Introduction
Gastric cancer (GC), the fourth most common type of
cancer, has been demonstrated to be the third leading cause of
cancer-associated mortality in males and the fifth leading cause of
cancer-associated mortality in females worldwide (1). In order to develop an effective
treatment for GC, the molecular mechanisms of its development and
progression must be elucidated. However, in the past decade,
limited progress has occurred in studying the progression of
GC.
Netrin-1 (NTN1) is a laminin-like protein that was
initially identified as one of the axonal guidance molecules in
neuronal cell development (2). NTN1
has several dependent receptors, including uncoordinated-5-homolog
(UNC5H), which consists of UNC5H1, UNC5H2, UNC5H3 and UNC5H4,
deleted in colorectal cancer (DCC), neogenin and Down syndrome cell
adhesion molecule. Previous studies have demonstrated that NTN1
mRNA and protein expression is associated with numerous types of
cancer, including colorectal (3,4),
hepatic (5), neuroblastoma
(6), breast (7), pancreatic (8,9),
prostate (10) and non-small cell
lung cancer (11). In addition,
NTN1 has been demonstrated to regulate cancer cell proliferation,
migration, invasion and apoptosis (5,8,9,12,13).
Our previous study revealed that NTN1 promoted GC cells growth via
its receptor neogenin (14);
however, the downstream signaling pathway remains to be
elucidated.
Numerous studies have reported that focal adhesion
kinase (FAK) may be one of the primary downstream effector
molecules of NTN1 in neural system development (15,16).
Previous findings have indicated that NTN1 induces mesenchymal stem
cell motility via the distinct activation of FAK (17). In addition, a number of studies
reported the proliferation-promoting effect of NTN1 in renal
tubular epithelial cells was induced via activation of the
extracellular signal-regulated kinase (ERK)/mitogen-activated
protein kinase (MAPK) signaling pathway (18,19).
However, the coordination of signal transduction cascades
downstream of NTN1 via FAK remains unclear in GC development. Thus,
investigating the mechanisms of the NTN1/FAK-mediated signaling
pathway in the regulation of GC cell proliferation is of
importance.
In the present study, the role of NTN1 in promoting
the growth of GC cells and its associated signaling pathways was
investigated. It was demonstrated that NTN1 significantly promoted
GC cells proliferation in vitro and in vivo.
Furthermore, the present results suggest that NTN1-induced GC cells
proliferation was mediated by the ERK/MAPK signaling pathway via
activation of FAK.
Materials and methods
Cell culture and reagents
The human GC cell lines (SGC7901 and MGC803) were
obtained from the Cell Bank of the Chinese Academy of Medical
Science (Shanghai, China). All cell lines were cultured in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.), penicillin (100 U/ml) and streptomycin (100
mg/ml) at 37°C in an atmosphere containing 5% CO2. MEK
inhibitor U0126 and FAK inhibitor PF562271 were purchased from
Selleck Chemicals (Houston, TX, USA). GC cells were pretreated with
PF562271 (10 µM) and U0126 (10 µM) for 24 h to inhibit MEK and FAK
expression.
RNA interference and lentivirus
transfection
The polybrene lentivirus transfection reagent was
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China). The
shRNAs targeting NTN1 (5′-CATGGAGCTCTACAAGCTT-3′) and scramble
shRNA (5′-GTTCTCCGAACGTGTCACGT-3′) were synthesized and ligated
into a retroviral vector (Shanghai GenePharma Co., Ltd.). DNA
fragments corresponding to NTN1, which were amplified from human
genomic DNA, were cloned into the lentivirus for NTN1
overexpression (Shanghai GenePharma Co., Ltd.). For lentivirus
transfection, 2×105 SGC7901 and MGC803 cells were plated
into 6-well plates. After 24 h, cells were washed with PBS 3 times,
and then cultured with 2 ml RPMI-1640, 100 µl NTN1 overexpression
or knockdown lentivirus (1×108 transducing units/ml) and
polybrene (5 µg/ml). Retrovirus packaging and transfection were
conducted according to the manufacturer's protocol.
Cell proliferation assay
Cell proliferation was determined using a CCK-8
assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan). GC
cells transfected with lentivirus were seeded into 96-well plates
at a density of 1×103 cells/well in triplicate and
incubated with RPMI-1640 supplemented with 10% FBS for 5 days.
CCK-8 solution (10 µl) was added to each plate at various time
points (24, 48, 72, 96 and 120 h) and cells were incubated for 2 h
at 37°C. The absorbance was measured according to the
manufacturer's protocol.
Cell cycle assay using flow cytometry
(FCM)
Transfected GC cells were digested with trypsin and
centrifuged at 1,000 × g for 5 min at room temperature.
Subsequently, the cells were washed with PBS 3 times and fixed in
75% ethanol at 4°C overnight. Prior to FCM detection, cells were
washed twice with PBS, incubated with RNAse at room temperature for
15 min, stained with 500 µl propidium iodide staining solution for
15 min at room temperature. Data was obtained using a FACScan flow
cytometer with BD Cell Quest Pro 5.0 software (both from BD
Biosciences, Franklin Lakes, NJ, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tumor xenograft tissues
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. cDNA was produced from RNA
by RT using a PrimeScript RT reagent kit (Takara Biotechnology Co.,
Ltd., Dalian, China). The RT temperature protocol used was as
follows: 15 min at 37°C; reverse transcriptase inactivation for 5
sec at 85°C; followed by storage at 4°C. The primer sequences were
as follows: β-actin forward, 5′-AGATCCTGACCGAGCGTGGC-3′ and
reverse, 5′-CCAGGGAGGAAGAGGATGCG-3′; NTN1 forward,
5′-AAGCAGGGCACAAGTCGTAT-3′ and reverse, 5′-TGCTCTTGTCTGCCACGATG-3′.
qPCR was performed using SYBR Green Real-time PCR Master mix (Roche
Diagnostics GmbH, Mannheim, Germany). Briefly, the qPCR was
performed on an Applied Biosystems 7500 Fast Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with the
following thermal profile: Hot-start DNA polymerase activation to
95°C for 10 min; 40 cycles of 95°C for 15 sec and 60°C for 1 min;
followed by melt curve analysis at 95°C for 15 sec, 60°C for 1 min
and 95°C for 15 sec. Relative expression of NTN1 mRNA in tissues
was analyzed according to the 2−ΔΔCq method (20). β-actin used as an internal control
gene.
