Introduction
The EGFR pathway was defined more than 20 years ago,
and was quickly shown to be associated with a variety of important
cellular pathways (1); including
those associated with cell proliferation and the cell cycle
(2). The potential role of EGFR2
antagonists (e.g., trastuzumab and lapatinib) in the treatment of
Neu-amplified breast cancer was immediately appreciated, and the
use of the monoclonal antibody trastuzumab and more recently
pertuzumab directed against Neu (EGFR2) has profoundly altered the
outcome for women with Neu-amplified breast cancer (3). The potential use of an EGFR1 inhibitor
appeared particularly appealing since EGFR1 is overexpressed in
multiple tumor types (2). EGFR1
small-molecule inhibitors are approved for the treatment of lung
cancer (4), particularly lung
tumors with EGFR1 mutations, and for pancreatic cancers (in
conjunction with standard therapy in an advanced setting). In
addition, small-molecule inhibitors or monoclonal antibodies
directed against EGFR1 have been employed in head and neck cancer
in conjunction with radiation, in colorectal cancer, and in second
line therapy of squamous cell carcinoma of the lung (5,6). In
the treatment of estrogen receptor-positive (ER+) breast
cancer, there have been two studies which demonstrate potential
efficacy based either on clinical outcome in a neoadjuvant setting
(7), or striking modulation of the
generally accepted biomarker Ki67 in a pre-surgical protocol
(8). Furthermore, recent data have
demonstrated efficacy in advanced ER+ breast cancer when
used in conjunction with an aromatase inhibitor (9). We previously reported the EGFR
inhibitors gefitinib (10) and
erlotinib (11), as well as the
Neu/EGFR2 inhibitor lapatinib (12), were effective in an MNU-induced
model of ER+ mammary cancer when these agents were
administered by daily dosing.
AKT, a serine threonine kinase, is the major
downstream substrate for PI3K and helps regulate cellular processes
that contribute to tumor progression; including cell proliferation,
survival, tissue invasion and angiogenesis. MK2206, a highly
selective allosteric inhibitor of AKT showed positive activity in a
phase I study, and is currently undergoing clinical investigation
in breast cancer patients (13).
Various animal models of breast cancer have
routinely been employed in the testing for preventive agents. The
ER+ rat mammary cancer model has been used for more than
30 years (14). These cancers are
histologically and genetically similar to well-differentiated
ER+ human breast cancers (15). Furthermore, as may be expected,
treatments that alter the hormonal axis (e.g., SERMS, aromatase
inhibitors) were found to be strong chemopreventive and even
therapeutic agents in this model (16,17).
MNU-induced mammary cancers, as mentioned above, respond to EGFR
inhibitors (10,11). The second model that was employed
was the mouse MMTV-Neu/P53KO that was initially developed by Muller
et al (18). These animals
develop ER− Neu-overexpressing mammary carcinomas. This
model was shown to be particularly sensitive to the effects of EGFR
inhibitors (19). We used a slight
modification of the model which includes a heterozygous KO of P53
(20). Cancers from this model
parallel human ER− Neu-amplified tumors which tend to
have P53 mutations.
Although (as will be shown below) weekly dosing with
TKI inhibitors is at least equally effective with daily dosing,
perhaps the main advantage relates to the observation that weekly
dosing with EGFR inhibitors has clearly been shown to be less
toxic. Although the targeted kinase inhibitors are clinically
important, questions of dosing schedule and toxicity (21) are likely to inhibit their use;
particularly in a prevention setting. In addition, there are
specific feedback loops (see Discussion) associated with daily
treatment with these classes of agents that may be partially
averted with weekly dosing. We recently showed in the MNU model
that erlotinib was effective when administered daily or weekly
(10). In the present study, we
expanded this observation to additional EGFR inhibitors (gefitinib
and lapatinib) to determine whether weekly dosing is effective in a
second mammary cancer model (MMTV-Neu/P53KO) which is
ER−. Specifically, we determined: i) the preventive
efficacy of daily and weekly dosing with gefitinib and lapatinib in
the MNU-induced ER+ model; ii) the efficacy of daily and
weekly therapeutic dosing in the MNU-induced ER+ model;
iii) the efficacy of daily and weekly gefitinib in the
MMTV-Neu/P53KO model; and iv) the modulation of biomarkers by
weekly dosing with gefitinib in the MNU model when examined 1 day
or 7 days after a weekly dose. These studies demonstrated that
weekly dosing was effective in both models, and that
proliferation-related biomarkers were modulated either 1 day or
even 7 days following a weekly dose with gefitinib. Finally, we
determined the more general relevance of the altered dosing to
other small-molecule kinase inhibitors. Specifically, the
allosteric inhibitor AKT MK2206 given alone or together with the
aromatase inhibitor (vorozole) was tested following daily or weekly
dosing.
Materials and methods
Chemicals and animals
Treatment of female Sprague-Dawley rats was
conducted as previously described (10,11).
Methylnitrosourea (MNU) was obtained from the NCI Chemical
Carcinogen Repository, and injected i.v. (75 mg/kg BW) via the
jugular vein when the animals were 50 days of age. Teklad diet and
rats were obtained from Harlan Sprague-Dawley, Inc. (Indianapolis,
IN, USA). All rats and mice were housed in IACUC approved (no.
09804) animal facilities at the University of Alabama at
Birmingham. The use of animals was necessary to obtain data that
would support a clinical trial of these agents in women. Ethical
approval was given bt the IACUC Committee. Gefitinib, lapatinib and
MK2206 were supplied by the NCI Cancer Prevention Repository. All
agents were administered by gavage on a daily or weekly basis.
Agents were administered in a volume of 0.5 ml/gavage for rat
studies, and 0.2 ml/gavage for mouse studies. The vehicle for both
agents was ethanol:polyethylene glycol 400 (10:90; v/v).
Data collection and analyses
In all studies, rats and mice were palpated for
mammary tumors twice weekly and weighed 1 time/week. Body weights
of the rats or mice did not differ >5% from the controls in
either the prevention or therapeutic studies. Statistical analyses
of cancer incidence and latency were determined using log-rank
analysis, while differences in final cancer multiplicity and in
final tumor weights were determined by Wilcoxon rank test (10,11).
Prevention studies with EGFR
inhibitors
In the prevention studies, treatment of the rats
with gefitinib or lapatinib was initiated 5 days after MNU
administration (or at 55 days of age). The number of rats/group was
15. We had previously determined effective daily doses for
gefitinib (11) and lapatinib
(12). Gefitinib was administered
daily at a dose of 10 mg/kg BW/day, while the weekly dose was 7
times the daily dose (70 mg/kg BW). Lapatinib was administered
daily at a dose of 75 mg/kg BW/day, while the weekly dose was 7
times the daily dose (525 mg/kg BW). These doses were all less than
the daily human equivalent dose (HED) based on FDA scaling; which
would be roughly 12 and 120 mg/kg BW/day for gefitinib and
lapatinib, respectively. Rats were examined twice a week for the
development of palpable mammary tumors. At the end of the study,
tumors were weighed and submitted for histological evaluation.
