Introduction
MicroRNAs (miRNAs) are evolutionarily conserved
small non-coding RNAs of 25 nucleotides in length that function as
negative regulators of gene expression by either inhibiting
translation or inducing degradation of specific messenger RNA
targets (1). miRNA expression could
be disturbed by carcinogenic agents, chemotherapy and diverse
external stimuli, which could impact genetic and epigenetic
programs contributing to the heterogeneous biological behavior of
tumors. For instance, long-term arsenic exposure of keratinocytes
resulted in the upregulation of miR-21, miR-200a and miR-141, which
were found to be involved in the development of melanoma, thus they
could be promising early biomarkers of skin cancer (2). Importantly, changes in the abundance
of miRNAs in tumors may correlates with clinical and pathological
features of patients. For example, the downregulation of
tumor-suppressor miR-198 and upregulation of MSLN, OCT-2, PBX-1 and
VCP in pancreatic tumors were associated with the poor survival of
patients (3). Consequently, miRNAs
represent novel prognostic biomarkers and promising translational
targets in cancer therapy. Particularly, the let-7 family of miRNAs
are frequently downregulated in diverse types of cancers. let-7 is
a major regulator of differentiation, pluripotency and apoptosis in
eukaryotic cells (4–6). In cancer cells, let-7 targets multiple
oncogenes involved in the deregulation of the cell cycle, cell
division, proliferation, angiogenesis and apoptosis (7). Markedly, experimental restoration of
normal expression levels of let-7 in cancer cells prevents
tumorigenesis indicating that it acts as a bona fide tumor
suppressor. These findings suggest that let-7 members can be used
as molecular tools and markers in cancer therapeutics.
Epithelial ovarian cancer (EOC) is a highly
metastatic disease with the highest mortality rate of all
gynecologic cancers (8). More than
90% ovarian cancers are classified as epithelial whereas the
remaining most frequent histotypes are serous, endometrioid,
clear-cell and mucinous. Until recently these malignancies were
considered as derived from ovarian surface epithelium. The
different ovarian cancer histotypes are characterized by altered
genomic and epigenetic patterns, which greatly impact oncogenic
signaling pathways, biological behavior and clinical outcome
(9). Conventional treatment of
ovarian cancer is based on surgery and chemotherapy. Platinum-based
agents including cisplatin and carboplatin represent the first-line
agents for patients with advanced ovarian cancer (10). Randomized controlled clinical trials
established that this therapeutic regimen yields 5-year survival
rate from 30 to 92%; and 40 to 60% complete responses depending on
the spread of disease at time of diagnosis (11). Although most patients with ovarian
cancer exhibit response to combination chemotherapy of platinum
salts, many patients develop resistance and relapse with a median
progression-free survival of only 18 months (12). However, although cisplatin
resistance mechanisms have been studied for decades, the genes and
factors involved in this adverse cellular event have not been fully
identified (13). The scenario is
worse as no molecular predictors of clinical response to therapy
are currently in use, although several cellular factors are
becoming increasingly studied (14).
Neoadjuvant chemotherapy has been recognized as a
reliable therapeutic strategy in patients with unresectable EOC.
Some advances in the study of cellular events leading to proper
response to neoadjuvant chemotherapy have been reported (15). However, the potential role of miRNAs
in neoadjuvant chemotherapy has not been fully explored in ovarian
cancer. In the present study, we investigated the changes in
expression of let-7 family members in order to evaluate whether
they have a prognostic role in EOC patients who received
neoadjuvant chemotherapy. In addition, we provide experimental
evidence concerning the role of let-7d-3p in apoptosis and
sensitization of ovarian cancer cells to chemotherapy.
Materials and methods
Cell lines
Human ovarian cancer cell line SKOV-3 was obtained
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and grown in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal bovine serum (FBS) and penicillin-streptomycin (50
U/ml; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Tissue collection
All molecular analyses were carried out on primary
biopsies. Tissues were formalin-fixed and embedded in paraffin.
Pathologists confirmed the existence of at least 80% tumor cells in
the clinical specimens.
Ethics statements
The Instituto Nacional de Cancerologia (Mexico)
provided the ovarian tumor and normal tissue collection. The
Instituto Nacional de Cancerologia (Mexico) ethics committee
approved the protocols concerning the use of human tissues. A
signed informed form consent was obtained from each participant or
a representative prior to release for research use.
RNA isolation from FFPE
Formalin-fixed paraffin-embedded (FFPE) tissues were
obtained from patients with ovarian cancer. Total RNA was isolated
using the RNeasy FFPE kit (Qiagen, Valencia, CA, USA) according to
the manufacture's protocol. Briefly, 5–10 FFPE sections of 10 µm
were incubated two times in xylene for 1 h at 63°C for
deparaffinization. Then, total RNA was extracted according to the
manufacturer's protocol. In addition, total RNA from SKOV-3 cells
was isolated using the TRIzol protocol (Ambion, Austin, TX, USA),
and concentration and purity were evaluated by spectrophotometry
(NanoDrop Technologies, Wilmington, DE, USA) followed by 1%
agarose-formaldehyde gel electrophoresis.
Reverse transcription and real-time
polymerase chain reaction
Quantitative real-time RT-PCR (qRT-PCR) analysis for
miRNA expression was performed using the TaqMan MicroRNA Assay kits
(Assay ID 001178; Thermo Fisher Scientific, Inc.). Total RNA (100
ng) was reverse transcribed using a looped-RT specific primer
targeting the let-7d-3p mature sequence CUAUACGACCUG CUGCCUUUCU,
dNTPs (100 mM; New England Biolabs, Ipswich, MA, USA), reverse
transcriptase MultiScribe (50 U/µl; Thermo Fisher Scientific,
Inc.), 10X buffer, RNase inhibitor (20 U/µl; Promega, Madison, WI,
USA) and 4.16 µl RNase-free water. Retrotranscription reaction
(1:15) was mixed with master mix TaqMan (Universal PCR Master Mix,
No AmpErase UNG, 2X; Thermo Fisher Scientific, Inc.), and the
corresponding specific TaqMan PCR probe. PCR reaction was performed
in a GeneAmp System 9700 (Applied Biosystems, Foster City, CA, USA)
as follows: 95°C for 10 min, and 40 cycles at 95°C for 15 sec and
60°C for 1 min. Tests were normalized using RNU44 as control.
let-7d-3p inhibition and scramble
transfection in SKOV-3 cells
Let-7d-3p (90 nM) inhibitor (MH10785; Thermo Fisher
Scientific, Inc.) and scramble (30 nM) sequence (AM17110, Thermo
Fisher Scientific, Inc.) were used as negative control (https://www.thermofisher.com/order/genome-database/details/mirna/MC10785).
Both, the inhibitor and scramble sequences were individually
transfected into SKOV-3 cells using siPORT amine transfection agent
(Ambion). Briefly, antagomiR let-7d-3p and scramble were added to
wells containing 1×107 SKOV-3 cells and incubated for 48
h. Then, total RNA was extracted using Trizol and efficacy of
antagomiR treatment in endogenous let-7d-3p downregulation was
evaluated by qRT-PCR using specific stem-looped RT oligonucleotide
and TaqMan probe (ID: 001178; Thermo Fisher Scientific, Inc.) as
implemented in the TaqMan MicroRNA Assays protocol.
