Introduction
Breast cancer is the primary cause of cancer
mortality in women (1), with bones
among the most common metastatic sites (2,3). Bone
metastasis increases skeletal-related events (SREs), which are
defined as pathological fractures, spinal cord compression and bone
pain requiring palliative radiotherapy and/or surgical treatment,
and worsens the activities of daily living and patient quality of
life. Bone destruction associated with cancer metastasis is caused
by activated osteoclast function, but not by the direct effects of
cancer cells in the bone (4). For
osteoclast activity, receptor activator of nuclear factor κ-B
ligand (RANKL) promotes osteoclast differentiation, formation and
bone-resorptive ability by binding to its receptor, RANK, which is
expressed in both hematopoietic osteoclast-precursor cells and
mature osteoclasts. In the area of bone metastasis, cancer cells
produce several osteolytic factors, including
parathyroid-hormone-related peptide (PTHrP), interleukin (IL)-1β,
IL-6 and IL-8, each of which can stimulate osteoblasts and/or
stromal cells to increase RANKL expression (5–7).
Additionally, RANKL is also expressed in metastatic cancer cells
(8,9), with its overexpression in the area of
bone metastasis inducing osteoclast activation (10). Recently, osteoclast function was
focused on as a therapeutic target in metastatic bone tumors, and
several inhibitors of osteoclast activity, such as bisphosphonates
and denosumab, have been developed and used to prevent metastatic
bone destruction. However, the effects of these treatment
modalities remain limited. In addition, osteonecrosis of the jaw
and hypocalcemia represent serious side-effects and long-term
prognosis associated with these treatments remains unknown
(11–14).
Hypoxia is a common feature of malignant tumors,
including metastatic bone tumors. Tumor hypoxia is caused by an
inadequate supply of oxygen that occurs when the tumor outgrows its
blood supply, resulting in chronic hypoxia and the inability of
oxygen to reach some cells due to the large diffusion distance
(15–17). Hypoxia strongly contributes to the
reduced efficacy of certain chemotherapeutic agents and/or
radiotherapy and represents a major cause of tumor propagation
(15,16,18).
Additionally, tumor-associated hypoxia plays a significant role in
tumor progression associated with bone metastasis (19,20).
Hypoxic conditions allow factors, such as hypoxia-inducible
factor-1 (HIF-1), to overexpress and trigger the production of
angiogenic proteins (17,21,22).
HIFs are essential factors for maintaining cellular oxygen
homeostasis and adaptation to hypoxic environments. HIF-1α, an
oxygen-dependent α subunit of HIF (23), is activated by hypoxia and induces
RANKL expression. Increased hypoxia accompanied by HIF-1
overexpression also promotes the progression of bone metastasis in
breast cancer (24,25). Therefore, tumor hypoxia can be
considered as an attractive therapeutic target involved with bone
metastases.
To obtain local oxygenation in tumor tissues, a
direct absorption of oxygen through the skin can be considered,
however oxygen is not easily absorbed through the skin (26). We previously demonstrated that
transcutaneous CO2 application could lead to increased
oxygen release from red blood cells, known as a physiological
phenomenon, the Bohr effect (27,28).
Oxygenation in treated tissues could be easily and effectively
induced by the transcutaneous CO2 treatment via the
oxygen dissociation from hemoglobins (28). Using the CO2 treatment,
we could induce mitochondrial apoptosis in human cancer xenografts
and improve hypoxia in cancer tissues in the absence of
side-effects (29–32). Based on these findings, we
hypothesized that hypoxia would promote bone destruction via the
activation of factors related to osteoclast differentiation and
osteolysis, and that recovery from hypoxia following transcutaneous
CO2 application would inhibit bone destruction by cancer
metastasis. In the present study, we examined whether oxygen
conditions affect the expression of osteoclast-differentiation and
osteolytic factors in vitro, as well as the effects of
recovery from hypoxia following transcutaneous CO2
application on bone destruction and osteoclast activity using an
in vivo bone metastatic model of breast cancer.
Materials and methods
Cell lines
The human breast cancer cell line MDA-MB-231 was
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA) (33), and cells were
grown as monolayers in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck KGaA, St. Louis, MO, USA) supplemented with
10% (v/v) fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA), 100
U/ml penicillin and 100 µg/ml streptomycin. The cultures were
maintained in a humidified atmosphere with 5% CO2 at
37°C.
In vitro experiments
To investigate the effect of altered oxygen
conditions on breast cancer cells in vitro, MDA-MB-231 cells
were incubated for 6 days in one of three different oxygen
conditions: normoxic (20% O2, 5% CO2),
hypoxic (2% O2, 5% CO2) or reoxygenated
conditions. For the reoxygenated condition, cells were incubated
under normoxic conditions (20% O2, 5% CO2)
for 3 days followed by 3 days of incubation under hypoxic
conditions (2% O2, 5% CO2), as previously
described (30,32). Following incubation, total RNA was
collected from the cells and the mRNA levels of RANKL, PTHrP,
IL-1β, IL-6 and IL-8 were evaluated by quantitative
real-time polymerase chain reaction (qPCR).
Animal models
Female BALB/c nude mice aged 5 weeks old were
obtained from CLEA Japan Inc. (Tokyo, Japan). Animals were
maintained under pathogen-free conditions in accordance with
institutional guidelines. Animals were fed pathogen-free laboratory
chow and allowed free access to autoclaved water in an
air-conditioned room with a 12-h light/dark cycle. All animal
experiments were approved by the Ethics Committee of the Kobe
University Animal Experimentation Regulations (permission no.
P120404-R1). To create the in vivo bone metastasis model of
breast cancer, 18 mice were randomly divided into two groups: a
CO2-treated group (CO2 group; n=9) and a
control group (n=9). MDA-MB-231 cells [1.0×106 cells in
10 µl phosphate-buffered saline (PBS)] were intramedullary
implanted into the proximal epiphysis of the tibia of all mice in
both groups (34). For surgical
procedures, mice were anesthetized by intraperitoneal injection of
20–30 mg/kg pentobarbital sodium for induction and isoflurane
inhalation at a concentration of 2% for maintenance.
Transcutaneous CO2
application
Transcutaneous CO2 application was
performed as previously described (30–32,35).
Briefly, the area of skin around the lower limb where MDA-MB-231
cells were implanted was treated with CO2 hydrogel. This
area was then sealed with a polyethylene bag, and 100%
CO2 gas was administered into the bag. Mice in the
control group were treated similarly, replacing CO2 with
room air.
In vivo studies
Examination of the in vivo effects of
transcutaneous CO2 application on breast cancer bone
metastasis was performed following treatment (CO2 or
air) at 4-week post-implantation of MDA-MB-231 cells and for 10
min/mouse, twice weekly for 2 weeks. The tumor volume and body
weight were monitored twice weekly until the end of the treatment.