Western blot analysis
SGC7901 and MGC803 cells were harvested for protein
extraction according to standard procedures. Cell extracts were
prepared for western blotting using a Total Protein Extraction kit
(cat. no. KGP2100; Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China). Subsequently, a BCA protein assay was used for protein
quantification. A total of 40 µg of protein extracts from each
group were resolved using 10% SDS-PAGE and transferred to
polyvinylidene fluoride membranes. Following blocking with 5%
skimmed milk at room temperature for 2 h, the membranes were
incubated with specific primary antibodies in dilution buffer at
4°C overnight. The following primary antibodies were used: NTN1
(cat. no. ab126729; anti-rabbit; 1:200 dilution; Abcam, Cambridge,
UK), GAPDH (cat. no. 51332; anti-mouse), phosphorylated (p)-FAK
(cat. no. 3284; anti-rabbit), FAK (cat. no. 13009; anti-rabbit),
p-c-Jun N-terminal kinase (JNK) (cat. no. 4668; anti-rabbit), JNK
(cat. no. 9252; anti-rabbit), p-ERK (cat. no. 4376; anti-rabbit),
ERK (cat. no. 4695; anti-rabbit), p-P38 (cat. no. 4631;
anti-rabbit) and P38 (cat. no. 8690; anti-rabbit) (all 1:1,000
dilution; all from Cell Signaling Technology, Inc., Danvers, MA,
USA). GAPDH was used as an internal control. Following this, the
membranes were washed 3 times with TBS-Tween-20 and incubated with
horseradish peroxidase (HRP)-conjugated anti-mouse (cat. no.,
GAM007; 1:1,000 dilution) or anti-rabbit IgG (cat. no. GAB007;
1:1,000 dilution) (both from Hangzhou Multi Sciences Biotech Co.,
Ltd., Hangzhou, China) at room temperature for 2 h. Bands were
visualized using ECL Plus (EMD Millipore, Billerica, MA, USA) with
a FluorChem E enhanced chemiluminescence detection system
(ProteinSimple, San Jose, CA, USA).
Tumor xenograft with human GC cells in
nude mice
A total of 15, 4-week-old male nude BALB/c mice
(weight range, 13–15 g) were purchased from the Department of
Laboratory Animal Centre of Yangzhou University (Yangzhou, China)
and divided into 3 groups at random. All mice were raised under
pathogen-free conditions, including at room temperature (21–26°C)
and 12 h light/dark cycle. The mice received 150 ml water and 5 g
food per 100 g body weight per day. SGC7901 and MGC803 cells (NTN1
knockdown, control and NTN1 overexpression) were implanted into the
right flank of the nude mice by subcutaneous injection
(2×106 cells in 100 µl of PBS) to promote tumor
formation. Bidimensional tumor diameters were measured using a
slide caliper every 7 days. Nude mice were euthanized after 4
weeks. Xenograft tumor volume was calculated using the following
formula: (ab)2 × (0.5)2, whereby a represents
the length of the tumor and b represents the width diameter of the
tumor (21). The present study was
approved by the Jiangsu University Animal Ethics Committee
(Zhenjiang, China).
Immunohistochemical staining
(IHC)