Cell migration and invasion
assays
SKOV-3 cells (1×105) treated with
let-7d-3p antagomiR (90 nM), or scramble sequence (30 nM) were
seeded in triplicate in a 6-well plate and grown to 80% confluence.
Twenty-four hours post-transfection a vertical wound was traced in
the cell monolayer. After 12 and 24 h, cells were fixed with 4%
paraformaldehyde and the scratched area was quantified. For
migration assays, SKOV-3 cells (1×105) were transfected
with let-7d-3p antagomiR or scramble sequence, and then transferred
to 0.5 ml serum-free medium and placed in the upper Transwell
chambers (Corning Inc., Corning, NY, USA), whereas the lower
chamber was loaded with 0.8 ml medium containing 10% FBS. The total
number of cells that migrated into the lower chamber was manually
counted after 24 h incubation at 37°C. Experiments were performed
three times by triplicate and results are expressed as mean ± SD.
P<0.05 was considered as statistically significant.
Cell proliferation assays
For cell proliferation studies, the MTT reagent
[(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was
added to SKOV-3 cells (1×105) and incubated for 3.5 h at
37°C. Then, dissolution buffer (99% isopropanol, 0.3% HCl, 0.7%
NP-40) was added to cells and incubated for 15 min. Absorbance was
recorded at 24 and 48 h using a spectrophotometer (570–630 nm).
Data were analyzed using the BioStat software.
Fluorescence-activated cell sorting
assays (FACS)
SKOV-3 cells (2×105) were seeded by
triplicate in a 6-well plate and treated for 48 h as follows: i)
siPORT transfection agent (mock); ii) scramble sequence (negative
control, 30 nM); and iii) let-7d-3p antagomiR (90 nM). Then,
carboplatin (50 µM) was added to let-7d-3p inhibitor-transfected
cells or alone in non-transfected cells and incubated for 24 h.
Then, cells were harvested, washed twice with PBS 1X, resuspended
in 100 µl buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM
CaCl2), and processed following the manufacturer's
instructions (Annexin V-FLUOS staining kit; Roche Diagnostics,
Basel, Switzerland). Briefly, cells were stained with 2 µl Annexin
V-FITC and 2 µl propidium iodide (PI) mixed with 100 µl incubation
buffer for 15 min, washed with 500 µl binding buffer and
resuspended in 300 µl PBS 1X. Apoptosis events were analyzed on the
FACSCalibur flow cytometer (BDIS; Becton-Dickinson, Franklin Lakes,
NJ, USA). Annexin V and PI emissions were detected in the FL-1 and
FL-2 channels, respectively. For each sample, data from 20,000
cells were acquired in list mode on logarithmic scales. Data were
analyzed using the Summit V4.3 software and results were
represented as the total percentage of apoptotic cells as the sum
of both early and late phases of apoptosis (Annexin
V-FITC-positive). Assays were performed by triplicate and data was
expressed as mean ± SD. P<0.05 was considered as statistically
significant.
Prediction of gene targets and gene
ontology (GO) analysis
miRNA target genes were predicted using TargetScan
and PicTar software. Only gene targets predicted by the two
algorithms were included in further analysis. Cellular pathways and
processes potentially affected by let-7c-3p were predicted using
DAVID 6.7 software.
Statistical analysis
Experiments were performed three times by triplicate
and results are represented as mean ± SD. One-way analysis of
variance (ANOVA) followed by Tukey's test were used to compare the
differences between means. A P<0.05 was considered as
statistically significant.
Results
Clinical and pathological
characteristics of the EOC patients
Ovarian tumor samples were collected between January
2010 and September 2012 from 34 patients diagnosed with EOC who
underwent carboplatin/paclitaxel neoadjuvant treatment followed by
debulking surgery at Instituto Nacional de Cancerologia. Tumor
tissues were histologically analyzed by a pathologist to confirm at
least 80% of tumor cells and then processed for total RNA isolation
for downstream analysis. An overview of the major clinical and
pathological features of tumors and patients included in the
present study is provided in Table
I. The average age of the patients at surgery was 52.1 years
(range 35–74). Disease stage and tumor grade were classified
according to the International Federation of Gynecology and
Obstetrics (FIGO) and the World Health Organization (WHO) criteria,
respectively. The majority of patients were diagnosed with stage
III (44.1%) and IV (44.1%) disease, whereas 11.7% were at stage I.
Tumor grade 1, 2 and 3 were found in 4, 3, and 17 women,
respectively. The most common histology was serous papillary high
grade (67.6%), and the remaining was endometrioid (17.6%), mucinous
(9.0%), serous papillary low grade (3.0%), and clear cell (3.0%).
At the time of diagnosis 16 (47%) patients showed no metastasis
whereas 18 (53.0%) had distal metastasis. The median follow-up time
was 34 months, ranging from 0.6 to 71 months. After neoadjuvant
chemotherapy 17 patients (50%) were classified as complete
responders and 2 (6%) as stable, whereas 12 (35.2%) had a partial
response. Response was monitored by measuring the levels of cancer
antigen 125 (CA125 or mucin 16) and CT scan. CA125 is a
well-accepted protein marker found on the surface of ovarian cancer
cells and diverse types of cancer. Serial measurements of CA125
were routinely used to monitor tumor response and survival during
chemotherapy.
 | Table I.Clinical and pathological features of
ovarian cancer patients treated with neoadjuvant chemotherapy
(n=34). |
Table I.
Clinical and pathological features of
ovarian cancer patients treated with neoadjuvant chemotherapy
(n=34).
