Introduction
The incidence of primary liver cancer continues to
increase in numerous countries, in part due to an increase in the
number of individuals infected with hepatitis C virus (1). Liver cancer is a malignant tumor with
a high mortality rate. Treatment modalities include surgery,
cryotherapy, hepatic arterial chemoembolization, radiofrequency
ablation, biological therapy, radiotherapy and radioactive seed
implantation (2), however, each of
these approaches has limitations. It is of clinical significance to
identify reasonable and effective treatments for patients with
advanced liver cancer (3,4).
1,25-Dihydroxy vitamin D3
[1,25(OH)2D3] is an important vitamin
required to sustain physiologic functions (5). It is important in cell proliferation,
differentiation and immune function, in addition to regulating bone
development and calcium and phosphorus metabolism (6). 1,25(OH)2D3 can
induce apoptosis in a variety of cells, with particularly
pronounced effects in tumor cells (7); however, the specific mechanism of
action remains to be fully elucidated.
Notch is a conserved cell membrane surface receptor,
which interacts with Notch ligands expressed on adjacent cell
surfaces (8). Normally, the ligand
can activate Notch signaling by inducing proteolysis, which
releases the intracellular region of Notch (ICN) from the cell
membrane. Upon entering the nucleus, the ICN activates the
suppressor of hairless/C-promoter binding factor 1 family of
transcription factors and regulates cell growth and development
(9). Notch signaling can either
promote or prevent cell differentiation, and it is critical in
tumor stem cell development (10).
The Notch pathway increases the activity of cancer stem cells,
resulting in tumor formation (11).
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl
ester (DAPT) is a synthetic γ secretase inhibitor, also known as a
Notch signal pathway inhibitor. DAPT does not induce toxicity or
cause side effects at a certain dose range, and has antitumor
effects (12,13), particularly with regard to
preventing hepatocellular carcinoma (HCC) invasion (14).
The present study applied
1,25(OH)2D3 in conjunction with the Notch
signaling pathway inhibitor DAPT to examine the anticancer effects
of these two drugs in liver cancer. The results may provide
experimental evidence supporting their use in the clinical
treatment of liver cancer.
Materials and methods
Cell culture
The HepG2 liver cancer cell line was purchased from
the Shanghai Cell Bank of Chinese Academy of Science (Shanghai,
China) and cultured in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Hyclone
Laboratories; GE Healthcare Life Sciences, Logan, UT, USA) in 5%
CO2 at 37°C.
The cells were divided into the following six
groups: Control, 10−10 M
1,25(OH)2D3, 10−8 M
1,25(OH)2D3, 10−6 M
1,25(OH)2D3, 1 µM DAPT, 5 µM DAPT, 10 µM
DAPT, and 10−6 M 1,25(OH)2D3 + 10
µM DAPT. Treatments were applied when the cell confluence had
reached 70%. Following treatment for 48 h at 37°C, Cell Counting
Kit-8 (CCK-8) assays, flow cytometry, Transwell assays and
wound-healing assays were performed to assess cell proliferation,
cell cycle, apoptosis, migration and invasion. The expression of
Notch and Jagged were detected by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analyses.
CCK-8 assay
The HepG2 cells were seeded in 96-well plates
(3×103 cells/ml) and treated with
1,25(OH)2D3 and/or DAPT for 48 h. Following
treatment, 10 µl medium with CCK-8 (Gibco; Thermo Fisher
Scientific, Inc.) was added into each well. Following an additional
incubation period of 4 h in a CO2 incubator at 37°C, the
absorbances were detected on a microplate reader (Thermo Fisher
Scientific, Inc.) at 560 nm. Cell viability was defined by the
optical density values.
Flow cytometry
The HepG2 cells were seeded in 6-well plates and
treated with 1,25(OH)2D3 and/or DAPT for 48
h. The cells were collected following digestion by trypsin (Gibco;
Thermo Fisher Scientific, Inc.). The cells were incubated with
Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide
(PI; cat. no. C1062; Beyotime Institute of Biotechnology, Ningbo,
China) for 30 min in the dark. Apoptosis and cell cycle
distribution were detected by flow cytometry (BD Biosciences,
Franklin Lakes, NJ, USA) and data were analyzed with FlowJo 10
software (FlowJo LLC, Ashland, OR, USA). The Cell Cycle platform in
FlowJo 10 software was used to analyze the cell cycle
distribution.
Transwell assay
The HepG2 cells were seeded in 6-well plates and
treated with 1,25(OH)2D3 and/or DAPT for 48
h. The cells were then digested, collected, seeded into the upper
chamber of Transwells, and subjected to serum starvation for 1 day.
The lower chamber contained DMEM with 10% FBS. The cells were
cultured in a CO2 incubator for 24 h, following which
the lower chamber was removed, fixed with polyformaldehyde for 20
min and stained with crystal violet (0.1%). Light microscopy was
used to capture images in at least five fields. The counted cell
numbers represented the cell invasion capacity.
Cell migration
The HepG2 cells were seeded in 6-well plates and
treated with 1,25(OH)2D3 and/or DAPT for 48
h. A pipette tip was used to create an even line across the center
of the confluent cell mass. Following incubation in a
CO2 incubator at 37°C for 48 h, images were captured
under a light microscope. The width at 0 h was divided by the width
at 48 h to calculate cell migration.
RT-qPCR analysis
The HepG2 cells were seeded in 6-well plates and
treated with 1,25(OH)2D3 and/or DAPT for 48
h. Total mRNA was extracted using a TRIzol assay kit (Baosheng
Science and Technology Innovation Co., Ltd., Shanghai, China).
Subsequently, the mRNA was transcribed into cDNA using a Reverse
Transcription kit according to the manufacturer's protocol (Takara
Biotechnology, Co., Ltd., Dalian, China). Fluorescence RT-qPCR
analysis was utilized to detect expression levels of the targeted
genes using cDNA as a template. mRNA was transcribed into cDNA
using a reverse transcription kit (cat. no. 639522; Takara
Biotechnology) at 37°C and qPCR was used to detect the expression
level of the target genes by using SYBR Green. The amplification
reactions were performed with initial denaturation at 95°C for 10
min, followed by 35 cycles of a two-step PCR at 95°C for 14 sec and
60°C for 1 min. The levels of Notch1, Notch2, Jagged1 and Jagged2
were normalized to GAPDH using 2−ΔΔCq method as
previously described (15). The
primers were as follows: Notch1, forward 5′-AATGTGGATGCCGCAGTTG-3′
and reverse 5′-ATCCGTGATGTCCCGGTTG-3′; Notch2, forward
5′-AGCTGCTACTCACAGGTGAACGAA-3′ and reverse
5′-CCAGCCTGCATCACAGAGACA-3′; Jagged1, forward
5′-CCAGGTCTTTGAGAACTCCAGATG-3′ and reverse
5′-TGACCAGAGCAGGCAGATGAA-3′; GAPDH, forward
5′-CAATGACCCCTTCATTGACC-3′ and reverse
5′-GAGAAGCTTCCCGTTCTCAG-3′.
