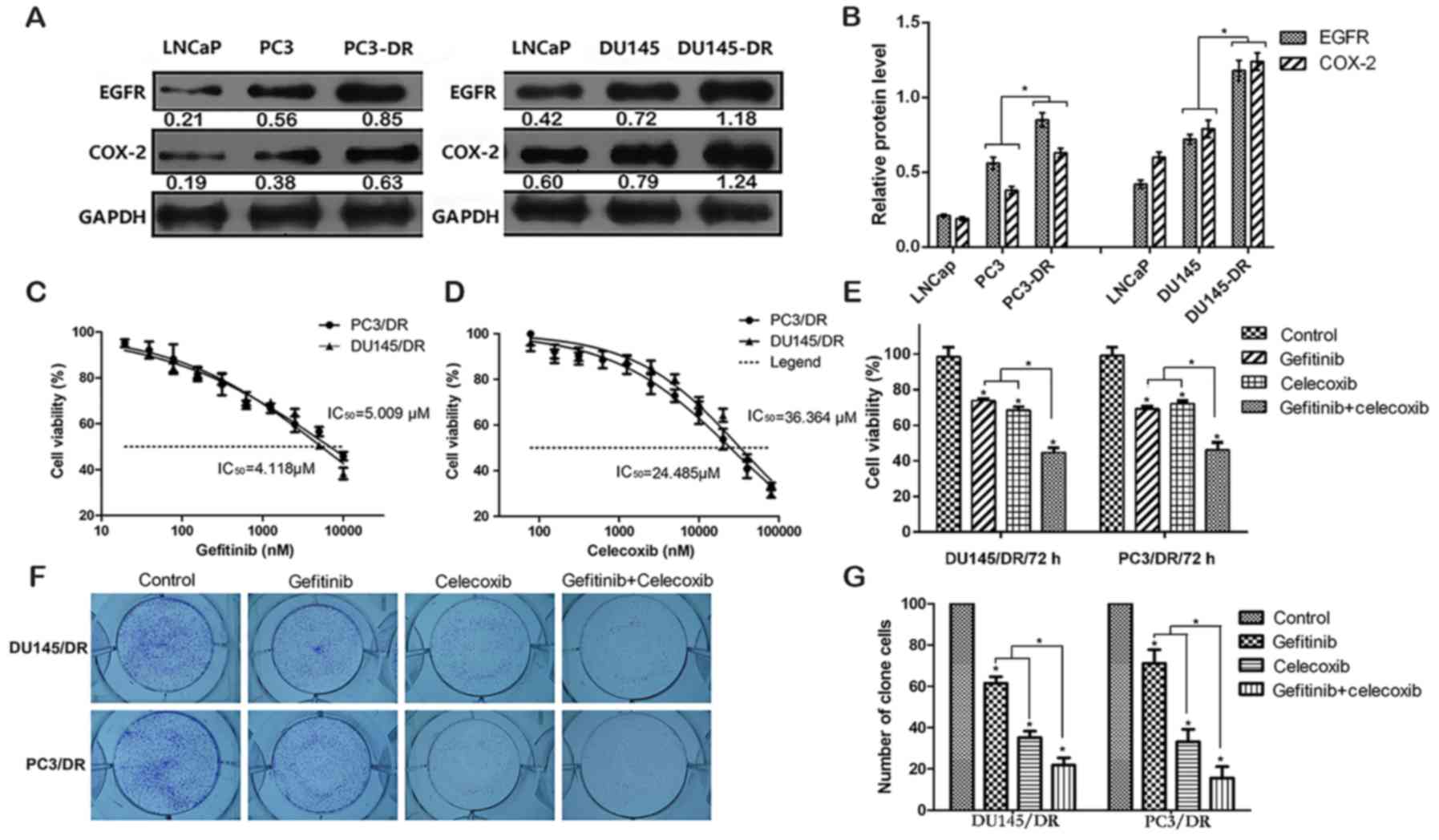
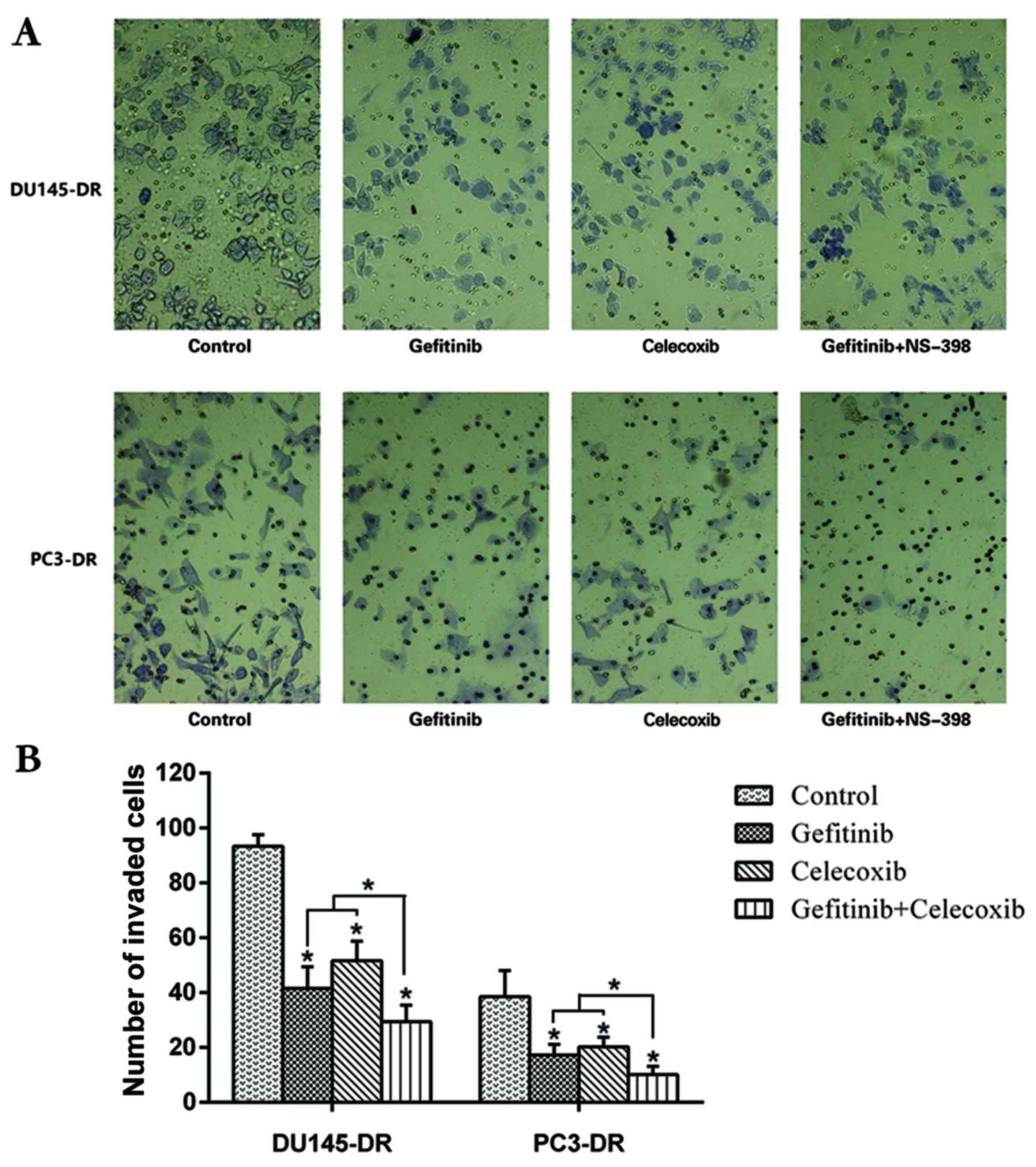
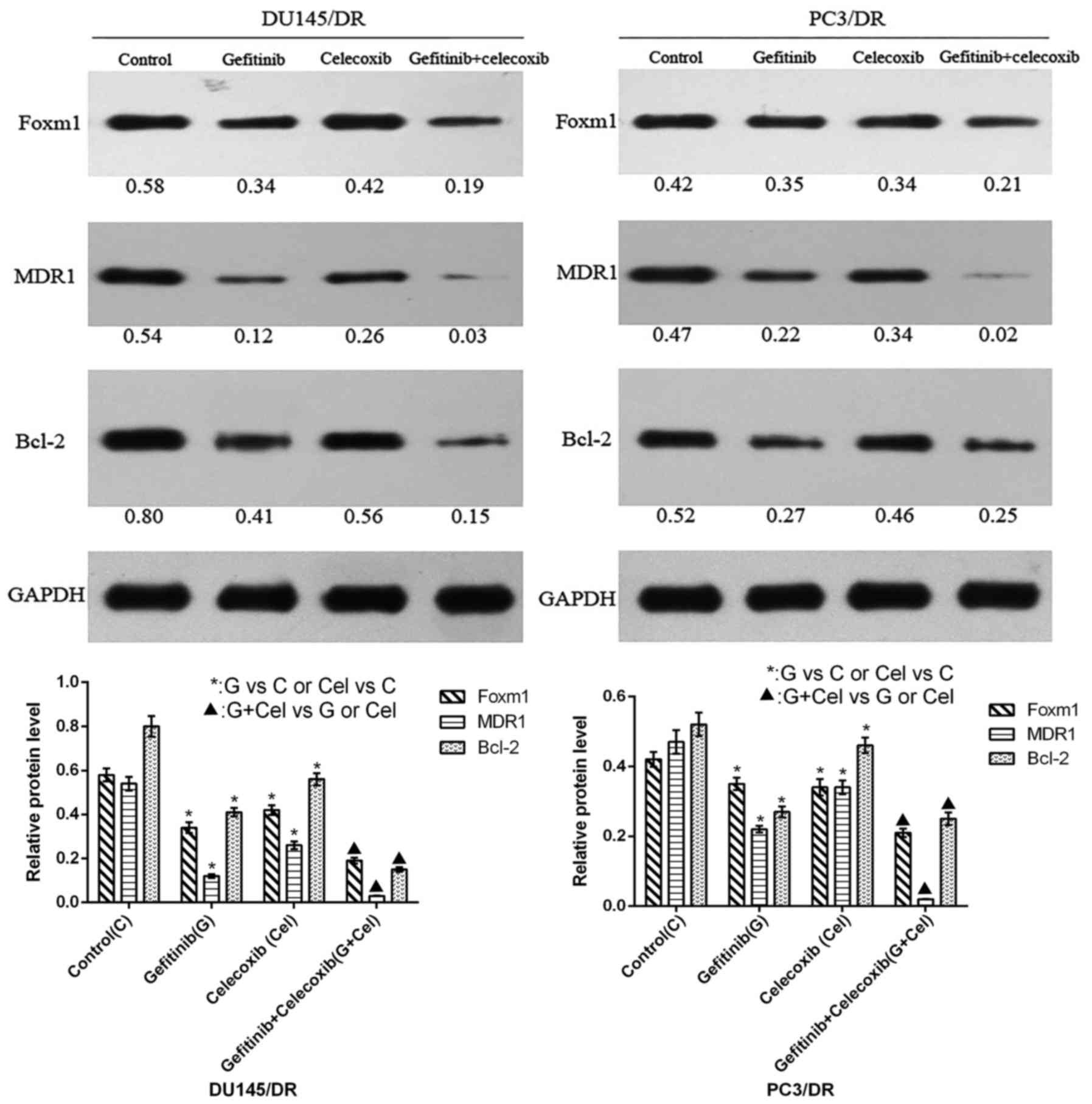
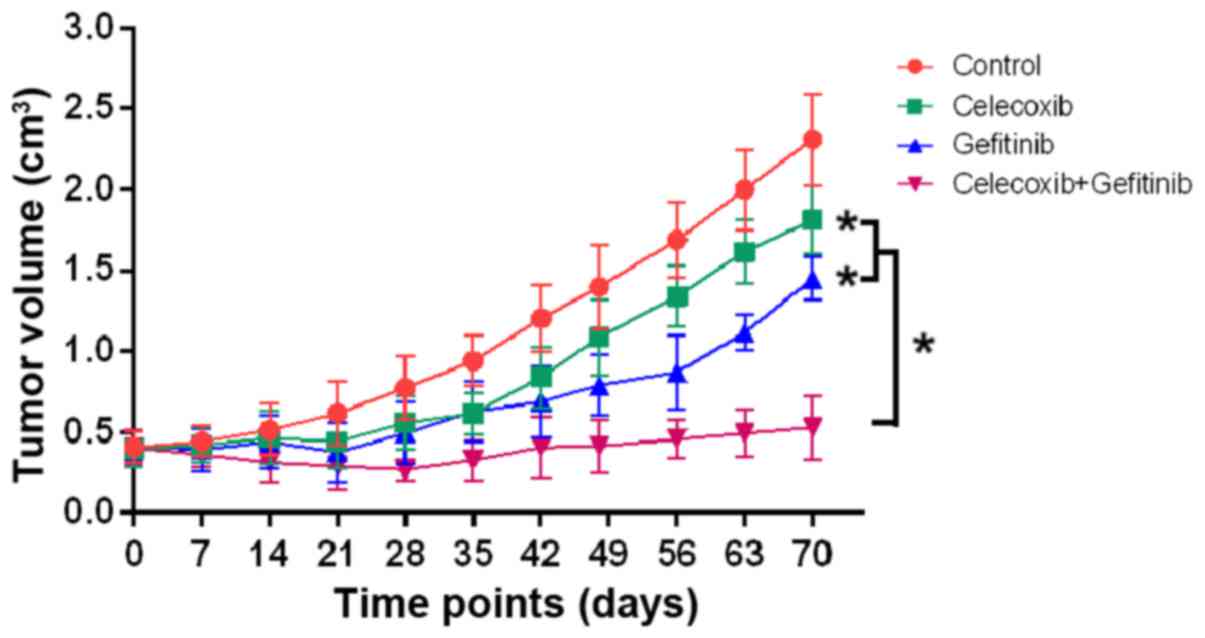
|
1
|
Magadoux L, Isambert N, Plenchette S,
Jeannin JF and Laurens V: Emerging targets to monitor and overcome
docetaxel resistance in castration resistant prostate cancer
(Review). Int J Oncol. 45:919–928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petrylak DP, Vogelzang NJ, Budnik N,
Wiechno PJ, Sternberg CN, Doner K, Bellmunt J, Burke JM, de Olza
MO, Choudhury A, et al: Docetaxel and prednisone with or without
lenalidomide in chemotherapy-naive patients with metastatic
castration-resistant prostate cancer (MAINSAIL): A randomised,
double-blind, placebo-controlled phase 3 trial. Lancet Oncol.
16:417–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scher HI, Jia X, Chi K, de Wit R, Berry
WR, Albers P, Henick B, Waterhouse D, Ruether DJ, Rosen PJ, et al:
Randomized, open-label phase III trial of docetaxel plus high-dose
calcitriol versus docetaxel plus prednisone for patients with
castration-resistant prostate cancer. J Clin Oncol. 29:2191–2198.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Araujo JC, Trudel GC, Saad F, Armstrong
AJ, Yu EY, Bellmunt J, Wilding G, McCaffrey J, Serrano SV, Matveev
VB, et al: Docetaxel and dasatinib or placebo in men with
