Introduction
Prostate cancer (PCa) remains among the most
frequently diagnosed solid tumors in men, and is the second-leading
cause of cancer-associated mortalities. The vast majority of PCa
patients treated with androgen ablation therapy eventually develop
castration-resistant PCa (CRPC) and bone metastasis. While
taxane-based chemotherapy regimens such as docetaxel and
cabazitaxel are widely used as first-line treatments for CRPC, the
associated improvement in survival is moderate (~2 months) and
patients typically experience significant side-effects (1). Multiple clinical trials have attempted
to improve the survival benefit of docetaxel treatment in CRPC by
combining it with other agents, however these attempts have so far
been unsuccessful. The tested agents include lenalidomide (2), calcitriol (3), dasatinib (4), vascular endothelial growth factor
(VEGF) inhibitors (5,6) and endothelin receptor antagonists
(7,8) as well as others (9–12). In
addition, docetaxel is only suitable for chemotherapy-naïve
patients, and resistance develops over time (13). While cabazitaxel is currently used
to treat these resistant patients, no chemotherapy regimens have
been successfully established for CRPC patients who have developed
tumor resistance to both docetaxel and cabazitaxel in randomized
clinical trials. Therefore, alternative therapeutic strategies with
greater long-term health benefits are required for CRPC
patients.
Accumulating evidence has indicated that targeting
epidermal growth factor receptor (EGFR) (14,15)
and cyclooxygenase-2 (COX-2) (16,17)
could be a promising strategy for preventing or delaying docetaxel
resistance. Furthermore, a direct interaction between EGFR
signaling and COX-2 activity has been suggested to occur in many
types of cancer (18,19). Our previous study also indicated
that the combination of COX-2 and EGFR inhibitors could
significantly improve the therapeutic effects of docetaxel in CRPC,
with lower toxicity (20). However,
the extent of their therapeutic effect and their mechanism in
docetaxel-resistant CRPC remain elusive.
In the present study, we determined the antitumor
efficacy of the tyrosine phosphorylated (p-)EGFR-selective
inhibitor gefitinib, and the COX-2 inhibitor celecoxib combination
therapy on two established docetaxel-resistant CRPC cell lines
(PC3/DR and DU145/DR) in vitro and in vivo. We also
studied the effect of this novel regimen on tumor regulating
proteins.
Materials and methods
Cell culture
LNCaP, PC-3 and DU-145 human CRPC cell lines were
purchased from the Chinese Academy of Sciences, Shanghai Institute
of Biochemistry and Cell Biology (Shanghai, China). These were
maintained at 37°C with 5% CO2 in an F12 and RPMI-1640
culture medium containing 10% fetal bovine serum (FBS; all from
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 26 mmol/l
NaH2CO3 (pH 7.4), 1% L-glutamine and
antibiotics. PC3/DR and DU145/DR cells were established as
described in our previous study (21).
Cell proliferation assay
Cells (2,000/well) were plated in triplicate in a
96-well plate. The anti-proliferative effect of treatment with
gefitinib and celecoxib (MedchemExpress, Monmouth Junction, NJ,
USA) on the cells was determined by an MTT assay. In brief, 2 mg/ml
MTT in PBS solution was added at 50 µl/well, and the cells were
incubated at 37°C for 2 h. Dimethyl sulfoxide (100 µl) was then
added to each well. The absorbance was measured at a wavelength of
490 nm with a microplate reader (BioTek Instruments, Inc. Winooski,
VT, USA).
Clone formation assay
Cells were seeded in 6-well plates at a density of
500 cells/well and incubated for 24 h. Then the cells were treated
with gefitinib, celecoxib or a combination of both. The cells were
incubated for 14 days, resulting in the formation of visible clonal
colonies. The colonies were fixed with 4% formaldehyde for 10 min
and dyed with 5 ml 0.5% crystal violet (Nantong Chem-Base Co.,
Ltd., Jiangsu, China) solution for 15 min. The number of
colonies/well was then counted.
Flow cytofluorometric analysis
The cells were treated with gefitinib, celecoxib or
a combination of both. Following 24 h of incubation, the growth
medium was removed and the cells were harvested. Supernatants were
discarded and pellets were resuspended in 400 µl propidium iodide
(PI) solution (50 µg/ml PI, 0.1% Triton X-100 and 0.1% sodium
citrate in PBS). Samples were then incubated at 4°C in the dark,
before being subjected to flow cytometric analysis to determine the
proportion of apoptotic and necrotic cells. Analyses were performed
using a FACSCalibur™ flow cytometer (Becton Dickinson, San Jose,
CA, USA).
In vitro invasion assays
The invasive potential of the PCa cells was assessed
by their ability to penetrate a Boyden chamber with an 8-µm pore
polyethylene terephthalate insert overlaid with a thin layer of
Matrigel. Cells were untreated (control), or pretreated with
gefitinib, celecoxib or both, for 24 h. For each condition,
5×104/ml cells/well were loaded into the top of either
the BD BioCoat Matrigel cell invasion chamber (BD Biosciences,
Franklin Lakes, NJ, USA) according to the manufacturer's
instructions. After incubation for 24 h at 37°C, the invasive cells
reaching the lower chamber were stained with crystal violet and
counted under phase-contrast microscopy (Leica IX51 microscope;
Leica Microsystems, Wetzlar, Germany). Cell invasion was expressed
as the mean number of invading cells in five random fields of
view.
Western blot analysis
Cells were treated with gefitinib, celecoxib or a
combination of both. The cells were harvested and lysed, following
which lysates were extracted with T-PER tissue protein extraction
reagent (Pierce, Rockford, IL, USA). A BCA Protein Assay kit was
used to determine total protein concentration, and lysates
containing equal amounts of protein (20 µg) were separated by 10%
SDS-PAGE and transferred to polyvinylidene difluoride membranes
using a Bio-Rad SemiDry apparatus. After blocking with closed
liquid containing 5% skimmed milk powder at room temperature for 2
h, the membranes were incubated with the following primary
antibodies: EGFR (cat. no. sc-71033), COX-2 (cat. no. sc-166475),
ABCB1 (cat. no. sc-55510) (all from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and Bcl-2 (cat. no. ab59348) and FOXM1 (cat. no.
ab180710) (both from Abcam, Cambridge, UK) and GAPDH (cat. no.