Western blot analysis
The HepG2 cells were seeded in 6-well plates and
treated with 1,25(OH)2D3 and/or DAPT for 48
h. Protein (20 µg) was extracted from the treated cells by a
protein isolation kit (cat. no. 28-9425-44; ReadyPrep; GE
Healthcare Life Sciences) and protein levels were quantified with a
bicinchoninic acid protein assay kit. Protein (25 µg/lane) wasrun
on sodium-dodecyl sulfate-polyacrylamide gels (10%), and
transferred onto membranes. The membranes were blocked with 5%
de-fat milk for 2 h at room temperature. The following primary
antibodies were used for overnight incubation at 4°C: Anti-Notch1
(dilution 1:1,000; cat. no. ab44986; Abcam, Cambridge, UK),
anti-Notch2 (dilution 1:2,000; cat. no. ab8926; Abcam),
anti-Jagged1 (dilution 1:1,000; cat. no. ab7771; Abcam) and
anti-Jagged2 (dilution 1:1,000; cat. no. ab109627; Abcam).
Following washing with PBST (0.2% Tween-20), the membranes were
incubated with the secondary antibody (dilution 1:100; cat. no.
ab131368; Abcam) for 2 h at room temperature. An Enhanced
Chemiluminescence kit (cat. no. RPN2133; GE Healthcare Life
Sciences) was added to the membrane prior to visualization with a
gel imaging system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
The data are presented as mean ± standard error of
mean (SEM). Statistical significance was calculated by one-way
analysis of variance (ANOVA) with Newman-Keuls as the post hoc test
(SPSS 17.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
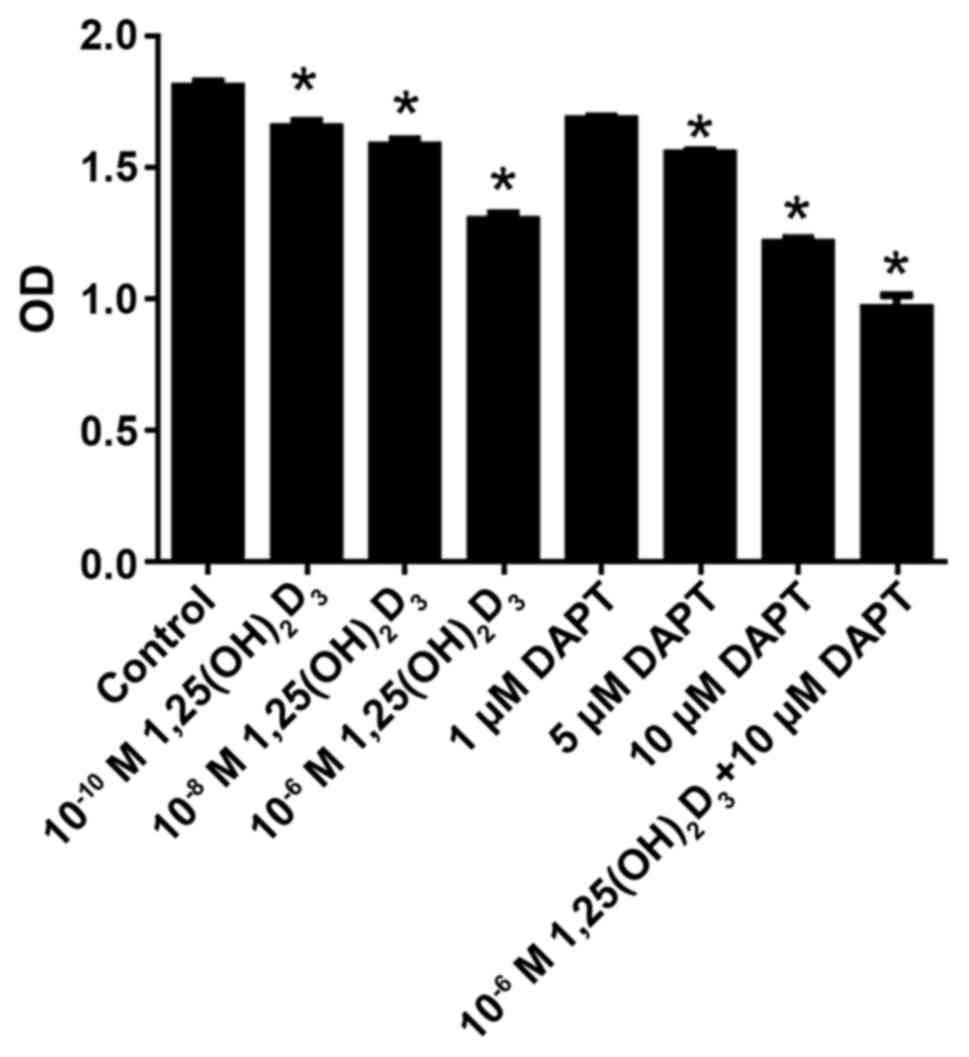
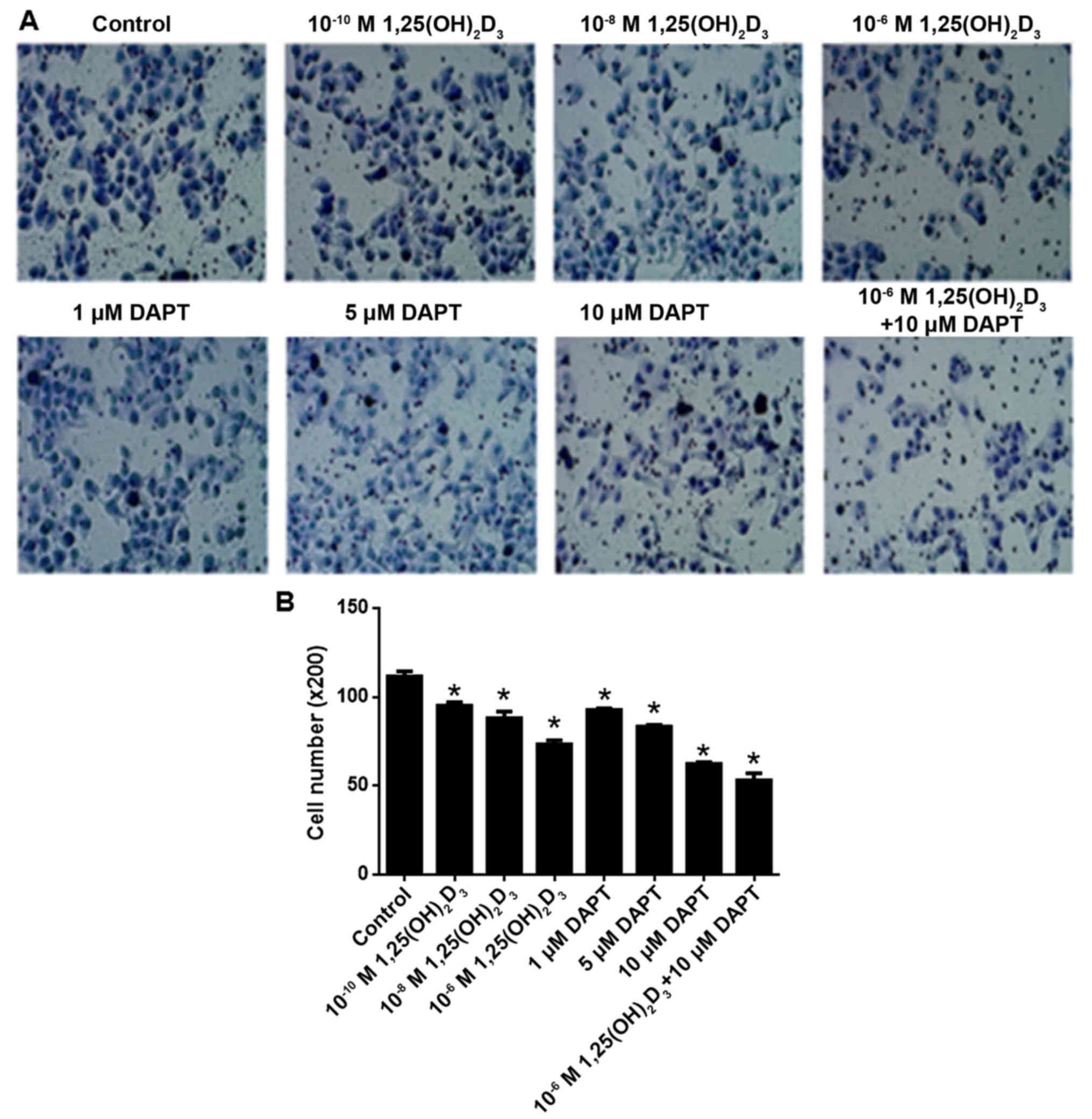
DAPT and
1,25(OH)2D3 inhibit HepG2 cell
proliferation
