Introduction
Liver cancer is the major type of primary liver
cancer as well as one of the most common malignant tumors worldwide
(1). China is a country with the
highest incidence of liver cancer in the world and its overall
morbidity and mortality show increasing trends (2). Although therapeutic approaches
targeted at liver cancer have been continuously advanced in recent
years, the overall therapeutic effects on liver cancer have shown
no obvious improvement, and recurrence and metastasis remain the
major factors affecting the prognosis of patients with liver cancer
(3).
Tumor metastasis is a multi-step, multi-stage and
multi-pathway complex process, which includes the detachment of
tumor cells from the primary lesion, invasion into blood vessels or
lymph vessels, migration and adhesion to appropriate sites,
induction of tumor angiogenesis, anti-host antitumor immunity, and
the eventual formation of distal metastasis (4). Multiple genes are involved during this
process. A vast majority of current studies have focused on the
effects and functions of related genes in tumor invasion and
metastasis, such as oncogenes, tumor-suppressor genes and
pro-metastatic genes (5). Research
has demonstrated that, apart from these protein-coding genes which
can regulate tumor invasion and metastasis, various non-coding
genes, particularly microRNA molecules, are also involved in
regulating tumor invasion and metastasis. MicroRNAs have been found
to be abnormally expressed in multiple malignant tumors, including
colorectal, lung, breast, liver, brain and prostate cancer, and
they play an important role in tumor proliferation,
differentiation, invasion, metastasis and treatment response
(6).
The PI3K/Akt signaling pathway plays a vital role in
the genesis, development and survival of tumors (7). With multiple biological activities,
activated Akt can catalyze the phosphorylation of a series of
proteins, promote tumor cell growth and proliferation, inhibit
apoptosis, facilitate invasion and metastasis, regulate endothelial
growth and angiogenesis, and increase sensitivity to radiation.
PTEN molecule is a significant inhibitory regulator of PI3K
(8).
As is indicated in research, PTEN gene deletions or
mutations can be observed in multiple human malignant solid tumors
(including prostate cancer, glioblastoma multiforme, melanoma,
thyroid cancer and bladder cancer) as well as hereditary tumor
susceptible syndrome (7). The PTEN
gene exerts certain effects on regulating phosphatase activities of
multiple intracellular protein molecules, rendering gene deletion
and mutation, which plays an important role in tumor genesis and
development (9).
As a type of nuclear transcription factor that is
extensively distributed in multiple cells, NF-κB plays an important
role in aspects of cell carcinogenesis and apoptosis regulation
(10). It has been indicated in
research that the NF-κB signal transduction pathway is involved in
the transcription expression of oncogenes and tumor-suppressor
genes in the liver, which can participate in the genesis and
development of liver cancer by inhibiting cell apoptosis. As a type
of polyphonic transcription factor with multi-function, NF-κB can
regulate cell apoptosis- and proliferation-associated genes, thus
playing an important role in cell carcinogenesis (11). The NF-κB protein family is comprised
of 5 distinct subunits, among which, the p50/p65 heterodimer is the
most common one. Abnormal regulation is induced when NF-κB is
inappropriately activated and located at the cell nucleus where it
cannot return to the cytoplasm (12). Furthermore, we examined the role of
microRNA-26b signaling in mediating the tumor growth of human liver
cancer.
Materials and methods
Liver cancer tissue
Written informed consent was obtained from all
patients according to the guidelines approved by the Institutional
Research Board of The First Affiliated Hospital of Dalian Medical
University and information regarding the clinical samples are shown
at Table I. Liver cancer tissue and
the adjacent normal tissue were collected from patients at The
First Affiliated Hospital of Dalian Medical University from May
2016 to August 2016. All specimens were immediately frozen in
liquid nitrogen and stored at −70°C until use.
 | Table I.Information concerning the clinical
samples from the patients with liver cancer. |
Table I.
Information concerning the clinical
samples from the patients with liver cancer.
| Variables | All patients
(N=54) | P-value |
|---|
| Age (years) |
| 0.592 |
| ≤60 | 32 |
|
|
>60 | 22 |
|
| Sex |
| 0.321 |
|
Female | 30 |
|
| Male | 24 |
|
| Tumor size (cm) |
|
|
| ≤3.0 | 25 | 0.832 |
|
>3.0 | 29 |
|
| Edmondson grade |
|
|
| I–II | 11 |
|
|
III–IV | 43 |
|
qRT-PCR detection
Total RNA isolation from tissues was conducted using
the TRIzol kit (Invitrogen, Carlsbad, CA, USA), followed by RNeasy
Mini kit (Qiagen, Inc., Valencia, CA, USA). RNA (100 ng) was
reverse-transcribed into cDNA using an iScript cDNA synthesis kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). MicroRNA-26b
expression was quantified using miR-qRT PCR using the Hairpin-it™
miRNA qPCR Quantitation kit (GenePharma Co., Ltd., Shanghai, China)
and measured as using the 2−ΔΔCt method.
HepG2 cell culture and controlled
microRNA-26 expression
HepG2 cells were obtained from the Cell Culture
Center of Chinese Peking Union Medical College (Beijing, China) and
cultured in Dulbeccos modified Eagles medium (DMEM) containing 10%
fetal bovine serum (FBS) (All from Thermo Fisher Scientific, Inc.,
Shanghai, China) at 37°C in 5% CO2. Anti-microRNA-26 and
control inhibitor mimics were purchased from RiboBio Co., Ltd.
(Guangzhou, China). MicroRNA-26 and control mimics were transfected
into HepG2 cells using Invitrogen™ Lipofectamine 2000 reagent
(Thermo Fisher Scientific). After cells were transfected for 6 h,
the cells was treated with 10 µM of 3-methyladenine (PI3K
inhibitor) or 5 µM of PDTC (NF-κB inhibitor) for 48 h.
Proliferation assay
Cells transfected with the mimics
(1×105/ml) were added to each well of a 96-well plate
for 24, 48 and 72 h. MTT (20 µl) (5 mg/ml; Sigma-Aldrich China,
Inc., Shanghai, China) was added and incubation was carried our at
37°C for 4 h and 150 µl DMSO was added and agitated for 20 min in
the dark for dissolution. The optical density (OD) was then
measured at an absorbance of 570 nm.
Apoptosis assay
Cells transfected with the mimics
(1×105/ml) were added to each well of a 6-well plate for
48 h. Cells were washed twice with PBS, and then centrifuged at
1,000 × g for 5 min. Annexin V/FITC (10 µl) and propidium iodide
(PI) (5 µl) (Becton-Dickinson, San Jose, CA USA) were added and
maintained at room temperature for 15 min in the dark. Apoptosis
were then analyzed by flow cytometry (BD FACSCalibur; BD
Biosciences, Franklin Lakes, NJ, USA).
Western blotting
Cells transfected with the mimics
(1×105/ml) were added to each well of a 6-well plate for
48 h, and then incubated with RIPA lysis buffer (Beyotime, Inc.,
Jiangsu, China) for 30 min. Protein contents were quantified using
the Bicinchoninic acid (BCA) protein kit (Beyotime, Inc.). Protein
(50 µg) was separated by 8–12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) and transferred into
a polyvinylidene fluoride (PVDF) membrane. The membrane was
incubated with anti-PI3K (4249), anti-p-Akt (4060), anti-NF-κB
(8242), anti-MMP-9 (13667), anti-pVEGF (2463) and anti-GAPDH (5174)
(Cell Signaling Technology, Inc., Danvers, MA, USA) antibodies
overnight at 4°C. The membrane was then incubated with the
anti-rabbit secondary antibody at 37°C for 1 h and protein bands
were visualized using an enhanced chemiluminesecence system.
Statistical analysis
Data are shown as mean ± SEM. The correlations were
analyzed using the analysis of variance (ANOVA). A probability
value of <0.05 was chosen for statistical significance.
