Introduction
Salivary adenoid cystic carcinoma (SACC) is one of
the most common salivary epithelium-derived malignant tumor types,
and is associated with persistent slow growth, perineural invasion,
a high recurrence rate, and distant metastases (1). SACC accounts for ~18% of all salivary
gland malignancies in China (2).
Due to neurotropic invasion and lung metastasis, surgery,
radiotherapy and chemotherapy are often unsuccessful, and the
recurrence rate is 16–85% (3).
Furthermore, the rate of lung metastasis may be up to 40%, with the
5–10-year survival rate as low as 37.4–41.8% (3). As the basic molecular mechanism of
SACC carcinogenesis remains unclear, it is important to elucidate
the malignant biological behaviors involved in SACC invasion and
metastasis.
In human tissues, the normal oxygen partial pressure
is 30–60 mmHg, while in the areas surrounding tumors, oxygen
partial pressure is often <5 mmHg (4,5). When
the tumor volume exceeds 3 mm3, a necrotic tumor center
(hypoxic zone) is usually formed as a result of the inadequate
oxygen, nutrients and energy supplied by new blood vessels
(6). Hypoxia preferentially
stimulates the overexpression of hypoxia-inducible factors (HIFs)
and induces the expression of downstream genes via signal
transduction pathways (7,8). Thus, the overexpression of HIFs allows
cells to adapt to hypoxic conditions and proliferate, resulting in
high levels of invasion, metastasis and tolerance to radiation and
chemotherapy (9–11). HIF-1 is a protein found in the
nuclear extracts of the hepatocarcinoma cell line Hep-3B when
cultured under hypoxic conditions. It specifically binds to the
hypoxia response element (HRG) of the erythropoietin (EPO)
gene (12–14). HIF-1 is a heterodimer consisting of
α and β subunits (15), and growing
evidence indicates that the α unit (HIF-1α) is the functional
subunit that is the unique limiting factor in oxygen regulation
(16). The blockade of the
degradation of HIF-1α leads to the accumulation and overexpression
of HIF-1α under hypoxia, promoting tumor growth,
neovascularization, distant metastases and chemoradiation
resistance (17,18). In addition, HIF-1α is frequently
overexpressed in various solid tumor tissues, and is associated
with tumor invasion and poor prognosis (6). Thus, blocking HIF-1α expression is a
potentially important strategy for targeting anoxic tumor cells.
Stoeltzing et al (9)
constructed a HIF1α-carrying plasmid (pHIF-1α) that, when
transfected into gastric cancer cells, was associated with
decreased tumor growth. Sun et al (19) reported that transfection with a
plasmid containing antisense HIF1α downregulated HIF-1α
expression, resulting in the inhibition of vascular endothelial
growth factor VEGF expression and a decrease in tumor microvessel
density. However, the mechanisms underlying the suppression of
cancer growth by HIF-1α are complicated, and the effect of HIF-1α
expression on SACC has not previously been discussed.
The present study aimed to investigate the effects
of HIF-1α on the proliferation, invasion, metastasis, apoptosis,
and angiogenesis of SACC-83 cells under hypoxia. The results
revealed that the downregulation of HIF-1α inhibited the
proliferation, growth, invasion, metastasis and angiogenesis of
SACC-83 cells, while inducing morphological changes and apoptosis
in these cells.
Materials and methods
Cells and reagents
SACC-83 cell lines were cultured in Dulbecco's
modified Eagle's medium (DMEM; HyClone Laboratories, Inc., Logan,
UT, USA) containing 10% fetal bovine serum (FBS; Biological
Industries, Kibbutz Beit Haemek, Israel) and antibiotics (100 U/ml
penicillin and 100 µg/ml streptomycin) in a 5% CO2
incubator at 37°C. Following subculture, cells were placed in an
anoxic incubator (5% CO2, 5% O2, and 90%
N2; Biospherix, Parish, NY, USA) for hypoxic treatment.
Lipofectamine 2000 and OPTI-MEM were purchased from Thermo Fisher
Scientific, Inc. (Invitrogen; Waltham, MA, USA). PBS, DMSO and
bovine serum albumin (BSA) were purchased from Beijing Solarbio
Science & Technology Co., Ltd. (Beijing, China). The Annexin
V/FITC kit and Matrigel were purchased from BD Biosciences
(Franklin Lakes, NJ, USA). The human VEGF ELISA (cat. no. EHC108)
kit was purchased from NeoBioscience (Shenzhen, China). Crystal
violet staining solution was purchased from Beyotime Institute of
Biotechnology (Haimen, China). Anti-HIF-1α (cat. no. ab16066),
anti-β-actin (cat. no. 8457), and anti-matrix metalloproteinase-2
(MMP-2; cat. no. ab92536) antibodies were purchased from Abcam
(Cambridge, UK), while horseradish peroxidase-conjugated anti-mouse
IgG (cat. no. ZB-2305) and anti-rabbit IgG (cat. no. ZB-2301)
secondary antibodies were purchased from OriGene Technologies, Inc.
(Beijing, China).
Transfection
The plasmid pGPU6/GFP/Neo, containing short hairpin
RNA (shRNA) complementary to HIF1α, was used to examine the
function of HIF-1α. The pGPU6/GFP/Neo-HIF-1α-homo plasmid (Shanghai
GenePharma, Shanghai, China) contained a promoter-driven green
fluorescent protein (GFP) reporter. The most effective
HIF1α-targeted small interfering RNA (siRNA) sequence
(5′-GCAGCTACTACATCACTTTCT-3′) was transformed into shRNA
(stem-loop-stem structure) and cloned into the pGPU6/GFP/Neo
plasmid. A non-targeting sequence (5′-GTTCTCCGAACGTGTCACGT-3′) was
used to generate a negative control plasmid. SACC-83 cells were
cultured in 12-well plates (2×105 cells/well) with DMEM
containing 10% FBS for 24 h at 37°C in 5% CO2 to 80%
confluence. Subsequently, cells were transfected with HIF1α
shRNA plasmid or empty plasmid using Lipofectamine 2000 reagent
according to the manufacturer's instructions. Cells were
transfected with 4 µg/ml HIF1α shRNA plasmid and 4 µg/ml
empty plasmid in OPTI-MEM. The culture medium was changed from
OPTI-MEM to fresh DMEM supplemented with 10% FBS 6 h after
transfection. The number of cells expressing GFP was determined
using a fluorescence microscope (Olympus IX71; Olympus Corporation,
Tokyo, Japan) 24 h after transfection, and the total cell number in
the same field of view was determined under brightfield
illumination. The transfected cell rate (%) was estimated as the
number of GFP-positive cells/total cell number ×100. GFP-positive
clones with G418 resistance were screened after 6–8 weeks of
culture by selecting cells transfected with the sequence resulting
in the best interference effect. G418-resistant cells were further
cultured and used in subsequent experiments.
