Introduction
The majority of patients with gastric cancer (GC)
already presents with advanced disease at the time of diagnosis or
develops metastases after treatment with curative intent. In these
patients, chemotherapy can provide palliation of symptoms, improved
survival and quality of life, compared to best supportive care
(BSC) (1,2).
Combination therapies have demonstrated
substantially higher response rates and survival compared to
monotherapy (3,4). Although the optimal regimen for
first-line chemotherapy of locally advanced/metastatic (a/m) GC
patients is well defined in tumors which overexpress human
epidermal growth factor receptor-2 (HER2) (5), this is not the case for HER2-negative
tumors, which are ~80% of the cases. In these patients the question
of whether a three-drug regimen is more effective than a
potentially less toxic doublet remains controversial.
According to the National Comprehensive Cancer
Network (NCCN) guidelines of 2016 two-drug regimens are preferred
and three-drug cytotoxic regimes should be reserved for medically
fit patients with good performance status (PS) and access to
frequent evaluation (6). The
European Society for Medical Oncology (ESMO) guidelines of 2016
state that ‘combination regimens incorporating a platinum agent and
a fluropyrimidine are generally used and it remains controversial
whether a triplet regimen is needed’ (7).
A recent systematic literature review and
meta-analysis has revealed a significant improvement in overall
survival (OS), progression-free survival (PFS) and objective
response rate (ORR) in favor of a triplet over a doublet
chemotherapy, especially when containing fluoropyrimidines,
cisplatin and taxane, at the expense of major incidence of grade
3–4 thrombocytopenia (6.2 vs. 3.7%), infections (10.2 vs. 6.4%) and
mucositis (9.7 vs. 4.7%) (8). A
real-world data analysis and a network meta-analysis have recently
revealed that anthracycline-based triplets do not improve the
efficacy of platinum-fluoropyrimidine doublets in terms of ORR,
median OS and median PFS as first-line treatment, and present with
greater toxicity and a major impact on the quality of life (QoL)
(9,10).
The combination of docetaxel, cisplatin and 5FU
(DCF) has been evaluated in randomized clinical trials. In the V325
trial, a randomized multinational phase III study, 445 untreated
a/m GC patients were randomized to receive either DCF every 3 weeks
or cisplatin and 5-FU (CF). At a median follow-up of 13.6 months,
median time to progression (5.6 vs. 3.7 months), median OS (9.2 vs.
8.6 months) and ORR (37 vs. 25%) were significantly higher with DCF
than CF, with a two-year survival rate of 18 and 9%, respectively.
The modified DCF (mDCF) regimen has been demonstrated to have at
least equal efficacy and lower toxicity compared to standard DCF
chemotherapy in a phase II trial (11). In addition, a recent phase II trial
(GATE trial) has evaluated the efficacy and tolerability of
docetaxel plus oxaliplatin with or without infusional 5-FU (TEF and
TE, respectively) or capecitabine (TEX) in a/m GC patients
(12). The addition of docetaxel to
oxaliplatin and 5-FU proved to have a better safety profile and was
also associated with higher ORR, longer median PFS and median OS
(47%, 7.7 and 14.6 months, respectively) compared to docetaxel,
oxaliplatin and capecitabine (26%, 5.6 and 11.3 months,
respectively). The frequency of grade 3 or 4 adverse events was
lower among patients treated with TEF (25%) compared to those
treated with TE (37%) or TEX (38%).
In addition, a triplet regimen with docetaxel
demonstrated efficacy in the neoadjuvant therapy setting in locally
advanced resectable esophago-gastric cancer, significantly
increasing the proportion of patients achieving pathological
complete regression compared with ECF/ECX in the multicenter,
open-label, randomised phase 2/3 trial FLOT4-AIO (13).
In order to increase the tolerability of 5-FU in
combination with irinotecan in metastatic colorectal cancer
patients, we have previously developed an alternative way of
administration of 5-FU, called timed-flat infusion (TFI), which
consisted in a 12-h nocturnal flat infusion (from 10:00 p.m. to
10:00 a.m.), without 5-FU bolus and folinic acid (14). To date no experimental evidence has
supported that the modulation of folinic acid enhanced the
antitumor activity of infusional 5-FU at its maximum tolerated dose
(15–17). TFI/5-FU, exploits the increased
activity in mononuclear cells of dihydropyrimidine dehydrogenase,
the enzyme involved in 5-FU intracellular catabolism, and the
reduced proliferation of the healthy tissue most damaged by 5-FU
(the bone marrow and oral/rectal mucosa) during the evening hours
(18–21). Subsequently, we developed a triplet
schedule, called FIr/FOx, containing irinotecan and oxaliplatin
administered every other week, associated with TFI/5-FU two nights
a week (22), and the FIrB/FOx
schedule, by adding bevacizumab to this triplet regimen (23), with manageable toxicity and high
received dose intensities (rDI).
On the basis of these previous findings, we designed
the FD/FOx regimen, a triplet schedule containing weekly alternated
docetaxel and oxaliplatin, associated with TFI/5-FU two nights a
week. In the present study we reported a single institution
retrospective analysis of a/m GC patients treated with first line
FD/FOx, in order to assess whether this alternative way of
administration of 5-FU, can allow to treat more patients with an
intensive regimen in common clinical practice.
Materials and methods
Patient eligibility
This retrospective analysis evaluated consecutive
a/m GC patients treated with FD/FOx regimen, from June 2007 to July
2017. Patients were eligible if they had histologically confirmed
diagnosis of GC (gastric or esophago-gastric adenocarcinoma); aged,
18–80 years; Eastern Cooperative Oncology Group Performance Status
(ECOG-PS) ≤2; adequate hematological, renal and hepatic functions.
The treatments were tailored according to patient fitness level
which was prevalently defined according to age [non-elderly (<65
years), young-elderly (≥65, <75 years) and old-elderly (≥75
years)], ECOG-PS and comorbidities. Comorbidities were evaluated by
the Cumulative Index Rating Scale (CIRS) (24) as follows: Primary CIRS stage
consisted of independent instrumental activity of daily living
(IADL) and absent or mild-grade comorbidities; intermediate CIRS
stage consisted of dependent or independent IADL and less than
three mild- or moderate-grade comorbidities; secondary CIRS stage,
consisting of more than three comorbidities or a severe
comorbidity, with or without dependent IADL. Patients with
performance status (PS) 3 were not eligible. All patients provided
written, informed consent to treatment. The procedures followed
were in accordance with the ethical standards of the local
responsible committees on human experimentation, namely the
Comitato Etico per le province di L'Aquila e Teramo.
