Introduction
Breast cancer is one of the most common types of
malignancy among women, and is associated with high mortality.
Globally, more than 1.7 million individuals are diagnosed with
breast cancer annually, and approximately 521,000 individuals
succumb to the disease (1). In
recent decades, the incidence of breast cancer has increased, with
almost one-tenth of all newly diagnosed cancers worldwide
originating in the breast (2).
Changes in reproductive and lifestyle characteristics are
contributing to the increased morbidity and mortality rates of
breast carcinoma. However, the exact molecular mechanisms
underlying breast carcinoma are not fully understood.
At present, three major protein markers: estrogen
receptor (ER), progesterone receptor (PR) and human epidermal
growth factor (EGF) receptor 2 (HER2), are used for determining the
classification, treatment and prognosis of breast carcinoma
(3). However, there are no
identified protein markers for the early diagnosis and treatment of
breast carcinoma (4). Therefore, it
is necessary to further investigate the molecular regulatory
mechanisms of breast carcinoma, and identify molecular markers that
can be used for early diagnosis and monitoring.
In recent years, DNA microarray analysis has been
developed as a rapid, high-throughput detection technology to
simultaneously monitor the differential expression of numerous
genes or miRNAs in oncology research, including in the field of
breast cancer (5–8). miRNAs are small ~21-nt RNAs involved
in post-transcriptional gene regulation. It has been demonstrated
that miRNAs pair with the mRNA 3′-untranslated region (UTR) of
target genes to regulate their expression, including in cancer. In
breast cancer, multiple miRNAs have been identified to regulate the
expression of target genes, including miRNA-155, miRNA-675,
miRNA-519a and miRNA-31 (9–12), among others.
Although DNA microarray application in oncology
research has been widely recognized, the test has high variability.
Therefore, in our research, we identified differentially expressed
genes and microRNAs by analyzing three breast cancer mRNA
microarray datasets, and one microRNA dataset. We then aimed to
identify the key genes in breast cancer with survival,
mRNA-microRNA interaction, ontology enrichment and network
analyses.
Materials and methods
Microarray data
The GEO (https://www.ncbi.nlm.nih.gov/gds. Accessed Jan. 26,
2018) is a free international public repository of high-throughput
functional genomics data, including microarray and next-generation
sequencing data. In this study, we used three gene expression
profiles (GSE26910, GSE42568 and GSE89116) and one miRNA expression
profile (GSE35412) from GEO.
The GSE26910 dataset was comprised of six breast
cancer and six para-carcinoma tissue sample mRNA expression
profiles (13); GSE42568 included
104 breast cancer and 17 para-carcinoma tissue sample mRNA
expression profiles (14); GSE89116
contained 30 breast cancer and nine para-carcinoma tissue sample
mRNA expression profiles (15); and
GSE35412 was comprised of 29 breast cancer and 21 para-carcinoma
tissue sample miRNA expression profiles (16). All datasets were downloaded in a
processed and normalized format.
Data processing
The GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r. Accessed Jan.
26, 2018) is an online data analysis tool that can be utilized to
analyze GEO data series obtained under the same experimental
conditions (17). In this study,
GEO2R was used to identify the differentially expressed miRNAs and
genes between the breast cancer and para-carcinoma tissue
expression profiles. Adjusted P-values (adj.p) were calculated
using the Benjamini and Hochberg false discovery rate method to
correct for the likelihood of false positive results. An
adj.P<0.01 and | logFC | >1 were set as the cut-off criteria
for differential expression.
Functional and pathway enrichment
analysis of the differentially expressed genes
The DAVID (https://david-d.ncifcrf.gov/summary.jsp. Accessed Jan.
26, 2018) online database is an online program that provides
comprehensive gene annotation tools (18). The database was used to perform gene
ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway enrichment analyses. P<0.05 was applied as the cut-off
criterion.
Protein-protein interaction (PPI)
network construction and module selection for the differentially
expressed genes
The construction of a network of interactions
between proteins can establish a framework for the study of
molecular mechanisms. In this study, a PPI network of the
differentially expressed genes was constructed using the Search
Tool for the Retrieval of Interacting Genes and Proteins (STRING)
database (https://string-db.org. Accessed Jan. 26,
2018) (19), followed by
visualization using Cytoscape software (20). The confidence score ≥0.7 was set as
the cut-off criterion. The PPI network module selection criteria
included a degree cut-off=2, node score cut-off=0.2, k-core=2 and
maximum depth=100 (21).
Prediction of miRNA target genes
The online databases TargetScan (http://www.targetscan.org. Accessed Jan. 26, 2018),
microT-CDS (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=microT_CDS.
Accessed Jan. 26, 2018) and Tarbase (http://diana.imis.athena-innovation.gr/DianaTools/index.php.
Accessed Jan. 26, 2018) were used to identify the target genes of
the differentially expressed miRNAs. These databases are
universally recognized for their ability to accurately predict
miRNA target genes.