|
Characteristics | No. of patients
(%) |
|---|
| Histological
subtypes |
|
| Serous
papillary-high grade | 23 (67.6) |
| Serous
papillary-low grade | 1 (3.0) |
|
Endometrioid | 6 (17.6) |
|
Mucinous | 3 (9.0) |
| Clear
cell | 1 (3.0) |
| FIGO staging |
|
| I | 4 (11.7) |
|
III | 15 (44.1) |
| IV | 15 (44.1) |
| Tumor grade |
|
| 1 | 4 (11.7) |
| 2 | 3 (9.0) |
| 3 | 27 (79.4) |
| Metastasis
status |
|
| No
metastasis | 16 (47.0) |
|
Metastasis | 18 (53.0) |
| Response to
therapy |
|
|
Partial | 12 (35.2) |
|
Stable | 2 (6.0) |
|
Complete | 17 (50) |
|
Clinical progression | 1 (3.0) |
|
Unknown | 2 (6.0) |
| Recurrence |
|
|
Yes | 24 (70.5) |
| No | 6 (17.6) |
|
Unknown | 4 (11.7) |
| Initial CA125,
U/ml |
|
|
<35 | 3 (9.0) |
|
35–65 | 3 (9.0) |
|
>65 | 28 (82.3) |
| After treatment
CA125, U/ml |
|
|
<35 | 13 (38.2) |
|
35–65 | 5 (14.7) |
|
>65 | 14 (41.1) |
|
Unknown | 2 (6) |
| Recurrence CA125,
U/ml |
|
|
<35 | 13 (38.2) |
|
35–65 | 6 (17.6) |
|
>65 | 14 (41.1) |
|
Unknown | 1 (3) |
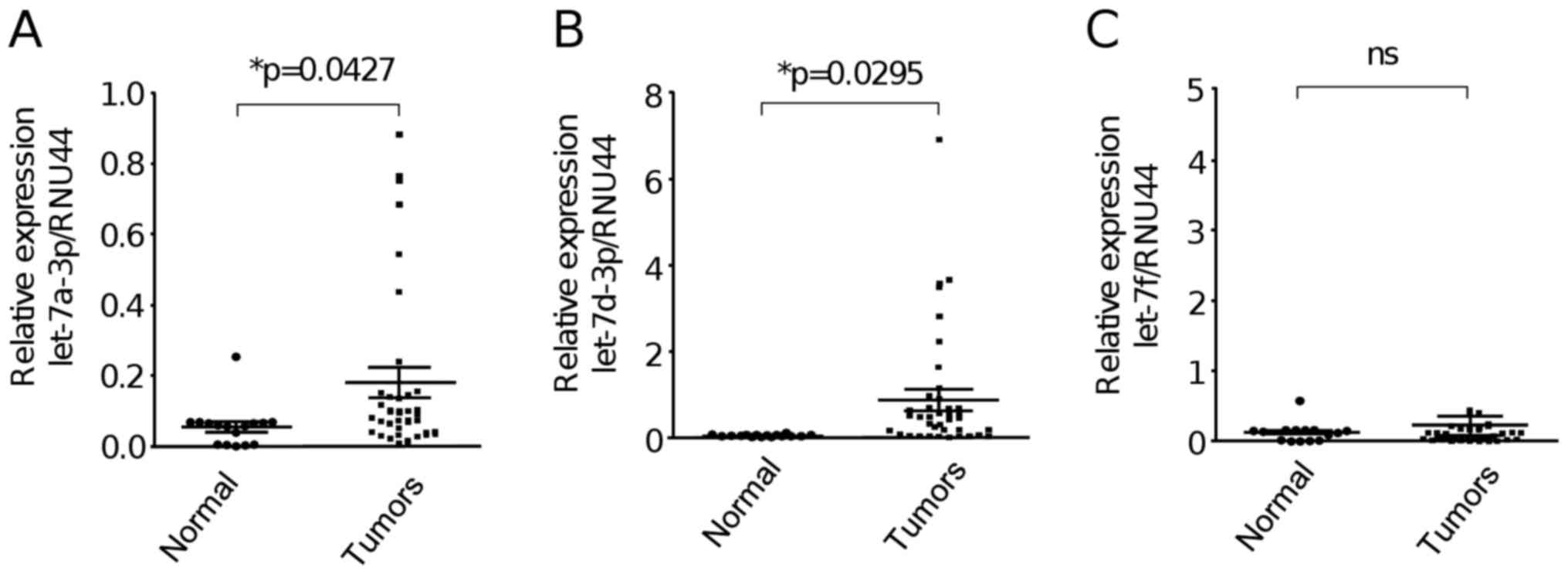
let-7 miRNA expression is deregulated
in ovarian tumors
In order to study the expression of members of the
let-7 family in primary EOC tumors and normal ovarian tissues, we
used stem-loop qRT-PCR as implemented in MicroRNA assay protocol
(Thermo Fisher Scientific, Inc.). After comparative
2−ΔΔCt analyses we found that let-7a-3p and let-7d-3p
were significantly (P<0.05) upregulated in ovarian tumors (n=40)
in comparison to normal ovarian tissues (n=18) (Fig. 1A and B). In contrast, no significant
change in the expression of let-7f between the groups was found
(Fig. 1C).
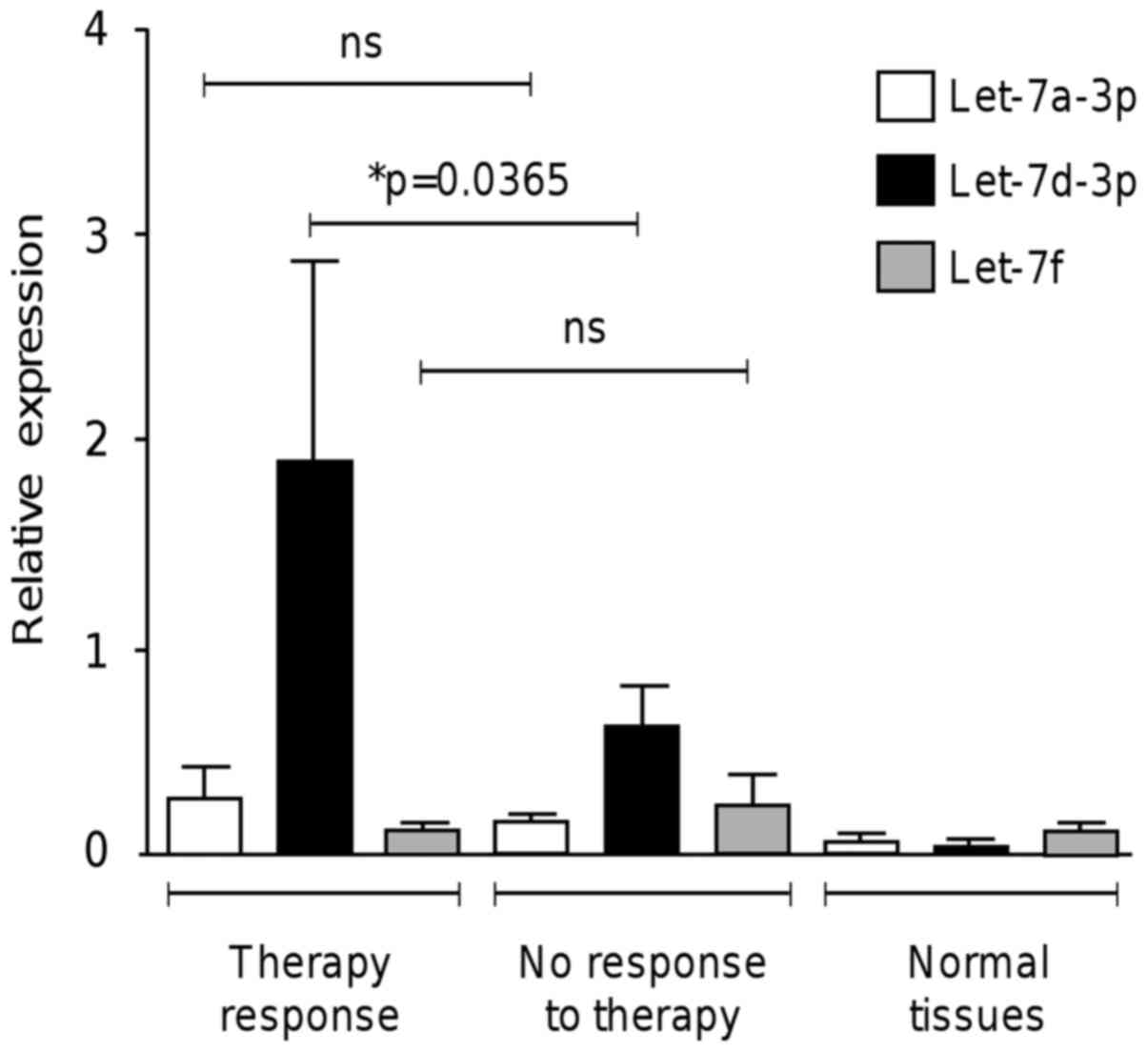
Upregulation of let-7d-3p is
associated with a response to neoadjuvant therapy
A high percentage of patients diagnosed with locally
advanced ovarian cancer have an unfavorable response to
conventional treatment. This situation is aggravated as no useful
molecular predictors for therapy response are currently used in
clinical practice (14). We aimed
to determine whether let-7 miRNA expression is associated with
response to neoadjuvant therapy. We analyzed by qRT-PCR the
expression of let-7 in ovarian cancer patients that had achieved a
positive response and no response to carboplatin/paclitaxel
regimen. Notably, data showed that the differential expression of
let-7d-3p in ovarian tumors was able to discriminate between the
patients that showed response to therapy and the non-responder
group (P<0.036) (Fig. 2). In
contrast, no significant differences in the expression of let-7a-3p
and let-7f between the responder and no-responder groups were
found.