Schedules of therapeutic regimens
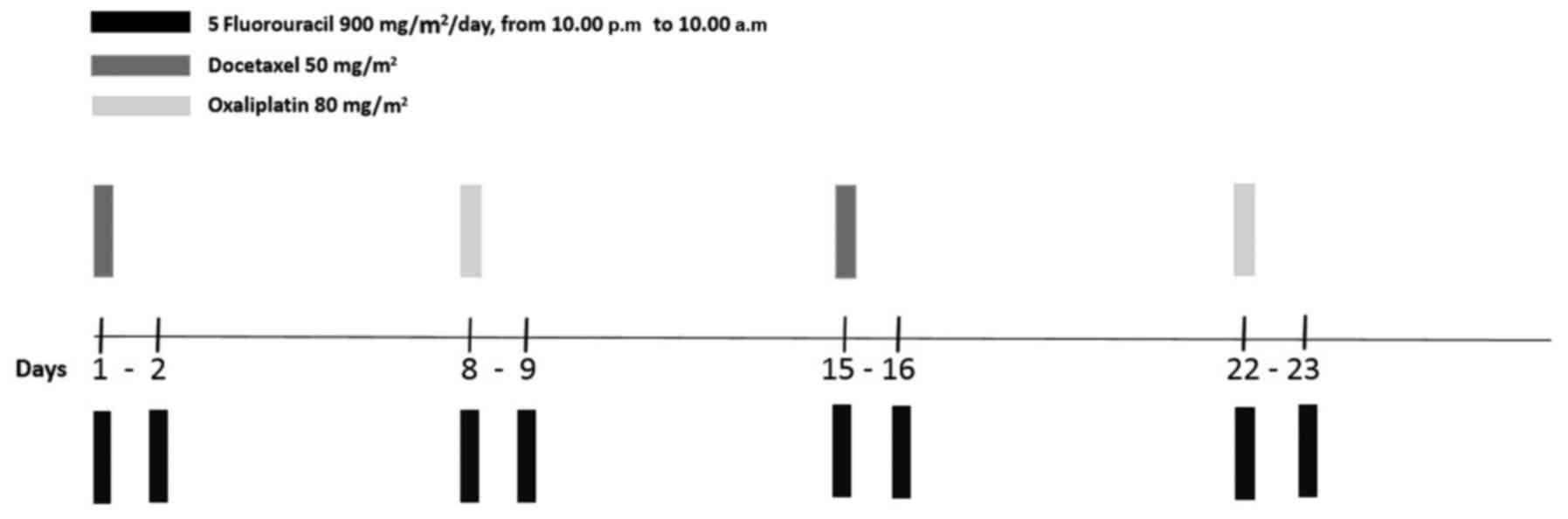
Standard (st) FD/FOx regimen (Fig. 1) is a schedule of weekly 12-h (from
10.00 p.m. to 10.00 a.m.) TFI/5-FU administered for two consecutive
nights at 900 mg/m2/day, associated to alternating
docetaxel 50 mg/m2 on days 1 and 15, and oxaliplatin 80
mg/m2 on days 8 and 22, with cycles repeated every four
weeks. 5-FU was administered by a portable pump (CADD Plus, SEVIT)
using a central venous access device (port-a-cath or peripherally
inserted central catheter). Modified (mod) FD/FOx was defined by
any projected dose reduction compared to the standard one, due to
age, PS and/or comorbidities. Dose level reductions were not
standardized; as a general rule, when a modulation was required,
doses of each drug were reduced within the following ranges: 5-FU
800–750 mg/m2/day, docetaxel 45–40 mg/m2 and
oxaliplatin 70 mg/m2. Specifically in patients with a
greater risk of gastrointestinal toxicity (peritoneal
carcinomatosis, irregular bowel motion, recent surgeries), priority
was given to the dose reduction of 5-FU. In patients with a greater
risk of malnutrition and leuco/neutropenia (un-resected primary
tumor, hypoalbuminemia, risk of infections), priority was given to
the dose reduction of docetaxel. In patients with a greater risk of
neurotoxicity and nephrotoxicity (diabetes, mild impaired renal
function), priority was given to the dose reduction of
oxaliplatin.
HER2 assessment
HER2 analysis was performed with
immunohistochemistry on paraffin embedded tissue from the primary
tumor and/or metastatic site (HercepTest; Dako Denmark A/S; Agilent
Technologies GmbH, Waldbronn, Germany).
Study design and statistical
analysis
A retrospective analysis of consecutive a/m GC
patients, treated with first line FD/FOx, was conducted to evaluate
the safety, activity and efficacy of the regimen in clinical
practice. This is a ‘regimen-oriented’ collection, in order to
assess the feasibility of FD/FOx in several stages of the disease.
Positive peritoneal cytology was considered metastatic disease
(25), therefore four patients,
treated with st FD/FOx, with peritoneal cytology-limited disease
were included in the efficacy and safety analysis, but not in the
activity one. One patient with locally advanced disease, treated
with st FD/FOx, was included in the activity and safety analysis,
but not in the efficacy one. Subgroup analysis was performed among
patients treated with standard and modified regimens. The clinical
evaluation of patient response was performed by computed tomography
scan. Positron-emission tomography was added based on the
investigators assessment. Follow-up was scheduled every three
months up to progression or death. The clinical criteria of
activity and efficacy were ORR, DCR, PFS and OS. ORR and DCR were
evaluated according to the RECIST criteria (26). Median PFS and median OS were
evaluated using the Kaplan-Meier method (27). ORR was defined as the rate of
patients experiencing an objective response [complete response
(CR), or partial response (PR)] as the best response. DCR was
defined as the rate of patients who experienced an objective
response or demonstrated stable disease (SD) as the best response
to treatment. PFS was defined as the length of time from the
beginning of the treatment until the disease progression or death
(from any cause) or until the last contact. OS was defined as the
length of time between the beginning of treatment until death or
upto the last contact. In the subgroup analysis Fisher's exact test
(28) was used to compare ORR and
log-rank test (29) was used to
compare median PFS and OS, according to first line regimens.
Toxicity was registered according to the National Cancer Institute
Common Toxicity Criteria (version 4.0; http://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf).
Median received dose intensities (rDI) were calculated ‘per cycle’
as mg/m2/week; percentage values were referred to
standard regimens for each agent. The data cut-off period was on
July 2017.
Results
Patient features
From June 2007 to July 2017, 32 consecutive a/m GC
patients, were treated with first line FD/FOx regimen: 22 patients
were treated with standard regimen and 10 patients were treated
with modified regimens due to age, ECOG-PS and/or comorbidities.
The clinical features of all 32 patients are presented in Table I: Male/Female ratio, 23/9; median
age, 60 years (range, 41–80); PS 0, 10 (31.2%), PS 1, 18 (56.3%),
PS 2, 4 (12.5%). The metastatic sites were as follows: Liver 11
(34.4%), peritoneum/ascites 21 (65.6%), lymph nodes 17 (53.1%),
others 5 (15.6%); 4 (12.5%) patients had peritoneal
cytology-limited disease. Two (6.3%) patients had primary tumor of
the gastroesophageal junction and 30 (93.8%) of the stomach.