Results
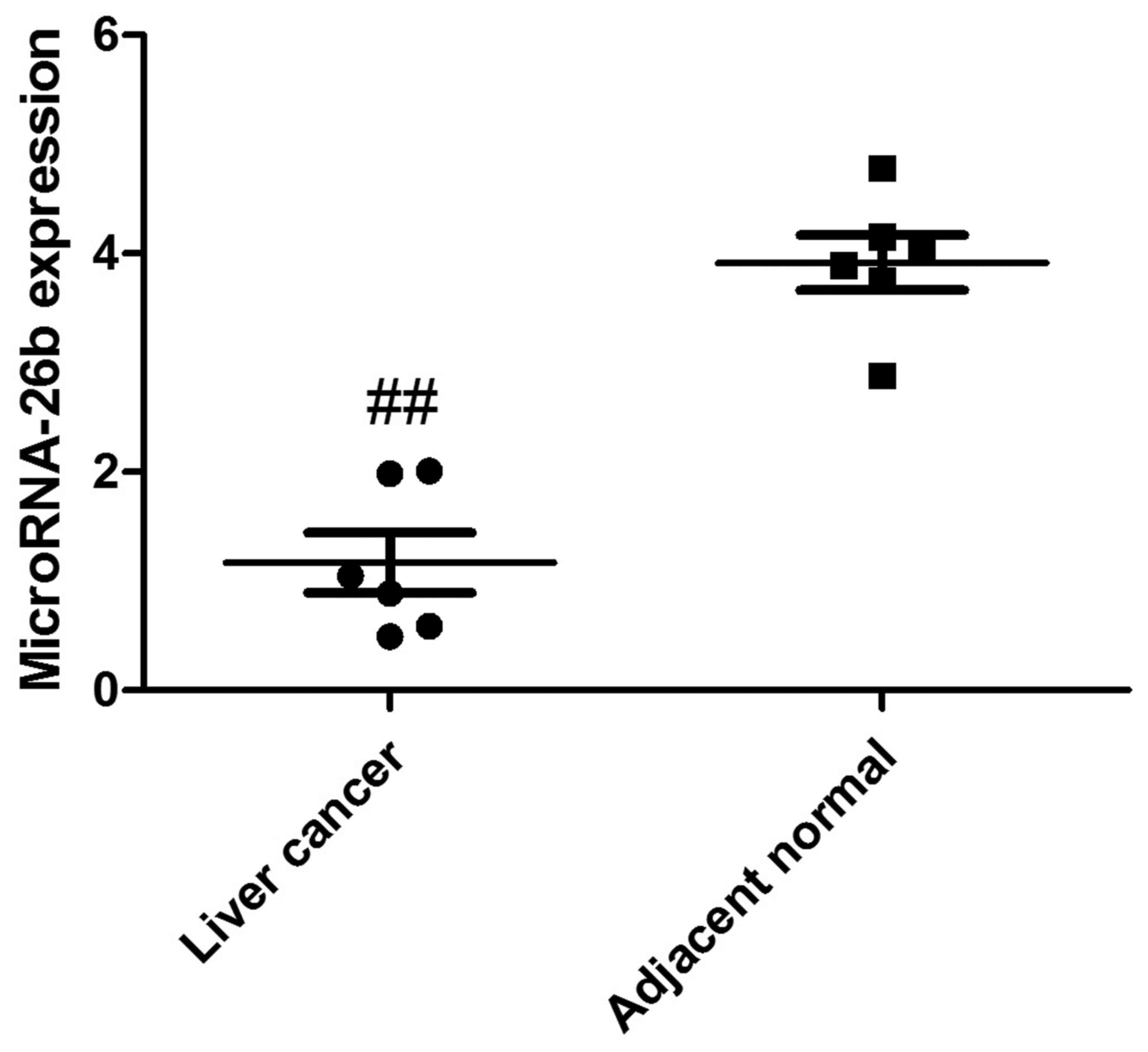
MicroRNA-26b expression in human liver
cancer tissue
To analyze the clinical samples of human liver
cancer tissue and the adjacent normal tissue, we performed qRT-PCR
to detect the expression of microRNA-26b. As shown in Fig. 1, microRNA-26b expression was
observably downregulated in yhe human liver cancer tissues,
compared with that noted in the adjacent normal tissues.
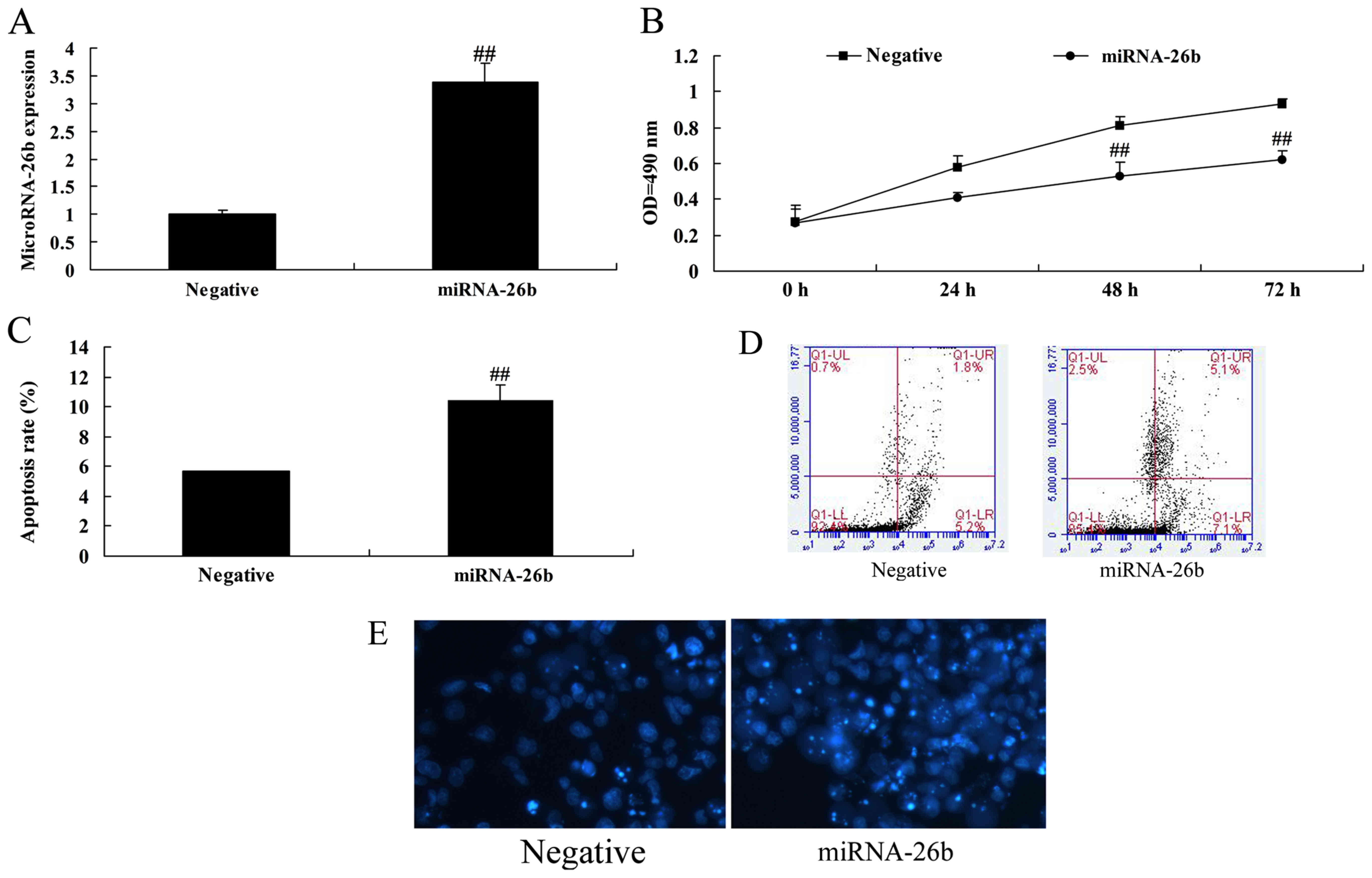
Correlation between microRNA-26b and
cell growth of liver cancer
In order to assess whether microRNA-26b possess
anticancer effects on liver cancer, microRNA-26b expression was
upregulated in HepG2 cells using microRNA-26b mimics. Fig. 2 indicates that microRNA-26b was
upregulated in the HepG2 cells using microRNA-26b mimics, and
upregulation of microRNA-26b significantly decreased cell
proliferation and induced apoptosis of the HepG2 cells.
