Introduction
Colorectal cancer (CRC) is a cancer type that has
high incidence and mortality worldwide. Rectal adenocarcinoma
(READ) is a type of CRC (1). Many
studies have focused on READ. However, its mortality remains high
due to a lack of efficient biomarkers. Previous studies have
demonstrated that various types of RNAs play important roles in
cancer development and progression by acting in multiple ways
(2,3). To further investigate the relationship
among various types of RNAs and to obtain more efficient
biomarkers, we identified cancer-specific RNAs and developed a
competing endogenous RNA (ceRNA) network based on three types of
RNAs, including long non-coding RNAs (lncRNAs), microRNAs (miRNAs)
and mRNAs, that are differentially expressed in READ.
Non-coding RNA (ncRNA) is a type of RNA molecule
that exists ubiquitously in organisms but lacks protein-coding
ability (4). lncRNAs, once viewed
as transcriptional ‘noise’, are one subtype of ncRNA and are
identified as ncRNAs with >200 bp. Previous studies have
revealed that lncRNAs play key roles in cancer processes including
proliferation, invasion and metastasis (5,6). For
instance, homeobox transcript antisense intergenic RNA (HOTAIR) has
been identified as having higher expression levels in the plasma of
CRC patients than in the plasma of healthy controls, and its high
expression level predicts a poor prognosis (7).
lncRNAs function in multiple ways including
interacting with mRNAs and miRNAs. In 2011, Salmena et al
(8) reported the ceRNA hypothesis
and demonstrated that RNA transcripts communicate with each other
by miRNA response elements (MREs). mRNAs and lncRNAs use MREs to
compete for miRNA-binding sites, further affecting the expression
of miRNAs and the competition between mRNAs, lncRNAs and pseudogene
transcripts and playing a crucial role in tumor processes (9,10).
Some studies regarding lncRNA profiles in CRC have
been performed, and the functions of some lncRNAs in CRC have
already been demonstrated (11–14),
however studies with large sample sizes and high throughput
detection methods on specific READ lncRNAs are still lacking. In
addition, few studies have focused on dysregulated lncRNAs that are
associated with sex, TNM, survival or other clinical features, and
even fewer studies have been designed to identify the potential
ceRNA network in READ. To provide answers to the questions
mentioned, we used bioinformatic tools and analysis data from The
Cancer Genome Atlas (TCGA), which is a public database with
expression data of lncRNAs, miRNAs and mRNAs of READ. TCGA contains
RNA sequencing data of a total of 167 READ tumor tissues and 10
adjacent non-tumor tissue samples. To the best of our knowledge,
the present study is the first to identify lncRNAs that can be
potential biomarkers and to further identify the ceRNA network in
READ. To ascertain the credibility of our results, we randomly
selected several lncRNAs from the ceRNA network and confirmed their
profiles by qRT-PCR. The relationship between the expression
pattern and the clinical features of seven lncRNAs were also
confirmed by qRT-PCR. This approach aided in revealing the
functions of lncRNAs and constructed a ceRNA network for READ.
Materials and methods
Data of patients and samples
RNA expression data with clinical data such as
pathological stage, sex, and TNM information were all downloaded
from TCGA database. We set exclusion criteria as follows: i)
histological diagnosis revealing that the tissue was not READ; ⅱ)
suffering malignancies other than READ; ⅲ) samples without enough
data for analysis; and ⅳ) patients who underwent preoperative
radiotherapy and chemotherapy. Ultimately, 167 tumor tissues and 10
adjacent non-tumor tissues were used in the study. The number of
tumor tissues in tumor stages I, II, III, and IV were 30, 51, 51,
and 24, respectively, based on the pathological stage. The study
followed the guidelines of TCGA, thus, the approval of an ethics
committee was not required.
The READ specimens and their paired adjacent
non-tumor tissues of 90 patients were selected from the First
Affiliated Hospital of Nanjing Medical University (Jiangsu, China)
for qRT-PCR analysis. Their ages ranged from 45–80 years, and they
were diagnosed as having rectal cancer based on histopathology and
clinical history. The tissues were stored in RNAlater (Ambion;
Thermo Fisher Scientific, Inc., Austin, TX, USA) at −80°C until RNA
extraction and further analyses were performed. The clinical
features of 60 patients were also obtained.
RNA sequence data and further
analysis
RNA expression pattern data (level 3) from patients
with READ was obtained from TCGA database (September 2017), which
provides normalized data from RNA sequencing by the RNASeqV2
system, including lncRNAs and mRNA expression profiles. We used an
Illumina HiSeq 2000 miRNA sequencing (miRNAseq) platform (Illumina,
Inc., Hayward, CA, USA) to obtain STAD level 3 miRNAseq data from
TCGA. The downloaded data included a number of individual data
files, with each file representing one tissue sample. We then
divided the tumor samples into four groups (tumor stages I, II,
III, IV) and analyzed the differences in the expression levels
between each tumor stage (tumor stage I, II, III, IV) and adjacent
non-tumor tissues, and between all tumor tissues and all adjacent
non-tumor tissues using the Empirical Analysis of Digital Gene
Expression Data package in R (edgeR, R version 3.4.1) [absolute
log2(fold-change)>2.0, FDR<0.01]. Then, we chose
an intersection of differentially expressed READ lncRNAs, mRNAs and
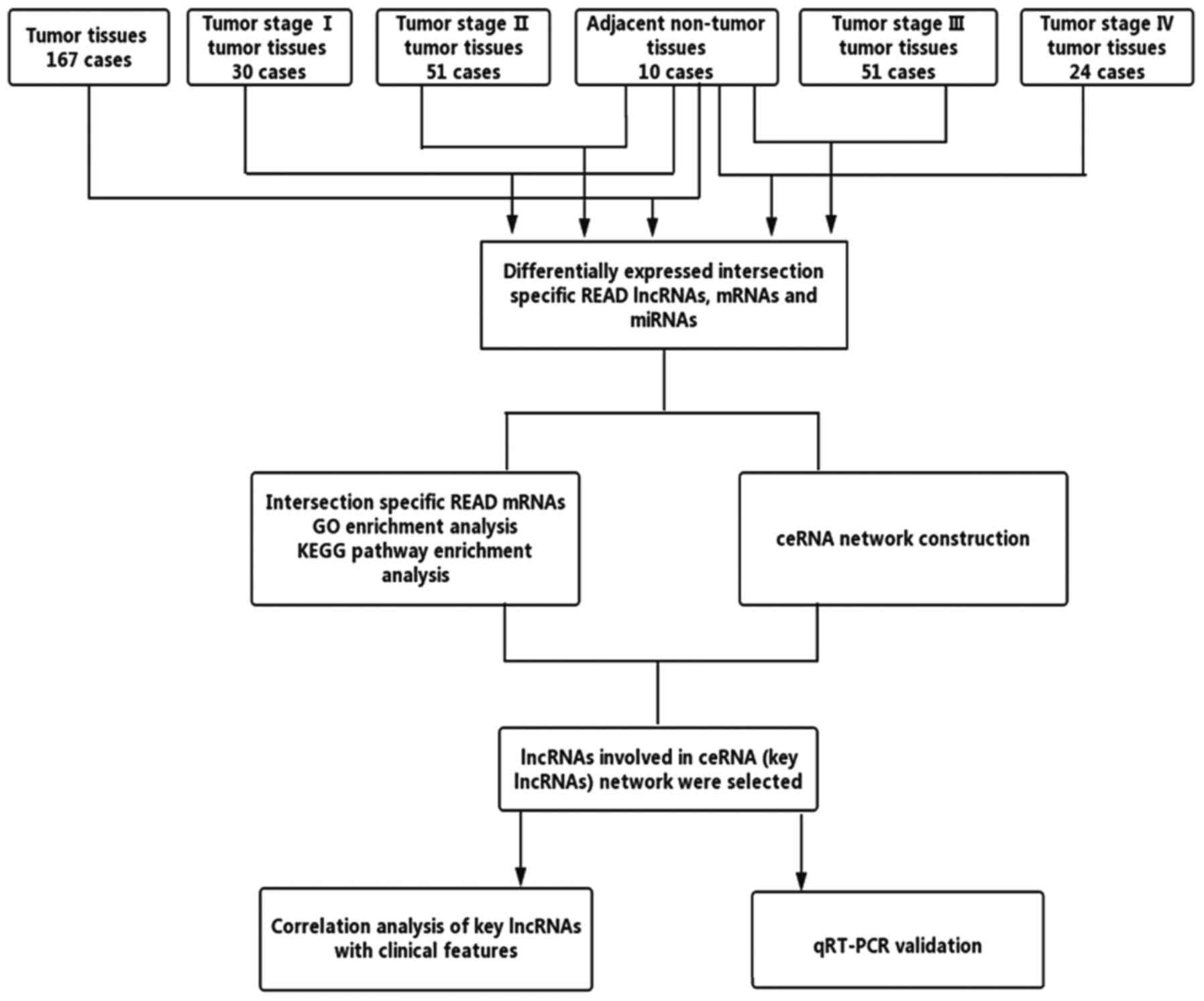
miRNAs for further analysis. The process is shown in Fig. 1.
Functional enrichment analysis
Database for Annotation, Visualization, and
Integrated Discovery (DAVID) bioinformatics resources (https://david.ncifcrf.gov/) was used for the
functional enrichment analysis, and we only researched the Gene
Ontology (GO) biological processes and the Kyoto Encyclopaedia of
Genes and Genomes (KEGG) pathways. The criteria were set as
P<0.05 and an enrichment score >1.5.