As shown in Fig. 1,
1,25(OH)2D3 inhibited cell proliferation in a
concentration-dependent manner at a range of
10−10−10−6 M. Following statistical analysis,
10−10, 10−8 and 10−6 M
1,25(OH)2D3 were found to significantly
inhibit cell proliferation compared with the control (P<0.05).
Similarly, DAPT inhibited cell proliferation in a
concentration-dependent manner at the range of 1–10 µM. Following
statistical analysis, 5 and 10 µM DAPT significantly inhibited cell
proliferation compared with the control (P<0.05). In addition
10−6 M 1,25(OH)2D3 and 10 µM DAPT
were co-applied. The results showed that 10 µM DAPT further
increased the anti-proliferative effect of 10−6 M
1,25(OH)2D3.
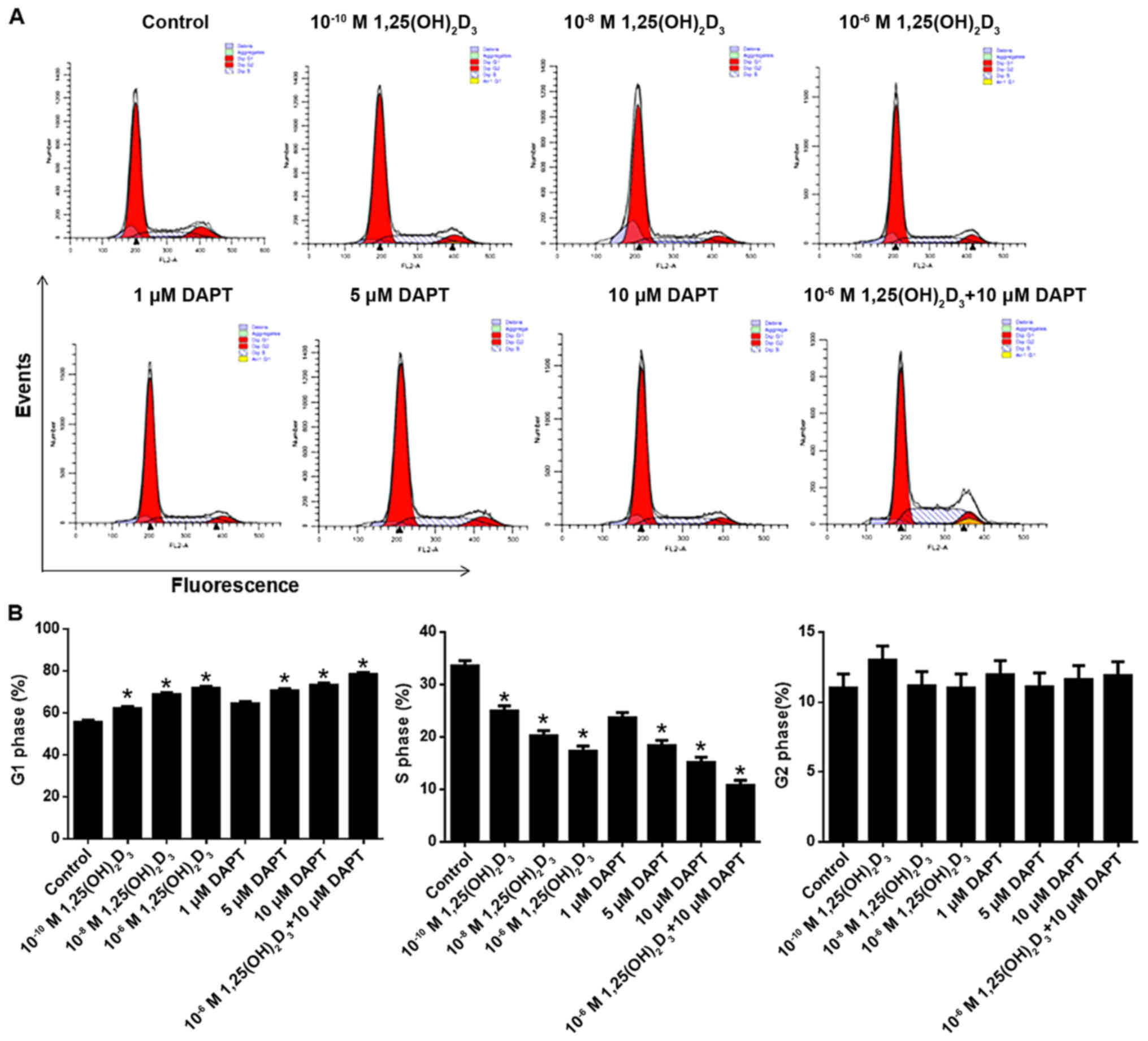
DAPT facilitates
1,25(OH)2D3-induced cell cycle arrest in
HepG2 cells
As shown in Fig. 2A and
B, 1,25(OH)2D3 increased and decreased
the numbers of cells in the G1 and S phases, respectively, in a
concentration-dependent manner at the range between
10−10 and 10−6 M. Statistical analysis showed
that the effects of 10−10, 10−8 and
10−6 M 1,25(OH)2D3 were
significant compared with the control (P<0.05). It was also
observed that DAPT increased the number of cells in the G1 phase
and decreased the number of cells in the S phase in a
concentration-dependent manner between 1 and 10 µM. Statistical
analysis showed that 5 and 10 µM DAPT significantly increased the
number of cells in the G1 phase and decreased those in the S phase
compared with the control (P<0.05). Notably, the co-application
of 10 µM DAPT further increased the cell cycle arrest observed
following treatment with 10−6 M
1,25(OH)2D3.