MB0077; Bioworld, Dublin, OH, USA). All primary antibodies were
diluted with QuickBlock™ Antibody Dilution Buffer (cat. no.
P0256FT; Shanghai, China) before use. Blots were incubated with
anti-rabbit IgG secondary antibody (cat. no. BS10650; Bioworld,
Dublin, OH, USA) diluted in skimmed milk powder for 1 h at room
temperature. The protein bands were detected with a
chemiluminescence detection system (Thermo Fisher Scientific, Inc.,
MA, USA). All western blotting was performed in triplicate and
quantified by densitometry using Gel-Pro 5.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
DU145/DR xenografts in nude mice
BALB/C nu/nu male mice (4–6 weeks old) weighing
18–22 g were obtained from the Shanghai SLAC Animal Laboratory
(Shanghai, China). The mice were maintained in a well-ventilated
enclosed system under controlled temperature (20–25°C) and humidity
(40–60%), with precautions to prevent pathogenic infestation. They
were housed under constant 12-h light/dark cycles (light duration
from 7:00 to 19:00) with food and water available ad
libitum. Animal experiments were performed in accordance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (NIH, Bethesda, MD, USA), and were approved by
the local Ethics Committee at Nanjing BenQ Hospital, Affiliated to
Nanjing Medical University (Nanjing, China). DU145/DR cells were
resuspended in PBS to a final density of 2×107 cells/ml,
and 100 µl cell suspension was transferred into the dorsal flank of
each mouse using a 27-gauge needle. The resulting tumor volumes
were determined with digital calipers and calculated according to
the equation V = (LxW2)/2, where V is the volume, L is
the length and W is the width. When well-established tumors of ~0.4
cm3 were detected, the mice were randomly allocated into
four groups (n=5 per group) and administered the following
treatment for a period of 10 weeks: i) control (PBS treatment); ii)
gefitinib (100 mg/kg/day); iii) celecoxib (4 mg/kg/day); iv)
gefitinib (100 mg/kg/day) plus celecoxib (4 mg/kg/day). At week 10,
the mice were sacrificed by cervical dislocation after the last
dosage and subcutaneous tumors were harvested.
Immunohistochemistry (IHC)
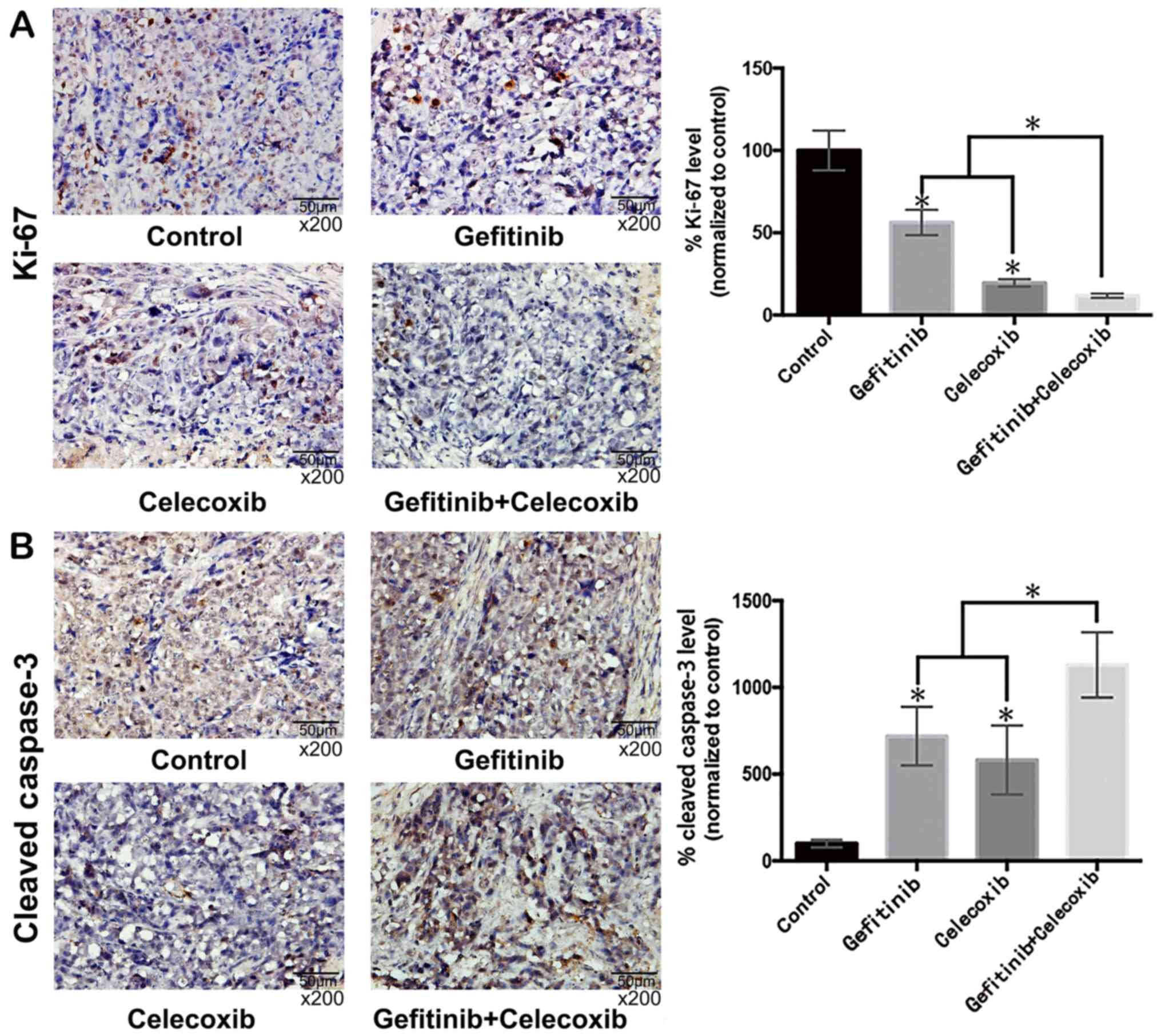
The expression levels of Ki-67 and cleaved
caspase-3, indices for proliferation and apoptosis, were determined
in the mouse tumor tissue by IHC. A section of each tumor was fixed
in 10% formalin. Antibodies against Ki-67 (dilution, 1:500; cat.
no. ab15580) and caspase-3 (dilution, 1:300; cat. no. ab2302; both
from Abcam) were used to stain 4-µm sections according to the
manufacturer's protocols. The sections were examined for positive
staining and vessel density that was quantified as previously
described (22,23). Representative fields were imaged
under an ×200 magnification.