metastatic castration-resistant prostate cancer (READY): A
randomised, double-blind phase 3 trial. Lancet Oncol. 14:1307–1316.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kelly WK, Halabi S, Carducci M, George D,
Mahoney JF, Stadler WM, Morris M, Kantoff P, Monk JP, Kaplan E, et
al: Randomized, double-blind, placebo-controlled phase III trial
comparing docetaxel and prednisone with or without bevacizumab in
men with metastatic castration-resistant prostate cancer: CALGB
90401. J Clin Oncol. 30:1534–1540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tannock IF, Fizazi K, Ivanov S, Karlsson
CT, Fléchon A, Skoneczna I, Orlandi F, Gravis G, Matveev V, Bavbek
S, et al: Aflibercept versus placebo in combination with docetaxel
and prednisone for treatment of men with metastatic
castration-resistant prostate cancer (VENICE): A phase 3,
double-blind randomised trial. Lancet Oncol. 14:760–768. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quinn DI, Tangen CM, Hussain M, Lara PN
Jr, Goldkorn A, Moinpour CM, Garzotto MG, Mack PC, Carducci MA,
Monk JP, et al: Docetaxel and atrasentan versus docetaxel and
placebo for men with advanced castration-resistant prostate cancer
(SWOG S0421): A randomised phase 3 trial. Lancet Oncol. 14:893–900.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fizazi K, Higano CS, Nelson JB, Gleave M,