Fourteen (43.7%) patients had unresected primary tumor.
 | Table I.Patient features. |
Table I.
Patient features.
|
| Total N (%) | Standard schedule N
(%) | Modified schedule N
(%) |
|---|
| Patients (N) | 32 | 22 | 10 |
| Age (years) |
| Range | 41–80 | 43–74 | 41–80 |
| Mean | 59.6 | 58.4 | 62.4 |
|
Median | 60 | 58.5 | 66.5 |
| Sex |
|
Male | 23 (71.9) | 16 (72.7) | 7 (70) |
|
Female | 9 (28.1) | 6 (27.3) | 3 (30) |
| Age |
| Non
elderly | 21 (65.6) | 16 (72.7) | 5 (50) |
| Young
elderly | 9 (28.1) | 6 (27.3) | 3 (30) |
| Old
elderly | 2 (6.3) | – | 2 (20) |
| ECOG-PS |
| 0 | 10 (31.2) | 9 (40.9) | 1 (10) |
| 1 | 18 (56.3) | 11 (50) | 7 (70) |
| 2 | 4 (12.5) | 2 (9.1) | 2 (20) |
| CIRS
(comorbidity) |
|
Primary | 11 (34.4) | 10 (45.4) | 1 (10) |
|
Intermediate | 20 (62.5) | 12 (54.6) | 8 (80) |
|
Secondary | 1 (3.1) | – | 1 (10) |
| Hercep test |
|
Positive | 2 (6.3) | 2 (9.1) | – |
|
Negative | 23 (71.9) | 15 (68.2) | 8 (80) |
|
N.V. | 7 (21.9) | 5 (22.7) | 2 (20) |
| Metastatic
disease |
|
Metachronous | 7 (21.9) | 7 (31.8) | – |
|
Synchronous | 25 (78.1) | 15 (68.2) | 10 (100) |
|
Previous ADJ/neoADJ | 2 (6.3) | 2 (9.1) | – |
| Primary tumor |
|
Gastroesophageal junction | 2 (6.3) | 2 (9.1) | – |
|
Stomach | 30 (93.8) | 20 (90.9) | 10 (100) |
| Locally
advanced disease | 1 (3.1) | 1 (4.5) | – |
| Sites of
metastasis |
|
Liver | 11 (34.4) | 6 (27.3) | 5 (50) |
|
Peritoneum, ascites | 21 (65.6) | 17 (77.3) | 4 (40) |
| Lymph
nodes | 17 (53.1) | 11 (50) | 6 (60) |
|
Others | 5 (15.6) | 3 (13.6) | 2 (20) |
|
Peritoneal cytology only | 4 (12.5) | 4 (18.2) | – |
