another study detailing that three 21T cell
lines grown in Matrigel exhibited morphological and functional
characteristics resembling in vivo behavior, including
colony morphology, cell polarization, acinar structure formation,
cell proliferation and invasion (29). The present results revealed that
A549 cells grown within semi-solid Matrigel exhibited some
characteristics similar to those found in dormant cancer cells,
including a decrease in cell proliferation, cell motility and
invasion, and an increase in chemoresistance. Cell cycle
distribution analysis revealed that growth of A549 cells within
semi-solid Matrigel matrix induced G0/G1 cell cycle arrest.
MAPK/ERK, PI3K/Akt and STAT3 have been implicated in
cellular growth, proliferation and survival. The results of the
present study revealed a marked reduction in p-ERK, p-Akt and
p-STAT3 expression, concurrently with a decreased proliferation
rate of A549 cells cultured in semi-solid Matrigel, compared with
A549 cells cultured on plastic plates. The reduction in ERK1/2
activation has been linked to tumor dormancy in vivo
(30,31). The activation of p38
stress-activated kinase induced cell cycle arrest via inhibition of
ERK1/2 signaling and uPAR expression (30), consistent with the enhanced
phosphorylation of p38 MAPK and the reduction of uPAR at both mRNA
and protein levels observed in our results. uPA and its receptor,
uPAR, have been associated with metastasis, tumor progression and
reduced overall survival of patients (32). Current evidence demonstrates that
downregulation of uPAR leads to a reduction in FAK phosphorylation
and deactivation of ERK1/2, resulting in dormancy in vivo
(22,30,33).
The decrease in FAK phosphorylation and uPA mRNA levels in our
study further support this notion. In support of a role for FAK and
MYLK in cell migration, the low-motility A549 cells cultured in
semi-solid Matrigel exhibited a decrease in both MYLK and p-FAK
protein levels. A recent study revealed that MYLK also has a
functional role in cell proliferation, and inhibition of MYLK
prevents the transition from dormant to proliferative state
(13). The expression level of
genes linked to cancer cell invasion (MMP2, MMP7, MMP9 and CXCR4)
were also decreased in cells cultured in semi-solid Matrigel
compared with cells cultured on plastic plates, which was
consistent with the decrease in the invasion rate of cells cultured
in semi-solid Matrigel.
Cyclin D1 facilitates cell cycle progression by
sequestering the CDK inhibitor p21, allowing S phase initiation and
progression. Consistent with the decrease in proliferative activity
of A549 cells embedded in semi-solid Matrigel, the protein level of
cyclin D1 was decreased concurrently with an increased p21 level,
thus preventing cells from reentering the cell division cycle.
According to a recent study, a decrease in p-STAT3 and p-ERK
expression led to downregulation of the downstream target
molecules, including cyclin D1 (34). p21 has been previously demonstrated
to be negatively regulated by Akt (35), so the inactivation of Akt further
promotes the inhibitory effect of p21 on cell cycle progression.
Changes in the levels of these signaling molecules are likely to be
responsible for dormancy of A549 cells cultured in semi-solid
Matrigel.
A number of studies have presented evidence for
ECM-mediated cytoprotection (36–38).
The response of metastases in different organ microenvironments to
chemotherapy differs markedly due to interaction of metastatic
cells with local environmental factors (39). Soluble cytokines secreted by the
tumor microenvironment may activate signal transduction pathways
that influence the response to cytotoxic drugs of tumor cells
(40). The concept of ECM-mediated
chemoresistance is also supported by the observation that adhesion
of small cell lung cancer (SCLC) cells to laminin enhances
resistance to several cytotoxic drugs (1). Etoposide treatment of SCLC cells grown
on fibronectin significantly reduced caspase-3 activation and
apoptosis (41). The ECM-mediated
chemoresistance in the present study was likely to be due to a
decrease in cell proliferation, since A549 cells embedded in
semi-solid Matrigel exhibited marked resistance to chemotherapy
drugs that were related to the cell proliferation rate, compared
with cells cultured on plastic plates. By contrast, the cells from
both culture conditions exhibited similar chemosensitivity for
drugs that were not related to the cell proliferation rate. Thus,
targeting ECM-suppressed cells with compounds that are more potent
against cells cultured in semi-solid Matrigel could represent a
possible direction for cancer treatment.