Tumor volume was calculated as previously described according to
the formula V = π/6 × a2 × b, where a and b represent
the shorter and longer diameters of the tumor cell implanted
proximal tibia, respectively (36).
qPCR analysis
Total RNA was extracted from cells and tumor tissues
by selective binding to a silica-gel-based membrane using an RNeasy
mini kit according to the manufacturer's protocol (Qiagen,
Valencia, CA, USA). Oligo (dT)-primed first-stand cDNA was
synthesized using a high-capacity cDNA transcription kit (Applied
Biosystems, Foster City, CA, USA). qPCR was performed in a 20-µl
reaction mixture using SYBR Green Master Mix reagent (Applied
Biosystems; Thermo Fisher Scientific, Inc., Foster City, CA, USA)
on an ABI Prism 7500 sequence-detection system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). PCR conditions were as follows:
one cycle at 95°C for 10 min, followed by 40 cycles at 95°C for 15
sec and 60°C for 1 min. Pre-designed primers specific for RANKL,
PTHrP, IL-1β, IL-6 and IL-8 were obtained from
Invitrogen Life Technologies; Thermo Fisher Scientific, Inc.
(Carlsbad, CA, USA). Primer sequences were as follows: RANKL
forward, 5′-CCCAGATCAAGGTGGTGTCT-3′ and reverse,
5′-TGCTGACCAATGAGAGCATC-3′; PTHrP forward,
5′-CATCAGCTCCTCCATGACAA-3′ and reverse, 5′-TCAGCTGTGTGGATTTCTGC-3′;
IL-1β forward, 5′-GGACAAGCTGAGGAAGATGC-3′ and reverse,
5′-TCGTTATCCCATGTGTCGAA-3′; IL-6 forward,
5′-AAAGAGGCACTGGCAGAAAA-3′ and reverse, 5′-TTTCACCAGGCAAGTCTCCT-3′;
IL-8 forward, 5′-GTTCCACTGTGCCTTGGTTT-3′ and reverse,
5′-GCTTCCACATGTCCTCACAA-3′. Relative expression of RANKL, PTHrP,
IL-1β, IL-6 and IL-8 was calculated using the ∆∆Cq
method (37), with normalization
against β-actin levels.
Micro-computed tomography (µCT)
analysis
Quantitative analysis of treated tibiae was
performed both before and after treatment using a µCT Scanner
(R_mCT; Rigaku Mechatronics Co., Ltd., Tokyo, Japan). Following the
end of treatment, all mice were euthanized by intraperitoneal
injection of 1 ml pentobarbital sodium (Kyoritsu Seiyaku, Tokyo,
Japan), and the treated tibiae were removed. The bone samples were
scanned by µCT, and using the constructed sagittal image from the
scanning data, histomorphometric analysis of the proximal epiphysis
of the tibia, excluding the cortical bone, was performed. Bone
volume was assessed using an image-analysis system (TRI/3D-BON;
Ratoc System Engineering, Tokyo, Japan). Following µCT scanning,
samples were fixed in 10% formalin for 48 h.
Hematoxylin and eosin (H&E)
staining
Formalin-fixed tibia samples were decalcified in 10%
ethylenediaminetetraacetic acid for 2 weeks and embedded in
paraffin. Paraffin-embedded tibia samples were sliced into
6-µm-thick sections and stained with H&E. Sections were
evaluated using a light microscope to confirm bone destruction and
the presence of cancer cells.
Immunohistochemical analysis
Paraffin-embedded tibia sections were pretreated
with citrate buffer for 40 min at 95°C and quenched with 0.05%
H2O2, followed by overnight incubation at 4°C
with the following primary antibodies in Can Get Signal Immunostain
Solution A (Toyobo, Osaka, Japan): mouse anti-HIF-1α antibody
(1:1,000; cat. no. sc-13515; Santa Cruz Biotechnology, Dallas, CA,
USA), rabbit anti-RANKL antibody (1:1,000; cat. no. ab9957; Abcam,
Tokyo, Japan), rabbit anti-IL-1β antibody (1:1,000; cat. no.
ab9722; Abcam), mouse anti-IL-6 antibody (1:1,000; cat. no.
sc-130326; Santa Cruz Biotechnology) and mouse anti-IL-8 antibody
(1:1,000; cat. no. sc-7303; Santa Cruz Biotechnology). Following
washes, the sections were incubated with horseradish
peroxidase-conjugated labeled anti-mouse (cat. no. 424131) or
anti-rabbit (cat. no. 424141) antibody (1:200; Histofine
Simplestain Max PO; Nichirei, Tokyo, Japan) for an additional 30
min at room temperature, counterstained with hematoxylin, and
examined with a BZ-X700 confocal microscope (Keyence Corporation,
Osaka, Japan). Immunopositive cells were counted in three random
fields under a high-power field (×200).
Tartrate-resistant acid phosphatase
(TRAP) staining
To evaluate the effect of transcutaneous
CO2 treatment on osteoclast activity in metastatic bone
tumors, TRAP staining was performed in paraffin-embedded
histological sections using the standard naphthol AS-BI phosphate
post-coupling method (38). Slides
were incubated at 37°C in sodium acetate buffer (pH 5.0) containing
0.01% naphthol AS-BI phosphate and 0.5 Ml- (+) tartaric acid. The
sections were then incubated in the same buffer containing
pararosaniline chloride for 20 min, followed by washing in
distilled water. The sections were counterstained with hematoxylin
for 30 sec, dehydrated with alcohol and penetrated with xylene.
TRAP-positive multinucleated cells containing more than three
nuclei were counted as osteoclasts in three random fields under a
light microscopy.
Statistical analysis
All experiments were performed independently in
triplicate, and data are presented as the mean ± standard error of
the mean (SEM) unless otherwise indicated. Differences between
groups were evaluated using the Mann-Whitney U test and also by
analysis of variance (ANOVA) with a Tukey's post hoc test to
compare continuous values. All tests were considered to indicate a
statistically significant difference at P<0.05.