Construction of a ceRNA network
According to the theory that lncRNAs can affect
miRNAs and can act as miRNA sponges to further regulate mRNAs, we
constructed a ceRNA network. The miRcode (http://www.mircode.org/) was used to predict the
lncRNA/miRNA interactions based on specific READ miRNAs. We
predicted miRNA-targeted mRNA by using TargetScan (http://www.targetscan.org/), miRDB (http://www.mirdb.org/) and miRanda (http://www.microrna.org/microrna/home.do). We retained
the intersection with the differentially expressed lncRNAs and
mRNAs. Cytoscape v3.0 was used to construct the lncRNA/miRNA/mRNA
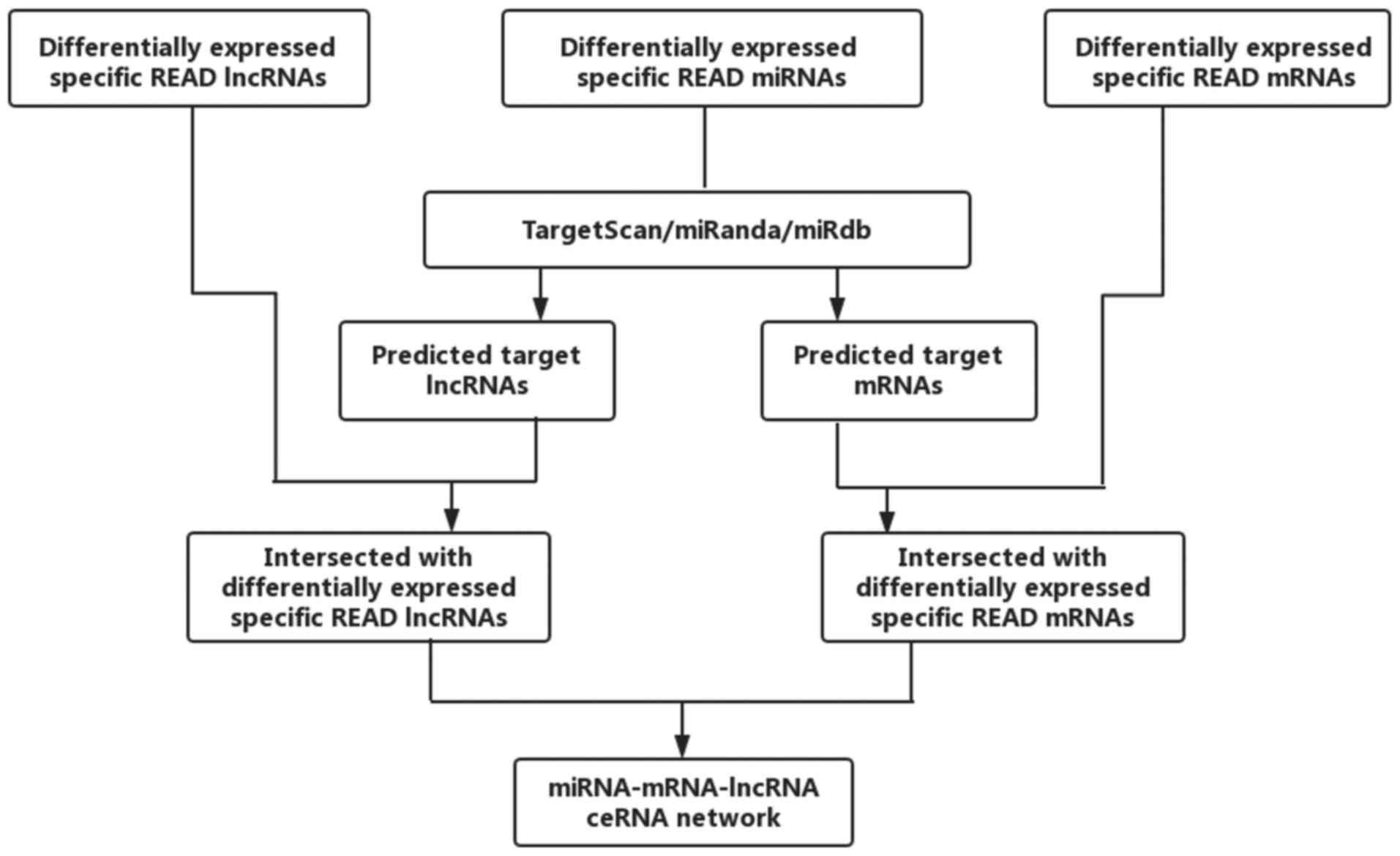
ceRNA network. The flowchart of the ceRNA network is presented in
Fig. 2.
Clinical feature analysis of key
lncRNAs
We extracted clinical information from TCGA
database. Based on the established ceRNA network in this study, we
selected lncRNAs from the network for further clinical analysis,
and clinical features such as sex, age, tumor staging, TNM staging
and lymphatic metastasis were chosen to analyze the correlation
between these features and key lncRNAs. We also investigated the
association between specific READ lncRNAs and the overall survival
time of patients.
RNA extraction and qRT-PCR
validation
We extracted RNA from tissue samples using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). Complementary DNA (cDNA)
was synthesized using the PrimeScript RT kit (Takara, Dalian,
China). qRT-qPCR was performed with a SYBR-Green PCR kit (Roche
Diagnostics, Indianapolis, IN, USA) via a StepOnePlus Real-Time PCR
system (Applied Biosystems, Foster City, CA, USA). The sequences of
the primers used for the PCR are presented in Table I. The PCR cycling conditions were as
follows: 95°C for 30 sec, 40 cycles of 95°C for 5 sec, 60°C for 30
sec, dissociation at 95°C for 15 sec, 60°C for 1 min and 95°C for
15 sec. The results were analysed using the 2−ΔΔCt
method. The qRT-PCR reactions were all repeated three times.
 | Table I.Primer sequences used for
qRT-PCR. |
Table I.
Primer sequences used for
qRT-PCR.
| Primer | Sequence |
|---|
| HULC | F
5′-ATCTGCAAGCCAGGAAGAGTC-3′ |
|
| R
5′-CTTGCTTGATGCTTTGGTCTGT-3′ |
| CRNDE | F
5′-TGGATGCTGTCAGCTAAGTTCAC-3′ |
|
| R
5′-TTCCAGTGGCATCCTCCTTATC-3′ |
| PVT1 | F
5′-TGAGAACTGTCCTTACGTGACC-3′ |
|
| R
5′-AGAGCACCAAGACTGGCTCT-3′ |
| ADAMTS9-AS2 | F
5′-TAAGACCCACGAACGACAGC-3′ |
|
| R
5′-CGTCATGCTTCGGCTTTCAG-3′ |
| GAPDH | F
5′-ACAGTCAGCCGCATCTTCTT-3′ |
|
| R
5′-GACAAGCTTCCCGTTCTCAG-3′ |
Statistical analysis
Statistical analysis was performed using R Studio (R
version 3.4.1), Statistical Programme for Social Sciences 20.0
(SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). The lncRNA data set
and the overall survival information were profiled using the
univariate Cox proportional hazards regression model. The results
were presented as Kaplan-Meier survival curves and multivariate Cox
regression analysis was applied for further study. Paired t-tests
were used to compare the differences in the qRT-PCR results.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Specific READ lncRNAs in patients
In total, 272 lncRNAs were identified that were
differentially expressed between READ tissues and adjacent
non-tumor tissues from TCGA database [absolute
log2(fold-change)>2, FDR<0.01], of which 143
lncRNAs were upregulated, and 129 lncRNAs were downregulated.
Further analysis of the differences between tumor tissues and
adjacent non-tumor tissues in patients with stage I, II, III and IV
cancer was performed. Then, 337, 323, 307 and 330 differentially
expressed lncRNAs were identified between adjacent non-tumor
tissues and READ stage I, II, III and IV tumor tissues,
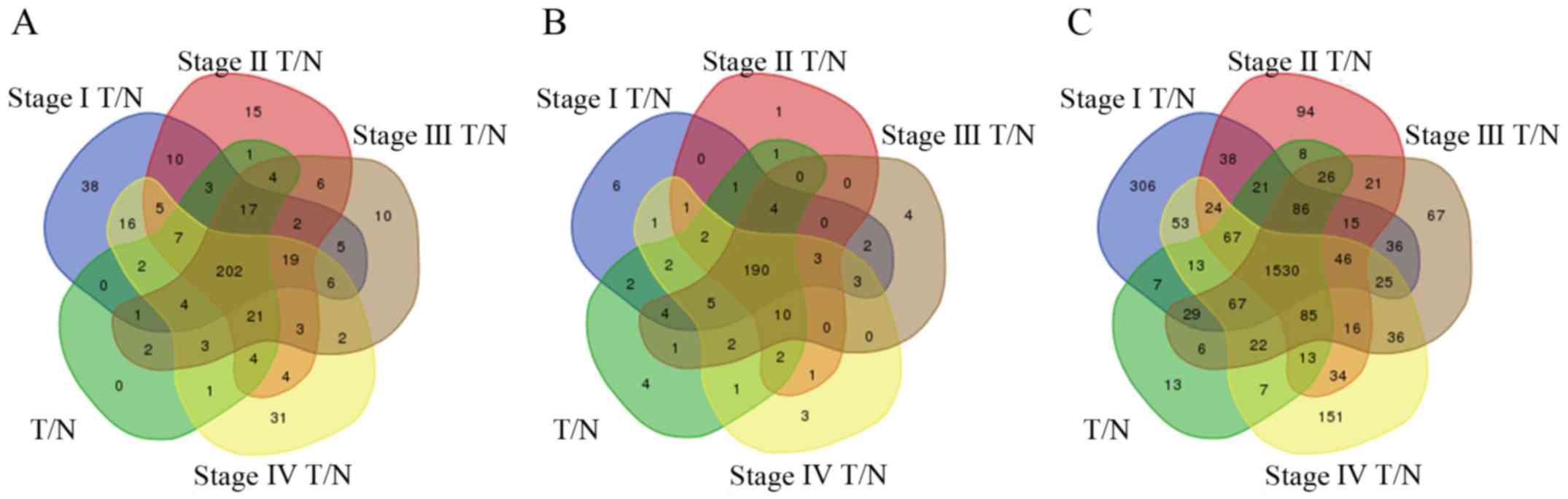
respectively. To enhance the credibility of the data, we used 202
differentially expressed lncRNAs from the intersection of the
aforementioned five groups for further analysis (Fig. 3A). These 202 lncRNAs were named
READ-specific lncRNAs. Finally, 34 lncRNAs were used to construct
the lncRNA/miRNA/mRNA ceRNA network (Table II).
 | Table II.Key lncRNAs involved in the ceRNA
network. |
Table II.