Analysis of the effect of the
differentially expressed genes on overall survival (OS)
In the online database, Kaplan-Meier (KM) Plotter
(http://www.kmplot.com. Accessed Jan. 26, 2018),
the impact of 54,675 genes on the survival time of cancer patients
was evaluated by analyzing the data from 10,188 cancer samples,
including 4,142 breast, 1,648 ovarian, 2,437 lung and 1,065 gastric
cancer sample microarray expression profiles (22). In the present study, we divided
breast carcinoma patients into two groups depending on the
expression of specific genes (high vs. low expression). The KM
Plotter database was utilized to analyze the OS of breast cancer
patients. Hazard ratios (HR) with 95% confidence intervals (CI) and
a log-rank P-value were calculated and displayed.
Results
Identification of differentially
expressed genes in three GEO datasets
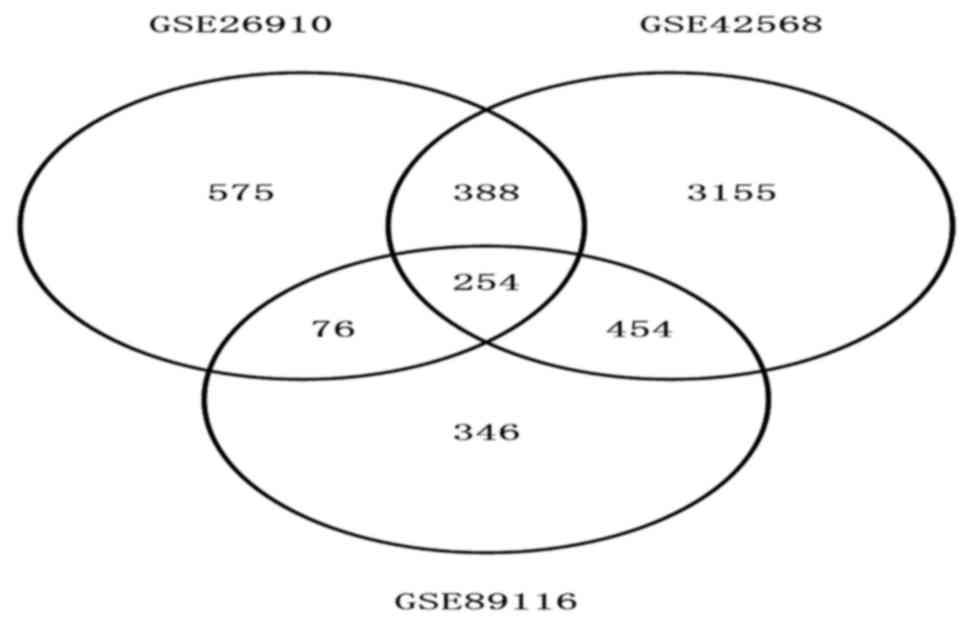
The total number of differentially expressed genes
was 1,293, 4,251 and 1,130 from GSE26910, GSE42568 and GSE89116,
respectively. A total of 254 genes showed the same expression trend
in all three data sets (Fig. 1).
This included 44 upregulated and 210 downregulated differentially
expressed genes.
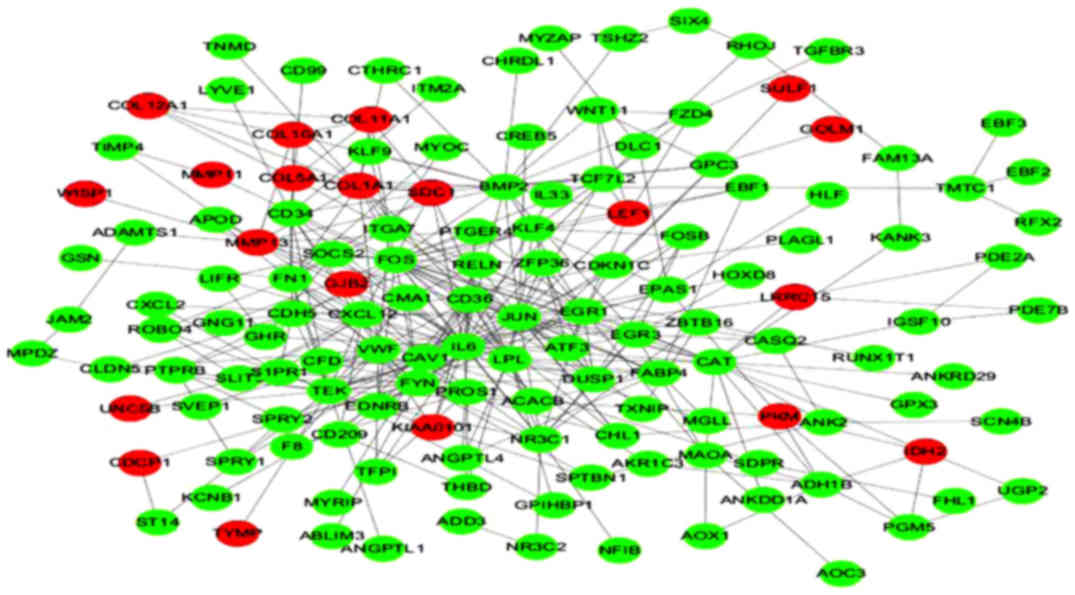
Construction of a PPI network of the
differentially expressed genes
The PPI network of the differentially expressed
genes consisted of 250 nodes and 375 edges, including 20
upregulated and 139 downregulated genes (Fig. 2).