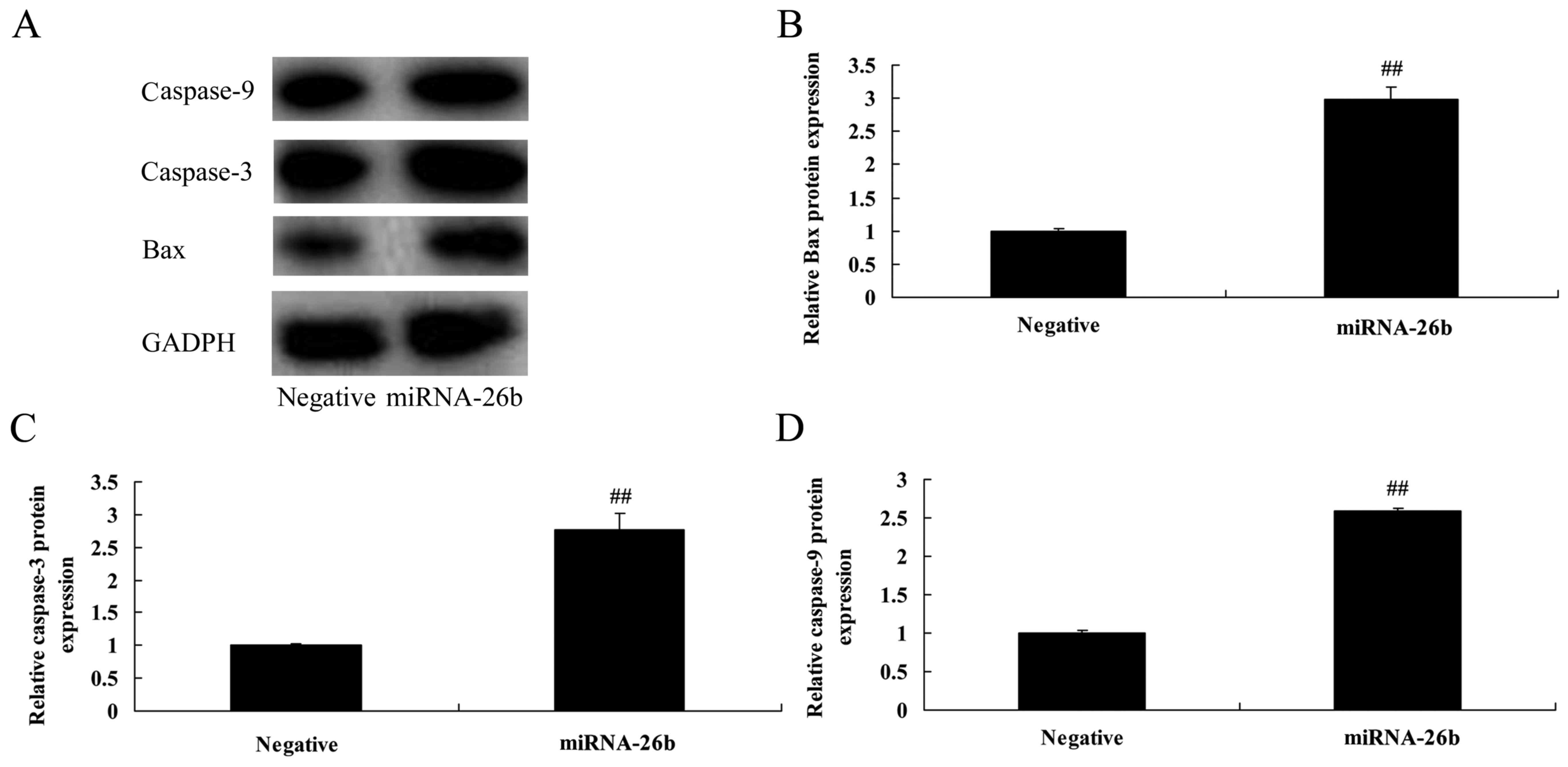
Correlation between microRNA-26b and
Bax and caspase-3/-9 protein expression in liver cancer
Then, we analyzed the correlation between
microRNA-26b and Bax and caspase-3/-9 protein expression in liver
cancer. As shown in Fig. 3,
statistical analyses demonstrated that microRNA-26b significantly
induced Bax and caspase-3/-9 protein expression in the HepG2
cells.
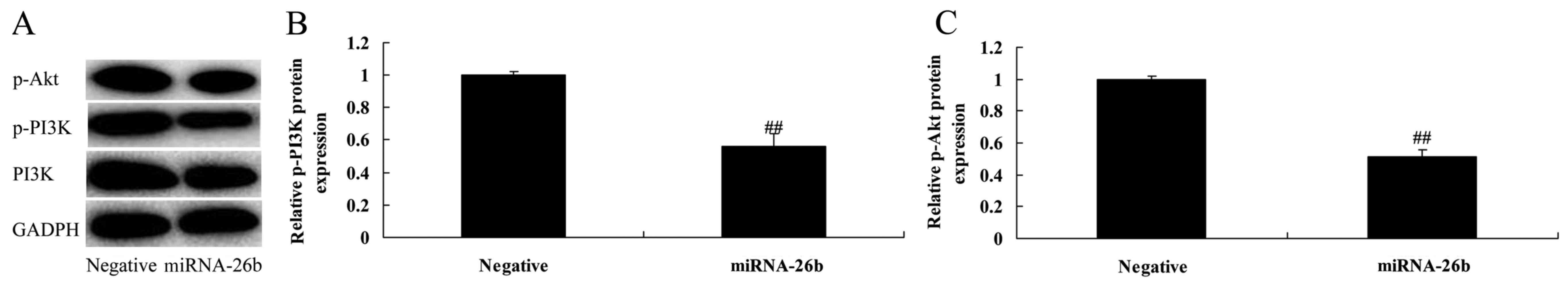
Correlation between microRNA-26b and
PI3K/Akt and NF-κB/MMP-9/VEGF pathways
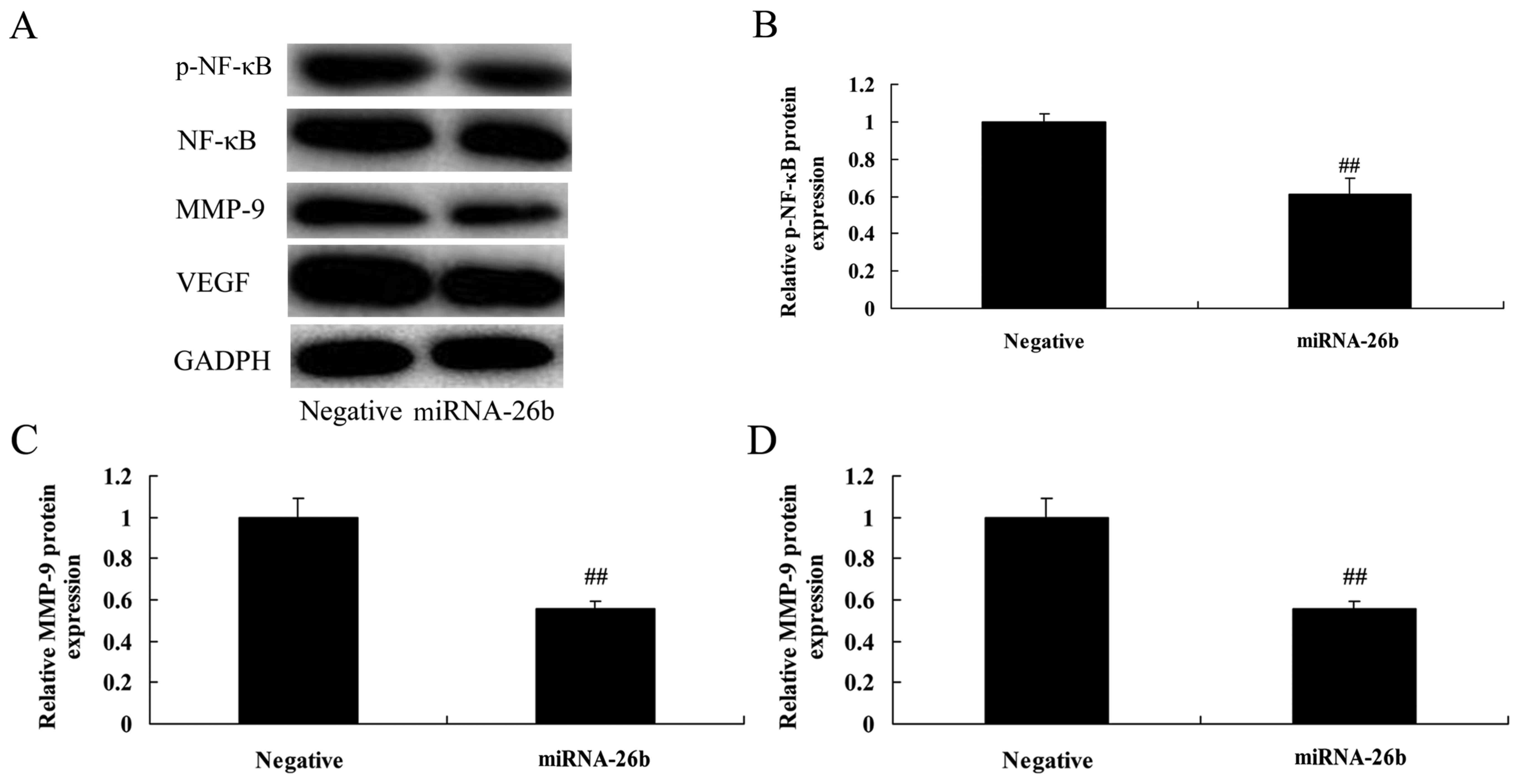
To investigate the correlation between microRNA-26b
and PI3K/Akt and NF-κB/MMP-9/VEGF pathways. PI3K, p-Akt, NF-κB,
MMP-9 and VEGF protein levels were significantly suppressed in the
HepG2 cells by the upregulation of microRNA-26b, compared with the
negative control group (Figs. 4 and
5). These results suggest that
microRNA-26b may have a functional role in the development of human
liver cancer.