Results
Hypoxic conditions increase mRNA
expression of osteoclast-differentiation and osteolytic factors in
MDA-MB-231 breast cancer cells in vitro
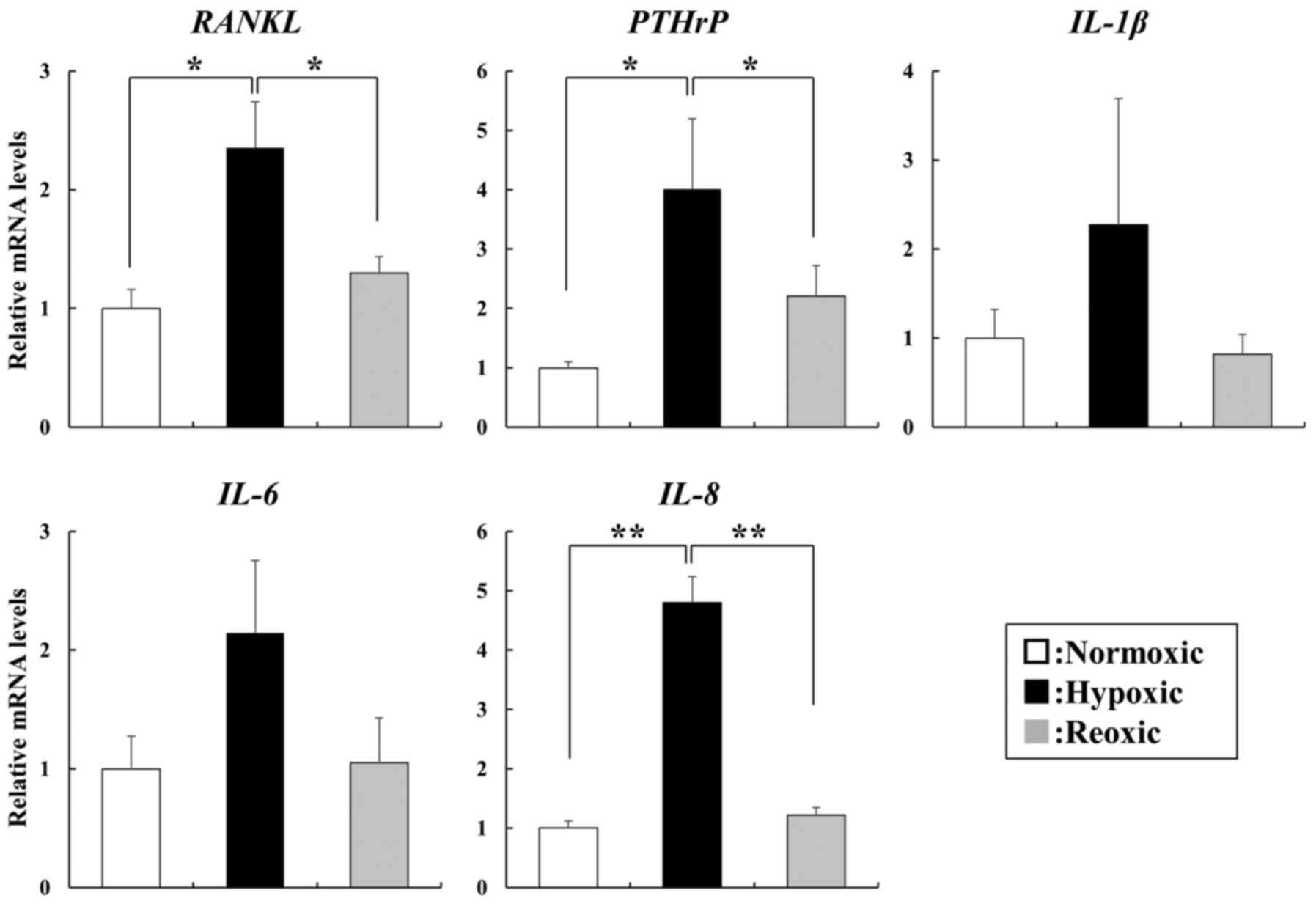
The mRNA expression of RANKL, PTHrP and IL-8 was
significantly increased in cells under hypoxic conditions compared
with their expression observed under normoxic conditions
(P<0.05), with the elevated expression of the three factors
subsequently decreased to the same levels as those under normoxic
conditions following reoxygenation (Fig. 1). We also observed elevated
expression of IL-1β and IL-6 under hypoxic conditions, however,
this increase was not statistically significant (Fig. 1).
Transcutaneous CO2
application suppresses tumor growth and prevents bone destruction
in a bone metastatic model of human breast cancer
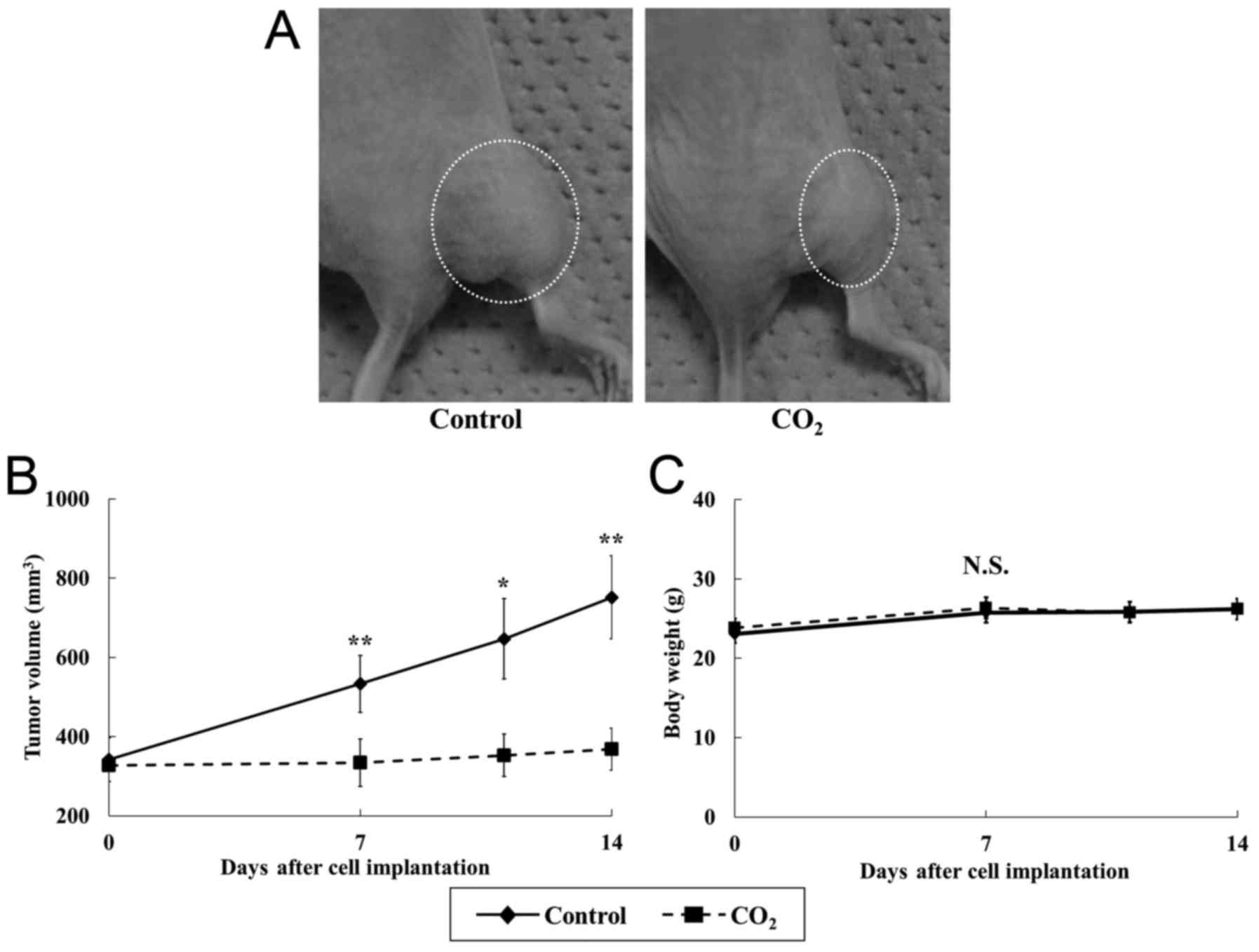
Transcutaneous CO2 treatment
significantly suppressed breast cancer tumor growth compared with
that observed in controls (Fig.
2A). In addition, at the end of the experiment, the tumor
volume in the CO2 group was 49% of that in the control
group (Fig. 2B) (P<0.05). There
was no significant difference in body weight between the groups
(Fig. 2C).