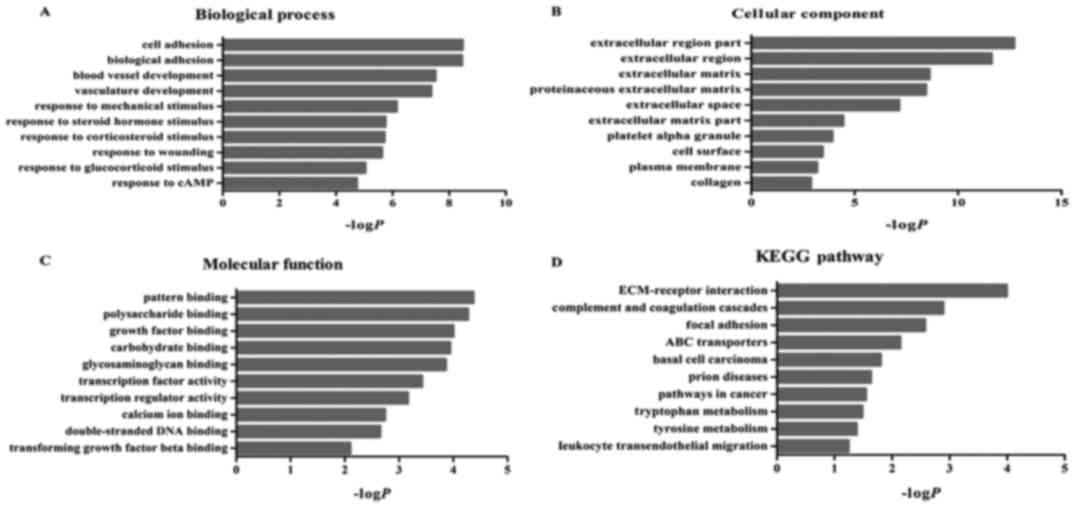
Function and pathway enrichment
analysis
We used the DAVID database to identify enriched
functions and pathways of the differentially expressed genes, in
order to further understand their function. The differentially
expressed genes were the most significantly enriched in cell
adhesion (biological process category), polysaccharide binding
(molecular function category) and extracellular region part
(cellular component category) (Fig.
3). In addition, these genes were significantly associated with
10 KEGG pathways, including ECM-receptor interactions, complement
and coagulation cascades and adhesion spots (Fig. 3).
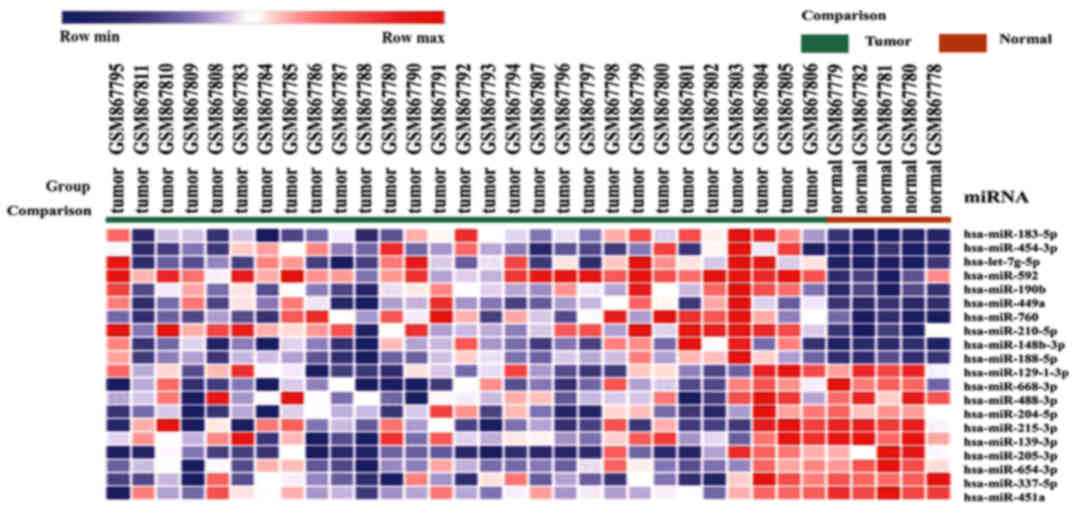
Prediction of the target genes of the
differentially expressed miRNAs
A total of 130 differentially expressed miRNAs were
detected in the GSE35412 dataset, including 17 upregulated and 113
downregulated miRNAs. miR-183-5p was the most significantly
upregulated miRNA, whereas miR-129-1-3p was the most significantly
downregulated (Fig. 4).