Western blot analysis
Cells from the three groups were collected after
hypoxic culture for 48 h and washed twice with cold PBS. Total
protein was isolated from cells using ice-cold RIPA for cell lysis
(1% Triton X-100, 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% sodium
dodecyl sulfate, 1 mM phenylmethylsulfonyl fluoride and 1 mM
ethylenediaminetetraacetic acid). Each mixture was centrifuged at
12,000 × g, for 20 min at 4°C, and the supernatant was collected
and boiled for 10 min at 100°C for immunoblotting. The
concentration of proteins was assessed using Bradford's method
(20). Proteins (30 µg) were
separated by 10% SDS-polyacrylamide gel electrophoresis at 100 V
for 2 h under reducing conditions and transferred to a
polyvinylidene fluoride (PVDF) membrane (Beijing Solarbio Science
& Technology Co., Ltd.) using the electrophoresis apparatus.
Membranes were blocked in 5% nonfat milk in Tris-buffered saline
containing 0.1% Tween-20 (TBST) for 1 h at room temperature.
Subsequently, PVDF membranes were incubated with antibodies against
HIF-1α (1:500), MMP-2 (1:1,000), and β-actin (1:2,000) overnight at
4°C. Then, the membranes were washed with TBST for 10 min three
times and incubated with the appropriate secondary antibody as
aforementioned (1:5,000) for 1 h at room temperature.
Immunoreactive proteins were detected using the SuperSignal™ West
Pico Chemiluminescent substrate (Thermo Fisher Scientific, Waltham,
MA, USA) followed by exposure to Bio-Rad ChemiDoc XRS+ image
analyzer (Hercules, CA, USA). Western blot signals were
semi-quantified by densitometry using Image-Pro Plus 6.0 software
(Media Cybernetics Inc., Bethesda, MD, USA). All western blot
experiments were repeated at least three times and then were used
to analyze the densitometry data.
Cell proliferation assay
Cell proliferation was determined by MTT assay
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). SACC-83 cells were
plated on 96-well plates (2×103 cells/well) and cultured
for 7 days under hypoxic conditions. Cell proliferation was
documented every 24 h according to the manufacturer's protocol.
Briefly, MTT solution (20 µl, 2.5 mg/ml) was added to each well and
incubated at 37°C. After 4 h, the medium was removed, and DMSO (150
µl) was added to each well to dissolve the formazan. Absorbance in
each well was measured at 490 nm using an automatic multi-well
spectrophotometer. The experiment was repeated at least three
times.
Cell apoptosis analysis
Cells were cultured under hypoxic conditions for 48
h. Subsequently, cells were collected and washed in ice-cold PBS
prior to staining with Annexin V/FITC and propidium iodide (PI)
solution for 15 min in the dark at room temperature. Cell apoptosis
was examined using a FACSCanto flow cytometer (BD Biosciences). All
experiments were repeated three times.
Cell cycle analysis
SACC-83 cells were seeded in 60-mm culture plates at
a density of 1×105 cells/well for observing the cell
cycle distribution. Cells were cultured under hypoxic conditions
for 48 h and were then harvested by trypsinization, fixed with 70%
ethanol and stored at 4°C overnight. After washing with PBS, cells
were incubated with 100 mg/ml RNase A and 50 mg/ml PI at room
temperature for 30 min in the dark. The distribution of the cells
was detected using a FACSCanto flow cytometer and analyzed using
FlowJo 10.0.7 software (TreeStar, Inc., Ashland, OR, USA).
ELISA
The concentration of VEGF in SACC-83 cell culture
medium was determined using human VEGF ELISA kit. SACC-83 cells
were cultured for 48 h to 90% confluency under hypoxic conditions.
Following treatment, the culture medium was collected and stored at
−80°C for use in the ELISA. All experiments were performed at least
three times, and the absorbance was measured at 450 nm.
Tube formation assay
SACC-83 cells were seeded in 60-mm plates. Following
adhesion, cells were cultured overnight in DMEM only and placed in
the hypoxic incubator for an additional 24 h. Human umbilical vein
endothelial cells (HUVECs; 3×104 cells/well) were seeded
on Matrigel-coated 96-well plates and incubated at 37°C. Serum-free
supernatant from shControl, shNC, and shHIF-1α cells were collected
and centrifuged at 716 × g, for 5 min at 25°C. The supernatants
were then incubated with HUVECs at 37°C for 24 h under hypoxic
conditions. The enclosed capillary structure of the tubes formed by
HUVECs was observed, and three different fields per well were
photographed (400× magnification) using an inverted microscope
(DMi1; Leica, Wetzlar, Germany). The total tube length of the tubes
in each field of view was measured using ImageJ 1.48 software
(National Institutes of Health, Bethesda, MD, USA).
Wound healing assay
The migration of SACC-83 cells was measured using
wound healing assays. SACC-83 cells (5×105 cells/well)
were seeded into a 6-well plate for 24 h. Next, 10-µl sterile
pipette tips were used to create linear scratch wounds on the
confluent cell monolayers. The three types of SACC-83 cells
(shControl, shNC, and shHIF-1α) were cultured in serum-free medium.
The scratch wounds were observed using an inverted microscope
(DMi1; Leica Microsystems GmbH), and images were acquired at 0, 24
and 48 h (×200 magnification). The migration of cells in the wound
area was quantified using ImageJ software. The scratch migration
rate (%) was estimated as [(Scratch width at 0 h - Scratch width at
24 or 48 h)/Scratch width at 0 h] × 100.
Matrigel invasion assay
A Transwell plate was pre-coated with 30 µl DMEM
containing 30% Matrigel and added to the membrane of the upper
chamber. The chamber was incubated at 37°C for 1 h. A suspension
(400 µl) of SACC-83 cells (2×105) at logarithmic growth
phase in serum-free medium only was seeded in the upper chamber.