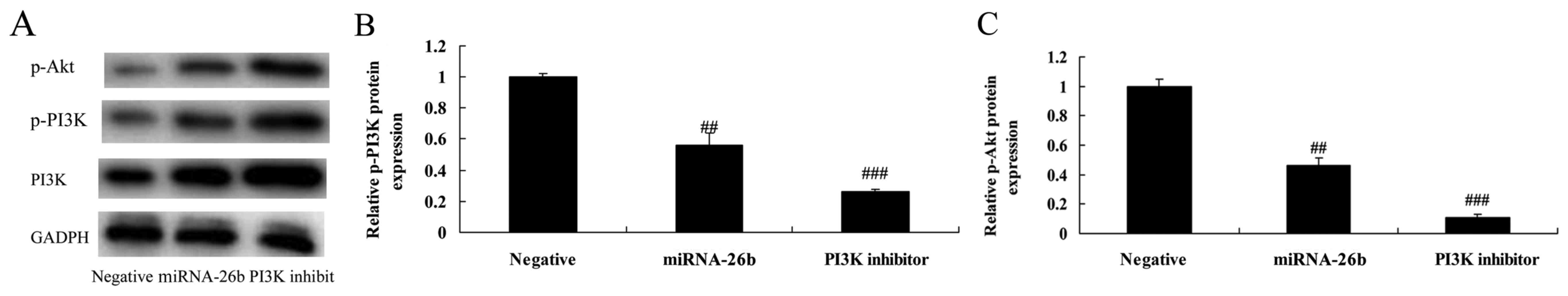
PI3K inhibitor regulates the
anticancer effects of microRNA-26b on PI3K/Akt pathways of liver
cancer
We examined whether the function of PI3K
participates in the anticancer effects of microRNA-26b on liver
cancer. PI3K inhibitor significantly further suppressed PI3K and
p-Akt protein expression in the HepG2 cells by microRNA-26b, when
compared with the negative control group (Fig. 6).
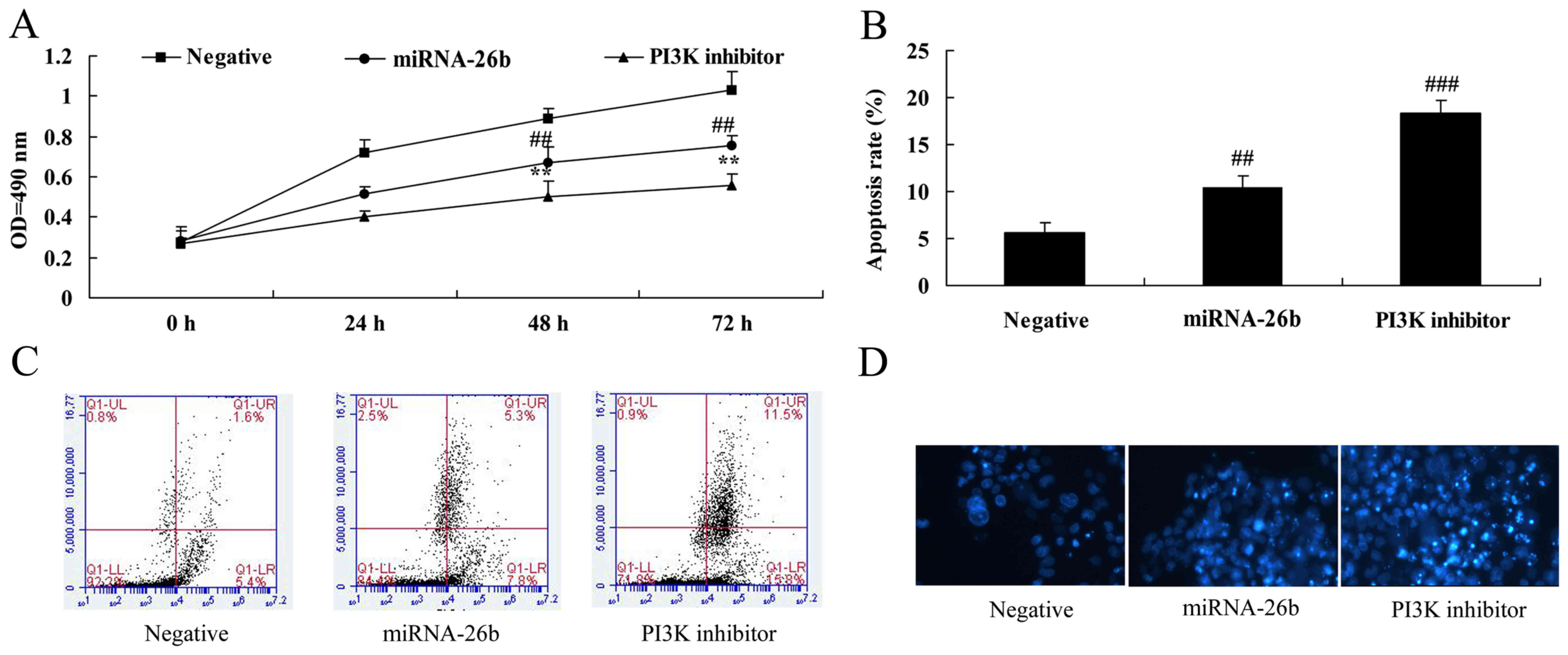
PI3K inhibitor regulates the
anticancer effects of microRNA-26b on liver cancer cell growth
We further examined the function of PI3K in the
anticancer effects of microRNA-26b on liver cancer cell growth and
apoptosis. Analysis of MTT assay and flow cytometry showed that, as
compared to the negative control, the PI3K inhibitor significantly
accelerated the anticancer effects of microRNA-26b on the
inhibition of liver cancer cell growth and induction of apoptosis
(Fig. 7).