| Unresected primary
tumor | 14 (43.7) | 10 (45.4) | 4 (40) |
Activity and efficacy
Among 32 patients who underwent first line FD/Fox
treatment, 8 were not evaluable concerning activity: Two patients
did not receive at least two cycles of treatment, so they had not
yet been evaluated for the disease; one died during the second
cycle (not treatment nor disease-related death); one was lost to
follow-up and four patients had peritoneal cytology-limited
disease. All the activity and efficacy details are listed in
Table II. In the ITT analysis, ORR
was 75% and DCR was 87.5%. One patient, in the st FD/FOx subgroup,
was not evaluable for efficacy, since he had undergone a
neo-adjuvant treatment for locally advanced disease. After a median
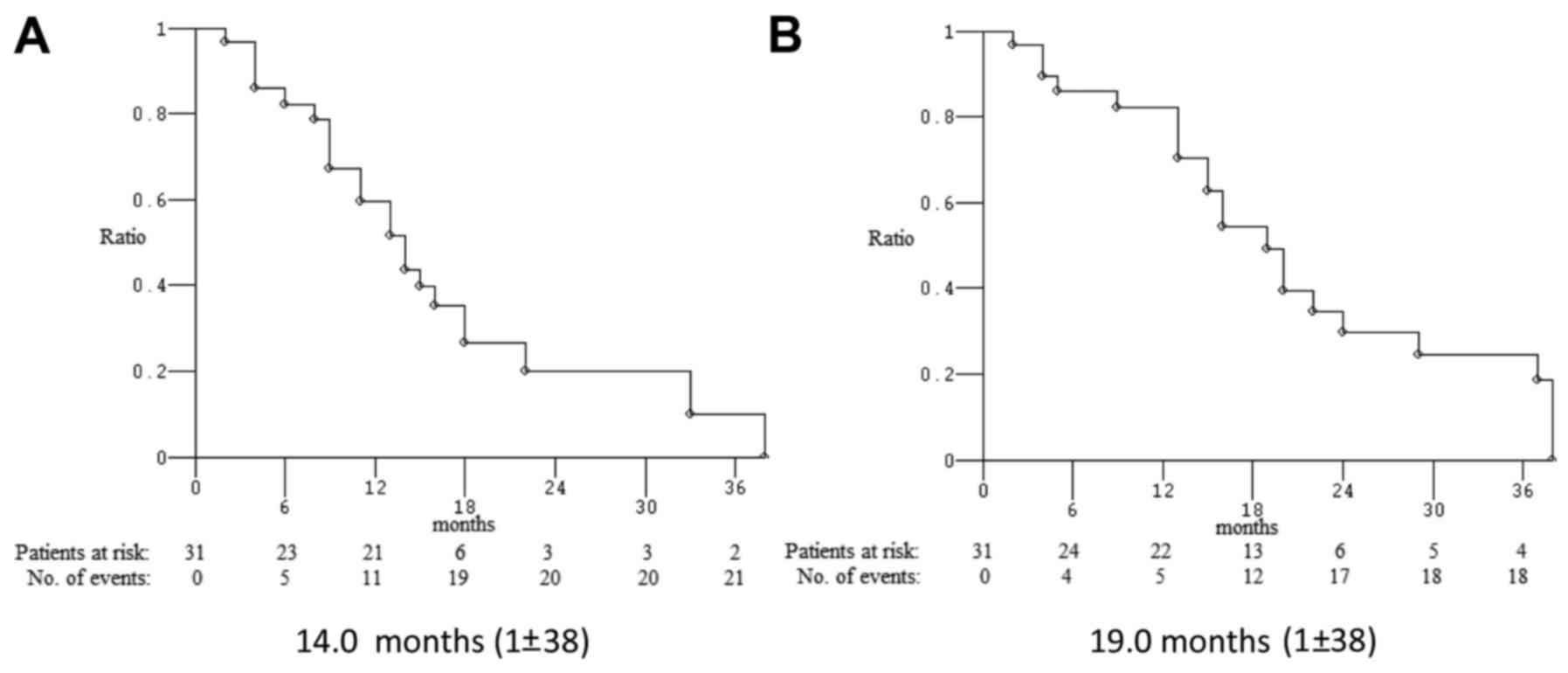
follow-up of 16 months, median PFS was 14.0 months and median OS
was 19.0 months (Fig. 2). Among 22
patients who underwent first line st FD/FOx regimen, 6 were not
evaluable for activity. ORR and DCR were 75 and 87.5%,
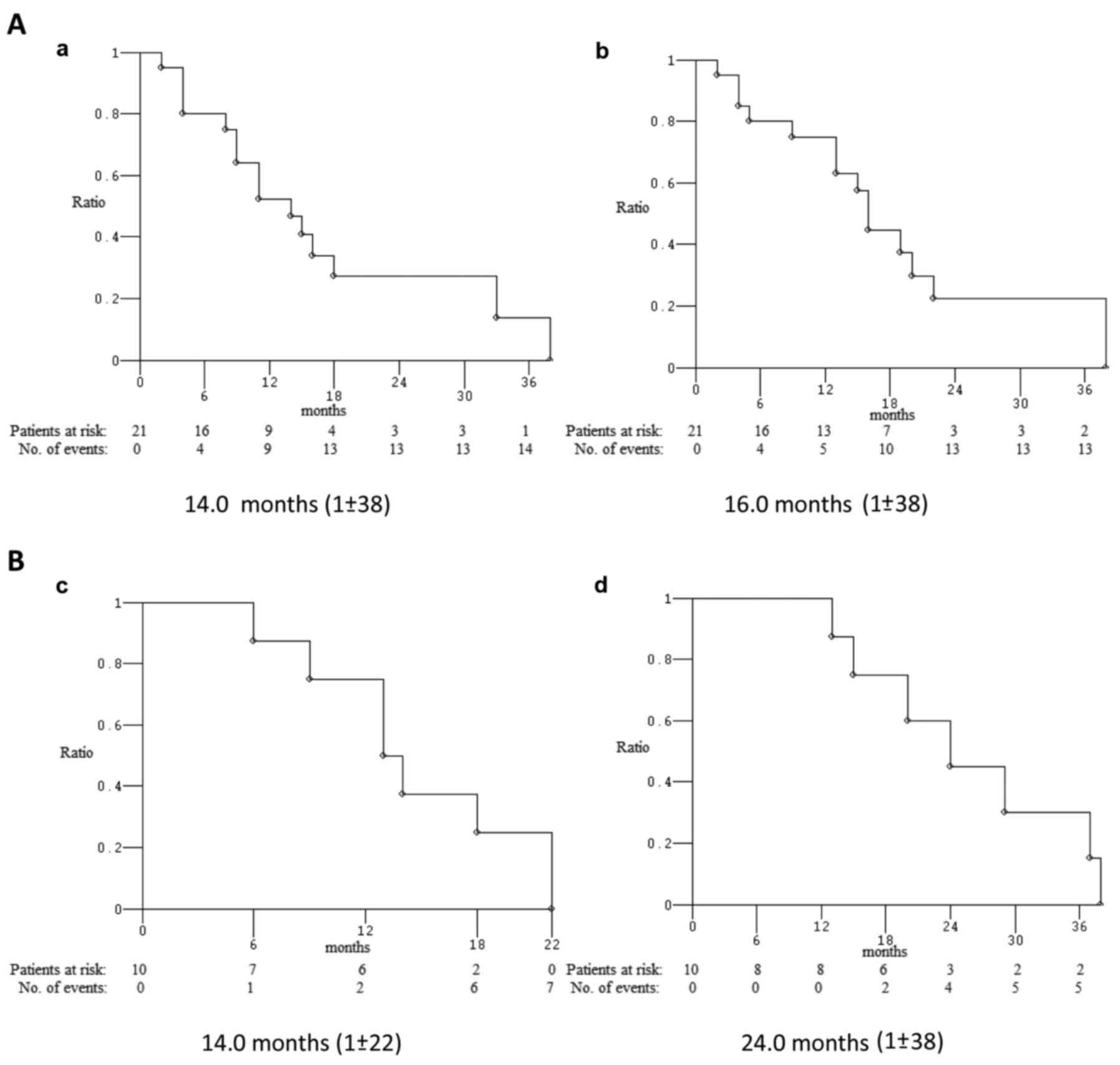
respectively; median PFS and median OS were 14 and 16.0 months,
respectively (Fig. 3). Among 10
patients who underwent first line mod FD/FOx regimens, 2 were not
evaluable for activity; ORR and DCR were 75% and DCR 87.5%; median
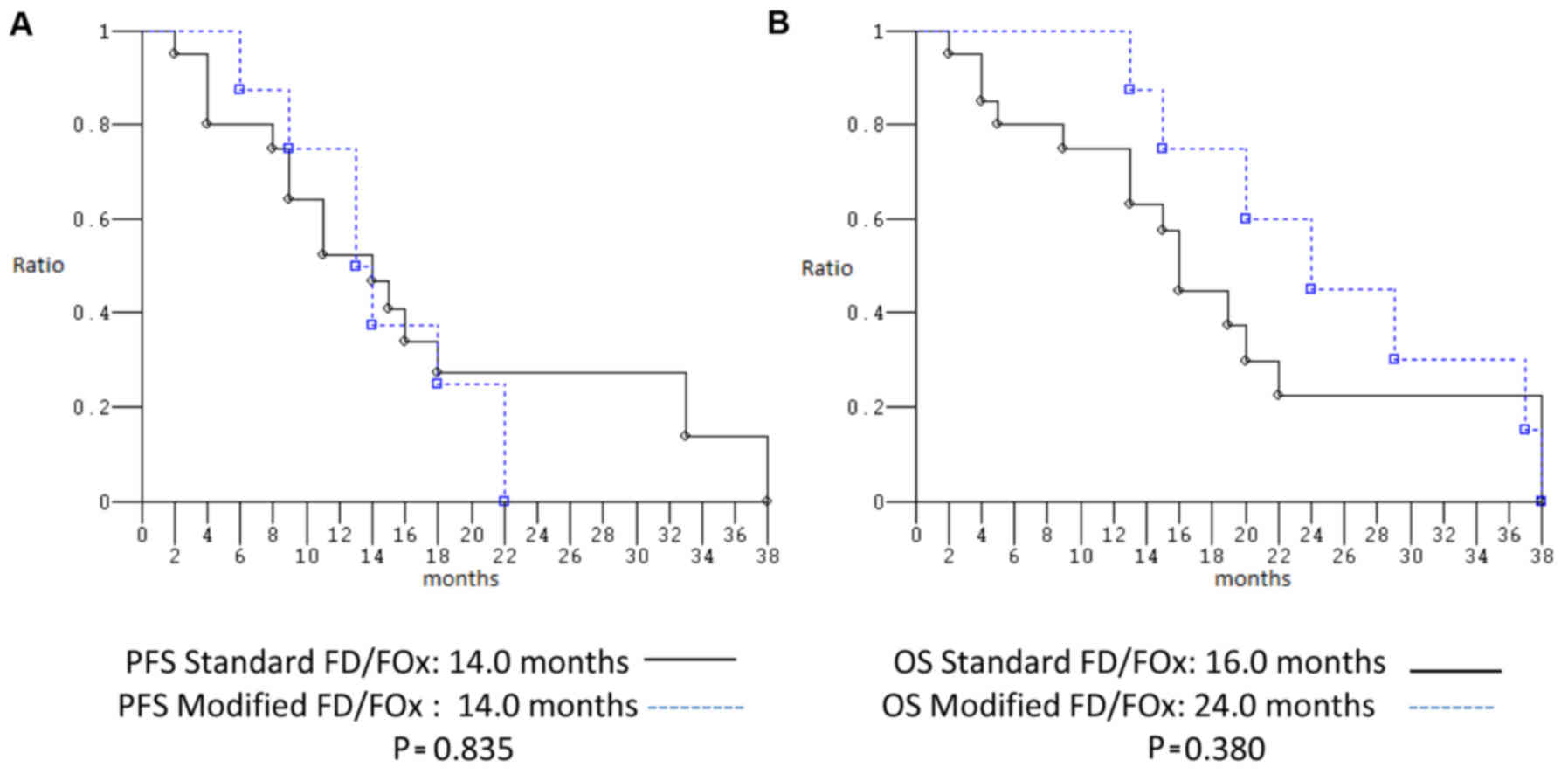
PFS and median OS were 14.0 and 24.0 months, respectively (Fig. 3). ORR, median PFS and median OS of
patients treated with st FD/FOx compared to those treated with
modified schedules were not significantly different: P=1.000,
P=0.835, and P=0.380, respectively (Fig. 4).