Key lncRNAs involved in the ceRNA
network.
| lncRNAs |
Log2(fold-change) | -Log(FDR) | lncRNAs |
Log2(fold-change) | -Log(FDR) |
|---|
| HULC |
8.48 |
2.92 | LINC00092 | −2.59 | 17.31 |
| ERVMER61-1 |
6.41 |
2.17 | LINC00402 | −2.85 | 9.49 |
| LINC00460 |
6.02 |
7.76 | LIFR-AS1 | −2.88 | 16.09 |
| CLDN10-AS1 |
5.37 |
4.35 | LINC00163 | −2.93 | 10.21 |
| POU6F2-AS1 |
5.20 |
2.65 | GDNF-AS1 | −2.96 | 16.35 |
| UCA1 |
4.84 |
5.31 | SFTA1P | −3.09 | 13.19 |
| CRNDE |
3.91 | 10.16 | HCG23 | −3.14 | 17.60 |
| DLX6-AS1 |
3.83 |
3.26 | CHL1-AS2 | −3.24 | 16.69 |
| GAS6-AS1 |
3.33 |
5.68 | RBMS3-AS3 | −3.34 | 25.42 |
| MIR17HG |
2.85 |
8.11 | LINC00473 | −3.48 | 17.51 |
| PVT1 |
2.76 | 17.27 | JAZF1-AS1 | −3.60 | 25.95 |
| PRSS30P |
2.70 |
3.94 | ADAMTS9-AS2 | −3.80 | 35.18 |
| PCAT1 |
2.13 |
4.21 | LINC00461 | −3.88 | 25.86 |
| SOX2-OT | −2.16 |
9.37 | C20orf166-AS1 | −4.30 | 35.28 |
| GRIK1-AS1 | −2.30 | 10.22 | LINC00507 | −4.33 | 17.01 |
| LINC00484 | −2.43 | 17.18 | FRMD6-AS2 | −4.61 | 23.38 |
| LINC00472 | −2.47 | 14.45 | ADAMTS9-AS1 | −5.09 | 40.40 |
GO enrichment and KEGG pathway
analyses of differentially expressed genes
For further study of the functions of the
differentially expressed genes, we selected intersecting mRNAs
across all READ stages from the differentially expressed mRNAs for
analysis. We first identified 2,000 differentially expressed mRNAs
between the tumor tissues of READ patients and the adjacent
non-tumor tissues [absolute log2(fold-change)>2,
FDR<0.01]. We also identified 2,363; 2,124; 2,113; and 2,189
differentially expressed mRNAs between adjacent non-tumor tissues
and READ tumor tissues (stage I, II, III and IV, respectively).
Then, we chose the mRNAs that were differentially expressed in all
five comparison groups, finally obtaining 1,530 differentially
expressed mRNAs that were used for further analysis (Fig. 3C). These mRNAs were identified as
READ-specific mRNAs.
The 1,530 differentially expressed mRNAs were
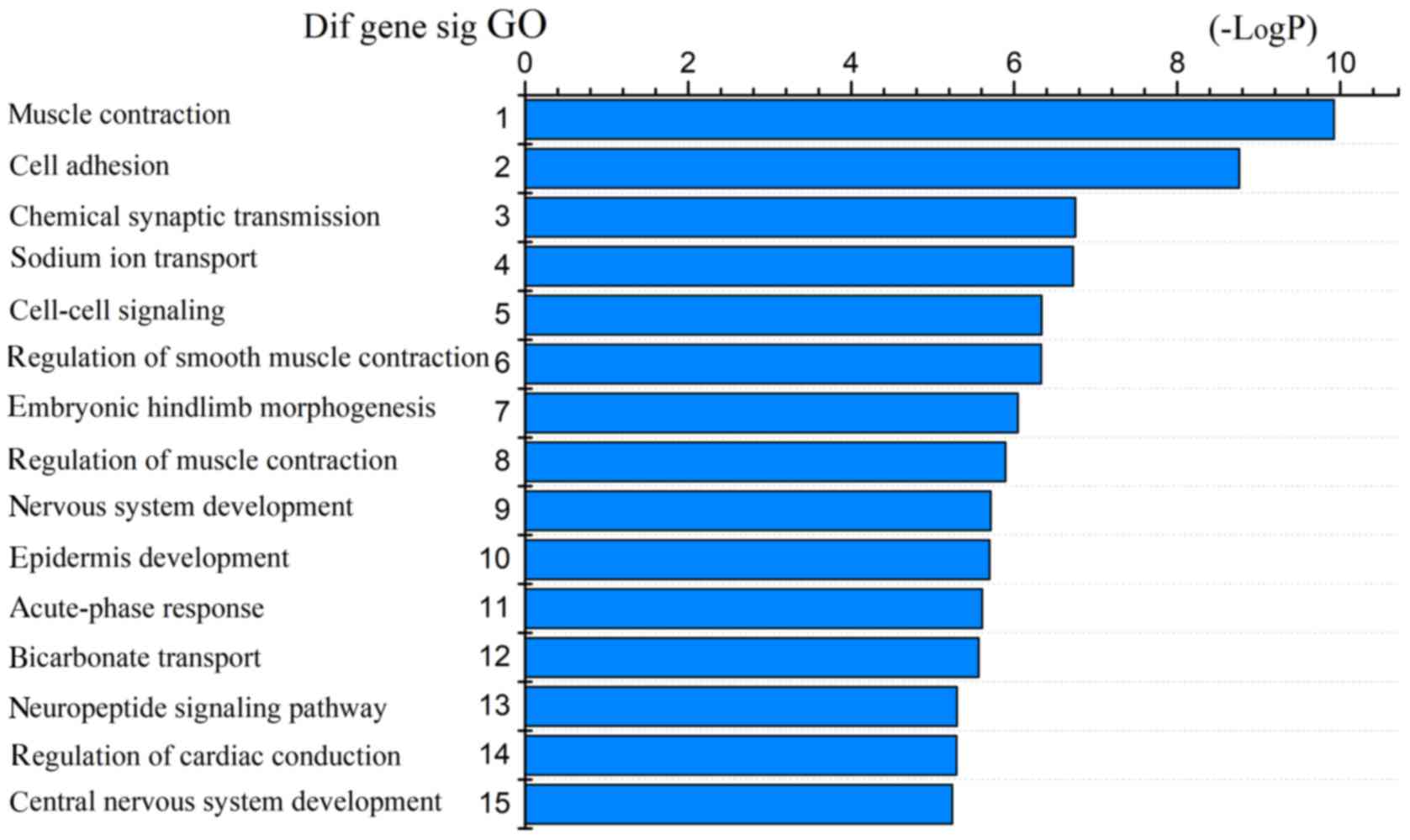
analyzed with DAVID bioinformatics resources. We chose to show the
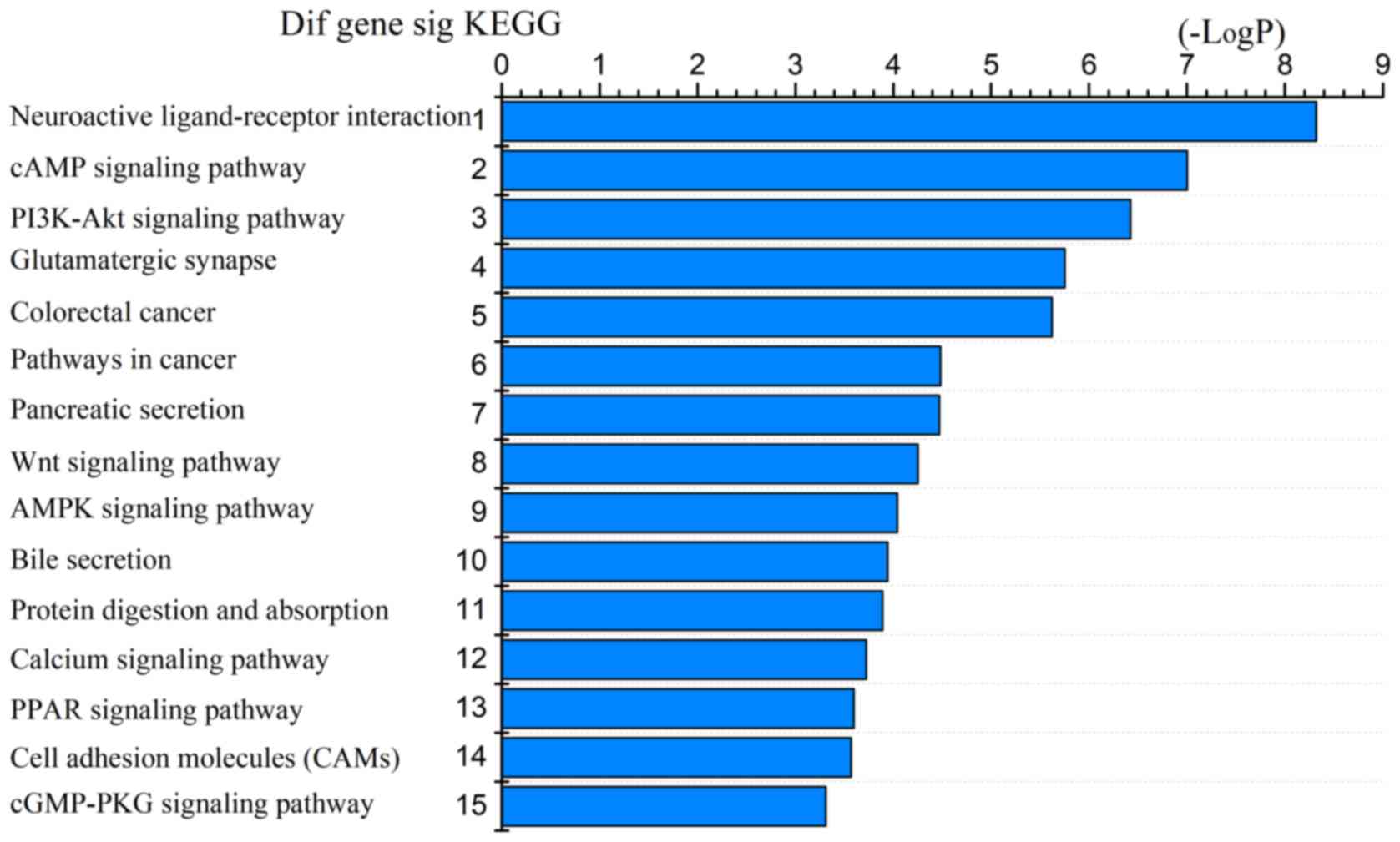
top 15 GO biological processes and 15 KEGG pathways of the
differentially expressed genes based on the P-values (Figs. 4 and 5). Among these pathways, the PI3K-Akt,
Wnt, AMPK, and cGMP-PKG signaling pathways and the cell adhesion
molecules (CAMs) were confirmed as CRC-associated pathways.
