Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of
the most aggressive forms of human pancreatic cancer. Although
in-depth knowledge of the disease has been elucidated and an
extensive amount of research has been conducted in the past few
decades, the effectiveness of treatment for this malignancy has not
substantially improved. PDAC remains the fourth leading cause of
cancer-related mortality in the United States, with a 5-year
survival rate of <8% (1). PDAC
may go undetected until patients have progressed to an advanced
stage. More than 80% of patients have already missed the
opportunity for radical surgery at the time of initial diagnosis
(2). Due to the aggressive nature
of this malignancy and its non-sensitivity to radiotherapy and
chemotherapy, local recurrence and distant metastasis after initial
‘curative’ resection is an unresolved clinical problem for PDAC
patients (3).
Active invasion and metastasis have been recognized
as hallmarks of cancer (4).
Accumulating evidence has indicated that epithelial-mesenchymal
transition (EMT), which initiates an invasion-metastasis cascade,
is a feature of aggressive tumors (5,6).
During EMT, cancer cells disassemble their epithelial junctions and
suppress the expression of junctional proteins. Consequently, the
cancer cells acquire the abilities to invade, resist apoptosis and
disseminate (7). E-cadherin, a
component of adherent junctions, is lost during EMT and cancer
progression. Conversely, another adherent junction protein,
N-cadherin is upregulated in cancer cells. A previous study
revealed that cadherin switching was a main event in EMT and was
necessary for the increased motility of cancer cells (8). Accumulating evidence has revealed that
EMT plays a crucial role in the invasion and metastasis of
pancreatic cancer (9,10). Recently, several studies have
revealed that many growth factors and cytokines, as well as
cellular signaling pathways, could induce EMT. One such cytokine is
transforming growth factor-β1 (TGF-β1) (11).
Compared with other treatments, metformin, the
first-line treatment for managing type 2 diabetes mellitus, has
been associated with reduced cancer burden in epidemiological
studies in diabetic patients (12,13).
There is also evidence that metformin use is associated with a
reduced risk of prostate (14),
colon (15) and pancreatic cancer
(16). Given the anticancer
properties and the safety profile of metformin, this treatment has
attracted great interest from cancer researchers worldwide
(17). In the past, exciting
preclinical studies have shown that metformin can inhibit cancer
cell growth both in vitro and in vivo (18–20).
Using the LSL-KrasG12D/+, Trp53fl/+ and Pdx1-Cre (KPC) mouse
models, our previous study indicated that metformin suppressed the
initiation and progression of pancreatic cancer (21). In another study (22), our data revealed that
metformin-mediated AMPK activation in cancer cells inhibited
pancreatic cancer progression by suppressing the desmoplastic
reaction in tumor tissues. Further mechanistic experiments revealed
that metformin reduced TGF-β1 production in cancer cells, thus
suppressing the TGF-β1-induced activation of pancreatic stellate
cells (PSCs) (22). Based on these
observations, we hypothesized that metformin could inhibit the
invasion and metastasis of pancreatic cancer cells by blocking the
autocrine TGF-β1 signaling. In the present study, we used the KPC
transgenic mouse model of pancreatic cancer to demonstrate that
metformin inhibited the invasion and metastasis of pancreatic
cancer by suppressing TGF-β1/Smad2/3 signaling.
Materials and methods
Cell culture and reagents
Human PDAC cell lines Panc-1 and BxPC-3 were
purchased from The Chinese Academy of Sciences Cell Bank of Type
Culture Collection (CBTCCCAS; Shanghai, China). The cells were
maintained in the appropriate medium (DMEM; HyClone Laboratories,
Logan, UT, USA) for Panc-1 and RPMI-1640 medium for BxPC-3 (HyClone
Laboratories) supplemented with 10% fetal bovine serum (FBS;
HyClone Laboratories; GE Healthcare Life Sciences, Logan, UT, USA)
and 1% penicillin/streptomycin in a humidified atmosphere
containing 5% CO2 at 37°C. Primary antibodies for
E-cadherin (1:1,000; cat. no. 3195), N-cadherin (1:800; cat. no.
13116), MMP-2 (1:1,000; cat. no. 40994) and vimentin (1:1,000; cat.
no. 5741) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Antibodies against β-actin (1:1,000; cat. no.
A5441) were obtained from Sigma-Aldrich; Merck (St. Louis, MO,
USA). Primary antibodies for TGF-β1 (1:800; cat. no. ab92486),
Smad2/3 (1:1,000; cat. no. ab202445) and p-Smad2/3 (1:1,000; cat.
no. ab63399) were purchased from Abcam (Cambridge, MA, USA) and
anti-α-SMA (1:800; cat. no. A03744) was obtained from Boster
Biological Technology (Wuhan, China). All secondary antibodies
(goat anti-rabbit IgG-HRP (1:10,000; cat. no. ssc-2004) and goat
anti-mouse IgG-HRP (1:10,000; cat. no. sc-2005) were purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). Recombinant TGF-β1
(T7039) was obtained from Sigma-Aldrich. Metformin (Sigma-Aldrich;
Merck) was dissolved in phosphate-buffered saline (PBS) as a stock
solution of 100 mM. Working dilutions of metformin were made in
culture medium immediately before use.
Wound healing assays
Wound healing assays were conducted to assess the
migration ability of cancer cells. Briefly, cancer cells were
seeded in 6-well plates and when they almost covered the well, the
cells were serum-starved and then treated with metformin (2 mM) for
24 h. Subsequently, the cells were scratched using a 200-ml sterile
pipette tip. Images of the matched-pair wound regions at 0 and 24 h
were obtained at a magnification of ×100 using a light microscope
(Nikon Instruments, Inc., Tokyo, Japan).