 | Table II.Activity and efficacy of overall
treated patients according to standard and modified regimens. |
Table II.
Activity and efficacy of overall
treated patients according to standard and modified regimens.
|
| Overall | St FD/FOx | Mod FD/FOx |
|---|
|
|
|
|
|
|---|
|
| N | % | N | % | N | % |
|---|
| Enrolled
patients | 32 | 100 | 22 | 100 | 10 | 100 |
| Evaluable
patients | 24 | 75 | 16 |
68.2 | 8 | 90 |
| Objective response
rate | 75 (95% CI,
53–90)a | 75 (95% CI,
48–93)a | 75 (95% CI,
35–97)a |
| Partial
response | 17 |
70.8 | 12 | 75 | 5 |
62.5 |
|
Complete response | 1 |
4.2 | – | – | 1 |
12.5 |
| Disease control
rate | 87.5 (95% CI,
67.6–97.3)a | 87.5 (95% CI,
61.6–98.4)a | 87.5 (95% CI,
47.3–99.6)a |
| Stable disease | 3 |
12.5 | 2 |
12.5 | 1 |
12.5 |
| Progression of
disease | 3 |
12.5 | 2 |
12.5 | 1 |
12.5 |
| Median PFS
(months) | 14 | 14 | 14 |
|
Range | 1±38 | 1±38 | 1±22 |
|
Progression events | 22 | 15 | 7 |
| Median OS
(months) | 19 | 16 | 24 |
|
Range | 1±38 | 1±38 | 1±38 |
|
Deaths | 22 | 15 | 7 |
Dose intensity
Among all patients, 149 cycles were administered and
the median number of administered cycles was 3 (range, 1–8), as
well as in st FD/FOx (range 1–8) and mod FD/FOx (range, 1–7). Seven
patients were treated with 5-FU 1,000 mg/m2/day for two
nights a week as initial dose. Median rDI per cycle in the overall
population was: Docetaxel 20 (8.5–25) mg/m2/w (80% of
the standard DI), oxaliplatin 32 (15–40) mg/m2/w (80% of
the standard DI), 5-FU 1,440 (375–1,800) mg/m2/w (80% of
the standard DI, referring to the initial dose of 900
mg/m2/day for two nights a week). All rDIs among
patients treated with st and mod FD/FΟx are listed in Table III.
 | Table III.Median received dose intensities in
FD/Fox regimens according to standard and modified regimens. |
Table III.
Median received dose intensities in
FD/Fox regimens according to standard and modified regimens.
|
| Overall FD/FOx | Standard
FD/FOx | Modified
FD/FOx |
|---|
|
|
|
|
|
|---|
|
| rDI/cycle
mg/m2/w | rDI/cycle
mg/m2/w | rDI/cycle
mg/m2/w |
|---|
|
|
|
|
|
|---|
|
| Median (range) | rDI
(%)a | Median (range) | rDI
(%)a | Median (range) | rDI
(%)a |
|---|
| Docetaxel | 20 (8.5–25) | 80 | 20 (8.5–25) | 80 | 20 (10–25) | 80 |
| Oxaliplatin | 32 (15–40) | 80 | 32 (17.5–40) | 80 | 32 (15–40) | 80 |
| 5FU | 1440
(375–1800) | 80 | 1500
(750–1800) | 80 | 1280
(375–1800) | 71.1 |
Toxicities
All patients were evaluable for safety analysis. The
only treatment-related grade 4 adverse event was neutropenia
(9.3%). Most relevant treatment-related grade 3 adverse events
were: Neutropenia (40.6%), diarrhea (18.7%), asthenia (18.7%). Most
relevant grade 2 toxicities were: Nausea (31.1%), anorexia (40.6%),
asthenia (62.5%), hypoalbuminemia (43.7%), peripheral neuropathy
(37.5%), alopecia (40.6%), leukopenia (31.2%), neutropenia (59.3%)
and anemia (34.3%) (Table IV). No
febrile neutropenia was observed and none of the patients died as a
result of adverse events. No significant differences appeared
between the toxicities of st and mod FD/FOx regimens (Table V).
 | Table IV.Toxicities of standard FD/FOx
regimen. |
Table IV.
Toxicities of standard FD/FOx
regimen.
