Introduction
Chondrosarcoma (CHS) is the second most common
primary bone malignancy; it accounts for 40% of all primary bone
malignancies and is characterized by a series of
clinicopathological signs (1,2). It
usually affects adults between the ages of 20 and 60 years
(3,4). Although chemotherapy and radiation
have been investigated for their efficacy against CHS, they are not
used as active treatments since these tumors are notoriously
resistant to both chemotherapy and radiation (3,5).
Therefore, there is an urgent need to identify new drugs and
therapeutic approaches to improve the clinical management of CHS
and prevent its recurrence.
Pyrroloquinoline quinone (PQQ) was first discovered
as a natural synergistic redox agent and a novel enzymatic
co-factor in bacteria. PQQ, as an essential nutrient, is a small
molecule that is water soluble and thermally stable and is present
in all types of plant and animal cells (6,7); in
particular, it is extensively distributed in mammalian cells as
well (8,9). It has been reported that PQQ can
inhibit the formation of peroxynitrite (an oxidant) and has
anti-lipid peroxidation and antioxidant properties (10–12).
Studies have also demonstrated that PQQ can protect nucleus
pulposus cells from hydrogen peroxide-induced apoptosis and
oxygen/glucose deprivation-induced apoptosis by suppressing the
mitochondrial-mediated apoptotic pathway and activating the
PI3K/AKT cell proliferation pathway in cardiomyocytes, respectively
(13,14). Moreover, PQQ was found to induce
apoptosis in multiple types of human cancer cells, such as
promonocytic leukemia U937 and lymphoma EL-4 cells, and induce
Jurkat cell programmed death (12)
and lung cancer cell apoptosis through mitochondrial-dependent
pathways, as well as decreased expression of the Bcl-2 protein
(involved in regulating cell death) (15). In addition, PQQ was found to have
little effect on normal cells, which means that it may be an ideal
drug for cancer therapy in the future (12,15,16).
In the case of CHS, similar results were also obtained. Our recent
study showed that the cell death rate of CHS cells increased with
an increase in PQQ concentration (at concentrations <120 µM),
and that PQQ did not show significant toxicity against normal cells
(17). However, the underlying
molecular mechanism of PQQ-induced apoptosis in CHS cells remains
to be elucidated.
In multicellular organisms, apoptosis plays a
crucial role in embryogenesis and homeostasis and is also
associated with neurodegenerative disorders and cancer (18,19).
There are two types of apoptosis pathways: caspase-dependent and
caspase-independent pathways (20).
Caspases, which comprise a family of cysteine-dependent
aspartate-guided proteases, play vital roles in the development of
apoptosis and its initiation and execution (21). The caspase-dependent apoptotic
pathways include caspase-3, procaspase-3, Smac and X-linked
inhibitor-of-apoptosis protein (XIAP), among others. In addition,
some of the proteins that are a part of the non-caspase-dependent
mitochondrial pathways are pro-caspase, endonuclease G (Endo G),
Apaf-1, apoptosis-inducing factor (AIF) and cytochrome c.
Based on previous studies that have shown the role of the
mitochondrial apoptosis pathways in the apoptotic mechanisms of PQQ
in various cancers, in the present study, we explored the level of
these proteins in CHS cells treated with various concentrations of
PQQ.
In the present study, we investigated the effect of
PQQ on the apoptosis of CHS cells and explored the potential
apoptotic pathways that may be involved. The findings indicated
that the apoptotic mechanism of PQQ involves caspase-dependent as
well as non-caspase-dependent pathways. Furthermore, PQQ was shown
to have in vivo effects as well on the growth of CHS.
Materials and methods
Cell culture and reagents
The chondrosarcoma cell line SW1353, osteosarcoma
cell line Saos-2 and 293 cells from the American Type Culture
Collection (ATCC; Manassas, VA, USA), as well as human XJH B
lymphocytes from the Institute of Biochemistry and Cell Biology
(Chinese Academy of Sciences, Shanghai, China) were cultured in
Dulbecco's modified Eagle's medium (DMEM) containing
Gibco® 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 1% penicillin/streptomycin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The cells were
grown in a 37°C incubator containing 5% CO2. PQQ was
purchased from Merck KGaA.
Cytotoxicity-induced cell death
analysis
Cytotoxic death was assessed using a CytoTox-Glo™
Cytotoxicity Assay (Promega Corp., Madison, WI, USA) according to
the manufacturer's instructions. Briefly, the cells were seeded at
a density of 1×104 cells/well in 3 ml of DMEM and
incubated at 37°C in 5% CO2 for 6 h. Then, different
concentrations of PQQ (0, 40, 80, 120 and 200 µM) were added to the
culture and the cells were incubated for 24 h under the same
conditions. Following this, 50 µl of CytoTox-Glo™ reagent (Promega)
was added, and the cells were incubated at 37°C for 15 min.
Luminescence was then measured as an indicator of the percentage of
dead cells. Finally, the cells were incubated with lysis reagent
for 15 min at room temperature and total cell luminescence was
measured by a microplate reader (ELx800; BioTek Instruments, Inc.,
Winooski, VT, USA).
