Introduction
Malignant melanoma (MM) is one of the most
aggressive forms of cutaneous neoplasms, and its incidence is
notably increasing (1). It is
estimated that there will be 87,110 newly diagnosed MM cases and
9,730 MM-associated mortalities in 2017 in the United States
(2). Despite substantial
improvement in the diagnosis and treatment of MM in previous years,
the prognosis remains poor for patients with MM diagnosed at
metastatic stages, with a median survival time of 6–9 months and a
5-year survival rate of <15% (3–6). Thus,
identifying effective biomarkers for the early detection and
efficient evaluation of prognosis of MM following surgery is
crucial.
MicroRNAs (miRNAs/miRs) comprise a group of small
non-coding RNAs (~22 nucleotides in length) (7). These miRNAs regulate the expression of
a wide variety of target genes through repressing translation or
inducing mRNA degradation by binding to complementary sites in
3′-untranslated regions (3′-UTRs) (8). Aberrantly expressing miRNAs may
function as tumor suppressors or oncogenes, depending on the
functions of their target genes (9–11).
Increasingly, miRNAs have been observed in various types of cancer,
and have been revealed to be involved in modulating cancer cell
behavior, including cell proliferation (12), cell apoptosis (13), cell cycle (14), cell migration (15) and cell invasion (16). Previously, a number of
aberrantly-expressed serum and tissue miRNAs have been employed as
diagnostic or prognostic indicators in MM (11,17,18).
The expression and function of miR-590-5p varies in
different types of tumor. miR-590-5p was previously demonstrated to
function as an oncogene, and promote the proliferation, migration
and G1-S phase transition by directly inhibiting the transforming
growth factor beta receptor II (TGF-βRII) in vulvar squamous cell
carcinoma (19). Conversely,
miR-590-5p serves a tumor suppressor role in breast cancer, and
inhibits cancer cell stemness and metastasis by targeting SRY-box 2
(20). Previously, it was revealed
that miR-590-5p was downregulated in human melanoma A375 cells, and
inhibited their migration and invasion ability (21). However, the precise functions and
underlying mechanisms of miR-590-5p on the proliferation and
apoptosis of MM cells remain unclear.
In the present study, a reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) was
performed to detect established oncogenic and tumor suppressor
miRNAs in MM cells and normal human melanocytes (HMs). Furthermore,
Cell Counting Kit-8 (CCK-8), flow cytometry and tumor xenograft
assays were performed to detect the effects of miR-590-5p on the
proliferation and apoptosis of MM cells in vitro, and tumor
growth in vivo. Finally, luciferase assays and western blot
analysis were performed to investigate whether Yes-associated
protein 1 (YAP1) was the functional mediator of miR-590-5p in MM
cells.
Materials and methods
Cell culture
Human MM cell lines A2058, A375, normal epidermal
melanocytes HEMa-LP and 293 cells were obtained from the Cell Bank
of the Chinese Academy of Sciences (Shanghai, China), where they
were characterized by mycoplasma detection, DNA-fingerprinting,
isozyme detection and cell vitality detection, performed by the
Cell Bank of the Chinese Academy of Sciences. A2058 and A375 cells
were cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) at 37°C in a humidified atmosphere
of 95% air and 5% CO2. HMs isolated from human foreskin
specimens from patients who received circumcision at the First
Affiliated Hospital of Xi'an Jiaotong University and provided
written informed consent for the use of their excised foreskin.
Ethical approval was obtained from the Ethics Committee of Xi'an
Jiaotong University (Xi'an, China). HM and HEMa-LP cells were
cultured in Medium 254 (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with HM growth supplement at 37°C in a humidified
atmosphere of 95% air and 5% CO2.
