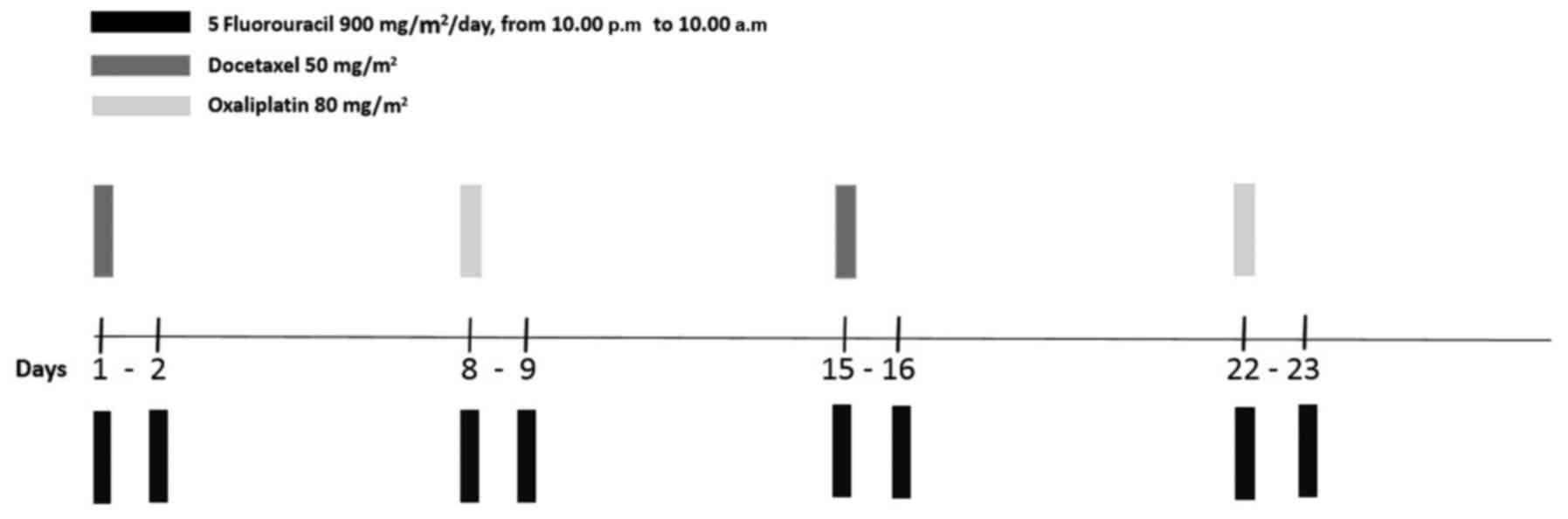
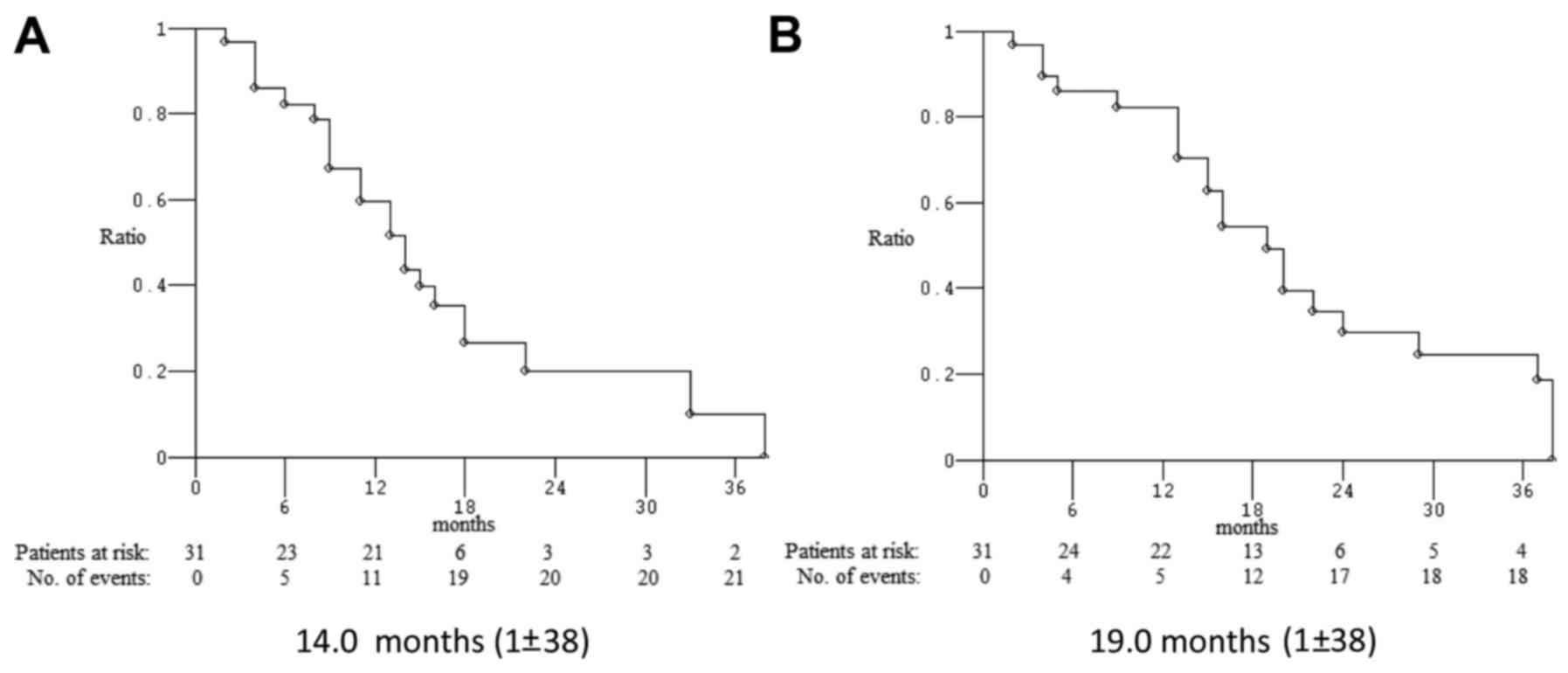
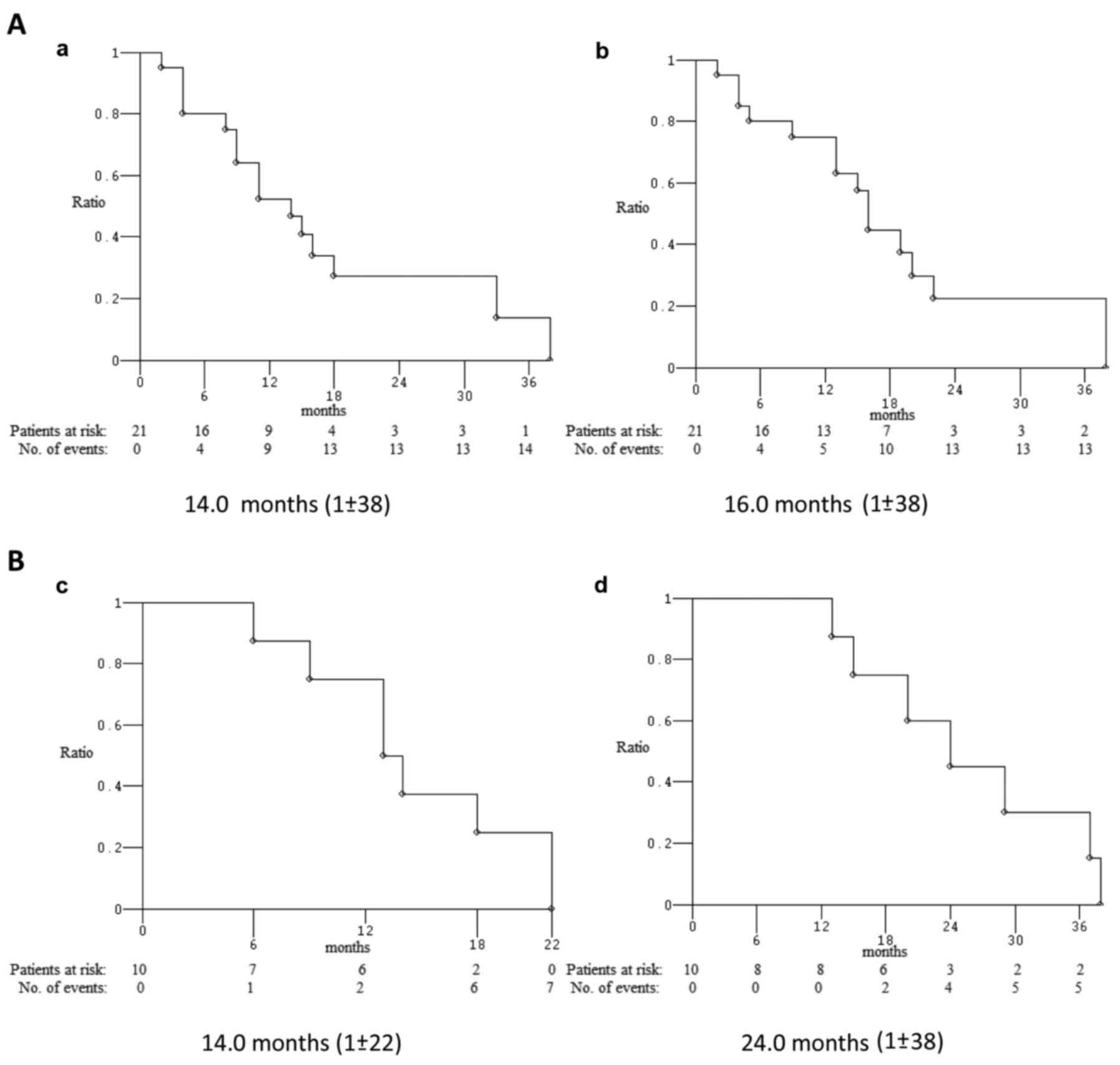
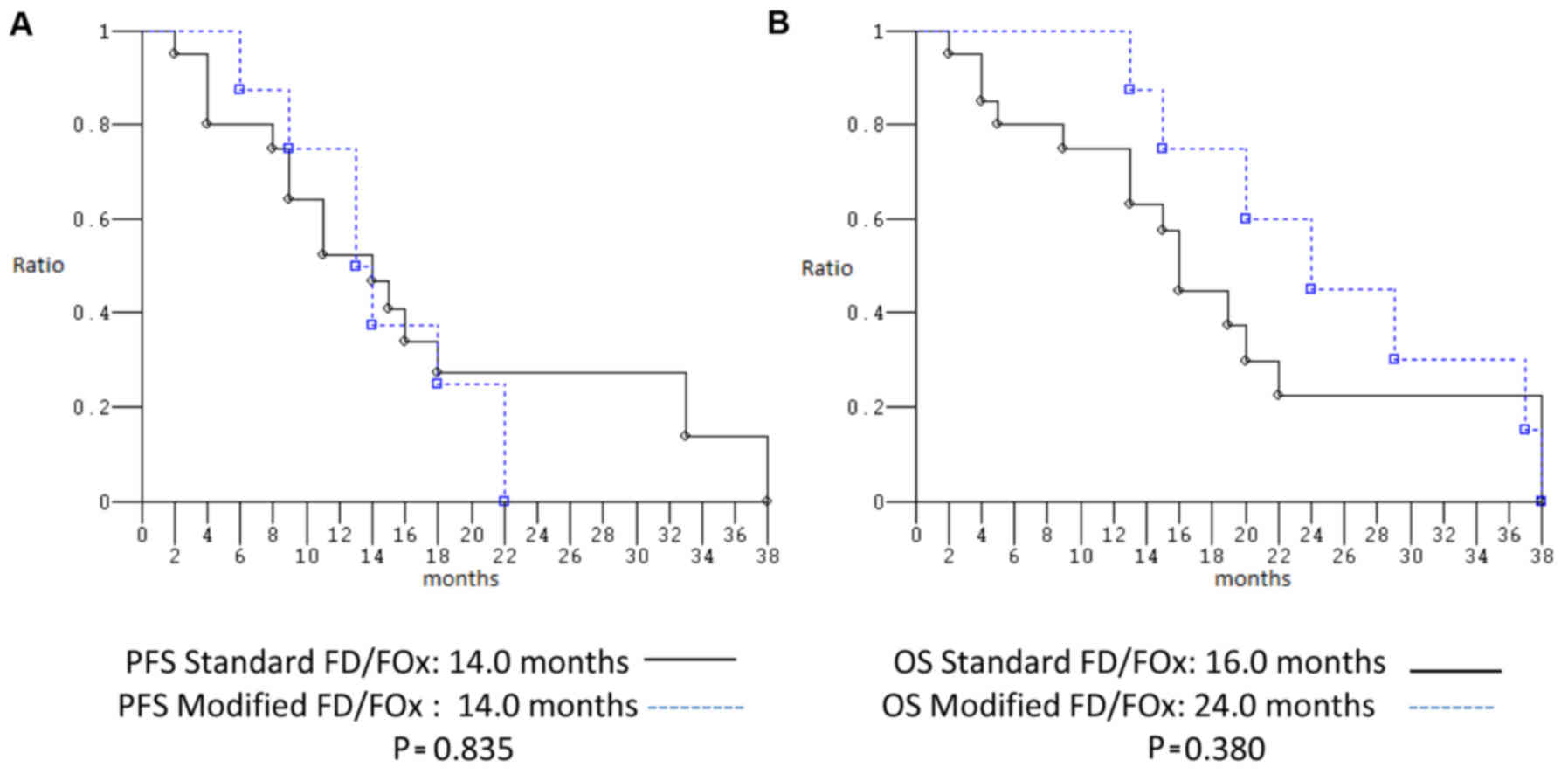
|
1
|
Murad AM, Santiago FF, Petroianu A, Rocha
PR, Rodrigues MA and Rausch M: Modified therapy with
5-fluorouracil, doxorubicin, and methotrexate in advanced gastric
cancer. Cancer. 72:37–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pyrhönen S, Kuitunen T, Nyandoto P and
Kouri M: Randomised comparison of fluorouracil, epidoxorubicin and
methotrexate (FEMTX) plus supportive care with supportive care
alone in patients with non-resectable gastric cancer. Br J Cancer.
71:587–591. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levi JA, Fox RM, Tattersall MH, Woods RL,
Thomson D and Gill G: Analysis of a prospectively randomized
comparison of doxorubicin versus 5-fluorouracil, doxorubicin, and
BCNU in advanced gastric cancer: Implications for future studies. J
Clin Oncol. 4:1348–1355. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemoterapy versus
chemoterapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3, open
label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ajani JA, D'Amico TA, Baggstrom M, Bentrem
DJ, Chao J, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, et
al: Clinical practice guidelines in oncology, gastric cancer.
National Comprehensive Cancer Network. version 4. 2017.
|
|
7
|
Smyth EC, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D; ESMO Guidelines Committee: Gastric
cancer: ESMO clinical practice guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 27 (Suppl 5):v38–v49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohammad NH, Ter Veer E, Ngai L, Mali R,
van Oijen MG and van Laarhoven HW: Optimal first-line
chemotherapeutic treatment in patients with locally advanced or
metastatic esophagogastric carcinoma: Triplet versus doublet
chemotherapy: A systematic literature review and meta-analysis.