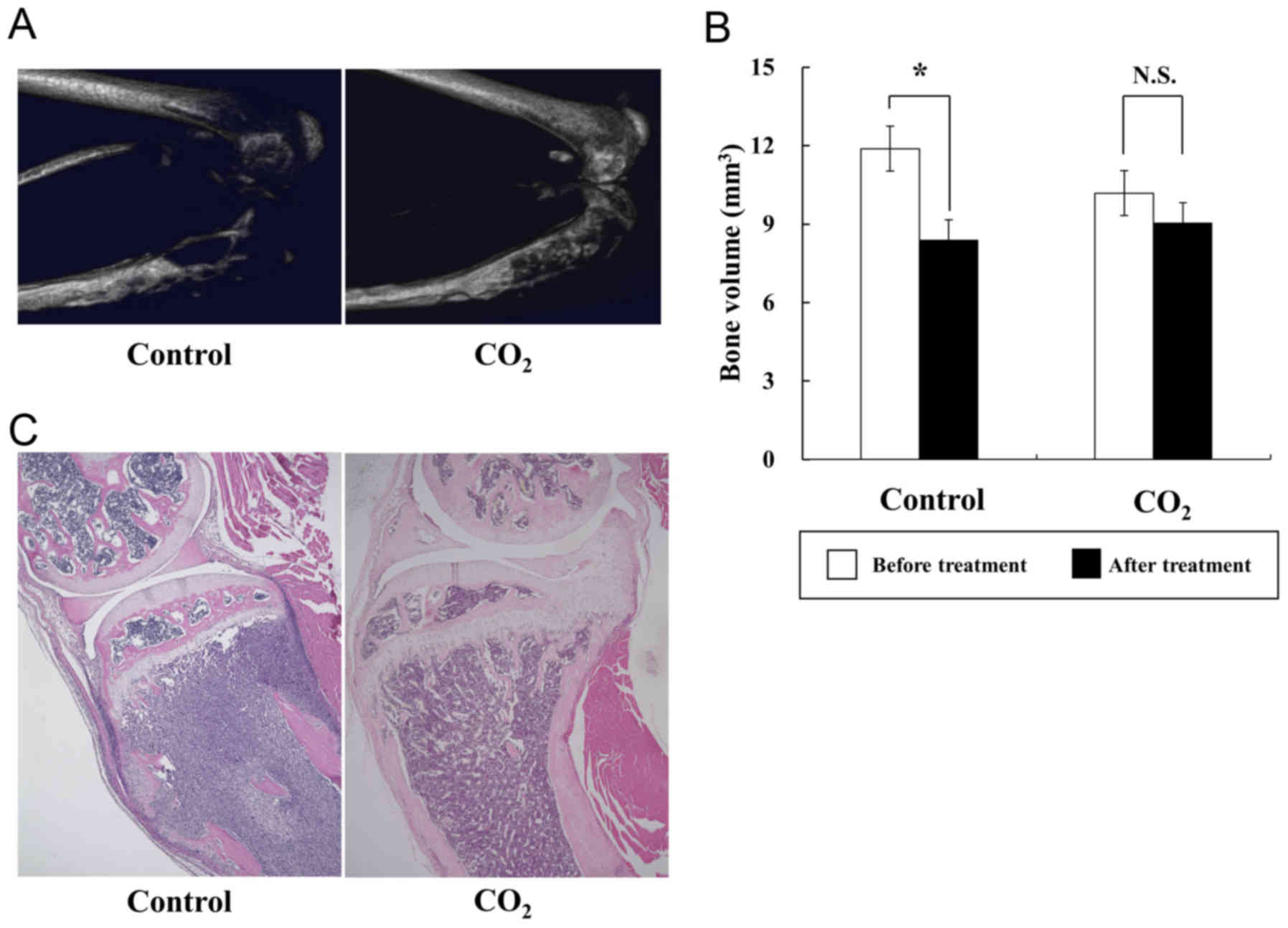
µCT analyses demonstrated that transcutaneous
CO2 application blocked bone destruction compared with
the control treatment, and that bone volume was significantly
preserved in the CO2 group (Fig. 3A and B). H&E staining of treated
tibiae also revealed that the area of cancer tissues in the
CO2 group was smaller than that in the control group
(Fig. 3C).
Osteoclast activity is significantly
inhibited by transcutaneous CO2 application and via
decreased expression of osteoclast-differentiation and osteolytic
factors along with decreased HIF-1α expression
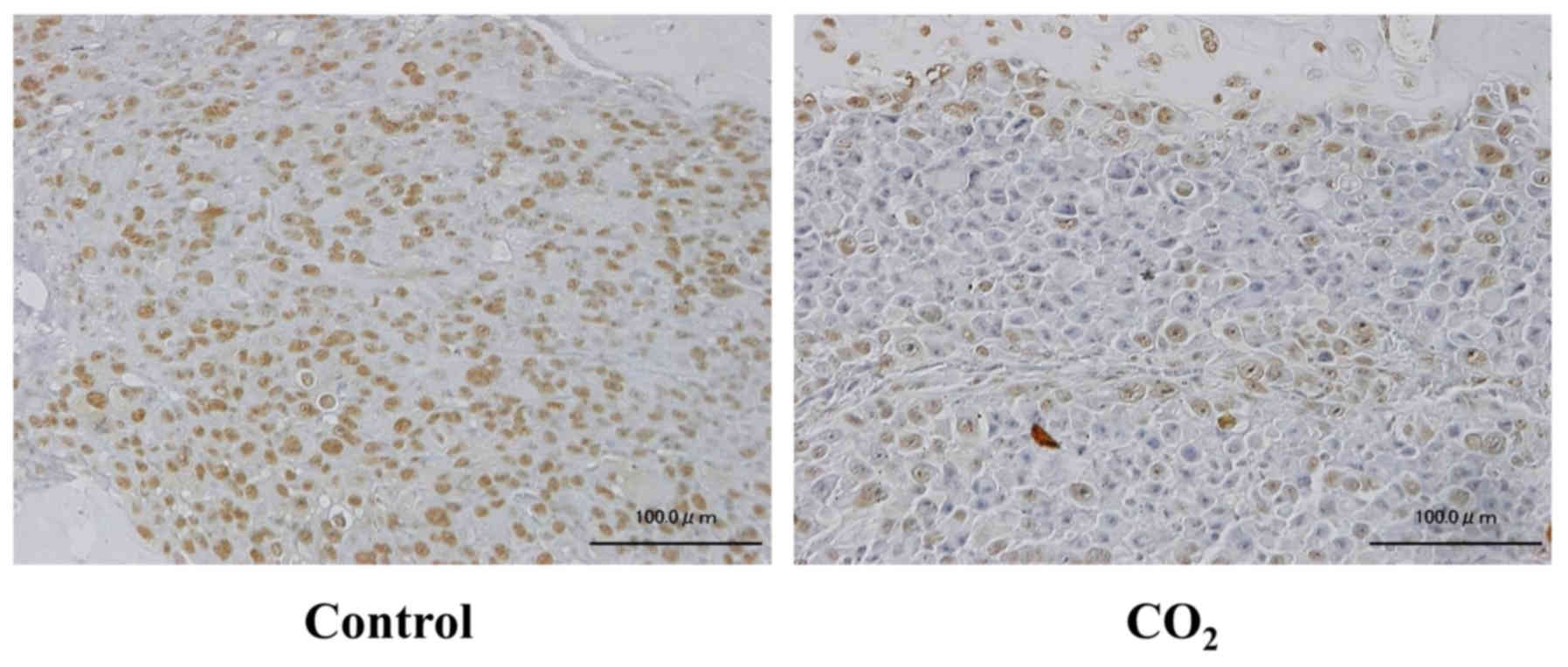
Immunopositive staining for HIF-1α was weakly