|
| Overall
patients |
|---|
|
|
|
|---|
| N | 32 |
|---|
| NCI-CTC
grade | 1 | 2 | 3 | 4 |
| Nausea (%) | 22 (68.5) | 10 (31.2) | – | – |
| Vomiting (%) | 12 (37.5) | 5 (15.6) | – | – |
| Diarrhea (%) | 23 (71.8) | 5 (15.6) | 6 (18.7) | – |
| Anorexia (%) | 18 (56.2) | 13 (40.6) | – | – |
| Constipation
(%) | 11 (34.3) | 2 (6.2) | – | – |
|
Stomatitis/mucositis (%) | 16 (50) | 4 (12.5) | – | – |
| Asthenia (%) | 25 (78.1) | 20 (62.5) | 6 (18.7) | – |
| Hypertransaminasemy
(%) | 14 (43.7) | 1 (3.1) | – | – |
| Cholestasis
(%) | 13 (40.6) | 2 (6.2) | – | – |
| Hypoalbuminemia
(%) | 17 (53.1) | 14 (43.7) | – | – |
| Peripheral
neuropathy (%) | 17 (53.1) | 12 (37.5) | – | – |
| Skin toxicity
(%) | 3 (9.3) | – | – | – |
| Onychodystrophy
(%) | 17 (53.1) | 5 (15.6) | – | – |
| Alopecia (%) | 14 (43.7) | 13 (40.6) | 3 (9.3) | – |
| Hand foot syndrome
(%) | 3 (9.3) | – | – | – |
| Conjunctivitis
(%) | 4 (12.2) | – | – | – |
| Leukopenia (%) | 20 (62.5) | 10 (31.2) | 3 (9.3) | – |
| Neutropenia
(%) | 20 (62.5) | 19 (59.3) | 10 (31.2) | 3 (9.3) |
| Anemia (%) | 26 (81.2) | 11 (34.3) | – | – |
| Thrombocytopenia
(%) | 10 (31.2) | 2 (6.2) | – | – |
 | Table V.Toxicities of the FD/FΟx regimen
according to standard/modified schedules. |
Table V.
Toxicities of the FD/FΟx regimen
according to standard/modified schedules.
|
| St FD/FOx | Mod FD/FOx |
|---|
|
|
|
|
|---|
| Number | 22 | 10 |
|---|
| NCI-CTC
grade | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Nausea (%) | 13 (59.1) | 4 (18.1) | – | – | 6 (60) | 3 (30) | – | – |
| Vomiting (%) | 9 (40.1) | 3 (13.6) | – | – | 3 (30) | 2 (20) | – | – |
| Diarrhea (%) | 16 (72.7) | 4 (18.1) | 5 (22.7) | – | 7 (70) | 1 (10) | 1 (10) | – |
| Anorexia (%) | 13 (59.1) | 8 (36.3) | – | – | 5 (50) | 5 (50) | – | – |
| Constipation
(%) | 8 (36.3) | 1 (4.5) | – | – | 3 (30) | 1 (10) | – | – |
|
Stomatitis/mucositis (%) | 12 (54.5) | 3 (13.6) | – | – | 4 (40) | 1 (10) | – | – |
| Asthenia (%) | 17 (77.2) | 14 (63.6) | 4 (18.1) | – | 8 (80) | 6 (60) | 2 (20) | – |
| Hypertransaminasemy
(%) | 11 (50) | 1 (4.5) | – | – | 3 (30) | – | – | – |
| Cholestasis
(%) | 7 (31.8) | 1 (4.5) | – | – | 6 (60) | 1 (10) | – | – |
| Hypoalbuminemia
(%) | 10 (45.4) | 9 (40.9) | – | – | 7 (70) | 5 (50) | – | – |
| Peripheral
neuropathy (%) | 19 (86.3) | 4 (18.1) | – | – | 8 (80) | – | – | – |
| Skin toxicity
(%) | 3 (13.6) | – | – | – | – | – | – | – |
| Onychodystrophy
(%) | 11 (50) | 5 (22.7) | – | – | 6 (60) | – | – | – |
| Alopecia (%) | 7 (31.8) | 11 (50) | 1 (4.5) | – | 7 (70) | 2 (20) | 2 (20) | – |
| Hand foot syndrome
(%) | 3 (13.6) | – | – | – | – | – | – | – |
| Conjunctivitis
(%) | 4 (18.1) | – | – | – | – | – | – | – |
| Leukopenia (%) | 14 (63.6) | 7 (31.8) | 2 (9.1) | – | 6 (60) | 3 (30) | 1 (10) | – |
| Neutropenia
(%) | 14 (63.6) | 14 (63.6) | 8 (36.3) | 2 (9.1) | 6 (60) | 5 (50) | 2 (20) | 1 (10) |
| Anemia (%) | 18 (81.8) | 7 (31.8) | – | – | 8 (80) | 4 (40) | – | – |
| Thrombocytopenia
(%) | 6 (27.2) | 1 (4.5) | – | – | 4 (40) | 1 (10) | – | – |
Subsequent treatments
Following the first line FD/FOx, 4 patients (12.5%)
underwent primary tumor resection. Among the 22 patients who
progressed at first line, 13 (59.1%) underwent a second line
chemotherapy as follows: 6 patients (46.1%) with a taxane-based
rechallenge (2 with paclitaxel-ramucirumab) and 4 patients (38.4%)
with irinotecan-based regimens. One patient underwent a third line
monotherapy with ramucirumab.
Discussion
A major problem concerning a triplet chemotherapy is
to achieve good clinical outcomes, ensuring an adequate rDI of each
drug and concurrently, limiting toxicities. Although DCF is a
standard treatment for a/m GC patients, its use is limited in
clinical practice, due to the unfavorable safety profile. In our
retrospective analysis, ORR was 75%, DCR was 87.5%, median PFS was
14 months and median OS was 19.0 months. The most relevant
treatment-related grade 3 adverse events were: Neutropenia (40.6%),
asthenia (18.7%) and diarrhea (18.7%), the only treatment-related
grade 4 adverse event was neutropenia (9.3%), no febrile
neutropenia was observed and no patients died as a result of
adverse events. In addition, no statistically significant
differences in ORR, median PFS and median OS, were observed between
st and mod FD/Fox regimens.
Despite the intrinsic limits of a retrospective
collection, and of the sample size, these data appeared to be
slightly better than those deriving from the phase 2 clinical
trials previously mentioned (11,12).
In the TEF arm of the GATE trial, the most similar regimen to
FD/FOx, ORR was 46.6%, median PFS was 7.7 months and median OS was
14.6 months. Furthermore, the most relevant treatment-related grade
3/4 adverse events were: Neutropenia (56%), leukopenia (30%)
fatigue (14%) and diarrhea (11%), whereas febrile neutropenia was
reported in 2% of patients. In a recent systematic review of
modified schedules of DCF chemotherapy the median pooled PFS and OS
were 7.2 months (95% CI, 5.9–8.8) and 12.3 months (95% CI,
10.6–14.3), respectively and the median pooled ORR was 49%. In
addition, grade 3/4 toxicities were: Neutropenia (29.1%),
thrombocytopenia (5.6%), anemia (8.9%), febrile neutropenia (7.6%),
diarrhea (6.6%), nausea/vomiting (4.9%) and neurotoxicity (9.9%)
(30). In this study median rDIs of
docetaxel, oxaliplatin and 5-FU were respectively 20, 32 and 1,440
mg/m2/week. These results are comparable to those
demonstrated in the TEF arm of GATE trial (20, 33 and 966
mg/m2/week) regarding docetaxel and oxaliplatin and even
higher regarding 5-FU (12). The
use of TFI/5-FU, and weekly-alternating docetaxel and oxaliplatin,
allowed us to reach these results, with an acceptable safety
profile, especially in terms of hematologic toxicity and
neurotoxicity. Since it was retrospective study of clinical
practice, even elderly patients with poor clinical conditions were
considered: 4 (12.5%) patients had an ECOG-PS 2 and 11 (34.4%)
patients were elderly. Dose modulations allowed us to treat these
patients as well. According to our opinion, patients with severe
comorbidities are not eligible for intensive regimens: Only one
patient (treated with mod FD/FOx) had a secondary CIRS stage. Even
though the weekly rate of the regimen represented a greater
engagement both for patients and their families, and a greater
workload for the outpatient clinic, it allowed us to carefully
monitor treatment and adverse events.