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD). All experimental data were compared using a Student's t-test.
Statistical analyses were performed using GraphPad Prism 5.01
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Gefitinib and celecoxib combination
therapy result in a greater cytotoxic effect in docetaxel-resistant
PCa cell lines
Two docetaxel-resistant PCa cell lines, PC3/DR and
DU145/DR, were established by culturing PC3 and DU145 cells in
docetaxel in a dose-escalating manner. Our previous study revealed
that resistant cell lines exhibited ~20- and ~200-fold higher
resistance, respectively to docetaxel, compared with the respective
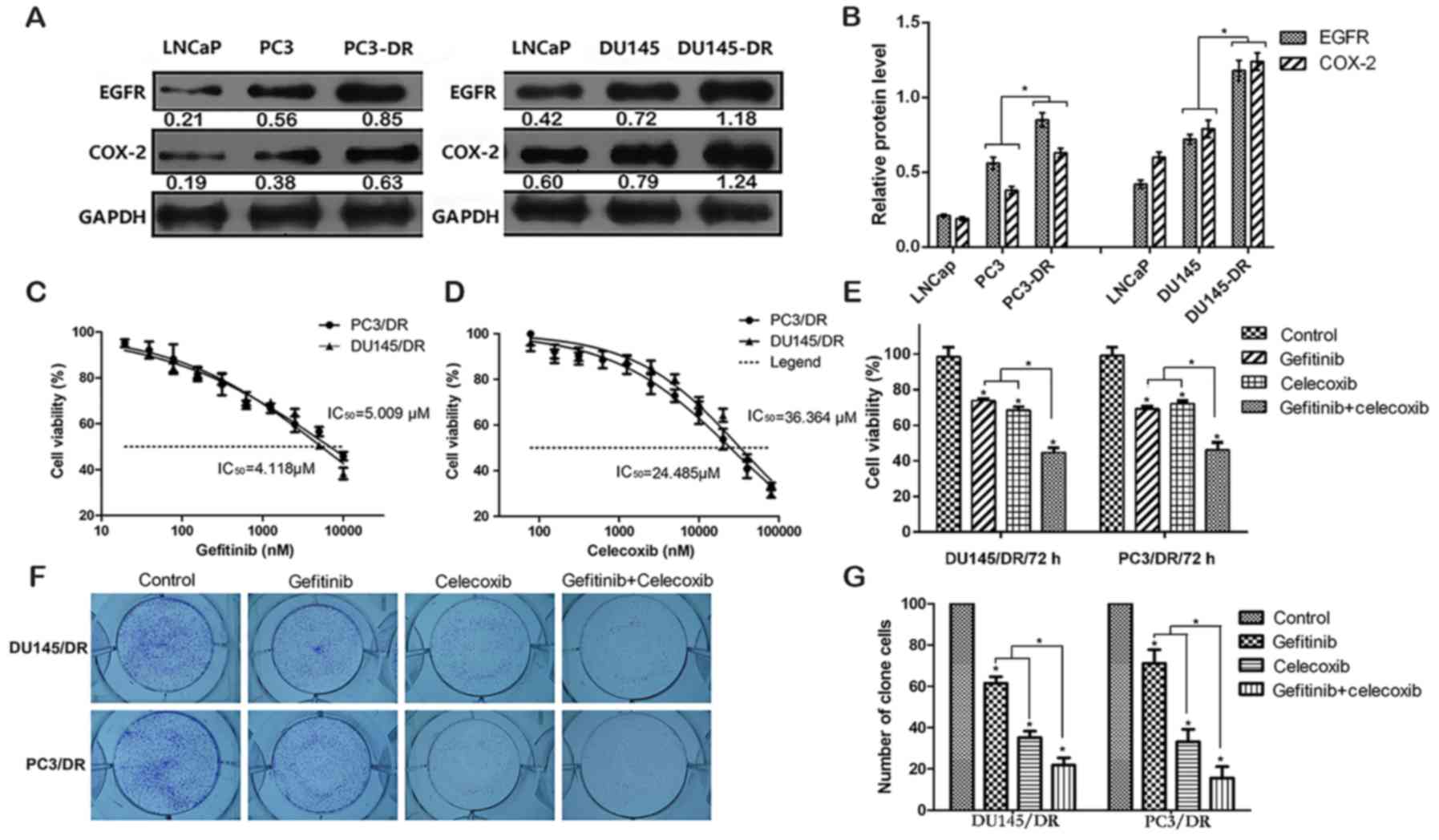
parental cells (21). The EGFR and
COX-2 protein levels were higher in the resistant cell lines than
in the parental lines (Fig. 1A and
B). An MTT assay demonstrated that the PC3/DR and DU145/DR
cells were sensitive to gefitinib and celecoxib treatment, with
half-maximal inhibitory concentration (IC50) values of
4.118 and 5.009 µM (gefitinib) and 36.364 and 24.485 µM
(celecoxib), respectively (Fig. 1C and
D). Concentrations of gefitinib and celecoxib that produced
~30% growth inhibition were selected for further experiments (0.625
and 5 µmol/l for PC3/DR cells, and 0.312 and 10 µmol/l for DU145/DR
cells). As displayed in Fig. 1E-G,
based on the data from MTT and clone formation assays, gefitinib or
celecoxib monotherapy induced mild cell growth inhibition
(P<0.05). However, co-treatment with both drugs resulted in a
supra-additive tumor cell growth inhibition which was stronger than
when either drug was used alone (P<0.05).
Gefitinib and celecoxib combination
therapy result in a greater induction of apoptosis and necrosis in
PC3/DR and DU145/DR cells
The exposure of PC3/DR and DU145/DR cells to either
gefitinib or celecoxib alone for 24 h significantly enhanced the
rate of apoptosis and necrosis compared with the untreated cells
(P<0.05). However, when both drugs were used in combination, an
even greater rate of cell death was observed than that of either
drug used alone (P<0.05) (Fig.