Xenograft tumor samples were fixed in 4% formalin at
room temperature overnight and embedded in paraffin. Then, the
sections were washed in PBS 3 times and blocked in 5% bovine serum
albumin (Servicebio Technology Co., Ltd., Wuhan, China) for 30 min
at room temperature. The 4-µm slices were incubated with ki-67
antibody (cat. no. ab15580; anti-rabbit; 1:100 dilution; Abcam) at
4°C overnight and washed with PBS 3 times. Following incubation
with HRP-polymer-conjugated secondary antibody (cat. no. ab6721;
goat anti-rabbit; 1:1,000 dilution; Abcam) at room temperature for
1 h, the slices were dyed with diaminobenzidine solution for 3 min
at room temperature and then stained with 0.2% hematoxylin at room
temperature for 3 min. The sections were observed with an inverted
microscope (original magnification, ×100; NIKON ECLIPSE TI-SR;
Nikon Corporation, Tokyo, Japan). Ki-67 expression levels were
evaluated according to the percentage of positively stained tumor
cells and cell staining intensity. The staining intensity was
graded as follows: 0 (no staining); 1 (weak); 2 (moderate); and 3
(strong). The following scores were used to describe the overall
proportion of Ki-67 positive cells: 0 (negative); 1 (<30%); 2
(30–60%); and 3 (>60%). The two scores were multiplied, with
scores ≥4 being defined as high expression, and scores <4 as low
expression.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 22; IBM, Corp., Armonk, NY, USA). Data are
presented as the mean ± standard error of the mean. Differences
between 2 groups were evaluated using paired student t-tests. Data
from >2 groups were compared with two-way analysis of variance
followed by a least-significant difference post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
NTN1 enhances GC cells proliferation
in vitro
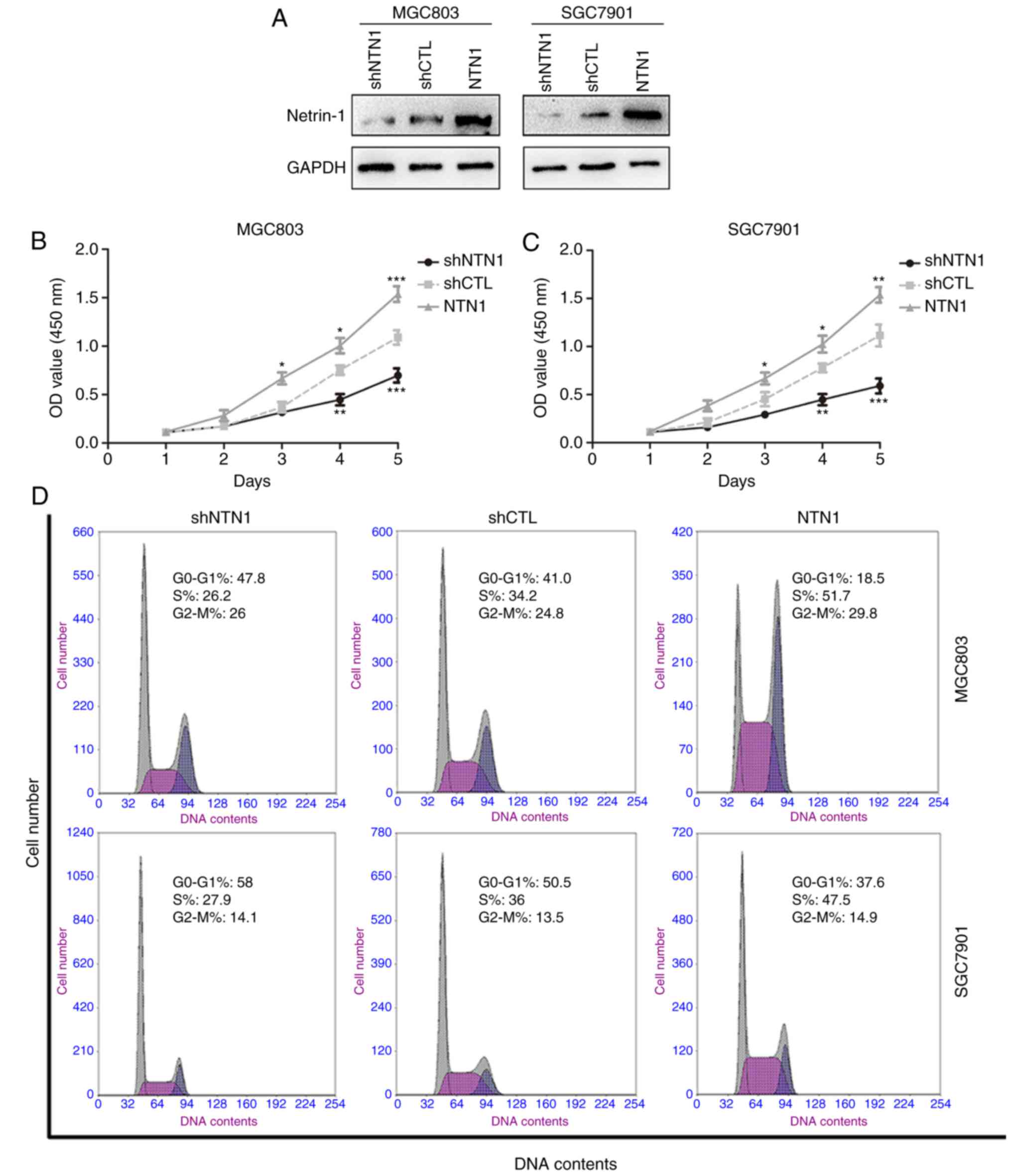
To investigate the role of NTN1 on GC cells
proliferation, MGC803 and SGC7901 cells were transfected with
lentivirus to overexpress or knock down NTN1. As shown in Fig. 1A, NTN1 protein expression was
markedly decreased following silencing and increased following
overexpression, indicating that transfection was successful. CCK-8
results revealed that NTN1 silencing significantly decreased the
proliferation of GC cells compared with the control group. By
contrast, NTN1 overexpression notably enhanced cell proliferation
in MGC803 and SGC7901 cells (Fig. 1B
and C). To further explore the mechanism responsible for the
NTN1-induced GC growth, the cell cycle distribution in each group
was investigated using FCM. Similarly, NTN1 knockdown in MGC803 and
SGC7901 cells significantly increased the percentage of cells in
G0/G1 phase, whereas the opposite was
observed in NTN1-overexpressed cells (Fig. 1D and E). Taken together, these
results suggest that NTN1 overexpression promoted GC cells
proliferation, while NTN1 knockdown induced cell cycle arrest at
the G0/G1 phases.