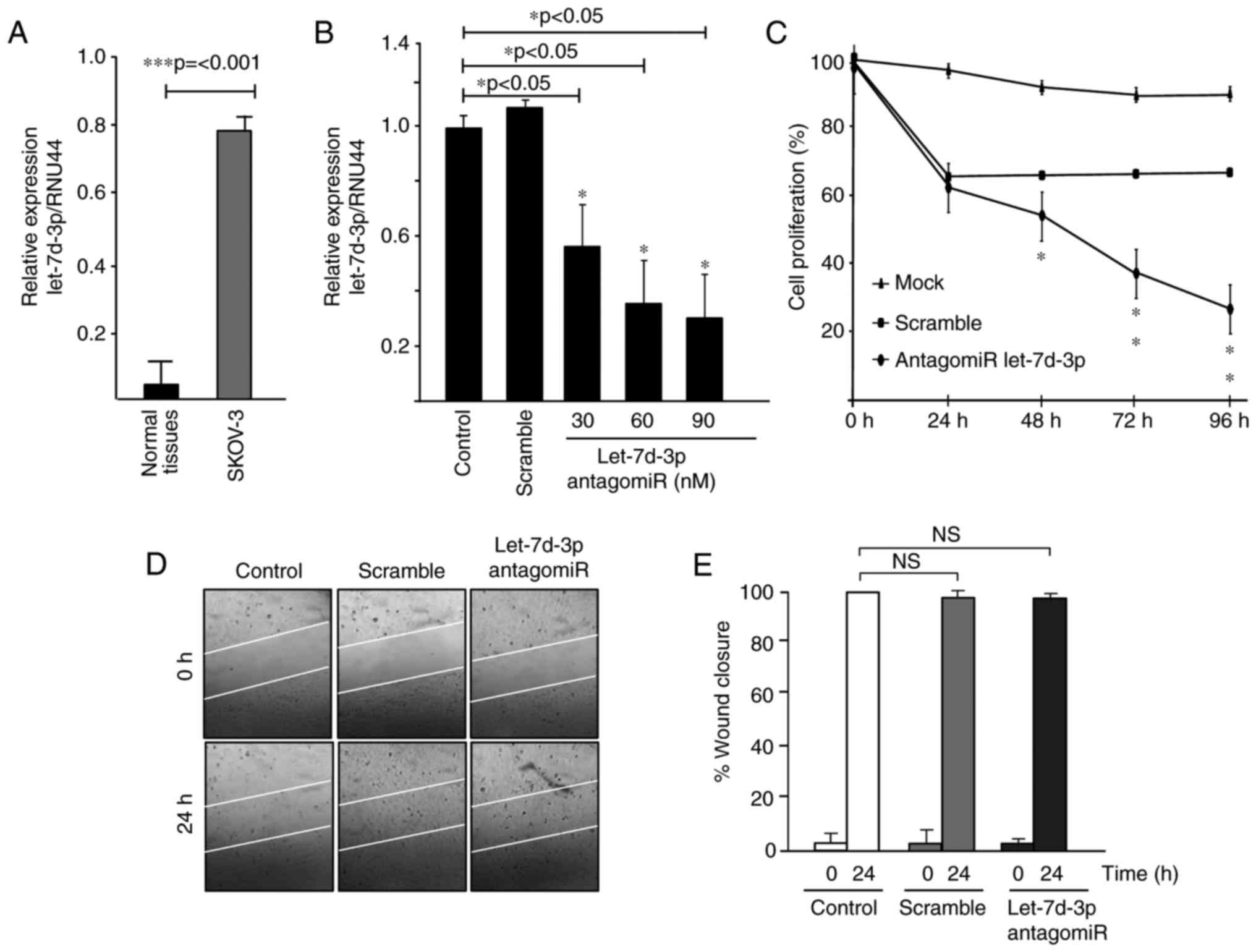
Effects of let-7d-3p on cell
proliferation and migration
Expression analysis of let-7 members allowed us to
evidence that let-7d-3p was significantly upregulated in ovarian
tumors in comparison to normal ovarian tissues. To study the
biological relevance of let-7d-3p we first confirmed its
upregulation in SKOV-3 ovarian cancer cells relative to ovarian
normal tissues (Fig. 3A). Then,
knockdown of let-7d-3p expression was performed using a specific
antagomiR. Data showed that transfection of increasing
concentrations (30, 60 and 90 nM) of let-7d-3p inhibitor
significantly downregulated the endogenous let-7d-3p expression in
a dose-dependent manner (Fig. 3B).
We next investigated whether the forced inhibition of let-7d-3p had
effects on cell proliferation in vitro. Data from the MTT
assays showed that the growth rate of SKOV-3 cells transfected with
let-7d-3p inhibitor (90 nM) was significantly (P<0.05) decreased
up to 80% in comparison with non-transfected control cells after 96
h (Fig. 3C). Then, we performed
scratch/wound-healing assays to evaluate the contribution of
let-7d-3p inhibition in cell migration. Unexpectedly, data
indicated no changes in the restoration of monolayers of cells
transfected with antagomiR let-7d-3p (90 nM) in comparison to
non-treated and scramble transfected control cells at 24 h
(Fig. 3D and E). Similar results in
SKOV-3 cell migration were obtained using Transwell chamber assays
at 24 h (data not shown).