2).
Cell invasive ability is reduced by
combined treatment with gefitinib and celecoxib in PC3/DR and
DU145/DR cells
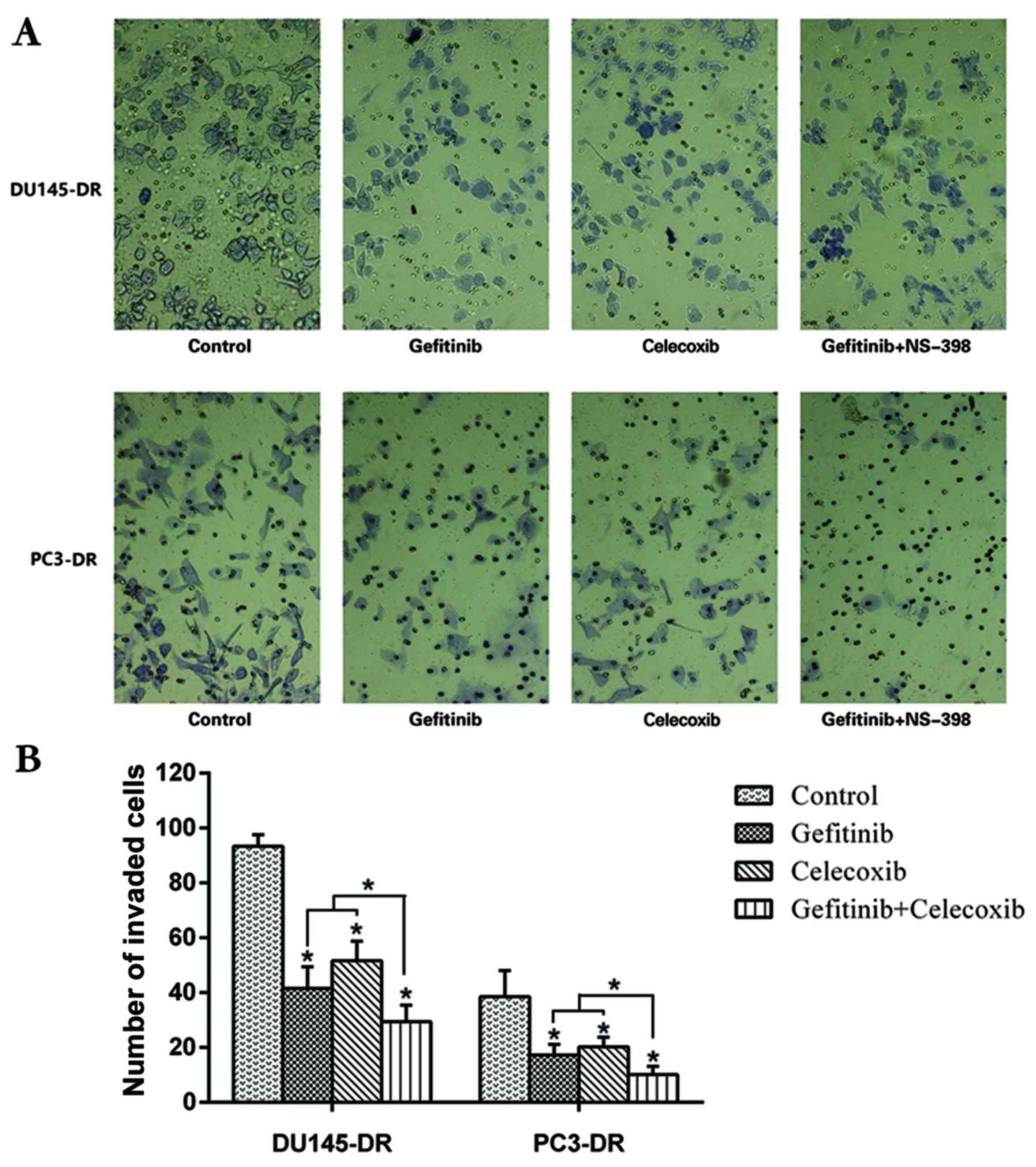
Analysis of the invasive potential of PC3/DR and
DU145/DR cells revealed that following treatment with gefitinib or
celecoxib, the invasive ability of the cells was significantly
inhibited (P<0.05) when compared with the untreated control.
Furthermore, when both drugs were used in combination, a
supra-additive inhibitory effect on cell invasion ability was
observed, with greater potency than when either drug was used alone
(P<0.05) (Fig. 3).