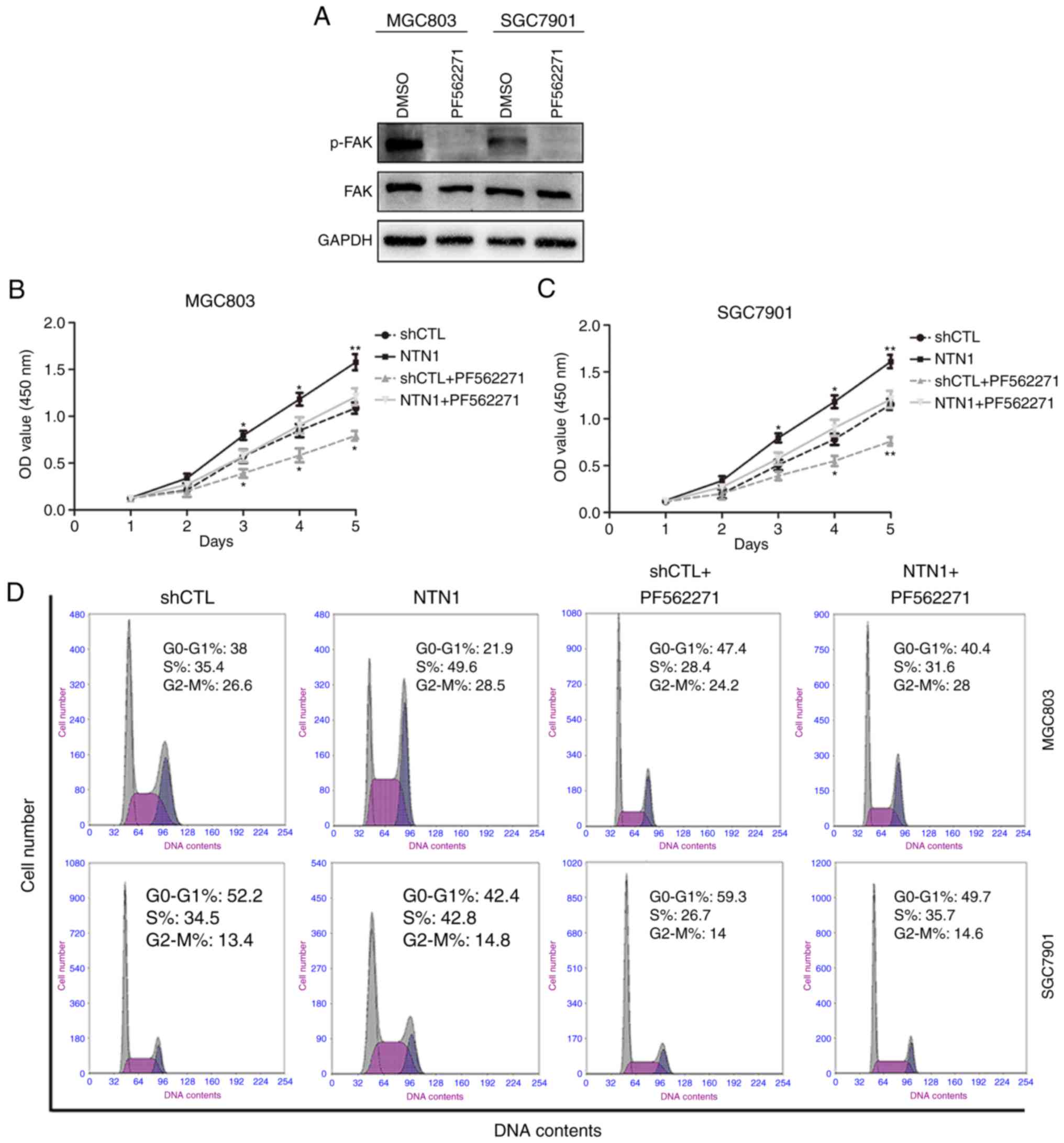
Enhanced GC cell proliferation induced
by NTN1 is FAK dependent
The effect of NTN1 on the phosphorylation of FAK,
which is considered to be essential in netrin signaling pathways
(16,22), was examined. The results indicated
that PF562271, a FAK inhibitor, markedly suppressed FAK
phosphorylation in MGC803 and SGC7901 cell lines (Fig. 2A). The results also demonstrated
that the proliferation of GC cells pretreated with PF562271 was
significantly inhibited (Fig. 2B and
C). In order to demonstrate that NTN1-induced GC cells growth
was mediated by FAK, FCM was used to assess the distribution of GC
cells in the cell cycle following NTN1 knockdown and
overexpression. As shown in Fig. 2D and
E, PF56227 significantly increased the percentage of cells in
the G0/G1 phases in MGC803 and SGC7901
cells.
Effects of NTN1 on the ERK/MAPK
signaling pathway
The ERK/MAPK signaling pathway is the primary
downstream pathway of FAK (22).
Whether ERK/MAPK signal cascades participate in NTN1-enhanced GC
cell proliferation was investigated. It was demonstrated that FAK,
ERK and JNK phosphorylation were reduced in MGC803 and SGC7901
cells following NTN1 knockdown compared with the negative control
group. By contrast, increased phosphorylation of FAK, ERK and JNK
was observed in the NTN1 overexpression group, while no changes
were detected in the total protein levels (Fig. 3A). However, another downstream
target of MAPK signaling pathway, P38, was not significantly
altered in GC cells following NTN1 knockdown and overexpression. To
verify the role of NTN1 in signaling pathways mediated by FAK,
MGC803 and SGC7901 cells with NTN1 overexpression were treated with
PF56227 for 24 h. The results revealed that PF56227 significantly
inhibited NTN1-induced phosphorylation of ERK and JNK (Fig. 3B).