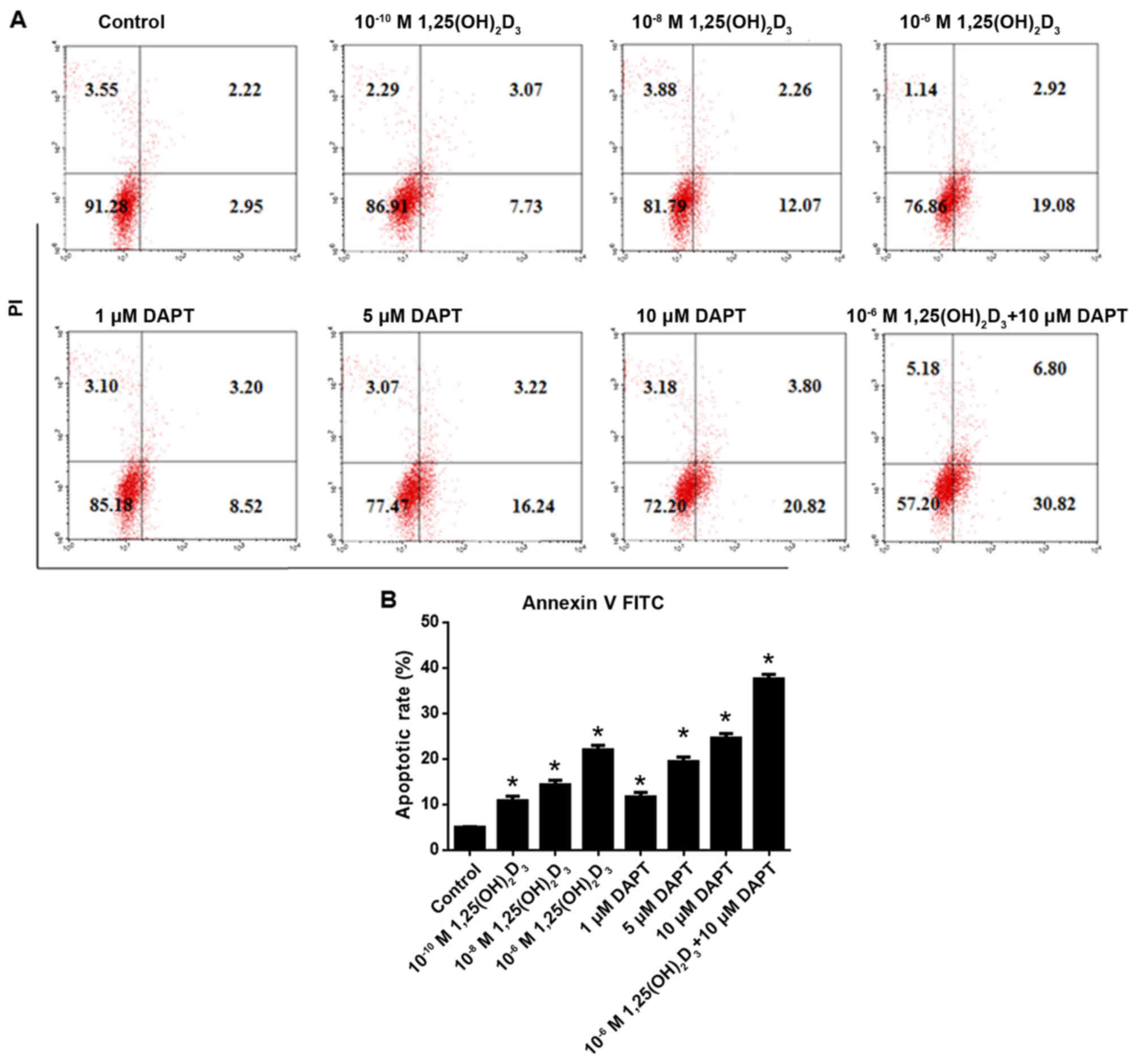
DAPT facilitates
1,25(OH)2D3-induced apoptosis
As shown in Fig. 3A and
B, 1,25(OH)2D3 induced HepG2 cell
apoptosis in a concentration-dependent manner between
10−10 and 10−6 M. Statistical analysis showed
that 10−10, 10−8 and 10−6 M
1,25(OH)2D3 significantly induced apoptosis
compared with that in the control (P<0.05). Similarly, DAPT
concentration-dependently induced apoptosis at a range of 1–10 µM.
Following statistical analysis, 1, 5 and 10 µM DAPT were found to
significantly induce HepG2 cell apoptosis compared with that in the
control (P<0.05). The co-application of 10−6 M
1,25(OH)2D3 and 10 µM DAPT revealed that
inhibiting γ secretase further increased the apoptosis induced by
10−6 M 1,25(OH)2D3.
