Introduction
Anaplastic thyroid cancer (ATC) represents ~1% of
all thyroid cancer (TC) cases, and is one of the most aggressive
human tumors, accounting for 15–40% of TC-related deaths (1,2). ATC
is classified as stage IV (American Joint Committee on Cancer),
regardless of tumor size, or presence of lymph node or distant
metastases (present in ~80% of patients at diagnosis) (3–5);
median survival is 6 months. The multimodal treatment [including
debulking, chemotherapy (doxorubicin, cisplatin, paclitaxel or
docetaxel), and hyperfractionated accelerated external beam
radiotherapy] is the most effective treatment, with a median
survival of 10 months (6,7). Several genetic alterations have been
shown in ATC molecular pathways, leading to tumor aggressiveness
and progression [p53, BRAF, RAS, RET/PTC, vascular endothelial
growth factor (VEGF) receptor (VEGFR)-1, VEGFR-2, epidermal growth
factor receptor (EGFR), PDGFRα, PDGFRβ, KIT, MET, PIK3Ca, PIK3Cb
and PDK1] (7,8). New drugs targeting these molecular
alterations have been recently evaluated in ATC (7), but to date no significant improvement
in patient survival has been observed.
Vandetanib (ZD6474, Caprelsa®) is an oral
once-daily multi-tyrosine kinase inhibitor (TKI), that inhibits the
activation of RET, EGFR, VEGFR-2, VEGFR-3, and slightly VEGFR-1,
and has potent antiangiogenic activity (9). Potent antineoplastic action of
vandetanib was shown against transplantable medullary thyroid
carcinoma (MTC) in nude mice (10).
In patients with aggressive MTC, a phase III clinical study showed
that vandetanib improved progression-free survival (30.5 vs. 19.3
months in the control group) (11).
It was approved by the Food and Drug Administration, and the
European Medicines Agency, in 2011, to treat locally advanced or
metastatic MTC (12). Vandetanib
has also shown promising results in aggressive differentiated TC
patients not responsive to the usual therapies (13,14).
In the present study, we aimed to evaluate the antineoplastic
activity of vandetanib in ATC continuous cell lines, and in primary
ATC cells, in vitro and in vivo.
Materials and methods
Drug
The effect of vandetanib (ZD6474,
Caprelsa®; Aurogene Srl, Rome, Italy; 1 and 100 nM; 1,
10, 25 and 50 µM) was investigated in vitro in primary ATC
cell cultures, in the 8305C continuous cell line, and AF cells; and
in vivo in 8305C cells in CD nu/nu mice.
Reagents
RPMI-1640 medium was obtained from Gibco (Thermo
Fisher Scientific, Inc., Waltham, MA, USA); PCR reagents for
quantitative real-time were obtained from Applied Biosystems
(Thermo Fisher Scientific, Inc.). The other chemicals and
supplements not reported in this section were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Patient source for thyroid tissue
We obtained surgical thyroid tissue samples from: i)
eight ATC patients at surgery; and ⅱ) six healthy subjects who
underwent parathyroidectomy. Recognized clinical, laboratory and
histological criteria were used to establish the diagnosis
(15–17). The absence of thyroglobulin (Tg),
thyroperoxidase (TPO), thyroid-stimulating hormone (TSH) receptor,
and sodium/iodide symporter (NIS) expression was shown by
immunohistochemistry, that was positive for cytokeratin (Fig. 1). DNA extraction and
microdissection, and the detection of BRAF mutation were
conducted by PCR Single-strand Conformation Polymorphism (by
accepted protocols); such as direct DNA sequencing (15–17).
All patients and controls agreed to enter the study, which was
approved by the local Ethics Committee of the University of
Pisa.
Primary ATC cell culture
We prepared ATC cells using protocols published
previously (15–17). Cancer tissues were divided in
fragments of 1–3 mm, and washed 3–5 times in M-199 media together
with streptomycin (500,000 U/l), penicillin (500,000 U/l) and
nystatin (1,000,000 U/l). Fragments were suspended in Dulbecco's
modified Eagle's medium (DMEM) with penicillin/streptomycin (50
mg/l), glutamine (1% w/v) and fetal calf serum (FCS) (20% v/v) and
then incubated at 37°C in 5% CO2 (all were from
Sigma-Aldrich; Merck KGaA).
As soon as the primary culture reached confluence,
the cells were transferred into primary tissue culture flasks
(Becton-Dickinson Labware, Bedford, MA, USA). To evaluate
colony-forming efficacy, cells on their 3rd passage were coated in
Methocel™(Dow Chemical Co., Milan, Italy) (18). The biggest colonies were spread in
tissue-culture flasks (15–17). At the 4th passage, the cells were
tested. The absence of Tg, TSH receptor (19), NIS (20), and TPO (21) expression was shown by
immunocytochemistry. Focal positivity for cytokeratin was observed
by immunocytochemistry (20). DNA
fingerprinting demonstrated a pattern similar to the original
cancer tissue (15–17).
Thyroid follicular cell (TFC)
culture
TFCs were established as previously reported
(22).
AF cells
Among the eight primary ATC cell cultures, one (the
AF cell line) grew >50 passages, and was also able to grow in
nu/nu mice when subcutaneously inoculated.
8305C continuous cell line
8305C cells (undifferentiated TC cell line, with
papillary component; DSMZ, Braunschweig, Germany) were maintained
in RPMI-1640 with 15% fetal bovine serum (FBS) with the addition of
2 mM L-glutamine.
Viability and proliferation assay
In order to investigate cell proliferation, we
conducted a WST-1 (Roche Diagnostics, Almere, The Netherlands)
(16,17,22).
We plated TFC, ATC, AF and 8305C cells (at 35,000 cells/ml in 100
µl/well, of 96-well plates), and treated them with vandetanib or
with vehicle alone for 24 h. To achieve IC50, the cells
were treated with a concentration range of vandetanib (in
quadruplicate), and IC50 was estimated by linear
interpolation. Triplicate experiments were conducted for each cell
preparation (16,17,22).
The absorbance was estimated at 450 nm after 1 and 2 h from the
beginning of tetrazolium reaction.
Cell number counting was used, as well, to estimate
the proliferation in all the considered cells, as previously
reported (16,17,22).
Apoptosis-Hoechst uptake
Firstly, we plated ATC, 8305C and AF cells (35,000
cells/ml in 100 µl/well; 96-well plates). Thereafter, the cells
were treated for 48 h with vandetanib (37°C, 5% CO2),
and dyed with Hoechst 33342 as previously described (22). The apoptotic cells/total cells ×100
ratio (apoptosis index) was evaluated.
Annexin V binding assay for
apoptosis
The assay was carried out on cells seeded in Lab-Tek
II Chamber Slide System (Nalge Nunc International, Penfield, NY,
USA), treated with vandetanib for 48 h, as previously reported
(22).