DMEM (500 µl) with 10% FBS was added to the lower chamber, and
cells were cultured at 37°C in 5% CO2, 5% O2,
and 90% N2 for 24 h. The liquid in the upper chamber and
non-invasive cells on the membrane surface were removed with a wet
cotton swab. The filter was fixed in 90% ethanol for 10 min and
stained with crystal violet. The degree of invasion of SACC-83
cells was estimated under a microscope (×400 magnification) in five
fields. Invasion was assessed quantitatively by counting the cells
invading the lower side of the filter. The experiments were
conducted in triplicate.
Statistical analysis
Data were analyzed using GraphPad Prism (version
5.0; GraphPad Software, Inc., La Jolla, CA, USA). Quantitative data
are expressed as mean ± standard error of the mean. Statistical
analysis was performed using one-way analysis of variance with
Tukey's post hoc test for ≥3 groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of stable cell
lines
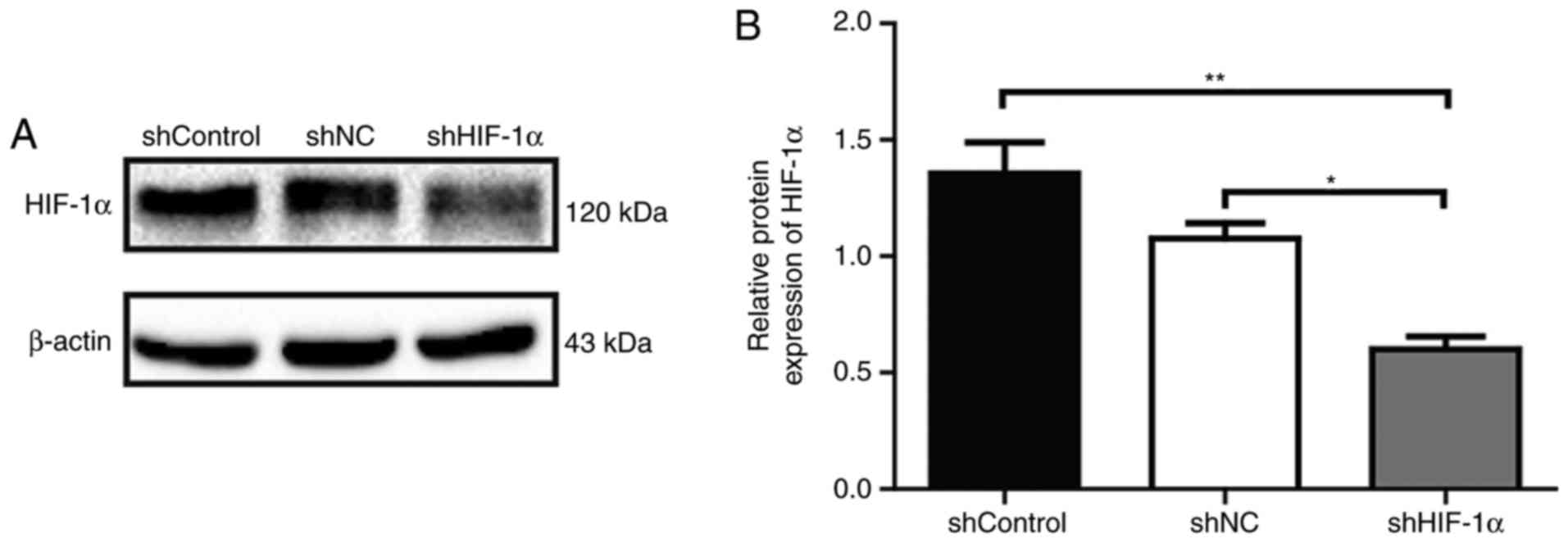
Western blot analysis was used to confirm the
inhibitory effects of HIF1α-targeted shRNA on the expression
of HIF-1α in SACC-83 cells. The results showed that the protein
expression of HIF-1α in SACC-83 cells was significantly reduced in
the shHIF-1α group compared with levels in the shControl and shNC
groups (P<0.01 and P<0.05, respectively; Fig. 1).
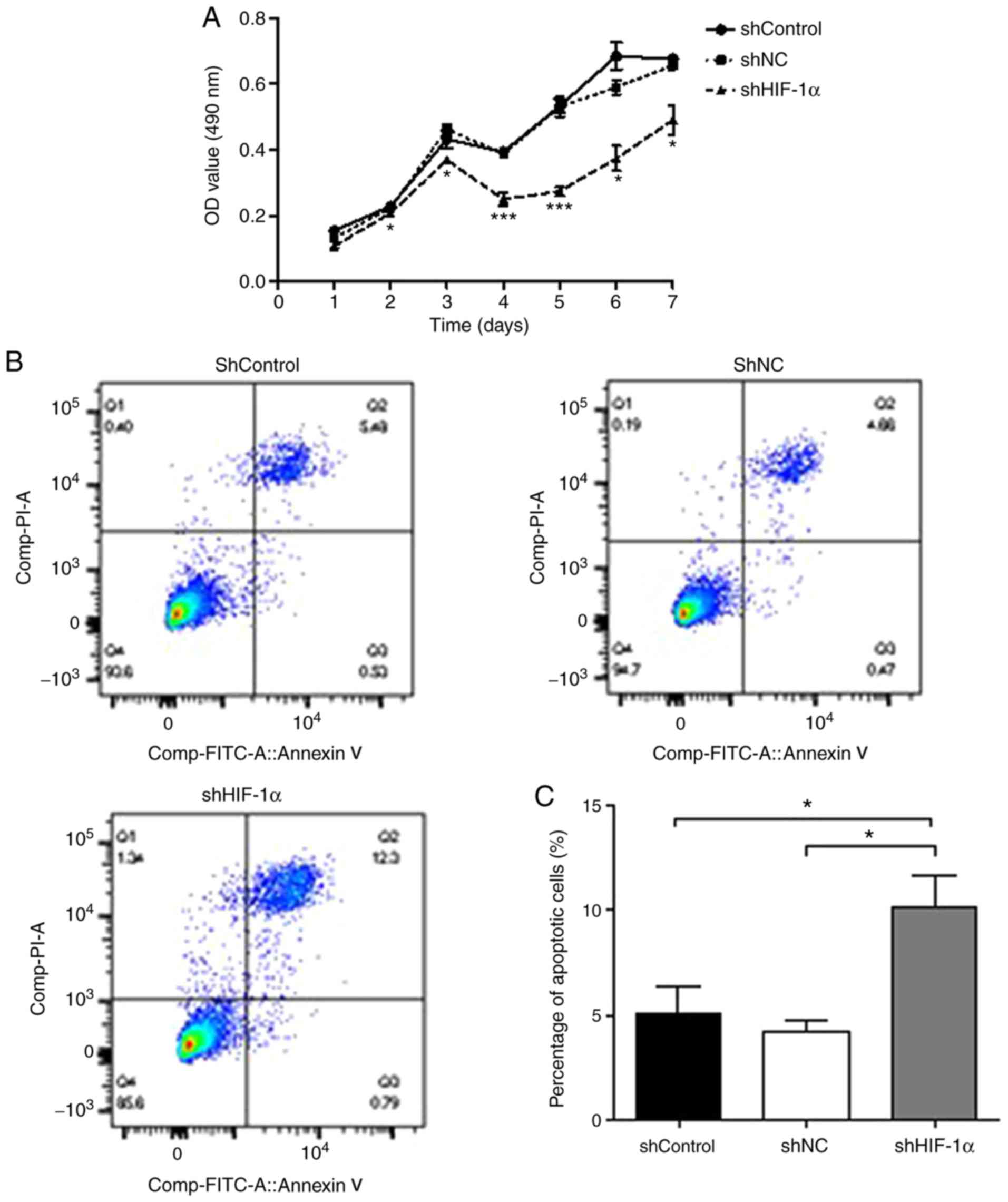
Downregulation of HIF-1α inhibits
SACC-83 cell growth and induces cell apoptosis but has no
significant effect on cell cycle progression under hypoxic
conditions
The effect of HIF-1α downregulation on the
proliferation of SACC-83 cells was assessed using a MTT assay. As
demonstrated in Fig. 2A, the rate
of proliferation of cells transfected with shHIF-1α was
significantly lower compared with that of cells transfected with
shControl or shNC. The effect of HIF-1α downregulation on SACC-83
cell apoptosis was also examined (Fig.
2B). Following shHIF-1α transfection, the percentage of
apoptotic SACC-83 cells increased to 13.09%, which was
significantly higher compared with the rates in the shControl
(6.01%) and shNC (5.13%) groups (P<0.05, Fig. 2C). Finally, the effect of HIF-1α
downregulation on cell cycle progression was examined using flow
cytometry with PI DNA staining (Fig.
2D). As shown in Fig. 2E, the