Matrigel invasion assay
Matrigel invasion assays were performed to
investigate the invasive ability of cancer cells. In brief, cancer
cells were serum-starved overnight and then pretreated with or
without metformin plus TGF-β1 (2 ng/ml) for 24 h. Subsequently, the
cells were trypsinized, and 5×104 cells and 200 µl of
serum-free medium were added to Matrigel-coated inserts (BD
Biosciences, Franklin Lakes, NJ, USA). Complete medium (500 µl) was
added to the lower chamber. Following incubation for 48 h, the
invaded cells were stained with 0.1% crystal violet solution for 15
min at room temperature, and 10 randomly selected fields were
photographed with a light microscope (Nikon Instruments, Inc.) at a
magnification of ×200.
Immunofluorescence analysis
Following the designated treatment,
immunohistochemical analyses were performed according to our
previously described protocol (23). Images were pseudocolored using a
fluorescence microscope (Nikon Eclipse Ti-s; Nikon Instruments,
Inc.) with the appropriate excitation and emission (blue, 440–450
nm; green, 530–550 nm; red, 630–650 nm) spectra at a magnification
of ×400. Image-Pro Plus software (version 6.0; Media Cybernetics
Inc., Rockville, MD, USA) was used for further analysis.
Western blotting
Total cellular protein was extracted using RIPA
lysis buffer (Beyotime Institute of Biotechnology, Guangzhou,
China). Protein concentrations were analyzed with a BCA protein
assay kit (Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. The same amounts of protein (150 µg)
from the samples were separated by 10% SDS-PAGE and then
transferred onto polyvinylidene difluoride (PVDF) membranes as
previously described (24). The
membranes were blocked with 5% non-fat dry milk in TBST [10 mM
Tris-HCl (pH 8.0), 150 mM NaCl, 0.05% Tween-20]. Then membranes
were incubated with primary antibodies overnight at 4°C. After
three washes of 10 min each in TBST, the membranes were incubated
with HRP-conjugated secondary antibodies for 1 h and subsequently
washed again. Protein expression was visualized using enhanced
