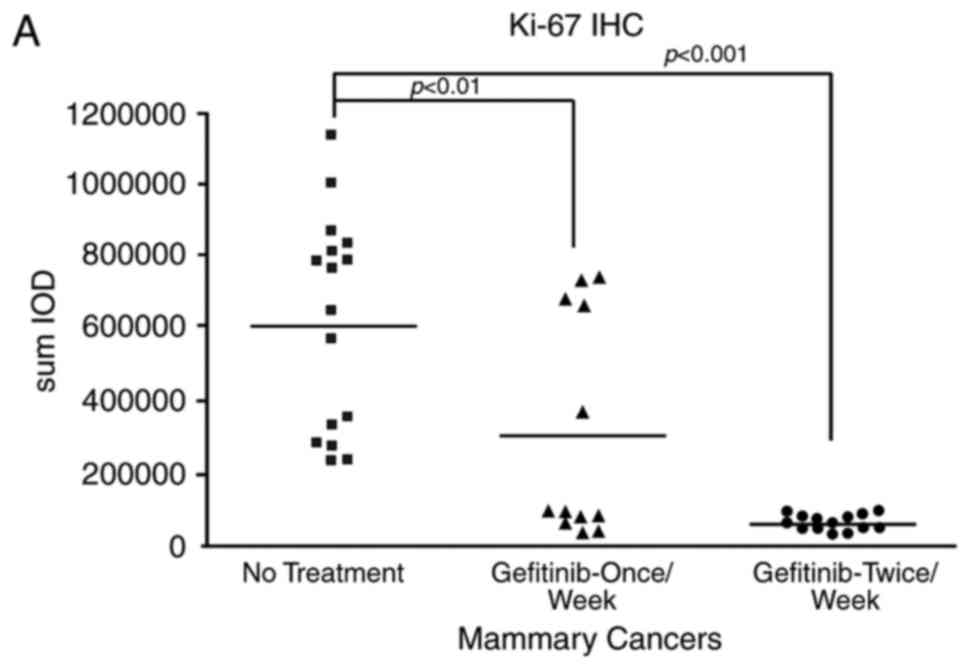
|
1
|
Salamone DS, Brandt R, Ciardiello F and
Normanno N: Epidermal growth factor-related peptides and their
receptors in human malignancies. Crit Rev Oncol Hematol.
19:183–232. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arteaga CT: Overview of epidermal growth
factor receptor biology and its role as therapeutic target in human
neoplasia. Semin Oncol. 29 5 Suppl 14:S3–S9. 2002. View Article : Google Scholar
|
|
3
|
Moasser MM and Krop IE: The evolving
landscape of HER2 targeting in breast cancer. JAMA Oncol.
1:1154–1161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hida L, Ogawa S, Park JC, Park JY, Shimazu
J, Horio Y and Yoshida K: Gefitinib for the treatment of non small
cell lung cancer. Expert Rev Anticancer Ther. 9:17–35. 2006.
View Article : Google Scholar
|
|
5
|
Wong SF: Cetuximab: An epidermal factor
receptor monoclonal antibody for treatment of colorectal cancer.