detected in CO2-treated tibiae, whereas positive
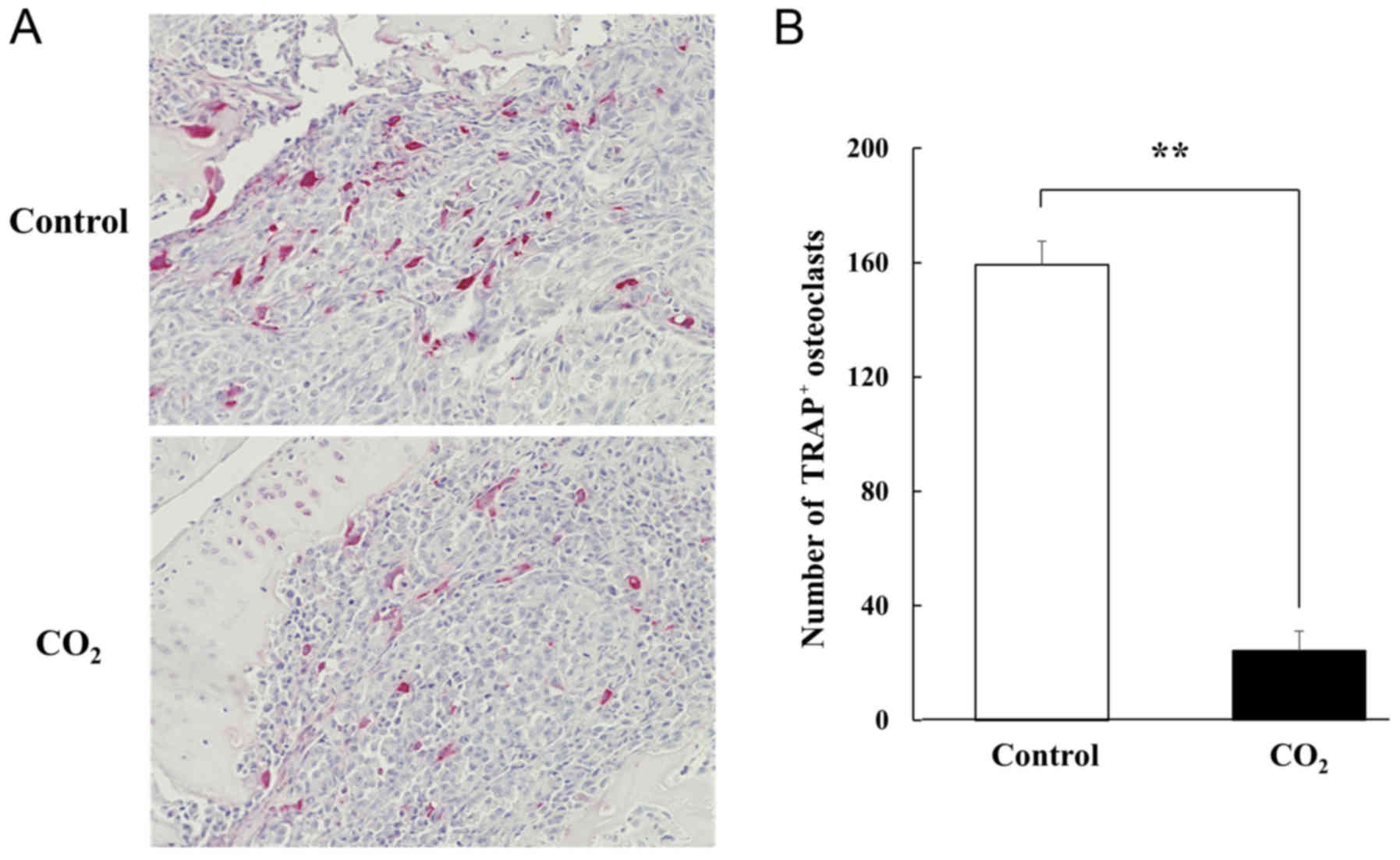
staining was extensively observed in the control tibiae (Fig. 4). For TRAP staining, the number of
TRAP-positive multinucleated osteoclasts was significantly reduced
following transcutaneous CO2 treatment compared with
that observed in the controls (Fig. 5A
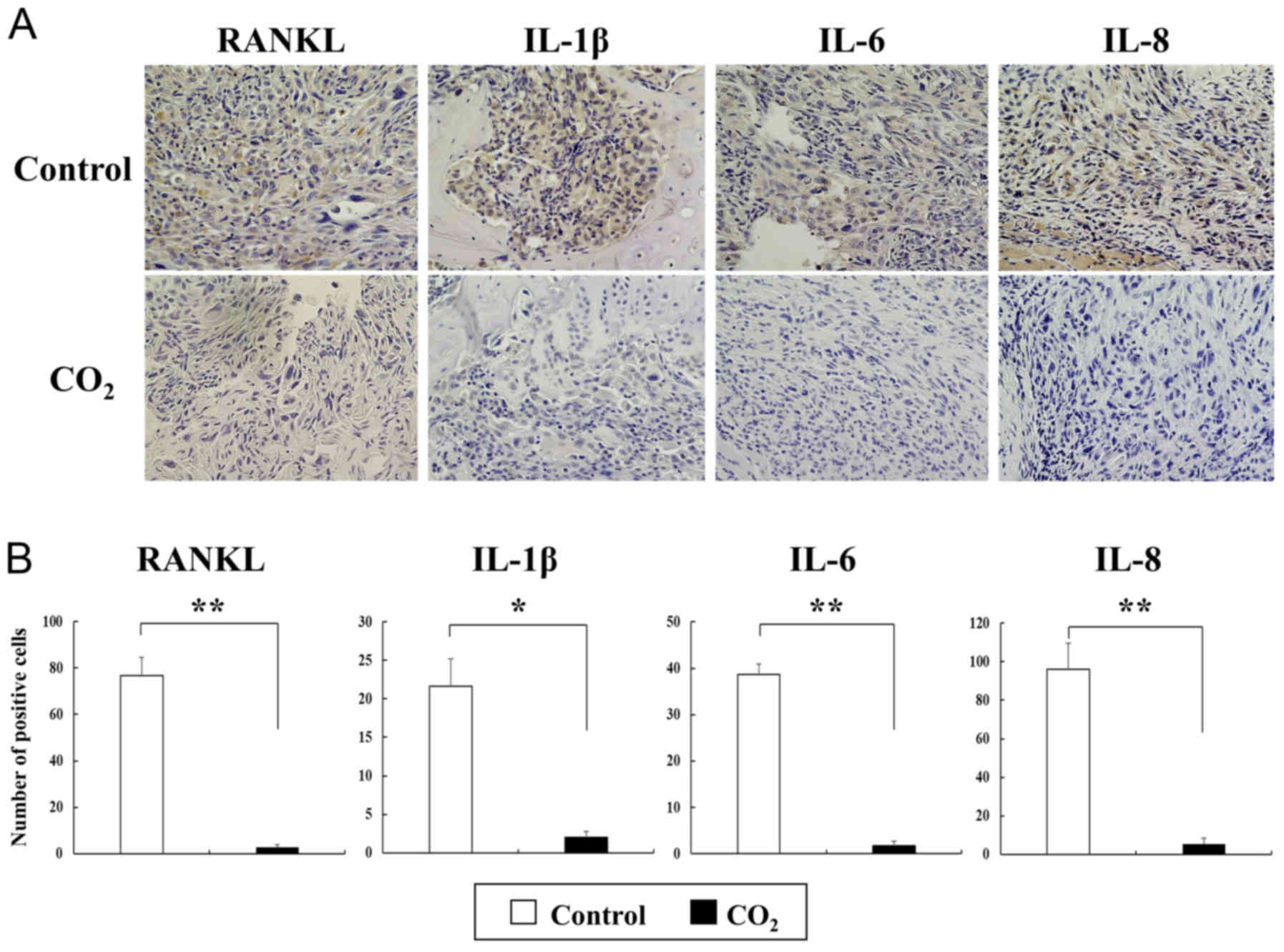
and B). Additionally, immunohistochemical staining for
osteoclast-differentiation and osteolytic factors revealed that
staining for RANKL, IL-1β, IL-6 and IL-8 was barely detectable in
the CO2-treated tibiae, but was strongly positive in the
control group (Fig. 6A).