Prediction of miRNA targets and
construction of a ceRNA network
The 31 miRNAs that were differentially expressed
between READ tumor tissues and adjacent non-tumor tissues were
identified. For further investigation, we then divided the tumor
tissues into four groups (stages I, II, III and IV) according to
the pathological stage, and then compared each group with adjacent
non-tumor tissues in turn. Subsequently, we obtained 190 specific
miRNAs by selecting the intersection of miRNAs differentially
expressed across the five comparison groups (Fig. 3B). These miRNAs were identified as
READ-specific miRNAs. In the next step, we investigated the
interactions between these intersecting miRNAs and the
READ-specific lncRNAs based on miRcode (http://www.mircode.org/), and 25 key miRNAs were
predicted to target 34 key lncRNAs (Table III).
 | Table III.miRNAs that may target READ
lncRNAs. |
Table III.
miRNAs that may target READ
lncRNAs.
| miRNAs | lncRNAs |
|---|
| hsa-mir-107 | C20orf166-AS1,
UCA1, DLX6-AS1, LINC00472, LINC00460, LINC00163, LINC00402,
LINC00484, ADAMTS9-AS2, LINC00461, LINC00507, FRMD6-AS2 |
| hsa-mir-141 | DLX6-AS1,
LINC00472, LINC00402, LINC00484, ADAMTS9-AS2, SOX2-OT,
LINC00461 |
| hsa-mir-143 | PRSS30P, UCA1,
CLDN10-AS1, SFTA1P, LINC00472, LINC00460, JAZF1-AS1, LINC00163,
LINC00402, LINC00484, ADAMTS9-AS2, SOX2-OT, LINC00461, CRNDE,
GDNF-AS1, PVT1, FRMD6-AS2 |
| hsa-mir-144 | POU6F2-AS1,
DLX6-AS1, ADAMTS9-AS1, ADAMTS9-AS2, LIFR-AS1, LINC00461, CRNDE |
| hsa-mir-150 | PRSS30P,
C20orf166-AS1, CLDN10-AS1, LINC00473, LINC00092, DLX6-AS1,
LINC00460, JAZF1-AS1, LINC00402, ADAMTS9-AS1, ADAMTS9-AS2,
LIFR-AS1, LINC00461, PVT1, HULC |
| hsa-mir-152 | DLX6-AS1,
LINC00484, ADAMTS9-AS2, PVT1 |
| hsa-mir-155 | DLX6-AS1,
LINC00472, LINC00402, ADAMTS9-AS1, ADAMTS9-AS2, LIFR-AS1, CRNDE,
HULC |
| hsa-mir-17 | C20orf166-AS1,
HCG23, DLX6-AS1, JAZF1-AS1, LINC00402, PVT1, PCAT1 |
| hsa-mir-182 | UCA1, SFTA1P,
ERVMER61-1, GAS6-AS1, LINC00402, RBMS3-AS3, ADAMTS9-AS1,
ADAMTS9-AS2, SOX2-OT, LIFR-AS1, PCAT1, FRMD6-AS2 |
| hsa-mir-183 | C20orf166-AS1,
CHL1-AS2, LINC00163, ADAMTS9-AS2, CRNDE, PVT1, LINC00507 |
| hsa-mir-192 | POU6F2-AS1, HCG23,
DLX6-AS1, SOX2-OT, LIFR-AS1, LINC00461, PCAT1 |
| hsa-mir-200a | DLX6-AS1,
LINC00472, LINC00402, LINC00484, ADAMTS9-AS2, SOX2-OT,
LINC00461 |
| hsa-mir-21 | PRSS30P,
ERVMER61-1, JAZF1-AS1, ADAMTS9-AS1, SOX2-OT, PVT1 |
| hsa-mir-215 | POU6F2-AS1, HCG23,
DLX6-AS1, SOX2-OT, LIFR-AS1, LINC00461, PCAT1 |
| hsa-mir-217 | LINC00402,
LINC00484, CRNDE, PVT1 |
| hsa-mir-22 | C20orf166-AS1,
DLX6-AS1, LINC00472, JAZF1-AS1, LINC00402, LINC00484, ADAMTS9-AS2,
LIFR-AS1, LINC00461, CRNDE, PCAT1, FRMD6-AS2 |
| hsa-mir-223 | DLX6-AS1, GAS6-AS1,
LINC00484, ADAMTS9-AS2, CRNDE |
| hsa-mir-32 | CLDN10-AS1,
POU6F2-AS1, GAS6-AS1, JAZF1-AS1, LINC00484, ADAMTS9-AS2, LIFR-AS1,
LINC00461, CRNDE, PCAT1 |
| hsa-mir-375 | C20orf166-AS1,
GRIK1-AS1, ADAMTS9-AS2, SOX2-OT, LIFR-AS1, PCAT1, LINC00507,
FRMD6-AS2 |
| hsa-mir-424 | PRSS30P, LINC00473,
LINC00092, SFTA1P, DLX6-AS1, LINC00472, LINC00484, LINC00461,
GDNF-AS1, PVT1, PCAT1 |
| hsa-mir-425 | C20orf166-AS1,
MIR17HG, LINC00472, LINC00460, LINC00461 |
| hsa-mir-429 | C20orf166-AS1,
DLX6-AS1, LINC00460, LINC00402, SOX2-OT |
| hsa-mir-454 | C20orf166-AS1,
ADAMTS9-AS1, ADAMTS9-AS2, SOX2-OT |
| hsa-mir-96 | UCA1, ERVMER61-1,
GAS6-AS1, RBMS3-AS3, ADAMTS9-AS1, ADAMTS9-AS2, SOX2-OT, LIFR-AS1,
LINC00461, FRMD6-AS2 |
| hsa-mir-98 | JAZF1-AS1,
LINC00484, ADAMTS9-AS2 |
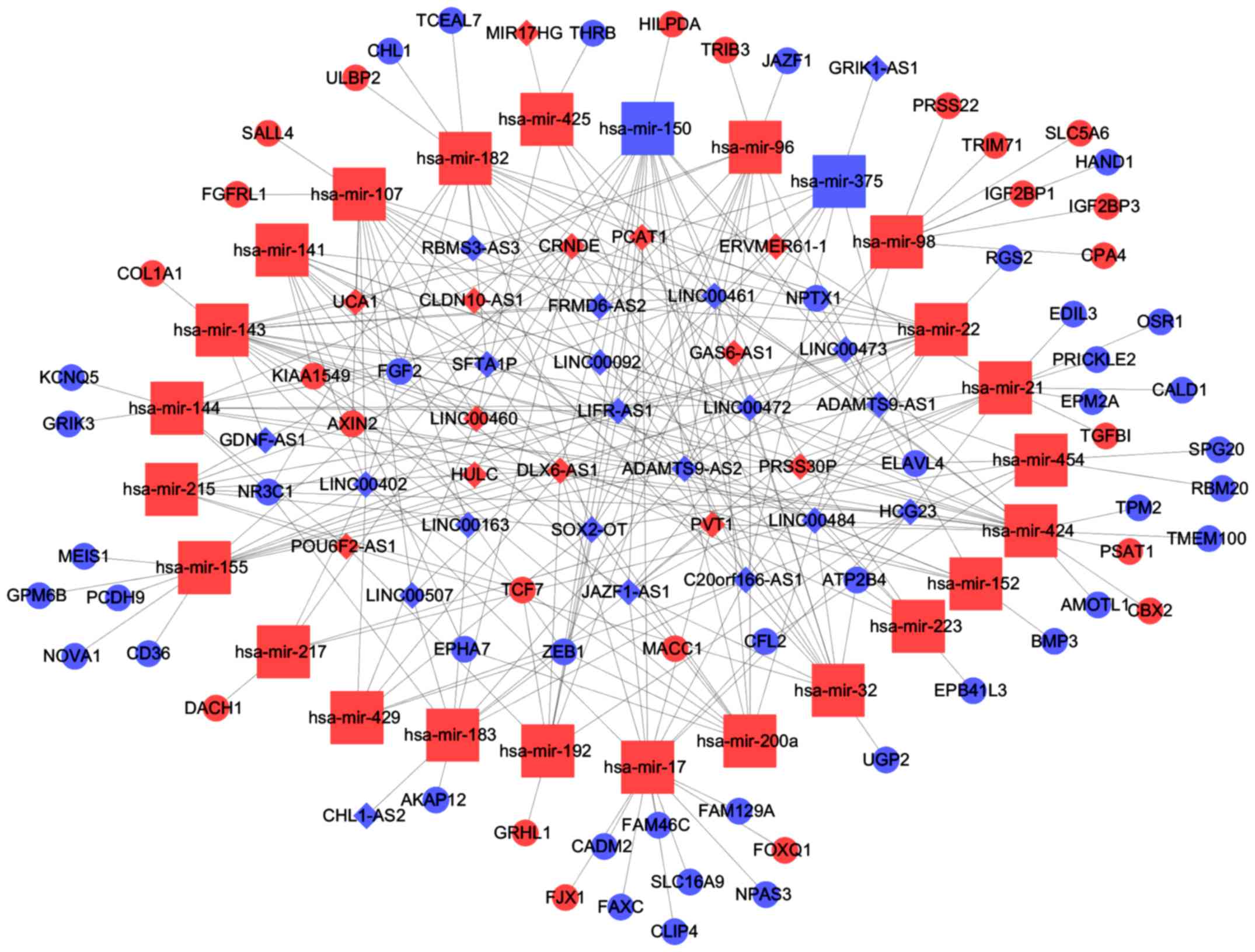
The 25 key miRNAs mentioned in Table III were then used to predict key
mRNAs using Targetscan (http://www.targetscan.org/), miRDB (http://www.mirdb.org/) and miRanda (http://www.microrna.org/microrna/home.do). We then
compared the predicted mRNAs with the 1,530 READ-specific mRNAs and
chose mRNAs that were found in both groups. Finally, 65 mRNAs were