cell cycle distribution of the shHIF-1α group was not significantly
different from those in the shControl and shNC groups
(P>0.05).
Downregulation of HIF-1α decreased
MMP-2 expression under hypoxic conditions
Western blot assays were performed to explore the
effect of HIF-1α downregulation on the expression of MMP-2, which
is closely linked with tumor invasion and metastasis (21). As shown in Fig. 3A and B, in correspondence with the
downregulation of HIF-1α, the expression of MMP-2 in the shHIF-1α
group was significantly lower compared with levels in the shControl
and shNC groups (P<0.01).
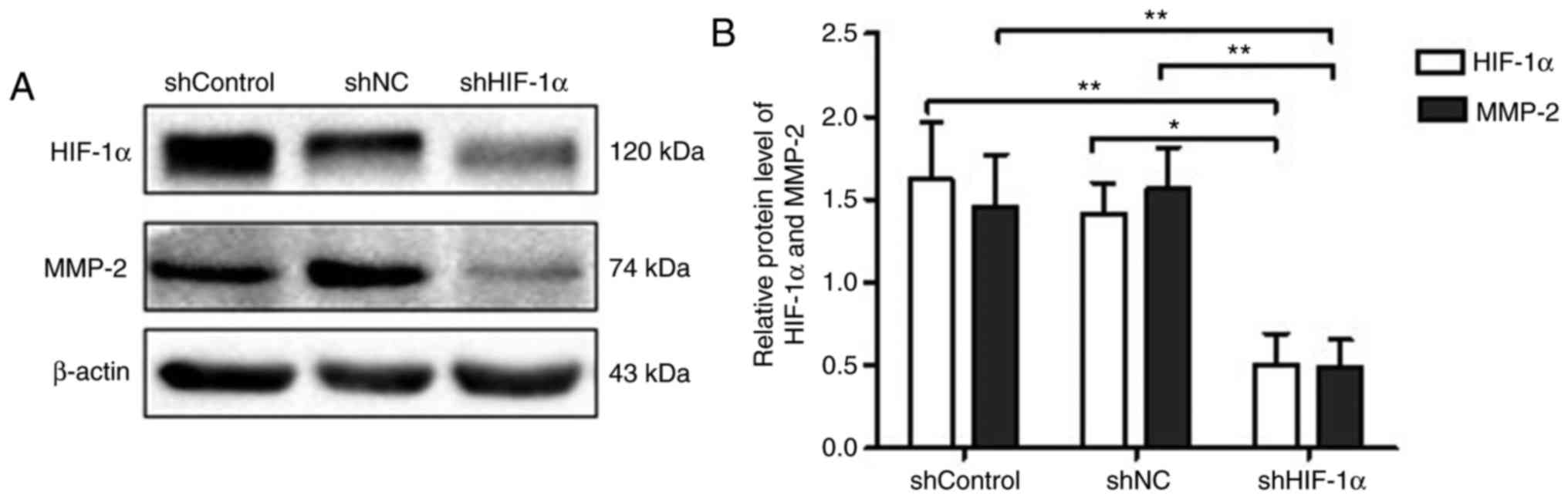 | Figure 3.Effects of downregulation of HIF-1α
on MMP-2 expression in SACC-83 cells under hypoxia. (A) Western
blot analysis of shControl, shNC and shHIF-1α groups. (B)
Quantification of MMP-2 and HIF-1α protein expression. All data are
expressed as the mean ± standard error of the mean. *P<0.05,
**P<0.01. shControl group, wild-type SACC-83 cells; shNC group,
empty plasmid transfection group; shHIF-1α group,
HIF1α-targeted shRNA transfection group; SACC, salivary
adenoid cystic carcinoma; shRNA, short hairpin RNA; HIF-1α,
hypoxia-inducible factor-1α; NC, negative control; MMP, matrix
metalloproteinase. |
Downregulation of HIF-1α suppresses
SACC-83 cell invasion and migration under hypoxic conditions
Cell migration and invasion are important processes
in tumor development and metastasis. Thus, the migration and
invasion rates of SACC-83 cells under hypoxic conditions were
assessed using wound healing and Matrigel Transwell assays. As
shown in Fig. 4A and B, the
downregulation of HIF-1α in SACC-83 cells significantly reduced
cell migration. The results indicated that the migration rate was
9.60% at 24 h and 22.08% at 48 h in the shHIF-1α group, and these
rates were significantly lower compared with those in the shControl
(23.71% at 24 h and 42.33% at 48 h) and shNC (23.35% at 24 h and
42.87% at 48 h) groups (P<0.05). In addition, the in
vitro Matrigel Transwell assay showed that the relative number
of SACC-83 cells/field was significantly lower in the shHIF-1α
group (51%) compared with in the shControl and shNC groups
(Fig. 4C and D; P<0.001),
suggesting that the downregulation of HIF-1α suppressed the
mobility and migration of SACC-83 cells.