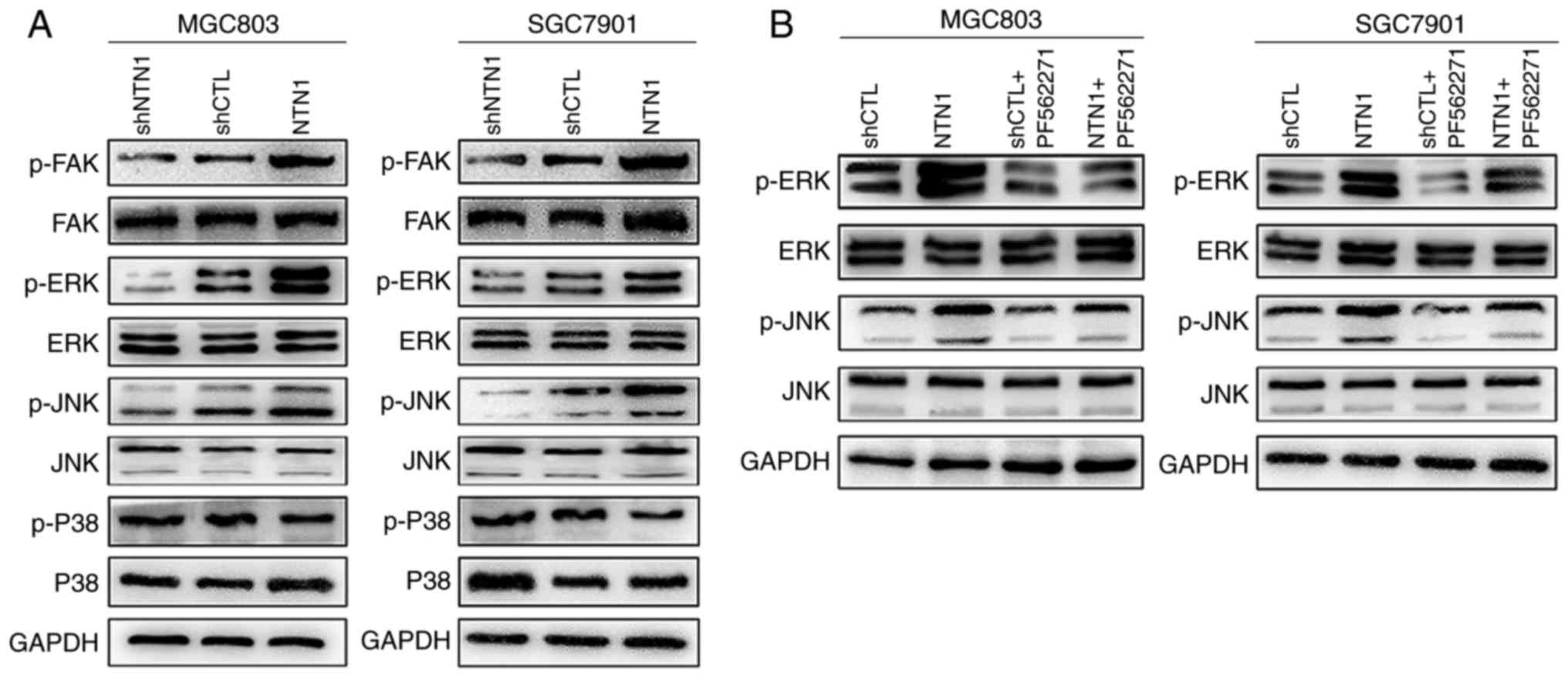 | Figure 3.Effects of NTN1 on the ERK/MAPK
signaling pathway are dependent on FAK. (A) MGC803 and SGC7901
cells were transfected with NTN1 knockdown or overexpression
lentivirus, and the expression levels of p-FAK/FAK, p-ERK/ERK,
p-JNK/JNK and p-P38/P38 were analyzed using western blot analysis.
(B) Western blot analysis revealed that pretreatment with FAK
inhibitor (PF562271) altered the expression levels of p-ERK/ERK and
p-JNK/JNK. FAK, focal adhesion kinase; NTN1, Netrin-1; sh, shRNA;
CTL, control; p, phosphorylated; JNK, -Jun N-terminal kinase; ERK,
extracellular signal-regulated kinase. |
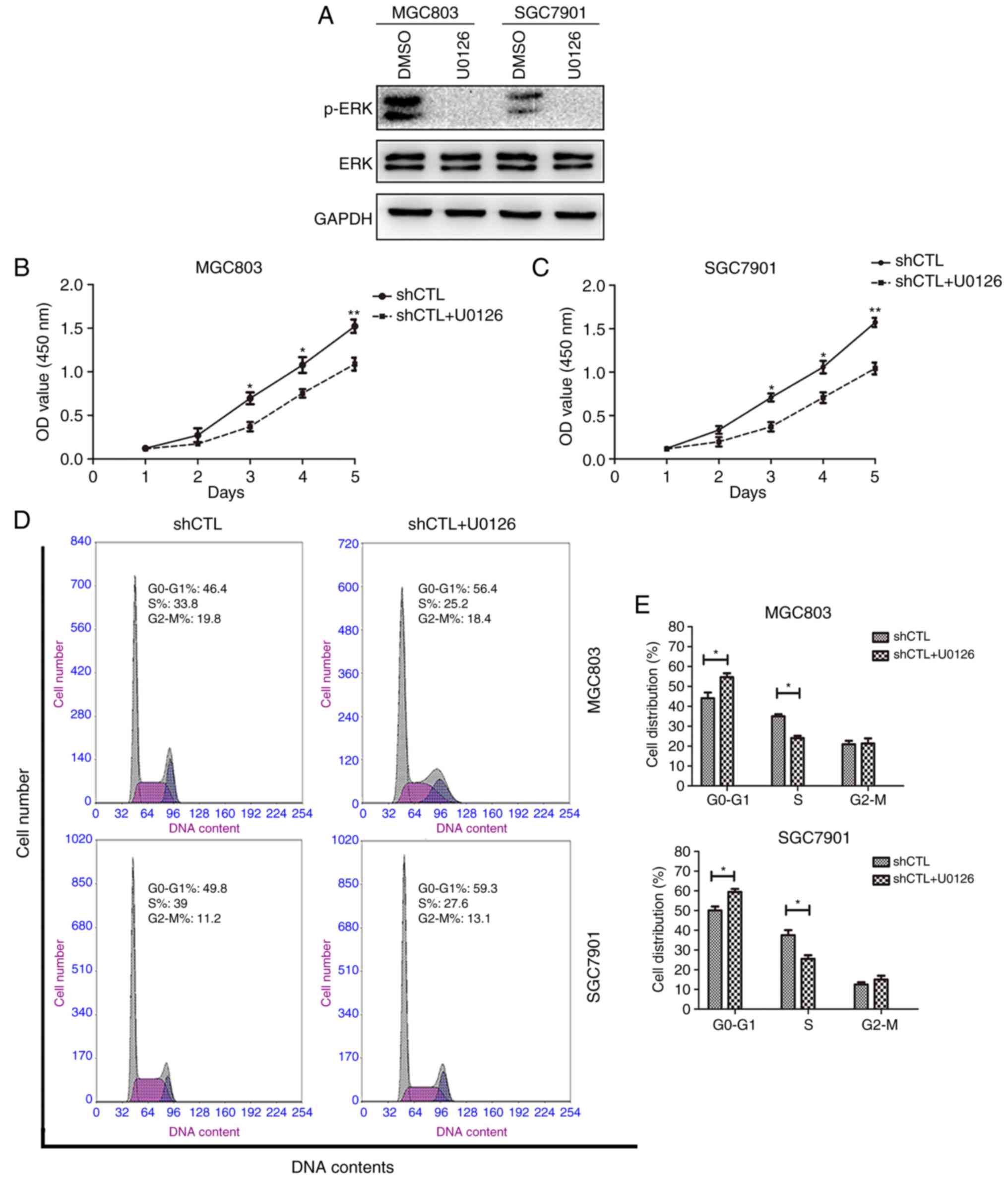
Effects of ERK on GC cells
proliferation ability in vitro
The effect of ERK on GC cell proliferation was
analyzed. ERK phosphorylation was suppressed in MGC803 and SGC7901
cell lines following treatment with MEK inhibitor U0126 (Fig. 4A). The results demonstrated that
pretreatment with the MEK inhibitor (U0126) also downregulated GC
cell proliferation (Fig. 4B and C)
and increased the percentage of cells in the
G0/G1 phases (Fig. 4D and E). Taken together, these
results demonstrated that the ERK/MAPK signaling pathway may be
involved in enhancing GC cell proliferation and that NTN1 exerted
this effect through the activation of FAK.