Cancer Metastasis Rev. 34:429–441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carmona-Bayonas A, Jiménez-Fonseca P,
Custodio A, Sánchez Cánovas M, Hernández R, Pericay C, Echavarria
I, Lacalle A, Visa L, Rodríguez Palomo A, et al:
Anthracycline-based triplets do not improve the efficacy of
platinum-fluoropyrimidine doublets in first-line treatment of
advanced gastric cancer: Real-world data from the AGAMEMON National
cancer Registry. Gastric Cancer. 21:96–105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ter Veer E, Haj Mohammad N, van Valkenhoef
G, Ngai LL, Mali RMA, Anderegg MC, van Oijen MGH and van Laarhoven
HWM: The efficacy and safety of first-line chemotherapy in advanced
esophagogastric cancer: A Network Meta-analysis. J Natl Cancer
Inst. 1082016.
|
|
11
|
Shah MA, Janjigian YY, Stoller R, Shibata
S, Kemeny M, Krishnamurthi S, Su YB, Ocean A, Capanu M, Mehrotra B,
et al: Randomized multicenter phase II study of modified docetaxel,
cisplatin, and fluorouracil (DCF) versus DCF plus growth factor
support in patients with metastatic gastric adenocarcinoma: A study
of the US gastric cancer consortium. J Clin Oncol. 33:3874–3879.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Cutsem E, Boni C, Tabernero J, Massuti
B, Middleton G, Dane F, Reichardt P, Pimentel FL, Cohn A, Follana
P, et al: Docetaxel plus oxaliplatin with or without fluorouracil
or capecitabine in metastatic or locally recurrent gastric cancer:
A randomized phase II study. Ann Oncol. 26:149–156. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Batran SE, Hofheinz RD, Pauligk C, Kopp
HG, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg
H, et al: Histopathological regression after neoadjuvant docetaxel,
oxaliplatin, fluorouracil, and leucovorin versus epirubicin,
cisplatin, and fluorouracil or capecitabine in patients with
resectable gastric or gastro-oesophageal junction adenocarcinoma
(FLOT4-AIO): Results from the phase 2 part of a multicentre,
open-label, randomised phase 2/3 trial. Lancet Oncol. 17:1697–1708.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ficorella C, Ricevuto E, Morelli MF,
Morese R, Cannita K, Cianci G, Porzio G, Di Rocco ZC, De Galitiis
F, De Tursi M, et al: Increased tolerability of bimonthly 12-hour
timed flat infusion 5-fluorouracil/irinotecan regimen in advanced
colorectal cancer: A dose-finding study. Oncol Rep. 15:1345–1350.
2006.PubMed/NCBI
|
|
15
|
Ficorella C, Bruera G, Cannita K, Porzio
G, Baldi PL, Tinari N, Natoli C and Ricevuto E: Triplet
chemotherapy in patients with metastatic colorectal cancer: Toward
the best way to safely administer a highly active regimen in
clinical practice. Clin Colorectal Cancer. 11:229–237. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Köhne CH1, Wils J, Lorenz M, Schöffski P,
Voigtmann R, Bokemeyer C, Lutz M, Kleeberg C, Ridwelski K, Souchon
R, et al: Randomized phase III study of high-dose fluorouracil
given as a weekly 24-hour infusion with or without leucovorin
versus bolus fluorouracil plus leucovorin in advanced colorectal
cancer: European organization of Research and Treatment of Cancer
Gastrointestinal Group Study 40952. J Clin Oncol. 21:3721–3728.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leichman CG, Fleming TR, Muggia FM, Tangen
CM, Ardalan B, Doroshow JH, Meyers FJ, Holcombe RF, Weiss GR,
Mangalik A, et al: A phase II study of fluorouracil and its
modulation in advanced colorectal cancer: A Southwest oncology
group study. J Clin Oncol. 13:1303–1311. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O'Dwyer PJ, Manola J, Valone FH, Ryan LM,
Hines JD, Wadler S, Haller DG, Arbuck SG, Weiner LM, Mayer RJ and
Benson AB III: Fluorouracil modulation in colorectal cancer: Lack
of improvement with N-phosphonoacetyl-l-aspartic acid or oral
leucovorin or interferon, but enhanced therapeutic index with
weekly 24-h infusion schedule-An eastern cooperative oncology
group/cancer and leukemia Group B study. J Clin Oncol.