Flow cytometric analysis
Flow cytometry combined with JC-1 staining was used
to detect the mitochondrial membrane potential. The chondrosarcoma
cell line SW1353 and osteosarcoma cell line Saos-2 were seeded in
6-well plates with complete medium and incubated for 24 h. After
incubation, the cells were treated with 120 µM PQQ or control (PBS)
for 48 h at 37°C in a 5% CO2 incubator. Then, SW1353
cells were treated with a trypsin-EDTA (0.25%) solution and
centrifuged at 1,000 × g for 5 min. Finally, the cells were washed
three times with PBS, and apoptosis was assessed using
Mitochondrial membrane potential detection kit (JC-1) staining
according to the manufacturer's instructions (Beyotime Institute of
Biotechnology, Shanghai, China) and flow cytometry with a
FACSCalibur system equipped with Cell Quest software (version 5.1;
BD Biosciences, Franklin Lakes, NJ, USA). FLH2/FLH1 ratio
represented the changes in the apoptotic cells. When apoptosis was
increased, FLH2/FLH1 decreased.
Western blot analysis
The cells were treated with PQQ or caspase inhibitor
Z-VAD-fmk for 48 h. For obtaining the whole-cell extract, the cells
were lysed with cell lysis buffer (Cell Signaling Technology,
Danvers, MA, USA) containing protease inhibitors (Sigma-Aldrich;
Merck KGaA). Then, the cells were centrifuged at 12,000 × g for 5
min at 4°C, and the supernatant was collected and assessed using a
BCA Protein assay kit (Sigma-Aldrich; Merck KGaA) to determine the
protein content. The proteins were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel (SDS-PAGE) with 20 mg of protein per
lane and transferred to polyvinylidene difluoride (PVDF) membranes
(Millipore, Billerica, MA, USA). Then, we used Tris-buffered saline
(TBS) and 0.1% Tween-20 (TBS/T) containing 5% bovine serum albumin
(BSA) to block the PVDF membranes at room temperature for 1 h.
Incubation with the primary antibodies was performed overnight at
4°C with antibodies against caspase-3 (cat. no. ab13847),
procaspase-3, Smac (cat. no. ab8115), and XIAP (cat. no. ab137392;
Abcam, Cambridge, MA, USA) diluted to 1:1,000 in TBS/T. After the
incubation, the membranes were washed three times for 5 min each
with TBS/T, and then incubated with the corresponding horseradish
peroxidase-labeled secondary antibodies (1:2,000; cat. no. ab97051;
Abcam) for 2 h at 37°C. The membranes were again washed three times
with TBS-T, and the protein bands formed were detected with the
enzyme-catalyzed chemiluminescent (ECL) kit (GE Healthcare,
Piscataway, NJ, USA) and quantified by densitometry using ImageJ
software (National Institutes of Health, Bethesda, MD, USA).
Co-immunoprecipitation assay
Cells were collected and lysed in 50 µl of cell
lysis buffer (Cell Signaling Technology) after treatment under
different conditions such as H2O2, cisplatin
and TNF-α for 48 h. A small portion of the cells was lysed in lysis
buffer containing SDS buffer solution, and the rest were lysed by
incubation in cell lysis buffer containing the appropriate
antibodies or beads at 4°C for 24 h. The immuno-precipitate was
separated by SDS-PAGE and transferred to PVDF membranes. Specific
signals were detected using anti-Smac, anti-caspase-3 or anti-XIAP
antibodies (Abcam). Protein expression was detected with the ECL
kit (cat. no. ab65623; Abcam).
Tumor xenograft implantation
All experiments on animals were performed according
to the Animal Care Guidelines of the First Affiliated Hospital of
Zhejiang University School of Medicine and were approved by the
National Institutes of Health Guide for Care and Use of Laboratory
Animals (NIH Publications, No. 8023, revised 1978). The animals
experiments comply with the ARRIVE guidelines and the AVMA
euthanasia guidelines 2013. A total of 10 female BALB/c nude mice
(4–5 weeks old) were purchased from Shanghai Experiment Animal
Centre (Shanghai, China). They were fed an irradiated pathogen-free
diet and were housed in a specific pathogen-free (SPF) environment
(a laboratory animal room maintained at 25±1°C with 65±5% humidity
on a 12-h light/dark cycle). We used SW1353 cells to establish the
mouse model. Briefly, 1×106 cells/mouse were injected
subcutaneously into the left flanks of BALB/c nude mice. The mice
were given a daily abdominal injection of 50 mg/kg PQQ (17) or PBS for 10 days. The mice were then
sacrificed by CO2 inhalation and the tumor xenografts
were collected. The tumor volume (V) was calculated using the
formula V = (width2 × length)/2 and the maximum tumor
size did not exceed a diameter of 2.0 cm.