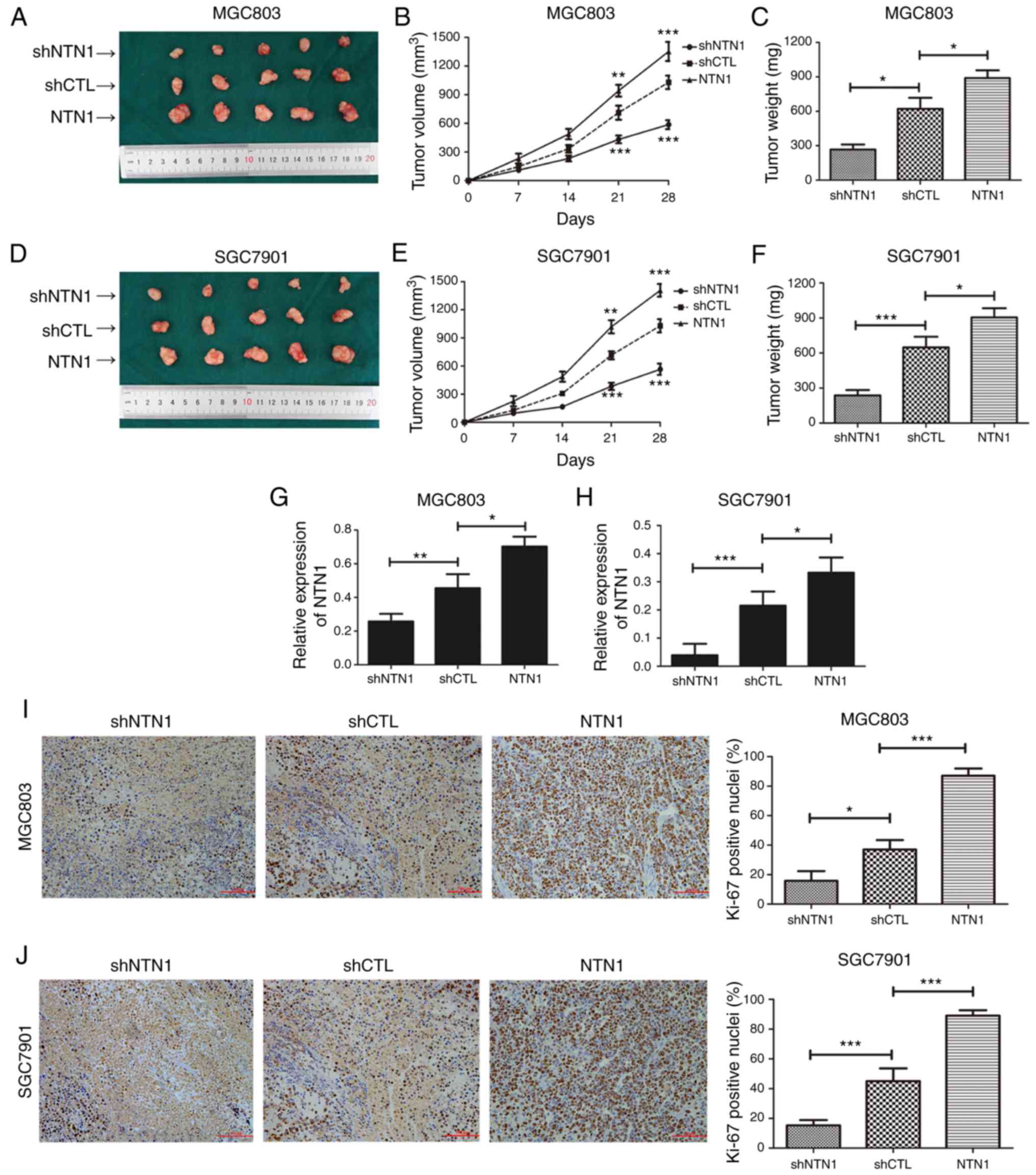
NTN1 promotes tumor growth in nude
mice inoculated with GC cells
In order to investigate the effect of NTN1 on tumor
growth in nude mice, GC cells (NTN1 knockdown, control and NTN1
overexpression) were injected into the right flank of nude mice to
form ectopic tumors. As shown in Fig.
5A-F, the tumor volume and weight in the NTN1 knockdown group
were decreased compared with the control group. Conversely, the
tumor volume and weight in the NTN1 overexpression group were
increased compared with the control group. Furthermore, the
relative expression of NTN1 in samples collected from nude mice was
measured using RT-qPCR. As shown in Fig. 5G and H, the expression of NTN1 was
downregulated in the NTN1 knockdown group, while it was upregulated
in the NTN1 overexpression group. Ki-67 IHC analysis was performed
to assess GC cell proliferation in vivo. The positive nuclei
rate of Ki-67 was lower in the NTN1 knockdown group and higher in
NTN1 overexpression group (Fig. 5I and
J). Taken together, these results indicate that proliferation
ability of GC cells was impaired following NTN1 knockdown, which
was consistent with the experimental results in vitro.