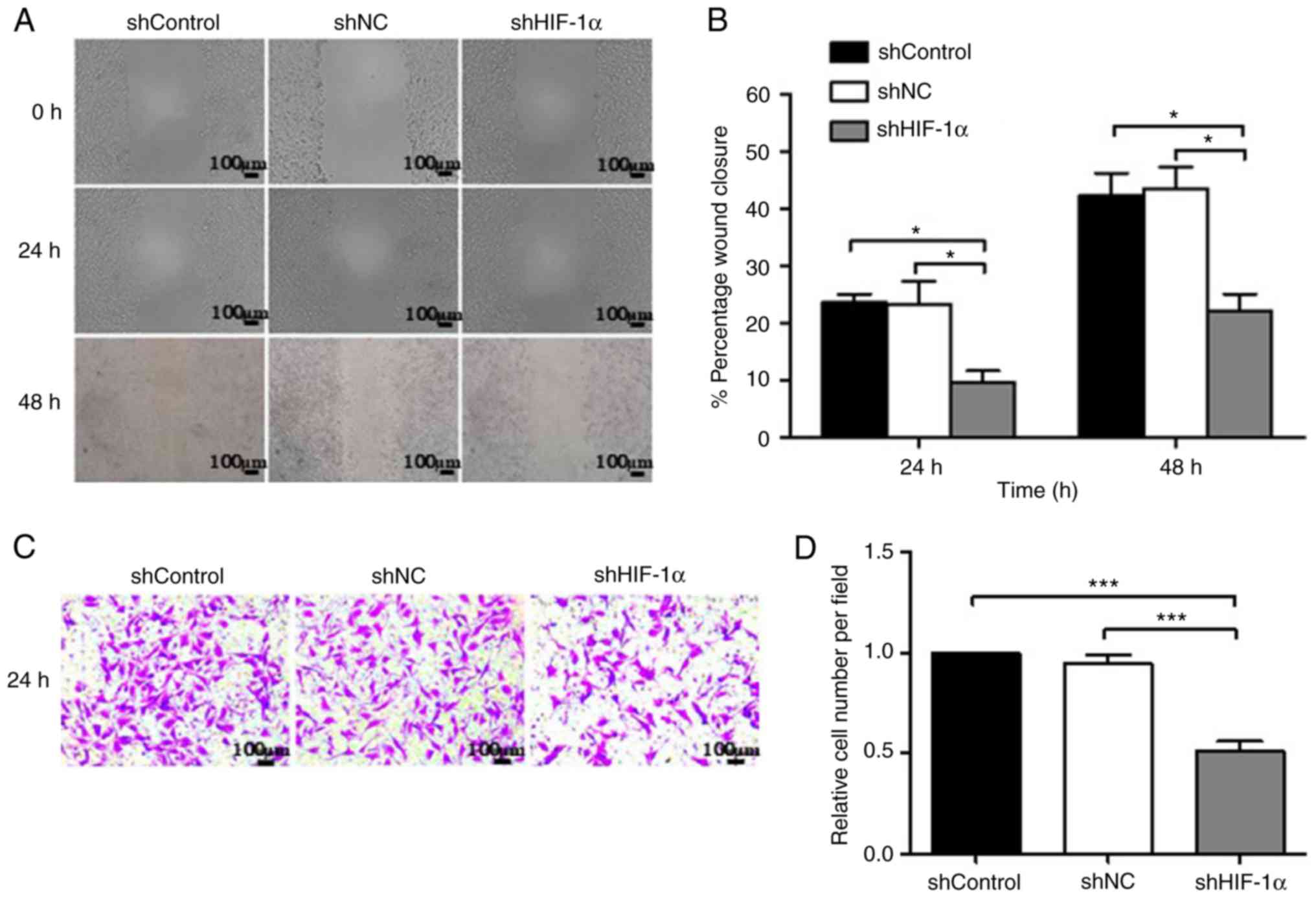 | Figure 4.Downregulation of HIF-1α inhibits the
invasion and migration of SACC-83 cells. (A) Wound-healing assay of
SACC-83 cells at 0, 24 and 48 h. Scale bar, 100 µm. (B)
Quantification of the effect of HIF-1α on SACC-83 cell migration in
the wound-healing assay. (C) The Transwell assay demonstrated the
number of invasive cells in the shControl, shNC and shHIF-1α
groups. Scale bar, 100 µm. (D) Quantification of the number of
invasive cells. The data are expressed as mean ± standard error of
the mean. *P<0.05, ***P<0.001. shControl group, wild-type
SACC-83 cells; shNC group, empty plasmid transfection group;
shHIF-1α group, HIF1α-targeted shRNA transfection group;
SACC, salivary adenoid cystic carcinoma; shRNA, short hairpin RNA;
HIF-1α, hypoxia-inducible factor-1α; NC, negative control. |
Downregulation of HIF-1α reduced
angiogenesis and VEGF expression in hypoxic SACC-83 cells
Tumor angiogenesis not only provides adequate
nutrition for the tumor, but also provides access for tumor
metastasis (22). To study the
effects of HIF-1α downregulation on the angiogenesis of hypoxic
SACC-83 cells, HUVECs were treated with cell culture medium derived
from each cell type for 24 h and then the tube formation rate was
evaluated in each group of HUVECs. Compared with the shControl and
shNC groups, the shHIF-1α group exhibited significantly shorter
tube lengths (Fig. 5A and B;
P<0.001).