Statistical analysis
All experiment results are expressed as mean and
standard deviation (SD) values. GraphPad Prism 6 (GraphPad
Software, Inc., La Jolla, CA, USA) was used for analysis of the
data. Statistical differences between two groups were analyzed with
the Student's t-test, and multiple group comparisons were conducted
using one-way analysis of variance (ANOVA) followed by Tukey's post
hoc test. P-values >0.05 were considered to indicate significant
differences.
Results
PQQ promotes cell death in CHS
cells
Following treatment of the cell lines SW1353,
Saos-2, 293 and XJH-B with 0, 40, 80, 120, or 200 µM PQQ for 24 h,
the cell death rate was higher in the SW1353 and Saos-2 cells than
the rate in normal human cell lines 293 and XJH B, Although 80 µM
PQQ also induced normal cell 293 and XJH-B cell death, the cell
death was <10%, and in Saos-2 and SW1353 cells, the cell death
was ~30–40% following treatment with 80 µM PQQ (Fig. 1A). Furthermore, the cell death rate
was higher with higher PQQ concentrations. With regard to the
effect of treatment time, we found that treatment with 120 µM PQQ
significantly enhanced cell death in a time-dependent manner
(Fig. 1B). Flow cytometric analysis
indicated that the percentage of apoptotic cells was higher among
the PPQ-treated SW1353 and Saos-2 cells than the PBS-treated SW1353
and Saos-2 cells (Fig. 1C). These
findings demonstrate that the cell death rate of the CHS cells
showed a dose- and time-dependent increase with PQQ treatment,
while the effect on normal cells was relatively small.
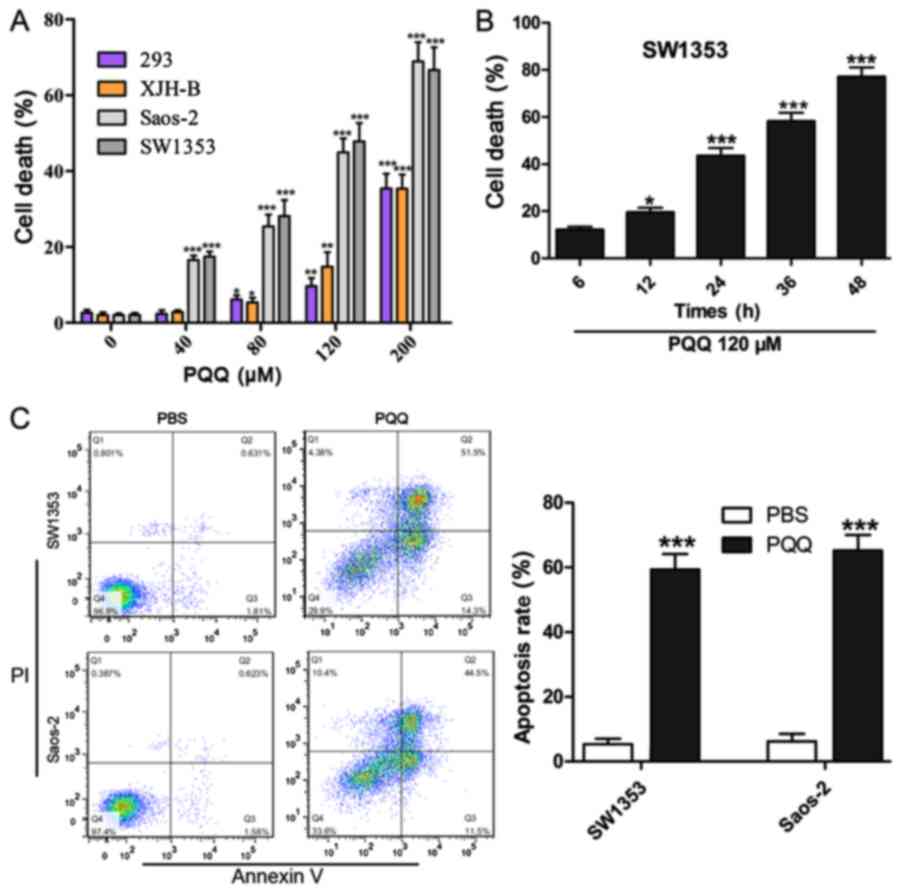 | Figure 1.Cytotoxic and apoptotic effects of PQQ
on CHS and normal cells. (A) Cytotoxicity of PQQ against SW1353,
Saos-2, 293 and XJH B cells according to the results of the cell
cytotoxicity assay. All cell types were incubated with PQQ (0, 40,
80, 120, or 200 µM) for 48 h. *P<0.05, **P<0.01,
***P<0.001 vs. 0 µM PQQ. (B) Cytotoxic effect of 120 mM PQQ on
SW1353 cells at different time points (6, 12, 24, 36, and 48 h).