Migration and invasion assays
A 96-well Transwell Permeable Support (Corning Life
Sciences, Corning, NY, USA) was used to achieve cell migration and
invasion, in agreement with the manufacturer's instructions,
applying minor modifications (23,24).
To assess intracellular fluorescence, we used a
96-well plate ELISA reader (excitation at 485 nm and emission at
520 nm). For the migration assay cells were incubated for 12 h; for
the invasion assay, 24 h. For the invasion experiments, the inserts
were coated with a basement membrane extract solution (Trevigen,
Gaithersburg, MD, USA) overnight (37°C, 5% CO2), before
plating cells. For each assay we constructed a standard curve to
transform the fluorescence data obtained to the number of invasive
or migrated cells. All the data were analyzed by StatView version
5.0 (SAS Institute, Inc., Cary, NC, USA).
ELISA tests in ATC cells
Phospho-EGFR inhibition cell-based
assay
ATC cells (5×104 cells/well) were plated
in 1% FBS medium for 24 h, and then treated for 72 h with
vandetanib at a concentration similar to the experimental
IC50 of cell proliferation (25 µM for ATC), and with a
higher (50 µM), or with a lower (1 µM) concentration of vandetanib,
or with vehicle. We collected cell lysates as previously reported
(25) and we assayed them with
PathScan phospo-EGFR (Tyr1173) and total EGFR sandwich ELISA kits
(Cell Signaling Technology, Inc., Danvers, MA, USA). Optical
density (OD) was estimated at 450 nm.
AKT (pThr308), or ERK1/2
(pTpY185/187)
ATC cells (5×104 cells/well) were exposed
for 72 h to vandetanib and subsequently lysed (25), and tested for human AKT or ERK1/2
phosphorylation by the PhosphoDetect AKT (pThr308) or by
PhosphoDetect ERK1/2 (pThr185/pTyr187) ELISA kits (Calbiochem; EMD
Millipore, Billerica, MA, USA). Data were normalized by total
protein AKT, or ERK1/2 concentrations evaluated by AKT, or ERK1/2
ELISA kits, respectively. OD was estimated at 450 nm.
Cyclin D1 protein expression
The ATC cells were exposed for 72 h to vandetanib at
the above considered concentrations or to vehicle, in order to
evaluate the vandetanib-modulated expression of the protein cyclin
D1. Cyclin D1 protein amount was measured by lysing cells with
(ice-cold 1X) lysis buffer (0.5 ml), as previously reported
(25). Lysates were gathered and
then sonicated on ice (for 10 sec). Subsequently, the samples were
microcentrifuged at 4°C for 10 min and the supernatant was
gathered. Cyclin D1 was measured in cancer cell lysates by the
human ELISA kit (Uscn Life Sciences, Inc., Wuhan, China). OD was
measured at 450 nm, and the obtained data were reported as cyclin
D1 ng/mg of total protein.
In vivo studies
Animals and treatment
Six-week-old CD nu/nu male mice weighing
20–25 g, supplied by Envigo (Milan, Italy), were housed in
microisolator cages on vented racks and manipulated using aseptic
techniques and were allowed unrestricted access to sterile food and
water. We proceeded according to the protocol approved by the
Academic Organization Responsible for Animal Welfare [Organismo
Preposto per il Benessere Animale (OPBA)] at the University of
Pisa, in agreement with the Italian law D.lgs. 26/2014, and by the
Italian Ministry of Health (authorization no. 613/2015-PR). In
order to obtain statistically meaningful results, each experiment
was conducted with the minimum necessary number of mice. In each
mouse we inoculated, subcutaneously, 2×106±5% viable
8305C cells, on day 0. Animal weights were monitored, and tumor
volume (mm3) was defined as: [(w1 × w1 × w2) × (π/6)],
where w1 refers to the smallest tumor diameter (mm), whereas w2 to
the largest one. We started the treatment (n=5 mice/group) after 30
days from cell inoculation, when the mean volume was ~100
mm3. All mice were randomized just before initiation of
treatment. Control mice received vehicle alone, or 25 or 12.5
mg/kg/day of vandetanib, injected intraperitoneally (i.p.) for 29
days. An anesthetic overdose of urethan was used to sacrifice mice,
and the tumors were then excised and measured.
Microvessel density in the cancer
tissue, and immunohistochemistry
Cancer tissues from the three treatment groups were
weighed, and then fixed in formalin and subsequently embedded in
paraffin. The sections (5 µm) were stained with hematoxylin and
eosin, and immunostaining was conducted as previously described
(23). An anti-VEGF rabbit
polyclonal antibody (diluted at 1:50; cat. no. sc-152; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) was used to estimate VEGF
expression, as the percentage of positive cells in ~1,000 tumor
cells. Anti-FVIII polyclonal antibody (cat. no. 760-2642; Ventana
Medical Systems, Inc.:Roche Group, Tucson, AZ, USA) was used to
determine microvascular count (MVC), as previously described
(23).
Statistical analysis
For normally distributed variables, the values are
expressed as mean (±SD), or as median (and interquartile range).
Experiments were conducted thrice with ATC from each subject, and
here we report the mean in the eight samples obtained by different
donors (for TFC and ATC). One-way ANOVA, or Mann-Whitney U or
Kruskal-Wallis test, were used to compare mean group values for
normally distributed variables. χ2 test was applied to
compare proportions. Post hoc comparisons on normally distributed
variables were conducted by Bonferroni-Dunn test. Apoptosis results
were analyzed by one-way ANOVA with Newman-Keuls multiple
comparisons test. All the data were analyzed by StatView version
5.0 (SAS Institute, Inc.).