Subsequently, target genes of the differentially expressed miRNAs
were obtained from online databases (TargetScan, microT-CDS and
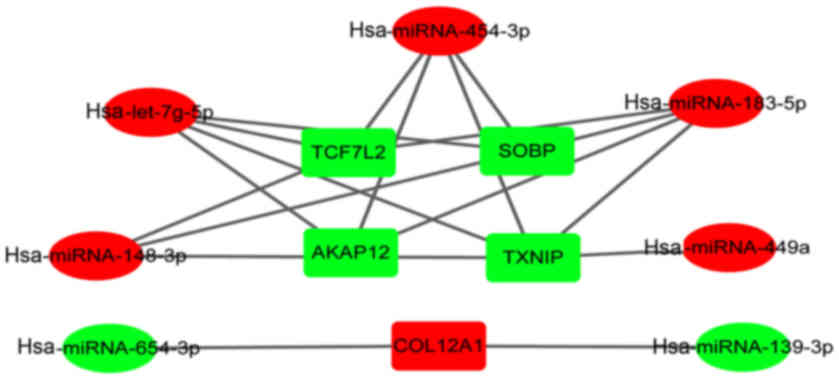
Tarbase). We determined that AKAP12, SOPB, TCF7L2, COL12A1
and TXNIP were the target genes for multiple differentially
expressed miRNAs (Fig. 5).
Survival analysis of key identified
differentially expressed genes
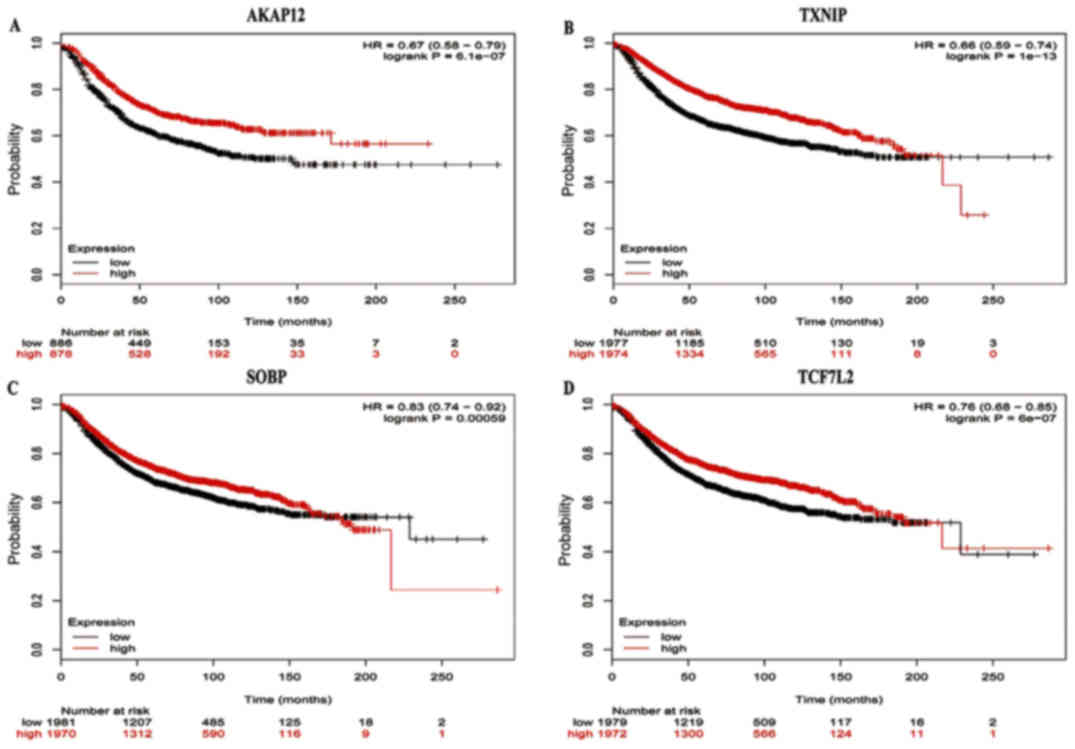
The prognostic values of five key genes in the PPI
network were assessed from KMplots. The OS rate for breast cancer
patients was analyzed based on the low and high expression of the
key genes. The results showed that high AKAP12 mRNA expression [HR
0.67 (95% CI, 0.58–0.79), P=6.1e-07] was associated with an
improved OS for breast carcinoma patients (Fig. 6), as was high expression of TXNIP
[HR 0.66 (95% CI, 0.59–0.74), P=1e-13], SOBP [HR 0.83 (95% CI,
0.74–0.92), P=0.00059] and TCF7L2 [HR 0.76 (95% CI, 0.68–0.85),
P=6e-07] (Fig. 6).
Discussion
Although our understanding of the pathogenesis and
clinical treatment of breast cancer has made significant progress,
the overall mortality rate for breast cancer has not improved
significantly, which can be attributed to the lack of molecular
markers for effective diagnosis and treatment. Therefore, it is
important to explore the molecular markers of breast carcinoma to
improve the survival rate and prevention of patients.
Microarray technology has been applied to detect the
genetic changes associated with the progression of various types of
malignancy. Microarray technology has also been widely used to
select molecular markers for determining the diagnosis, treatment
and prognosis of tumors. In this study, we screened a total of 254
differentially expressed genes through analyzing three mRNA
datasets, which included 44 upregulated genes and 210 downregulated
genes. These differentially expressed genes were significantly
enriched in cell adhesion, polysaccharide binding, extracellular
region part and ECM-receptor interaction, which are all associated
with the pathogenesis of carcinomas.