*P<0.05, ***P<0.001 vs. 6-h treatment. (C) Rate of cell
apoptosis in SW1353 and Saos-2 cells after treatment with 120 µM
PQQ for 24 h, according to flow cytometry results. ***P<0.001
vs. PBS treatment for 24 h. PQQ, pyrroloquinoline quinone. |
PQQ induced apoptosis of CHS cells by
activation of the mitochondrial caspase-dependent pathway
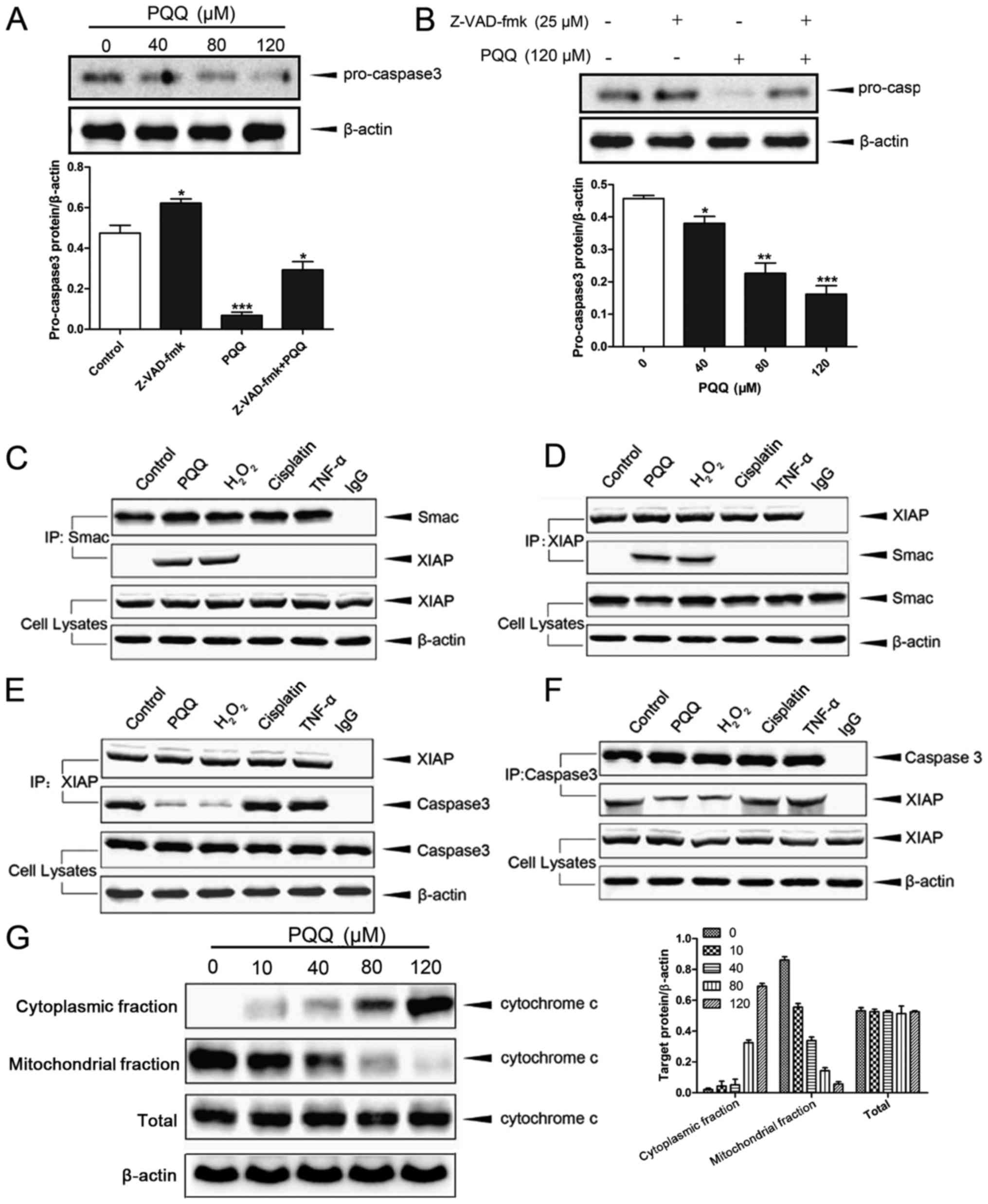
Western blot analysis showed that the level of
caspase-3 in the chondrosarcoma cells gradually decreased with an
increase in the concentration of PQQ (Fig. 2A). Upon combined treatment with
Z-VAD-fmk and PQQ, the level of procaspase-3 was significantly
inhibited in the chondrosarcoma cells (Fig. 2B). Co-immunoprecipitation analysis
showed that the binding of Smac to XIAP was significantly increased
and the binding of XIAP with caspase-3 was significantly decreased
in the combined PQQ- and H2O2-treated cells
compared with the control cells. This difference between the
control and treatment groups was not observed in the case of
treatment with only H2O2, only TNF-α or only
cisplatin (Fig. 2C-F). Furthermore,
we found that the protein level of cytochrome c in the cytoplasm
gradually increased with an increase in the concentration of PQQ,
while its level in the mitochondria gradually decreased. Notably,
the amount of total cytochrome c protein was not affected by the
increased PQQ concentration (Fig.
2G). These results demonstrate that PQQ can induce apoptosis of
CHS cells by activating the mitochondrial caspase-dependent
apoptosis pathway.