Results
In vitro studies in primary cell
cultures
Cell proliferation
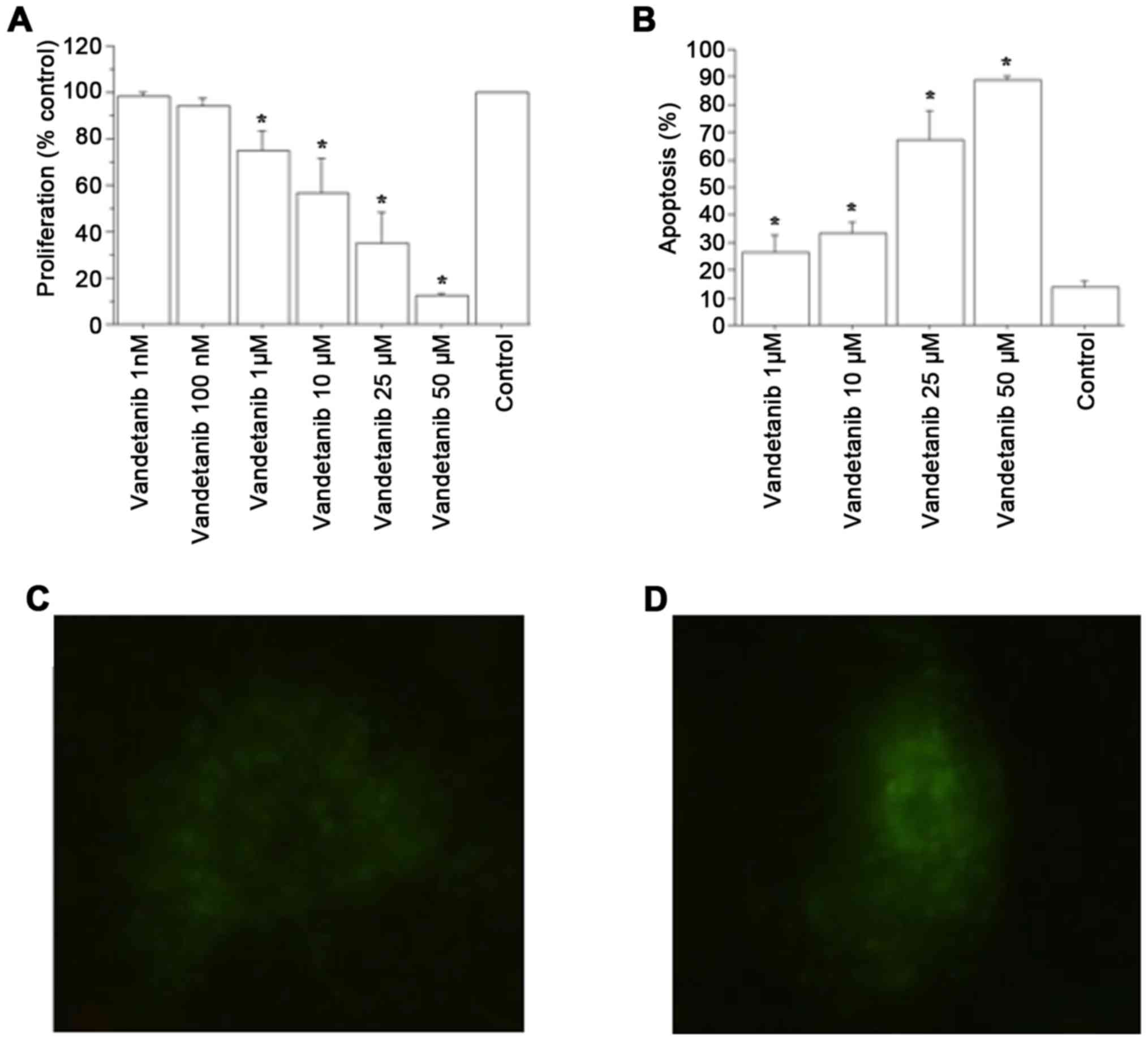
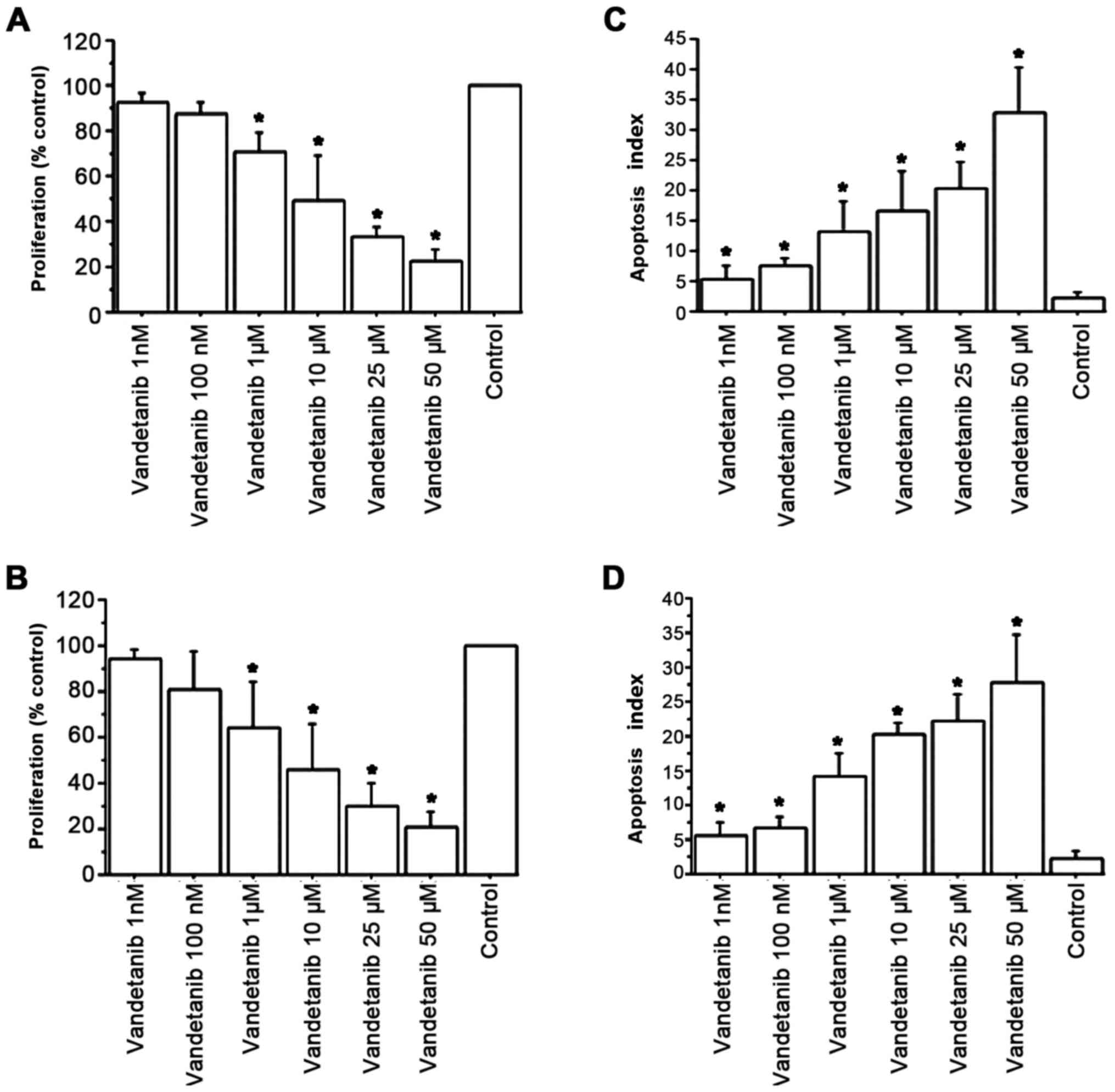
Vandetanib significantly reduced ATC cell
proliferation vs. the control, at 1 and 2 h (P<0.01, by ANOVA,
for both) (Fig. 2A). Cell counting
confirmed these results: after 1 h, the cell number was
12,120±680/100 µl/well in the ATC control group; 11,998±710 (99%)
with vandetanib 1 nM; 11,635±780 (96%) with vandetanib 100 nM;
10,787±690 (89%) with vandetanib 1 µM; 9,332±710 (77%) with
vandetanib 10 µM; 8,610±580 (71%) with vandetanib 25 µM; and
3,878±490 (32%) with vandetanib 50 µM (P<0.01, ANOVA); after 2
h, cell number was 18,950±910/100 µl/well; 18,382±920 (97%) with
vandetanib 1 nM; 17,623±990 (93%) with vandetanib 100 nM;
14,592±1,010 (77%) with vandetanib 1 µM; 10,423±1,180 (55%) with
vandetanib 10 µM; 6,443±1,190 (34%) with vandetanib 25 µM; and
2,274±750 (12%) with vandetanib 50 µM; (P<0.01, ANOVA). The
IC50 value, obtained with linear interpolation, was
13±2.9 µM for vandetanib.