We also analyzed a miRNA dataset, in which we
identified 130 differentially expressed miRNAs, including 17
upregulated and 113 downregulated miRNAs, in breast carcinoma.
miR-183-5p was the most significantly upregulated miRNA, while
miR-129-3p was the most markedly downregulated miRNA. miRNAs are
small, non-coding RNAs of ~22 nucleotides in length, which regulate
gene expression by targeting the 3′UTR of target mRNAs, resulting
in their degradation or the inhibition of translation. Previous
research has suggested that the dysregulation of miRNAs is involved
in the pathogenesis of many types of cancer, including breast
carcinoma. For example, it has been demonstrated that miR-21,
miR-210 and miR-221 are upregulated in triple-negative primary
breast carcinomas (23). In
addition, the overexpression of miR-301 is considered a negative
prognostic index for lymph node-negative invasive ductal breast
carcinoma. Shi et al identified that miR-301 is a crucial
oncogene in breast carcinoma that promotes nodal and distant
relapse via multiple pathways and mechanisms (24).
As miRNAs negatively regulate the expression of
their target genes, we analyzed the correlation of upregulated
genes with downregulated miRNAs, downregulated genes and
upregulated miRNAs. Notably, we identified that AKAP12, SOPB,
TCF7L2 and TXNIP were potentially the common targets of
hsa-miRNA-183-5p, hsa-miRNA-454-3p and hsa-let-7g-5p among the
downregulated genes. COL12A1 was potentially the common target of
hsa-miRNA-139-3p and hsa-miRNA-654-3p among the upregulated genes.
Subsequently, survival analysis of the relationship between the
postoperative survival of patients and the expression of these
genes suggested that four genes were closely associated with
improved OS of breast carcinoma patients, namely AKAP12, SOPB,
TCF7L2 and TXNIP.
Tumor progression is induced by genetic mutations
and epigenetic changes. Protein kinase A anchor 12 (AKAP12) is a
scaffold protein that plays a major regulatory role in cell
proliferation, migration, apoptosis and angiogenesis (25). Studies have also shown that AKAP12
is involved in multiple signaling pathways through regulating
protein kinases A and C. AKAP12 is a tumor metastasis inhibitory
factor, which is associated with carcinoma susceptibility and tumor
cell behavior in a variety of tumor types, including breast
carcinoma (26–28). AKAP12 has also been associated with
miRNAs in various types of disease. In liver cirrhosis, the
interaction of miRNA-183 or miRNA-186 can downregulate the
expression of AKAP12 (29). In
addition, Xia et al indicated that the overexpression of
miR-103 can promote cell proliferation and inhibit apoptosis by
downregulating AKAP12 expression in hepatocellular carcinoma cell
lines (30).
SopB (also known as SigD) is an effector of the
Salmonella typhimurium Type III secretion system that acts
on phospholipids in the host cell membrane (31,32).
SopB may induce epithelial-mesenchymal transition (EMT), which is
also associated with malignant disease. SopB plays a central role
in activating Wnt/β-catenin signaling, which can induce cell
transformation and Wnt/β-linked regulatory signaling transduction.
SopB-dependent activation of Akt kinases can lead to the inhibitory
phosphorylation of GSK3β, which further induces cytosolic β-catenin
and Wnt/β-catenin-mediated EMT (33).
The transcription factor 7-like 2 (TCF7L2) gene is
located on the long arm of chromosome 10q25.2 (previously called
TCF-4). TCF7L2 is a part of the Wnt/β-catenin signaling pathway,
which plays an important role in the regulation of cell development
and growth (34,35). In addition, epidemiological studies
have shown that TCF7L2 gene polymorphisms are associated with
increased susceptibility to carcinomas, including of the breast
(35,36). Additionally, TCF7L2 can increase the
expression of genes involved in the proliferation, apoptosis,
invasion and metastasis of tumor cells (37,38).
TCF7L2 synthesis is directly regulated by miR-21 transcription
(39). In cervical carcinoma, the
expression levels of miR-328 and TCF7L2 are negatively correlated.
Furthermore, miR-328 can reduce the expression of TCF7L2 to affect
the treatment of cervical carcinoma (40). Cervical carcinoma metastasis and
progression may also be inhibited by miR-212, through its direct
targeting of TCF7L2 expression (41). In addition, miR-181a-5p may regulate
the Wnt signaling pathway through the direct targeting of TCF7L2,
promoting 3T3-L1 preadipocyte differentiation and adipogenesis
(42).
TXNIP (also known as VDUP-1 or TBP-2) is
proapoptotic, and inhibits growth and metastasis (43). TXNIP has a variety of functions,
including an important regulatory role in the redox equilibrium,
and can increase the production of reactive oxygen species (ROS) to
induce apoptosis through oxidative stress. TXNIP is a major tumor
suppressor gene that is downregulated in a variety of solid tumors,
including breast carcinoma (44–46).