Clin Ther. 27:684–694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan WL and Ng QS: The continuing role of
epidermal growth factor receptor tyrosine kinase inhibitors in
advanced squamous cell carcinoma of the lung. Transl Lung Cancer
Res. 5:106–109. 2016.PubMed/NCBI
|
|
7
|
Polychronis A, Sinnett HD, Hadjiminas D,
Singhal H, Masnsi JL, Shivapatham D, Shousha S, Jiang J, Peston D,
Barrett N, et al: Preoperative gefitinib versus gefitinib and
anastrozole in postmenopausal patients with oestrogen-receptor
positive and epidermal-growth-factor-receptor-positive primary
breast cancer: A double blind placebo controlled phase II
randomized trial. Lancet Oncol. 6:383–391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baselga J, Albanell J, Ruiz A, Lluch A,
Gascon P, Guillém V, González S, Sauleda S, Marimón I, Tabernero
JM, et al: Phase II and tumor pharmacodynamic study of gefitinib in
patients with advanced breast cancer. J Clin Oncol. 23:5323–5333.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Massarweh S, Thann YL, Huang J, Sexton K,
Weiss H, Tsimelzon A, Beyer A, Rimawi M, Cai WY, Hilsenbeck S, et
al: A phase II neoadjvant trial of anastrozole, fulvestrant, and
gefitinib in patients with newly diagnosed estrogen receptor
positive breast cancer. Breast Cancer Res Treat. 129:819–827. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
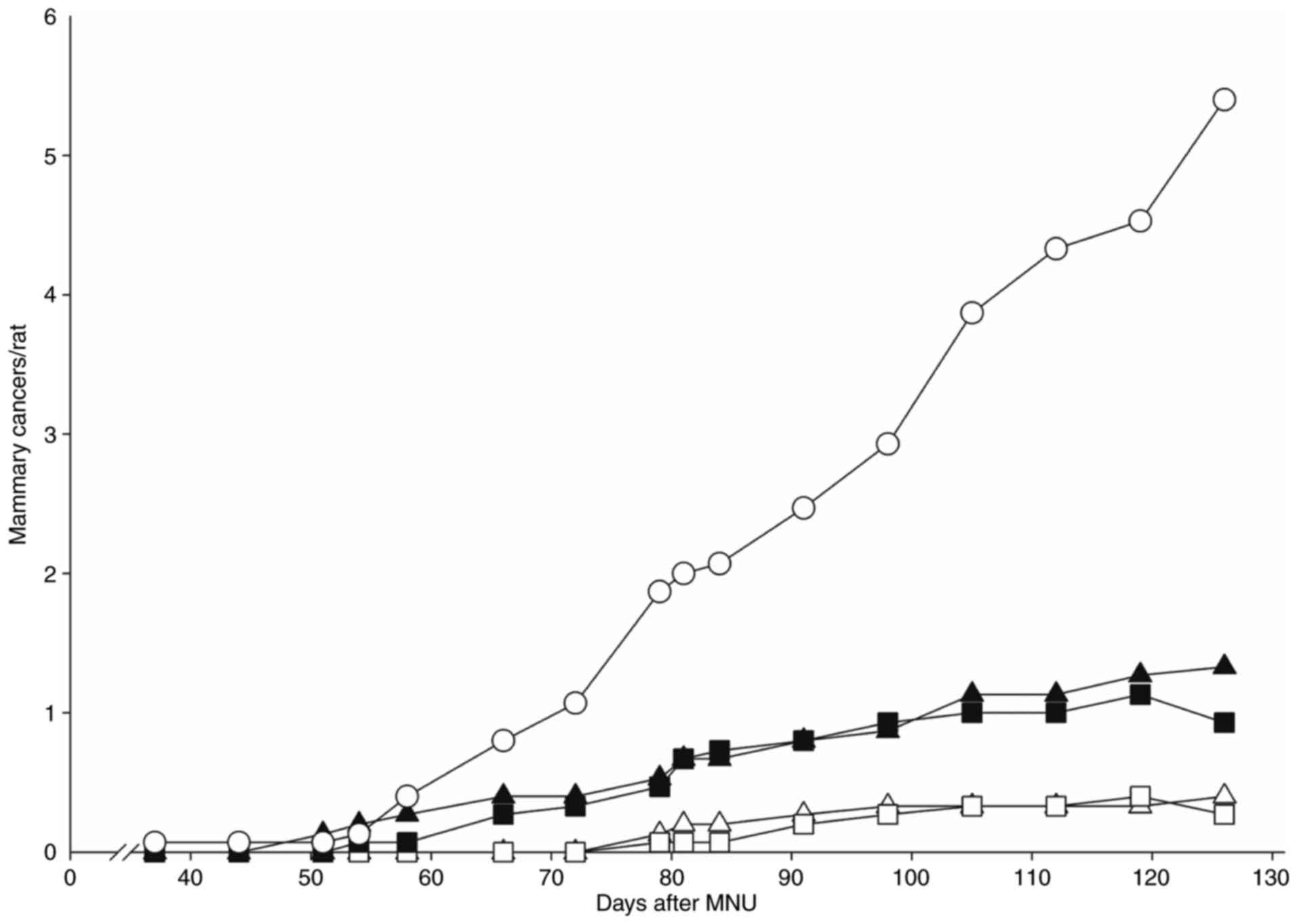
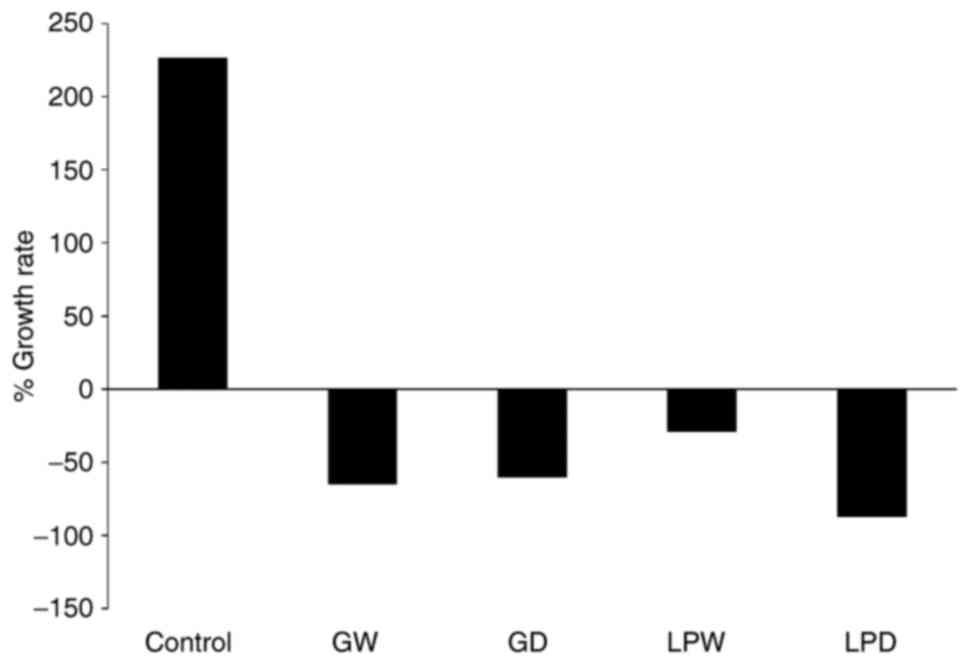
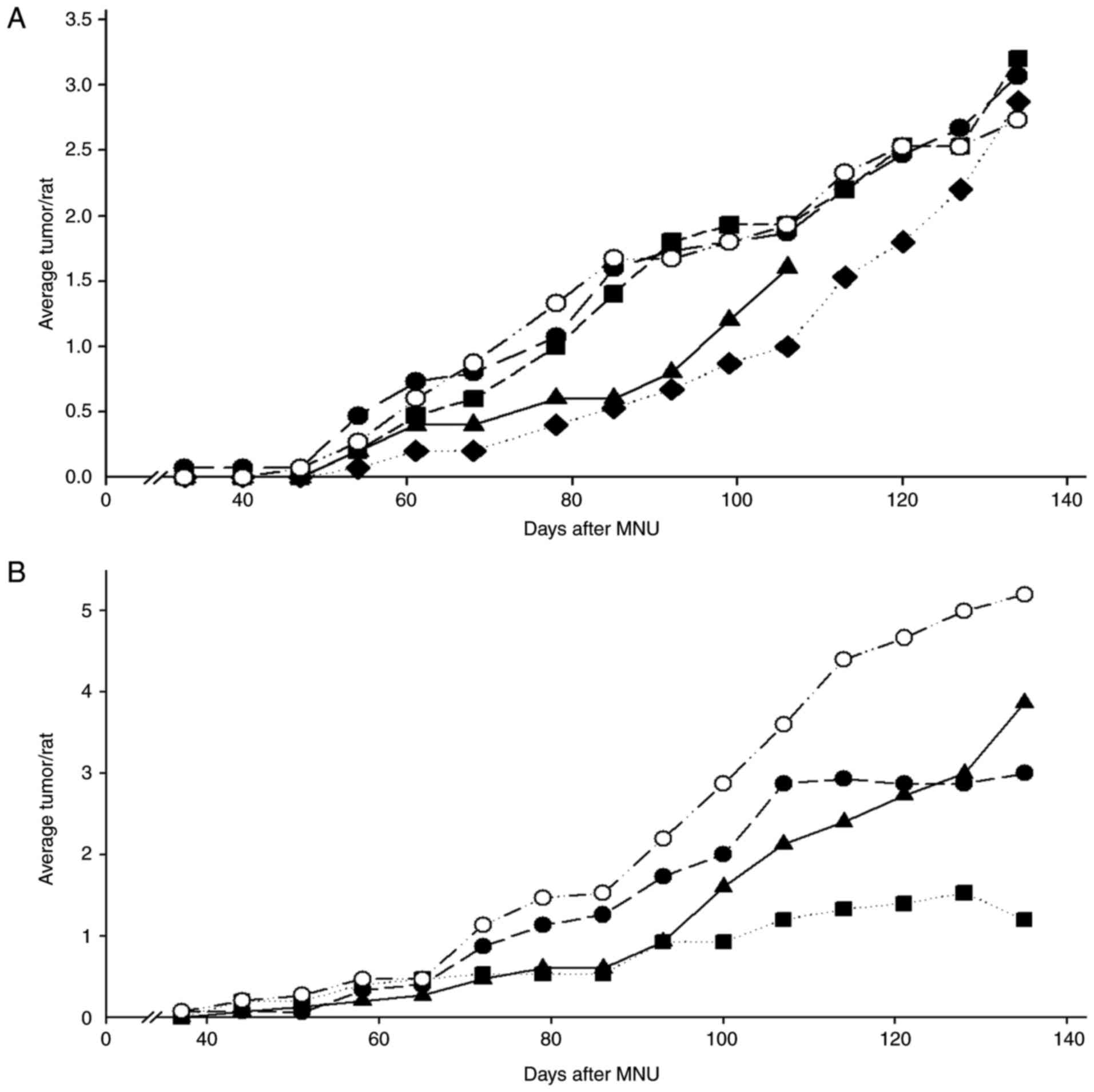
Lubet RA, Szabo E, Iwata KK, Gill SC,
Tucker C, Bode A, Steele VE, Juliana MM, Nicastro HL and Grubbs CJ:
Effect of intermittent dosing regimens of erlotinib on
methylnitrosourea-induced mammary carcinogenesis. Cancer Prev Res.
6:448–454. 2013. View Article : Google Scholar
|
|
11
|
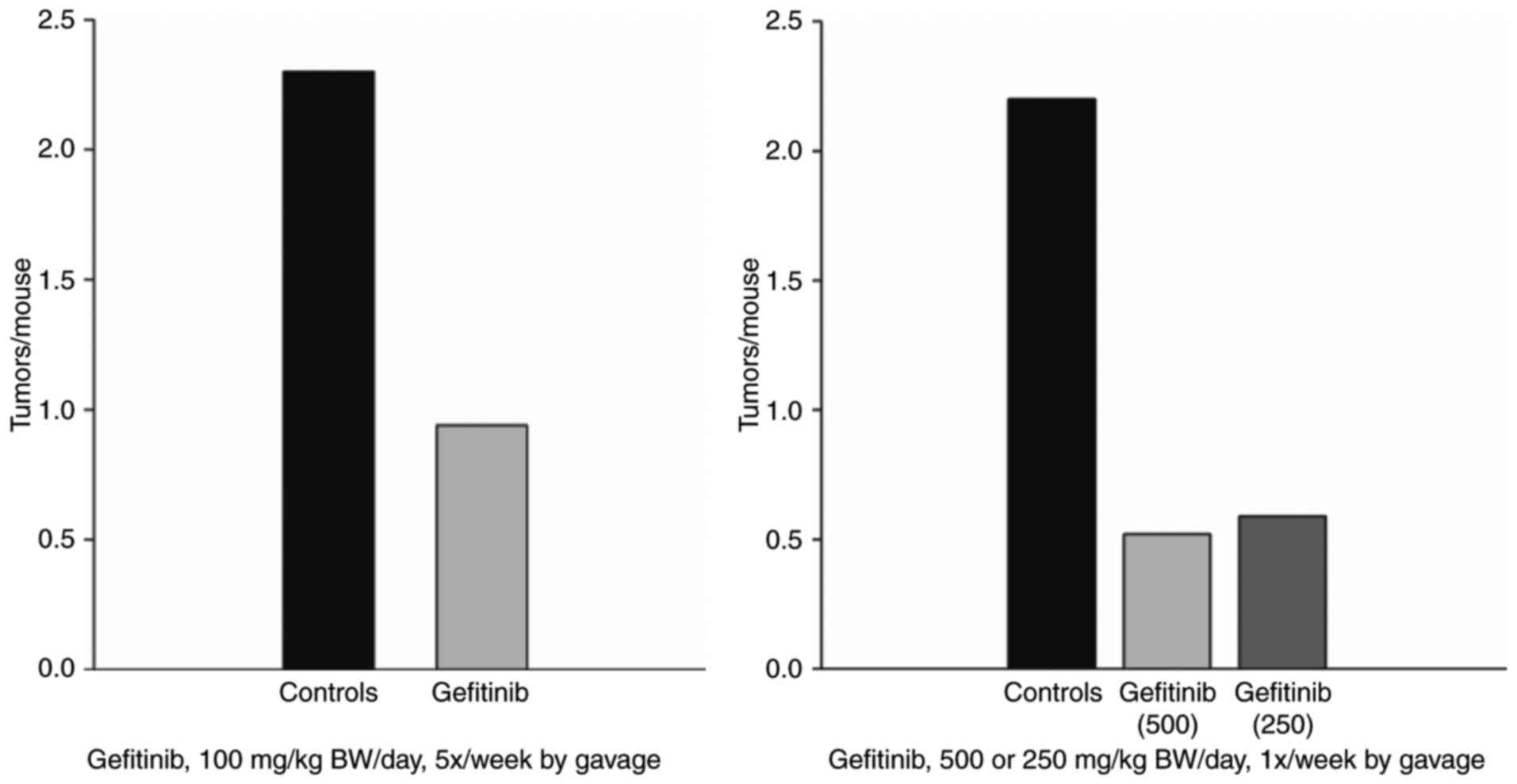
Lubet RA, Szabo E, Christov K, Bode AM,
Ericson ME, Steele VE, Juliana MM and Grubbs CJ: Effects of
gefitinib (Iressa) on mammary cancers: Preventive studies with