chemiluminescence (EMD Millipore, Billerica, MA, USA) and the
ChemiDoc XRS imaging system (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). β-actin was used to ensure equivalent protein
loading.
In vivo study
All experimental protocols were approved by the
Ethical Committee of The First Affiliated Hospital of Medical
College, Xi'an Jiaotong University (Xi'an, China).
LSL-KrasG12D/+; Trp53fl/+;
Pdx1-Cre (KPC) mice were generated and maintained as
previously described (21). A total
of 20, 6-week-old mice (female; average weight, 20±1.79 g) were
divided into two groups of 10 mice each. Mice in the metformin
group were gavaged with vehicle (sterile water) or metformin (200
mg/kg) daily for 4 weeks. All mice were housed under pathogen-free
conditions with a 12:12-h dark/light cycle under constant
temperature (24°C). At the termination of the experiment, the mice
were euthanized by 30% CO2 asphyxiation, followed by
cervical dislocation. Tissues were harvested and fixed in formalin.
Tumor samples were prepared for further histological analysis.
Statistical analysis
Each experiment was performed at least three times.
All quantitative data were analyzed using SPSS version 15.0 (SPSS,
Inc., Chicago, IL, USA) and are expressed as the mean ± standard
deviation (SD). Two-tailed unpaired Student's t-tests were used to
analyze the data between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
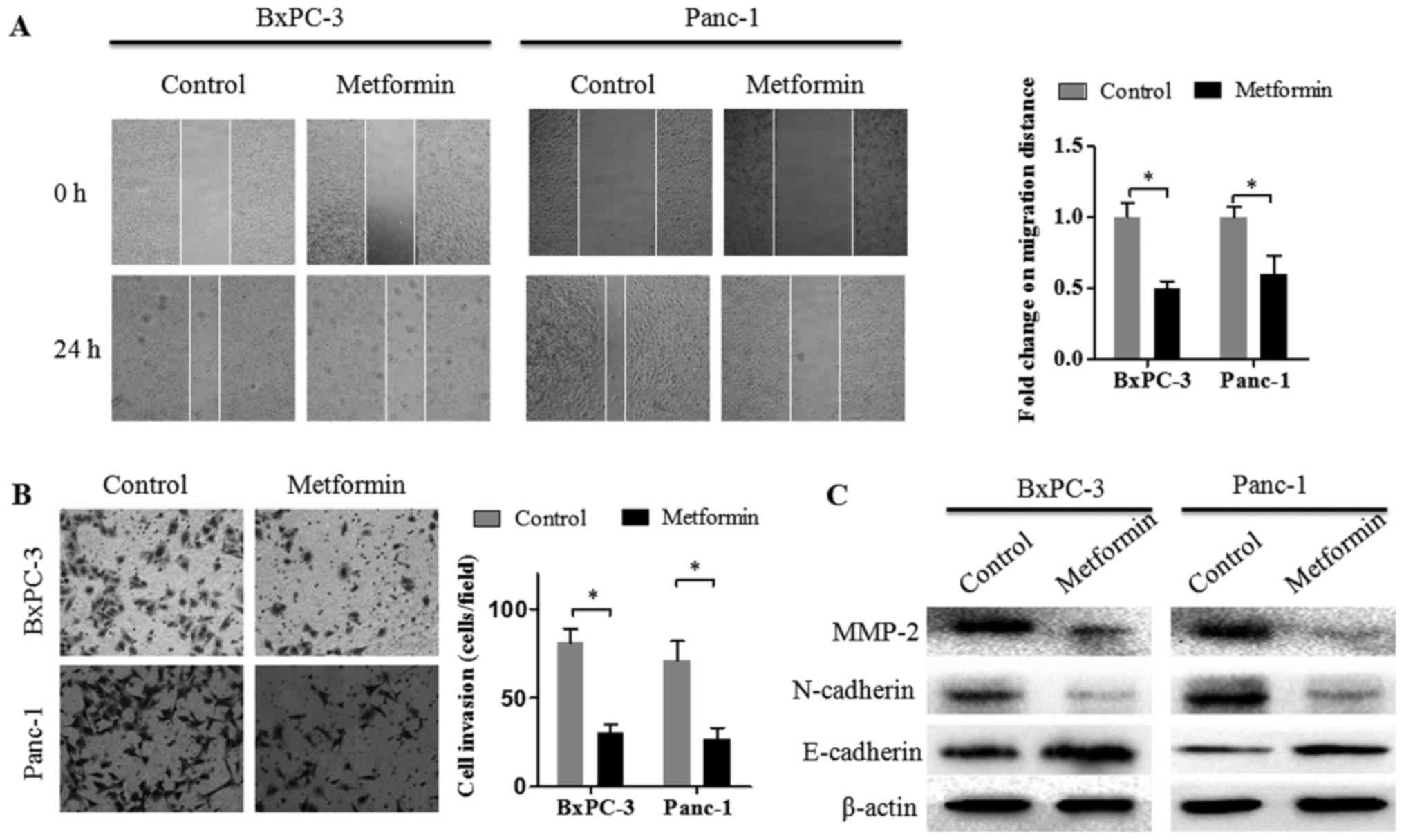
Metformin attenuates the migration and
invasion abilities of pancreatic cancer cells
First, the effect of metformin on the migration
ability of pancreatic cancer cells was assessed by wound healing
assays. Similarly, with our previous study, 2 mM metformin was also
used in the present study (22). As
displayed in Fig. 1A, the migration
ability of pancreatic cancer cells was significantly suppressed by
2 mM metformin treatment. To investigate the effect of metformin on
the invasion ability of pancreatic cancer cells, Matrigel invasion
assays were performed. The results revealed that the invasion
ability of pancreatic cancer cells was decreased by 2 mM metformin