found to interact with 25 miRNAs (Table IV). Among these mRNAs, some have
been verified to be transcribed from cancer-related genes.
 | Table IV.miRNAs that may target READ
mRNAs. |
Table IV.
miRNAs that may target READ
mRNAs.
| miRNAs | mRNAs |
|---|
| hsa-mir-107 | SALL4, AXIN2, FGF2,
FGFRL1 |
| hsa-mir-141 | MACC1, ZEB1, EPHA7,
KIAA1549, ELAVL4 |
| hsa-mir-143 | COL1A1 |
| hsa-mir-144 | FGF2, GRIK3, NR3C1,
KCNQ5 |
| hsa-mir-150 | HILPDA, ZEB1 |
| hsa-mir-152 | BMP3, NPTX1 |
| hsa-mir-155 | MEIS1, CD36, GPM6B,
NOVA1, PCDH9 |
| hsa-mir-17 | FAM46C, CLIP4,
CFL2, FAXC, NPAS3, FOXQ1, SLC16A9, FJX1, FAM129A, CADM2 |
| hsa-mir-182 | NR3C1, NPTX1,
ULBP2, CHL1, TCEAL7 |
| hsa-mir-183 | ZEB1, NR3C1,
AKAP12 |
| hsa-mir-192 | GRHL1, TCF7 |
| hsa-mir-200a | EPHA7, ZEB1, MACC1,
KIAA1549 |
| hsa-mir-21 | ATP2B4, PRICKLE2,
CALD1, EDIL3, EPM2A, OSR1, TGFBI |
| hsa-mir-215 | TCF7 |
| hsa-mir-217 | DACH1 |
| hsa-mir-22 | NR3C1, RGS2 |
| hsa-mir-223 | EPB41L3 |
| hsa-mir-32 | ATP2B4, UGP2, |
| hsa-mir-375 | ELAVL4 |
| hsa-mir-424 | AMOTL1, TPM2, CBX2,
TMEM100, AXIN2, PSAT1, FGF2 |
| hsa-mir-425 | THRB |
| hsa-mir-429 | ZEB1 |
| hsa-mir-454 | SPG20, CFL2,
RBM20 |
| hsa-mir-96 | JAZF1, TRIB3,
ZEB1 |
| hsa-mir-98 | PRSS22, IGF2BP1,
HAND1, SLC5A6, IGF2BP3, TRIM71, CPA4 |
According to information provided in Tables III and IV, we constructed a miRNA/lncRNA/mRNA
ceRNA network using Cytoscape 3.0. In conclusion, 25 miRNAs, 65
mRNAs and 34 lncRNAs were involved in the network (Fig. 6). We called lncRNAs involved in the
ceRNA network key lncRNAs.
Clinical feature analysis of key
READ-specific lncRNAs
To further study the lncRNAs, the correlation
between the lncRNAs involved in the ceRNA network and clinical
features including sex, age, tumor stage, TNM stage and lymphatic
metastasis status in TCGA database were analysed. We identified 7
lncRNAs associated with clinical features (P<0.05). The results
revealed that UCA1 and HULC were age-related, CHL1-AS2, LINC00484
and HULC were sex-related, LINC00484 and ADAMTS9-AS1 were
associated with TNM stage, CLDN10-AS1 was associated with tumor
stage, and UCA1 was associated with lymphatic metastasis (Table V).
 | Table V.The correlation between COAD key
lncRNAs involved in the ceRNA network and their clinical
features. |
Table V.
The correlation between COAD key
lncRNAs involved in the ceRNA network and their clinical
features.
| Comparisons | Upregulated | Downregulated |
|---|
| Age (<50 vs.
>50 years) |
| UCA1, HULC |
| Sex (female vs.
male) | CHL1-AS2,
LINC00484 | HULC |
| Lymphatic
metastasis (no vs. yes) |
| UCA1 |
| Tumor stage (stage
I, II vs. stage III, IV) | CLDN10-AS1 | DLX6-AS1 |
| TNM staging system
(T1+T2 vs. T3+T4) | LINC00484,
ADAMTS9-AS1 |
|
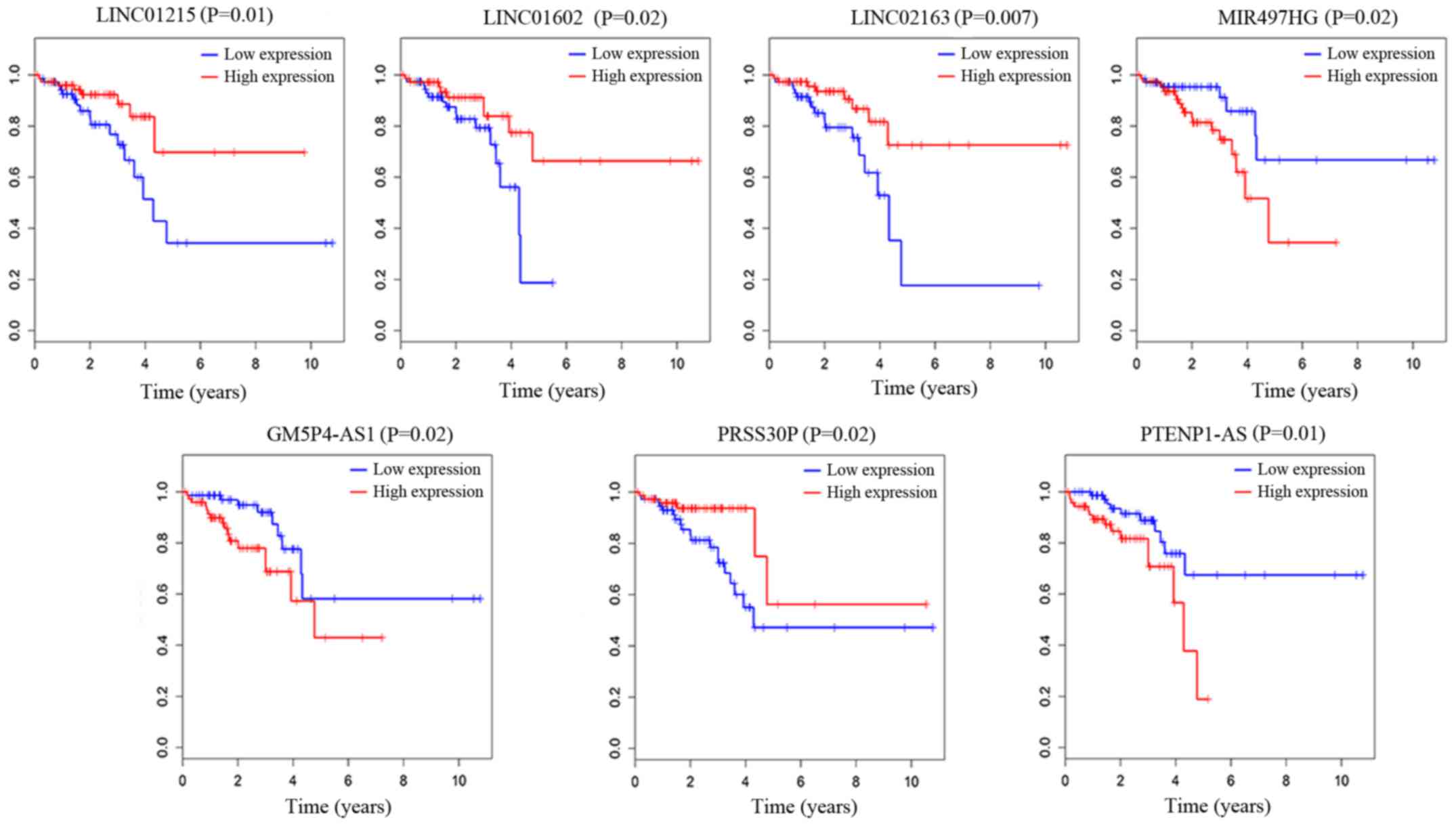
We also analyzed the association of the overall
survival of patients with the 202 specific lncRNAs based on the
clinical data of 177 samples from TCGA database. To carry out this
research, we used a univariate Cox proportional hazards regression
model and finally found 7 lncRNAs that were significantly
associated with the overall survival of READ patients (log-rank
P<0.05). As described in Fig. 7,
LINC01215, LINC01602, LINC02163 and PRSS30P were positively
correlated with overall survival (P<0.05). MIR497HG, PGM5P4-AS1
and PTENP1-AS were negatively correlated with overall survival
(P<0.05). Based on the results of the univariate Cox regression
analysis, 7 lncRNAs were associated with overall survival. We
performed a multivariate Cox regression analysis with these 7
lncRNAs. LINC01602, LINC02163 and MIR497HG were found to be
independent influencing factors of survival time, LINC01602 and
MIR497HG were negatively related to overall survival, and LINC02163
was positively related to overall survival. We present these
results in Table VI.