Changes in the expression of Bcl-2,
FOXM1 and ABCB1 (MDR1) are induced by gefitinib and celecoxib
treatment of PC3/DR and DU145/DR cells
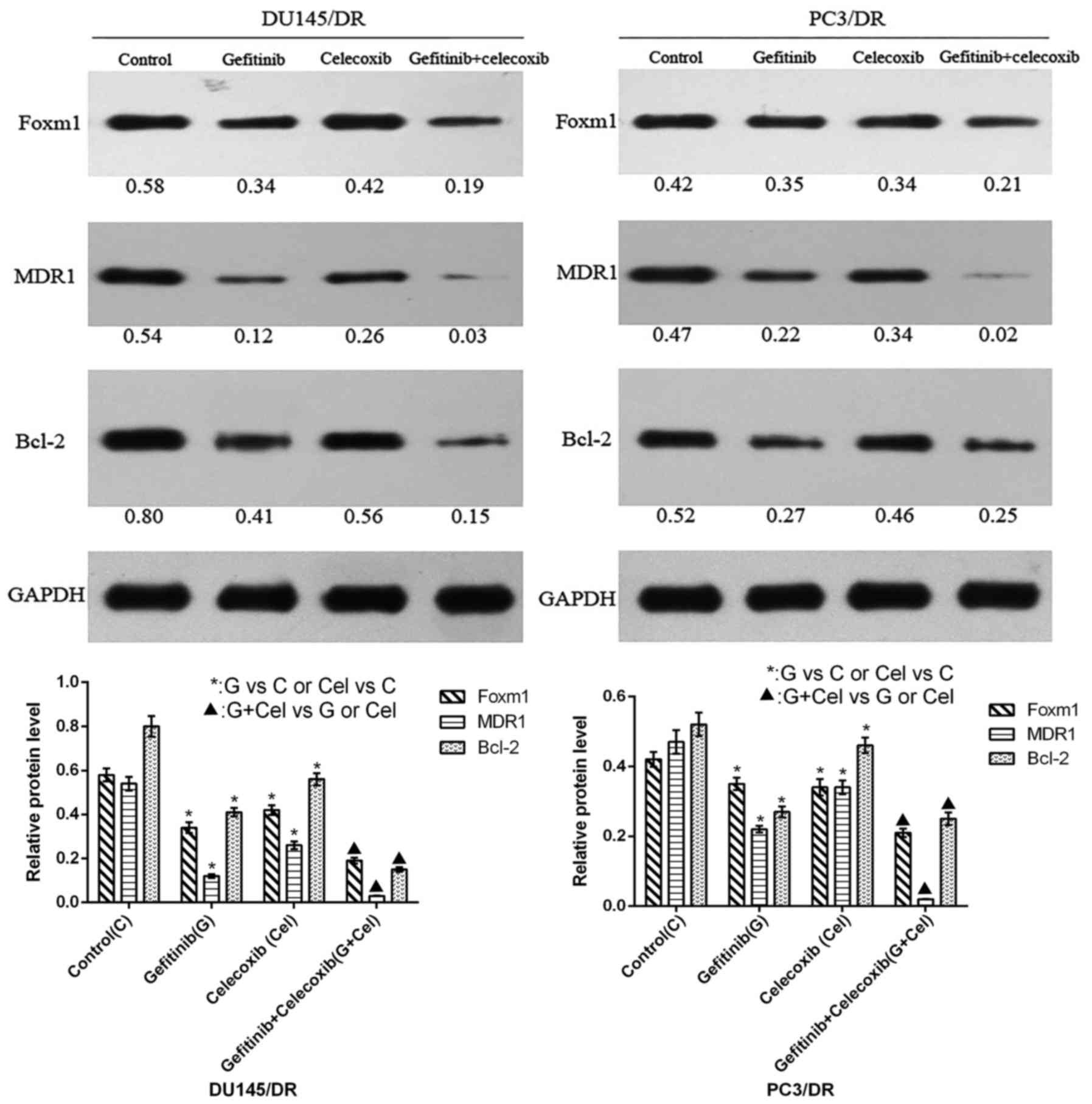
The results of western blotting demonstrated that
gefitinib or celecoxib monotherapy reduced the expression of Bcl-2,
FOXM1 and ABCB1 (MDR1) in the resistant cell lines (Fig. 4). Their levels were further
decreased when the cells were subjected to combination therapy
(P<0.05).
Gefitinib and celecoxib combination
therapy inhibits DU145/DR tumor growth in vivo
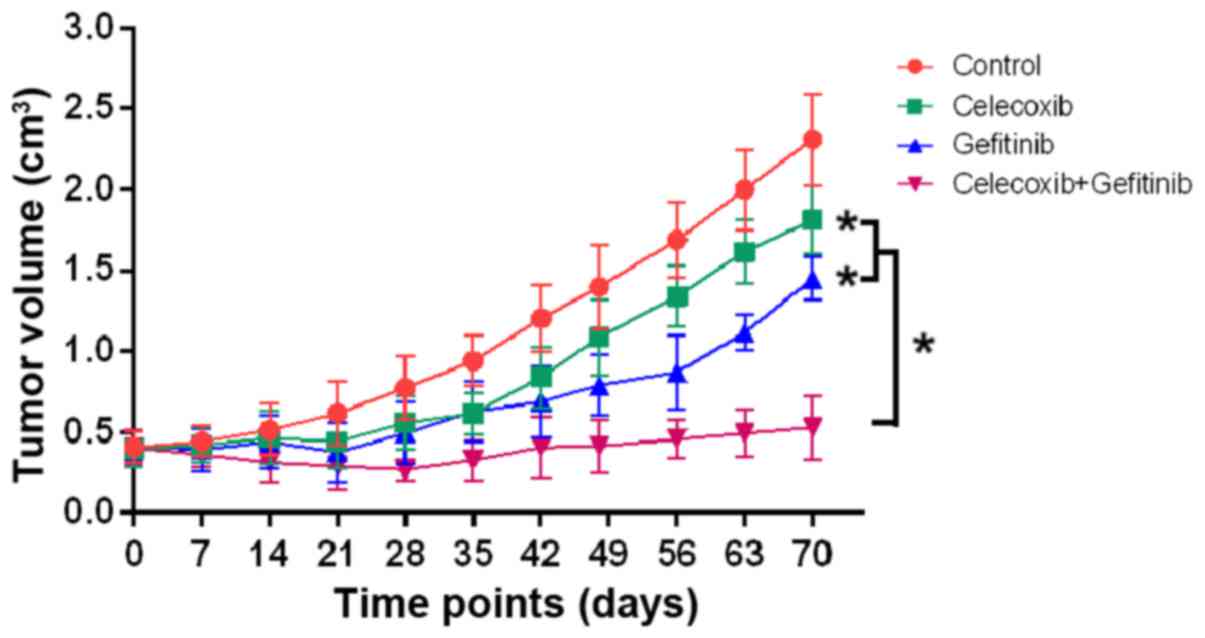
The antitumor activities of the control, gefitinib,
celecoxib and gefitinib in combination with celecoxib were
analyzed. A linear mixed model with random intersects was fitted to
the log-transformed data to compare tumor growth over time in the
different treatment groups. The results indicated that although
gefitinib and celecoxib alone moderately inhibited tumor growth, no
significant differences were found compared with the control
(P>0.05). However, the combination of gefitinib with celecoxib
significantly inhibited tumor growth when compared with the control
or monotherapies (P<0.05) (Fig.
5). In addition, co-treatment with both drugs was well
tolerated, as no weight loss or other signs of acute or delayed
toxicity were observed.
Ki-67 and caspase-3 immunostaining of
treated xenograft tumors
As displayed in Fig.
6A, the tumor Ki-67 index was significantly decreased in the
gefitinib and celecoxib monotherapy groups compared with the
control group. However, the tumor Ki-67 index of the combination
therapy group with both drugs was significantly lower than that of
either monotherapy group (P<0.05). In addition, the
cleaved-caspase-3 levels of the tumors from the combination group
were significantly higher than in the other groups (P<0.05)
(Fig. 6B).