Discussion
The proto-oncogene NTN1 has been reported to promote
cancer cell proliferation during tumor development (7,8,23). In
the present study, the in vivo and in vitro results
identified that the nerve-derived molecule NTN1 has the ability to
stimulate GC cell proliferation, suggesting that NTN1 has an
oncogenic effect. Furthermore, it was revealed that NTN1 induces
the distinct activation of FAK, subsequently leading to the
induction of the ERK/MAPK signaling pathway.
FAK is a cytoplasmic protein tyrosine kinase, which
has been demonstrated to be involved in cancer cell progression,
including cell proliferation and migration (24). Previous studies have reported that
FAK may be one of the primary downstream effectors of NTN1,
functioning through the receptors DCC and neogenin (15,16).
Furthermore, it has been reported that NTN1-induced cell
proliferation and migration is mediated by the activation of FAK
(17,25). The results of the present study
suggest that FAK phosphorylation was decreased following NTN1
knockdown in GC cells, whereas NTN1 overexpression had the opposite
effect. GC cell proliferation was significantly inhibited following
pretreatment with FAK inhibitor, which is consistent with previous
results (26,27). These results indicate that
dysfunction of FAK signaling pathway may be an important mechanism
involved in GC cell proliferation.
The ERK/MAPK signaling pathway has been demonstrated
to serve important roles in the proliferation of various cells
(28). In addition, the results of
the present study revealed that ERK and JNK phosphorylation levels
were increased following NTN1 overexpression, which has been
confirmed to be a downstream effect of NTN1 in other studies
(5,18). Notably, the mechanism by which NTN1
induces renal proximal tubular epithelial cells proliferation has
been proposed as stimulation of the UNC5B, which triggers the
activation of the ERK/MAPK signaling pathway (18). The results in the current study
indicated that NTN1 knockdown decreased the phosphorylation of ERK
and JNK, while P38 phosphorylation was unchanged. Furthermore, the
present results suggest that the addition of the MEK inhibitor
suppressed the proliferation ability of NTN1 in GC cells. However,
it has been reported that NTN1 stimulates p38/MAPK, but not
ERK/MAPK signaling pathways in Schwann cells (29). Notably, it was also reported that
NTN1 suppressed the growth of pancreatic cancer via inhibition of
the ERK/MAPK signaling pathway through its receptor UNC5B (30). In addition, NTN1 has been
demonstrated to induce the inhibition of the ERK/MAPK signaling
pathway during lung branching morphogenesis (31). These findings suggest that NTN1
possibly has bidirectional effects on the ERK/MAPK signaling
pathway depending on the receptor types or ligand structure.
In conclusion, the present study demonstrated that
the NTN1-induced promotion of GC cell proliferation was mediated
via activation of the FAK/ERK/MAPK signaling pathway in vivo
and in vitro. Considering the role of NTN1 on GC
progression, the therapeutic potential of NTN1 in GC requires
further investigation and evaluation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Jiangsu
Province Fund for ‘Six talent summits’ high-level talent (grant no.
2016-WSN-007) and Zhenjiang Key Research and Development Plan
(grant nos. SH2015066 and SH2017023).
Availability of data and materials
All data generated or analyzed during this study are
available upon reasonable request from the corresponding
author.
Authors' contributions
ZX and JC conceived and designed the study. KY, MS,
YX and SD performed the experiments and acquired data. LW, JQ, LC
and XF analyzed the data. KY drafted and edited the manuscript. All
authors have given final approval of the version to be
published.
Ethics approval and consent to
participate
The present study was approved by the Jiangsu
University Animal Ethics Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lai Wing Sun K, Correia JP and Kennedy TE:
Netrins: Versatile extracellular cues with diverse functions.
Development. 138:2153–2169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mazelin L, Bernet A, Bonod-Bidaud C, Pays
L, Arnaud S, Gespach C, Bredesen DE, Scoazec JY and Mehlen P:
Netrin-1 controls colorectal tumorigenesis by regulating apoptosis.
Nature. 431:80–84. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ko SY, Blatch GL and Dass CR: Netrin-1 as
a potential target for metastatic cancer: Focus on colorectal
cancer. Cancer Metastasis Rev. 33:101–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han P, Fu Y, Liu J, Wang Y, He J, Gong J,
Li M, Tan Q, Li D, Luo Y, et al: Netrin-1 promotes cell migration
and invasion by down-regulation of BVES expression in human
hepatocellular carcinoma. Am J Cancer Res. 5:1396–1409.
2015.PubMed/NCBI
|
|
6
|
Delloye-Bourgeois C, Fitamant J, Paradisi
A, Cappellen D, Douc-Rasy S, Raquin MA, Stupack D, Nakagawara A,
Rousseau R, Combaret V, et al: Netrin-1 acts as a survival factor
for aggressive neuroblastoma. J Exp Med. 206:833–847. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fitamant J, Guenebeaud C, Coissieux MM,
Guix C, Treilleux I, Scoazec JY, Bachelot T, Bernet A and Mehlen P:
Netrin-1 expression confers a selective advantage for tumor cell
survival in metastatic breast cancer. Proc Natl Acad Sci USA.