Upregulation of Bcl-xL and downregulation of Bax may
also be involved, at least in part, in the evasion of apoptosis of
A549 cells cultured in semi-solid Matrigel. In addition, p-p65 has
been reported to confer resistance to chemotherapy (42). An increased level of p-p65,
concurrent with a decreased level of IκB, could also be responsible
for the acquired chemoresistance of A549 cells cultured in
semi-solid Matrigel. Overexpression of drug efflux pumps has been
associated with chemoresistance (43). However, the mRNA expression levels
of MDR-1 (encoding P-glycoprotein) and MRP3 (encoding multi-drug
resistance protein) was unaffected by ECM proteins in the present
study. Recent studies have established that cancer stem cells
(CSCs), a distinct population of cancer cells possessing stem-like
properties, are responsible for tumor initiation and progression,
metastasis, therapy/apoptosis resistance and relapse (44–49).
CSCs and dormant cells are closely related, since stem cells remain
dormant to prevent cell exhaustion and acquire mutations until they
become activated in response to injury or stress (50). A549 cells embedded in semi-solid
Matrigel in the present study acquired some stem-like properties,
in terms of quiescence and resistance to therapy. In addition, CSCs
and cells embedded in semi-solid Matrigel share some mechanisms for
dormancy and chemotherapeutic resistance, including cell cycle
modifications and the overexpression of anti-apoptotic proteins.
Cell cycle progression analysis and cytotoxicity assays indicated
that blockade of ERK1/2 and Akt in A549 cells induced G0/G1 phase
cell cycle arrest, accompanied by chemoresistance, similar to that
observed in cells cultured in semi-solid Matrigel. These results
are in agreement with those of previous studies, which reported
that the inhibition of ERK1/2 and Akt induced dormancy and was
associated with acquired resistance to chemotherapy drugs (51,52).
In conclusion, our results revealed that MAPK/ERK
and PI3K/Akt pathways may be possible mechanisms underlying
Matrigel-mediated dormancy and the chemoresistance of A549 cells.
The semi-solid Matrigel culture environment used in the present
study induced a dormant-like phenotype that may mimic in
vivo dormancy and could be used to predict responses to
chemotherapy. This semi-solid Matrigel-embedded cell culture system
could provide a simple means for screening and developing new
therapeutic approaches to improve the response to chemotherapeutic
agents and eliminate dormant micrometastatic cells.
Acknowledgements
This study was supported by the Chulabhorn Research
Institute (Bangkok, Thailand).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fridman R, Giaccone G, Kanemoto T, Martin
GR, Gazdar AF and Mulshine JL: Reconstituted basement membrane
(matrigel) and laminin can enhance the tumorigenicity and the drug
resistance of small cell lung cancer cell lines. Proc Natl Acad Sci
USA. 87:pp. 6698–6702. 1990; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weaver VM, Fischer AH, Peterson OW and
Bissell MJ: The importance of the microenvironment in breast cancer
progression: Recapitulation of mammary tumorigenesis using a unique
human mammary epithelial cell model and a three-dimensional culture
assay. Biochem Cell Biol. 74:833–851. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Romero-López M, Trinh AL, Sobrino A, Hatch
MM, Keating MT, Fimbres C, Lewis DE, Gershon PD, Botvinick EL,
Digman M, et al: Recapitulating the human tumor microenvironment:
Colon tumor-derived extracellular matrix promotes angiogenesis and
tumor cell growth. Biomaterials. 116:118–129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cramer GM, Jones DP, El-Hamidi H and Celli
JP: ECM composition and rheology regulate growth, motility, and
response to photodynamic therapy in 3D models of pancreatic ductal
adenocarcinoma. Mol Cancer Res. 15:15–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin CQ and Bissell MJ: Multi-faceted
regulation of cell differentiation by extracellular matrix. FASEB
J. 7:737–743. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Streuli CH, Bailey N and Bissell MJ:
Control of mammary epithelial differentiation: Basement membrane
induces tissue-specific gene expression in the absence of cell-cell
interaction and morphological polarity. J Cell Biol. 115:1383–1395.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muschler J, Lochter A, Roskelley CD,
Yurchenco P and Bissell MJ: Division of labor among the alpha6beta4
integrin, beta1 integrins, and an E3 laminin receptor to signal
morphogenesis and beta-casein expression in mammary epithelial
cells. Mol Biol Cell. 10:2817–2828. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakajima M, Morikawa K, Fabra A, Bucana CD
and Fidler IJ: Influence of organ environment on extracellular
matrix degradative activity and metastasis of human colon carcinoma
cells. J Natl Cancer Inst. 82:1890–1898. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gohji K, Nakajima M, Boyd D, Dinney CP,
Bucana CD, Kitazana S, Kamidono S and Fidler IJ: Organ-site
dependence for the production of urokinase-type plasminogen
activator and metastasis by human renal cell carcinoma cells. Am J
Pathol. 151:1655–1661. 1997.PubMed/NCBI
|
|
10
|
Luzzi KJ, MacDonald IC, Schmidt EE,
Kerkvliet N, Morris VL, Chambers AF and Groom AC: Multistep nature
of metastatic inefficiency: Dormancy of solitary cells after
successful extravasation and limited survival of early
micrometastases. Am J Pathol. 153:865–873. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naumov GN, MacDonald IC, Weinmeister PM,
Kerkvliet N, Nadkarni KV, Wilson SM, Morris VL, Groom AC and
Chambers AF: Persistence of solitary mammary carcinoma cells in a
secondary site: A possible contributor to dormancy. Cancer Res.