A/m GC patients and particularly those with
unresected primary tumor and/or peritoneal carcinomatosis (44 and
66%, respectively of our population) typically dropped out of
treatment precociously, due to the deterioration of the clinical
conditions, as well as developing nutritional problems such as
anorexia, dysphagia, vomiting and slimming. Considering that these
patients often do not reach a second line of treatment, they
probably need rapid tumor shrinkage in order to palliate symptoms.
Concurrently, an adequate nutritional support and a careful
management of toxicities, which can be particularly severe in
malnourished patients, are mandatory. In our case-series, among 14
patients with unresected primary tumors, 4 (28.5%) underwent
resection of the primary tumor and among the 22 patients who
progressed on the FD/FOx regimen, 13 (59%) underwent a second line
of treatment, 46% of which taxane-based, with or without
ramucirumab.
In the second-line setting of a/m GC patients, the
advent of ramucirumab, a fully human monoclonal antibody against
the vascular endothelial growth factor receptor (VEGFR)-2, set a
new standard of care in patients progressing after a platinum-based
first-line therapy. Ramucirumab in two large international phase
III multicenter studies demonstrated benefits in median OS, median
PFS and DCR compared to BSC (REGARD trial). It also demonstrated
benefits in median OS, median PFS, ORR and DCR in association with
paclitaxel compared to paclitaxel alone (RAINBOW trial) (31,32).
Ultimately, a triplet chemotherapy cannot always be
used and it is crucial to select patients eligible for an intensive
regimen. The features of patients (age, expected QoL, prolonged
survival) and those of the disease (tumor burden, symptoms) play a
central role in the decision-making process, and the use of
validate prognostic factors such as the Royal Marsden Hospital
prognostic index or similar could aid clinicians (33,34).
Therefore, a taxane could be used in the first-line setting, in
combination with platinum compounds and fluoropyrimidines in ‘high
risk’ patients. These patients will probably not reach a second
line (i.e. with ECOG-PS 2, liver metastasis, peritoneal metastasis,
increase of alkaline phosphatase), and frequently need a rapid
relief of symptoms due to high tumor burden. However, a triplet
regimen should be excluded in cases of severe (or uncontrolled)
comorbidities. Otherwise a taxane should be used in second line,
associated with ramucirumab, in ‘low risk’ patients (i.e. with none
of the risk factors mentioned above), with a better prognosis,
following progression to a first line treatment based on platinum
compounds and fluoropyrimidines, by modulating the treatment in
view of this potential second line. Both choices could be correct
in ‘intermediate risk’ patients (i.e. patients with 1–2 of the risk
factors mentioned above).
In conclusion, the retrospecive nature, the small
sample size and the mixed population (4 patients with peritoneal
cytology-limited disease) were strong limitations, which did not
allow us to reach any conclusive considerations, nevertheless the
FD/FOx regimen has become a common clinical practice in our
institute. In our opinion it appears to be a feasible option for
first-line treatment of a/m GC patients, especially in case of high
tumour burden, when the patient needs rapid tumour shrinkage and
disease-related symptoms control. TFI/5-FU and dose modulations
have probably allowed us to use an intensive regimen in clinical
practice even in elderly and ‘frail’ patients, with comorbidities
and ECOG-PS 1–2. TFI could be an alternative and an easy modality
of administration of 5-FU, which still represents the backbone of
the first-line regimen for treating a/m GC patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
AC, KC, GP and CF conceived and designed the study.
AP, RV, VV, BDB, OV, PLB and LV collected the data. AC and AP wrote
the paper. CF and GP reviewed and edited the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All patients provided written, informed consent to
treatment. The procedures followed were in accordance with the
ethical standards of the local responsible committees on human
experimentation (Comitato etico per le province di L'Aquila e
Teramo).
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Murad AM, Santiago FF, Petroianu A, Rocha
PR, Rodrigues MA and Rausch M: Modified therapy with
5-fluorouracil, doxorubicin, and methotrexate in advanced gastric
cancer. Cancer. 72:37–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pyrhönen S, Kuitunen T, Nyandoto P and
Kouri M: Randomised comparison of fluorouracil, epidoxorubicin and
methotrexate (FEMTX) plus supportive care with supportive care
alone in patients with non-resectable gastric cancer. Br J Cancer.
71:587–591. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levi JA, Fox RM, Tattersall MH, Woods RL,
Thomson D and Gill G: Analysis of a prospectively randomized
comparison of doxorubicin versus 5-fluorouracil, doxorubicin, and
BCNU in advanced gastric cancer: Implications for future studies. J
Clin Oncol. 4:1348–1355. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemoterapy versus
chemoterapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3, open
label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ajani JA, D'Amico TA, Baggstrom M, Bentrem
DJ, Chao J, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, et
al: Clinical practice guidelines in oncology, gastric cancer.
National Comprehensive Cancer Network. version 4. 2017.
|
|
7
|
Smyth EC, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D; ESMO Guidelines Committee: Gastric
cancer: ESMO clinical practice guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 27 (Suppl 5):v38–v49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohammad NH, Ter Veer E, Ngai L, Mali R,
van Oijen MG and van Laarhoven HW: Optimal first-line
chemotherapeutic treatment in patients with locally advanced or
metastatic esophagogastric carcinoma: Triplet versus doublet
chemotherapy: A systematic literature review and meta-analysis.