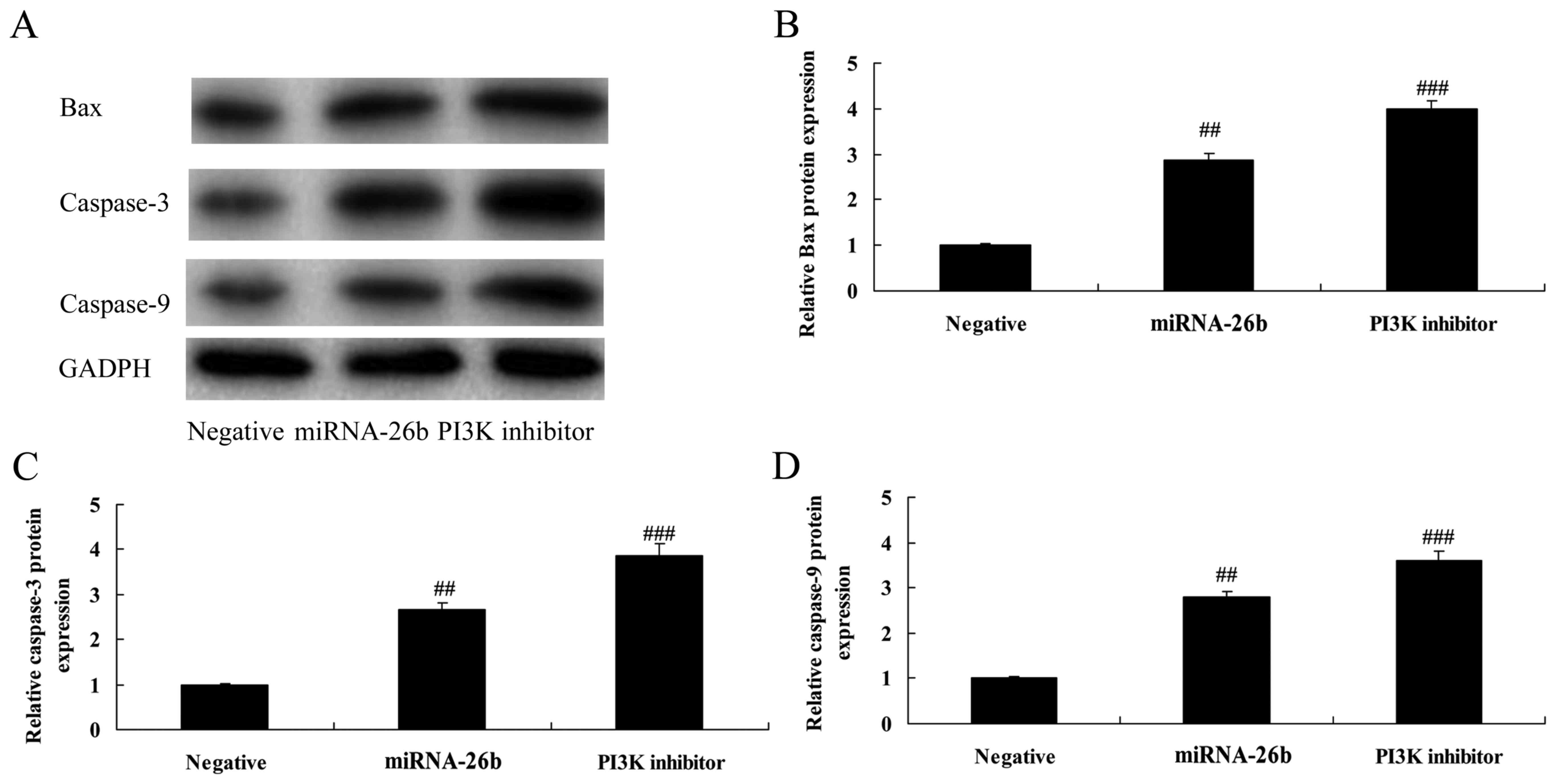
PI3K inhibitor regulates the
anticancer effects of microRNA-26b on Bax and caspase-3/-9 protein
expression in liver cancer
We analyzed the effects of PI3K on Bax and
caspase-3/-9 protein levels in the HepG2 cells by microRNA-26b
using western blotting. The PI3K inhibitor further significantly
induced Bax and caspase-3/-9 protein expression in the liver cancer
cells by microRNA-26b, compared with the negative control group
(Fig. 8). These data demonstrated
that PI3K is correlated with the anticancer effects of microRNA-26b
on liver cancer.
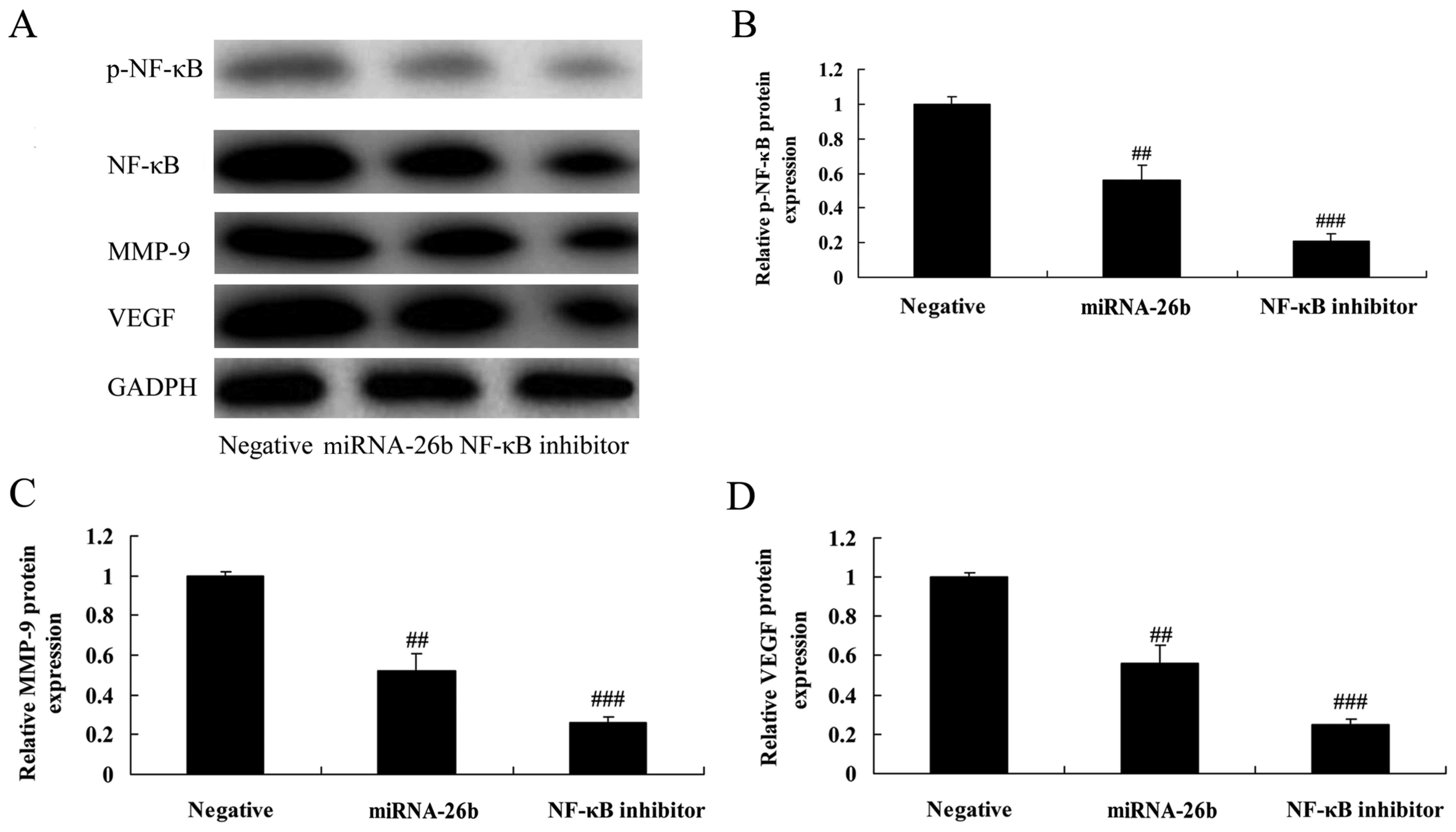
NF-κB inhibitor regulates the
anticancer effects of microRNA-26b on NF-κB/MMP-9/VEGF pathways in
liver cancer
We examined whether NF-κB influences the anticancer
effects of microRNA-26b in liver cancer. The NF-κB inhibitor
significantly further suppressed NF-κB, MMP-9 and VEGF protein
expression in liver cancer cells by microRNA-26b, compared with the
negative control group (Fig.
9).