Therapeutic study
Rats received MNU at 50 days of age and were
observed for the appearance of mammary cancers. When an animal
developed a tumor of ~100–200 mm2, the rat received
lapatinib (75 mg/kg BW/day or 525 mg/kg BW, once/week) or gefitinib
(10 mg/kg BW/day or 70 mg/kg BW, once/week) for 6 weeks. Tumor size
was determined with calipers before initiation of treatment and
twice each week during the course of treatment. The largest
diameter of the cancer was measured and this value was multiplied
by the perpendicular diameter (size expressed in mm2).
The gefitinib and lapatinib treated rats as well as the control
group had an n of 10 (10,11,22).
MMTV-Neu/P53KO (ER−) mouse
mammary cancer model
MMTV-Neu+/−/p53 KO+/− mice
were generated and maintained as previously described (23). MMTV-Neu transgenic mice [strain
FVB/N-Tg (MMTV-Neu) 202 Mul/J] were purchased from the Jackson
Laboratory (Bar Harbor, ME, USA). A p53 deficient line (p53 NS-T)
was purchased on a C57BL background (Taconic) and back-crossed at
least 5 times onto a FBV/N background. For generation of
MMTV-Neu+/−/p53 KO+/− females,
p53−/− males were crossed with MMTV-Neu+/+
females. MMTV-Neu females were generated by crossing
MMTV-Neu+/+ males with FVB/NJ females. The animal rooms
were lighted 12 h/day in a facility specially designed for
administering chemical carcinogens to animals. Mice were given diet
(Teklad 4% mash) and water ad libitum. Mice were treated
with gefitinib by gavage either daily (5 times/week) or weekly
beginning at 6 weeks of age. Mice were examined weekly for the
development of palpable mammary tumors beginning at 4 months of
age. Mice were sacrificed when a large palpable mammary tumor
developed or at termination when mice were 11 months of age.
Prevention studies of MK2206 alone or
with vorozole in the MNU-induced rat model
In the first study, rats were treated with MK2206
administered daily (33,100 or 300 mg/kg BW/day) or weekly (700
mg/kg BW) initiated 5 days after MNU administration (or at 55 days
of age). Based on these initial studies showing the efficacy of
weekly dosing with MK2206, weekly dosing of this agent together
with a low dose of the aromatase inhibitor vorozole (0.12 mg/kg
BW/day) was evaluated. We had previously shown that vorozole at
this dose was highly effective in this model (17).
Biomarker levels in treated
MNU-induced tumors
Cancers were allowed to develop in MNU-treated rats.
When a palpable tumor developed in an animal, it was treated with
gefitinib (70 mg/kg BW). All tumor-bearing rats were administered
gefitinib or vehicle on day 0. Seven days later, one set of rats
was sacrificed. The second group of rats was administered a second
dose of gefitinib on day 6 and sacrificed 24 h later. At the time
of sacrifice, mammary cancers were excised and fixed for
histological classification and immunohistochemistry (IHC).
IHC detection of phosphorylated EGFR
or Ki67
Paraffin-embedded tissue samples were heated, and
paraffin was removed with xylene. Rehydration of the samples was
performed using ethanol, and antigen retrieval was performed using
10 mM sodium citrate buffer (pH 6.0). Endogenous peroxidase was
quenched using 3% H2O2/methanol. Specimens
were blocked using 50% goat serum/1X PBS for 1 h. Ki-67 (ab-16667;
Abcam, Cambridge, UK) was diluted 1:100 in 50% goat serum/1X PBS.
The antibody to detect p-EGFR (Tyr.1173; 4407; Cell Signaling
Technology, Beverly, MA, USA) was diluted 1:100 in 50% goat
serum/1X PBS. The primary antibody was incubated overnight at 4°C,
and washed with PBS for 5 min. The biotinylated anti-rabbit
secondary antibody (Ba-1000; Vector Laboratories, Inc., Burlingame,
CA, USA) was prepared at a 1:150 dilution. Samples were incubated
with the secondary antibody for 60 min. The ABC complex/HRP
solution (Pk-6100; Vector Laboratories) was applied and incubated
for 30 min at room temperature. Slides were then washed with 1X PBS
for 3 min twice. Slides were incubated until staining was
sufficient and then rinsed in deionized water. Counterstaining was
performed by placing the slides in hematoxylin for 1 min.
Dehydration was performed by dipping slides in ethanol followed by
xylene for 7 min each. The images of the slides were acquired using
a Leica Microscope DMIRB (type 090–132.701) and Image Pro 6.3, and
then analyzed using Image Pro Premier 9.0 software (Media
Cybernetics, Inc., Bethesda, MD, USA).
Results
Preventive efficacy of weekly dosing
with gefitinib or lapatinib in the MNU model
Our previous studies showed that gefitinib at a dose
of 10 mg/kg BW/day and lapatinib at a dose of 75 mg/kg BW/day were
each highly effective in preventing ER+ mammary cancers
(11,12). We, therefore, compared the efficacy
of daily dosing with weekly dosing at 7 times the daily dose. Daily
lapatinib (75 mg/kg BW/day) was highly effective, reducing tumor
multiplicity by 85%. A weekly dose of 525 mg/kg BW reduced tumor
multiplicity ~75%. Results with both doses were significantly
different from the MNU controls (P<0.02), and the outcomes of
the two treatments did not differ from each other. Similarly,
gefitinib was tested either daily (10 mg/kg BW/day) or weekly (70
mg/kg BW). The daily dose inhibited tumor multiplicity by 80%,
whereas the weekly dose was 70% effective (Fig. 1). Measurements of final tumor
weights (Table I) showed that
either daily or weekly dosing with gefitinib or lapatinib reduced
the average tumor weight by 90% when compared to the tumors from
the control rats.
 | Table I.Effect of daily or weekly treatment
with gefitinib or lapatinib on development of MNU-induced mammary
tumors in rats. |
Table I.