PQQ induces the apoptosis of CHS cells
by activating the mitochondrial non-caspase-dependent pathway
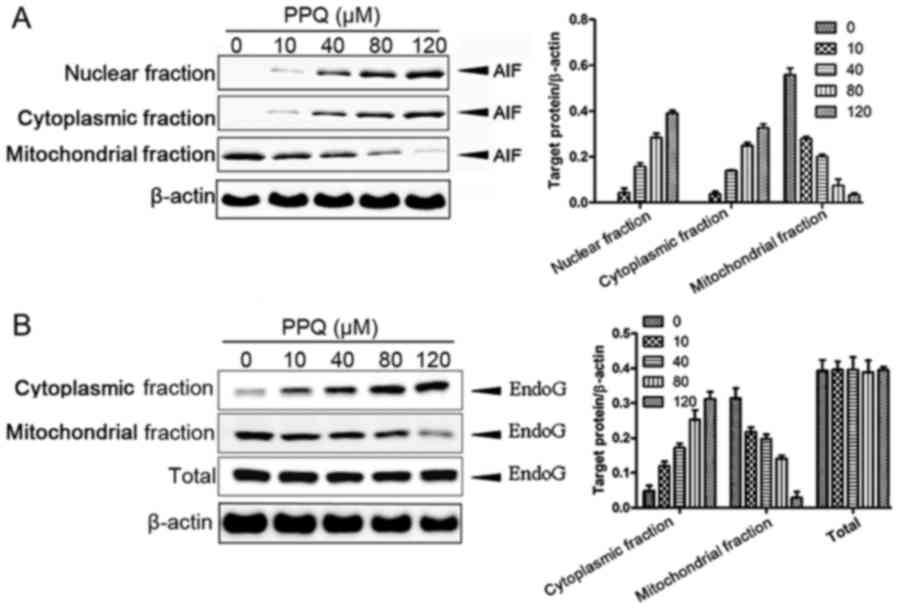
Western blot analysis showed that the level of AIF
protein in the cytoplasm and nucleus was gradually upregulated with
an increase in the concentration of PQQ, while the level of AIF
protein in the mitochondria was gradually decreased (Fig. 3A). Furthermore, the level of the
EndoG protein was gradually increased with an increase in the
concentration of PQQ in the cytoplasm, but its level showed a
gradual decrease in the mitochondria (Fig. 3B). However, the total amount of
EndoG did not change in correspondence with the PQQ concentration.
These results indicate that PQQ could induce apoptosis in the CHS
SW135 cells by activating mitochondrial non-caspase-dependent
pathways.
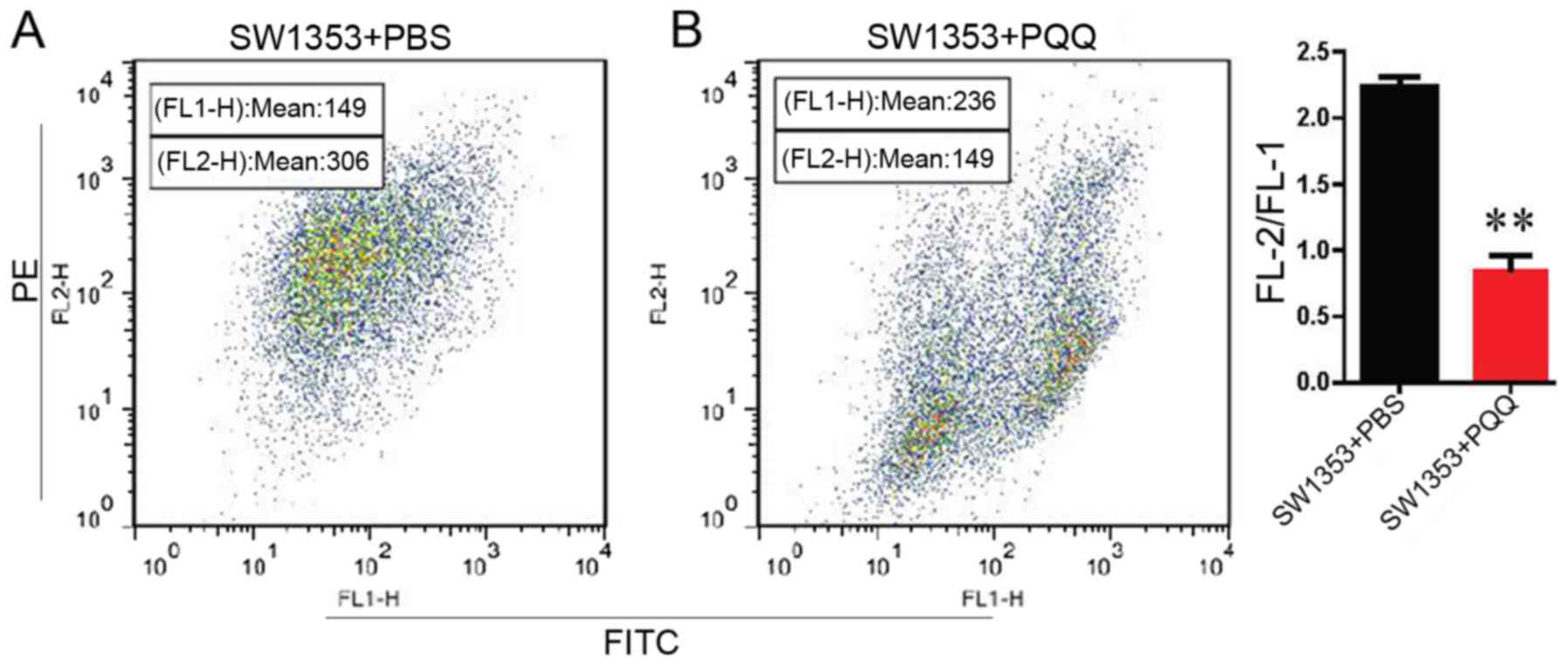
PQQ reduces the membrane potential of