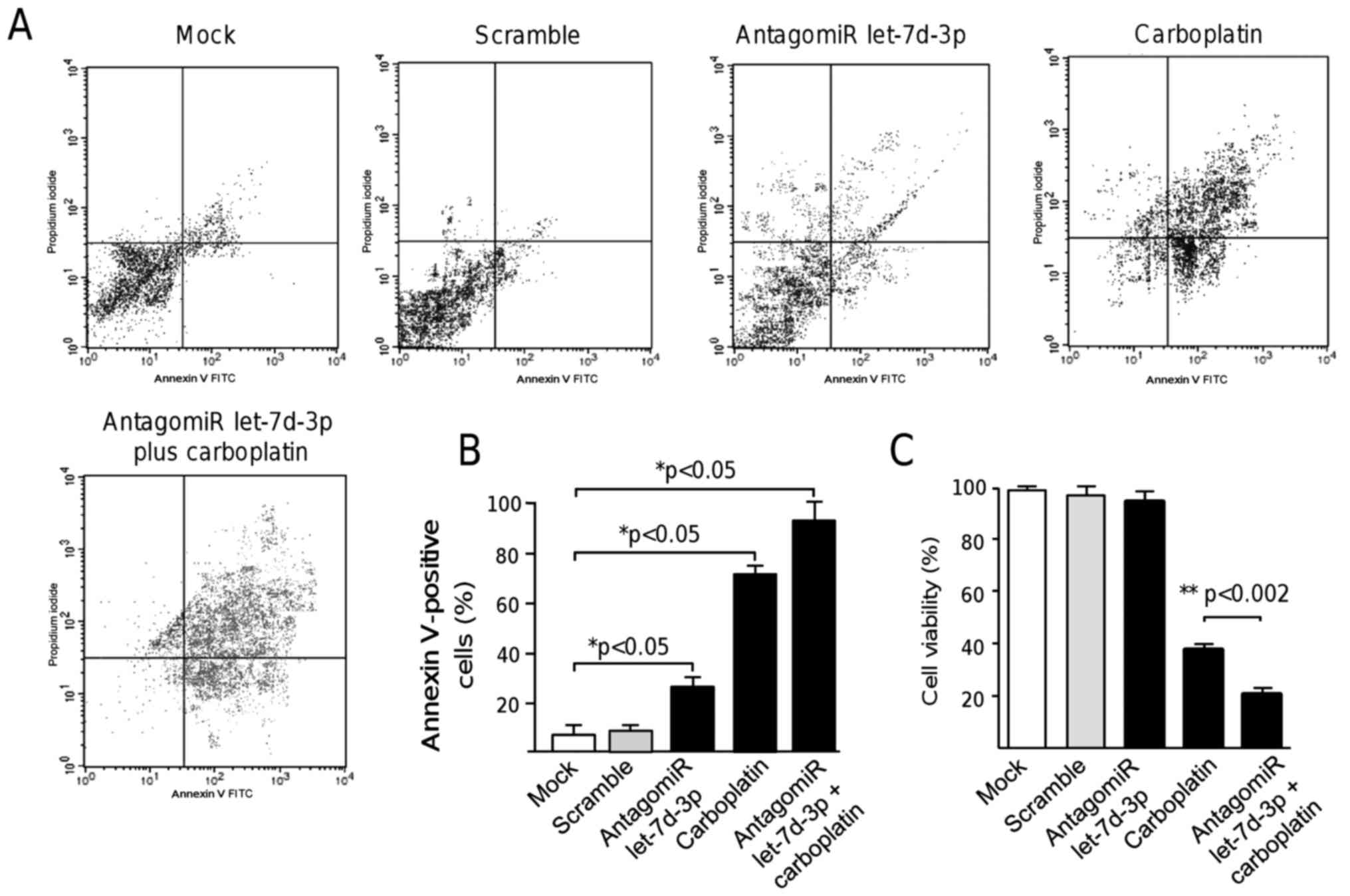
Inhibition of let-7d-3p induces
apoptosis
Standard treatment options for ovarian cancer
patients include the use of platinum salt-based therapy. However,
the effectiveness of this regimen is poor and additional
therapeutic strategies are needed to improve clinical outcome.
Therefore, we investigated whether the inhibition of let-7d-3p may
induce apoptosis resulting in the potential sensitization of cancer
cells to platinum chemotherapy. The number of apoptotic cancer
cells in cultures treated with the let-7d-3p inhibitor was assessed
using Annexin V-FITC assays. Our results showed that the percentage
of apoptotic cells was significantly increased (P<0.05) from
9.8% in the non-treated control cells to 29.6% in cells treated
with let-7d-3p inhibitor for 48 h (Fig.
4A and B). As expected, carboplatin monotherapy used as a
control of cell death resulted in a marked increase in apoptotic
cells (64.5%) in comparison to mock and scramble transfected
control cells. Notably, a significant increase (P<0.05) in cell
death up to 93.8% was found in let-7d-3p-deficient cells treated
with carboplatin in comparison to the controls indicating a
synergistic effect in apoptosis exerted by antagomiR therapy
(Fig. 4A and B).
let-7d-3p inhibition sensitizes
ovarian cancer cells to chemotherapy
To evaluate the potential chemosensitizing effect of
let-7d-3p, we next analyzed the cell viability effects of its
inhibition in combination with carboplatin cytotoxic therapy. Data
showed that while treatment with the let-7d-3p inhibitor (90 nM)
alone slightly affected ovarian cancer cell viability, a
combination of let-7d-3p plus carboplatin (5 mM) resulted in a
marked increase in cell cytotoxicity (Fig. 4C). These data suggested that
let-7d-3p sensitizes SKOV-3 cells to carboplatin therapy, at least
in part, by cell death induction.