There is a correlation between the expression of TXNIP and the
metastasis and survival prognosis of breast carcinoma (45). TXNIP also plays a critical role in
the treatment of HER-1/HER-2-positive tumors, and is a potential
prognostic indicator in breast carcinoma (47). TXNIP has been associated with a
variety of miRNAs and has been demonstrated as a target of miR-342
(48), miR-135a (49) and miR-20a (50). The miR-373 expression is negatively
correlated with the expression of TXNIP, and activation of the
miR-373-TXNIP signal transduction axis is associated with a poor
outcome in breast carcinoma (51).
This may be mediated through an effect on the invasion and
migration of breast carcinoma cells, which is associated with the
prognosis of breast carcinoma patients (52).
In summary, the present study intended to identify
the differentially expressed genes in breast carcinoma and thus
find the potential biomarkers for predicting disease progression
using comprehensive bioinformatic analyses. In this study, a total
of 254 differentially expressed genes and 130 differentially
expressed miRNAs were screened; AKAP12, SOPB, TCF7L2 and
TXNIP, and several miRNAs, including miR-183-5p, let-7g-5p
and miR-454-3p, may be key breast carcinoma-associated genes. Our
results suggested that data mining and integration is a useful tool
to predict the progression of breast carcinoma, and to identify the
mechanisms of the occurrence and development of tumors. To apply
these gene expression profiles in clinical practice, it is
necessary to improve the reliability and reproducibility of this
model within dependent datasets in the future. Nevertheless, our
research may provide new information for the diagnosis and
treatment of breast carcinoma patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
GMZ and LLS conceived and designed the study. HG was
involved in the conception of the study. GMZ and LLS wrote the
paper. GMZ reviewed and edited the manuscript. GMZ, HG and LLS read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work is appropriately investigated and
resolved.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board of the Department of Shuyang People's Hospital (Shuyang,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Albain KS, André F,
Bergh J, et al: Panel members: Personalizing the treatment of women
with early breast cancer: Highlights of the St Gallen International
Expert Consensus on the Primary Therapy of Early Breast Cancer
2013. Ann Oncol. 24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coates AS, Winer EP, Goldhirsch A, Gelber
RD, Gnant M, Piccart-Gebhart M, Thürlimann B and Senn HJ: Panel
Members: Tailoring therapies - improving the management of early
breast cancer: St Gallen International Expert Consensus on the
Primary Therapy of Early Breast Cancer 2015. Ann Oncol.
26:1533–1546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mohammed ZM, McMillan DC, Edwards J,
Mallon E, Doughty JC, Orange C and Going JJ: The relationship
between lymphovascular invasion and angiogenesis, hormone
receptors, cell proliferation and survival in patients with primary
operable invasive ductal breast cancer. BMC Clin Pathol. 13:312013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mok SC, Bonome T, Vathipadiekal V, Bell A,
Johnson ME, Wong KK, Park DC, Hao K, Yip DK, Donninger H, et al: A
gene signature predictive for outcome in advanced ovarian cancer
identifies a survival factor: Microfibril-associated glycoprotein
2. Cancer Cell. 16:521–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bowen NJ, Walker LD, Matyunina LV, Logani
S, Totten KA, Benigno BB and McDonald JF: Gene expression profiling
supports the hypothesis that human ovarian surface epithelia are
multipotent and capable of serving as ovarian cancer initiating
cells. BMC Med Genomics. 2:712009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elgaaen Vilming B, Olstad OK, Haug KB,
Brusletto B, Sandvik L, Staff AC, Gautvik KM and Davidson B: Global
miRNA expression analysis of serous and clear cell ovarian
carcinomas identifies differentially expressed miRNAs including
miR-200c-3p as a prognostic marker. BMC Cancer. 14:802014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elgaaen BV, Olstad OK, Sandvik L, Odegaard
E, Sauer T, Staff AC and Gautvik KM: ZNF385B and VEGFA are strongly
differentially expressed in serous ovarian carcinomas and correlate
with survival. PLoS One. 7:e463172012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kong W, He L, Richards EJ, Challa S, Xu
CX, Permuth-Wey J, Lancaster JM, Coppola D, Sellers TA, Djeu JY, et
al: Upregulation of miRNA-155 promotes tumour angiogenesis by
targeting VHL and is associated with poor prognosis and
triple-negative breast cancer. Oncogene. 33:679–689. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vennin C, Spruyt N, Dahmani F, Julien S,
Bertucci F, Finetti P, Chassat T, Bourette RP, Le Bourhis X and
Adriaenssens E: H19 non coding RNA-derived miR-675 enhances
tumorigenesis and metastasis of breast cancer cells by
downregulating c-Cbl and Cbl-b. Oncotarget. 6:29209–29223. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ward A, Shukla K, Balwierz A, Soons Z,
König R, Sahin O and Wiemann S: MicroRNA-519a is a novel oncomir
conferring tamoxifen resistance by targeting a network of
tumour-suppressor genes in ER+ breast cancer. J Pathol.