CHS cells and inhibits the growth of CHS cell xenografts
We used JC-1 staining combined with flow cytometry
to detect mitochondrial membrane potential in response to treatment
with 120 µM PQQ. The results indicated that PQQ reduced the
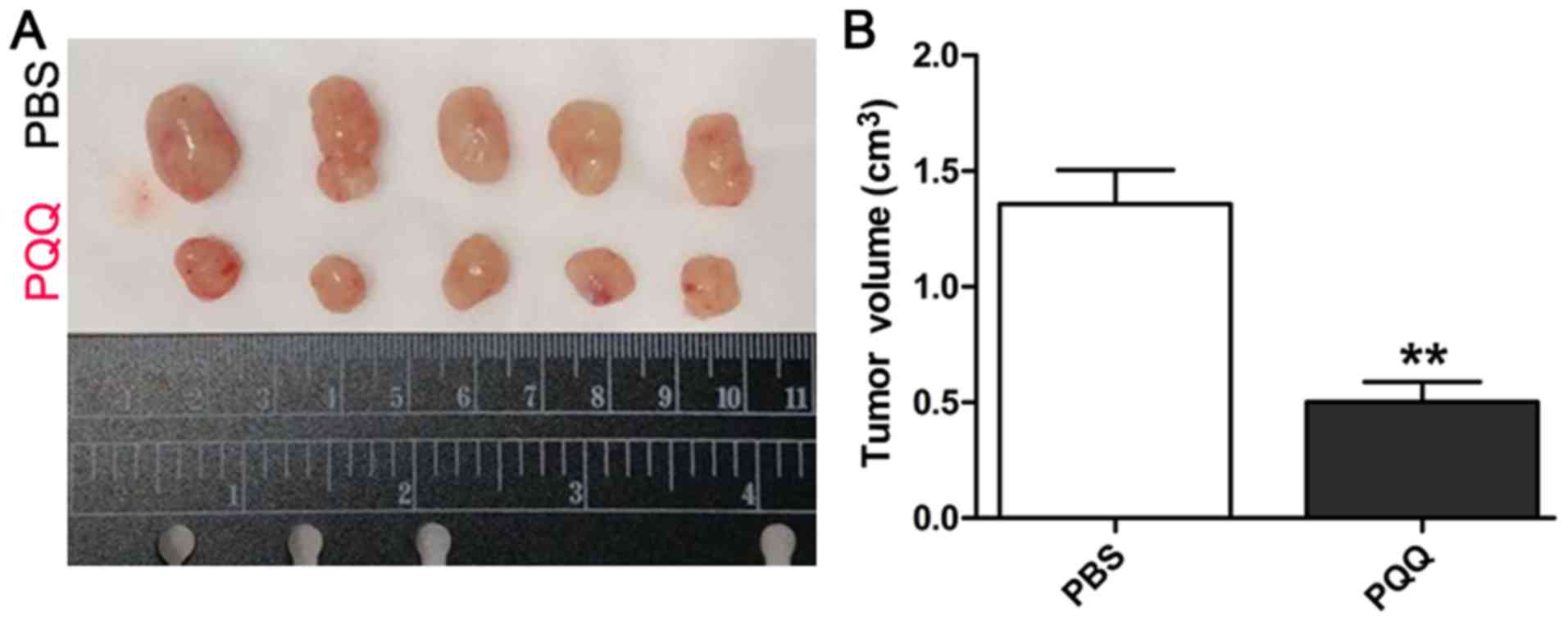
membrane potential compared with the PBS group (Fig. 4A and B). To investigate whether PQQ
had similar effects in in vivo conditions, SW1353 cells were
xenografted into BALB/c nude mice. The tumor volume was
significantly smaller after treatment with PQQ than after control
treatment (Fig. 5A and B). These
findings indicate that PQQ can inhibit the growth of chondrosarcoma
cell xenografts by inhibiting proliferation and promoting apoptosis
of these tumor cells.
Discussion
In the present study, we provide intitial evidence
for the role of mitochondrial apoptotic pathways in the antitumor
effect of pyrroloquinoline quinone (PQQ) against chondrosarcoma
(CHS).