Miller K, Morris T, Nathan FE, McIntosh S, Pemberton K and Moul JW:
Phase III, randomized, placebo-controlled study of docetaxel in
combination with zibotentan in patients with metastatic
castration-resistant prostate cancer. J Clin Oncol. 31:1740–1747.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferrero JM, Chamorey E, Oudard S, Dides S,
Lesbats G, Cavaglione G, Nouyrigat P, Foa C and Kaphan R: Phase II
trial evaluating a docetaxel-capecitabine combination as treatment
for hormone-refractory prostate cancer. Cancer. 107:738–745. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hahn NM, Marsh S, Fisher W, Langdon R, Zon
R, Browning M, Johnson CS, Scott-Horton TJ, Li L and Sweeney CJ:
Hoosier oncology group randomized phase II study of docetaxel,
vinorelbine, and estramustine in combination in hormone-refractory
prostate cancer with pharmacogenetic survival analysis. Clin Cancer
Res. 12:6094–6099. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Petrioli R, Paolelli L, Francini E,
Manganelli A, Salvestrini F and Francini G: Weekly docetaxel and
epirubicin in treatment of advanced hormone-refractory prostate
cancer. Urology. 69:142–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ross RW, Beer TM, Jacobus S, Bubley GJ,
Taplin ME, Ryan CW, Huang J and Oh WK: Prostate Cancer Clinical
Trials Consortium: A phase 2 study of carboplatin plus docetaxel in