treatment (Fig. 1B). These results
indicated that metformin inhibited the invasion and migration
capacities of pancreatic cancer cells in vitro. Available
evidence has indicated that active EMT in cancer cells enhances
their invasion and metastasis abilities. To explore whether
metformin has an effect on EMT-related molecules, the protein
expression levels of E-cadherin, N-cadherin and MMP-2 were detected
in pancreatic cancer cells following exposure to 2 mM metformin for
48 h. The western blotting results revealed that metformin
treatment decreased the protein expression levels of N-cadherin and
MMP-2 and increased the protein expression levels of E-cadherin in
both BxPC-3 and Panc-1 cells (Fig.
1C).
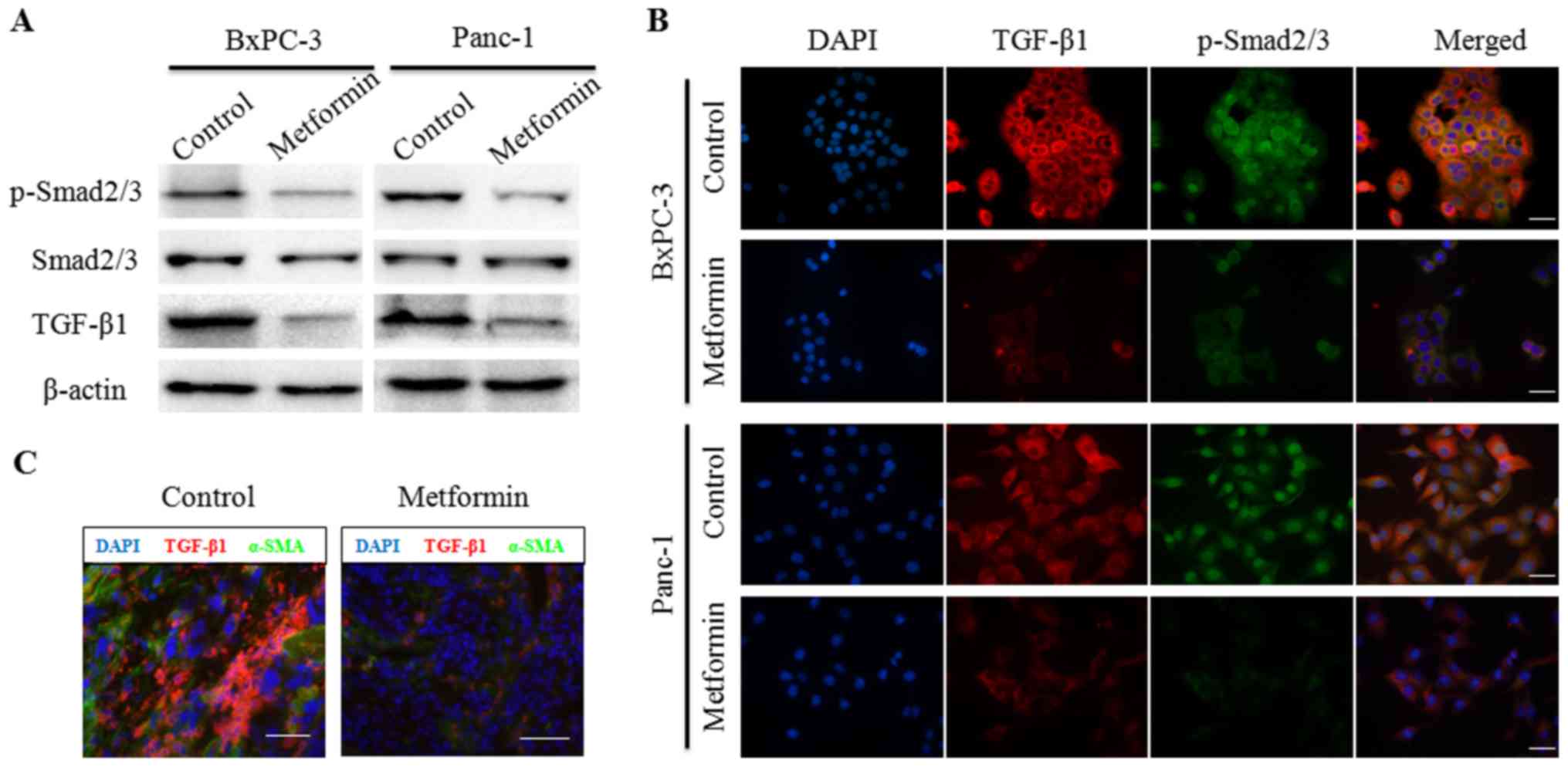
Metformin suppresses TGF-β1/Smad2/3
signaling in pancreatic cancer cells
Canonical TGF-β1/Smad2/3 signaling has been revealed
to play an important role in EMT in various epithelial cells
(11). Our previous study (22) revealed that metformin inhibited
TGF-β1 production in pancreatic cancer cells by inducing AMPK
activation. To further explore the effects of metformin on
TGF-β1/SMAD2/3 signaling in pancreatic cancer cells, BxPC-3 and
Panc-1 cells were treated with 2 mM metformin. Following 48 h,
total cell protein was extracted and subjected to western blot
analysis. As anticipated, the protein expression levels of TGF-β1
were decreased following metformin treatment. Additionally, the
protein expression levels of p-Smad2/3 were also decreased by
metformin treatment in both BxPC-3 and Panc-1 cells (Fig. 2A). In addition, this phenomenon was
confirmed by immunofluorescence assays (Fig. 2B). The results revealed that the
expression levels of TGF-β1 and p-Smad2/3 were decreased by
metformin treatment in pancreatic cancer cells. Notably, p-Smad2/3
was observed primarily in the cytoplasm following metformin
treatment. In a subcutaneous tumor model, the immunofluorescence
data revealed that TGF-β1 staining in tumor tissue, as well as
α-SMA expression, was reduced by the oral administration of 200