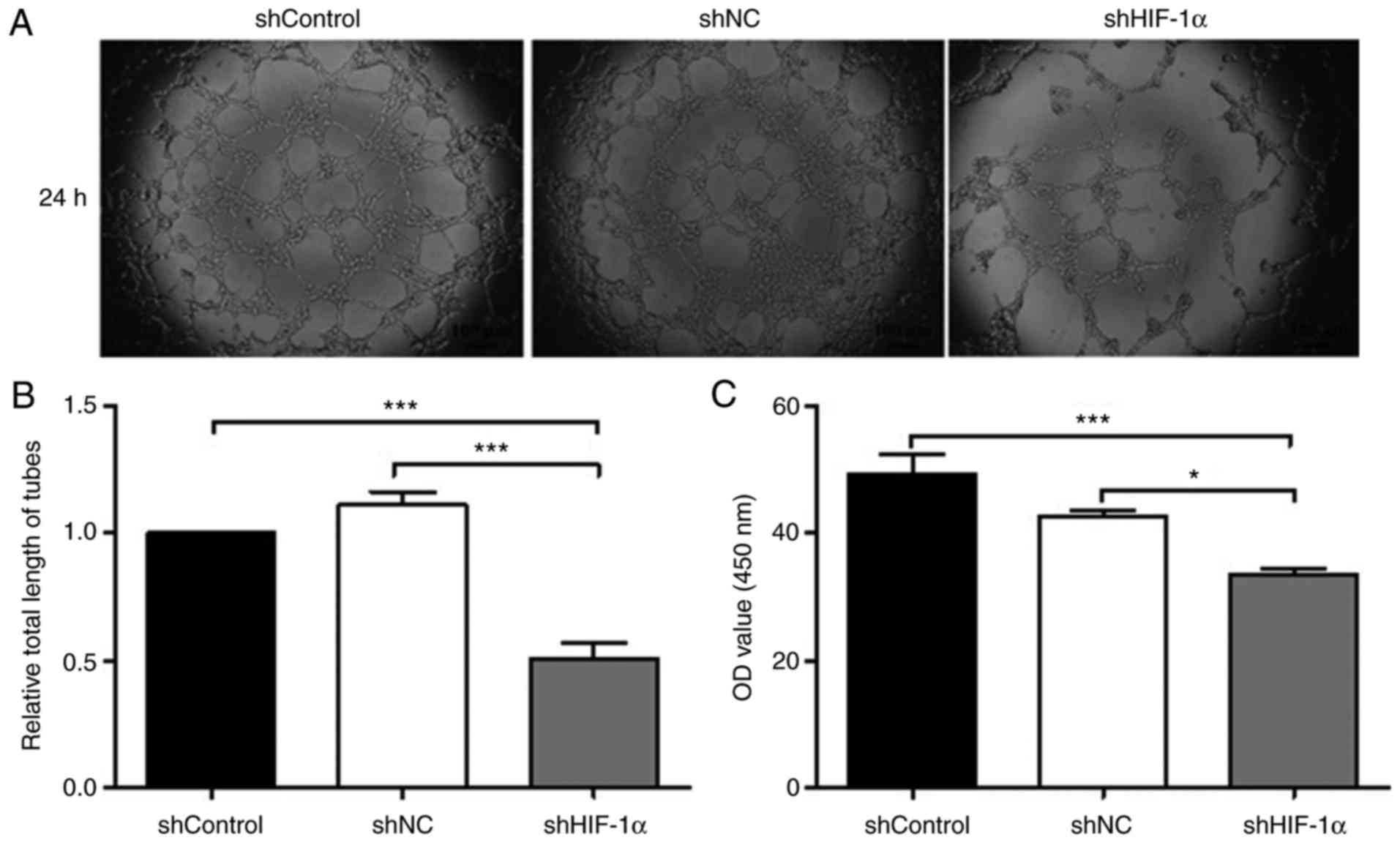 | Figure 5.Downregulation of HIF-1α inhibits
tube formation and VEGF expression in SACC-83 cells. (A) Effects of
downregulation of HIF-1α on angiogenesis were verified by human
umbilical vein endothelial cell tube-formation assays.
Representative images of tube formation (magnification, ×400). (B)
Relative total length of tubes in each group of SACC-83 cells. (C)
VEGF levels were determined by ELISA and the absorbance at 450 nm
was measured. All data are expressed as the mean ± standard error
of the mean. *P<0.05, ***P<0.001. shControl group, wild-type
SACC-83 cells; shNC group, empty plasmid transfection group;
shHIF-1α group, HIF1α-targeted shRNA transfection group;
SACC, salivary adenoid cystic carcinoma; shRNA, short hairpin RNA;
HIF-1α, hypoxia-inducible factor-1α; NC, negative control; VEGF,
vascular endothelial growth factor. |
A large number of clinical studies have reported
that high expression of VEGF is associated with tumor microvessel
density, the degree of malignancy and poor patient prognosis
(23,24). To investigate whether HIF-1α
downregulation affected the protein expression of VEGF,
double-antibody ELISA was used. The results of the ELISA revealed
that the protein expression level of VEGF was lower in the shHIF-1α
group compared with in the shControl and shNC groups (Fig. 5C; P<0.001 and P<0.05,
respectively). The optical density values of the shControl and shNC
groups were 49.27 and 42.53, respectively, whereas that of the
shHIF-1α group was significantly lower (33.62). Together, these
results suggest that a reduction in HIF-1α expression significantly
weakened tube-formation ability and reduced VEGF expression in
SACC-83 cells.