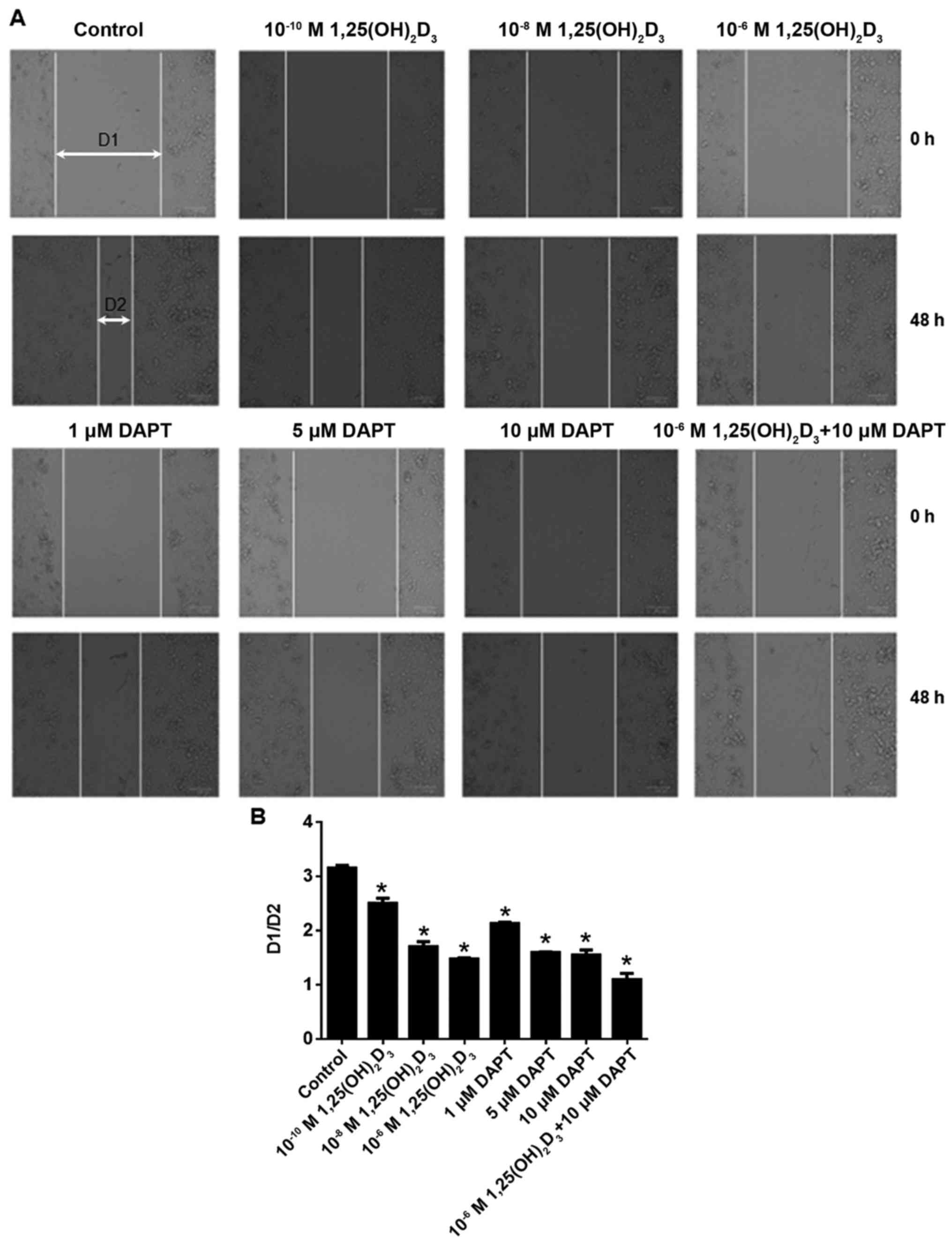
DAPT facilitates the anti-migration
effect of 1,25(OH)2D3
As shown in Fig. 4A and
B, 1,25(OH)2D3 inhibited HepG2 cell
migration at the range of 10−10−10−6 M. It
was also shown that DAPT inhibited HepG2 cell migration at the
range of 1–10 µM. The results showed that the combination of 10 µM
DAPT with 10−6 M 1,25(OH)2D3
further inhibited cell migration.
DAPT facilitates the anti-invasion
effect of 1,25(OH)2D3
As shown in Fig. 5A and
B, 1,25(OH)2D3 inhibited cell invasion in
a concentration-dependent manner at the range of
10−10−10−6 M. Statistical analysis showed
that 10−10, 10−8 and 10−6 M
1,25(OH)2D3 significantly inhibited cell
invasion compared with the that in the control (P<0.05). It was
also shown that DAPT inhibited cell invasion in a
concentration-dependent manner at the range of 1–10 µM. Statistical
analysis revealed that 1, 5 and 10 µM DAPT significantly inhibited
cell invasion compared with that in the control (P<0.05). The
results also showed that 10 µM DAPT further increased the
anti-invasion effect of 10−6 M
1,25(OH)2D3.
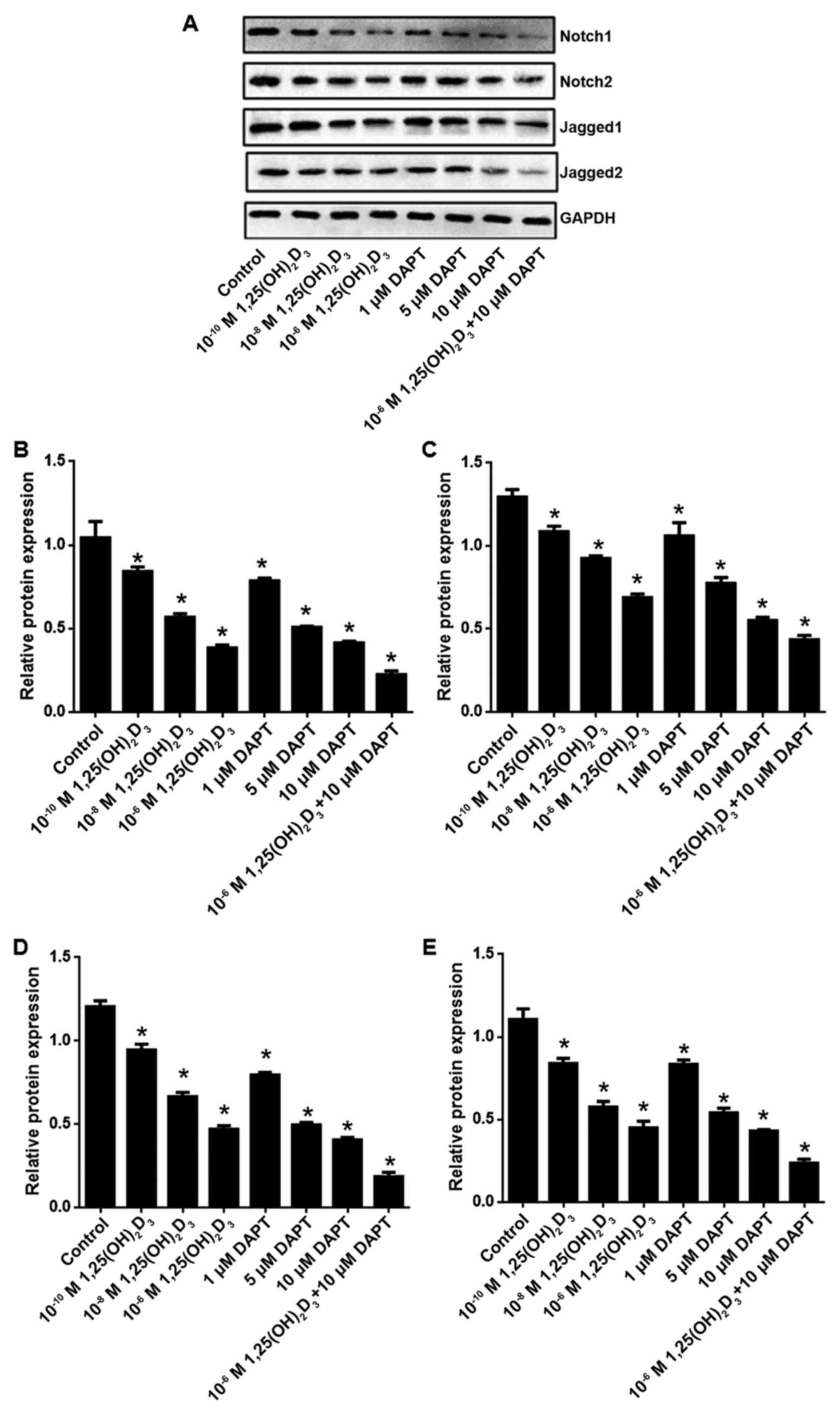
1,25(OH)2D3
and/or reduces the expression of Notch1, Notch2, Jagged1 and
Jagged2
Compared with the control group, the mRNA levels of
Notch1, Notch2, Jagged1 and Jagged2 were significantly reduced
following 1,25(OH)2D3 and/or DAPT treatment
(Fig. 6A-D). It was also confirmed
that 1,25(OH)2D3 and/or DAPT reduced the
protein levels of Notch1, Notch2, Jagged1 and Jagged2 (Fig. 7A-E).