62:2162–2168. 2002.PubMed/NCBI
|
|
12
|
Naumov GN, Townson JL, MacDonald IC,
Wilson SM, Bramwell VH, Groom AC and Chambers AF: Ineffectiveness
of doxorubicin treatment on solitary dormant mammary carcinoma
cells or late-developing metastases. Breast Cancer Res Treat.
82:199–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barkan D, Kleinman H, Simmons JL, Asmussen
H, Kamaraju AK, Hoenorhoff MJ, Liu ZY, Costes SV, Cho EH, Lockett
S, et al: Inhibition of metastatic outgrowth from single dormant
tumor cells by targeting the cytoskeleton. Cancer Res.
68:6241–6250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boyerinas B, Zafrir M, Yesilkanal AE,
Price TT, Hyjek EM and Sipkins DA: Adhesion to osteopontin in the
bone marrow niche regulates lymphoblastic leukemia cell dormancy.
Blood. 121:4821–4831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghajar CM, Peinado H, Mori H, Matei IR,
Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY,
et al: The perivascular niche regulates breast tumour dormancy. Nat
Cell Biol. 15:807–817. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oridate N, Lotan R and Lotan D:
Reconstituted basement membrane (Matrigel): A useful semisolid
medium for growth of tumor cell colonies. In Vitro Cell Dev Biol
Anim. 32:192–193. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kleinman HK and Martin GR: Matrigel:
Basement membrane matrix with biological activity. Semin Cancer
Biol. 15:378–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alley MC, Scudiero DA, Monks A, Hursey ML,
Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH and Boyd
MR: Feasibility of drug screening with panels of human tumor cell
lines using a microculture tetrazolium assay. Cancer Res.
48:589–601. 1988.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meltzer A: Dormancy and breast cancer. J
Surg Oncol. 43:181–188. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karrison TG, Ferguson DJ and Meier P:
Dormancy of mammary carcinoma after mastectomy. J Natl Cancer Inst.
91:80–85. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aguirre-Ghiso JA: Models, mechanisms and
clinical evidence for cancer dormancy. Nat Rev Cancer. 7:834–846.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu W, Kim J and Ossowski L: Reduction in
surface urokinase receptor forces malignant cells into a protracted
state of dormancy. J Cell Biol. 137:767–777. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Radinsky R: Modulation of tumor cell gene
expression and phenotype by the organ-specific metastatic
environment. Cancer Metastasis Rev. 14:323–338. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fidler IJ: Seed and soil revisited:
Contribution of the organ microenvironment to cancer metastasis.
Surg Oncol Clin N Am. 10:257–269. 2001.PubMed/NCBI
|
|
26
|
Henriet P, Zhong ZD, Brooks PC, Weinberg
KI and DeClerck YA: Contact with fibrillar collagen inhibits
melanoma cell proliferation by up-regulating p27KIP1. Proc Natl
Acad Sci USA. 97:pp. 10026–10031. 2000; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roth JM, Akalu A, Zelmanovich A,
Policarpio D, Ng B, MacDonald S, Formenti S, Liebes L and Brooks
PC: Recombinant alpha2(IV)NC1 domain inhibits tumor
cell-extracellular matrix interactions, induces cellular
senescence, and inhibits tumor growth in vivo. Am J Pathol.