Discussion
SACC is a common malignant tumor of the head and
neck that exhibits distinct potential for invasion and distant
migration (25). It has long been
recognized that tumors are hypoxic, tumor hypoxia is a poor
prognostic factor, and established tumors are protected by hypoxia
and HIF-1α-mediated biochemical pathways (26). A previous study showed that the
inhibition of HIF-1α expression using specifically targeted siRNA
could increase apoptosis and reduce migration in clear cell renal
cell carcinoma 786-O cells (27).
Consistent with these results, in the present study, we confirmed
that HIF-1α downregulation induced proliferation and apoptosis in
hypoxic SACC cells.
As an important target of HIF-1α, VEGF is the most
potent and selective angiogenesis-promoting factor, increasing
microvascular permeability and inducing endothelial cell division,
proliferation, and migration (28).
HIF-1α is regulated by the phosphatidylinositol 3-kinase and
mitogen-activated protein kinase signaling pathways, which
ultimately promote its expression (29). In this study, SACC-83 cells were
cultured in a simulated hypoxic environment (5% O2) and
a RNA interference technique was used to downregulate HIF-1α
expression in SACC-83 cells. The results demonstrated that the
downregulation of HIF-1α significantly reduced the expression of
VEGF in SACC-83 cells and that VEGF-associated tumor angiogenesis
was significantly suppressed in HUVECs. HIF-1α serves an important
role in regulating the VEGF signal transduction pathway under
hypoxic conditions. In the process of early angiogenesis, HIF-1α
not only increases the stability of VEGF mRNA but also
increases the transcriptional activity of VEGF, resulting in
the transport of more oxygen and nutrients to the tumor and
ultimately promoting tumor invasion and distant transfer (30). Reducing the expression of HIF-1α in
tumor cells may thus decrease the production of VEGF and inhibit
tumor growth. HIF-1α also serves a key role in the autocrine
VEGF-VEGF receptor (VEGFR) signal transduction pathway, which may
be another mechanism by which the downregulation of HIF-1α inhibits
tumor angiogenesis (9,31,32)
HIFs induce a variety of factors and enzymes
associated with tumor invasion and metastasis, including MMPs
(33). MMP-2 is the most widely
distributed proteolytic enzyme in the MMP family, and is associated
with tumor invasion and metastasis (34). Certain studies have reported that
HIF-1α promotes the invasion and metastasis of cervical cancer by
downregulating E-cadherin expression and upregulating the
expression of MMP-2 (35,36). MMP-2 increases the extent of tumor
vascularization and is an important component of the angiogenesis
initiation system (9,37). In hypoxic environments, tumor cells
secrete large amounts of HIF-1α, inducing the secretion of MMP-2 by
endothelial cells (9,37). In the present study, it was
demonstrated that the downregulation of HIF-1α significantly
attenuated MMP-2 expression. Furthermore, as expected, the results
of wound healing and Transwell assays revealed that HIF-1α
significantly suppressed the migration and invasion of SACC
cells.
In summary, the present study demonstrated that the
downregulation of HIF-1α significantly inhibited proliferation,
invasion, migration and angiogenesis in hypoxic SACC-83 cells, and
promoted apoptosis in this cell type. Additionally, HIF-1α
downregulation significantly attenuated the expression of MMP-2 and
VEGF. The inhibition of angiogenesis may be useful for the
treatment of neoplastic lesions and pro-angiogenic therapies may be
important for future treatment of ischemic diseases (38). In the future, these findings should
be confirmed in xenograft models and the signaling molecules
underlying the effects of HIF-1α on SACC should be elucidated
through further research.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Research
and Development Program of Jiangxi Province (grant no.
20171BBG70120) and the Graduate Innovation Foundation of Nanchang
University (grant no. cx2016357).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CX, YP, XZ, LW, ZL, SY and HW contributed to the
conception, design and completion of the present study. CX and YP
contributed equally to the experimental work and drafting of the
manuscript. XZ analyzed the data and revised the manuscript
critically for important intellectual content. LW and ZL performed
the experiments and interpreted the results. SY produced the
figures and HW contributed to study design.
Ethics approval and consent
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
Glossary
Abbreviations
Abbreviations:
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
ECM
|
extracellular matrix
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
FBS
|
fetal bovine serum
|
|
GFP
|
green fluorescent protein
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
PI
|
propidium iodine
|
|
PVDF
|
polyvinylidene fluoride
|
|
SACC
|
salivary adenoid cystic carcinoma
|
|
SEM
|
standard error of the mean
|
|
shRNA
|
short hairpin RNA
|
|
TBS
|
Tris-buffered saline
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Gondivkar SM, Gadbail AR, Chole R and
Parikh RV: Adenoid cystic carcinoma: A rare clinical entity and
literature review. Oral Oncol. 47:231–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li LJ, Li Y, Wen YM, Liu H and Zhao HW:
Clinical analysis of salivary gland tumor cases in West China in
past 50 years. Oral Oncol. 44:187–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Umeda M, Nishimatsu N, Masago H, Ishida Y,
Yokoo S, Fujioka M, Shibuya Y and Komori T: Tumor-doubling time and
onset of pulmonary metastasis from adenoid cystic carcinoma of the
salivary gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
88:473–478. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rampling R, Cruickshank G, Lewis AD,
Fitzsimmons SA and Workman P: Direct measurement of pO2
distribution and bioreductive enzymes in human malignant brain
tumors. Int J Radiat Oncol Biol Phys. 29:427–431. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kayama T, Yoshimoto T, Fujimoto S and
Sakurai Y: Intratumoral oxygen pressure in malignant brain tumor. J
Neurosurg. 74:55–59. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
7
|
Semenza GL: HIF-1: Mediator of
physiological and pathophysiological responses to hypoxia. J Appl
Physiol. 88:1474–1480. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Covello KL and Simon MC: HIFs, hypoxia,
and vascular development. Curr Top Dev Biol. 62:37–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stoeltzing O, McCarty MF, Wey JS, Fan F,
Liu W, Belcheva A, Bucana CD, Semenza GL and Ellis LM: Role of
hypoxia-inducible factor 1alpha in gastric cancer cell growth,
angiogenesis, and vessel maturation. J Natl Cancer Inst.