Reduction in mitochondrial membrane potential is
considered to be an indicator of mitochondrial damage and early
apoptosis. In the present study, the mitochondrial membrane
potential was significantly reduced with PQQ treatment in
comparison with the control groups. These findings indicate that
PQQ treatment causes a change in mitochondrial membrane
permeability, which is one of the key events in initiating
mitochondrial pathways. Mitochondrial apoptosis is a well-known
apoptotic signaling pathway that is accompanied by mitochondrial
depolarization, cytochrome c over-release and caspase-3
activation (22), and the
mitochondrial apoptotic pathways can be caspase-dependent or
caspase-independent apoptotic pathways (23). In the caspase-dependent cytochrome
c pathway, mitochondrial instability results in the
redistribution of cytochrome c into the cytoplasm, which
triggers apoptosis via the continuous activation of caspase-9 and
caspase-3 (24,25). In the caspase-dependent Smac
pathway, Smac is released into the cytoplasm during the onset of
programmed apoptosis, where it specifically binds to XIAPs and
prevents the inhibitory effect of XIAP on caspase precursors
(26). In the present study, the
level of caspase-3 in the chondrosarcoma cells was gradually
decreased with increase in the concentration of PQQ. Further, the
binding of Smac to XIAP was significantly increased while the
binding of XIAP with caspase-3 was significantly decreased.
Finally, the level of cytochrome c in the cytoplasm was
gradually increased with an increase in the concentration of PQQ,
and it was accompanied by a decrease in the mitochondrial
cytochrome c level. All the findings indicate that the
anticancer effects of PQQ in chondrosarcomas may involve the
induction of apoptosis via regulation of caspase-dependent
pathways.
Caspase-independent mitochondrial apoptotic pathways
mainly include AIF- and EndoG-induced apoptosis pathways. AIF is a
flavoprotein which is usually confined to the mitochondrial
intermembrane space. New evidence indicates that the transport of
mitochondrial AIF into the cytoplasm and then the nucleus is an
indicator of caspase-independent apoptosis (27). In this study, PQQ treatment was
found to result in increased levels of AIF protein in the cytoplasm
and nucleus and decreased levels in the mitochondria. EndoG,
another mitochondrial factor, is also transported from the
mitochondria to the cytoplasm and then the nucleus upon induction
of apoptosis (28). Similar to the
results for AIF, the levels of EndoG protein were also increased in
the cytoplasm and decreased in the mitochondria with an increase in
the concentration of PQQ. In the case of EndoG, the total amount in
the cells did not change with PQQ treatment; this confirms that the
higher cytoplasmic levels observed were a result of the transport
of this protein from the mitochondria and not an increase in its
expression. Thus, these findings indicate that PQQ induces
apoptosis of the CHS cell line SW1353 by activating mitochondrial
caspase-independent pathways.
Finally, we confirmed the in vitro effects by
showing that PQQ had in vivo inhibitory effects on
tumorigenesis and caused a decrease in tumor size. Thus, PQQ may be
able to inhibit the proliferation of CHS cells. The in vivo
mechanisms are probably similar to the in vitro ones, but
they need to be studied in future investigations.
In conclusion, we established that the mechanism
underlying PQQ-induced cancer cell apoptosis in CHS involves the
activation of mitochondrial caspase-dependent as well as
caspase-independent pathways. Thus, the proteins identified in
these pathways could be potential targets for the treatment of
chondrosarcoma.
Acknowledgments
Not applicable.
Funding
This study was supported by the Zhejiang Province
Natural Science Foundation of China (no. LY15H160060).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CX and RW conceived the research idea; JP and MS
performed the experiments; CX, RW and JP analyzed the data; CX
wrote the manuscript. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All experiments on animals were performed according
to the Animal Care Guidelines of the First Affiliated Hospital of
Zhejiang University School of Medicine and were approved by the
National Institutes of Health Guide for Care and Use of Laboratory
Animals (NIH Publications, No. 8023, revised 1978). The animals
experiments comply with the ARRIVE guidelines and the AVMA
euthanasia guidelines 2013.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wunder JS, Nielsen TO, Maki RG, O'Sullivan
B and Alman BA: Opportunities for improving the therapeutic ratio
for patients with sarcoma. Lancet Oncol. 8:513–524. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leddy LR and Holmes RE: Chondrosarcoma of
bone. Cancer Treat Res. 162:117–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gelderblom H, Hogendoorn PC, Dijkstra SD,
van Rijswijk CS, Krol AD, Taminiau AH and Bovée JV: The clinical
approach towards chondrosarcoma. Oncologist. 13:320–329. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fiorenza F, Abudu A, Grimer RJ, Carter SR,
Tillman RM, Ayoub K, Mangham DC and Davies AM: Risk factors for
survival and local control in chondrosarcoma of bone. J Bone Joint
Surg Br. 84:93–99. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Onishi AC, Hincker AM and Lee FY:
Surmounting chemotherapy and radioresistance in chondrosarcoma:
Molecular mechanisms and therapeutic targets. Sarcoma.