19:2413–2421. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lévi F: Chronopharmacology of anticancer
agents. Redfern PH and Lemmer B: Handbook of Exeperimental
pharmacology: Physiology and pharmacology of biological
Rhythms-cancer chemotherapy. Springer-Verlag; Berlin: pp. 299–301.
1997, View Article : Google Scholar
|
|
20
|
Harris BE, Song R, Soong SJ and Diasio RB:
Relationship between dihydropyrimidine dehidrogenase activity and
plasma 5-fluorouracil levels with evidence for circadian varation
of enzyme activity and plasma drug levels in cancer patients
receiving 5-fluorouracil by protracted continuous infusion. Cancer
Res. 50:197–201. 1990.PubMed/NCBI
|
|
21
|
Smaaland R, Abrahamsen JF, Svardal AM,
Lote K and Ueland PM: DNA cell cycle distrubution and glutathione
(GSH) content according to circadian stage in bone marrow of cancer
patients. Br J Cancer. 66:39–45. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morelli MF, Santomaggio A, Ricevuto E,
Cannita K, De Galitiis F, Tudini M, Bruera G, Mancini M,
Pelliccione M, Calista F, et al: Triplet schedule of weekly
5-Fluorouracil and alternating irinotecan or oxaliplatin in
advanced colorectal cancer: A dose-finding and phase II study.
Oncol Rep. 23:1635–1640. 2010.PubMed/NCBI
|
|
23
|
Bruera G, Santomaggio A, Cannita K, Baldi
PL, Tudini M, De Galitiis F, Mancini M, Marchetti P, Antonucci A,
Ficorella C and Ricevuto E: ‘Poker’ association of weekly
alternating 5-fluorouracil, irinotecan, bevacizumab and oxaliplatin
(FIr-B/FOx) in first line treatment of metastatic colorectal
cancer: A phase II study. BMC Cancer. 10:5672010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Extermann M, Overcash J, Lyman GH, Parr J
and Balducci L: Comorbidity and functional status are independent
in older cancer patients. J Clin Oncol. 16:1582–1587. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors: European
organization for research and treatment of cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaplan EL and Meier P: Nonparametric
estimation of incomplete observations. J Am Stat Assoc. 53:457–481.
1958. View Article : Google Scholar
|
|
28
|
Fisher RA: On the interpretation of χ2
from contingency tables, and the calculation of P. J R Stat Soc.
85:87–94. 1922. View
Article : Google Scholar
|
|
29
|
Peto R and Peto J: Asymptomatically
efficient rank invariant test procedures. J R Stat Soc A.
135:185–207. 1972. View
Article : Google Scholar
|
|
30
|
Petrelli F, Tomasello G, Ghidini M,
Passalacqua R and Barni S: Modified schedules of DCF chemotherapy
for advanced gastric cancer: A systematic review of efficacy and
toxicity. Anticancer Drugs. 28:133–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry
DR, et al: Ramucirumab monotherapy for previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chau I, Ashley S and Cunningham D:
Validation of the Royal Marsden hospital prognostic index in
advanced esophagogastric cancer using individual patient data from
the REAL 2 study. J Clin Oncol. 27:e3–e4. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fuchs CS, Muro K, Tomasek J, Van Cutsem E,
Cho JY, Oh SC, Safran H, Bodoky G, Chau I, Shimada Y, et al:
Prognostic factor analysis of overall survival in gastric cancer
from two phase III studies of Second-line Ramucirumab (REGARD and
RAINBOW) using pooled patient data. J Gastric Cancer. 17:132–144.
2017. View Article : Google Scholar : PubMed/NCBI
|