Discussion
Taxane-based chemotherapy regimens remain the
first-line treatment for CRPC as they are associated with the
highest tumor regression and prostate-specific antigen (PSA)
response of the existing regimens. The survival benefit is also
superior to other chemotherapies currently in clinical use.
Docetaxel is primarily useful for patients who have received no
prior chemotherapy and is administered with prednisone to minimize
the side-effects. However, docetaxel resistance ultimately
develops, prompting the switch to a cabazitaxel-prednisone regimen
(24). However, the survival
benefit of this regimen is also moderate, as resistance develops
rapidly. In addition, numerous side-effects are associated with
this regimen, including treatment-related mortality. The present
study explored the efficacy of an alternative treatment for
docetaxel-resistant CRPC.
It has been suggested that EGFR and COX-2 play an
important role in the development of docetaxel resistance in PCa.
Their inhibition enhances the efficacy of docetaxel treatment
(14–17,20).
However, these studies were conducted in docetaxel-sensitive CRPC
cells, rather than docetaxel-resistant. In the present study, we
established two docetaxel-resistant CRPC cell lines (PC3/DR and
DU145/DR) by culturing PC3 and DU145 cells in docetaxel with an
escalating dose, and found that EGFR and COX-2 expression was
significantly elevated in the resistant cells compared with the
parental cell lines, indicating their possible role in docetaxel
resistance. Therefore, we hypothesized that EGFR and COX-2
inhibition could have therapeutic potential in docetaxel-resistant
CRPC.
Gefitinib and celecoxib are specific inhibitors for
EGFR and COX-2, respectively. Gefitinib inhibits the
phosphorylation of EGFR and has been applied for the treatment of
advanced lung cancer. Previous studies have shown that gefitinib
treatment can inhibit EGFR activity in CRPC cells to enhance their
sensitivity to docetaxel (14,15).
Celecoxib is a COX-2-selective nonsteroidal anti-inflammatory drug
used to treat pain and inflammation in osteoarthritis and
rheumatoid arthritis. It was demonstrated that celecoxib
significantly increased chemotherapeutic drug-induced apoptosis in
PCa cells (16) and increased the
efficacy of androgen withdrawal in vivo (25). However, to provide a curative
benefit, such an inhibitor must be used long-term or at a high
dosage, which can lead to an increase in dose-related side-effects.
Consequently, dose reduction strategies to provide similar
therapeutic benefit through the appropriate combination with other
drugs, may be more clinically viable. In the present study, we
explored the effect of targeting EGFR and COX-2 with gefitinib and
celecoxib, respectively, in docetaxel-resistant PCa cells.
To select appropriate concentrations for the in
vitro experiments, in our preliminary experiments, we
established concentration-response curves for both drugs to
determine the individual concentrations of each drug that yielded
~30% growth inhibition in the PC3/DR and DU145/DR cells. Following
this, to reasonably evaluate the combined effect of these two
drugs, based on their concentration-response curves, we determined
their relative appropriate concentrations for the subsequent
combination experiments as follows: 0.625 µmol/l (gefitinib) and 5
µmol/l (celecoxib) in PC3/DR cells, and 0.312 µmol/l (gefitinib)
and 10 µmol/l (celecoxib) in DU145/DR cells. We took into account
two main considerations for determining drug dosages: Whether the
dosages were able to effectively inhibit cell growth and whether
the effects of a combination of the two drugs result in possible
lethal inhibition. Based on the aforementioned lines of enquiry,
drug concentrations that led to 30% growth inhibition were deemed
appropriate. If dosages that led to 40 or 50% growth inhibition
were selected for each drug, a combination of the two drugs may
have resulted in lethal growth inhibition, which would have led to
an ineffective evaluation of the additive effects of the drugs. We
observed a greater effect on cell growth inhibition when EGFR and
COX-2 were simultaneously inhibited by the combination of gefitinib
and celecoxib, rather than individually. Furthermore, our results
regarding the induction of apoptosis and inhibition of cell
invasion by combination therapy were consistent with the MTT and
clone formation assay results. In the apoptosis experiment,
treatment with gefitinib and celecoxib alone or a combination of
the two drugs did not result in a high apoptosis rate. In other
words, most of the cells still maintained their own activity.
Although we did not completely exclude the effects of apoptosis
from the invasion assay, the effect of such concentrations of
different drug treatments on cell invasiveness is reasonably
expected. A significant decrease in tumor volume was also observed
in mice treated with the combination of both drugs compared with
each monotherapy group. Based on the aforementioned results, it is
possible that this novel combination may be clinically effective in
preventing prostate tumor growth and metastasis.
The upregulation of the multidrug resistance protein
ABCB1 (also known as MDR1) has been verified as a mechanism
underlying docetaxel resistance in PCa. Its synthesis may be
induced by docetaxel treatment, and it diminishes the efficacy of
docetaxel by actively removing it from cells across the membrane
(26,27). Similarly, FOXM1 was revealed to
mediate resistance to docetaxel in gastric and lung cancers, and
its inhibition enhanced the docetaxel sensitivity of
docetaxel-resistant cells (28–30).
Bcl-2 expression was also revealed to be associated with docetaxel
resistance in PCa (31–33). In our study, we found that the
antitumor effects of gefitinib and celecoxib combination therapy
may stem from the inhibition of ABCB1, FOXM1 and Bcl-2 expression.
Western blotting data from both the mono- and combination therapy
experiments demonstrated that the expression levels of these
proteins were significantly reduced. Furthermore, we also used IHC
to detect the expression of Ki-67 and cleaved-caspase-3 in
vivo, as is widely performed to assess the proliferative and
apoptotic potential of a tumor, respectively (34–36).
Our findings revealed that EGFR and COX-2 inhibitor combination
therapy had a beneficial effect on these factors as well, as Ki-67
was downregulated, and cleaved caspase-3 was upregulated. However,
further studies are warranted to evaluate the exact and detailed
mechanism of these factors in the context of CRPC pathogenesis and
therapy. Future studies are also warranted to determine whether
inhibiting EGFR and COX-2 improves the efficacy of docetaxel in
treating docetaxel-resistant CRPC by e.g. studying the effect of
the combination of their inhibition and docetaxel.