Effect of daily or weekly treatment
with gefitinib or lapatinib on development of MNU-induced mammary
tumors in rats.
|
| Mammary
cancers |
|---|
|
|
|
|---|
| Treatment (no. of
rats/group=15) | Incidence (%) | Multiplicity | Tumor weight
(g) |
|---|
| Vehicle | 94 | 4.9 | 8.49 |
| Gefitinib (daily,
10 mg/kg BW/day) | 27b | 0.27b | 0.81b |
| Gefitinib (weekly,
70 mg/kg BW) | 56a | 1.0b | 1.07b |
| Lapatinib (daily,
75 mg/kg BW/day) | 40b | 0.4b | 0.26b |
| Lapatinib (weekly,
525 mg/kg BW) | 66 | 1.3b | 1.2b |
Therapeutic efficacy of weekly dosing
with gefitinib or lapatinib in the MNU model
The MNU mammary cancer model was also used to
examine the therapeutic efficacy of lapatinib or gefitinib by
allowing small palpable tumors to develop before initiating
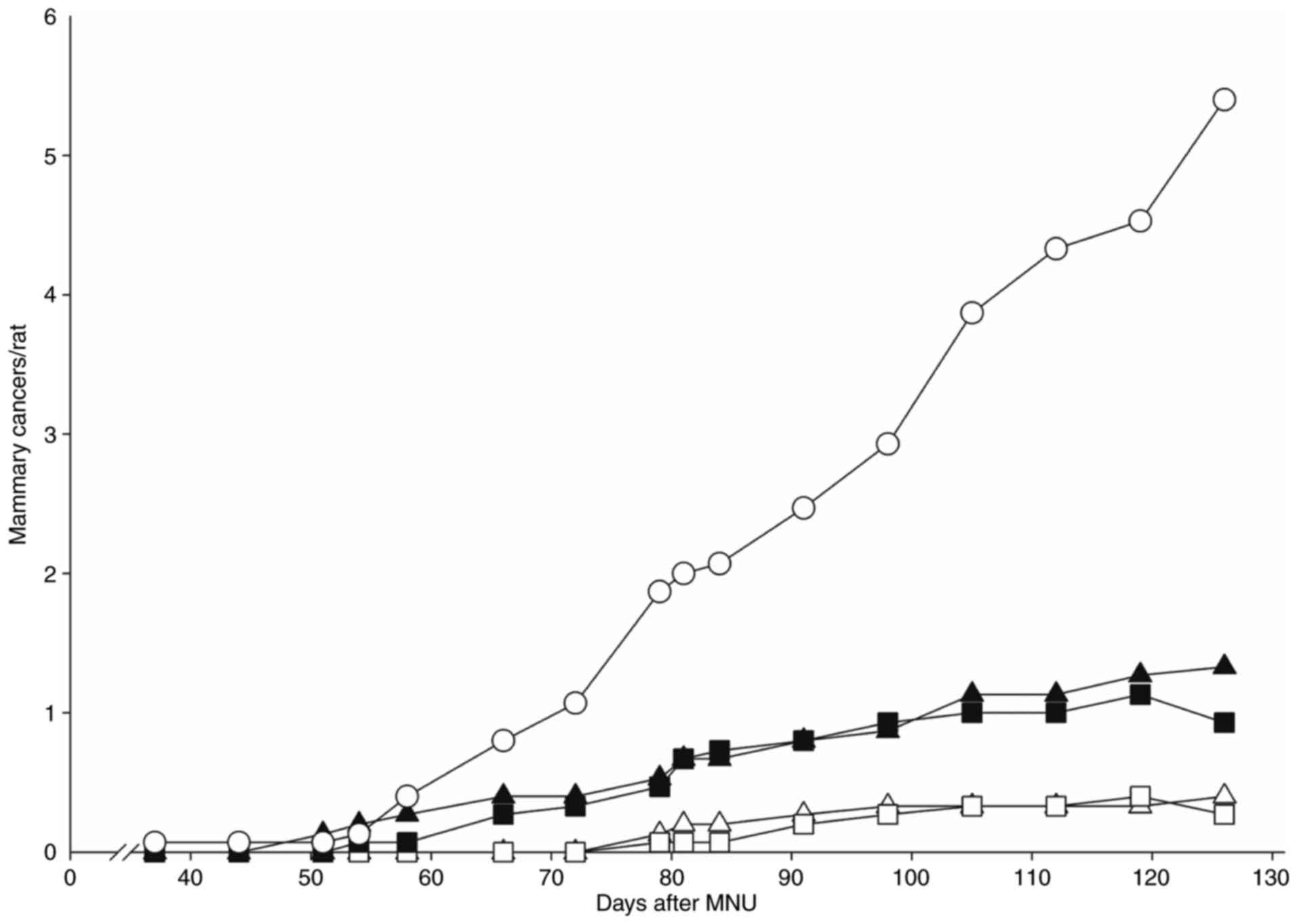
treatment (Fig. 2). Tumor-bearing
rats (10/group) were treated with lapatinib (75 mg/kg BW/day) or
gefitinib (10 mg/kg BW/day) on a daily basis, or with lapatinib
(525 mg/kg BW) or gefitinib (70 mg/kg BW) on a weekly basis, or
received the vehicle. Cancers in the vehicle-treated group
increased in size by 250% over 6 weeks. Daily or weekly treatments
with gefitinib or lapatinib reduced tumor size on average by
35–50%. Daily or weekly dosing of either inhibitor resulted in at
least half of the treated mammary tumors decreasing by 60% in size.
We considered a 60% decrease as a clear regression; implying that
either daily or weekly dosing was highly effective.