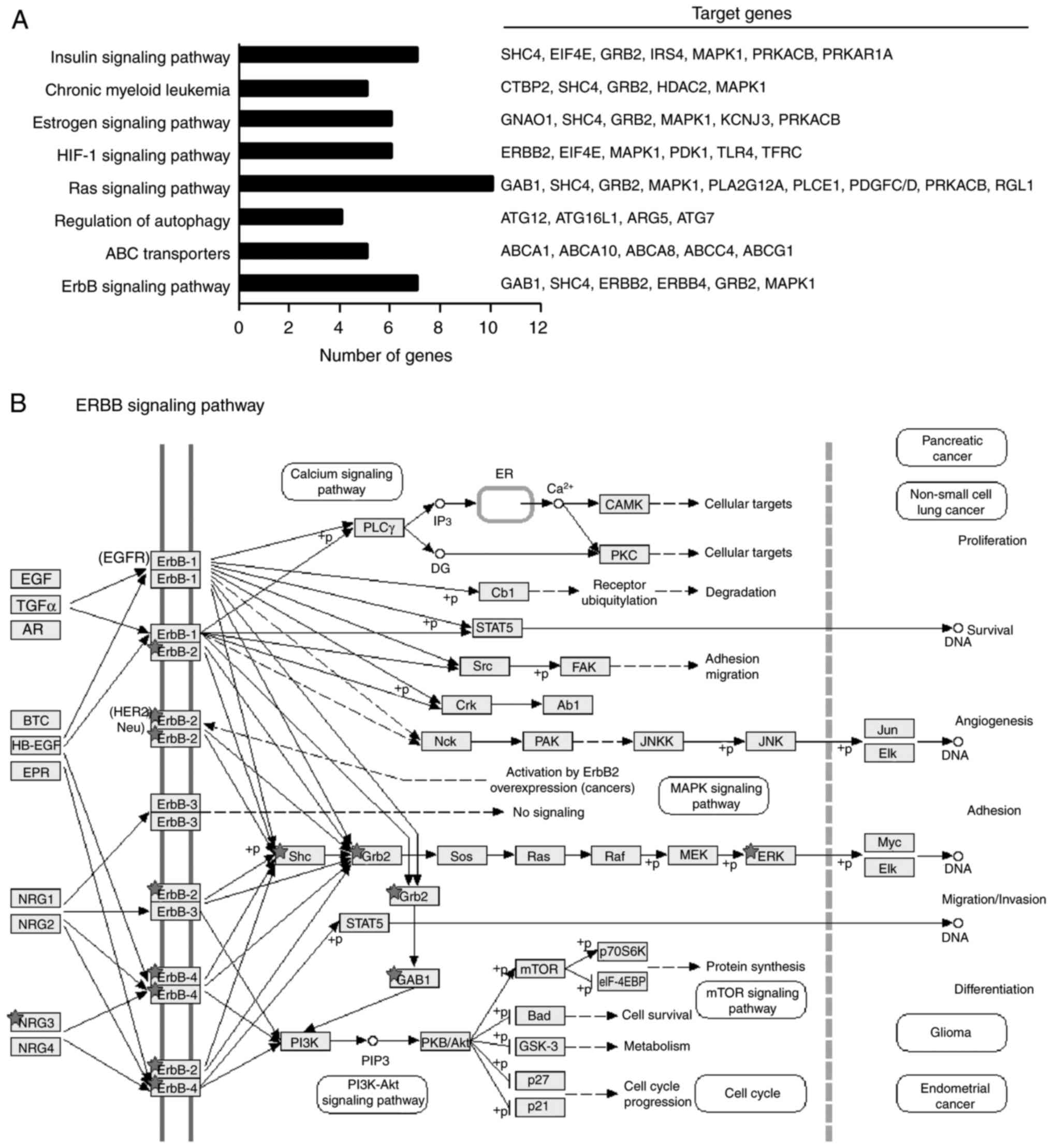
Overview of the signaling pathways
modulated by let-7d-3p
Prediction of let-7d-3p targets based on GO
categories identified several genes involved in key cellular
processes and signaling pathways related to tumor development,
progression and drug-resistance including ABC transporters, ErbB,
RAS and HIF-1 pathways (Fig. 5A and
B). For example, activation of ErbB signaling contributes to
chemoradiotherapy resistance phenotypes in ovarian, breast and
cervical cancer, suggesting that let-7d-3p overexpression could be
associated with a complete response to therapy in ovarian cancer
through similar modulation of ErbB signaling (16–18).
However, additional experimental data is needed to confirm this
hypothesis.
Discussion
Chemotherapy, radiotherapy and surgery are the most
frequently used treatment modalities for ovarian cancer (9). In ovarian cancer, surgery followed by
a combination of paclitaxel and carboplatin therapy are used as the
first-line agents yielding response rates of 80% (10). Unfortunately, the majority of
ovarian cancer patients relapse within the first 18 months, and
recurrent disease is frequently much more resistant to conventional
therapy than primary tumors (13).
Therefore, alternative therapeutic approaches are needed to improve
patient survival and outcome. Tumor suppressor let-7 miRNAs are
frequently downregulated in human cancers, and they may be useful
for the prediction of the clinical response to therapy and outcome
(7,9). Hence, restoration of normal expression
levels of let-7 may be exploited for cancer therapeutics. Here, we
analyzed the expression of three members of the let-7 miRNA family
(let-7a-3p, let-7d-3p and let-7f), and explored the functions of
let-7d-3p in apoptosis and therapeutic response. Our data showed
that let-7d-3p overexpression was able to discriminate between the
ovarian cancer patients that showed response to therapy from the
non-responder group. Moreover, data indicate that let-7d-3p
efficiently sensitized SKOV-3 cells to carboplatin therapy, at
least in part, by cell death induction. Taken altogether, these
data highlighted the potential role of let-7d-3p as a novel
predictor of response to platinum-based chemotherapy.
Genetic and epigenetic alterations leading to
aberrant regulation of miRNA expression is known to be involved in
the development of resistance to chemotherapy of human cancers
(19). Notably, the prediction of
let-7d-3p targets identified a number of genes involved in
signaling pathways related to drug resistance including ABC
transporters, ErbB, RAS and HIF-1 pathways which may be related to
the chemosensitizing effect of let-7d-3p in SKOV-3 cells (Fig. 5A and B). Tumor cells acquire
resistance to chemotherapeutic drugs through various mechanisms
including upregulation of members of the ABC transporter family
(20). The relatively rapid
acquisition of resistance to chemotherapeutic agents may be
mediated by ABC transporters, MDR/MRP and P-glycoprotein; mainly
they can increase efflux of drugs from cancer cells, thereby
decreasing intracellular drug concentrations. Markedly, several
studies have shown that miRNAs, such as miR-200c and others, can
attenuate the effects of these drug-resistance components (21,22).
In addition, miRNAs play a critical role in the drug-resistance of
tumor cells and the clinical response to cytotoxic chemotherapy in
cancer (23–34). The inhibition of ABC transporters by
miRNAs has been reported in several studies. For instance it was
reported that both let-7i and let-7g reduced ABCC10 expression in
esophageal carcinoma (24). In
addition ABCG2 was downregulated by miR-222 in squamous cell
carcinoma (25). In addition, ABCA1
suppression by miR106a in lung cancer promoted cisplatin
sensitivity (26). Similarly,
knockdown of miR-127 enhanced the adriamycin sensitivity in glioma
cells through modulation of MDR1 and MRP1 expression (27). MDR1 expression also was reduced by
miR-27a through the FZD7/β-catenin pathway resulting in increased
5-fluorouracil toxicity in hepatocellular carcinoma cells (28). On the other hand, increased miR-124
expression in renal cell carcinoma was found to promote
chemosensitivity to doxorubicin by decreased of P-glycoprotein
expression levels via targeting FZD5/protein kinase C (PKC)
signaling (29). In addition,
miR-145 upregulation enhanced the effect chemotherapeutic
inhibiting P-glycoprotein through decreased activity of Fas
signaling (30). On the other hand,
miR-21 silencing in lung cancer A549/DDP cells reversed MDR by
modulation of MDR-related gene expression and inhibition of AKT
signaling (31).
Moreover, PI3K-AKT pathway activation could
contribute to pertuzumab resistance through miR-150 downregulation
in ovarian cancer (32); miR-21,
miR-542-3p, miR-205 downregulation may decrease the response to
trastuzumab and chemotherapy also through the PI3K pathway in
breast cancer (33–35). In addition, MYC expression has been
linked to tamoxifen-resistant via transcriptional regulation of the
HOXB7 repressor miR-196a (36).
Similarity, miR-217 low-expression levels may increase the
resistance of EGFR and HER2 inhibitors through an inverse
modulation of CAGE in melanoma (37).
We also observed that the Ras pathway could be
impacted by let-7d-3p. The Ras pathway is also involved with
acquisition of resistance to therapy and is extensively modulated
by miRNAs in diverse types of cancer. For instance, miR-122
downregulation contributed to sorafenib resistance through of
Ras/Raf/Erk signaling in hepatocellular carcinoma (38). In prostate cancer, miR-143
downregulation decreased the sensitivity to docetaxel by targeting
the EGFR/RAS/MAPK pathway (39).