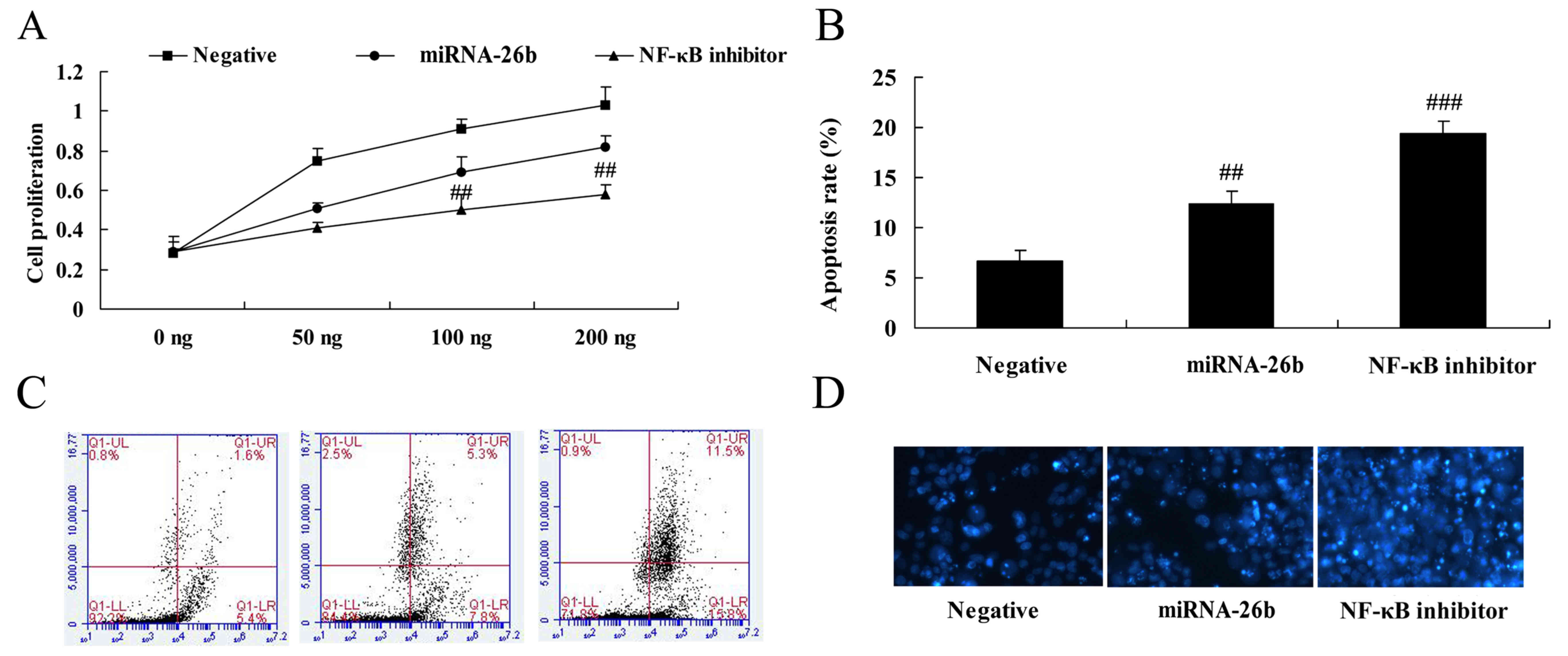
NF-κB inhibitor regulates the
anticancer effects of microRNA-26b on liver cancer cell growth
The results of analyses for liver cancer cell growth
and apoptosis using MTT assay and flow cytometry in HepG2 cells by
microRNA-26b and NF-κB inhibitor are shown in Fig. 10. It was observed that NF-κB
inhibitor significantly facilitated the anticancer effects of
microRNA-26b on liver cancer cell growth inhibitor and apoptosis
induction (Fig. 10).
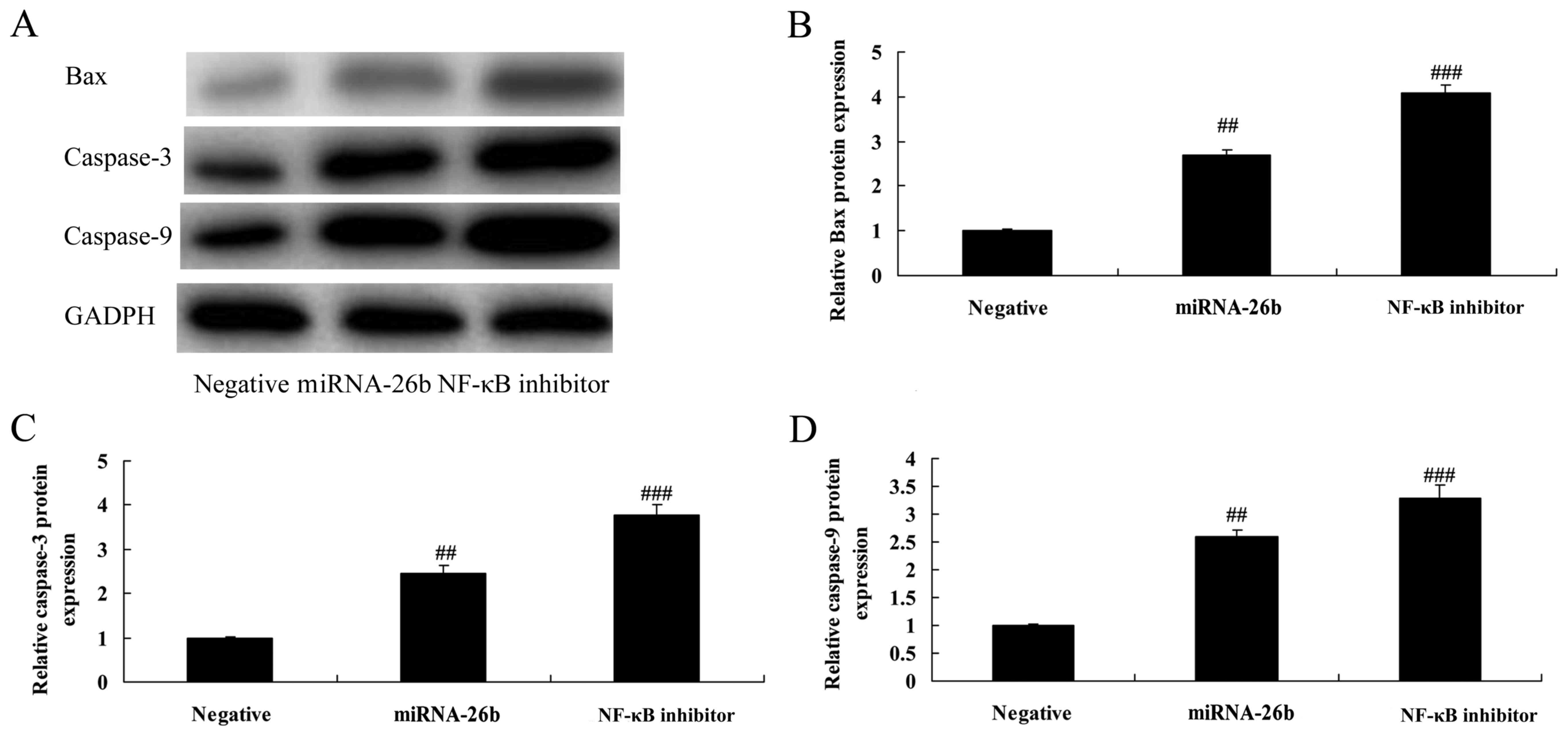
NF-κB inhibitor regulates the
anticancer effects of microRNA-26b on Bax and caspase-3/-9 protein
expression in liver cancer
In addition, we analyzed Bax and caspase-3/-9
protein expression in liver cancer by microRNA-26b. As shown in
Fig. 11, NF-κB inhibitor
significantly further induced Bax and caspase-3/-9 protein
expression in the HepG2 cells by microRNA-26b, compared with the
negative control group.
Discussion
Liver cancer is a major disease that severely
threatens human health. Liver cancer shows an annually increasing
trend due to the influence of factors such as viral hepatitis and
environment (13). Although liver
cancer is treated by conventional surgery combined with drugs
(chemotherapy) in the clinic, recurrence and metastasis, together
with the resistance of cancer cells to drugs has resulted in bleak
prospects for treating liver cancer. Consequently, unveiling the
molecular mechanisms of the drug resistance of liver cancer will
bring new hopes for treating liver cancer (14). In the present study, we showed that
microRNA-26b expression was observably downregulation in human
liver cancer tissue.
microRNAs exert effects that are analogous to tumor
suppressors or oncogenes by binding with oncogenes or tumor
suppressor genes, and are closely related to tumor genesis and
development (15). Participating in
tumor invasion and metastasis, microRNAs play a regulatory role in
multiple steps during tumor metastasis (16). Abnormal microRNA expression has been
reported e to exist in human cancers, and the differential
expression profile has been reported in multiple cancers
successively, including breast, colorectal and liver cancer
(17). microRNAs are located in
tumor-associated fragile sites in the genome, prone to develop
deletion, amplification or translocation. microRNAs exert effects
that are analogous to tumor suppressors or oncogenes through
locating in oncogene or tumor suppressor gene (18). These data demonstrated that
microRNA-26b has anticancer effects, by inhibiting cell
proliferation and inducing apoptosis of liver cancer cells.
Bcl-2 protein can form a heterodimer with the Bax
protein (the pro-apoptotic factor), which inhibits tumor apoptosis,
prolongs the cell lifespan, and thus participates in tumor genesis
and development (14).