men with metastatic hormone-refractory prostate cancer who are
refractory to docetaxel. Cancer. 112:521–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Basch E, Loblaw DA, Oliver TK, Carducci M,
Chen RC, Frame JN, Garrels K, Hotte S, Kattan MW, Raghavan D, et
al: Systemic therapy in men with metastatic castration-resistant
prostate cancer: American Society of Clinical Oncology and Cancer
Care Ontario Clinical Practice Guideline. J Clin Oncol.
32:3436–3448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borghese C, Cattaruzza L, Pivetta E,
Normanno N, De Luca A, Mazzucato M, Celegato M, Colombatti A and
Aldinucci D: Gefitinib inhibits the cross-talk between mesenchymal
stem cells and prostate cancer cells leading to tumor cell
proliferation and inhibition of docetaxel activity. J Cell Biochem.
114:1135–1144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hour TC, Chung SD, Kang WY, Lin YC, Chuang
SJ, Huang AM, Wu WJ, Huang SP, Huang CY and Pu YS: EGFR mediates
docetaxel resistance in human castration-resistant prostate cancer
through the Akt-dependent expression of ABCB1 (MDR1). Arch Toxicol.
89:591–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dandekar DS, Lopez M, Carey RI and
Lokeshwar BL: Cyclooxygenase-2 inhibitor celecoxib augments
chemotherapeutic drug-induced apoptosis by enhancing activation of