2011:3815642011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumazawa T, Sato K, Seno H, Ishii A and
Suzuki O: Levels of pyrroloquinoline quinone in various foods.
Biochem J. 307:331–333. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noji N, Nakamura T, Kitahata N, Taguchi K,
Kudo T, Yoshida S, Tsujimoto M, Sugiyama T and Asami T: Simple and
sensitive method for pyrroloquinoline quinone (PQQ) analysis in
various foods using liquid chromatography/electrospray-ionization
tandem mass spectrometry. J Agric Food Chem. 55:7258–7263. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salisbury SA, Forrest HS, Cruse WB and
Kennard O: A novel coenzyme from bacterial primary alcohol
dehydrogenases. Nature. 280:843–844. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fluckiger R, Paz M, Mah J, Bishop A and
Gallop PM: Characterization of the glycine-dependent redox-cycling
activity in animal fluids and tissues using specific inhibitors and
activators: evidence for presence of PQQ. Biochem Biophys Res
Commun. 196:61–68. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y and Rosenberg PA: The essential
nutrient pyrroloquinoline quinone may act as a neuroprotectant by
suppressing peroxynitrite formation. Eur J Neurosci. 16:1015–1024.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyauchi K, Urakami T, Abeta H, Shi H,
Noguchi N and Niki E: Action of pyrroloquinolinequinol as an
antioxidant against lipid peroxidation in solution. Antioxid Redox
Signal. 1:547–554. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He K, Nukada H, Urakami T and Murphy MP:
Antioxidant and pro-oxidant properties of pyrroloquinoline quinone
(PQQ): Implications for its function in biological systems. Biochem
Pharmacol. 65:67–74. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang L, Rong Z, Zeng M, Cao Y, Gong X, Lin
L, Chen Y, Cao W, Zhu L and Dong W: Pyrroloquinoline quinone
protects nucleus pulposus cells from hydrogen peroxide-induced
apoptosis by inhibiting the mitochondria-mediated pathway. Eur
Spine J. 24:1702–1710. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu F, Yu H, Liu J and Cheng L:
Pyrroloquinoline quinone inhibits oxygen/glucose
deprivation-induced apoptosis by activating the PI3K/AKT pathway in
cardiomyocytes. Mol Cell Biochem. 386:107–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Min Z, Wang L, Jin J, Wang X, Zhu B, Chen
H and Cheng Y: Pyrroloquinoline quinone induces cancer cell
apoptosis via mitochondrial-dependent pathway and down-regulating
cellular bcl-2 protein expression. J Cancer. 5:609–624. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato K and Toriyama M: Effect of
pyrroloquinoline quinone (PQQ) on melanogenic protein expression in
murine B16 melanoma. J Dermatol Sci. 53:140–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wen L, Lu X, Wang R, Jin X, Hu L and You
C: Pyrroloquinoline quinone induces chondrosarcoma cell apoptosis
by increasing intracellular reactive oxygen species. Mol Med Rep.
17:7184–7190. 2018.PubMed/NCBI
|
|
18
|
Strauss HW, Blankenberg F, Vanderheyden JL
and Tait J: Translational imaging: Imaging of apoptosis. Handb Exp
Pharmacol. 1–275. 2008.
|
|
19
|
Lockshin RA and Zakeri Z: Cell death in
health and disease. J Cell Mol Med. 11:1214–1224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu PP, Kuo SC, Huang WW, Yang JS, Lai KC,
Chen HJ, Lin KL, Chiu YJ, Huang LJ and Chung JG:
(−)-Epigallocatechin gallate induced apoptosis in human adrenal
cancer NCI-H295 cells through caspase-dependent and
caspase-independent pathway. Anticancer Res. 29:1435–1442.
2009.PubMed/NCBI
|
|
21
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Chen Y, Zhang X, Cai G, An S, Wang
X, Teng L and Wang D: Tricholoma matsutake aqueous extract induces
hepatocellular carcinoma cell apoptosis via caspase-dependent
mitochondrial pathway. Biomed Res Int. 2016:90143642016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
24
|
Jiang X and Wang X: Cytochrome c promotes
caspase-9 activation by inducing nucleotide binding to Apaf-1. J
Biol Chem. 275:31199–31203. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C,
Karasuyama H, Su MS, Rakic P and Flavell RA: Reduced apoptosis and
cytochrome c-mediated caspase activation in mice lacking caspase 9.
Cell. 94:325–337. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chai J, Du C, Wu JW, Kyin S, Wang X and
Shi Y: Structural and biochemical basis of apoptotic activation by
Smac/DIABLO. Nature. 406:855–862. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cande C, Cohen I, Daugas E, Ravagnan L,
Larochette N, Zamzami N and Kroemer G: Apoptosis-inducing factor
(AIF): A novel caspase-independent death effector released from
mitochondria. Biochimie. 84:215–222. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li LY, Luo X and Wang X: Endonuclease G is
an apoptotic DNase when released from mitochondria. Nature.
412:95–99. 2001. View
Article : Google Scholar : PubMed/NCBI
|