Furthermore, the number of cells immunopositive for these factors
in the CO2-treated tumors was significantly decreased
compared with that observed in the control tumors (Fig. 6B).
Discussion
In the area of bone metastasis, osteolysis is mainly
caused by activated osteoclast function and not by the direct
effects of metastatic cancer cells (4,39). In
response to factors associated with osteoclast differentiation
(RANKL) and osteolysis (PTHrP, IL-1β, IL-6 and IL-8), which are
produced by cancer cells, osteoblast-mediated cross-talk between
RANKL and its receptor, RANK, which is expressed in hematopoietic
osteoclast-precursor cells and mature osteoclasts, is promoted,
followed by stimulation of osteoclast activity, especially
bone-resorptive functions (5–7,40–42).
In particular, PTHrP induced osteoclast formation through the
upregulation of RANKL (42) and
represented a specific mediator of osteolysis in metastatic breast
cancer (43,44). Previous studies reported that breast
cancer cells secreted high levels of RANKL, IL-1β and IL-6 under
hypoxic conditions (45–47). In the present study using MDA-MB-231
breast cancer cells, we observed that the in vitro
expression of factors, including RANKL, PTHrP, IL-1β, IL-6 and
IL-8, was increased under hypoxic conditions and subsequently
decreased by reoxygenation. These findings were consistent with
previous studies (45–47) and indicated that hypoxic conditions
affected the expression of osteoclast-differentiation and
osteolytic factors, which play essential roles in bone destruction
associated with cancer metastasis. Based on these findings, we
hypothesized that improving hypoxia could suppress the stimulation
of osteoclast activity, and that recovery from hypoxia would
inhibit bone destruction by cancer metastasis.
Hypoxia is a common feature of solid tumors,
including metastatic bone tumors, and is mainly a consequence of
poor perfusion in solid tumors. In bone metastasis, abnormal blood
vessels are insufficient to supply oxygen to metastatic tumor
tissues, causing intratumoral hypoxia (48). The median tumor oxygen concentration
in breast cancer is reportedly ≤1.3%, which is substantially lower
than that in normal tissues (25).
Hypoxia negatively affects clinical outcomes, including overall
patient survival, motility, and resistance to chemotherapy and/or
radiotherapy by impacting tumor-cell functions. The transcription
factor HIF-1 is a key regulator of the cellular response promoting
survival in the hostile environment of hypoxic tumors (23). In human malignancies, HIFs
critically contribute to chemoresistance and/or radioresistance
(49) and are associated with
histological grade, stage and local or distant recurrence (50–53).
Recently, a role for HIF-1α in promoting lung and bone metastases
was described using a transgenic model of breast cancers (25). Therefore, improvement of hypoxic
conditions by regulating HIF-1α in metastatic bone tumors may
control not only tumor progression, but also bone destruction.
Although several therapeutic strategies to overcome hypoxia and/or
to specifically target hypoxic cells have been investigated,
attempts to improve tumor oxygen levels have not yielded clinically
compelling results (54–56), and metastatic bone tumors are still
considered an incurable disease. Recent therapeutic strategies for
treating bone metastasis include surgery, radiotherapy and
osteoclast inhibitors, such as bisphosphonates and denosumab, the
human monoclonal antibody used to neutralize RANKL. Regardless of
the wide use of these treatments, radiotherapy should still be
administered for symptom palliation (57), although bisphosphonates and
denosumab can cause unfavorable side-effects, such as osteonecrosis
of the jaw (11–14). Based on these findings, new
strategies for the treatment of bone metastases with fewer
side-effects are required.
The benefits of CO2 therapy have been
realized for therapeutic purposes, especially in the treatment of
peripheral vascular disorders (58), and carbonated spa therapy has
historically been used in Europe as an effective treatment for
cardiac diseases and skin problems (59,60).
The therapeutic effects of CO2 are mediated by an
increase in blood flow and microcirculation, nitric oxide-dependent
neocapillary formation and an increase in the partial pressure of
O2 in local tissue, known as the Bohr effect (27). The Bohr effect is represented by a
rightward shift in the O2-hemoglobin dissociation curve
accompanied with increased pCO2 or decreased pH
(27). We previously demonstrated
that transcutaneous CO2 application increased
O2 pressure in treated tissues, through the absorption
of CO2, potentially causing an artificial Bohr effect
(28). Transcutaneous
CO2 application suppressed the in vivo human
undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma
tumor growth through induction of mitochondrial apoptosis
accompanied by mitochondrial proliferation (29). Additionally, animal models of
osteosarcoma (30) and oral
squamous cell carcinoma (31)
demonstrated that CO2 treatment decreased metastatic
potential along with decreased expression of both HIF-1α and
vascular endothelial growth factor (30,31).
These findings strongly indicated that transcutaneous
CO2 application exhibited antitumor effects by improving
hypoxic conditions within tumor tissues (29–31).
In the present study, we revealed that transcutaneous
CO2 application in a bone metastatic model of human
breast cancer, significantly improved intratumoral hypoxia along
with decreased expression of HIF-1α, RANKL and osteolytic factors,
as well as suppressed osteoclast function along with decreased TRAP
activity, resulting in blockage of progressive bone destruction
without observable side-effects.
However, the present study had several limitations.
Firstly, we have not examined the influence of CO2
application on osteoblasts in the surrounding tissues. We
previously reported that CO2 application accelerated
fracture repair in a rat-fracture model by increasing osteoblast
activity (61). In addition,
several studies revealed that, in the presence of osteolytic bone
metastasis, tumor cells increased the RANKL production of
osteoblasts in the surrounding bone stroma of the host with
decreased osteoprotegerin, by releasing a variety of factors
including interleukins and PTHrP, resulting in more osteoclast
formation and bone degradation (62,63).
Based on these previous studies, CO2 application may
inhibit the tumor-induced RANKL production of osteoblasts,
resulting in decreased bone destruction via decreased osteoclast
activity. Secondly, we observed that transcutaneous CO2
application could inhibit metastatic bone destruction along with
decreased osteoclast activity in a well-established bone metastatic
model using MDA-MB-231 human breast cancer cells, however we used
only one breast cancer cell line in the present study.
Additionally, we did not directly assess the oxygen conditions in
the metastatic bone during transcutaneous CO2 treatment.