caspase-3 and −9 in prostate cancer cells. Int J Cancer.
115:484–492. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kattan J, Bachour M, Farhat F, El Rassy E,
Assi T and Ghosn M: Phase II trial of weekly docetaxel, zoledronic
acid, and celecoxib for castration-resistant prostate cancer.
Invest New Drugs. 34:474–480. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buchanan FG, Holla V, Katkuri S, Matta P
and DuBois RN: Targeting cyclooxygenase-2 and the epidermal growth
factor receptor for the prevention and treatment of intestinal
cancer. Cancer Res. 67:9380–9388. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Z, Zhang X, Li M, Wang Z, Wieand HS,
Grandis JR and Shin DM: Simultaneously targeting epidermal growth
factor receptor tyrosine kinase and cyclooxygenase-2, an efficient
approach to inhibition of squamous cell carcinoma of the head and
neck. Clin Cancer Res. 10:5930–5939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin J, Wu H, Shi H, Pan W, Yu H and Zhu J:
Combined inhibition of epidermal growth factor receptor and
cyclooxygenase-2 leads to greater anti-tumor activity of docetaxel
in advanced prostate cancer. PLoS One. 8:e761692013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin JZ, Wang ZJ, De W, Zheng M, Xu WZ, Wu
HF, Armstrong A and Zhu JG: Targeting AXL overcomes resistance to
docetaxel therapy in advanced prostate cancer. Oncotarget.