In conclusion, our study revealed, for the first
time, the application of the combination of gefitinib and celecoxib
therapy in docetaxel-resistant CRPC, confirming their improved
tumor inhibitory effect relative to gefitinib or celecoxib
monotherapies. The inhibition of the EGFR and COX-2 pathways by
gefitinib-celecoxib co-therapy represents a potential treatment for
docetaxel-resistant CRPC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Planning Project of Nanjing Medical University (grant
no. 2013NJMU148), the Jiangsu Natural Science Foundation, China
(grant no. BK20161108) and the Medical Science and Technology
Development Project of Nanjing, China (grant no. YKK15092).
Availability of data and material
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JZL, ZX and ZYR conceived and designed the study. IH
and YY analyzed the results and were major contributors in writing
the manuscript. JZL and JGZ performed the experiments. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participation
The animal experiments were conducted in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals (NIH, Bethesda, MD, USA), and approved by the
local Ethics Committee of Nanjing BenQ Hospital, Affiliated to
Nanjing Medical University, China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Magadoux L, Isambert N, Plenchette S,
Jeannin JF and Laurens V: Emerging targets to monitor and overcome
docetaxel resistance in castration resistant prostate cancer
(Review). Int J Oncol. 45:919–928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petrylak DP, Vogelzang NJ, Budnik N,
Wiechno PJ, Sternberg CN, Doner K, Bellmunt J, Burke JM, de Olza
MO, Choudhury A, et al: Docetaxel and prednisone with or without
lenalidomide in chemotherapy-naive patients with metastatic
castration-resistant prostate cancer (MAINSAIL): A randomised,
double-blind, placebo-controlled phase 3 trial. Lancet Oncol.
16:417–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scher HI, Jia X, Chi K, de Wit R, Berry
WR, Albers P, Henick B, Waterhouse D, Ruether DJ, Rosen PJ, et al:
Randomized, open-label phase III trial of docetaxel plus high-dose
calcitriol versus docetaxel plus prednisone for patients with
castration-resistant prostate cancer. J Clin Oncol. 29:2191–2198.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Araujo JC, Trudel GC, Saad F, Armstrong
AJ, Yu EY, Bellmunt J, Wilding G, McCaffrey J, Serrano SV, Matveev
VB, et al: Docetaxel and dasatinib or placebo in men with
metastatic castration-resistant prostate cancer (READY): A
randomised, double-blind phase 3 trial. Lancet Oncol. 14:1307–1316.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kelly WK, Halabi S, Carducci M, George D,
Mahoney JF, Stadler WM, Morris M, Kantoff P, Monk JP, Kaplan E, et
al: Randomized, double-blind, placebo-controlled phase III trial
comparing docetaxel and prednisone with or without bevacizumab in
men with metastatic castration-resistant prostate cancer: CALGB
90401. J Clin Oncol. 30:1534–1540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tannock IF, Fizazi K, Ivanov S, Karlsson
CT, Fléchon A, Skoneczna I, Orlandi F, Gravis G, Matveev V, Bavbek
S, et al: Aflibercept versus placebo in combination with docetaxel
and prednisone for treatment of men with metastatic
castration-resistant prostate cancer (VENICE): A phase 3,
double-blind randomised trial. Lancet Oncol. 14:760–768. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quinn DI, Tangen CM, Hussain M, Lara PN
Jr, Goldkorn A, Moinpour CM, Garzotto MG, Mack PC, Carducci MA,
Monk JP, et al: Docetaxel and atrasentan versus docetaxel and
placebo for men with advanced castration-resistant prostate cancer
(SWOG S0421): A randomised phase 3 trial. Lancet Oncol. 14:893–900.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fizazi K, Higano CS, Nelson JB, Gleave M,
Miller K, Morris T, Nathan FE, McIntosh S, Pemberton K and Moul JW:
Phase III, randomized, placebo-controlled study of docetaxel in
combination with zibotentan in patients with metastatic
castration-resistant prostate cancer. J Clin Oncol. 31:1740–1747.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferrero JM, Chamorey E, Oudard S, Dides S,
Lesbats G, Cavaglione G, Nouyrigat P, Foa C and Kaphan R: Phase II
trial evaluating a docetaxel-capecitabine combination as treatment
for hormone-refractory prostate cancer. Cancer. 107:738–745. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hahn NM, Marsh S, Fisher W, Langdon R, Zon
R, Browning M, Johnson CS, Scott-Horton TJ, Li L and Sweeney CJ:
Hoosier oncology group randomized phase II study of docetaxel,
vinorelbine, and estramustine in combination in hormone-refractory
prostate cancer with pharmacogenetic survival analysis. Clin Cancer
Res. 12:6094–6099. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Petrioli R, Paolelli L, Francini E,
Manganelli A, Salvestrini F and Francini G: Weekly docetaxel and
epirubicin in treatment of advanced hormone-refractory prostate
cancer. Urology. 69:142–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ross RW, Beer TM, Jacobus S, Bubley GJ,
Taplin ME, Ryan CW, Huang J and Oh WK: Prostate Cancer Clinical
Trials Consortium: A phase 2 study of carboplatin plus docetaxel in
men with metastatic hormone-refractory prostate cancer who are
refractory to docetaxel. Cancer. 112:521–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Basch E, Loblaw DA, Oliver TK, Carducci M,
Chen RC, Frame JN, Garrels K, Hotte S, Kattan MW, Raghavan D, et
al: Systemic therapy in men with metastatic castration-resistant
prostate cancer: American Society of Clinical Oncology and Cancer
Care Ontario Clinical Practice Guideline. J Clin Oncol.
32:3436–3448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borghese C, Cattaruzza L, Pivetta E,
Normanno N, De Luca A, Mazzucato M, Celegato M, Colombatti A and
Aldinucci D: Gefitinib inhibits the cross-talk between mesenchymal
stem cells and prostate cancer cells leading to tumor cell
proliferation and inhibition of docetaxel activity. J Cell Biochem.
114:1135–1144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hour TC, Chung SD, Kang WY, Lin YC, Chuang
SJ, Huang AM, Wu WJ, Huang SP, Huang CY and Pu YS: EGFR mediates
docetaxel resistance in human castration-resistant prostate cancer
through the Akt-dependent expression of ABCB1 (MDR1). Arch Toxicol.