Therefore, further investigations are required to clarify the
effects of transcutaneous CO2 application on cancer
cells and bone destruction in metastatic bone tumors. In
conclusion, our findings strongly indicated that oxygen conditions
affected the expression of osteoclast-differentiation and
osteolytic factors, which play essential roles in bone destruction
by breast cancer cells, and that transcutaneous CO2
application blocked metastatic bone destruction by improving
hypoxic conditions along with decreased expression of osteolytic
factors and TRAP activity. Although further studies are required to
elucidate the mechanisms associated with the effects of
CO2 application, transcutaneous CO2
application can be considered a potential therapeutic strategy for
the treatment of metastatic bone destruction in breast cancer
patients.
Acknowledgements
We thank Mrs. Minako Nagata, Mrs. Maya Yasuda and
Mrs. Kyoko Tanaka for their expert technical assistance. The
abstract was presented at the 2018 Annual Meeting of the
Orthopaedic Research Society Mar. 10-13, 2018 in New Orleans, LA,
USA and was published as abstract no. 2073.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
TT, TK, TU, MT, NF, HH, TN, RK and TA conceived and
designed the study. TT performed the experiments and wrote the
initial draft of the manuscript. TT, TU, MT, MM, SF and EK
performed the analyses and interpretation of data. TK is the
principal investigator and was involved in the conceptualization,
experimental design, discussion of the data, and writing of the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee of Kobe University Animal Experimentation Regulations
(permission no: P120404-R1).
Patient consent for publication
Not applicable.
Competing interests
The hydro-gel was donated from Neochemir, Inc. TU is
a full-time employee of Neochemir, Inc. The international patent
publication number is WO2004/002393, with a publication date of
January 8, 2004. This does not alter the author's adherence to all
journal policies on sharing data and materials.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:6243s–6249s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steinauer K, Huang DJ, Eppenberger-Castori
S, Amann E and Güth U: Bone metastases in breast cancer: Frequency,
metastatic pattern and non-systemic locoregional therapy. J Bone
Oncol. 3:54–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas RJ, Guise TA, Yin JJ, Elliott J,
Horwood NJ, Martin TJ and Gillespie MT: Breast cancer cells
interact with osteoblasts to support osteoclast formation.
Endocrinology. 140:4451–4458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kearns AE, Khosla S and Kostenuik PJ:
Receptor activator of nuclear factor kappaB ligand and
osteoprotegerin regulation of bone remodeling in health and
disease. Endocr Rev. 29:155–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blair JM, Zhou H, Seibel MJ and Dunstan
CR: Mechanisms of disease: Roles of OPG, RANKL and RANK in the
pathophysiology of skeletal metastasis. Nat Clin Pract Oncol.
3:41–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suda T, Takahashi N, Udagawa N, Jimi E,
Gillespie MT and Martin TJ: Modulation of osteoclast
differentiation and function by the new members of the tumor
necrosis factor receptor and ligand families. Endocr Rev.
20:345–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gonzalez-Suarez E, Jacob AP, Jones J,
Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D and
Dougall WC: RANK ligand mediates progestin-induced mammary
epithelial proliferation and carcinogenesis. Nature. 468:103–107.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Canon JR, Roudier M, Bryant R, Morony S,
Stolina M, Kostenuik PJ and Dougall WC: Inhibition of RANKL blocks
skeletal tumor progression and improves survival in a mouse model
of breast cancer bone metastasis. Clin Exp Metastasis. 25:119–129.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roodman GD and Dougall WC: RANK ligand as
a therapeutic target for bone metastases and multiple myeloma.
Cancer Treat Rev. 34:92–101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Von Hoff DD, Layard MW, Basa P, Davis HL
Jr, Von Hoff AL, Rozencweig M and Muggia FM: Risk factors for
doxorubicin-induced congestive heart failure. Ann Intern Med.
91:710–717. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luu T, Chung C and Somlo G: Combining
emerging agents in advanced breast cancer. Oncologist. 16:760–771.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vyas S, Hameed S and Murugaraj V:
Denosumab-associated osteonecrosis of the jaw - a case report. Dent
Update. 41:449–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Halloran M, Boyd NM and Smith A:
Denosumab and osteonecrosis of the jaws - the pharmacology,
pathogenesis and a report of two cases. Aust Dent J. 59:516–519.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vaupel P and Harrison L: Tumor hypoxia:
Causative factors, compensatory mechanisms, and cellular response.
Oncologist. 9 Suppl 5:4–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
19
|
Rundqvist H and Johnson RS: Tumour
oxygenation: Implications for breast cancer prognosis. J Intern
Med. 274:105–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siclari VA, Mohammad KS, Tompkins DR,
Davis H, McKenna CR, Peng X, Wessner LL, Niewolna M, Guise TA,
Suvannasankha A, et al: Tumor-expressed adrenomedullin accelerates
breast cancer bone metastasis. Breast Cancer Res. 16:4582014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Castro Junior G, Puglisi F, de Azambuja
E, El Saghir NS and Awada A: Angiogenesis and cancer: A cross-talk
between basic science and clinical trials (the ‘do ut des’
paradigm). Crit Rev Oncol Hematol. 59:40–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pugh CW and Ratcliffe PJ: Regulation of
angiogenesis by hypoxia: Role of the HIF system. Nat Med.
9:677–684. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang ZN, Zhang F, Tang P, Qi XW and Jiang
J: Hypoxia induces RANK and RANKL expression by activating HIF-1α
in breast cancer cells. Biochem Biophys Res Commun. 408:411–416.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kallergi G, Markomanolaki H, Giannoukaraki
V, Papadaki MA, Strati A, Lianidou ES, Georgoulias V, Mavroudis D
and Agelaki S: Hypoxia-inducible factor-1alpha and vascular
endothelial growth factor expression in circulating tumor cells of
breast cancer patients. Breast Cancer Res. 11:R842009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hiraga T, Kizaka-Kondoh S, Hirota K,
Hiraoka M and Yoneda T: Hypoxia and hypoxia-inducible factor-1
expression enhance osteolytic bone metastases of breast cancer.
Cancer Res. 67:4157–4163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hansen TN, Sonoda Y and McIlroy MB:
Transfer of oxygen, nitrogen, and carbon dioxide through normal
adult human skin. J Appl Physiol. 49:438–443. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bohr C, Hasselbach K and Krogh A: Ueber
emen in biologischen Bezuehung wichtigen Einfluss, den die Kohlen
saurespannung des Blutes anf dessen Samerstoffbinding ubt. Arch
Physiol. 16:402–412. 1904.
|
|
28
|
Sakai Y, Miwa M, Oe K, Ueha T, Koh A,
Niikura T, Iwakura T, Lee SY, Tanaka M and Kurosaka M: A novel
system for transcutaneous application of carbon dioxide causing an
‘artificial Bohr effect’ in the human body. PLoS One. 6:e241372011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Onishi Y, Kawamoto T, Ueha T, Kishimoto K,
Hara H, Fukase N, Toda M, Harada R, Minoda M, Sakai Y, et al:
Transcutaneous application of carbon dioxide (CO2)
induces mitochondrial apoptosis in human malignant fibrous
histiocytoma in vivo. PLoS One. 7:e491892012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harada R, Kawamoto T, Ueha T, Minoda M,
Toda M, Onishi Y, Fukase N, Hara H, Sakai Y, Miwa M, et al:
Reoxygenation using a novel CO2 therapy decreases the
metastatic potential of osteosarcoma cells. Exp Cell Res.