The results of WST-1 assay in TFC cells with
vandetanib showed a slight but significant reduction in
proliferation with respect to the control both at 1 h (P<0.01,
ANOVA) with vandetanib 10 µM (94% vs. control), 25 µM (87% vs.
control), and 50 µM (81% vs. control), and at 2 h (P<0.01, for
both, ANOVA) with vandetanib 10 µM (87% vs. control), 25 µM (81%
vs. control), and 50 µM (76% vs. control). Cell counting supported
the previously reported results: after 1 h, the cell number was
11,290±730/100 µl/well in the TFC control; 10,612±1,080 (94%) with
vandetanib 10 µM; 9,822±940 (87%) with vandetanib 25 µM; and
9,145±880 (81%) with vandetanib 50 µM; (P<0.01, ANOVA); after 2
h, the cell number was 17,950±910/100 µl/well; 15,615±1,090 (87%)
with vandetanib 10 µM; 14,540±950 (81%) with vandetanib 25 µM; and
13,640±890 (76%) with vandetanib 50 µM (P<0.01, ANOVA).
Proliferation and BRAF
Three considered ATCs had
V600EBRAF mutation, while RET/PTC1 and RET/PTC3,
H-RAS or N-RAS mutations were not reported in primary ATC cells by
real-time PCR. Proliferation was inhibited similarly in ATC from
cancers with or without V600EBRAF mutation (data
not shown).
Apoptosis
Vandetanib increased the number of apoptotic ATC
cells dose-dependently (P<0.001; by ANOVA; Fig. 2B). The Annexin V assay corroborated
these results (Fig. 2C and D).
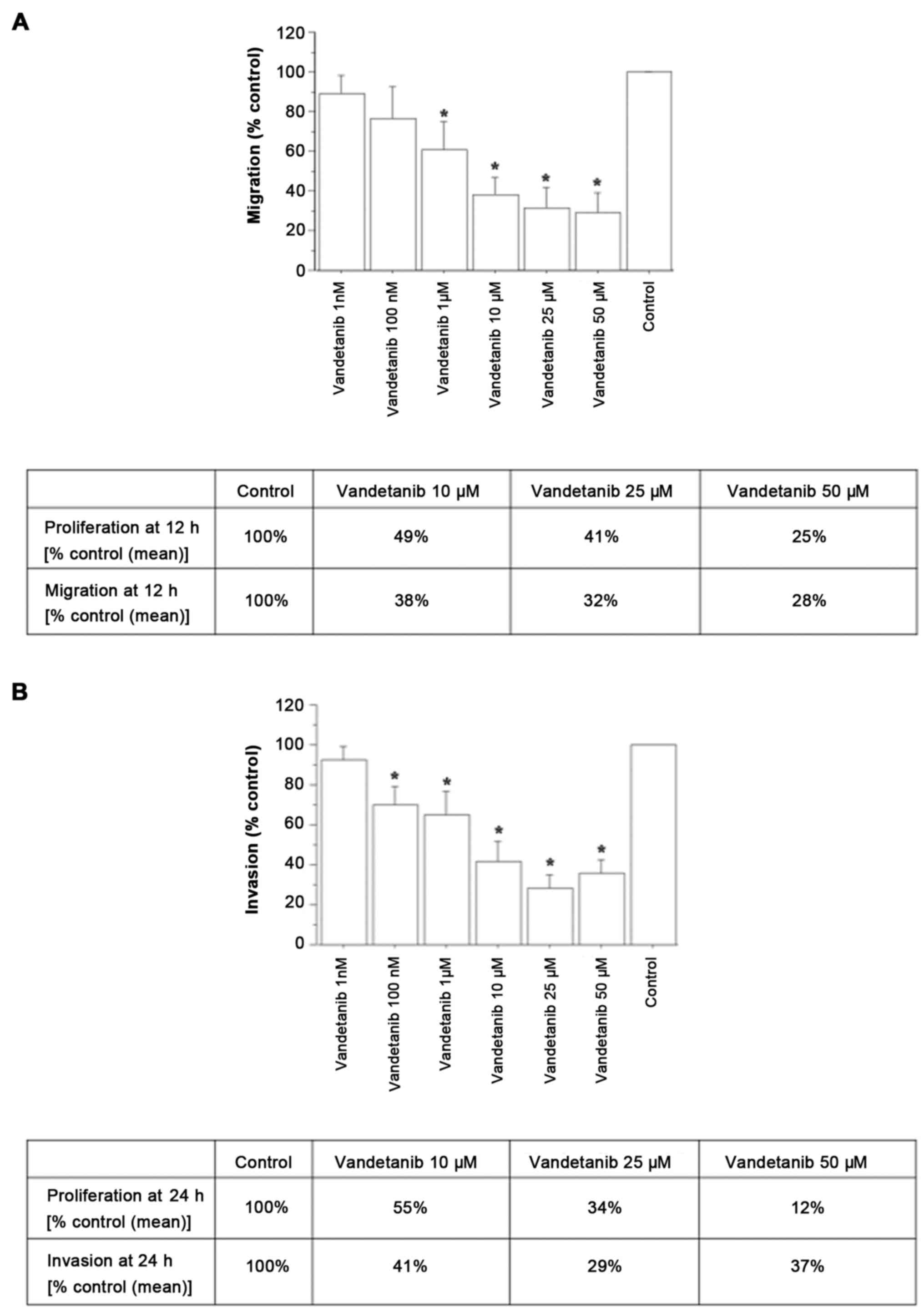
Migration and invasion
The effect of vandetanib on migration and invasion
was evaluated in ATC cells showing a reduction in both migration
(Fig. 3A) and invasion (Fig. 3B).