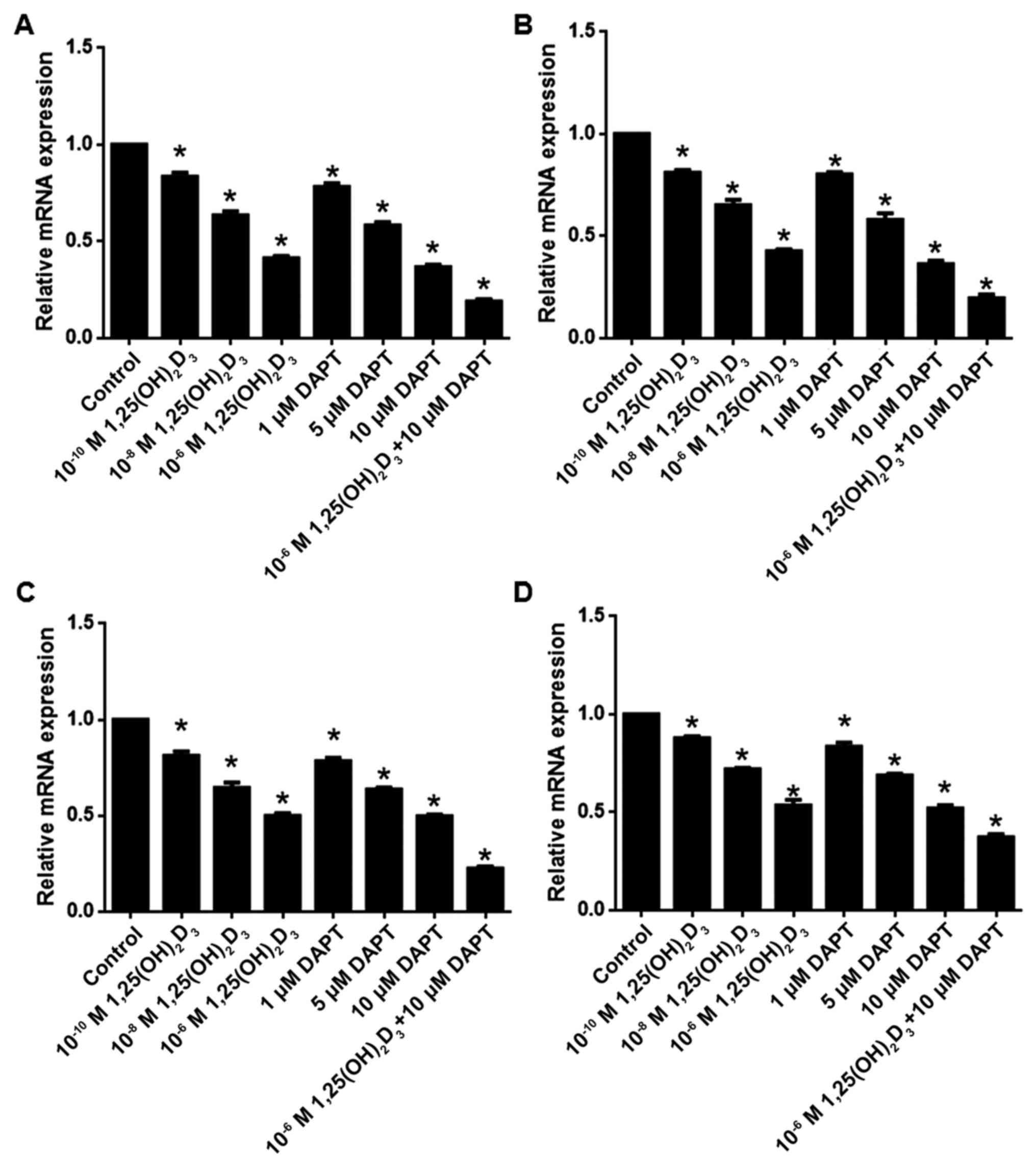 | Figure 6.1,25(OH)2D3
and/or DAPT reduces the mRNA levels of Notch1, Notch2, Jagged1 and
Jagged2. mRNA levels of (A) Notch1, (B) Notch2, (C) Jagged1 and (D)
Jagged2. Results are presented as the mean ± standard error of the
mean of six replicates. *P<0.05, vs. control. DAPT,
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl
ester; 1,25(OH)2D3, 1,25-dihydroxy vitamin
D3. |
Discussion
Several studies have reported the anticancer effect
of 1,25(OH)2D3 (14,16,17),
including in the context of liver cancer (18,19).
However, the mechanisms involved remain to be fully elucidated. HCC
and hepatoblastoma are types of primary liver cancer, which occur
predominantly in adults and children, respectively (20). In the present study, the HepG2
hepatoblastoma cell line was selected, which was initially
considered to be a HCC cell line (19) but was later identified as a
hepatoblastoma-derived cell line (21). The present study investigated the
antitumor effect of 1,25(OH)2D3, which is the
active form of vitamin D, and examined its synergistic effects with
DAPT. The HepG2 cell line was selected to assess
1,25(OH)2D3 cytotoxicity over a wide
concentration range to provide a therapeutic index for this agent
as an anticancer drug in humans. At
1,25(OH)2D3 of >1,000 nM cell viability
was significantly reduced. The physiological range of
1,25(OH)2D3 in human plasma is 36–150 pM. A
dose of 165 µg (~2.75 µg/kg) is clinically safe, even when
administered weekly (Cmax 14.9±4.78 nM) (22). As the intracellular concentration is
usually higher than that in serum, the present study used 100–1,000
nM for in vitro experiments, which was similar to the
approach adopted in other studies (23,24).
It was previously reported that 1,25(OH)2D3
did not affect normal gastric cells in this dose range (25), suggesting the safety of clinical
1,25(OH)2D3 use. In addition, DAPT
co-application promoted the action of
1,25(OH)2D3. In future investigations, a
lower dose of 1,25(OH)2D3 requires assessment
to confirm the synergistic effect of DAPT.