mg/kg metformin (Fig. 2C). These
results were consistent with those of our previous study (22). These findings revealed that
metformin suppressed TGF-β1/Smad2/3 signaling in pancreatic cancer
cells.
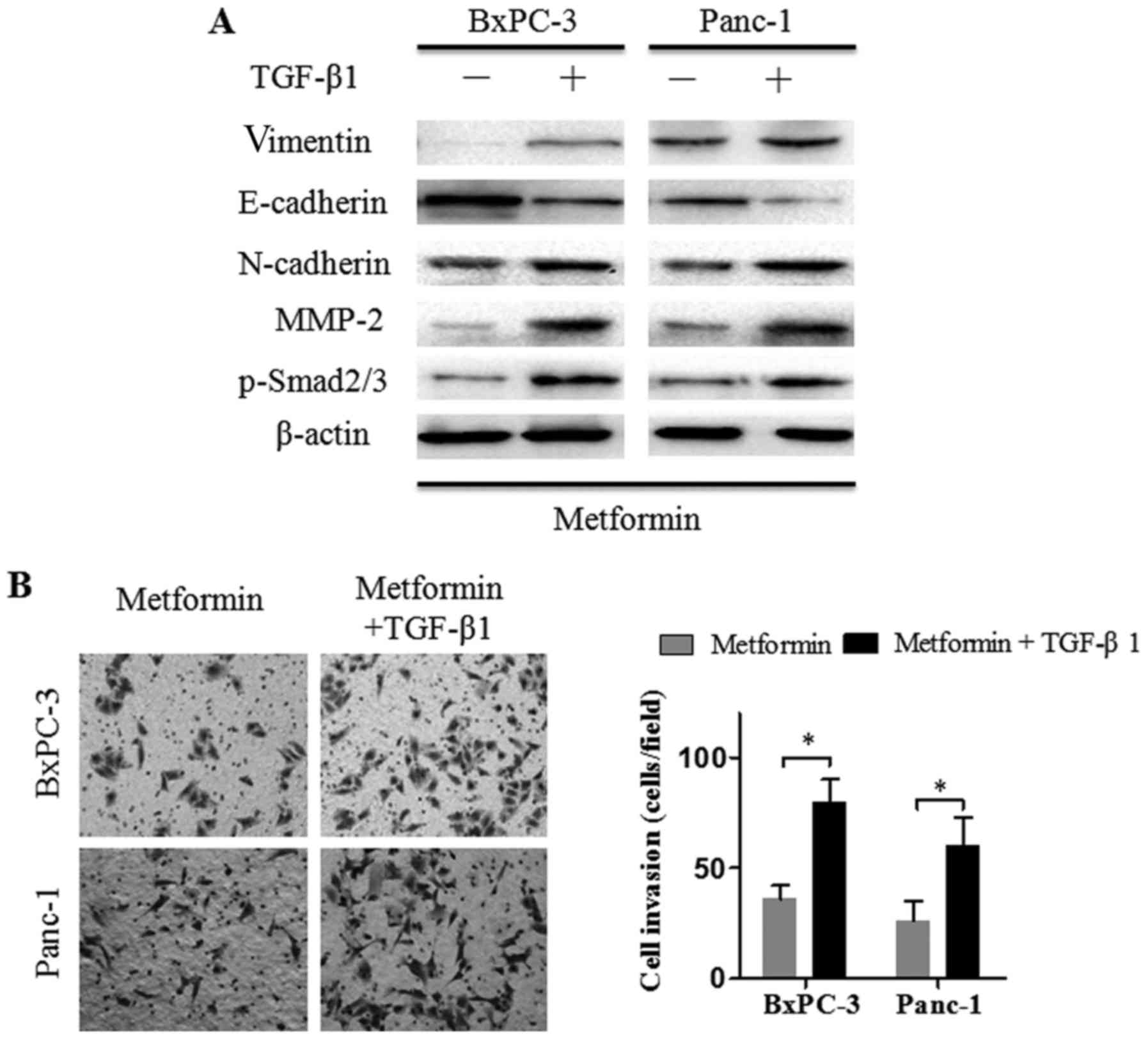
Exogenous TGF-β1 reverses the effects
of metformin on the invasion ability of pancreatic cancer
cells
The aforementioned observations revealed that
metformin suppressed the invasion ability and TGF-β1/Smad2/3
signaling in pancreatic cancer cells. To ascertain that TGF-β1
signaling inhibition attenuated cancer cell invasion, Panc-1 and
BxPC-3 cells were treated with metformin (2 mM) alone or metformin
plus recombinant TGF-β1 (2 ng/ml) for 24 h and then, EMT- and
invasion-related markers were detected by western blot assays. The
results revealed that the addition of recombinant TGF-β1 to the
culture medium induced Smad2/3 phosphorylation (Fig. 3A). Furthermore, N-cadherin,
vimentin, and MMP-2 protein expression levels were increased, and
E-cadherin protein expression levels were decreased in Panc-1 and
BxPC-3 cells treated with metformin plus TGF-β1, compared to cells
treated with metformin alone. Matrigel invasion assays revealed
that the metformin-mediated suppression of cancer cell invasion was
almost recovered in the presence of TGF-β1. Collectively, these
observations indicated that TGF-β1 downregulation may be
responsible for the metformin-mediated invasion and EMT changes in
pancreatic cancer cells.
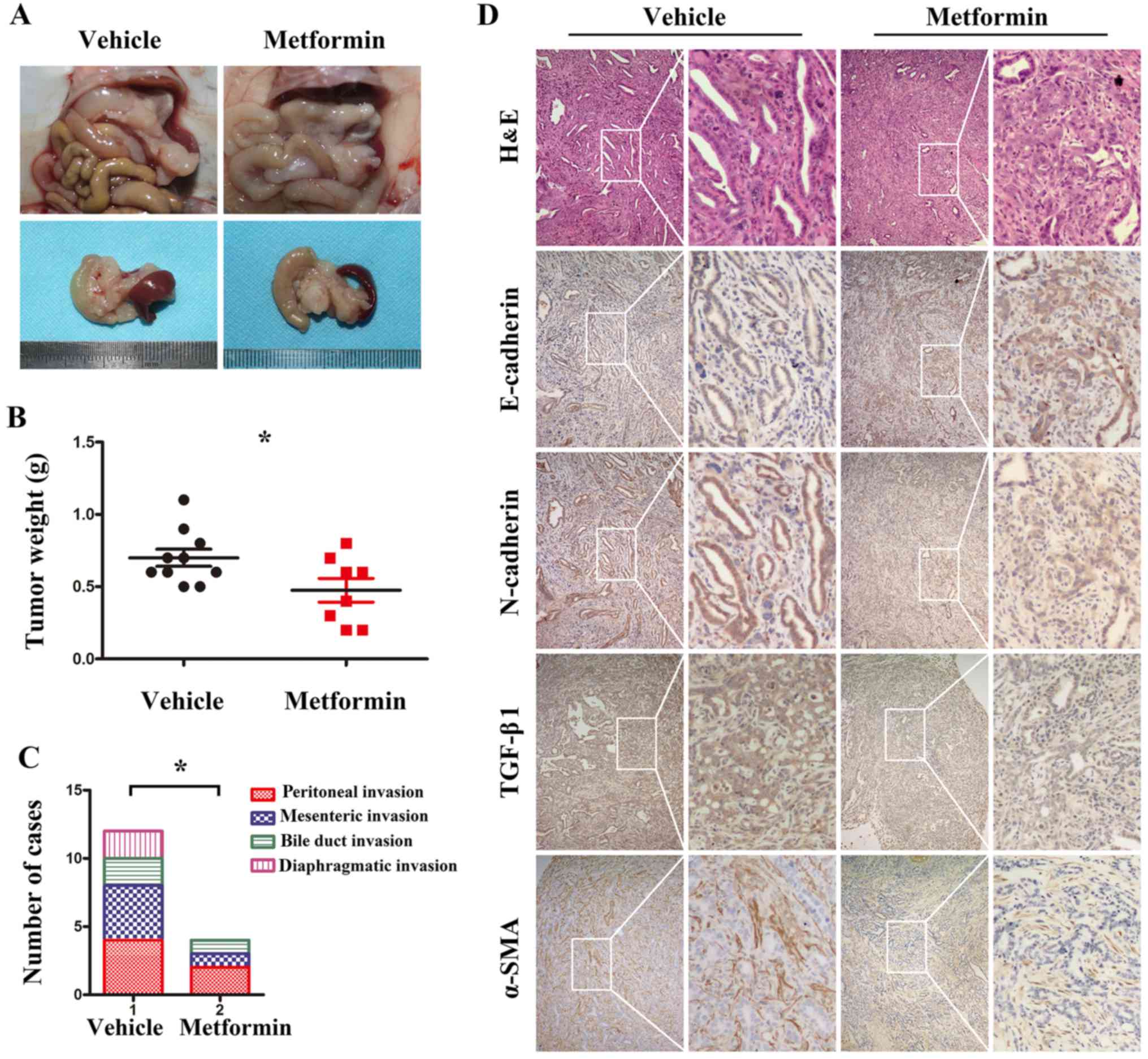
Metformin suppresses pancreatic cancer
tumor growth and metastasis in KPC transgenic mice
The KPC model is a well-validated, clinically
relevant model of PDAC that recapitulates the spectrum of
pancreatic cancer from pancreatic intraepithelial neoplasia (PanIN)
to invasive PDAC (21). To
determine the effects of metformin in vivo, KPC transgenic
mice were generated and treated orally with vehicle (sterile water,
daily for 4 weeks) or metformin (200 mg/kg, daily for 4 weeks). At
the end of the experiment, KPC mice were sacrificed, and the tumor
samples were prepared (Fig. 4A).