Preventive efficacy of weekly dosing
with gefitinib in the MMTV/Neu transgenic model
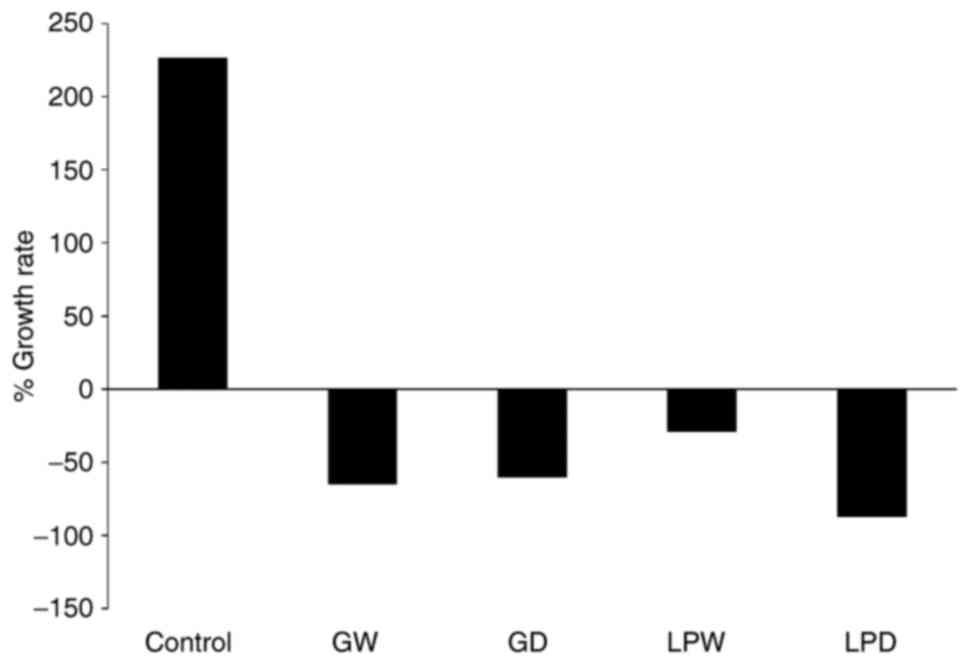
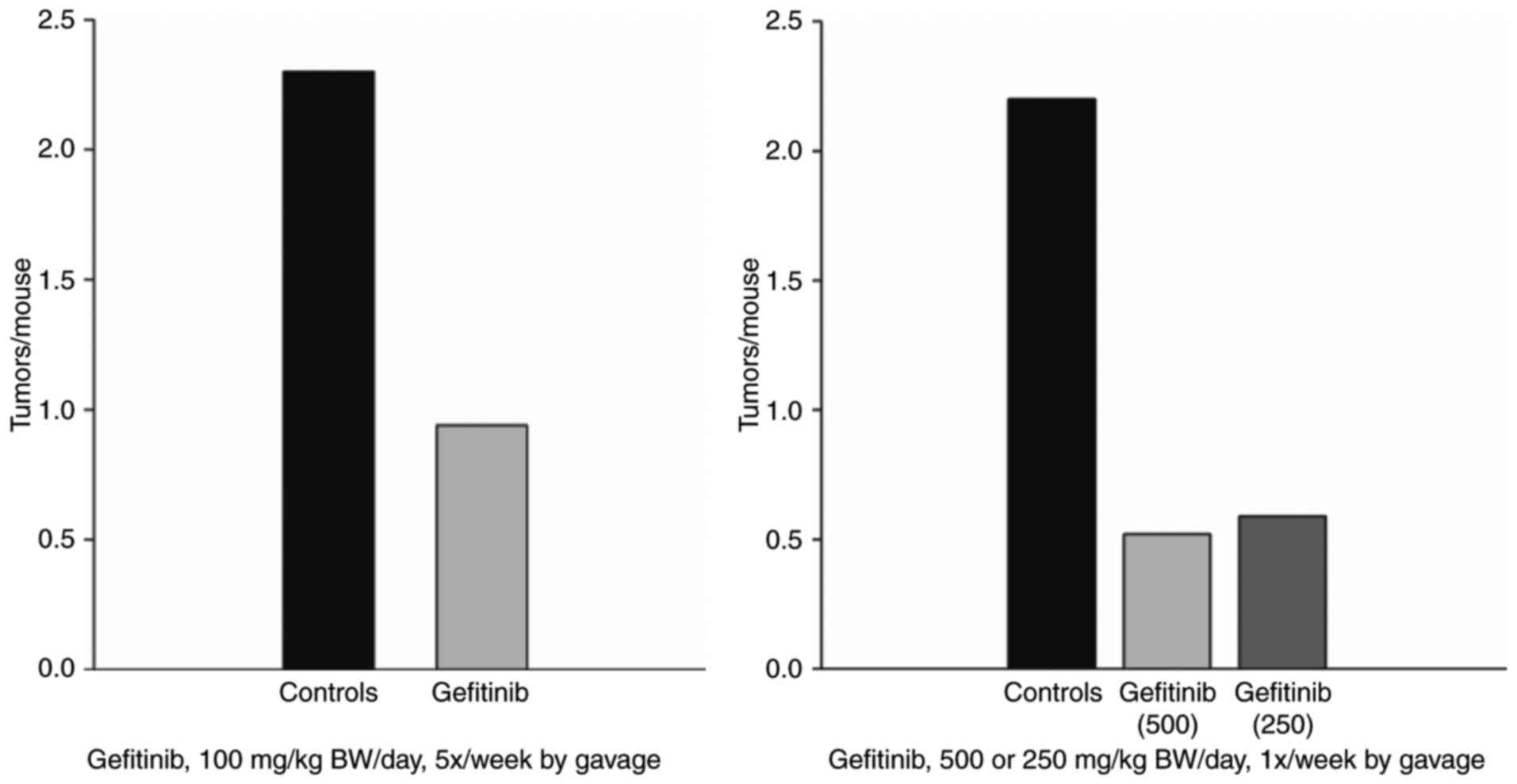
The efficacy of gefitinib in the MMTV-Neu model was
evaluated. Gefitinib was either administered at 100 mg/kg BW/day, 5
times/week or administered weekly at 500 or 250 mg/kg BW beginning
when the mice were 35 days of age. The studies examining daily and
weekly treatments were performed at different times. Daily dosing
with gefitinib yielded a 65% decrease in tumor multiplicity, and
the weekly dosing at 500 or 250 mg/kg BW both reduced tumor
multiplicity 75–80% (Fig. 3). There
is probably no significant difference from the daily dosing;
however, since the studies were performed at different times a
direct comparison could not be made.
Preventive efficacy of weekly dosing
with the AKT-inhibitor MK2206 in the MNU rat model
In the initial studies, the preventive effects of
MK2206 alone were determined in the MNU model. Daily doses of 30,
100 and 300 mg/kg BW/day or a weekly dose of 700 mg/kg BW were
administered. The daily dose of 300 mg/kg BW/day was toxic, while
the two lower daily doses were ineffective in preventing mammary
cancers. In contrast, the single weekly dose of 700 mg/kg BW was
moderately effective; substantially increasing tumor latency
[log-rank P<0.05 (Fig. 4A)] and
decreasing final tumor weights (Table
IIA). This weekly dosing did not cause any observable toxicity.
In the second study, we examined the effects of weekly dosing of
MK2206, daily dosing with a low dose of the aromatase inhibitor
vorozole, or the combination of these agents (Fig. 4B). Weekly MK2206 or vorozole reduced
tumor multiplicity 40–50%, and the combination was effective in
reducing multiplicity by 70% (P<.05) and reducing tumor weights
(Table IIB).