Overexpression of Bcl-2 is significantly correlated with
radiotherapy resistance of laryngeal squamous cell carcinoma, and
it can also prompt the proliferation of tumor cells whose DNA has
been damaged by radiation (8). In
the present study, microRNA-26b promoted Bax and caspase-3/-9
protein expressions in liver cancer cells. MicroRNA-26b had
anticancer effects by inhibiting cell proliferation, inducing
apoptosis and promoting Bax and caspase-3/-9 protein expressions in
a liver cancer cell line. MicroRNA-26b significantly suppressed
PI3K/Akt and NF-κB/MMP-9/VEGF pathways.
The PI3K/Akt signaling pathway plays a vital role
during tumor genesis and development. Possessing multiple
biological activities, the activated Akt catalyzes the
phosphorylation of a series of proteins, promotes tumor cell growth
and proliferation, inhibits apoptosis, enhances invasion and
metastasis, regulates endothelial growth and angiogenesis, and
increases sensitivity to radiation (8). AKT, an important substance for
transmitting growth signals, plays a role of central information
substance during the growth of tumor cells; therefore, AKT
activation predicts exuberant tumor cell growth. PTEN is considered
to be an extremely important tumor-suppressor gene, the C2 domain
of which plays a crucial role in the process of inhibiting tumor
growth and metastasis (19). The
results indicated that microRNA-26b significantly suppressed the
PI3K/Akt pathway. Suppression of PI3K significantly facilitated the
effects of microRNA-26b on suppression of the PI3K/Akt pathway,
inhibition of cell proliferation, induction of apoptosis and
increases in Bax and caspase-3/-9 protein expression in a liver
cancer cell line. Zhu et al demonstrated that microRNA-26b
participates in regulating the chemotactic response of mesenchymal
stem cells (MSCs) toward hepatocyte growth factor through
activation of Akt and FAK (20).
NF-κB is a type of heterodimer composed by 50 ku
and65 ku protein, which forms a compound with its inhibitor IκBs
under normal conditions, and remains in the resting state (21). When stimulated by external factors,
such as hypoxia, cytokine, viral protein, mitogen and ultraviolet,
IκBs may be degraded, and IκBs in the trimer compound will be
phosphorylated, thus leading to dissociation with NF-κB;
subsequently, NF-κB enters the cell nucleus, acts on the target
factor and exerts its function (21,22).
NF-κB is a type of transcription factor with multidirectional
regulatory function, which can regulate the expression of multiple
genes, such as apoptosis-associated genes, oncogenes, tumor
metastasis-associated adhesion molecules, and extracellular matrix
protease; particularly, it can upregulate the expression of VEGF
and MMP-9 genes, and is closely associated with tumor genesis,
infiltration and metastasis (22).
In addition, we demonstrated that the growth, infiltration and
metastasis of malignant tumors require angiogenesis. As an
important multi-functional angiogenesis factor, VEGF exerts its
function by specifically acting on receptors on the vascular
endothelial cell surface, which cannot only promote mitosis and
proliferation of vascular endothelial cells but also enhance
capillary permeability (23).
Upregulated VEGF expression can be observed in all malignant
tumors, and thus it plays a vital role in angiogenesis,
infiltration and metastasis of tumors (24). To our surprise, we found that
microRNA-26b significantly suppressed the NF-κB/MMP-9/VEGF
pathway.
Matrix metalloproteinases (MMPs) are a class of
zinc-dependent proteases which can degrade the extracellular matrix
(ECM) as well as a majority of proteins on the basement membrane,
and they play important roles in the invasion and metastasis of
malignant tumors (11). MMP-9
belongs to the gelatinase family, which mainly degrades gelatin,
along with type IV, V, VII and X basement membrane collagen
(25). Its high expression can
result in accelerated degradation of ECM and the basement membrane
of blood vessels, stimulates tumor cells to move out of the
carcinoma nest along with the injured basement membrane and
infiltrate to surrounding tissues, which is benefit to tumor cells
going in and out of blood vessels, thus promoting distal metastasis
(26). High MMP-9 expression is
related to the enhanced invasion and metastatic abilities of
multiple tumors, including gastric, colon and liver cancer
(22). Therefore, our results
suggest that the inhibition of NF-κB significantly enhanced the
effects of microRNA-26b on suppression of the NF-κB/MMP-9/VEGF
pathway, inhibition of cell proliferation, induction of apoptosis,
and promotion of Bax and caspase-3/-9 protein expression in a liver
cancer cell line. Li et al revealed that microRNA-26b
suppresses non-small cell lung cancer metastasis through the
NF-κB/MMP-9/VEGF pathway (27).
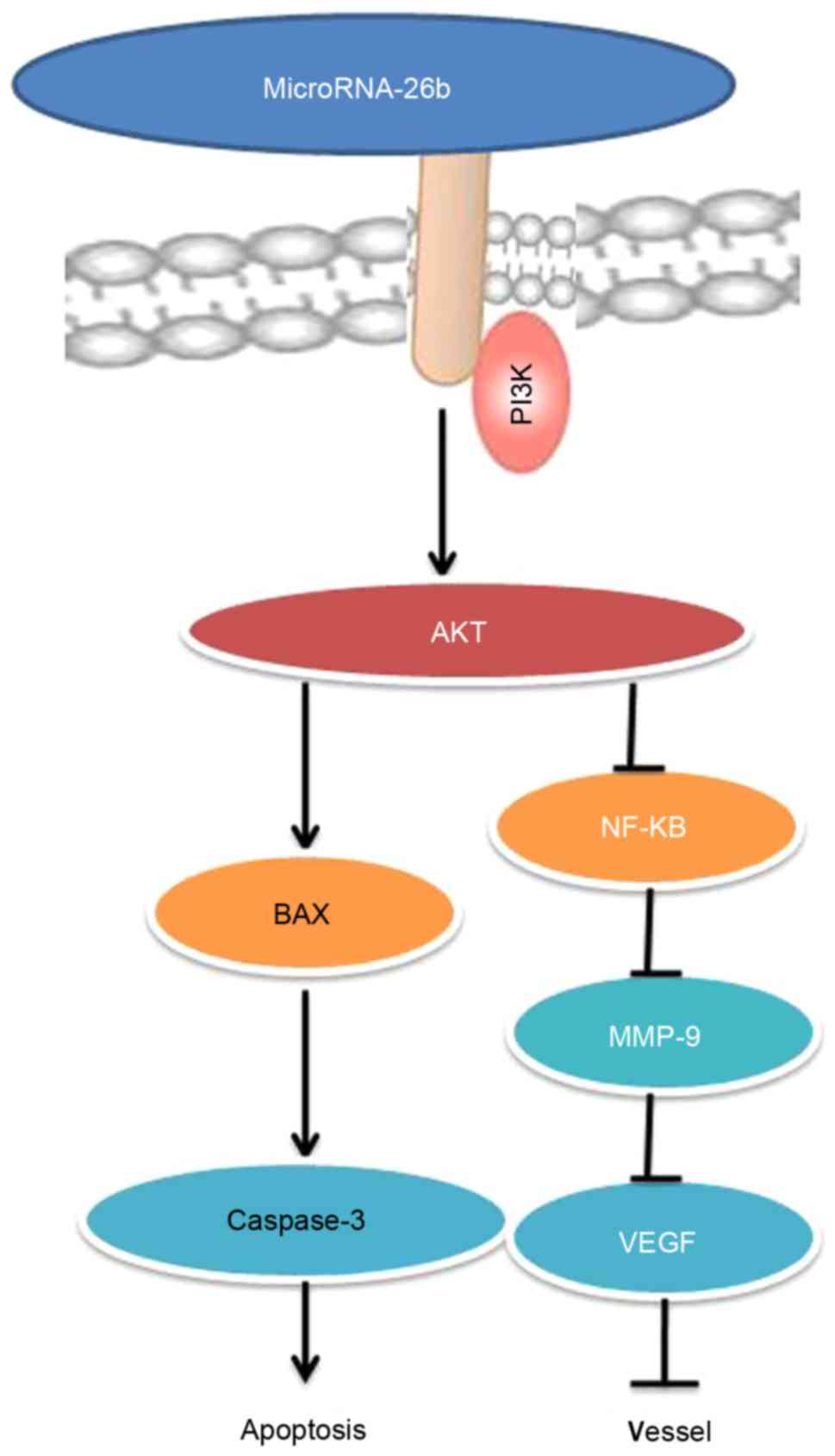
In conclusion, our findings indicate that
microRNA-26b has anticancer effects, inhibits cell proliferation,
induces apoptosis and promotes Bax and caspase-3/-9 protein
expression in liver cancer cells mainly through the PI3K/Akt and
NF-κB/MMP-9/VEGF pathways (Fig.