Cancer Metastasis Rev. 34:429–441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carmona-Bayonas A, Jiménez-Fonseca P,
Custodio A, Sánchez Cánovas M, Hernández R, Pericay C, Echavarria
I, Lacalle A, Visa L, Rodríguez Palomo A, et al:
Anthracycline-based triplets do not improve the efficacy of
platinum-fluoropyrimidine doublets in first-line treatment of
advanced gastric cancer: Real-world data from the AGAMEMON National
cancer Registry. Gastric Cancer. 21:96–105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ter Veer E, Haj Mohammad N, van Valkenhoef
G, Ngai LL, Mali RMA, Anderegg MC, van Oijen MGH and van Laarhoven
HWM: The efficacy and safety of first-line chemotherapy in advanced
esophagogastric cancer: A Network Meta-analysis. J Natl Cancer
Inst. 1082016.
|
|
11
|
Shah MA, Janjigian YY, Stoller R, Shibata
S, Kemeny M, Krishnamurthi S, Su YB, Ocean A, Capanu M, Mehrotra B,
et al: Randomized multicenter phase II study of modified docetaxel,
cisplatin, and fluorouracil (DCF) versus DCF plus growth factor
support in patients with metastatic gastric adenocarcinoma: A study
of the US gastric cancer consortium. J Clin Oncol. 33:3874–3879.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Cutsem E, Boni C, Tabernero J, Massuti
B, Middleton G, Dane F, Reichardt P, Pimentel FL, Cohn A, Follana
P, et al: Docetaxel plus oxaliplatin with or without fluorouracil
or capecitabine in metastatic or locally recurrent gastric cancer:
A randomized phase II study. Ann Oncol. 26:149–156. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Batran SE, Hofheinz RD, Pauligk C, Kopp
HG, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg
H, et al: Histopathological regression after neoadjuvant docetaxel,
oxaliplatin, fluorouracil, and leucovorin versus epirubicin,
cisplatin, and fluorouracil or capecitabine in patients with
resectable gastric or gastro-oesophageal junction adenocarcinoma
(FLOT4-AIO): Results from the phase 2 part of a multicentre,
open-label, randomised phase 2/3 trial. Lancet Oncol. 17:1697–1708.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ficorella C, Ricevuto E, Morelli MF,
Morese R, Cannita K, Cianci G, Porzio G, Di Rocco ZC, De Galitiis
F, De Tursi M, et al: Increased tolerability of bimonthly 12-hour
timed flat infusion 5-fluorouracil/irinotecan regimen in advanced
colorectal cancer: A dose-finding study. Oncol Rep. 15:1345–1350.
2006.PubMed/NCBI
|
|
15
|
Ficorella C, Bruera G, Cannita K, Porzio
G, Baldi PL, Tinari N, Natoli C and Ricevuto E: Triplet
chemotherapy in patients with metastatic colorectal cancer: Toward
the best way to safely administer a highly active regimen in
clinical practice. Clin Colorectal Cancer. 11:229–237. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Köhne CH1, Wils J, Lorenz M, Schöffski P,
Voigtmann R, Bokemeyer C, Lutz M, Kleeberg C, Ridwelski K, Souchon
R, et al: Randomized phase III study of high-dose fluorouracil
given as a weekly 24-hour infusion with or without leucovorin
versus bolus fluorouracil plus leucovorin in advanced colorectal
cancer: European organization of Research and Treatment of Cancer
Gastrointestinal Group Study 40952. J Clin Oncol. 21:3721–3728.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leichman CG, Fleming TR, Muggia FM, Tangen
CM, Ardalan B, Doroshow JH, Meyers FJ, Holcombe RF, Weiss GR,
Mangalik A, et al: A phase II study of fluorouracil and its
modulation in advanced colorectal cancer: A Southwest oncology
group study. J Clin Oncol. 13:1303–1311. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O'Dwyer PJ, Manola J, Valone FH, Ryan LM,
Hines JD, Wadler S, Haller DG, Arbuck SG, Weiner LM, Mayer RJ and
Benson AB III: Fluorouracil modulation in colorectal cancer: Lack
of improvement with N-phosphonoacetyl-l-aspartic acid or oral
leucovorin or interferon, but enhanced therapeutic index with
weekly 24-h infusion schedule-An eastern cooperative oncology
group/cancer and leukemia Group B study. J Clin Oncol.
19:2413–2421. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lévi F: Chronopharmacology of anticancer
agents. Redfern PH and Lemmer B: Handbook of Exeperimental
pharmacology: Physiology and pharmacology of biological
Rhythms-cancer chemotherapy. Springer-Verlag; Berlin: pp. 299–301.
1997, View Article : Google Scholar
|
|
20
|
Harris BE, Song R, Soong SJ and Diasio RB:
Relationship between dihydropyrimidine dehidrogenase activity and
plasma 5-fluorouracil levels with evidence for circadian varation
of enzyme activity and plasma drug levels in cancer patients
receiving 5-fluorouracil by protracted continuous infusion. Cancer
Res. 50:197–201. 1990.PubMed/NCBI
|
|
21
|
Smaaland R, Abrahamsen JF, Svardal AM,
Lote K and Ueland PM: DNA cell cycle distrubution and glutathione
(GSH) content according to circadian stage in bone marrow of cancer
patients. Br J Cancer. 66:39–45. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morelli MF, Santomaggio A, Ricevuto E,
Cannita K, De Galitiis F, Tudini M, Bruera G, Mancini M,
Pelliccione M, Calista F, et al: Triplet schedule of weekly
5-Fluorouracil and alternating irinotecan or oxaliplatin in
advanced colorectal cancer: A dose-finding and phase II study.
Oncol Rep. 23:1635–1640. 2010.PubMed/NCBI
|
|
23
|
Bruera G, Santomaggio A, Cannita K, Baldi
PL, Tudini M, De Galitiis F, Mancini M, Marchetti P, Antonucci A,
Ficorella C and Ricevuto E: ‘Poker’ association of weekly
alternating 5-fluorouracil, irinotecan, bevacizumab and oxaliplatin
(FIr-B/FOx) in first line treatment of metastatic colorectal
cancer: A phase II study. BMC Cancer. 10:5672010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Extermann M, Overcash J, Lyman GH, Parr J
and Balducci L: Comorbidity and functional status are independent
in older cancer patients. J Clin Oncol. 16:1582–1587. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors: European
organization for research and treatment of cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaplan EL and Meier P: Nonparametric
estimation of incomplete observations. J Am Stat Assoc. 53:457–481.
1958. View Article : Google Scholar
|
|
28
|
Fisher RA: On the interpretation of χ2
from contingency tables, and the calculation of P. J R Stat Soc.
85:87–94. 1922. View
Article : Google Scholar
|
|
29
|
Peto R and Peto J: Asymptomatically
efficient rank invariant test procedures. J R Stat Soc A.
135:185–207. 1972. View
Article : Google Scholar
|
|
30
|
Petrelli F, Tomasello G, Ghidini M,
Passalacqua R and Barni S: Modified schedules of DCF chemotherapy
for advanced gastric cancer: A systematic review of efficacy and
toxicity. Anticancer Drugs. 28:133–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry
DR, et al: Ramucirumab monotherapy for previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chau I, Ashley S and Cunningham D:
Validation of the Royal Marsden hospital prognostic index in
advanced esophagogastric cancer using individual patient data from
the REAL 2 study. J Clin Oncol. 27:e3–e4. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fuchs CS, Muro K, Tomasek J, Van Cutsem E,
Cho JY, Oh SC, Safran H, Bodoky G, Chau I, Shimada Y, et al:
Prognostic factor analysis of overall survival in gastric cancer
from two phase III studies of Second-line Ramucirumab (REGARD and
RAINBOW) using pooled patient data. J Gastric Cancer. 17:132–144.
2017. View Article : Google Scholar : PubMed/NCBI
|