96:946–956. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Samanta D, Gilkes DM, Chaturvedi P, Xiang
L and Semenza GL: Hypoxia-inducible factors are required for
chemotherapy resistance of breast cancer stem cells. Proc Natl Acad
Sci USA. 111:E5429–E5438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harada H: How can we overcome tumor
hypoxia in radiation therapy? J Radiat Res. 52:545–556. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Semenza GL: Development of novel
therapeutic strategies that target HIF-1. Expert Opin Ther Targets.
10:267–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Biol. 12:5447–5454. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee FS and Percy MJ: The HIF pathway and
erythrocytosis. Annu Rev Pathol. 6:165–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang GL and Semenza GL: Purification and
characterization of hypoxia-inducible factor 1. J Biol Chem.
270:1230–1237. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pugh CW, O'Rourke JF, Nagao M, Gleadle JM
and Ratcliffe PJ: Activation of hypoxia-inducible factor-1;
definition of regulatory domains within the alpha subunit. J Biol
Chem. 272:11205–11214. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meijer TW, Kaanders JH, Span PN and
Bussink J: Targeting hypoxia, HIF-1, and tumor glucose metabolism
to improve radiotherapy efficacy. Clin Cancer Res. 18:5585–5594.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Łuczak MW, Roszak A, Pawlik P, Kędzia H,
Lianeri M and Jagodziński PP: Increased expression of HIF-1A and
its implication in the hypoxia pathway in primary advanced uterine
cervical carcinoma. Oncol Rep. 26:1259–1264. 2011.PubMed/NCBI
|
|
19
|
Sun X, Vale M, Jiang X, Gupta R and
Krissansen GW: Antisense HIF-1alpha prevents acquired tumor
resistance to angiostatin gene therapy. Cancer Gene Ther.
17:532–540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen W, Xi H, Wei B and Chen L: The
prognostic role of matrix metalloproteinase 2 in gastric cancer: A
systematic review with meta-analysis. J Cancer Res Clin Oncol.
140:1003–1009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deryugina EI and Quigley JP: Tumor
angiogenesis: MMP-mediated induction of intravasation- and
metastasis-sustaining neovasculature. Matrix Biol 44–46. 1–112.
2015.
|
|
23
|
Chekhonin VP, Shein SA, Korchagina AA and
Gurina OI: VEGF in tumor progression and targeted therapy. Curr
Cancer Drug Targets. 13:423–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong J, Yang Q, Li J and Zhou S: Effects
of MDM2 inhibitors on vascular endothelial growth factor-mediated
tumor angiogenesis in human breast cancer. Angiogenesis. 17:37–50.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan F, Wang C, Li T, Cai W and Sun J: Role
of miR-21 in the growth and metastasis of human salivary adenoid
cystic carcinoma. Mol Med Rep. 17:4237–4244. 2018.PubMed/NCBI
|
|
26
|
Zimna A and Kurpisz M: Hypoxia-inducible
factor-1 in physiological and pathophysiological angiogenesis:
Applications and therapies. Biomed Res Int. 2015:5494122015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song T, Zhang X, Wang C, Wu Y, Cai W, Gao
J and Hong B: MiR-138 suppresses expression of hypoxia-inducible
factor 1α (HIF-1 α) in clear cell renal cell carcinoma 786-O cells.
Asian Pac J Cancer Prev. 12:1307–1311. 2011.PubMed/NCBI
|
|
28
|
Roslavtceva VV, Salmina AB, Prokopenko SV,
Pozhilenkova EA, Kobanenko IV and Rezvitskaya GG: The role of
vascular endothelial growth factor in the regulation of development
and functioning of the brain: New target molecules for
pharmacotherapy. Biomed Khim. 62:124–133. 2016.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu B, Miao ZH, Jiang Y, Li MH, Yang N, Li
T and Ding J: c-Jun protects hypoxia-inducible factor-1alpha from
degradation via its oxygen-dependent degradation domain in a
nontranscriptional manner. Cancer Res. 69:7704–7712. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qi L, Zhu F, Li SH, Si LB, Hu LK and Tian
H: Retinoblastoma binding protein 2 (RBP2) promotes
HIF-1α-VEGF-induced angiogenesis of non-small cell lung cancer via
the Akt pathway. PLoS One. 9:e1060322014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ravi R, Mookerjee B, Bhujwalla ZM, Sutter
CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL and Bedi A:
Regulation of tumor angiogenesis by p53-induced degradation of
hypoxia-inducible factor 1alpha. Genes Dev. 14:34–44.
2000.PubMed/NCBI
|
|
32
|
Tang N, Wang L, Esko J, Giordano FJ, Huang
Y, Gerber HP, Ferrara N and Johnson RS: Loss of HIF-1alpha in
endothelial cells disrupts a hypoxia-driven VEGF autocrine loop
necessary for tumorigenesis. Cancer Cell. 6:485–495. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee YA, Choi HM, Lee SH, Hong SJ, Yang HI,
Yoo MC and Kim KS: Hypoxia differentially affects IL-1β-stimulated
MMP-1 and MMP-13 expression of fibroblast-like synoviocytes in an
HIF-1α-dependent manner. Rheumatology. 51:443–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang YL, Liu X, Gao SY, Feng H, Jiang YP,
Wang SS, Yang J, Jiang J, Ma XR, Tang YJ, et al: WIP1 stimulates
migration and invasion of salivary adenoid cystic carcinoma by
inducing MMP-9 and VEGF-C. Oncotarget. 6:9031–9044. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wucherpfennig AL, Li YP, Stetler-Stevenson
WG, Rosenberg AE and Stashenko P: Expression of 92 kD type IV
collagenase/gelatinase B in human osteoclasts. J Bone Miner Res.
9:549–556. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nagase H: Matrix metalloproteinases. A
mini-review. Contrib Nephrol. 107:85–93. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu CT, Bi KW, Huang CC, Wu HT, Ho HY, S
Pang JH and Huang ST: Davallia bilabiata exhibits anti-angiogenic
effect with modified MMP-2/TIMP-2 secretion and inhibited VEGF
ligand/receptors expression in vascular endothelial cells. J
Ethnopharmacol. 196:213–224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang F, Chang M, Shi Y, Jiang L, Zhao J,
Hai L, Sharen G and Du H: Down-regulation of hypoxia-inducible
factor-1 suppresses malignant biological behavior of
triple-negative breast cancer cells. Int J Clin Exp Med.
7:3933–3940. 2014.PubMed/NCBI
|