89:591–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dandekar DS, Lopez M, Carey RI and
Lokeshwar BL: Cyclooxygenase-2 inhibitor celecoxib augments
chemotherapeutic drug-induced apoptosis by enhancing activation of
caspase-3 and −9 in prostate cancer cells. Int J Cancer.
115:484–492. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kattan J, Bachour M, Farhat F, El Rassy E,
Assi T and Ghosn M: Phase II trial of weekly docetaxel, zoledronic
acid, and celecoxib for castration-resistant prostate cancer.
Invest New Drugs. 34:474–480. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buchanan FG, Holla V, Katkuri S, Matta P
and DuBois RN: Targeting cyclooxygenase-2 and the epidermal growth
factor receptor for the prevention and treatment of intestinal
cancer. Cancer Res. 67:9380–9388. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Z, Zhang X, Li M, Wang Z, Wieand HS,
Grandis JR and Shin DM: Simultaneously targeting epidermal growth
factor receptor tyrosine kinase and cyclooxygenase-2, an efficient
approach to inhibition of squamous cell carcinoma of the head and
neck. Clin Cancer Res. 10:5930–5939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin J, Wu H, Shi H, Pan W, Yu H and Zhu J:
Combined inhibition of epidermal growth factor receptor and
cyclooxygenase-2 leads to greater anti-tumor activity of docetaxel
in advanced prostate cancer. PLoS One. 8:e761692013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin JZ, Wang ZJ, De W, Zheng M, Xu WZ, Wu
HF, Armstrong A and Zhu JG: Targeting AXL overcomes resistance to
docetaxel therapy in advanced prostate cancer. Oncotarget.
8:41064–41077. 2017.PubMed/NCBI
|
|
22
|
Shen SJ, Zhang YH, Gu XX, Jiang SJ and Xu
LJ: Yangfei Kongliu Formula, a compound Chinese herbal medicine,
combined with cisplatin, inhibits growth of lung cancer cells
through transforming growth factor-β1 signaling pathway. J Integr
Med. 15:242–251. 2017.PubMed/NCBI
|
|
23
|
Abedinpour P, Baron VT, Welsh J and
Borgström P: Regression of prostate tumors upon combination of
hormone ablation therapy and celecoxib in vivo. Prostate.
71:813–823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bahl A, Oudard S, Tombal B, Ozgüroglu M,
Hansen S, Kocak I, Gravis G, Devin J, Shen L, de Bono JS, et al:
Impact of cabazitaxel on 2-year survival and palliation of
tumour-related pain in men with metastatic castration-resistant
prostate cancer treated in the TROPIC trial. Ann Oncol.
24:2402–2408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang Y, Dai J, Zhang H, Sottnik JL,
Keller JM, Escott KJ, Sanganee HJ, Yao Z, McCauley LK and Keller
ET: Activation of the Wnt pathway through AR79, a GSK3β inhibitor,
promotes prostate cancer growth in soft tissue and bone. Mol Cancer
Res. 11:1597–1610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sánchez C, Mercado A, Contreras HR,
Mendoza P, Cabezas J, Acevedo C, Huidobro C and Castellón EA:
Chemotherapy sensitivity recovery of prostate cancer cells by
functional inhibition and knock down of multidrug resistance
proteins. Prostate. 71:1810–1817. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu Y, Liu C, Armstrong C, Lou W, Sandher
A and Gao AC: Antiandrogens inhibit ABCB1 efflux and ATPase
activity and reverse docetaxel resistance in advanced prostate
cancer. Clin Cancer Res. 21:4133–4142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X1, Yao R, Yue L, Qiu W, Qi W, Liu S,
Yao Y and Liang J: FOXM1 mediates resistance to docetaxel in
gastric cancer up-regulating stathmin. J Cell Mol Med. 18:811–823.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okada K, Fujiwara Y, Takahashi T, Nakamura
Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Mori
M, et al: Overexpression of forkhead box M1 transcription factor
(FOXM1) is a potential prognostic marker and enhances
chemoresistance for docetaxel in gastric cancer. Ann Surg Oncol.
20:1035–1043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang K, Zhu X, Zhang K, Zhu L and Zhou F:
FoxM1 inhibition enhances chemosensitivity of docetaxel-resistant
A549 cells to docetaxel via activation of JNK/mitochondrial
pathway. Acta Biochim Biophys Sin. 48:804–809. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Souza R, Zahedi P, Badame RM, Allen C
and Piquette-Miller M: Chemotherapy dosing schedule influences drug
resistance development in ovarian cancer. Mol Cancer Ther.
10:1289–1299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kordezangeneh M, Irani S, Mirfakhraie R,
Esfandyari-Manesh M, Atyabi F and Dinarvand R: Regulation of
BAX/BCL2 gene expression in breast cancer cells by
docetaxel-loaded human serum albumin nanoparticles. Med Oncol.
32:2082015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kubo T, Kawano Y, Himuro N, Sugita S, Sato
Y, Ishikawa K, Takada K, Murase K, Miyanishi K, Sato T, et al: BAK
is a predictive and prognostic biomarker for the therapeutic effect
of docetaxel treatment in patients with advanced gastric cancer.
Gastric Cancer. 19:827–838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bokhorst LP, Roobol MJ, Bangma CH and van
Leenders GJ: Effect of pathologic revision and Ki67 and ERG
immunohistochemistry on predicting radical prostatectomy outcome in
men initially on active surveillance. Prostate. 77:1137–1143. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cui D, Dai J, Keller JM, Mizokami A, Xia S
and Keller ET: Notch pathway inhibition using PF-03084014, a
γ-secretase inhibitor (GSI), enhances the antitumor effect of
docetaxel in prostate cancer. Clin Cancer Res. 21:4619–4629. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Richardsen E, Andersen S, Al-Saad S,
Rakaee M, Nordby Y, Pedersen MI, Ness N, Grindstad T, Movik I,
Dønnem T, et al: Evaluation of the proliferation marker Ki-67 in a
large prostatectomy cohort. PLoS One. 12:e01868522017. View Article : Google Scholar : PubMed/NCBI
|