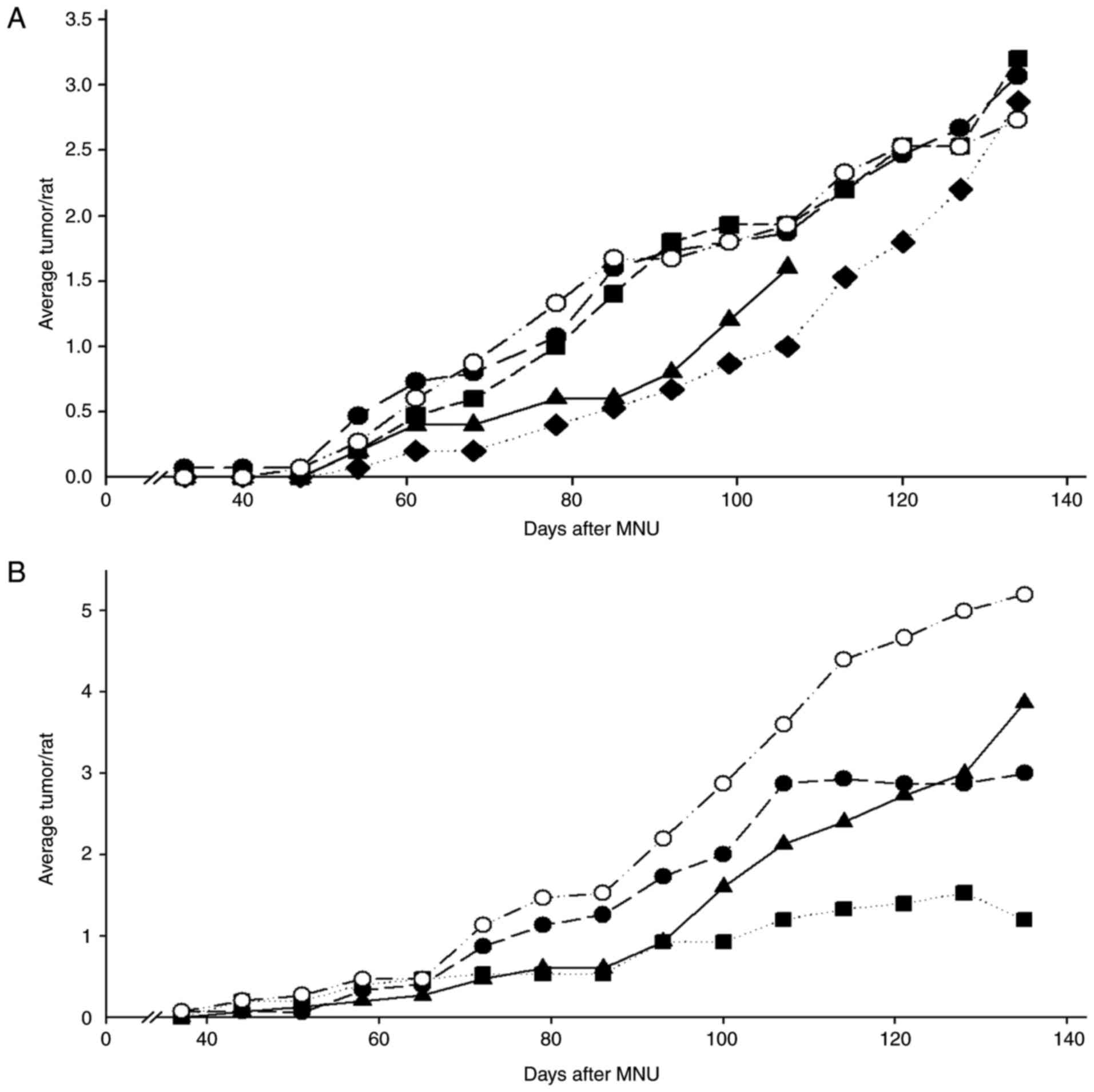 | Figure 4.(A and B) Effects of daily or weekly
dosing with MK2206 and the effects of weekly dosing with MK2206 and
vorozole on the development of ER+ tumors. Rats
(n=15/group) were treated with MNU at 50 days of age and, beginning
5 days later, treated with the indicated agents. (A) Rats were
treated with MK2206 (30, 100 or 300 mg/kg BW/day or 700 mg/kg BW, 1
time/week). Daily doses of 33 or 100 mg/kg BW/day were ineffective,
while the 300 mg/kg BW/day was toxic. The dose of 700 mg/kg BW, 1
time/week reduced the development of cancers (log-rank, P<0.05).
○ MK2206 (30 mg/kg BW/day); ● MK2206 (100 mg/kg BW/day); ▲ MK2206
(300 mg/kg BW/day); ♦ MK2206 (700 mg/kg BW/week); and ■ controls.
Tumor weights in this group were decreased ~70%; Wilcoxon rank,
P<0.05 (Table IIA). (B) Rats
were treated with MK2206 (700 mg/kg BW, 1 time/week) and/or a
suboptimal dose of the aromatase inhibitor vorozole (0.12 mg/kg
BW/day). Each agent alone reduced tumor multiplicity 30–40%, while
the combination decreased the development of tumors ~70% (Wilcoxon
rank, P<0.05). ▲ MK2206 (700 mg/kg BW/week); ● vorozole (0.12
mg/kg BW/day); ■ MK2206 + vorozole; ○ controls. Tumor weights in
this group were also decreased; Wilcoxon rank, *P<0.05 (Table IIB). |
 | Table II.Effect of AKT inhibitor MK2206 and/or
vorozole on the development of MNU-induced mammary cancers in
rats. |
Table II.
Effect of AKT inhibitor MK2206 and/or
vorozole on the development of MNU-induced mammary cancers in
rats.
| A, Effect of daily
or weekly treatment with the AKT inhibitor MK2206 |
|---|
|
|---|
|
| Mammary
cancers |
|---|
|
|
|
|---|
| Treatment (no. of
rats/group=15) | Incidence (%) | Multiplicity | Weight (g) |
|---|
| Vehicle | 94 | 2.7 | 6.6 |
| MK2206 (daily, 33
mg/kg/day) | 87 | 3.0 | 6.6 |
| MK2206 (daily, 100
mg/kg/day) | 87 | 3.2 | 3.25 |
| MK2206 (weekly, 700
mg/kg) | 80 | 2.8 | 1.57a |
|
| B, Effect of
treatment with the AKT inhibitor MK2206 and/or daily treatment with
vorozole |
|
| Vehicle | 100 | 4.5 | 9.1 |
| Vorozole (daily,
0.12 mg/kg/day) | 87 | 3.0 | 6.8 |
| MK2206 (weekly, 700
mg/kg) | 87 | 3.2 | 5.9 |
| MK2206 (weekly, 700
mg/kg) + vorozole (daily, 0.12 mg/kg day) | 60 | 1.7a | 3.6a |
Modulation of biomarkers in
tumor-bearing rats treated short term with gefitinib
Palpable tumors were treated with gefitinib (70
mg/kg BW, 1X/week) or vehicle on day 0. Mammary cancers were
collected from animals administered one weekly dose of gefitinib
and sacrificed on day 7, or from rats administered a second dose of
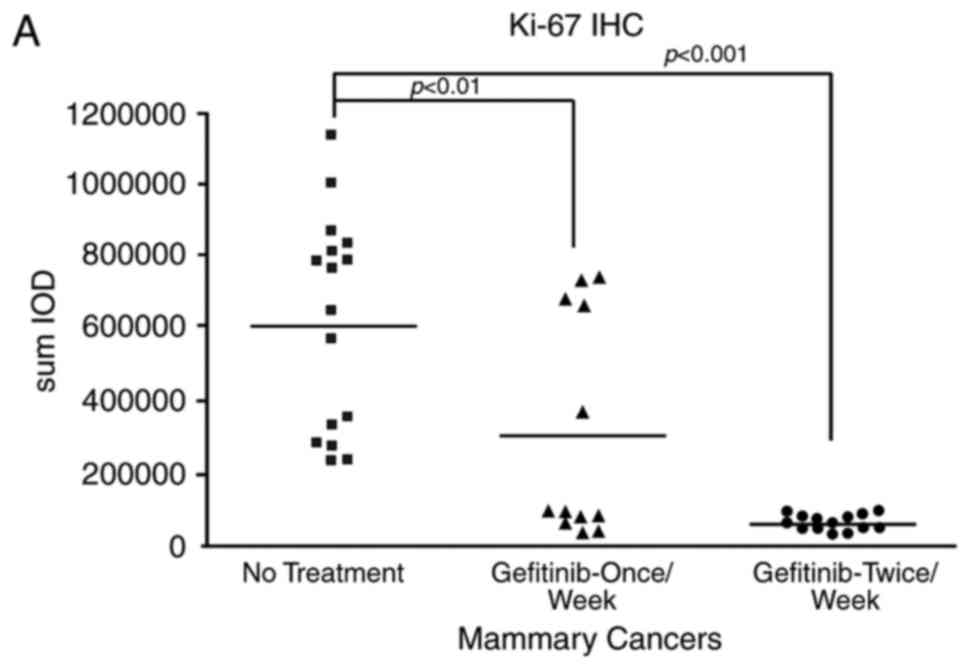
gefitinib on day 6 and sacrificed 24 h later. We initially
determined Ki67 levels in the cancers and found that in rats
sacrificed 1 day or 7 days following a weekly dose of gefitinib
significantly decreased levels were noted relative to the
vehicle-treated rats. The results were more striking at 24 h after
a weekly dose (Fig. 5A).