The results revealed that the average tumor weight in the
metformin-treated group was lower than that in the vehicle-treated
group (Fig. 4B). In addition,
compared with vehicle treatment, metformin treatment significantly
decreased the incidence of abdominal invasion, including peritoneal
invasion, mesenteric invasion and bile duct invasion as well as
diaphragmatic invasion (Fig. 4C).
The immunohistochemistry results (Fig.
4D) revealed that the TGF-β1 immunoreactivity in the
metformin-treated group was lower than that in the vehicle-treated
group. In addition, increased E-cadherin and decreased N-cadherin
expression levels were observed in the metformin-treated group.
Furthermore, compared with vehicle treatment, metformin treatment
reduced the area of α-SMA-positive staining. Collectively, these
results indicated that metformin inhibited pancreatic cancer tumor
growth, EMT and metastasis in vivo.
Discussion
PDAC is typically associated with a poor prognosis
even after curative resection with postoperative adjuvant
chemotherapy. Despite advances in our understanding of the
molecular and genetic mechanisms of pancreatic cancer, managing the
disease remains a clinical challenge due to its poor response to
most chemotherapeutic agents (25).
Local recurrence and metastasis are the primary causes of treatment
failure in cancer patients and of cancer-related deaths. Recently,
mounting evidence from both observational and laboratory studies
indicated that metformin treatment may be associated with a
decreased risk of developing cancer and a better response to
chemotherapy (12,26). The potential mechanisms underlying
the antitumor properties of metformin remain unclear. Our previous
studies have indicated that metformin can block the interaction
between cancer cells and PSCs by reducing cancer cell-derived
cytokines (22). In the present
study, we found that metformin treatment significantly attenuated
the migration and invasion properties of pancreatic cancer cells.
In addition, metformin suppressed EMT in pancreatic cancer cells.
This outcome was also produced in KPC transgenic mice. Furthermore,
our data revealed that these effects of metformin occurred in part
via the downregulation of TGF-β1/Smad2/3 signaling in pancreatic
cancer.
TGF-β1 is a potent cytokine with marked
functionalities, including the regulation of cell apoptosis,
proliferation, differentiation and extracellular matrix production
(27). TGF-β1 binding to its
receptor results in the phosphorylation and activation of the
transcription factors Smad2/3. Once activated, Smad2/3 translocates
into the nucleus to govern gene transcription. The role of TGF-β1
during cancer initiation and progression is complex and
paradoxical. TGF-β1 functions as a tumor suppressor in normal and
early-stage cancers by inducing cell-cycle arrest and apoptotic
reactions and as a tumor promoter in late-stage cancers by
promoting cancer growth, invasion and metastasis (28,29).
Parallel studies have suggested that TGF-β1 is a potent inducer of
EMT (11,30). Adding TGF-β1 to epithelial cells in
culture is a convenient way to induce EMT in various epithelial
cells (31). During EMT induction,
TGF-β1 rapidly activates PI3K, Akt, mTOR complex 1 (mTORC1) and S6
kinase, leading to increases in protein synthesis, cell motility
and invasion (32). A previous
study has indicated that TGF-β1 acted in an autocrine manner to
enhance tumor cell invasion in pancreatic cancer by upregulating
MMP-2 (33). In another study, a
significant association was found between the expression of TGF-β1
and lymph node involvement and the depth of invasion in pancreatic
cancer (34). In the present study,
we found that TGF-β1 expression in pancreatic cancer cells was
decreased by metformin. Notably, metformin treatment inhibited
TGF-β1-induced Smad2/3 phosphorylation in pancreatic cancer
cells.
The desmoplastic reaction, which is a result of the
proliferation of activated PSCs and the increased deposition of
extracellular matrix (ECM) components, is a prominent pathological
characteristic of pancreatic cancer (35). Accumulating evidence has indicated
that the desmoplastic reaction in pancreatic cancer contributes to
the aggressive nature of this malignancy by fostering tumor growth
and metastatic spread, as well as enhancing chemoradiotherapy
resistance (36–39). Thus, strategies targeting
cancer-stroma interactions may serve as a potential approach for
pancreatic cancer treatment (8). In
pancreatic cancer, PSCs are activated by tumor-stromal
interactions, including direct contact with pancreatic cancer cells
and paracrine growth factors, such as TGF-β1, secreted from
pancreatic cancer cells (40,41).