319:1988–1997. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takeda D, Hasegawa T, Ueha T, Imai Y,
Sakakibara A, Minoda M, Kawamoto T, Minamikawa T, Shibuya Y, Akisue
T, et al: Transcutaneous carbon dioxide induces mitochondrial
apoptosis and suppresses metastasis of oral squamous cell carcinoma
in vivo. PLoS One. 9:e1005302014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Onishi Y, Kawamoto T, Ueha T, Hara H,
Fukase N, Toda M, Harada R, Sakai Y, Miwa M, Nishida K, et al:
Transcutaneous application of Carbon Dioxide (CO2)
enhances chemosensitivity by reducing hypoxic conditions in human
malignant fibrous histiocytoma. J Cancer Sci Ther. 4:174–181. 2012.
View Article : Google Scholar
|
|
33
|
Bobrova TS, Kriukova IN, Voronina AN and
Bassalyk LS: Study of the ‘continuous cell antigen’ and the human
gastric mucosa. Biull Eksp Biol Med. 86:744–747. 1978.(In Russian).
PubMed/NCBI
|
|
34
|
Okada Y, Ueno H, Katagiri M, Oneyama T,
Shimomura K, Sakurai S, Mataga I, Moride M and Hasegawa H:
Experimental study of antiangiogenic gene therapy targeting VEGF in
oral cancer. Odontology. 98:52–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oe K, Ueha T, Sakai Y, Niikura T, Lee SY,
Koh A, Hasegawa T, Tanaka M, Miwa M and Kurosaka M: The effect of
transcutaneous application of carbon dioxide (CO2) on
skeletal muscle. Biochem Biophys Res Commun. 407:148–152. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Okada Y, Akisue T, Hara H, Kishimoto K,
Kawamoto T, Imabori M, Kishimoto S, Fukase N, Onishi Y and Kurosaka
M: The effect of bevacizumab on tumour growth of malignant fibrous
histiocytoma in an animal model. Anticancer Res. 30:3391–3395.
2010.PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−∆∆C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Modderman WE, Raap Tuinenburg-Bol AC and
Nijweide PJ: Tartrate-resistant acid phosphatase is not an
exclusive marker for mouse osteoclasts in cell culture. Bone.
12:81–87. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boyde A, Maconnachie E, Reid SA, Delling G
and Mundy GR: Scanning electron microscopy in bone pathology:
Review of methods, potential and applications. Scan Electron
Microsc. 4:1537–1554. 1986.
|
|
40
|
Kozlow W and Guise TA: Breast cancer
metastasis to bone: Mechanisms of osteolysis and implications for
therapy. J Mammary Gland Biol Neoplasia. 10:169–180. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bendre MS, Montague DC, Peery T, Akel NS,
Gaddy D and Suva LJ: Interleukin-8 stimulation of
osteoclastogenesis and bone resorption is a mechanism for the
increased osteolysis of metastatic bone disease. Bone. 33:28–37.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Roodman GD: Biology of osteoclast
activation in cancer. J Clin Oncol. 19:3562–3571. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bryden AA, Hoyland JA, Freemont AJ, Clarke
NW and George NJ: Parathyroid hormone related peptide and receptor
expression in paired primary prostate cancer and bone metastases.
Br J Cancer. 86:322–325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miki T, Yano S, Hanibuchi M and Sone S:
Bone metastasis model with multiorgan dissemination of human
small-cell lung cancer (SBC-5) cells in natural killer
cell-depleted SCID mice. Oncol Res. 12:209–217. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rattigan Y, Hsu JM, Mishra PJ, Glod J and
Banerjee D: Interleukin 6 mediated recruitment of mesenchymal stem
cells to the hypoxic tumor milieu. Exp Cell Res. 316:3417–3424.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Filippi I, Carraro F and Naldini A:
Interleukin-1β affects MDAMB231 breast cancer cell migration under
hypoxia: Role of HIF-1α and NFκB transcription factors. Mediators
Inflamm. 2015:7894142015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tang ZN, Zhang F, Tang P, Qi XW and Jiang
J: Hypoxia induces RANK and RANKL expression by activating HIF-1α
in breast cancer cells Biochem Biophys Res Commun. 408:411–416.
2011.
|
|
48
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vaupel P and Mayer A: Hypoxia in cancer:
Significance and impact on clinical outcome. Cancer Metastasis Rev.
26:225–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Miyazawa M, Yasuda M, Fujita M, Hirasawa
T, Kajiwara H, Hirabayashi K, Ogane N, Shimizu M, Asanuma H,
Murakami M, et al: Association of hypoxia-inducible factor-1
expression with histology in epithelial ovarian tumors: A
quantitative analysis of HIF-1. Arch Gynecol Obstet. 279:789–796.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zeng W, Wan R, Zheng Y, Singh SR and Wei
Y: Hypoxia, stem cells and bone tumor. Cancer Lett. 313:129–136.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rohwer N and Cramer T: Hypoxia-mediated
drug resistance: Novel insights on the functional interaction of
HIFs and cell death pathways. Drug Resist Updat. 14:191–201. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Schwab LP, Peacock DL, Majumdar D, Ingels
JF, Jensen LC, Smith KD, Cushing RC and Seagroves TN:
Hypoxia-inducible factor 1α promotes primary tumor growth and
tumor-initiating cell activity in breast cancer. Breast Cancer Res.
14:R62012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Teicher BA: Combination of
perfluorochemical emulsions and carbogen breathing with cancer
chemotherapy. Artif Cells Blood Substit Immobil Biotechnol.
22:1109–1120. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kawasoe Y, Yokouchi M, Ueno Y, Iwaya H,
Yoshida H and Komiya S: Hyperbaric oxygen as a chemotherapy
adjuvant in the treatment of osteosarcoma. Oncol Rep. 22:1045–1050.
2009.PubMed/NCBI
|
|
56
|
Kaanders JH, Bussink J and van der Kogel
AJ: Clinical studies of hypoxia modification in radiotherapy. Semin
Radiat Oncol. 14:233–240. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Budach W: Radiotherapy in patients with
metastatic breast cancer. Eur J Cancer. 47 Suppl 3:S23–S27. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Blair DA, Glover WE and McArrdle L: The
mechanism of the peripheral vasodilation following carbon dioxide
inhalation in man. Clin Sci. 19:407–423. 1960.
|
|
59
|
Matz H, Orion E and Wolf R: Balneotherapy
in dermatology. Dermatol Ther. 16:132–140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hartmann BR, Bassenge E, Pittler M and
Hartmann BR: Effect of carbon dioxide-enriched water and fresh
water on the cutaneous microcirculation and oxygen tension in the
skin of the foot. Angiology. 48:337–343. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Koga T, Niikura T, Lee SY, Okumachi E,
Ueha T, Iwakura T, Sakai Y, Miwa M, Kuroda R and Kurosaka M:
Topical cutaneous CO2 application by means of a novel
hydrogel accelerates fracture repair in rats. J Bone Joint Surg Am.
96:2077–2084. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Trinkaus M, Ooi WS, Amir E, Popovic S,
Kalina M, Kahn H, Singh G, Gainford MC and Clemons M: Examination
of the mechanisms of osteolysis in patients with metastatic breast
cancer. Oncol Rep. 21:1153–1159. 2009.PubMed/NCBI
|
|
63
|
Chen YC, Sosnoski DM and Mastro AM: Breast
cancer metastasis to the bone: Mechanisms of bone loss. Breast
Cancer Res. 12:2152010. View Article : Google Scholar : PubMed/NCBI
|