12). Those findings suggest the microRNA-26b may be a novel
biomarker for the early diagnosis or may aid in developing new
clinical treatments for liver cancer.
References
|
1
|
Bush DA, Smith JC, Slater JD, Volk ML,
Reeves ME, Cheng J, Grove R and de Vera ME: Randomized clinical
trial comparing proton beam radiation therapy with transarterial
chemoembolization for hepatocellular carcinoma: Results of an
interim analysis. Int J Radiat Oncol Biol Phys. 95:477–482. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma M, Tuaine J, McLaren B, Waters DL,
Black K, Jones LM and McCormick SP: Chemotherapy agents alter
plasma lipids in breast cancer patients and show differential
effects on lipid metabolism genes in liver cells. PLoS One.
11:e01480492016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo JR, Shen HC, Liu Y, Xu F, Zhang YW,
Shao Y and Su YJ: Effect of acute normovolemic hemodilution
combined with controlled low central venous pressure on blood
coagulation function and blood loss in patients undergoing
resection of liver cancer operation. Hepatogastroenterology.
62:992–996. 2015.PubMed/NCBI
|
|
4
|
Han S, Tang Q, Lu X, Chen R, Li Y, Shu J,
Zhang X and Cao J: Dysregulation of hepatic microRNA expression
profiles with Clonorchis sinensis infection. BMC Infect Dis.
16:7242016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He S, Zhang DC and Wei C: MicroRNAs as
biomarkers for hepatocellular carcinoma diagnosis and prognosis.
Clin Res Hepatol Gastroenterol. 39:426–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan Y, Lin B, Ye Y, Wen D, Chen L and Zhou
X: Differential expression of serum microRNAs in cirrhosis that
evolve into hepatocellular carcinoma related to hepatitis B virus.
Oncol Rep. 33:2863–2870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng X, Jiang J, Shi S, Xie H, Zhou L and
Zheng S: Knockdown of miR-25 increases the sensitivity of liver
cancer stem cells to TRAIL-induced apoptosis via PTEN/PI3K/Akt/Bad
signaling pathway. Int J Oncol. 49:2600–2610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sui Y, Zheng X and Zhao D: Rab31 promoted
hepatocellular carcinoma (HCC) progression via inhibition of cell
apoptosis induced by PI3K/AKT/Bcl-2/BAX pathway. Tumour Biol.
36:8661–8670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Palian BM, Rohira AD, Johnson SA, He L,
Zheng N, Dubeau L, Stiles BL and Johnson DL: Maf1 is a novel target
of PTEN and PI3K signaling that negatively regulates oncogenesis
and lipid metabolism. PLoS Genet. 10:e10047892014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Zhao B, Huang C, Meng XM, Bian EB
and Li J: Melittin restores PTEN expression by down-regulating
HDAC2 in human hepatocelluar carcinoma HepG2 cells. PLoS One.
9:e955202014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin SY, Kim CG, Jung YJ, Lim Y and Lee
YH: The UPR inducer DPP23 inhibits the metastatic potential of
MDA-MB-231 human breast cancer cells by targeting the
Akt-IKK-NF-κB-MMP-9 axis. Sci Rep. 6:341342016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manna K, Khan A, Das Kr D, Kesh Bandhu S,
Das U, Ghosh S, Dey Sharma R, Saha Das K, Chakraborty A,
Chattopadhyay S, et al: Protective effect of coconut water
concentrate and its active component shikimic acid against
hydroperoxide mediated oxidative stress through suppression of
NF-κB and activation of Nrf2 pathway. J Ethnopharmacol.
155:132–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moulton CA, Gu CS, Law CH, Tandan VR, Hart
R, Quan D, Smith Fairfull RJ, Jalink DW, Husien M, Serrano PE, et
al: Effect of PET before liver resection on surgical management for
colorectal adenocarcinoma metastases: A randomized clinical trial.
JAMA. 311:1863–1869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Melucci E, Cosimelli M, Carpanese L, Pizzi
G, Izzo F, Fiore F, Golfieri R, Giampalma E, Sperduti I, Ercolani
C, et al: Italian Society of Locoregional Therapies in Oncology
(S.I.T.I.L.O.): Decrease of survivin, p53 and Bcl-2 expression in
chemorefractory colorectal liver metastases may be predictive of
radiosensivity radiosensivity after radioembolization with
yttrium-90 resin microspheres. J Exp Clin Cancer Res. 32:132013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gnoni A, Santini D, Scartozzi M, Russo A,
Licchetta A, Palmieri V, Lupo L, Faloppi L, Palasciano G, Memeo V,
et al: Hepatocellular carcinoma treatment over sorafenib:
Epigenetics, microRNAs and microenvironment. Is there a light at
the end of the tunnel? Expert Opin Ther Targets. 19:1623–1635.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun J, Zhou M, Yang H, Deng J, Wang L and
Wang Q: Inferring potential microRNA-microRNA associations based on
targeting propensity and connectivity in the context of protein
interaction network. PLoS One. 8:e697192013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He J, Zhao K, Zheng L, Xu Z, Gong W, Chen
S, Shen X, Huang G, Gao M, Zeng Y, et al: Upregulation of
microRNA-122 by farnesoid X receptor suppresses the growth of
hepatocellular carcinoma cells. Mol Cancer. 14:1632015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park KU, Seo YS, Lee YH, Park J, Hwang I,
Kang KJ, Nam J, Kim SW and Kim JY: Altered microRNA expression
profile in hepatitis B virus-related hepatocellular carcinoma.
Gene. 573:278–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Zheng L, Ding Y, Li Q, Wang R,
Liu T, Sun Q, Yang H, Peng S, Wang W, et al: MiR-20a induces
cell radioresistance by activating the PTEN/PI3K/Akt
signaling pathway in hepatocellular carcinoma. Int J Radiat Oncol
Biol Phys. 92:1132–1140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu A, Kang N, He L, Li X, Xu X and Zhang
H: miR-221 and miR-26b regulate chemotactic migration of MSCs
toward HGF through activation of Akt and FAK. J Cell Biochem.
117:1370–1383. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo J, Zhou H, Wang F, Xia X, Sun Q, Wang
R and Cheng B: The hepatitis B virus X protein downregulates NF-κB
signaling pathways through decreasing the Notch signaling pathway
in HBx-transformed L02 cells. Int J Oncol. 42:1636–1643. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsieh SC, Tsai JP, Yang SF, Tang MJ and
Hsieh YH: Metformin inhibits the invasion of human hepatocellular
carcinoma cells and enhances the chemosensitivity to sorafenib
through a downregulation of the ERK/JNK-mediated NF-κB-dependent
pathway that reduces uPA and MMP-9 expression. Amino Acids.
46:2809–2822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alidzanovic L, Starlinger P, Schauer D,
Maier T, Feldman A, Buchberger E, Stift J, Koeck U, Pop L,
Gruenberger B, et al: The VEGF rise in blood of bevacizumab
patients is not based on tumor escape but a host-blockade of VEGF
clearance. Oncotarget. 7:57197–57212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo LY, Zhu P and Jin XP: Association
between the expression of HIF-1α and VEGF and prognostic
implications in primary liver cancer. Genet Mol Res. 15:152016.
View Article : Google Scholar
|
|
25
|
Zheng CG, Chen R, Xie JB, Liu CB, Jin Z
and Jin C: Immunohistochemical expression of Notch1, Jagged1, NF-κB
and MMP-9 in colorectal cancer patients and the relationship to
clinicopathological parameters. Cancer Biomark. 15:889–897. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grünwald B, Vandooren J, Locatelli E,
Fiten P, Opdenakker G, Proost P, Krüger A, Lellouche JP, Israel LL,
Shenkman L, et al: Matrix metalloproteinase-9 (MMP-9) as an
activator of nanosystems for targeted drug delivery in pancreatic
cancer. J Control Release. 239:39–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li D, Wei Y, Wang D, Gao H and Liu K:
MicroRNA-26b suppresses the metastasis of non-small cell lung
cancer by targeting MIEN1 via NF-κB/MMP-9/VEGF pathways. Biochem
Biophys Res Commun. 472:465–470. 2016. View Article : Google Scholar : PubMed/NCBI
|