 | Table VI.Results of multivariate cox
regression analysis. |
Table VI.
Results of multivariate cox
regression analysis.
| lncRNAs | β | OR (95CI) | P-value |
|---|
| LINC01215 | −0.016 | 0.984
(0.951–1.019) | 0.368 |
| LINC01602 | 0.004 | 1.004
(1.001–1.007) | 0.011 |
| LINC02163 | −0.042 | 0.959
(0.929–0.991) | 0.011 |
| PRSS30P | −0.005 | 0.995
(0.989–1.002) | 0.144 |
| MIR497HG |
0.046 | 1.048
(1.006–1.090) | 0.023 |
| PGM5P4-AS1 | −0.036 | 0.965
(0.837–1.112) | 0.621 |
| PTENP1-AS | 0.320 | 1.377
(0.968–1.960) | 0.075 |
qRT-PCR validation
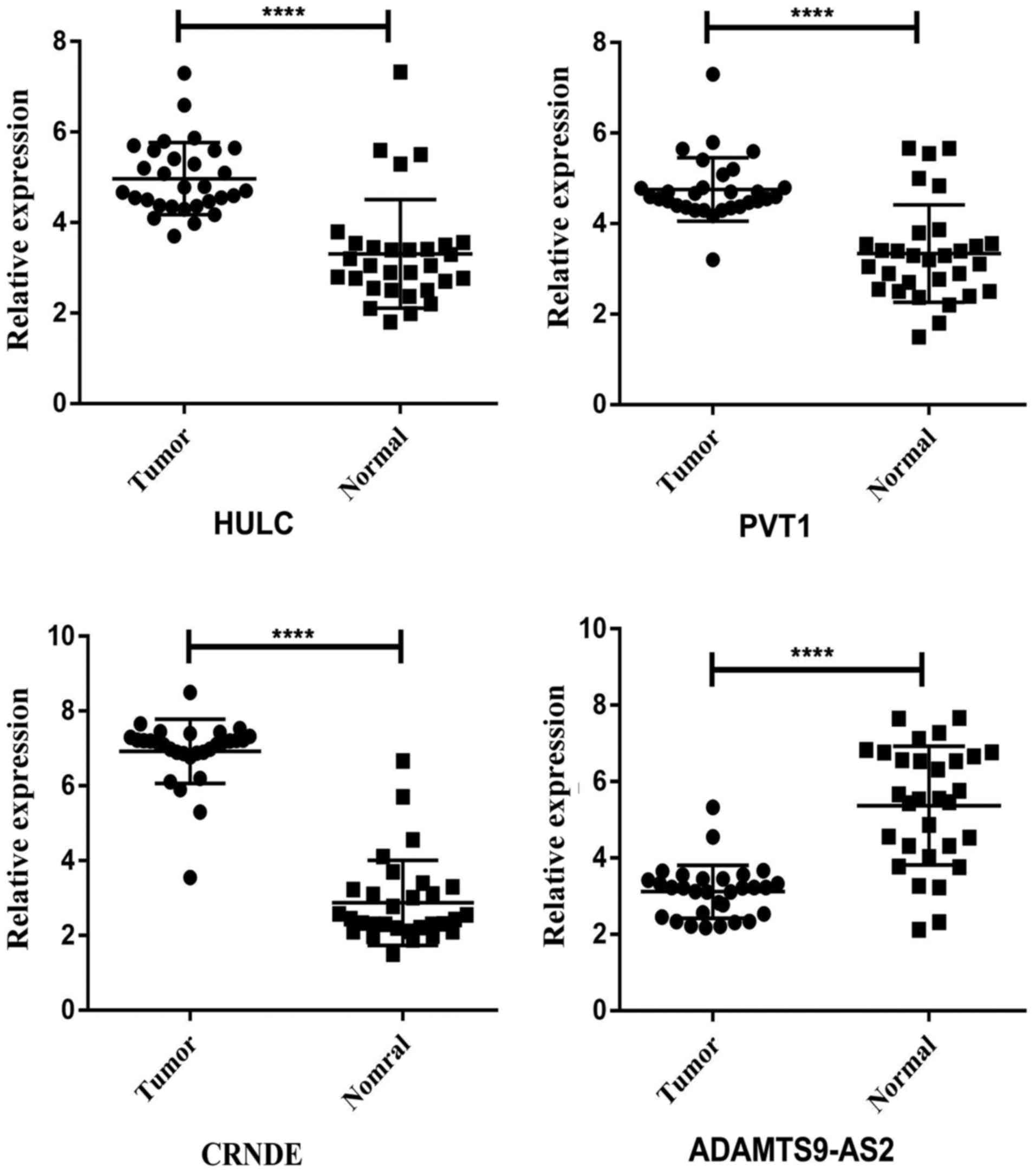
To check the credibility of the bioinformatics
results, we randomly selected 4 key lncRNAs (HULC, CRNDE, PVT1 and
ADAMTS9-AS2) from the network. Paired t-tests were applied to
analyse the differences in expression of the selected lncRNAs
between READ tumor tissues and the adjacent non-tumor tissues.
HULC, CRNDE and PVT1 expression levels were increased, and the
expression level of ADAMTS9-AS2 was decreased in READ tumor tissues
compared to adjacent non-tumor tissues. The qRT-PCR results from 30
READ patients were consistent with the bioinformatics results
(Fig. 8). We also confirmed the
correlation between the expression pattern and clinical features of
7 lncRNAs. Sixty patients were divided into two groups according to
the expression level of the lncRNA concerned. Patients with higher
and lower than the median expression of the lncRNA concerned were
allocated into high and low expression groups, respectively. The
results are displayed in Table
VII. Thus, our bioinformatics analysis was reliable.
 | Table VII.Expression of lncRNAs related to
clinical features according to the clinicopathological
characteristics of patients. |
Table VII.
Expression of lncRNAs related to
clinical features according to the clinicopathological
characteristics of patients.