Liver cancer is one of the most common primary
malignant tumors and is the third leading cause of
cancer-associated mortality globally (26). Effective treatments are required to
improve patient quality of life and prolong survival rates,
therefore, it is of clinical significance to identify effective
therapeutic compounds and clarify their mechanisms of action. DAPT
is a synthetic secretory enzyme inhibitor, which can inhibit Notch
signaling (27). As previously
described, signaling dysfunction is a hallmark of multiple types of
cancer (28,29). The Notch pathway is closely
associated with tumor occurrence and development. Therefore,
inhibitors of tumor-related signaling pathways are considered
candidates for treating tumors (30). In addition, the Notch signaling
pathway inhibitor has low cytotoxicity and does not induce side
effects. These characteristics provide a novel approach for the
investigation and development of novel antitumor drugs (31). The present study provided in
vitro evidence that DAPT inhibited liver cancer cell
proliferation, migration and invasion. In a previous study, long
intergenic non-coding RNA-p21 was found to inhibit HCC invasion and
metastasis through its effects on Notch signaling (7). The results of the present study also
demonstrated that 1,25(OH)2D3 enhanced the
anti-invasive effects of DAPT in liver cancer cells. However, the
antitumor effects of 1,25(OH)2D3 + DAPT
require validation in an in vivo model in the future to
understand its antitumor effects.
Although the cytotoxicity of DAPT is low, high doses
can elicit adverse effects. The dose of DAPT requires strict
control, however, a single low dose may not effectively prevent
cancer progression. 1,25(OH)2D3 has several
biological activities, including maintaining a stable calcium
environment, regulating immune function and affecting tumor
biological activity, which may affect cell proliferation and
apoptosis (32).
1,25(OH)2D3 functions mainly through binding
to a specific vitamin D receptor to inhibit myeloid leukemia cell
proliferation (33). Previous
studies have shown that 1,25(OH)2D3 can
inhibit tumor cell proliferation, reduce tumor cell migration and
invasion, and induce apoptosis of prostate cancer cells (34). The results of the present study
confirmed that 1,25(OH)2D3 exerted anticancer
activity in liver cancer cells. Critically, the combined
application of 1,25(OH)2D3 with DAPT had more
pronounced effects, compared with either
1,25(OH)2D3 or DAPT alone. In the present
study, Annexin V-FITC/PI were applied to stain the cells and
apoptosis was detected by flow cytometry. Although the combined
application of 1,25(OH)2D3 with DAPT had more
pronounced effects on apoptosis, typical apoptotic pathways require
evaluation, including caspase pathways (35). The present study also quantified the
cell cycle distribution following drug treatments. The combined
application of 1,25(OH)2D3 with DAPT further
arrested the cells at the G1 phase. Cyclin D1 is an important cell
cycle regulatory protein (36),
which requires detection to confirm the effects on cell cycle
arrest.
Notch is a conserved pathway in biological evolution
and reflects the complex mechanisms of intercellular communication.
It is important in cell fate determination during the development
of several organs (37). Notch
signaling also occurs in several mature mammalian tissues (38), regulating cell growth,
differentiation, tissue regeneration and intracellular environment
stability. It is involved in normal and pathological states of
liver development; the major molecules are Notch1-4 and their
ligands are Jagged1 and δ-like 4 (39). Abnormal Notch expression and
signaling are associated with various malignancies. In the present
study, RT-qPCR and western blot analyses were performed, and it was
found that Notch1, Notch2, Jagged1 and Jagged2 were expressed at
low levels in liver cancer cells following treatment with DAPT
and/or 1,25(OH)2D3. These results suggested
that Notch signaling is involved in promoting liver cancer cell
progression by regulating cell cycle and apoptosis. As Notch
signaling is also associated with autophagy (40), whether
1,25(OH)2D3 and/or DAPT affects autophagy in
liver cancer cells requires further investigation.
In conclusion, the findings of the present study
demonstrated that treatment of liver cancer with
1,25(OH)2D3 inactivated Notch signaling,
prevented proliferation, migration and invasion, and promoted
apoptosis. The combined application of
1,25(OH)2D3 with the synthetic γ secretase
inhibitor DAPT had more marked effects. However, the present study
selected a type of liver cancer cell with hepatoblastoma origin. In
a future study, the anti-liver cancer effect of the combination
application of 1,25(OH)2D3 with DAPT on HCC
also requires confirmation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LC, ZT and XM conceived and designed the study. LC
and LL performed the experiments. LC and XM wrote the manuscript.
LC, LL, ZT and XM reviewed and edited the manuscript. All authors
read and approved the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Institutional Review Board of Jiangxi Provincial People's Hospital
(Jiangxi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Cevik O, Li D, Baljinnyam E, Manvar D,
Pimenta EM, Waris G, Barnes BJ and Kaushik-Basu N: Interferon
regulatory factor 5 (IRF5) suppresses hepatitis C virus (HCV)
replication and HCV-associated hepatocellular carcinoma. J Biol
Chem. 292:21676–21689. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thein HH, Qiao Y, Zaheen A, Jembere N,
Sapisochin G, Chan KK, Yoshida EM and Earle CC: Cost-effectiveness
analysis of treatment with non-curative or palliative intent for
hepatocellular carcinoma in the real-world setting. PLoS One.
12:e01851982017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ballotari P, Vicentini M, Manicardi V,
Gallo M, Chiatamone Ranieri S, Greci M and Giorgi Rossi P: Diabetes
and risk of cancer incidence: Results from a population-based
cohort study in northern Italy. BMC Cancer. 17:7032017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo M, Kitano M, Sakurai T and Nishida N:
General rules for the clinical and pathological study of primary
liver cancer, nationwide follow-up survey and clinical practice
guidelines: The outstanding achievements of the liver cancer study
group of Japan. Dig Dis. 33:765–770. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jia X, Gu Y, Groome LJ, Al-Kofahi M,
Alexander JS, Li W and Wang Y: 1,25(OH)2D3 induces placental
vascular smooth muscle cell relaxation by phosphorylation of myosin
phosphatase target subunit 1Ser507Potential beneficial
effects of Vitamin D on placental vasculature in humans. Biol
Reprod. 94:1162016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang CJ, Zhao D, Yin X, Zhang H, Ma L,
Chen JP, Liu C and Yang XP: Effects of 1,25(OH)2D3 on proliferation
and apoptosis of human glomerular mesangial cells. Am J Transl Res.
8:2659–2666. 2016.PubMed/NCBI
|
|
7
|
He XJ, Ding Y, Xiang W and Dang XQ: Roles
of 1,25(OH)2D3 and Vitamin D receptor in the pathogenesis of
rheumatoid arthritis and systemic lupus erythematosus by regulating
the activation of CD4+ T cells and the PKCδ/ERK
signaling pathway. Cell Physiol Biochem. 40:743–756. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fazio C and Ricciardiello L: Inflammation
and Notch signaling: A crosstalk with opposite effects on
tumorigenesis. Cell Death Dis. 7:e25152016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo J, Wang P, Wang R, Wang J, Liu M,
Xiong S, Li Y and Cheng B: The Notch pathway promotes the cancer
stem cell characteristics of CD90+ cells in
hepatocellular carcinoma. Oncotarget. 7:9525–9537. 2016.PubMed/NCBI
|
|
10
|
Kim SJ, Lee HW, Baek JH, Cho YH, Kang HG,
Jeong JS, Song J, Park HS and Chun KH: Activation of nuclear PTEN
by inhibition of Notch signaling induces G2/M cell cycle arrest in
gastric cancer. Oncogene. 35:251–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsai Y: Social security income and the
utilization of home care: Evidence from the social security notch.