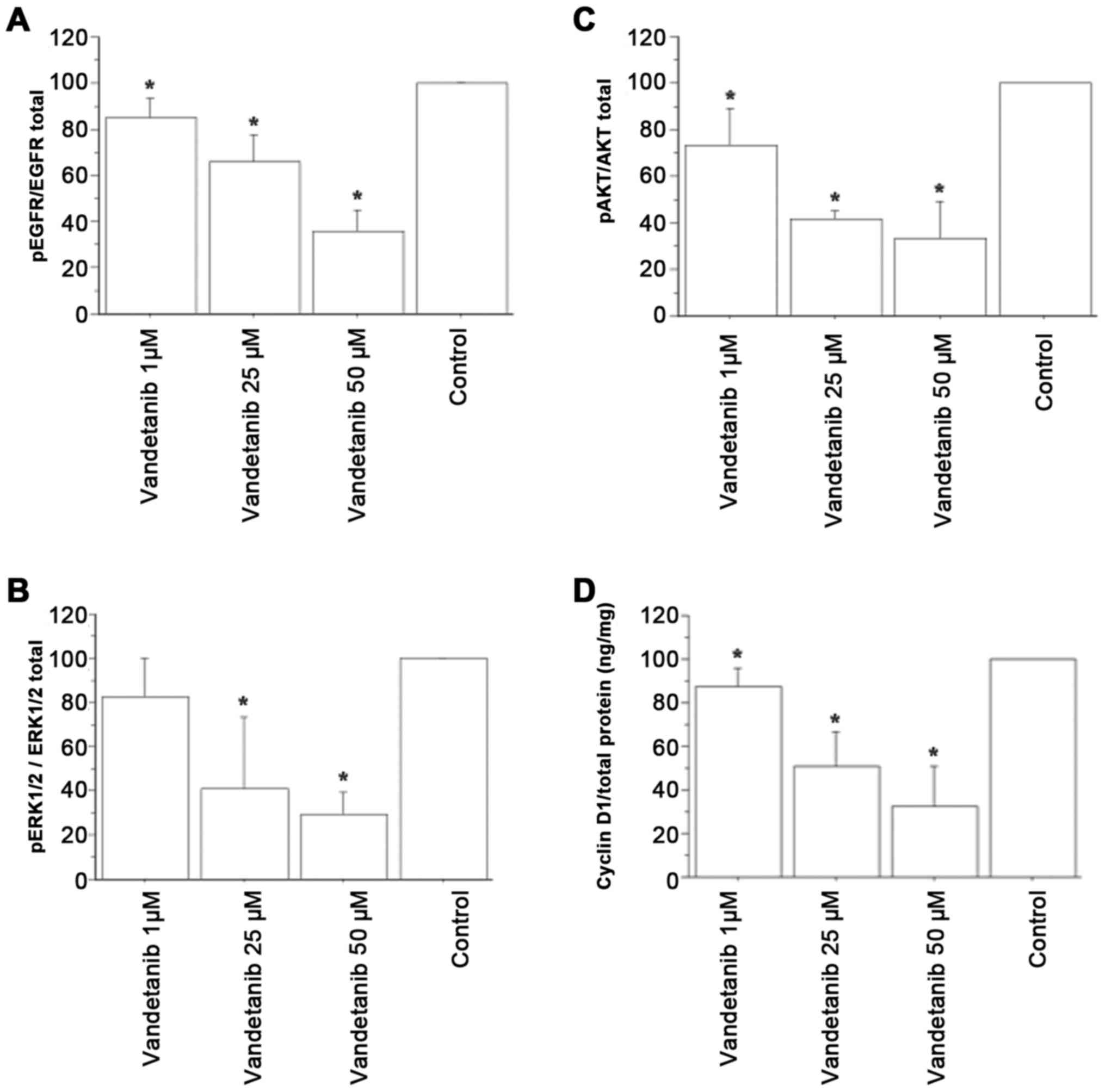
EGFR inhibition in ATC cells by
vandetanib
Vandetanib significantly reduced the phosphorylated
(p)EGFR/total EGFR ratio in the ATC cell lysates in a
dose-dependent manner (Fig.
4A).
Inhibition of AKT or ERK1/2
phosphorylation in ATC cells by vandetanib
Vandetanib significantly reduced the pAKT/total AKT
and pERK1/2/total ERK1/2 protein ratios in the ATC cells (Fig. 4B and C).
Vandetanib decreases cyclin D1 protein
levels in ATC cells
Lower cyclin D1 concentrations were detected in ATC
cells treated with vandetanib vs. those treated with vehicle, and
vandetanib inhibited cyclin D1 gene expression in a
dose-dependent manner (P<0.05; Fig.
4D).
Studies in vitro in AF and 8305C
cells
Vandetanib demonstrated a dose-dependent
antiproliferative activity in the 8305C cell line (IC50
of 9.6±3.4 µM) (Fig. 5A), and in AF
cells (IC50 of 4.7±1.8 µM) (Fig. 5B). Vandetanib dose-dependently
induced the apoptosis of the 8305C cells: apoptotic cells were
16.8% with vandetanib 10 µM, and 20 and 33.2% with vandetanib 25
and 50 µM, respectively (P<0.001; by ANOVA; Fig. 5C). In AF cells, vandetanib
dose-dependently increased apoptosis: apoptotic cells were 19.9%
with vandetanib 10 µM, and 22.7 and 27.8% with vandetanib 25 and 50
µM, respectively (P<0.001; by ANOVA; Fig. 5D).