Moreover, miR-3127-5p expression levels were associated with
dasatinib resistance in lung cancer through the c-Abl/Ras/ERK
pathway (40). Low levels of let-7b
diminish the cytotoxicity of paclitaxel and gemcitabine through
K-Ras mutant in several cancer types (41). Similarly, K-Ras was found to
increase the 5-fluorouracil resistance through miR-224 expression
in colorectal cancer (42). In
ovarian cancer, miR-634 is involved with cisplatin resistance via
Ras-MAPK activation (43). Finally,
some reports indicate that hypoxic conditions can contribute to
radiation therapy and chemotherapy resistance, and miRNAs can
regulate drug-resistance through direct downregulation of hypoxia
inducible factor (HIF-1α) (44–47).
For instance, restoration of Numb expression by inhibition of
miR-182 caused HIF-1α inhibition in breast cancer cells, resulting
in trastuzumab resistance (45). In
addition, hypoxic aggressiveness of prostate cancer cells was
linked with increased expression of VEGF, IL-6 and miR-21 (46). In addition, in hepatocarcinoma,
miR-338-5p sensitized cancer cells to sorafenib by targeting HIF-1α
(47).
In conclusion, we found that let-7d-3p, an miRNA
with no previous characterized functions in ovarian cancer, was
associated with apoptosis and positive response to
carboplatin/paclitaxel chemotherapy in patients. Therefore, we
propose that let-7d-3p could be useful as a potential molecular
biomarker of the clinical response to neoadjuvant therapy in
ovarian cancer patients.
Acknowledgements
We acknowledge the Autonomous University of Mexico
City and CONACyT for support. RGV was recipient of a CONACYT
fellowship (no. 441111).
Funding
The present study was partly funded by CONACyT
(grant nos. 222335 and 233370), Mexico.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
CLC, DGR and ERG conceived and designed the study.
RGV, ONHDLC, YMSV, HADLV and RRP performed the experiments. ACR and
SLG assisted with FACS analysis. DGR, ERG, AMG and DIO provide the
tumor and normal tissues and clinical data. LAM reactived purchase
and conceived the project. CLC, LAM and HAV wrote the paper.
Ethics approval and consent to
participate
The Instituto Nacional de Cancerologia at Mexico
provided the ovarian tumor and normal tissues collection. The
corresponding ethics committee approved the protocols concerning
the use of human tissues. A signed informed form consent was
obtained from each participant or a representative prior to release
for research use.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gonzalez H, Lema C, Kirken RA, Maldonado
RA, Varela-Ramirez A and Aguilera RJ: Arsenic-exposed keratinocytes
exhibit differential microRNAs expression profile; potential
implication of miR-21, miR-200a and miR-141 in melanoma pathway.
Clin Cancer Drugs. 2:138–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marin-Muller C, Li D, Bharadwaj U, Li M,
Chen C, Hodges SE, Fisher WE, Mo Q, Hung MC and Yao Q: A
tumorigenic factor interactome connected through tumor suppressor
microRNA-198 in human pancreatic cancer. Clin Cancer Res.
19:5901–5913. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ibarra I, Erlich Y, Muthuswamy SK,
Sachidanandam R and Hannon GJ: A role for microRNAs in maintenance
of mouse mammary epithelial progenitor cells. Genes Dev.
21:3238–3243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson CD, Esquela-Kerscher A, Stefani G,
Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J,
Shingara J, et al: The let-7 microRNA represses cell proliferation
pathways in human cells. Cancer Res. 15:7713–7722. 2007. View Article : Google Scholar
|
|
6
|
Kumar MS, Erkeland SJ, Pester RE, Chen CY,
Ebert MS, Sharp PA and Jacks T: Suppression of non-small cell lung
tumor development by the let-7 microRNA family. Proc Natl Acad Sci
USA. 105:3903–3908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barh D, Malhotra R, Ravi B and Sindhurani
P: MicroRNA let-7: An emerging next-generation cancer therapeutic.
Curr Oncol. 17:70–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sandercock J, Parmar MK, Torri V and Qian
W: First-line treatment for advanced ovarian cancer: Paclitaxel,
platinum and the evidence. Br J Cancer. 87:815–824. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R: Gynecologic Oncology Group: Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
Gynecologic Oncology Group study. J Clin Oncol. 21:3194–3200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ledermann JA, Marth C, Carey MS, Birrer M,
Bowtell DD, Kaye S, McNeish I, Oza A, Scambia G, Rustin G, et al:
Role of molecular agents and targeted therapy in clinical trials
for women with ovarian cancer. Int J Gynecol Cancer. 21:763–770.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Petrillo M, Zannoni GF, Beltrame L,
Martinelli E, DiFeo A, Paracchini L, Craparotta I, Mannarino L,
Vizzielli G, Scambia G, et al: Identification of high-grade serous
ovarian cancer miRNA species associated with survival and drug
response in patients receiving neoadjuvant chemotherapy: A
retrospective longitudinal analysis using matched tumor biopsies.
Ann Oncol. 27:625–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Zhang Y, Gao Y, Cui Y, Liu H, Li M
and Tian Y: Downregulation of HNF1 homeobox B is associated with
drug-resistance in ovarian cancer. Oncol Rep. 32:979–988. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye XM, Zhu HY, Bai WD, Wang T, Wang L,
Chen Y, Yang AG and Jia LT: Epigenetic silencing of miR-375 induces
trastuzumab resistance in HER2-positive breast cancer by targeting
IGF1R. BMC Cancer. 14:1342014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pedroza-Torres A, Fernández-Retana J,
Peralta-Zaragoza O, Jacobo-Herrera N, de Leon Cantu D, Cerna-Cortés
JF, Lopez-Camarillo C and Pérez-Plasencia C: A microRNA expression
signature for clinical response in locally advanced cervical
cancer. Gynecol Oncol. 142:557–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Çalışkan M, Güler H and Çetintaş Bozok V:
Current updates on microRNAs as regulators of chemoresistance.
Biomed Pharmacother. 95:1000–1012. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun YL, Patel A, Kumar P and Chen ZS: Role
of ABC transporters in cancer chemotherapy. Chin J Cancer.
31:51–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sui H, Cai GX, Pan SF, Deng WL, Wang YW,
Chen ZS, Cai SJ, Zhu HR and Li Q: miR-200c attenuates P-gp-mediated
MDR and metastasis by targeting JNK2/c-Jun signaling pathway in
colorectal cancer. Mol Cancer Ther. 13:3137–3151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sorrentino A, Liu CG, Addario A, Peschle
C, Scambia G and Ferlini C: Role of microRNAs in drug-resistant
ovarian cancer cells. Gynecol Oncol. 111:478–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Samuel P, Pink RC, Brooks SA and Carter
DR: miRNAs and ovarian cancer: A miRiad of mechanisms to induce
cisplatin drug-resistance. Expert Rev Anticancer Ther. 16:57–70.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boyerinas B, Park SM, Murmann AE, Gwin K,
Montag AG, Zillhardt M, Hua YJ, Lengyel E and Peter ME: Let-7
modulates acquired resistance of ovarian cancer to Taxanes via
IMP-1-mediated stabilization of multidrug-resistance 1. Int J
Cancer. 130:1787–1797. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu K, Yang Y, Zhao J and Zhao S:
BAG3-mediated miRNA let-7g and let-7i inhibit proliferation and
enhance apoptosis of human esophageal carcinoma cells by targeting
the drug transporter ABCC10. Cancer Lett. 371:125–133. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao L, Ren Y, Tang H, Wang W, He Q, Sun
J, Zhou X and Wang A: Deregulation of the miR-222-ABCG2 regulatory
module in tongue squamous cell carcinoma contributes to
chemoresistance and enhanced migratory/invasive potential.