varied dosages, combinations with vorozole or targretin, and
biomarker changes. Mol Cancer Ther. 7:972–979. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Cho YY, Langfald A, Carper A, Lubet
RA, Grubbs CJ, Ericson ME and Bode AM: Lapatinib, a
preventive/therapeutic agent against mammary cancer, suppresses
RTK-mediated signaling through multiple signaling pathways. Cancer
Prev Res. 4:1190–1197. 2011. View Article : Google Scholar
|
|
13
|
Ma CX, Sanchez C, Gao F, Crowder R,
Naughton M, Pluard T, Creekmore A, Guo Z, Hoog J, Lockhart AC, et
al: A Phase I Study of the AKT inhibitor MK-2206 in combination
with hormonal therapy in postmenopausal women with estrogen
receptor-positive metastatic breast cancer. Clin Cancer Res.
22:2650–2658. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grubbs CJ, Peckham JC and McDonnough KD:
Effect of ovarian hormones on the induction of
1-methyl-1nitrosourea induced mammary cancer. Carcinogenesis.
4:495–497. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chan MM, Lu X, Merchant FM, Inglehart JD
and Miron PL: Gene expression profiling of NMU-induced rat mammary
tumors: Cross species comparison with human breast cancer.
Carcinogenesis. 26:1343–1353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gottardis MM and Jordan VC: Antitumor
actions of keoxifene and tamoxifen in the
N-nitrosomethylurea-induced rat mammary carcinoma model. Cancer
Res. 47:4020–4024. 1987.PubMed/NCBI
|
|
17
|
Lubet RA, Steele VE, Casebolt TL, Eto I,
Kelloff GJ and Grubbs CJ: Chemopreventive effects of the aromatase
inhibitors vorozole (R-83842) and 4-hydroxyandrostenedione in the
methylnitrosourea (MNU)-induced mammary tumor model in
Sprague-Dawley rats. Carcinogenesis. 15:2775–2780. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muller WJ, Sinn E, Pattengale PK, Wallace
R and Leder P: Single-step induction of mammary adenocarcinoma in
transgenic mice bearing the activiated c-neu oncogene. Cell.