The effects of gefitinib treatment on endpoints more
directly related to its mechanism of action were also examined;
specifically, phosphorylation of EGFR. Levels of phosphorylated
EGFR were profoundly reduced in mammary cancers obtained within 1
day of the last weekly dosing (Fig.
5B), but were not significantly reduced 6 days after a weekly
dosing.
Discussion
The EGFR pathway is integral to various pathways of
cell signaling and cell replication, and is overexpressed in
various tumors including lung, head and neck, urinary bladder and
triple-negative breast cance (1,2). The
primary focus of inhibiting this pathway has been the development
of small-molecule inhibitors (gefitinib, erlotinib) or monoclonal
antibodies (e.g., cetuximab, trastuzumab). EGFR1 inhibitors
(gefitinib and erlotinib) have proven to be highly effective
against a subset of lung adenocarcinomas that have mutations in the
EGFR1 gene (4). In early
ER+ breast cancer, EGFR1 inhibitors have proven
effective when administered together with aromatase inhibitors in
ER+ breast cancers (9).
Notably, there are at least two studies implying the efficacy of
EGFR inhibitors in earlier stages of ER+ breast cancer.
One was a true neoadjuvant study in which Iressa®
administered for 12–16 weeks (7)
induced regression in 40% of tumors. The second study (8) showed significant decreases in
proliferation in tumors treated short-term with Iressa. Inhibition
of Ki67 has proven to be a highly predictive endpoint in the
therapeutic setting of ER+ breast cancer (24). The efficacy of EGFR inhibitors in
ER+ breast cancer is surprising since ER+
tumors generally have low expression of EGFR1. EGFR2 (Neu)
inhibitors have been shown to be a striking target for
ER+ or ER− breast cancers with amplification
and overexpression of the Neu gene (3).
Previously we found that MNU-induced ER+
rat mammary cancers are highly sensitive to the EGFR1 inhibitors
erlotinib and gefitinib (10,11),
and the EGFR 2/1 inhibitor lapatinib (12). In our earlier prevention studies,
these EGFR inhibitors caused a dose-dependent decrease in tumor
multiplicity and final tumor weights at dose levels below their
human equivalents. Furthermore, these agents proved to be
therapeutic in this model. All 3 agents exhibit toxicity in humans
causing both acneiform skin rashes and diarrhea. However, these
toxicities are not seen in either mice or rats at the effective
doses. The primary impetus for examining the potential of weekly
dosing with gefitinib or erlotinib is that weekly dosing with these
agents at doses up to 7 times the daily dose has been shown to
significantly decrease the incidence of toxicities, i.e., rash and
diarrhea (25,26). Thus, whether weekly dosing achieves
significant efficacy it may allow for the use of these targeted
agents in a prevention or adjuvant setting. One specific example is
the recent finding that the combination of erlotinib plus sulindac
was highly effective in individuals with familial adenomatous
polyposis (27). A weekly dosing of
the EGFR inhibitor which may be expected to reduce toxicity may
make such a protocol more tolerable.
In the present experiments, we found in the MNU
model that lapatinib and gefitinib are highly effective when
administered weekly, as contrasted with daily dosing. This result
may not be as surprising regarding gefitinib since the
mechanistically similar EGFR1 inhibitor erlotinib was highly
effective (10). The results with
lapatinib which preferentially inhibits EGFR2 (Neu) and has a
relatively short half-life in rodents (T1/2 ≤4 h) is
more surprising. Thus, lapatinib may be expected to achieve optimal
serum levels at ≤48 h; even with a weekly dose 7 times higher than
the daily dose.
Weekly dosing of this class of agents was highly
effective in the MMTV-Neu model of ER− mammary cancer in
transgenic mice. Notably, Brown et al previously
demonstrated that the EGFR inhibitor gefitinib was profoundly
effective in this model (19). The
present data confirmed these results, and furthermore showed that
weekly dosing at either 5 times or even 2.5 times the daily dose
was as effective as daily dosing.
The effects of weekly dosing with gefitinib on
expression of Ki67 in MNU-induced mammary cancers were examined. We
previously showed that daily dosing with gefitinib decreased the
levels of Ki67 in mammary tumors (11). When the effects of weekly dosing
with gefitinib were determined, significant decreases in Ki67 at
either 1 day or 7 days following a weekly dose were observed. Our
specific interest in Ki67 labeling in mammary cancer is based on
the fact that it appears to be indicative of agent efficacy in a
preoperative setting in humans (24). When EGFR phosphorylation was
examined 1 day after a weekly dose of gefitinib, a profound
inhibition of EGFR1 phosphorylation was observed as expected. In
contrast, most of this inhibitory activity was not observed 7 days
following a single weekly dose. This would make sense if serum and
cancer tissue levels of the agents were lower after this time
period.
Given the efficacy of weekly dosing with the EGFR
inhibitors, it was determined whether such an effect may be
observed with other kinase inhibitors. The efficacy of MK2206, an
inhibitor of the serine-threonine kinase AKT, was evaluated with or
without the aromatase inhibitor vorozole in the MNU-induced model.