J Health Econ. 43:45–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z, Wang J, Zhao C, Ren K, Xia Z, Yu H
and Jiang K: Acute blockage of Notch signaling by DAPT induces
neuroprotection and neurogenesis in the neonatal rat brain after
stroke. Transl Stroke Res. 7:132–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Ye Z, Zheng S, Chen L, Wan Y, Deng
Y and Yang R: Lingo-1 shRNA and Notch signaling inhibitor DAPT
promote differentiation of neural stem/progenitor cells into
neurons. Brain Res. 1634:34–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jia M, Jiang L, Wang YD, Huang JZ, Yu M
and Xue HZ: lincRNA-p21 inhibits invasion and metastasis of
hepatocellular carcinoma through Notch signaling-induced
epithelial-mesenchymal transition. Hepatol Res. 46:1137–1144. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng N, Salker MS, Zhang S, Singh Y, Shi
B, Stournaras C and Lang F: 1α,25(OH)2D3 Induces actin
depolymerization in endometrial carcinoma cells by targeting RAC1
and PAK1. Cell Physiol Biochem. 40:1455–1464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Osafi J, Hejazi A, Stutz DD, Keiserman MA,
Bergman CJ and Kingsley K: Differential effects of
1,25-dihydroxyvitamin D3 on oral squamous cell carcinomas in vitro.
J Diet Suppl. 11:145–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu HQ and Zheng J: Synergistic inhibitory
effect of all-trans retinoic acid and 1,25-dihydroxy vitamin D3 on
growth of human hepatoma cell line HepG2. Ai Zheng. 25:1470–1476.
2006.(In Chinese). PubMed/NCBI
|
|
19
|
Huang J, Yang G, Huang Y and Zhang S:
Inhibitory effects of 1,25(OH)2D3 on the proliferation of
hepatocellular carcinoma cells through the downregulation of HDAC2.
Oncol Rep. 38:1845–1850. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taniguchi K, Roberts LR, Aderca IN, Dong
X, Qian C, Murphy LM, Nagorney DM, Burgart LJ, Roche PC, Smith DI,
et al: Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in
hepatocellular carcinomas and hepatoblastomas. Oncogene.
21:48632002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
22
|
Takiishi T, Ding L, Baeke F, Spagnuolo I,
Sebastiani G, Laureys J, Verstuyf A, Carmeliet G, Dotta F, Van
Belle TL, et al: Dietary supplementation with high doses of regular
vitamin D3 safely reduces diabetes incidence in NOD mice when given
early and long term. Diabetes. 63:2026–2036. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tangpricha V, Spina C, Yao M, Chen TC,
Wolfe MM and Holick MF: Vitamin D deficiency enhances the growth of
MC-26 colon cancer xenografts in Balb/c mice. J Nutr.
135:2350–2354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang J, Yang G, Huang Y and Zhang S:
1,25(OH)2D3 induced apoptosis of human hepatocellular carcinoma
cells in vitro and inhibited their growth in a nude mouse xenograft
model by regulating histone deacetylase 2. Biochimie. 146:28–34.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li M, Li L, Zhang L, Hu W, Shen J, Xiao Z,
Wu X, Chan FL and Cho CH: 1,25-Dihydroxyvitamin D3 suppresses
gastric cancer cell growth through VDR- and mutant p53-mediated
induction of p21. Life Sci. 179:88–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin YC, Lee BH, Alagie J and Su CH:
Combination treatment of ergosterol followed by amphotericin B
induces necrotic cell death in human hepatocellular carcinoma
cells. Oncotarget. 8:72727–72738. 2017.PubMed/NCBI
|
|
27
|
Liu X, Sheng HB, Ma R, Yang JM, Luo WW,
Yang XY, Ren DD and Chi FL: Notch signaling is active in normal
mouse middle ear epithelial cells. Exp Ther Med. 11:1661–1667.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Q, Chen X and Hay N: Akt as a target
for cancer therapy: More is not always better (lessons from studies
in mice). Br J Cancer. 117:159–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Melo AC, Paulino E and Garces ÁH: A
review of mTOR pathway inhibitors in gynecologic cancer. Oxid Med
Cell Longev. 2017:48097512017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murta D, Batista M, Silva E, Trindade A,
Henrique D, Duarte A and Lopes-da-Costa L: Notch signaling in the
epididymal epithelium regulates sperm motility and is transferred
at a distance within epididymosomes. Andrology. 4:314–327. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abad M, Hashimoto H, Zhou H, Morales MG,
Chen B, Bassel-Duby R and Olson EN: Notch inhibition enhances
cardiac reprogramming by increasing MEF2C transcriptional activity.
Stem Cell Reports. 8:548–560. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao D, Zhang CJ, Yang R, Chen JP, Ma L,
Liu G and Yang XP: Effect of 1,25(OH2D3 on the proliferation of
human mesangial cells and their expression of Ki67. Genet Mol Res.
16:2017.doi: 10.4238/gmr16029191. View Article : Google Scholar
|
|
33
|
Song JH, Park E, Kim MS, Cho KM, Park SH,
Lee A, Song J, Kim HJ, Koh JT and Kim TS: l-Asparaginase-mediated
downregulation of c-Myc promotes 1,25(OH)2 D3-induced myeloid
differentiation in acute myeloid leukemia cells. Int J Cancer.
140:2364–2374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abu El Maaty MA, Alborzinia H, Khan SJ,
Buttner M and Wölfl S: 1,25(OH)2D3 disrupts glucose metabolism in
prostate cancer cells leading to a truncation of the TCA cycle and
inhibition of TXNIP expression. Biochim Biophys Acta.
1864:1618–1630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fesik SW and Shi Y: Structural biology.
Controlling the caspases. Science. 294:1477–1478. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stacey DW: Cyclin D1 serves as a cell
cycle regulatory switch in actively proliferating cells. Curr Opin
Cell Biol. 15:158–163. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Geisler F and Strazzabosco M: Emerging
roles of Notch signaling in liver disease. Hepatology. 61:382–392.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu J, Chi F, Guo T, Punj V, Lee WN, French
SW and Tsukamoto H: NOTCH reprograms mitochondrial metabolism for
proinflammatory macrophage activation. J Clin Invest.
125:1579–1590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
D'Angelo RC, Ouzounova M, Davis A, Choi D,
Tchuenkam SM, Kim G, Luther T, Quraishi AA, Senbabaoglu Y, Conley
SJ, et al: Notch reporter activity in breast cancer cell lines
identifies a subset of cells with stem cell activity. Mol Cancer
Ther. 14:779–787. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song BQ, Chi Y, Li X, Du WJ, Han ZB, Tian
JJ, Li JJ, Chen F, Wu HH, Han LX, et al: Inhibition of Notch
signaling promotes the adipogenic differentiation of mesenchymal
stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR
pathway. Cell Physiol Biochem. 36:1991–2002. 2015. View Article : Google Scholar : PubMed/NCBI
|