105:4850–4855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dumartin L, Quemener C, Laklai H, Herbert
J, Bicknell R, Bousquet C, Pyronnet S, Castronovo V, Schilling MK,
Bikfalvi A and Hagedorn M: Netrin-1 mediates early events in
pancreatic adenocarcinoma progression, acting on tumor and
endothelial cells. Gastroenterology. 138(1595–1606): 1606.
e1591–1598. 2010.
|
|
9
|
Huang Q, Hua HW, Jiang F, Liu DH and Ding
G: Netrin-1 promoted pancreatic cancer cell proliferation by
upregulation of Mdm2. Tumour Biol. 35:9927–9934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong CZ, Liu J, Liu L, Zhang Z and Guo KF:
Interactional expression of netrin-1 and its dependence receptor
UNC5B in prostate carcinoma. Tumour Biol. 34:2765–2772. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Delloye-Bourgeois C, Brambilla E,
Coissieux MM, Guenebeaud C, Pedeux R, Firlej V, Cabon F, Brambilla
C, Mehlen P and Bernet A: Interference with netrin-1 and tumor cell
death in non-small cell lung cancer. J Natl Cancer Inst.
101:237–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akino T, Han X, Nakayama H, McNeish B,
Zurakowski D, Mammoto A, Klagsbrun M and Smith E: Netrin-1 promotes
medulloblastoma cell invasiveness and angiogenesis, and
demonstrates elevated expression in tumor tissue and urine of
patients with pediatric medulloblastoma. Cancer Res. 74:3716–3726.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimizu A, Nakayama H, Wang P, König C,
Akino T, Sandlund J, Coma S, Italiano JE Jr, Mammoto A, Bielenberg
DR, et al: Netrin-1 promotes glioblastoma cell invasiveness and
angiogenesis by multiple pathways including activation of RhoA,
cathepsin B, and cAMP-response element-binding protein. J Biol
Chem. 288:2210–2222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin K, Wang LJ, Zhang X, He Z, Xia Y, Xu
J, Wei S, Li B, Li Z, Sun G, et al: Netrin-1 promotes gastric
cancer cell proliferation and invasion via the receptor neogenin
through PI3K/AKT signaling pathway. Oncotarget. 8:51177–51189.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu G, Beggs H, Jurgensen C, Park HT, Tang
H, Gorski J, Jones KR, Reichardt LF, Wu J and Rao Y: Netrin
requires focal adhesion kinase and Src family kinases for axon
outgrowth and attraction. Nat Neurosci. 7:1222–1232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moore SW, Zhang X, Lynch CD and Sheetz MP:
Netrin-1 attracts axons through FAK-dependent mechanotransduction.
J Neurosci. 32:11574–11585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee SJ, Jung YH, Oh SY, Yong MS, Ryu JM
and Han HJ: Netrin-1 induces MMP-12-dependent E-cadherin
degradation via the distinct activation of PKCα and FAK/Fyn in
promoting mesenchymal stem cell motility. Stem Cells Dev.
23:1870–1882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Reeves WB and Ramesh G: Netrin-1
increases proliferation and migration of renal proximal tubular
epithelial cells via the UNC5B receptor. Am J Physiol Renal
Physiol. 296:F723–F729. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mohamed R, Jayakumar C, Ranganathan PV,
Ganapathy V and Ramesh G: Kidney proximal tubular
epithelial-specific overexpression of netrin-1 suppresses
inflammation and albuminuria through suppression of COX-2-mediated
PGE2 production in streptozotocin-induced diabetic mice. Am J
Pathol. 181:1991–2002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naito S, von Eschenbach AC, Giavazzi R and
Fidler IJ: Growth and metastasis of tumor cells isolated from a
human renal cell carcinoma implanted into different organs of nude
mice. Cancer Res. 46:4109–4115. 1986.PubMed/NCBI
|
|
22
|
Li W, Lee J, Vikis HG, Lee SH, Liu G,
Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, et al: Activation of
FAK and Src are receptor-proximal events required for netrin
signaling. Nat Neurosci. 7:1213–1221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodrigues S, De Wever O, Bruyneel E,
Rooney RJ and Gespach C: Opposing roles of netrin-1 and the
dependence receptor DCC in cancer cell invasion, tumor growth and
metastasis. Oncogene. 26:5615–5625. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ying X, Huang A, Xing Y, Lan L, Yi Z and
He P: Lycorine inhibits breast cancer growth and metastasis via
inducing apoptosis and blocking Src/FAK-involved pathway. Sci China
Life Sci. 60:417–428. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang X, Li S, Zhong J, Zhang W, Hua X, Li
B and Sun H: CD151 mediates netrin-1-induced angiogenesis through
the Src-FAK-Paxillin pathway. J Cell Mol Med. 21:72–80. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng Z, Liu F, Zhang H, Li X, Li Y, Li J,
Liu F, Cao Y, Cao L and Li F: miR-135a inhibits tumor metastasis
and angiogenesis by targeting FAK pathway. Oncotarget.
8:31153–31168. 2017.PubMed/NCBI
|
|
27
|
Zang M, Zhang Y, Zhang B, Hu L, Li J, Fan
Z, Wang H, Su L, Zhu Z, Li C, et al: CEACAM6 promotes tumor
angiogenesis and vasculogenic mimicry in gastric cancer via FAK
signaling. Biochim Biophys Acta. 1852:1020–1028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Razidlo GL, Kortum RL, Haferbier JL and
Lewis RE: Phosphorylation regulates KSR1 stability, ERK activation,
and cell proliferation. J Biol Chem. 279:47808–47814. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lv J, Sun X, Ma J, Ma X, Zhang Y, Li F, Li
Y and Zhao Z: Netrin-1 induces the migration of Schwann cells via
p38 MAPK and PI3K-Akt signaling pathway mediated by the UNC5B
receptor. Biochem Biophys Res Commun. 464:263–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
An XZ, Zhao ZG, Luo YX, Zhang R, Tang XQ,
Hao D, Zhao X, Lv X and Liu D: Netrin-1 suppresses the MEK/ERK
pathway and ITGB4 in pancreatic cancer. Oncotarget. 7:24719–24733.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Stein E, Oliver T, Li Y, Brunken
WJ, Koch M, Tessier-Lavigne M and Hogan BL: Novel role for Netrins
in regulating epithelial behavior during lung branching
morphogenesis. Curr Biol. 14:897–905. 2004. View Article : Google Scholar : PubMed/NCBI
|