54:105–115. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu C, Speers C, Zhang Y, Xu X, Hill J,
Steinbis E, Celestino J, Shen Q, Kim H, Hilsenbeck S, et al: Effect
of epidermal growth factor receptor inhibitor on development of
estrogen receptor-negative mammary tumors. J Natl Cancer Inst.
95:1825–1833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zelazny E, Li B, Anagnostopoulos AM,
Coleman A and Perkins AS: Cooperating oncogenic events in murine
mammary tumorigenesis: Assessment of ErbB2, mutant p53, and mouse
mammary tumor virus. Exp Mol Pathol. 70:183–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guttman-Yassky E, Mita A, De Jonge M,
Matthews L, McCarthy S, Iwata KK, Verweij J, Rowinsky EK and
Krueger JG: Characterization of the cutaneous pathology in (NSCLC)
patients treated with (EGFR) tyrosine kinase inhibitor erlotinib.
Eur J Cancer. 46:2010–2019. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Christov K, Shilkaitis A, Green A, Mehta
RG, Grubbs C, Kelloff G and Lubet R: Cellular responses of mammary
carcinomas to aromatase inhibitors: Effects of vorozole. Breast
Cancer Res Treat. 60:117–128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lubet RA, Boring D, Steele VE, Ruppert JM,
Juliana MM and Grubbs CJ: Lack of efficacy of the statins
atorvastatin and lovastatin in rodent mammary carcinogenesis.
Cancer Prev Res. 2:161–167. 2009. View Article : Google Scholar
|
|
24
|
Dowsett M, Smith IE, Ebbs SR, Dixon JM,
Skene A, Griffith C, Boeddinghaus I, Salter J, Detre S, Hills M, et
al: Short-term changes in Ki-67 during neoadjuvant treatment of
primary breast cancer with anastrozole or tamoxifen alone or
combined correlate with recurrence-free survival. Clin Cancer Res.
11:951S–958S. 2005.PubMed/NCBI
|
|
25
|
Grommes C, Oxnard GR, Kris MG, Miller VA,
Pao W, Holodny AI, Clarke JL and Lassman AB: ‘Pulsatile’ high-dose
weekly erlotinib for CNS metastases from EGFR mutant non-small cell
lung cancer. Neuro Oncol. 13:1364–1369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Milton DT, Azzoli CG, Heelan RT,
Venkatraman EM, Gomez JE, Kris MG, Krug LM, Pao W, Rizvi NA, Dunne
M and Miller VA: A phase I/II study of weekly high-dose erlotinib
in previously treated patients with nonsmall cell lung cancer.
Cancer. 107:1034–1041. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Samadder NJ, Neklason DW, Boucher KM,
Byrne KR, Kanth P, Samowitz W, Jones D, Tavtigian SV, Done MW,
Berry T, et al: Effects of Sulindac and erlotinib vs placebo on
duodenal neoplasia in familial adenomatous polyposis: A randomized
clinical trial. JAMA. 315:1266–1275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chandarlapaty S, Sawai A, Scaltriti M,
Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK,
Baselga J and Rosen N: AKT inhibition relieves feedback suppression
of receptor tyrosine kinase expression and activity. Cancer Cell.
19:58–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ercan D, Xu C, Yanaita M, Monast CS,
Pratilas CA, Montero J, Butaney M, Shimamura T, Sholl L, Ivanova
EV, et al: Reactivation of ERK signaling causes resistance to EGFR
kinase inhibitors. Cancer Discov. 2:934–947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang W, Hosford SR, Dillon LM, Shee K, Liu
SC, Bean JR, Salphati L, Pang J, Zhang X, Nannini MA, et al:
Strategically timing inhibition of phosphatidylinositol 3-kinase to
maximize therapeutic index in estrogen receptor alpha-positive
PIK3CA-mutant breast cancer. Clin Cancer Res. 22:2250–2260. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Q, Li R, Chen X, Lee SB, Pan J,
Xiong D, Hu J, Miller MS, Szabo E, Lubet RA, et al: Effects of
weekly or daily dosing regimen of Gefitinib in mouse models of lung
cancer. Oncotarget. 8:72447–72456. 2017.PubMed/NCBI
|