233:368–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rasheed SA, Teo CR, Beillard EJ, Voorhoeve
PM, Zhou W, Ghosh S and Casey PJ: MicroRNA-31 controls G protein
alpha-13 (GNA13) expression and cell invasion in breast cancer
cells. Mol Cancer. 14:672015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Planche A, Bacac M, Provero P, Fusco C,
Delorenzi M, Stehle JC and Stamenkovic I: Identification of
prognostic molecular features in the reactive stroma of human
breast and prostate cancer. PLoS One. 6:e186402011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clarke C, Madden SF, Doolan P, Aherne ST,
Joyce H, O'Driscoll L, Gallagher WM, Hennessy BT, Moriarty M, Crown
J, et al: Correlating transcriptional networks to breast cancer
survival: A large-scale coexpression analysis. Carcinogenesis.
34:2300–2308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wasson MK, Chauhan PS, Singh LC, Katara D,
Sharma Dev J, Zomawia E, Kataki A, Kapur S and Saxena S:
Association of DNA repair and cell cycle gene variations with
breast cancer risk in Northeast Indian population: A multiple
interaction analysis. Tumour Biol. 35:5885–5894. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Romero-Cordoba S, Rodriguez-Cuevas S,
Rebollar-Vega R, Quintanar-Jurado V, Maffuz-Aziz A, Jimenez-Sanchez
G, Bautista-Piña V, Arellano-Llamas R and Hidalgo-Miranda A:
Identification and pathway analysis of microRNAs with no previous
involvement in breast cancer. PLoS One. 7:e319042012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets - update. Nucleic Acids Res. 41:D991–D995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC Bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gyorffy B, Lánczky A and Szállási Z:
Implementing an online tool for genome-wide validation of
survival-associated biomarkers in ovarian-cancer using microarray
data from 1287 patients. Endocr Relat Cancer. 19:197–208. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Radojicic J, Zaravinos A, Vrekoussis T,
Kafousi M, Spandidos DA and Stathopoulos EN: MicroRNA expression
analysis in triple-negative (ER, PR and Her2/neu) breast cancer.
Cell Cycle. 10:507–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi W, Gerster K, Alajez NM, Tsang J,
Waldron L, Pintilie M, Hui AB, Sykes J, P'ng C, Miller N, et al:
MicroRNA-301 mediates proliferation and invasion in human breast
cancer. Cancer Res. 71:2926–2937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hayashi M, Nomoto S, Kanda M, Okamura Y,
Nishikawa Y, Yamada S, Fujii T, Sugimoto H, Takeda S and Kodera Y:
Identification of the A kinase anchor protein 12 (AKAP12) gene as a
candidate tumor suppressor of hepatocellular carcinoma. J Surg
Oncol. 105:381–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Flotho C, Paulun A, Batz C and Niemeyer
CM: AKAP12, a gene with tumour suppressor properties, is a target
of promoter DNA methylation in childhood myeloid malignancies. Br J
Haematol. 138:644–650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su B, Zheng Q, Vaughan MM, Bu Y and Gelman
IH: SSeCKS metastasis-suppressing activity in MatLyLu prostate
cancer cells correlates with vascular endothelial growth factor
inhibition. Cancer Res. 66:5599–5607. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi MC, Jong HS, Kim TY, Song SH, Lee DS,
Lee JW, Kim TY, Kim NK and Bang YJ: AKAP12/Gravin is inactivated by
epigenetic mechanism in human gastric carcinoma and shows growth
suppressor activity. Oncogene. 23:7095–7103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goeppert B, Schmezer P, Dutruel C, Oakes
C, Renner M, Breinig M, Warth A, Vogel MN, Mittelbronn M, Mehrabi
A, et al: Down-regulation of tumor suppressor A kinase anchor
protein 12 in human hepatocarcinogenesis by epigenetic mechanisms.
Hepatology. 52:2023–2033. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia W, Ni J, Zhuang J, Qian L, Wang P and
Wang J: MiR-103 regulates hepatocellular carcinoma growth by
targeting AKAP12. Int J Biochem Cell Biol. 71:1–11. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ruan HH, Li Y, Zhang XX, Liu Q, Ren H,
Zhang KS and Zhao H: Identification of TRAF6 as a ubiquitin ligase
engaged in the ubiquitination of SopB, a virulence effector protein
secreted by Salmonella typhimurium. Biochem Biophys Res
Commun. 447:172–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perrett CA and Zhou D: Erratum:
Salmonella type III effector SopB modulates host cell
exocytosis. Emerg Microbes Infect. 2:e392013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tahoun A, Mahajan S, Paxton E, Malterer G,
Donaldson DS, Wang D, Tan A, Gillespie TL, O'Shea M, Roe AJ, et al:
Salmonella transforms follicle-associated epithelial cells
into M cells to promote intestinal invasion. Cell Host Microbe.