Initially, the preventive agent showed that daily dosing of 100
mg/kg BW/day or less was ineffective, while a weekly dose of 700
mg/kg BW increased tumor latency and substantially decreased final
tumor weights. In the second prevention study, the combination of
MK2206 (700 mg/kg BW/week) was evaluated together with a low (0.12
mg/kg BW/day) dose of the aromatase inhibitor vorozole. We
performed numerous studies with vorozole in this model, and
demonstrated it to have dose-dependent preventive (17) and therapeutic effects (22). The individual dosing with suboptimal
vorozole and/or weekly MK2206 reduced tumor multiplicity 40–50%,
while the combination yielded a 70% decrease in tumor multiplicity
and final tumor weights. This result has two important aspects.
First, it confirms the general finding that this agent or class of
agents has limited activity on its own, but appears more effective
in combination with agents that inhibit estrogen activity.
Additionally, it showed that weekly dosing was, in fact, more
effective than daily dosing using the same total dose. Perhaps most
importantly, this models clinical trials in tamoxifen-resistant
ER+ tumors combining an aromatase inhibitor and MK-2206
(13).
The other major question raised by the
chemopreventive efficacy of weekly dosing is related to the
responsible mechanism. One possibility is that the high dose level
may hit additional targets. However, these agents are relatively
target specific and, even if the higher dose hits additional
targets for a short time, one is likely to achieve the higher serum
levels only transiently. The second possibility may be that the
pharmacokinetics of weekly dosing is markedly different. Certainly
our prior study with erlotinib (10) showed a similar half-life for the
drug; employing either daily or weekly doses (7 times the daily
dose). Furthermore, our present data examining EGFR phosphorylation
following gefitinib treatment imply that the primary pharmacologic
effect (inhibition of EGFR phosphorylation) is lost over a weekly
period. The third possibility is that there are feedback mechanisms
that inhibit activity following continual exposure to an inhibitor.
Thus, the increased efficacy of weekly dosing with the AKT
inhibitor has a strong mechanistic rationale. Daily dosing with an
AKT inhibitor produces a negative feedback loop that results in
increased expression and phosphorylation of multiple proteins,
including HER3, IGF1R and MEK (28). This negative feedback is likely to
suppress the activity of AKT inhibitors. The weekly dosing with the
AKT inhibitor (resulting in intermittent inhibition of AKT
phosphorylation) is likely to partially ameliorate this feedback
loop and, thereby, may actually increase efficacy. These negative
feedback loops may not be unique to AKT inhibitors. Since continual
exposure to EGFR inhibitors in cell culture may elicit a heightened
downstream activation of ERK phosphorylation, weekly dosing may
similarly help to overcome these feedback loops (29).
There are a number of important questions raised by
these results. i) Are the effects of weekly dosing with TKIs
reproducible? The results with multiple EGFR inhibitors and the
allosteric AKT inhibitor argues that it is reproducible.
Furthermore, the recent preclinical data of Miller et al
(30) with the PI3K inhibitor
pictilisib argues that this may be a generalized property.
Additionally, we recently found that gefitinib administered weekly
was in fact more effective than daily dosing in both a
carcinogen-induced model of lung adenocarcinomas as well as a human
tumor xenograft employing an EGFR-mutant lung adenocarcinoma
(31). ii) Although it is likely to
be influenced by PK characteristics of individual agents, will the
altered dosing regimen decrease toxicity? A reduction in toxicity
may encourage the use of these agents in an adjuvant and even allow
their use in a prevention setting. This question cannot be
addressed in animal models since the rash or diarrhea observed in
humans with these agents are not observed in rats or mice. However,
effective weekly dosing with erlotinib for brain metastases of
EGFR-mutant lung tumors is associated with a striking decrease in
the incidence and severity of rash (25). Furthermore, a relatively large dose
range study with erlotinib demonstrated the reduced toxicity of
weekly dosing (26). This toxicity
question can only be properly addressed clinically. However, the
high prevalence of these symptoms would suggest that the toxicity
aspects could be evaluated in relatively small clinical trials.
iii) Is this greatly altered dosing applicable to multiple organ
sites? Similarly, weekly dosing with gefitinib, erlotinib or
lapatinib was as effective as daily dosing with the same agents in
a rat urinary bladder cancer model (Lubet RA and Grubbs CJ,
unpublished data). Finally, our studies found that weekly dosing of
the PI3K inhibitor XL-147 or gefitinib also appeared more effective
than daily dosing in a chemically induced lung cancer model (Wang
Y, Lubet RA and You M, unpublished data).
Acknowledgements
The present study was supported by the NCI Contract
No. HHSN261200433001C.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Salamone DS, Brandt R, Ciardiello F and
Normanno N: Epidermal growth factor-related peptides and their
receptors in human malignancies. Crit Rev Oncol Hematol.
19:183–232. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arteaga CT: Overview of epidermal growth
factor receptor biology and its role as therapeutic target in human
neoplasia. Semin Oncol. 29 5 Suppl 14:S3–S9. 2002. View Article : Google Scholar
|
|
3
|
Moasser MM and Krop IE: The evolving
landscape of HER2 targeting in breast cancer. JAMA Oncol.
1:1154–1161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hida L, Ogawa S, Park JC, Park JY, Shimazu
J, Horio Y and Yoshida K: Gefitinib for the treatment of non small
cell lung cancer. Expert Rev Anticancer Ther. 9:17–35. 2006.
View Article : Google Scholar
|
|
5
|
Wong SF: Cetuximab: An epidermal factor
receptor monoclonal antibody for treatment of colorectal cancer.
Clin Ther. 27:684–694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan WL and Ng QS: The continuing role of
epidermal growth factor receptor tyrosine kinase inhibitors in
advanced squamous cell carcinoma of the lung. Transl Lung Cancer
Res. 5:106–109. 2016.PubMed/NCBI
|
|
7
|
Polychronis A, Sinnett HD, Hadjiminas D,
Singhal H, Masnsi JL, Shivapatham D, Shousha S, Jiang J, Peston D,
Barrett N, et al: Preoperative gefitinib versus gefitinib and
anastrozole in postmenopausal patients with oestrogen-receptor
positive and epidermal-growth-factor-receptor-positive primary
breast cancer: A double blind placebo controlled phase II
randomized trial. Lancet Oncol. 6:383–391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baselga J, Albanell J, Ruiz A, Lluch A,
Gascon P, Guillém V, González S, Sauleda S, Marimón I, Tabernero
JM, et al: Phase II and tumor pharmacodynamic study of gefitinib in
patients with advanced breast cancer. J Clin Oncol. 23:5323–5333.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Massarweh S, Thann YL, Huang J, Sexton K,
Weiss H, Tsimelzon A, Beyer A, Rimawi M, Cai WY, Hilsenbeck S, et
al: A phase II neoadjvant trial of anastrozole, fulvestrant, and
gefitinib in patients with newly diagnosed estrogen receptor
positive breast cancer. Breast Cancer Res Treat. 129:819–827. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lubet RA, Szabo E, Iwata KK, Gill SC,
Tucker C, Bode A, Steele VE, Juliana MM, Nicastro HL and Grubbs CJ:
Effect of intermittent dosing regimens of erlotinib on
methylnitrosourea-induced mammary carcinogenesis. Cancer Prev Res.