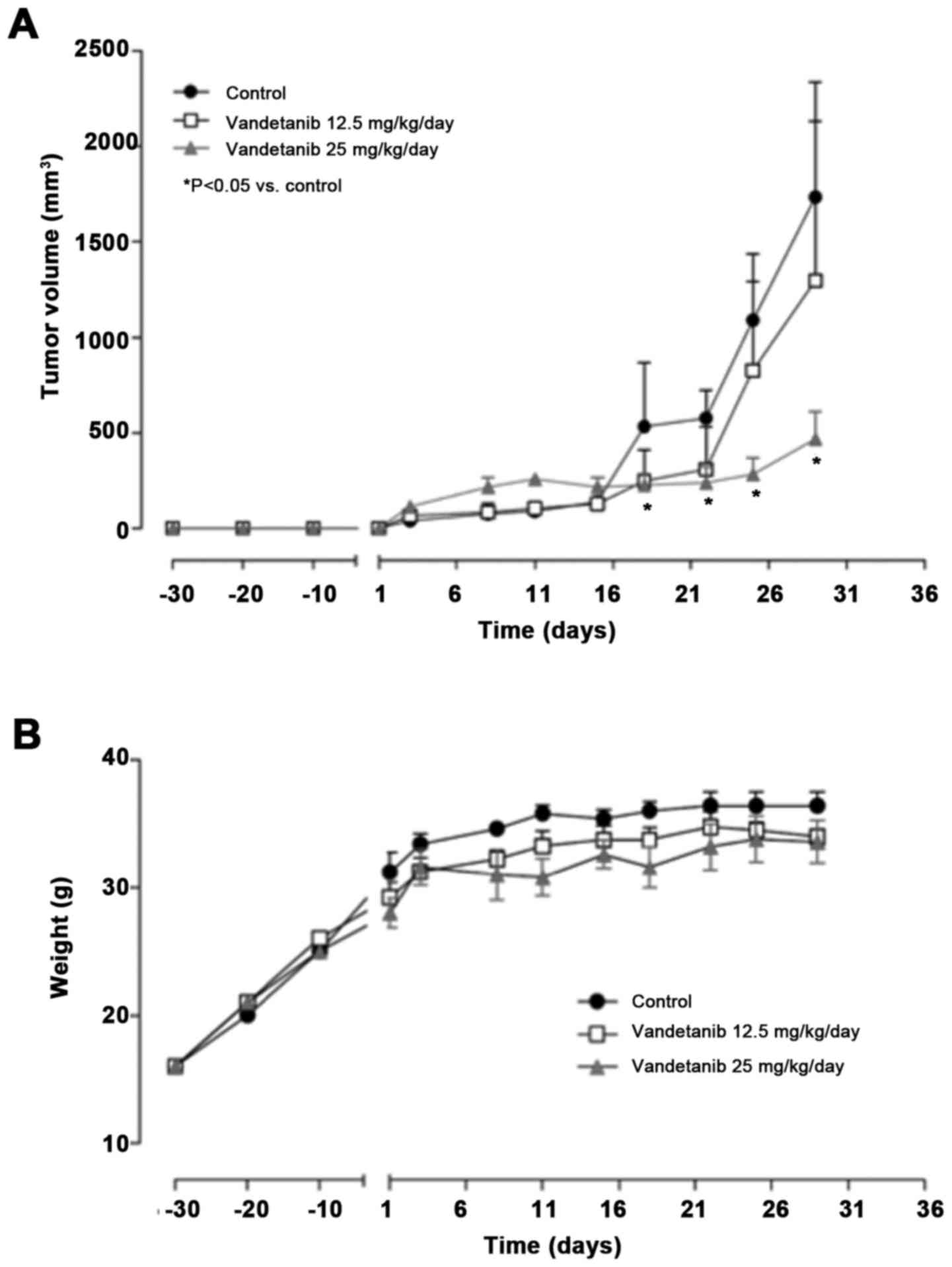
In vivo studies
Vandetanib inhibits 8305C tumor growth
in mice with no body weight loss
Thirty days after subcutaneous xenotransplantation
of 8305C cells in CD nu/nu mice, tumor masses reached the
average volume of 100 mm3 and treatment with vandetanib
was initiated. In order to establish the optimal antitumor dose,
two different doses were administered. At the lower dose of 12.5
mg/kg a slight, but not significant, antitumor activity was
recorded (overall from days 16 to 22), and no statistical
differences were obtained when tumor volumes in the treatment group
were compared to the control group (Fig. 6A). In contrast, at the highest dose
of 25 mg/kg/day i.p., vandetanib significantly inhibited tumor
growth (e.g., at day 25, 282.1 vs. 1,086.9 mm3 of
controls P<0.05; Fig. 6A).
Notably, no loss of mouse body weight throughout the course of the
experiment was observed at both the administered doses, suggesting
that the vandetanib treatment was well tolerated even at its
optimal antitumor dose (Fig.
6B).
Vandetanib decreases VEGF-A expression and
microvessel density in 8305C tumor tissues. 8305C cells led to the
formation of a tumor histologically similar to ATC. Vandetanib
significantly reduced VEGF-A and FVIII immunostaining. A localized
immunoreactivity for VEGF-A was present in cells of the control
cancer mass, and vandetanib reduced it (51±9 vs. 37±7; P<0.05),
with a concurrent reduction in microvessel density (15±4 vs.
controls 26±7; P<0.05).
Discussion
Vandetanib belongs to the TKIs, that are under
evaluation for ATC treatment (26).
With the present study, we contributed to the
understanding of the anticancer activity of vandetanib,
demonstrating that: i) it inhibited primary ATC cell proliferation
in vitro, through increased apoptosis, and suppressed the
migration and invasion abilities as well; and ⅱ) it blocked 8305C
cell proliferation in vitro, increasing apoptosis and
reducing 8305C tumor growth in CD nu/nu mice as well, with
no toxicity.
Our results are in line with those of another study
that reported how vandetanib is able to inhibit 8305C cell growth
in vivo, and to block angiogenesis, decreasing vascular
permeability (27). We also
observed the important antiangiogenic activity of vandetanib in
8305C xenotransplants.
The antiproliferative action of vandetanib was
observed in all the used primary ATC cells, independently from the
absence/presence of V600EBRAF mutation.
Our data support the concept that vandetanib could
be used for a multiple signal inhibition (involving RET, VEGFR,
EGFR, ERK, AKT, and others), and it also exhibits antiangiogenic
activity (28).
It is suggested that AKT plays a crucial role in ATC
oncogenesis, and it has been demonstrated that pharmacological and
molecular inhibition of PI3K or AKT isoforms are able to reduce the
in vitro growth and motility of human TC cell lines
(29,30). Moreover, both RAS/RAF/MAPK, ERK and
PI3K pathways are implicated in the carcinogenesis of TCs, and
mutations in such genes have been reported in ATC (31). Since AKT and ERK proteins are
activated, once phosphorylated, in ATC, these proteins have been
suggested as potential targets of therapy. In the present study in
ATC cells, vandetanib significantly inhibited ERK1/2 and AKT
phosphorylation.
Furthermore, EGFR phosphorylation in ATC cells was
significantly reduced by vandetanib treatment, according to the
results obtained by Di Desidero et al (32) and our previous results (33), reporting that TKI suppressed EGFR
phosphorylation in ATC.
Cyclin D1 regulates cell cycle progression (34), and its expression was observed at
different levels in ~67% of ATC cells by Lee et al (35). In addition, overexpression of cyclin
D1 was reported in 77% of ATC by Wiseman et al (36). Vandetanib, a TKI of both VEGFR-2,
and EGFR, inhibited cell growth downregulating cyclin E and D1
expression (37). Notably, we
showed that vandetanib downregulated the cyclin D1 protein in ATC
cells.
We found a significant 8305C cell-derived tumor
growth inhibition in CD nu/nu mice by vandetanib, without
body weight loss, suggesting a minimal toxicity profile, whereas
other compounds are known to provoke different adverse effects in
humans and animals (38).