It has recently been reported that metformin reduces desmoplasia in
pancreatic cancer by reducing the expression of inflammatory
cytokines, resulting in reduced disease progression (42). Our previous study also indicated
that metformin suppressed desmoplasia in pancreatic cancer by
reducing TGF-β1 production in pancreatic cancer cells and
inhibiting paracrine-mediated PSC activation both in vitro
and in vivo (22). The
anti-stromal behavior of metformin has been previously verified in
a genetically engineered mouse model of pancreatic cancer (21). In the present study, we also
observed that α-SMA-positive cells were markedly reduced in cancer
tissues from KPC mice following metformin treatment. Collectively,
this evidence indicated the anti-stromal properties of metformin,
and that reduced TGF-β1 production by cancer cells may be the
underlying mechanism.
In the past, we conducted a series of studies on the
anticancer effect of metformin on pancreatic cancer. Our previous
study revealed that metformin suppressed desmoplasic reaction in
pancreatic cancer by reducing TGF-β1 production in cancer cells and
suppressing paracrine TGF-β1-induced PSC activation (22). In addition, both our in vitro
and in vivo experiments revealed that metformin inhibited
the proliferation, invasion and migration of pancreatic cancer
cells (22,43). Furthermore, by using a genetic mouse
model of pancreatic cancer, our results confirmed the inhibitory
effect of metformin on pancreatic cancer initiation and progression
(21). However, whether the
inhibitory effect of metformin on tumor invasion and metastasis is
mediated by targeting the autocrine TGF-β1/Smad pathway remains
unknown. Based on the aforementioned research, in the present
study, we investigated the effect of metformin on TGF-β1/Smad in
pancreatic cancer cells. Our results indicated that blocking
autocrine TGF-β1 signaling was partially responsible for
metformin-reduced cell invasion properties. Therefore, the present
study is a confirmation and supplement to our previous studies.
In conclusion, the results from the present study
indicated that metformin suppressed the invasion, EMT and
metastasis of pancreatic cancer by preventing autocrine
TGF-β1/Smad2/3 signaling both in vitro and in vivo.
Thus, our study revealed a new possible mechanism for the antitumor
effects of metformin.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81402971,
81672434 and 81702916).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
QM and WD designed the experiments. WQ, CZ and JC
performed the majority of the experiments. TQ, YX, LC and JL
analyzed the data. KC and XL organized the figures. JM wrote the
manuscript. KC, XL and JM were also involved in the conception of
the study. QM and WD reviewed it. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were authorized by the
Ethics Committee of the First Affiliated Hospital of Medical
College, Xi'an Jiaotong University (Xi'an, China). The protocols
also complied with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiu J and Yau T: Metastatic pancreatic
cancer: Are we making progress in treatment? Gastroenterol Res
Pract 2012. 8989312012.
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Micalizzi DS, Haber DA and Maheswaran S:
Cancer metastasis through the prism of epithelial-to-mesenchymal
transition in circulating tumor cells. Mol Oncol. 11:770–780. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morandi A, Taddei ML, Chiarugi P and
Giannoni E: Targeting the metabolic reprogramming that controls
epithelial-to-mesenchymal transition in aggressive tumors. Front
Oncol. 7:402017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maeda M, Johnson KR and Wheelock MJ:
Cadherin switching: Essential for behavioral but not morphological
changes during an epithelium-to-mesenchyme transition. J Cell Sci.
118:873–887. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Q, Miele L, Sarkar FH and Wang Z: The
role of EMT in pancreatic cancer progression. Pancreat Disord Ther.
2(pii): e1212012.PubMed/NCBI
|
|
10
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK,
Vonderheide RH, et al: EMT and dissemination precede pancreatic
tumor formation. Cell. 148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lamouille S, Connolly E, Smyth JW, Akhurst
RJ and Derynck R: TGF-β-induced activation of mTOR complex 2 drives
epithelial-mesenchymal transition and cell invasion. J Cell Sci.
125:1259–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Decensi A, Puntoni M, Goodwin P, Cazzaniga
M, Gennari A, Bonanni B and Gandini S: Metformin and cancer risk in
diabetic patients: A systematic review and meta-analysis. Cancer
Prev Res. 3:1451–1461. 2010. View Article : Google Scholar
|
|
13
|
Noto H, Goto A, Tsujimoto T and Noda M:
Cancer risk in diabetic patients treated with metformin: A
systematic review and meta-analysis. PLoS One. 7:e334112012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wright JL and Stanford JL: Metformin use
and prostate cancer in Caucasian men: Results from a
population-based case-control study. Cancer Causes Control.
20:1617–1622. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Currie CJ, Poole CD and Gale EA: The
influence of glucose-lowering therapies on cancer risk in type 2
diabetes. Diabetologia. 52:1766–1777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li D, Yeung SC, Hassan MM, Konopleva M and
Abbruzzese JL: Antidiabetic therapies affect risk of pancreatic
cancer. Gastroenterology. 137:482–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aljada A and Mousa SA: Metformin and
neoplasia: Implications and indications. Pharmacol Ther.