Oncotarget. 6:44538–44550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma Y, Li X, Cheng S, Wei W and Li Y:
MicroRNA-106a confers cisplatin resistance in non-small cell lung
cancer A549 cells by targeting adenosine triphosphatase-binding
cassette A1. Mol Med Rep. 11:625–632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng R and Dong L: Knockdown of
microRNA-127 reverses adriamycin resistance via cell cycle arrest
and apoptosis sensitization in adriamycin-resistant human glioma
cells. Int J Clin Exp Pathol. 8:6107–6116. 2015.PubMed/NCBI
|
|
29
|
Chen Z, Ma T, Huang C, Zhang L, Lv X, Xu
T, Hu T and Li J: MiR-27a modulates the MDR1/P-glycoprotein
expression by inhibiting FZD7/β-catenin pathway in hepatocellular
carcinoma cells. Cell Signal. 25:2693–2701. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Long QZ, Du YF, Liu XG, Li X and He DL:
miR-124 represses FZD5 to attenuate P-glycoprotein-mediated
chemo-resistance in renal cell carcinoma. Tumour Biol.
36:7017–7026. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng H, Liu Z, Liu T, Cai Y, Wang Y, Lin
S, Chen J, Wang J, Wang Z and Jiang B: Fas signaling promotes
chemoresistance in gastrointestinal cancer by up-regulating
P-glycoprotein. Oncotarget. 15:10763–10777. 2014.
|
|
32
|
Dong Z, Ren L, Lin L and Li J, Huang Y and
Li J: Effect of microRNA-21 on multidrug-resistance reversal in
A549/DDP human lung cancer cells. Mol Med Rep. 11:682–690. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wuerkenbieke D, Wang J, Li Y and Ma C:
miRNA-150 downregulation promotes pertuzumab resistance in ovarian
cancer cells via AKT activation. Arch Gynecol Obstet.
292:1109–1116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De Mattos-Arruda L, Bottai G, Nuciforo PG,
Di Tommaso L, Giovannetti E, Peg V, Losurdo A, Perez-Garcia J,
Masci G, Corsi F, et al: MicroRNA-21 links
epithelial-to-mesenchymal transition and inflammatory signals to
confer resistance to neoadjuvant trastuzumab and chemotherapy in
HER2-positive breast cancer patients. Oncotarget. 10:37269–37280.
2015.
|
|
35
|
Ma T, Yang L and Zhang J: miRNA-542-3p
downregulation promotes trastuzumab resistance in breast cancer
cells via AKT activation. Oncol Rep. 33:1215–1220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iorio MV, Casalini P, Piovan C, Di Leva G,
Merlo A, Triulzi T, Ménard S, Croce CM and Tagliabue E:
microRNA-205 regulates HER3 in human breast cancer. Cancer Res.
69:2195–2200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin K, Park S, Teo WW, Korangath P, Cho
SS, Yoshida T, Győrffy B, Goswami CP, Nakshatri H, Cruz LA, et al:
HOXB7 Is an ERα cofactor in the activation of HER2 and multiple ER
target genes leading to endocrine resistance. Cancer Discov.
5:944–959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim Y, Kim H, Park D, Han M, Lee H, Lee
YS, Choe J, Kim YM and Jeoung D: miR-217 and CAGE form feedback
loop and regulates the response to anti-cancer drugs through EGFR
and HER2. Oncotarget. 7:10297–10321. 2016.PubMed/NCBI
|
|
39
|
Xu Y, Huang J, Ma L, Shan J, Shen J, Yang
Z, Liu L, Luo Y, Yao C and Qian C: MicroRNA-122 confers sorafenib
resistance to hepatocellular carcinoma cells by targeting IGF-1R to
regulate RAS/RAF/ERK signaling pathways. Cancer Lett. 371:171–181.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu B, Niu X, Zhang X, Tao J, Wu D, Wang Z,
Li P, Zhang W, Wu H, Feng N, et al: miR-143 decreases prostate
cancer cells proliferation and migration and enhances their
sensitivity to docetaxel through suppression of KRAS. Mol Cell
Biochem. 350:207–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun Y, Chen C, Zhang P, Xie H, Hou L, Hui
Z, Xu Y, Du Q, Zhou X, Su B and Gao W: Reduced miR-3127-5p
expression promotes NSCLC proliferation/invasion and contributes to
dasatinib sensitivity via the c-Abl/Ras/ERK pathway. Sci Rep.
4:65272014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dai X, Jiang Y and Tan C: Let-7
sensitizes KRAS mutant tumor cells to chemotherapy. PLoS
One. 10:e01266532015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Amankwatia EB, Chakravarty P, Carey FA,
Weidlich S, Steele RJ, Munro AJ, Wolf CR and Smith G: MicroRNA-224
is associated with colorectal cancer progression and response to
5-fluorouracil-based chemotherapy by KRAS-dependent and
-independent mechanisms. Br J Cancer. 112:1480–1490. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
van Jaarsveld MT, van Kuijk PF, Boersma
AW, Helleman J, van IJcken WF, Mathijssen RH, Pothof J, Berns EM,
Verweij J and Wiemer EA: miR-634 restores drug sensitivity
in resistant ovarian cancer cells by targeting the Ras-MAPK
pathway. Mol Cancer. 14:1962015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ajduković J: HIF-1 - a big chapter in the
cancer tale. Exp Oncol. 38:9–12. 2016.PubMed/NCBI
|
|
46
|
Sajadimajd S, Yazdanparast R and Akram S:
Involvement of Numb-mediated HIF-1α inhibition in
anti-proliferative effect of PNA-antimiR-182 in
trastuzumab-sensitive and -resistant SKBR3 cells. Tumour Biol.
37:5413–5426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bao B, Ahmad A, Kong D, Ali S, Azmi AS, Li
Y, Banerjee S, Padhye S and Sarkar FH: Hypoxia induced
aggressiveness of prostate cancer cells is linked with deregulated
expression of VEGF, IL-6 and miRNAs that are attenuated by CDF.
PLoS One. 7:e437262012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu H, Zhao L, Fang Q, Sun J, Zhang S, Zhan
C, Liu S and Zhang Y: MiR-338-3p inhibits hepatocarcinoma cells and
sensitizes these cells to sorafenib by targeting hypoxia-induced
factor 1α. PLoS One. 9:e1155652014. View Article : Google Scholar : PubMed/NCBI
|