Nevertheless, we did not collect data concerning the kidney, liver,
or other biochemical tests, that will be provided in future
studies.
Antineoplastic activity of vandetanib in ATC is the
result of different effects on tumoral cells, that include: i)
antiproliferative activity; ii) increased apoptosis; ⅲ) inhibition
of both migration, and invasion; and ⅳ) inhibition of cancer
neovascularization.
New therapeutic attempts for the treatment of ATC
are ongoing, even if various limitations are still present for the
selective use of new molecules. Even if neoplastic tissue has a
potential target (as BRAF), the tumor response is present in only a
few patients. As we achieve target inhibition, any response may
occur owing to the increased activity of other compensatory
pathways, that rescue cancer cell growth. The efficacy of treatment
could be increased by assessing the sensitivity of primary ATC
cells from each subject to different TKIs. In fact, in vitro
chemosensitivity tests are able to predict in vivo
effectiveness in 60% of cases (39), while a negative chemosensitivity
test in vitro is associated with a 90% of ineffectiveness of
the treatment in vivo (39,40),
thus avoiding the administration of inactive chemotherapeutics to
these patients (15,16,24,26).
In the present study, we first showed an antitumoral
activity of vandetanib (a multi-targeted kinase inhibitor, with
antiangiogenic effect) in human primary ATC cell cultures
established directly from patients, paving the way for personalized
TKI and for future clinical trials.
Acknowledgements
Not applicable.
References
|
1
|
Hundahl SA, Fleming ID, Fremgen AM and
Menck HR: A National Cancer Data Base report on 53,856 cases of
thyroid carcinoma treated in the U.S., 1985–1995 [see comments].
Cancer. 83:2638–2648. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kitamura Y, Shimizu K, Nagahama M, Sugino
K, Ozaki O, Mimura T, Ito K, Ito K and Tanaka S: Immediate causes
of death in thyroid carcinoma: Clinicopathological analysis of 161
fatal cases. J Clin Endocrinol Metab. 84:4043–4049. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: ThyroidAmerican Joint Committee
on Cancer: AJCC Cancer Staging Manual. 6th. Springer-Verlag; New
York, NY: pp. 772002
|
|
4
|
Miccoli P, Materazzi G, Antonelli A,
Panicucci E, Frustaci G and Berti P: New trends in the treatment of
undifferentiated carcinomas of the thyroid. Langenbecks Arch Surg.
392:397–404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kebebew E: Anaplastic thyroid cancer:
Rare, fatal, and neglected. Surgery. 152:1088–1089. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Crevoisier R, Baudin E, Bachelot A,
Leboulleux S, Travagli JP, Caillou B and Schlumberger M: Combined
treatment of anaplastic thyroid carcinoma with surgery,
chemotherapy, and hyperfractionated accelerated external
radiotherapy. Int J Radiat Oncol Biol Phys. 60:1137–1143. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smallridge RC, Ain KB, Asa SL, Bible KC,
Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal
MS, et al: American Thyroid Association Anaplastic Thyroid Cancer
Guidelines Taskforce: American Thyroid Association guidelines for
management of patients with anaplastic thyroid cancer. Thyroid.
22:1104–1139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Antonelli A, Fallahi P, Ferrari SM,
Ruffilli I, Santini F, Minuto M, Galleri D and Miccoli P: New
targeted therapies for thyroid cancer. Curr Genomics. 12:626–631.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jasim S, Ozsari L and Habra MA:
Multikinase inhibitors use in differentiated thyroid carcinoma.
Biologics. 8:281–291. 2014.PubMed/NCBI
|
|
10
|
Johanson V, Ahlman H, Bernhardt P, Jansson
S, Kölby L, Persson F, Stenman G, Swärd C, Wängberg B, Stridsberg
M, et al: A transplantable human medullary thyroid carcinoma as a
model for RET tyrosine kinase-driven tumorigenesis. Endocr Relat
Cancer. 14:433–444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wells SA Jr, Robinson BG, Gagel RF, Dralle
H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR,
et al: Vandetanib in patients with locally advanced or metastatic
medullary thyroid cancer: A randomized, double-blind phase III
trial. J Clin Oncol. 30:134–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cooper MR, Yi SY, Alghamdi W, Shaheen DJ
and Steinberg M: Vandetanib for the treatment of medullary thyroid
carcinoma. Ann Pharmacother. 48:387–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leboulleux S, Bastholt L, Krause T, de la
Fouchardiere C, Tennvall J, Awada A, Gómez JM, Bonichon F,
Leenhardt L, Soufflet C, et al: Vandetanib in locally advanced or
metastatic differentiated thyroid cancer: A randomised,
double-blind, phase 2 trial. Lancet Oncol. 13:897–905. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
AstraZeneca, . Evaluation of Efficacy,
Safety of Vandetanib in Patients With Differentiated Thyroid Cancer
(VERIFY) [ClinicalTrials.gov Identifier: NCT01876784].
2016.Available from. https://www.clinicaltrials.gov/ct2/show/NCT01876784Last
Update Posted: September 6, 2017.
|
|
15
|
Antonelli A, Ferrari SM, Fallahi P, Berti
P, Materazzi G, Barani L, Marchetti I, Ferrannini E and Miccoli P:
Primary cell cultures from anaplastic thyroid cancer obtained by
fine-needle aspiration used for chemosensitivity tests. Clin
Endocrinol (Oxf). 69:148–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Antonelli A, Ferrari SM, Fallahi P, Berti
P, Materazzi G, Marchetti I, Ugolini C, Basolo F, Miccoli P and
Ferrannini E: Evaluation of the sensitivity to chemotherapeutics or
thiazolidinediones of primary anaplastic thyroid cancer cells
obtained by fine-needle aspiration. Eur J Endocrinol. 159:283–291.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Antonelli A, Ferrari SM, Fallahi P, Berti
P, Materazzi G, Minuto M, Giannini R, Marchetti I, Barani L, Basolo
F, et al: Thiazolidinediones and antiblastics in primary human
anaplastic thyroid cancer cells. Clin Endocrinol (Oxf). 70:946–953.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fiore L, Pollina LE, Fontanini G, Casalone
R, Berlingieri MT, Giannini R, Pacini F, Miccoli P, Toniolo A,
Fusco A, et al: Cytokine production by a new undifferentiated human
thyroid carcinoma cell line, FB-1. J Clin Endocrinol Metab.