133:108–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhalla K, Hwang BJ, Dewi RE, Twaddel W,
Goloubeva OG, Wong KK, Saxena NK, Biswal S and Girnun GD: Metformin
prevents liver tumorigenesis by inhibiting pathways driving hepatic
lipogenesis. Cancer Prev Res. 5:544–552. 2012. View Article : Google Scholar
|
|
19
|
Kato K, Gong J, Iwama H, Kitanaka A, Tani
J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y, et al:
The antidiabetic drug metformin inhibits gastric cancer cell
proliferation in vitro and in vivo. Mol Cancer Ther. 11:549–560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Courtois S, Durán RV, Giraud J, Sifré E,
Izotte J, Mégraud F, Lehours P, Varon C and Bessède E: Metformin
targets gastric cancer stem cells. Eur J Cancer. 84:193–201. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen K, Qian W, Jiang Z, Cheng L, Li J,
Sun L, Zhou C, Gao L, Lei M, Yan B, et al: Metformin suppresses
cancer initiation and progression in genetic mouse models of
pancreatic cancer. Mol Cancer. 16:1312017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duan W, Chen K, Jiang Z, Chen X, Sun L, Li
J, Lei J, Xu Q, Ma J, Li X, et al: Desmoplasia suppression by
metformin-mediated AMPK activation inhibits pancreatic cancer
progression. Cancer Lett. 385:225–233. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duan W, Li R, Ma J, Lei J, Xu Q, Jiang Z,
Nan L, Li X, Wang Z, Huo X, et al: Overexpression of Nodal induces
a metastatic phenotype in pancreatic cancer cells via the Smad2/3
pathway. Oncotarget. 6:1490–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang Z, Chen X, Chen K, Sun L, Gao L,
Zhou C, Lei M, Duan W, Wang Z, Ma Q and Ma J: YAP inhibition by
resveratrol via activation of AMPK enhances the sensitivity of
pancreatic cancer cells to gemcitabine. Nutrients. 8(pii):
E5462016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Collisson EA and Olive KP: Pancreatic
cancer: Progress and challenges in a rapidly moving field. Cancer
Res. 77:1060–1062. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang HH and Guo XL: Combinational
strategies of metformin and chemotherapy in cancers. Cancer
Chemother Pharmacol. 78:13–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tian M, Neil JR and Schiemann WP:
Transforming growth factor-β and the hallmarks of cancer. Cell
Signal. 23:951–962. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morrison CD, Parvani JG and Schiemann WP:
The relevance of the TGF-β Paradox to EMT-MET programs. Cancer
Lett. 341:30–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ikushima H and Miyazono K: TGFbeta
signalling: A complex web in cancer progression. Nat Rev Cancer.
10:415–424. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pirozzi G, Tirino V, Camerlingo R, Franco
R, La Rocca A, Liguori E, Martucci N, Paino F, Normanno N and Rocco
G: Epithelial to mesenchymal transition by TGFβ-1 induction
increases stemness characteristics in primary non small cell lung
cancer cell line. PLoS One. 6:e215482011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lamouille S and Derynck R: Cell size and
invasion in TGF-beta-induced epithelial to mesenchymal transition
is regulated by activation of the mTOR pathway. J Cell Biol.
178:437–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ellenrieder V, Hendler SF, Ruhland C,
Boeck W, Adler G and Gress TM: TGF-beta-induced invasiveness of
pancreatic cancer cells is mediated by matrix metalloproteinase-2
and the urokinase plasminogen activator system. Int J Cancer.
93:204–211. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yin T, Wang C, Liu T, Zhao G and Zhou F:
Implication of EMT induced by TGF-beta1 in pancreatic cancer. J
Huazhong Univ Sci Technol Med Sci. 26:700–702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feig C, Gopinathan A, Neesse A, Chan DS,
Cook N and Tuveson DA: The pancreas cancer microenvironment. Clin
Cancer Res. 18:4266–4276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Apte MV, Park S, Phillips PA, Santucci N,
Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA,
et al: Desmoplastic reaction in pancreatic cancer: Role of
pancreatic stellate cells. Pancreas. 29:179–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang D, Wang D, Yuan Z, Xue X, Zhang Y, An
Y, Chen J, Tu M, Lu Z, Wei J, et al: Persistent activation of
pancreatic stellate cells creates a microenvironment favorable for
the malignant behavior of pancreatic ductal adenocarcinoma. Int J
Cancer. 132:993–1003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lonardo E, Frias-Aldeguer J, Hermann PC
and Heeschen C: Pancreatic stellate cells form a niche for cancer
stem cells and promote their self-renewal and invasiveness. Cell
Cycle. 11:1282–1290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mantoni TS, Lunardi S, Al-Assar O,
Masamune A and Brunner TB: Pancreatic stellate cells radioprotect
pancreatic cancer cells through β1-integrin signaling. Cancer Res.
71:3453–3458. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Löhr M, Schmidt C, Ringel J, Kluth M,
Müller P, Nizze H and Jesnowski R: Transforming growth factor-beta1
induces desmoplasia in an experimental model of human pancreatic
carcinoma. Cancer Res. 61:550–555. 2001.PubMed/NCBI
|
|
41
|
Vonlaufen A, Phillips PA, Xu Z, Goldstein
D, Pirola RC, Wilson JS and Apte MV: Pancreatic stellate cells and
pancreatic cancer cells: An unholy alliance. Cancer Res.
68:7707–7710. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Incio J, Suboj P, Chin SM, Vardam-Kaur T,
Liu H, Hato T, Babykutty S, Chen I, Deshpande V, Jain RK and
Fukumura D: Metformin reduces desmoplasia in pancreatic cancer by
reprogramming stellate cells and tumor-associated macrophages. PLoS
One. 10:e01413922015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen K, Qian W, Li J, Jiang Z, Cheng L,
Yan B, Cao J, Sun L, Zhou C, Lei M, et al: Loss of AMPK activation
promotes the invasion and metastasis of pancreatic cancer through
an HSF1-dependent pathway. Mol Oncol. 11:1475–1492. 2017.
View Article : Google Scholar : PubMed/NCBI
|