|
|
| UCA1
expression |
| HULC
expression |
|
|---|
|
|
|
|
|
|
|
|---|
|
Characteristics | No. | High group | Low group | P-value | High group | Low group | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
|
<50 | 18 | 5 | 13 | 0.024 | 5 | 13 | 0.024 |
|
>50 | 42 | 25 | 17 |
| 25 | 17 |
|
| Sex |
|
|
|
|
|
|
|
|
Female | 32 | 17 | 15 | 0.605 | 12 | 20 | 0.038 |
|
Male | 28 | 13 | 15 |
| 18 | 10 |
|
| Lymphatic
metastasis |
|
|
|
|
|
|
|
| No | 36 | 14 | 22 | 0.035 | 15 | 21 | 0.113 |
|
Yes | 24 | 16 | 8 |
| 15 | 9 |
|
| Tumor stage |
|
|
|
|
|
|
|
| Stage
I, II | 33 | 12 | 21 | 0.020 | 15 | 18 | 0.436 |
| Stage
III, IV | 27 | 18 | 9 |
| 15 | 12 |
|
| TNM staging
system |
|
|
|
|
|
|
|
|
T1+T2 | 32 | 17 | 15 | 0.605 | 14 | 18 | 0.301 |
|
T3+T4 | 28 | 13 | 15 |
| 16 | 12 |
|
|
|
|
| CHL1-AS2
expression |
| LINC00484
expression |
|
|
|
|
|
|
|
|
|
Characteristics | No. | High group | Low group | P-value | High group | Low group | P-value |
| Age (years) |
|
|
|
|
|
|
|
|
<50 | 18 | 8 | 10 | 0.573 | 9 | 9 | 1.000 |
|
>50 | 42 | 22 | 20 |
| 21 | 21 |
|
| Sex |
|
|
|
|
|
|
|
|
Female | 32 | 20 | 12 | 0.038 | 21 | 11 | 0.009 |
|
Male | 28 | 10 | 18 |
| 9 | 19 |
|
| Lymphatic
metastasis |
|
|
|
|
|
|
|
| No | 36 | 17 | 19 | 0.598 | 19 | 17 | 0.598 |
|
Yes | 24 | 13 | 11 |
| 11 | 13 |
|
| Tumor stage |
|
|
|
|
|
|
|
| Stage
I, II | 33 | 16 | 17 | 0.796 | 18 | 15 | 0.436 |
| Stage
III, IV | 27 | 14 | 13 |
| 12 | 15 |
|
| TNM staging
system |
|
|
|
|
|
|
|
|
T1+T2 | 32 | 13 | 19 | 0.120 | 20 | 12 | 0.038 |
|
T3+T4 | 28 | 17 | 11 |
| 10 | 18 |
|
|
|
|
| CLDN10-AS1
expression |
| DLX6-AS1
expression |
|
|
|
|
|
|
|
|
|
Characteristics | No. | High group | Low group | P-value | High group | Low group | P-value |
| Age (years) |
|
|
|
|
|
|
|
|
<50 | 18 | 8 | 10 | 0.573 | 7 | 11 | 0.260 |
|
>50 | 42 | 22 | 20 |
| 23 | 19 |
|
| Sex |
|
|
|
|
|
|
|
|
Female | 32 | 16 | 16 | 1.000 | 15 | 17 | 0.605 |
|
Male | 28 | 14 | 14 |
| 15 | 13 |
|
| Lymphatic
metastasis |
|
|
|
|
|
|
|
| No | 36 | 21 | 15 | 0.113 | 20 | 16 | 0.292 |
|
Yes | 24 | 9 | 15 |
| 10 | 14 |
|
| Tumor stage |
|
|
|
|
|
|
|
| Stage
I, II | 33 | 21 | 12 | 0.020 | 11 | 22 | 0.004 |
| Stage
III, IV | 27 | 9 | 18 |
| 19 | 8 |
|
| TNM staging
system |
|
|
|
|
|
|
|
|
T1+T2 | 32 | 18 | 14 | 0.301 | 15 | 17 | 0.605 |
|
T3+T4 | 28 | 12 | 16 |
| 15 | 13 |
|
|
|
| ADAMTS9-AS1
expression |
|
|
|
|
|
|
|
Characteristics | No. | High group | Low group | P-value |
| Age (years) |
|
|
|
|
|
<50 | 18 | 6 | 12 | 0.091 |
|
>50 | 42 | 24 | 18 |
|
| Sex |
|
|
|
|
|
Female | 32 | 17 | 15 | 0.605 |
|
Male | 28 | 13 | 15 |
|
| Lymphatic
metastasis |
|
|
|
|
| No | 36 | 20 | 16 | 0.291 |
|
Yes | 24 | 10 | 14 |
|
| Tumor stage |
|
|
|
|
| Stage
I, II | 33 | 18 | 15 | 0.436 |
| Stage
III, IV | 27 | 12 | 15 |
|
| TNM staging
system |
|
|
|
|
|
T1+T2 | 32 | 21 | 11 | 0.010 |
|
T3+T4 | 28 | 9 | 19 |
|
Discussion
CRC is one of the most common cancers around the
world, and its incidence and mortality remain high. READ is a type
of CRC and has a higher incidence than the other types of colon
adenocarcinoma (COAD) (15).
Although great progress in the treatment, prognosis and diagnosis
of READ has been achieved through studies, its mortality still
remains high, which may be due to the lack of efficient biomarkers
and the unclear mechanisms underlying READ.
Recent studies have demonstrated that lncRNAs play
vital roles during tumor progression, and some of them may be
biomarkers for better prognosis and diagnosis (16–18).
lncRNAs function in many ways. The ceRNA hypothesis postulates that
lncRNAs may compete with mRNAs for the binding sites of miRNAs and
may further affect mRNA expression through MREs (8). The hypothesis makes the relationship
of miRNAs, mRNAs and lncRNAs more complicated and better explains
the interaction among a variety of types of RNAs at the genetic
level.
There are many studies on lncRNAs in CRC (19,20),
but few of them have focused on READ. Additionally, the sample
sizes of previous studies were not large enough, and almost none of
the studies focused on the potential ceRNA network. In the present
study, we aimed to research the lncRNAs that may have the ability
to be better biomarkers and further explored the interaction among
lncRNAs, miRNAs and mRNAs by constructing a ceRNA network in
READ.
In this study, we identified READ-specific lncRNAs,
mRNAs and miRNAs based on the intersecting differential expression
between tumor tissues from all four stages and adjacent non-tumor
tissues. We further analyzed the functions and pathways involving
the differentially expressed genes by GO and KEGG. Then, with the
use of bioinformatics tools, we constructed a ceRNA network with
READ-specific mRNAs, miRNAs and lncRNAs. We further analyzed the
lncRNAs involved in the ceRNA network, examining their correlations
with clinical features, and we identified specific lncRNAs that
were correlated with overall survival. We finally confirmed our
findings in the tissues of 30 READ patients using qRT-PCR.
Some lncRNAs from the READ-specific lncRNAs have
been reported as playing vital roles in cancers. CCAT1 has been
reported to promote gallbladder cancer development (21), and CCAT2 has been identified to play
a significant role in the progression of colon cancer (22). This also supported the reliability
of our analysis. Through univariate and multivariate Cox regression
analyses, 3 lncRNAs (LINC01602, LINC02163 and MIR497HG) from the
READ-specific lncRNAs were identified as being associated with
overall survival. LINC01602 and MIR497HG were negatively related to
overall survival, and LINC02163 was positively related to overall
survival. These 3 lncRNAs were not involved in the ceRNA network
but may still play vital roles in READ and be potential indicators
of the prognosis of READ since they were related to overall
survival.
Using GO and KEGG, we analysed the functions and
pathways of READ-specific mRNAs. The GO results revealed that the
functions enriched, involved aspects of immune function, metabolism
and cellular functions. Among the results of the KEGG pathway
analysis, several were confirmed to be cancer-associated. PI3K/AKT
signaling plays crucial roles in reducing apoptosis, stimulating
cell growth and increasing proliferation, as reported in previous
studies (23). It has also been
reported that many lncRNAs including PlncRNA-1 and AB073614 affect
CRC through the PI3K/AKT signaling pathway (24,25).
Studies have demonstrated that AMPK can also be activated by some
lncRNAs. Li et al revealed that liver kinase B1 (LKB1)
phosphorylates and promotes AMPK and then reduces cancer cell
proliferation and metabolism (26).
Available data indicate that Wnt signaling substantially impacts
non-small cell lung cancer (NSCLC) tumorigenesis, prognosis, and
resistance to therapy (27).
Previous studies have reported that cell adhesion molecules (CAMs)
play a significant role in the progression of metastasis (28). Li et al found that by
activating the cGMP/PKG pathway, Wnt/β-catenin signaling can be
suppressed (29). Some other
cancer-associated pathways such as those known to be involved in
CRC were also revealed by our results, further demonstrating the
reliability of the results. These pathways and functions may also
be related to READ-specific lncRNAs due to different interactions
between lncRNAs and mRNAs in READ.
With the ceRNA network in READ, we can further
research the underlying mechanism of the intersections among
lncRNAs, mRNAs and miRNAs in READ. Several interactions between
RNAs in our network have been previously confirmed. UCA1 interacts
with miR-182 to modulate glioma proliferation (30), and UCA1 regulates miR-143 to promote
the invasion and EMT of bladder cancer (31). These previous studies strongly
demonstrate that our analysis was reliable. In addition, the
network can aid in understanding the interactions of miRNAs,
lncRNAs and mRNAs in READ from various perspectives. Some lncRNAs
that exist in the ceRNA network were previously reported to be
READ-related lncRNAs. For example, Yang et al discovered
that HULC promotes colorectal carcinoma progression by
epigenetically repressing NKD2 expression (32). Han et al demonstrated that
the lncRNA CRNDE can regulate the progression and chemoresistance
of CRC via modulation of the expression levels of miR-181a-5p and
Wnt/β-catenin signaling activity (33). High expression levels of PCAT-1 were
involved in CRC progression and it can be a novel biomarker of poor
prognosis in patients with CRC (34). PVT1 may be a new oncogene
co-amplified with c-Myc in CRC tissues and extracellular vesicles
and functionally correlated with the proliferation and apoptosis of
CRC cells (35). Most lncRNAs that
interacted with other RNAs may play significant roles in READ
processes Through further investigation of the lncRNAs involved in
the network, 7 lncRNAs were identified as being associated with
clinical features. Among these 7 lncRNAs, UCA1, HULC and LINC00484
appeared often, and these 3 lncRNAs may be the most clinically
relevant lncRNAs, potentially acting as biomarkers. Further study
still needs to be performed to understand their correlation with
clinical features and their potential as efficient biomarkers.
To verify our bioinformatic analysis, we randomly
selected 4 lncRNAs from the network to assess their expression
levels in 30 paired READ tumor tissues and adjacent non-tumor
tissues. We also confirmed the correlation between the expression
pattern and the clinical features of 7 lncRNAs using qRT-PCR. The
results revealed that our analysis was credible.
Our analysis has great meaning in the study of a
ceRNA network in READ and some results were confirmed by qRT-PCR.