166:901–911. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Tsai P, Wieder ED, Kribben A, Van
Putten V, Schrier RW and Nemenoff RA: Vascular smooth muscle cells
grown on Matrigel. A model of the contractile phenotype with
decreased activation of mitogen-activated protein kinase. J Biol
Chem. 269:19653–19658. 1994.PubMed/NCBI
|
|
29
|
Souter LH, Andrews JD, Zhang G, Cook AC,
Postenka CO, Al-Katib W, Leong HS, Rodenhiser DI, Chambers AF and
Tuck AB: Human 21T breast epithelial cell lines mimic breast cancer
progression in vivo and in vitro and show stage-specific gene
expression patterns. Lab Invest. 90:1247–1258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aguirre Ghiso JA, Kovalski K and Ossowski
L: Tumor dormancy induced by downregulation of urokinase receptor
in human carcinoma involves integrin and MAPK signaling. J Cell
Biol. 147:89–104. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aguirre Ghiso JA, Liu D, Mignatti A,
Kovalski K and Ossowski L: Urokinase receptor and fibronectin
regulate the ERKMAPK to p38MAPK activity
ratios that determine carcinoma cell proliferation or dormancy in
vivo. Mol Biol Cell. 12:863–879. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pavón MA, Arroyo-Solera I, Céspedes MV,
Casanova I, León X and Mangues R: uPA/uPAR and SERPINE1 in head and
neck cancer: Role in tumor resistance, metastasis, prognosis and
therapy. Oncotarget. 7:57351–57366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aguirre Ghiso JA: Inhibition of FAK
signaling activated by urokinase receptor induces dormancy in human
carcinoma cells in vivo. Oncogene. 21:2513–2524. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Modi PK, Komaravelli N, Singh N and Sharma
P: Interplay between MEK-ERK signaling, cyclin D1, and
cyclin-dependent kinase 5 regulates cell cycle reentry and
apoptosis of neurons. Mol Biol Cell. 23:3722–3730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rössig L, Jadidi AS, Urbich C, Badorff C,
Zeiher AM and Dimmeler S: Akt-dependent phosphorylation of
p21Cip1 regulates PCNA binding and proliferation of
endothelial cells. Mol Cell Biol. 21:5644–5657. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hodkinson PS, Mackinnon AC and Sethi T:
Extracellular matrix regulation of drug resistance in small-cell
lung cancer. Int J Radiat Biol. 83:733–741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Said G, Guilbert M, Morjani H, Garnotel R,
Jeannesson P and El Btaouri H: Extracellular matrix proteins
modulate antimigratory and apoptotic effects of Doxorubicin.
Chemother Res Pract. 2012:2686812012.PubMed/NCBI
|
|
38
|
Majidinia M and Yousefi B: Breast tumor
stroma: A driving force in the development of resistance to
therapies. Chem Biol Drug Des. 89:309–318. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fidler IJ, Wilmanns C, Staroselsky A,
Radinsky R, Dong Z and Fan D: Modulation of tumor cell response to
chemotherapy by the organ environment. Cancer Metastasis Rev.
13:209–222. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dalton WS: The tumor microenvironment as a
determinant of drug response and resistance. Drug Resist Updat.
2:285–288. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Buttery RC, Rintoul RC and Sethi T: Small
cell lung cancer: The importance of the extracellular matrix. Int J
Biochem Cell Biol. 36:1154–1160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Godwin P, Baird AM, Heavey S, Barr MP,
O'Byrne KJ and Gately K: Targeting nuclear factor-kappa B to
overcome resistance to chemotherapy. Front Oncol. 3:1202013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ughachukwu P and Unekwe P: Efflux
pump-mediated resistance in chemotherapy. Ann Med Health Sci Res.
2:191–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu H, Patel MR, Prescher JA, Patsialou A,
Qian D, Lin J, Wen S, Chang YF, Bachmann MH, Shimono Y, et al:
Cancer stem cells from human breast tumors are involved in
spontaneous metastases in orthotopic mouse models. Proc Natl Acad
Sci USA. 107:pp. 18115–18120. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pajonk F, Vlashi E and McBride WH:
Radiation resistance of cancer stem cells: The 4 R's of
radiobiology revisited. Stem Cells. 28:639–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
D'Andrea FP: Intrinsic radiation
resistance of mesenchymal cancer stem cells and implications for
treatment response in a murine sarcoma model. Dan Med J.
59:B43882012.PubMed/NCBI
|
|
47
|
LaBarge MA: The difficulty of targeting
cancer stem cell niches. Clin Cancer Res. 16:3121–3129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lacerda L, Pusztai L and Woodward WA: The
role of tumor initiating cells in drug resistance of breast cancer:
Implications for future therapeutic approaches. Drug Resist Updat.
13:99–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang Z, Li Y, Ahmad A, Azmi AS, Kong D,
Banerjee S and Sarkar FH: Targeting miRNAs involved in cancer stem
cell and EMT regulation: An emerging concept in overcoming drug
resistance. Drug Resist Updat. 13:109–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Trumpp A, Essers M and Wilson A: Awakening
dormant haematopoietic stem cells. Nat Rev Immunol. 10:201–209.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Endo H, Okuyama H, Ohue M and Inoue M:
Dormancy of cancer cells with suppression of AKT activity
contributes to survival in chronic hypoxia. PLoS One. 9:e988582014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li Q, Chow AB and Mattingly RR:
Three-dimensional overlay culture models of human breast cancer
reveal a critical sensitivity to mitogen-activated protein kinase
kinase inhibitors. J Pharmacol Exp Ther. 332:821–828. 2010.
View Article : Google Scholar : PubMed/NCBI
|