82:4094–4100. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agretti P, De Marco G, De Servi M,
Marcocci C, Vitti P, Pinchera A and Tonacchera M: Evidence for
protein and mRNA TSHr expression in fibroblasts from patients with
thyroid-associated ophthalmopathy (TAO) after adipocytic
differentiation. Eur J Endocrinol. 152:777–784. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Copland JA, Marlow LA, Williams SF, Grebe
SK, Gumz ML, Maples WJ, Silverman VE and Smallridge RC: Molecular
diagnosis of a BRAF papillary thyroid carcinoma with multiple
chromosome abnormalities and rare adrenal and hypothalamic
metastases. Thyroid. 16:1293–1302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Christensen L, Blichert-Toft M, Brandt M,
Lange M, Sneppen SB, Ravnsbaek J, Mollerup CL, Strange L, Jensen F,
Kirkegaard J, et al: Thyroperoxidase (TPO) immunostaining of the
solitary cold thyroid nodule. Clin Endocrinol (Oxf). 53:161–169.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Antonelli A, Ferrari SM, Fallahi P,
Frascerra S, Piaggi S, Gelmini S, Lupi C, Minuto M, Berti P,
Benvenga S, et al: Dysregulation of secretion of CXC
alpha-chemokine CXCL10 in papillary thyroid cancer: Modulation by
peroxisome proliferator-activated receptor-gamma agonists. Endocr
Relat Cancer. 16:1299–1311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Antonelli A, Bocci G, La Motta C, Ferrari
SM, Fallahi P, Fioravanti A, Sartini S, Minuto M, Piaggi S, Corti
A, et al: Novel pyrazolopyrimidine derivatives as tyrosine kinase
inhibitors with antitumoral activity in vitro and in vivo in
papillary dedifferentiated thyroid cancer. J Clin Endocrinol Metab.
96:E288–E296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Antonelli A, Bocci G, La Motta C, Ferrari
SM, Fallahi P, Ruffilli I, Di Domenicantonio A, Fioravanti A,
Sartini S, Minuto M, et al: CLM94, a novel cyclic amide with
anti-VEGFR-2 and antiangiogenic properties, is active against
primary anaplastic thyroid cancer in vitro and in vivo. J Clin
Endocrinol Metab. 97:E528–E536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bocci G, Fioravanti A, La Motta C, Orlandi
P, Canu B, Di Desidero T, Mugnaini L, Sartini S, Cosconati S, Frati
R, et al: Antiproliferative and proapoptotic activity of CLM3, a
novel multiple tyrosine kinase inhibitor, alone and in combination
with SN-38 on endothelial and cancer cells. Biochem Pharmacol.
81:1309–1316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Antonelli A, Fallahi P, Ulisse S, Ferrari
SM, Minuto M, Saraceno G, Santini F, Mazzi V, D'Armiento M and
Miccoli P: New targeted therapies for anaplastic thyroid cancer.
Anticancer Agents Med Chem. 12:87–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gule MK, Chen Y, Sano D, Frederick MJ,
Zhou G, Zhao M, Milas ZL, Galer CE, Henderson YC, Jasser SA, et al:
Targeted therapy of VEGFR2 and EGFR significantly inhibits growth
of anaplastic thyroid cancer in an orthotopic murine model. Clin
Cancer Res. 17:2281–2291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
La Motta C, Mugnaini L, Sartini S, Da
Settimo F, Bocci G, Fioravanti A, Del Tacca M and Martini C: 2007
Derivati a nucleo pirazolo (3,4-d) pirimidinico quali inibitori di
proteina tirosina chinasi. RM2007A000480 del 14/9/2007.
|
|
29
|
Liu Z, Hou P, Ji M, Guan H, Studeman K,
Jensen K, Vasko V, El-Naggar AK and Xing M: Highly prevalent
genetic alterations in receptor tyrosine kinases and
phosphatidylinositol 3-kinase/akt and mitogen-activated protein
kinase pathways in anaplastic and follicular thyroid cancers. J
Clin Endocrinol Metab. 93:3106–3116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shinohara M, Chung YJ, Saji M and Ringel
MD: AKT in thyroid tumorigenesis and progression. Endocrinology.
148:942–947. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Santarpia L, El-Naggar AK, Cote GJ, Myers
JN and Sherman SI: Phosphatidylinositol 3-kinase/akt and
ras/raf-mitogen-activated protein kinase pathway mutations in
anaplastic thyroid cancer. J Clin Endocrinol Metab. 93:278–284.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Di Desidero T, Fioravanti A, Orlandi P,
Canu B, Giannini R, Borrelli N, Man S, Xu P, Fontanini G, Basolo F,
et al: Antiproliferative and proapoptotic activity of sunitinib on
endothelial and anaplastic thyroid cancer cells via inhibition of
Akt and ERK1/2 phosphorylation and by down-regulation of cyclin-D1.
J Clin Endocrinol Metab. 98:E1465–E1473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Antonelli A, Bocci G, Fallahi P, La Motta
C, Ferrari SM, Mancusi C, Fioravanti A, Di Desidero T, Sartini S,
Corti A, et al: CLM3, a multitarget tyrosine kinase inhibitor with
antiangiogenic properties, is active against primary anaplastic
thyroid cancer in vitro and in vivo. J Clin Endocrinol Metab.
99:E572–E581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Klein EA and Assoian RK: Transcriptional
regulation of the cyclin D1 gene at a glance. J Cell Sci.
121:3853–3857. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee JJ, Au AY, Foukakis T, Barbaro M, Kiss
N, Clifton-Bligh R, Staaf J, Borg A, Delbridge L, Robinson BG, et
al: Array-CGH identifies cyclin D1 and UBCH10 amplicons in
anaplastic thyroid carcinoma. Endocr Relat Cancer. 15:801–815.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wiseman SM, Masoudi H, Niblock P, Turbin
D, Rajput A, Hay J, Bugis S, Filipenko D, Huntsman D and Gilks B:
Anaplastic thyroid carcinoma: Expression profile of targets for
therapy offers new insights for disease treatment. Ann Surg Oncol.
14:719–729. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sarkar S, Mazumdar A, Dash R, Sarkar D,
Fisher PB and Mandal M: ZD6474, a dual tyrosine kinase inhibitor of
EGFR and VEGFR-2, inhibits MAPK/ERK and AKT/PI3-K and induces
apoptosis in breast cancer cells. Cancer Biol Ther. 9:592–603.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ye L, Santarpia L and Gagel RF: The
evolving field of tyrosine kinase inhibitors in the treatment of
endocrine tumors. Endocr Rev. 31:578–599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Blumenthal RD and Goldenberg DM: Methods
and goals for the use of in vitro and in vivo chemosensitivity
testing. Mol Biotechnol. 35:185–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Antonelli A, Ferri C, Ferrari SM,
Sebastiani M, Colaci M, Ruffilli I and Fallahi P: New targeted
molecular therapies for dedifferentiated thyroid cancer. J Oncol.
2010:9216822010. View Article : Google Scholar : PubMed/NCBI
|