However, there were still several limitations in our study.
Firstly, the sample number of normal tissues was not very large
although previous studies with a small sample size also exist
(36). Secondly the follow-up
information of our patients was not enough to study the overall
survival, most follow-up information after surgery was ~2–3 years.
In the future, we will validate our analysis results as soon as we
obtain enough follow-up information. Thirdly, further research is
also warranted on the functions of key lncRNAs in vivo and
in vitro.
In conclusion, our study revealed READ-specific
lncRNAs using bioinformatics analysis and studied their
associations with clinical features based on data from TCGA
database. To the best of our knowledge, studies about lncRNA
profiles with such a large sample size are rare. Some key lncRNAs
may become efficient biomarkers for READ diagnosis and prognosis.
In addition, we constructed a ceRNA network with READ-specific
lncRNAs, miRNAs and mRNAs, and it revealed the relationship among
these three types of RNAs and aided in elucidating the mechanisms
underlying READ on the genetic level.
Acknowledgements
Not applicable.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee YJ, Kang YR, Lee SY, Jin Y, Han DC and
Kwon BM: Ginkgetin induces G2-phase arrest in HCT116 colon cancer
cells through the modulation of b-Myb and miRNA34a expression. Int
J Oncol. 51:1331–1342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu S, Wu F and Jiang Z: Identification of
hub genes, key miRNAs and potential molecular mechanisms of
colorectal cancer. Oncol Rep. 38:2043–2050. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamamura S, Imai-Sumida M, Tanaka Y and
Dahiya R: Interaction and cross-talk between non-coding RNAs. Cell
Mol Life Sci. 75:467–484. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sana J, Faltejskova P, Svoboda M and Slaby
O: Novel classes of non-coding RNAs and cancer. J Transl Med.
10:1032012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takahashi K, Yan I, Haga H and Patel T:
Long noncoding RNA in liver diseases. Hepatology. 60:744–753. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Svoboda M, Slyskova J, Schneiderova M,
Makovicky P, Bielik L, Levy M, Lipska L, Hemmelova B, Kala Z,
Protivankova M, et al: HOTAIR long non-coding RNA is a negative
prognostic factor not only in primary tumors, but also in the blood
of colorectal cancer patients. Carcinogenesis. 35:1510–1515. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bassett AR, Azzam G, Wheatley L, Tibbit C,
Rajakumar T, McGowan S, Stanger N, Ewels PA, Taylor S, Ponting CP,
et al: Understanding functional miRNA-target interactions in vivo
by site-specific genome engineering. Nat Commun. 5:46402014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang L, Qiu M, Xu Y, Wang J, Zheng Y, Li
M, Xu L and Yin R: Upregulation of long non-coding RNA PRNCR1 in
colorectal cancer promotes cell proliferation and cell cycle
progression. Oncol Rep. 35:318–324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao Y, Lin M, Bu Y, Ling H, He Y, Huang C,
Shen Y, Song B and Cao D: p53-inducible long non-coding RNA PICART1
mediates cancer cell proliferation and migration. Int J Oncol.
50:1671–1682. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen DL, Chen LZ, Lu YX, Zhang DS, Zeng
ZL, Pan ZZ, Huang P, Wang FH, Li YH, Ju HQ, et al: Long noncoding
RNA XIST expedites metastasis and modulates epithelial-mesenchymal
transition in colorectal cancer. Cell Death Dis. 8:e30112017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding J, Li J, Wang H, Tian Y, Xie M, He X,
Ji H, Ma Z, Hui B, Wang K, et al: Long noncoding RNA CRNDE promotes
colorectal cancer cell proliferation via epigenetically silencing
DUSP5/CDKN1A expression. Cell Death Dis. 8:e29972017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turati F, Bravi F, Di Maso M, et al:
Adherence to the World Cancer Research Fund/American Institute for
Cancer Research recommendations and colorectal cancer risk. Eur J
Cancer. 85:86–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Liang X and Li Y: Long non-coding
RNA MEG3 inhibits cell growth of gliomas by targeting miR-93 and
inactivating PI3K/AKT pathway. Oncol Rep. 38:2408–2416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Huang S, Li Y, Zhang W, He K, Zhao
M, Lin H, Li D, Zhang H, Zheng Z, et al: Decreased expression of
LncRNA SLC25A25-AS1 promotes proliferation, chemoresistance, and
EMT in colorectal cancer cells. Tumor Biol. 37:14205–14215. 2016.
View Article : Google Scholar
|
|
18
|
Qiu JJ, Zhang XD, Tang XY, Zheng TT, Zhang
Y and Hua KQ: ElncRNA1, a long non-coding RNA that is
transcriptionally induced by oestrogen, promotes epithelial ovarian
cancer cell proliferation. Int J Oncol. 51:507–514. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ozawa T, Matsuyama T, Toiyama Y, Takahashi
N, Ishikawa T, Uetake H, Yamada Y, Kusunoki M, Calin G and Goel A:
CCAT1 and CCAT2 long noncoding RNAs, located within the 8q.24.21
‘gene desert’, serve as important prognostic biomarkers in
colorectal cancer. Ann Oncol. 28:1882–1888. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang L, Xu L, Wang Q, Wang M and An G:
Dysregulation of long non-coding RNA profiles in human colorectal
cancer and its association with overall survival. Oncol Lett.
12:4068–4074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma MZ, Chu BF, Zhang Y, Weng MZ, Qin YY,
Gong W and Quan ZW: Long non-coding RNA CCAT1 promotes gallbladder
cancer development via negative modulation of miRNA-218-5p. Cell
Death Dis. 6:e15832015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ling H, Spizzo R, Atlasi Y, Nicoloso M,
Shimizu M, Redis RS, Nishida N, Gafà R, Song J, Guo Z, et al:
CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic
progression and chromosomal instability in colon cancer. Genome
Res. 23:1446–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Danielsen SA, Eide PW, Nesbakken A, Guren
T, Leithe E and Lothe RA: Portrait of the PI3K/AKT pathway in
colorectal cancer. Biochim Biophys Acta. 1855:104–121.
2015.PubMed/NCBI
|
|
24
|
Song W, Mei JZ and Zhang M: LncRNA
PlncRNA-1 promotes colorectal cancer cell progression by regulating
PI3K/Akt signaling pathway. Oncol Res. Aug 23–2017.(Epub ahead of
print).
|
|
25
|
Wang Y, Kuang H, Xue J, Liao L, Yin F and
Zhou X: LncRNA AB073614 regulates proliferation and metastasis of
colorectal cancer cells via the PI3K/AKT signaling pathway. Biomed
Pharmacother. 93:1230–1237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li N, Huang D, Lu N and Luo L: Role of the
LKB1/AMPK pathway in tumor invasion and metastasis of cancer cells
(Review). Oncol Rep. 34:2821–2826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saadatmand S, de Kruijf EM, Sajet A,
Dekker-Ensink NG, van Nes JG, Putter H, Smit VT, van de Velde CJ,
Liefers GJ and Kuppen PJ: Expression of cell adhesion molecules and
prognosis in breast cancer. Br J Surg. 100:252–260. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li N, Xi Y, Tinsley HN, Gurpinar E, Gary
BD, Zhu B, Li Y, Chen X, Keeton AB, Abadi AH, et al: Sulindac
selectively inhibits colon tumor cell growth by activating the
cGMP/PKG pathway to suppress Wnt/β-catenin signaling. Mol Cancer
Ther. 12:1848–1859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He Z, Wang Y, Huang G, Wang Q, Zhao D and
Chen L: The lncRNA UCA1 interacts with miR-182 to modulate glioma
proliferation and migration by targeting iASPP. Arch Biochem
Biophys. 623–624:1–8. 2017. View Article : Google Scholar
|
|
31
|
Luo J, Chen J, Li H, Yang Y, Yun H, Yang S
and Mao X: LncRNA UCA1 promotes the invasion and EMT of bladder
cancer cells by regulating the miR-143/HMGB1 pathway. Oncol Lett.
14:5556–5562. 2017.PubMed/NCBI
|
|
32
|
Yang XJ, Huang CQ, Peng CW, Hou JX and Liu
JY: Long noncoding RNA HULC promotes colorectal carcinoma
progression through epigenetically repressing NKD2 expression.
Gene. 592:172–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han P, Li JW, Zhang BM, Lv JC, Li YM, Gu
XY, Yu ZW, Jia YH, Bai XF, Li L, et al: The lncRNA CRNDE promotes
colorectal cancer cell proliferation and chemoresistance via
miR-181a-5p-mediated regulation of Wnt/β-catenin signaling. Mol
Cancer. 16:92017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ge X, Chen Y, Liao X, Liu D, Li F, Ruan H
and Jia W: Overexpression of long noncoding RNA PCAT-1 is a novel
biomarker of poor prognosis in patients with colorectal cancer. Med
Oncol. 30:5882013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo K, Yao J, Yu Q, Li Z, Huang H, Cheng
J, Wang Z and Zhu Y: The expression pattern of long non-coding RNA
PVT1 in tumor tissues and in extracellular vesicles of colorectal
cancer correlates with cancer progression. Tumor Biol.
39:10104283176991222017. View Article : Google Scholar
|
|
36
|
Wang S, Zhang C, Zhang Z, Qian W, Sun Y,
Ji B, Zhang Y, Zhu C, Ji D, Wang Q, et al: Transcriptome analysis
in primary colorectal cancer tissues from patients with and without
liver metastases using next-generation sequencing. Cancer Med.
6:1976–1987. 2017. View Article : Google Scholar : PubMed/NCBI
|