8:41064–41077. 2017.PubMed/NCBI
|
|
22
|
Shen SJ, Zhang YH, Gu XX, Jiang SJ and Xu
LJ: Yangfei Kongliu Formula, a compound Chinese herbal medicine,
combined with cisplatin, inhibits growth of lung cancer cells
through transforming growth factor-β1 signaling pathway. J Integr
Med. 15:242–251. 2017.PubMed/NCBI
|
|
23
|
Abedinpour P, Baron VT, Welsh J and
Borgström P: Regression of prostate tumors upon combination of
hormone ablation therapy and celecoxib in vivo. Prostate.
71:813–823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bahl A, Oudard S, Tombal B, Ozgüroglu M,
Hansen S, Kocak I, Gravis G, Devin J, Shen L, de Bono JS, et al:
Impact of cabazitaxel on 2-year survival and palliation of
tumour-related pain in men with metastatic castration-resistant
prostate cancer treated in the TROPIC trial. Ann Oncol.
24:2402–2408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang Y, Dai J, Zhang H, Sottnik JL,
Keller JM, Escott KJ, Sanganee HJ, Yao Z, McCauley LK and Keller
ET: Activation of the Wnt pathway through AR79, a GSK3β inhibitor,
promotes prostate cancer growth in soft tissue and bone. Mol Cancer
Res. 11:1597–1610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sánchez C, Mercado A, Contreras HR,
Mendoza P, Cabezas J, Acevedo C, Huidobro C and Castellón EA:
Chemotherapy sensitivity recovery of prostate cancer cells by
functional inhibition and knock down of multidrug resistance
proteins. Prostate. 71:1810–1817. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu Y, Liu C, Armstrong C, Lou W, Sandher
A and Gao AC: Antiandrogens inhibit ABCB1 efflux and ATPase
activity and reverse docetaxel resistance in advanced prostate
cancer. Clin Cancer Res. 21:4133–4142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X1, Yao R, Yue L, Qiu W, Qi W, Liu S,
Yao Y and Liang J: FOXM1 mediates resistance to docetaxel in
gastric cancer up-regulating stathmin. J Cell Mol Med. 18:811–823.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okada K, Fujiwara Y, Takahashi T, Nakamura
Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Mori
M, et al: Overexpression of forkhead box M1 transcription factor
(FOXM1) is a potential prognostic marker and enhances
chemoresistance for docetaxel in gastric cancer. Ann Surg Oncol.
20:1035–1043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang K, Zhu X, Zhang K, Zhu L and Zhou F:
FoxM1 inhibition enhances chemosensitivity of docetaxel-resistant
A549 cells to docetaxel via activation of JNK/mitochondrial
pathway. Acta Biochim Biophys Sin. 48:804–809. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Souza R, Zahedi P, Badame RM, Allen C
and Piquette-Miller M: Chemotherapy dosing schedule influences drug
resistance development in ovarian cancer. Mol Cancer Ther.
10:1289–1299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kordezangeneh M, Irani S, Mirfakhraie R,
Esfandyari-Manesh M, Atyabi F and Dinarvand R: Regulation of
BAX/BCL2 gene expression in breast cancer cells by
docetaxel-loaded human serum albumin nanoparticles. Med Oncol.
32:2082015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kubo T, Kawano Y, Himuro N, Sugita S, Sato
Y, Ishikawa K, Takada K, Murase K, Miyanishi K, Sato T, et al: BAK
is a predictive and prognostic biomarker for the therapeutic effect
of docetaxel treatment in patients with advanced gastric cancer.
Gastric Cancer. 19:827–838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bokhorst LP, Roobol MJ, Bangma CH and van
Leenders GJ: Effect of pathologic revision and Ki67 and ERG
immunohistochemistry on predicting radical prostatectomy outcome in
men initially on active surveillance. Prostate. 77:1137–1143. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cui D, Dai J, Keller JM, Mizokami A, Xia S
and Keller ET: Notch pathway inhibition using PF-03084014, a
γ-secretase inhibitor (GSI), enhances the antitumor effect of
docetaxel in prostate cancer. Clin Cancer Res. 21:4619–4629. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Richardsen E, Andersen S, Al-Saad S,
Rakaee M, Nordby Y, Pedersen MI, Ness N, Grindstad T, Movik I,
Dønnem T, et al: Evaluation of the proliferation marker Ki-67 in a
large prostatectomy cohort. PLoS One. 12:e01868522017. View Article : Google Scholar : PubMed/NCBI
|