12:645–656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ravindranath A, O'Connell A, Johnston PG
and El-Tanani MK: The role of LEF/TCF factors in neoplastic
transformation. Curr Mol Med. 8:38–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Connor AE, Baumgartner RN, Baumgartner KB,
Kerber RA, Pinkston C, John EM, Torres-Mejia G, Hines L, Giuliano
A, Wolff RK, et al: Associations between TCF7L2 polymorphisms and
risk of breast cancer among Hispanic and non-Hispanic white women:
The Breast Cancer Health Disparities Study. Breast Cancer Res
Treat. 136:593–602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu XP, Hu GN, Du JQ and Li HQ: TCF7L2 gene
polymorphisms and susceptibility to breast cancer: A meta-analysis.
Genet Mol Res. 14:2860–2867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Naidu R, Yip CH and Taib NA: Genetic
variations in transcription factor 7-like 2 (TCF7L2) gene:
Association of TCF7L2 rs12255372(G/T) or rs7903146(C/T) with breast
cancer risk and clinico-pathological parameters. Med Oncol.
29:411–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Min W, Liu X, Lu Y, Gong Z, Wang M, Lin S,
Kang H, Jin T, Wang X, Ma X, et al: Association of transcription
factor 7-like 2 gene polymorphisms with breast cancer risk in
northwest Chinese women. Oncotarget. 7:77175–77182. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lan F, Yue X, Han L, Shi Z, Yang Y, Pu P,
Yao Z and Kang C: Genome-wide identification of TCF7L2/TCF4 target
miRNAs reveals a role for miR-21 in Wnt-driven epithelial cancer.
Int J Oncol. 40:519–526. 2012.PubMed/NCBI
|
|
40
|
Wang X and Xia Y: microRNA-328 inhibits
cervical cancer cell proliferation and tumorigenesis by targeting
TCF7L2. Biochem Biophys Res Commun. 475:169–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou C, Tan DM, Chen L, Xu XY, Sun CC,
Zong LJ, Han S and Zhang YZ: Effect of miR-212 targeting TCF7L2 on
the proliferation and metastasis of cervical cancer. Eur Rev Med
Pharmacol Sci. 21:219–226. 2017.PubMed/NCBI
|
|
42
|
Ouyang D, Xu L, Zhang L, Guo D, Tan X, Yu
X, Qi J, Ye Y, Liu Q, Ma Y, et al: MiR-181a-5p regulates 3T3-L1
cell adipogenesis by targeting Smad7 and Tcf7l2. Acta Biochim
Biophys Sin (Shanghai). 48:1034–1041. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim SY, Suh HW, Chung JW, Yoon SR and Choi
I: Diverse functions of VDUP1 in cell proliferation,
differentiation, and diseases. Cell Mol Immunol. 4:345–351.
2007.PubMed/NCBI
|
|
44
|
Zhou J, Yu Q and Chng WJ: TXNIP (VDUP-1,
TBP-2): A major redox regulator commonly suppressed in cancer by
epigenetic mechanisms. Int J Biochem Cell Biol. 43:1668–1673. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cadenas C, Franckenstein D, Schmidt M,
Gehrmann M, Hermes M, Geppert B, Schormann W, Maccoux LJ, Schug M,
Schumann A, et al: Role of thioredoxin reductase 1 and thioredoxin
interacting protein in prognosis of breast cancer. Breast Cancer
Res. 12:R442010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou J and Chng WJ: Roles of thioredoxin
binding protein (TXNIP) in oxidative stress, apoptosis and cancer.
Mitochondrion. 13:163–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nie W, Huang W, Zhang W, Xu J, Song W,
Wang Y, Zhu A, Luo J, Huang G, Wang Y, et al: TXNIP interaction
with the Her-1/2 pathway contributes to overall survival in breast
cancer. Oncotarget. 6:3003–3012. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cittelly DM, Das PM, Spoelstra NS,
Edgerton SM, Richer JK, Thor AD and Jones FE: Downregulation of
miR-342 is associated with tamoxifen resistant breast tumors. Mol
Cancer. 9:3172010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhu HJ, Wang DG, Yan J and Xu J:
Up-regulation of microRNA-135a protects against myocardial
ischemia/reperfusion injury by decreasing TXNIP expression in
diabetic mice. Am J Transl Res. 7:2661–2671. 2015.PubMed/NCBI
|
|
50
|
Li XF, Shen WW, Sun YY, Li WX, Sun ZH, Liu
YH, Zhang L, Huang C, Meng XM and Li J: MicroRNA-20a negatively
regulates expression of NLRP3-inflammasome by targeting TXNIP in
adjuvant-induced arthritis fibroblast-like synoviocytes. Joint Bone
Spine. 83:695–700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen D, Dang BL, Huang JZ, Chen M, Wu D,
Xu ML, Li R and Yan GR: MiR-373 drives the
epithelial-to-mesenchymal transition and metastasis via the
miR-373-TXNIP-HIF1α-TWIST signaling axis in breast cancer.
Oncotarget. 6:32701–32712. 2015.PubMed/NCBI
|
|
52
|
Wang JG, Zhang LK, Chen YB, Zhang T, Yuan
PF and Liu DC: Influence of miR-373 on the invasion and migration
of breast cancer and the expression level of target genes TXNIP. J
Biol Regul Homeost Agents. 29:367–372. 2015.PubMed/NCBI
|