6:448–454. 2013. View Article : Google Scholar
|
|
11
|
Lubet RA, Szabo E, Christov K, Bode AM,
Ericson ME, Steele VE, Juliana MM and Grubbs CJ: Effects of
gefitinib (Iressa) on mammary cancers: Preventive studies with
varied dosages, combinations with vorozole or targretin, and
biomarker changes. Mol Cancer Ther. 7:972–979. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Cho YY, Langfald A, Carper A, Lubet
RA, Grubbs CJ, Ericson ME and Bode AM: Lapatinib, a
preventive/therapeutic agent against mammary cancer, suppresses
RTK-mediated signaling through multiple signaling pathways. Cancer
Prev Res. 4:1190–1197. 2011. View Article : Google Scholar
|
|
13
|
Ma CX, Sanchez C, Gao F, Crowder R,
Naughton M, Pluard T, Creekmore A, Guo Z, Hoog J, Lockhart AC, et
al: A Phase I Study of the AKT inhibitor MK-2206 in combination
with hormonal therapy in postmenopausal women with estrogen
receptor-positive metastatic breast cancer. Clin Cancer Res.
22:2650–2658. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grubbs CJ, Peckham JC and McDonnough KD:
Effect of ovarian hormones on the induction of
1-methyl-1nitrosourea induced mammary cancer. Carcinogenesis.
4:495–497. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chan MM, Lu X, Merchant FM, Inglehart JD
and Miron PL: Gene expression profiling of NMU-induced rat mammary
tumors: Cross species comparison with human breast cancer.
Carcinogenesis. 26:1343–1353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gottardis MM and Jordan VC: Antitumor
actions of keoxifene and tamoxifen in the
N-nitrosomethylurea-induced rat mammary carcinoma model. Cancer
Res. 47:4020–4024. 1987.PubMed/NCBI
|
|
17
|
Lubet RA, Steele VE, Casebolt TL, Eto I,
Kelloff GJ and Grubbs CJ: Chemopreventive effects of the aromatase
inhibitors vorozole (R-83842) and 4-hydroxyandrostenedione in the
methylnitrosourea (MNU)-induced mammary tumor model in
Sprague-Dawley rats. Carcinogenesis. 15:2775–2780. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muller WJ, Sinn E, Pattengale PK, Wallace
R and Leder P: Single-step induction of mammary adenocarcinoma in
transgenic mice bearing the activiated c-neu oncogene. Cell.
54:105–115. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu C, Speers C, Zhang Y, Xu X, Hill J,
Steinbis E, Celestino J, Shen Q, Kim H, Hilsenbeck S, et al: Effect
of epidermal growth factor receptor inhibitor on development of
estrogen receptor-negative mammary tumors. J Natl Cancer Inst.
95:1825–1833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zelazny E, Li B, Anagnostopoulos AM,
Coleman A and Perkins AS: Cooperating oncogenic events in murine
mammary tumorigenesis: Assessment of ErbB2, mutant p53, and mouse
mammary tumor virus. Exp Mol Pathol. 70:183–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guttman-Yassky E, Mita A, De Jonge M,
Matthews L, McCarthy S, Iwata KK, Verweij J, Rowinsky EK and
Krueger JG: Characterization of the cutaneous pathology in (NSCLC)
patients treated with (EGFR) tyrosine kinase inhibitor erlotinib.
Eur J Cancer. 46:2010–2019. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Christov K, Shilkaitis A, Green A, Mehta
RG, Grubbs C, Kelloff G and Lubet R: Cellular responses of mammary
carcinomas to aromatase inhibitors: Effects of vorozole. Breast
Cancer Res Treat. 60:117–128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lubet RA, Boring D, Steele VE, Ruppert JM,
Juliana MM and Grubbs CJ: Lack of efficacy of the statins
atorvastatin and lovastatin in rodent mammary carcinogenesis.
Cancer Prev Res. 2:161–167. 2009. View Article : Google Scholar
|
|
24
|
Dowsett M, Smith IE, Ebbs SR, Dixon JM,
Skene A, Griffith C, Boeddinghaus I, Salter J, Detre S, Hills M, et
al: Short-term changes in Ki-67 during neoadjuvant treatment of
primary breast cancer with anastrozole or tamoxifen alone or
combined correlate with recurrence-free survival. Clin Cancer Res.
11:951S–958S. 2005.PubMed/NCBI
|
|
25
|
Grommes C, Oxnard GR, Kris MG, Miller VA,
Pao W, Holodny AI, Clarke JL and Lassman AB: ‘Pulsatile’ high-dose
weekly erlotinib for CNS metastases from EGFR mutant non-small cell
lung cancer. Neuro Oncol. 13:1364–1369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Milton DT, Azzoli CG, Heelan RT,
Venkatraman EM, Gomez JE, Kris MG, Krug LM, Pao W, Rizvi NA, Dunne
M and Miller VA: A phase I/II study of weekly high-dose erlotinib
in previously treated patients with nonsmall cell lung cancer.
Cancer. 107:1034–1041. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Samadder NJ, Neklason DW, Boucher KM,
Byrne KR, Kanth P, Samowitz W, Jones D, Tavtigian SV, Done MW,
Berry T, et al: Effects of Sulindac and erlotinib vs placebo on
duodenal neoplasia in familial adenomatous polyposis: A randomized
clinical trial. JAMA. 315:1266–1275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chandarlapaty S, Sawai A, Scaltriti M,
Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK,
Baselga J and Rosen N: AKT inhibition relieves feedback suppression
of receptor tyrosine kinase expression and activity. Cancer Cell.
19:58–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ercan D, Xu C, Yanaita M, Monast CS,
Pratilas CA, Montero J, Butaney M, Shimamura T, Sholl L, Ivanova
EV, et al: Reactivation of ERK signaling causes resistance to EGFR
kinase inhibitors. Cancer Discov. 2:934–947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang W, Hosford SR, Dillon LM, Shee K, Liu
SC, Bean JR, Salphati L, Pang J, Zhang X, Nannini MA, et al:
Strategically timing inhibition of phosphatidylinositol 3-kinase to
maximize therapeutic index in estrogen receptor alpha-positive
PIK3CA-mutant breast cancer. Clin Cancer Res. 22:2250–2260. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Q, Li R, Chen X, Lee SB, Pan J,
Xiong D, Hu J, Miller MS, Szabo E, Lubet RA, et al: Effects of
weekly or daily dosing regimen of Gefitinib in mouse models of lung
cancer. Oncotarget. 8:72447–72456. 2017.PubMed/NCBI
|