Oligonucleotide transfection
miR-590-5p negative control (NC; sequence,
GUCCAGUGAAUUCCCAG), miR-590-5p inhibitors (sequence,
GACGUAAAAUACUUAUUCGAG), miR-590-5p mimics (sequence,
GAGCUUAUUCAUAAAAUGCAG), YAP1 NC (antisense,
CCGGTAAATTTCTGAAATTTATTTCAAGAGATTTCTAAATCTCATCCTGAGTCTCTCTTTTTG and
sense,
AATTCAAAAAGACAGGACTTTAGAAATTCTCTTGAAATCCATCAGGAAGAGGACCTGTTTG) and
YAP1 small interfering RNA (siRNA; antisense,
CCGGCAGGCCTCCTCTTCCTGATGGATTTCAGAGAATCCATCAGGAAGAGGACCTGTTTG and
sense,
AATTCAAAACAGGTCCTCTTCCTGATGGATTCTCTTGAAATCCATCAGGAAGAGGACCTGTTTTG)
were purchased from Shanghai GenePharma Co., Ltd. (Shanghai,
China). When the confluence of A375 and A2058 cells reached 50–60%
in a 6-well plate, oligonucleotides (50 nmol) were mixed with 5 µl
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) in
500 µl serum-free DMEM (Life Technologies; Thermo Fisher
Scientific, Inc.). The transfection solutions were added to each
well containing 500 µl serum-free DMEM (Life Technologies; Thermo
Fisher Scientific, Inc.). Once the cells were incubated with
oligonucleotides for 24 h, the culture medium was changed to to
DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (HyClone; GE Healthcare Life Sciences).
Following transfection, cell samples were collected at 48 h for
further analyses.
RT-qPCR
For miR590-5p quantification, total miRNA was
extracted from A375 and A2058 cells using the miRNeasy RNA
isolation kit (Qiagen GmbH, Hilden, Germany) according to the
manufacturer's protocol. Total miRNA samples were
reverse-transcribed into cDNA using the miScript Reverse
Transcription kit (Qiagen GmbH, Hilden, Germany) according to the
manufacturer's protocol with a miR-590-5p specific primer and
universal small nuclear U6 RNA was used as an internal loading
control. RT was performed at 45°C for 60 min and 70°C for 10 min.
TaqMan miRNA RT-qPCR (Applied Biosystems; Thermo Fisher Scientific,
Inc.) were used to detect and quantify miR-590-5p and U6
expression. For YAP1 quantification, total RNA was extracted from
the cells using TRIzol reagent (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) according to the manufacturer's protocol. RNA
samples were then reverse-transcribed into cDNA using PrimeScript™
RT Master Mix (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's protocol with a YAP1 specific primer and U6 RNA was
used as an internal loading control. SYBR Mix (Takara Bio, Inc.)
was used to detect and quantify YAP1 and β-actin expression.
RT-qPCR assays were performed under the following thermocycling
conditions: 95°C for 5 min, followed by 45 cycles of 95°C for 15
sec, 60°C for 30 sec and 72°C for 30 sec. Data were analyzed with
7500 software v.2.0.1 (Applied Biosystems; Thermo Fisher
Scientific, Inc.), with the automatic Cq setting for adapting the
baseline and threshold for Cq determination (22). Each sample was examined in
triplicate. The primer sequences used in the present study are
listed in Table I.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene name | Forward primer
5′-3′ | Reverse primer
5′-3′ |
|---|
| Yes-associated
protein 1 |
ACCCACAGCTCAGCATCTTCG |
TGGCTTGTTCCCATCCATCAG |
| β-actin |
CGTCTTCCCCTCCATCGT |
GAAGGTGTGGTGCCAGATTT |
|
microRNA-590-5p |
GGAATTCTTCAGTTGTAACCCAG |
CGGGATCCTTGAGATGTCACCAA |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
CCK-8
A2058 cells transfected with miR-590-5p NC and
miR-590-5p mimics (used 24 h after transfection) and A375 cells
transfected with miR-590-5p NC and miR-590-5p inhibitors (used 24 h
after transfection) were seeded in a 96-well culture plate at a
density of 2,000 cells per well. Each group was established in nine
wells. CCK-8 reagents (MedChemExpress, Monmouth Junction, NJ, USA)
were added into each well 24, 48, 72, 96 and 120 h after seeding,
and each group was cultured for 50 min at 37°C in a humidified
atmosphere of 95% air and 5% CO2. The OD values were
measured at 490 nm in a Microplate Reader.
Flow cytometry
A375 and A2058 cells in 6 well culture plate were
harvested by trypsinization at 37°C for 5 min, and wash three times
with PBS. For cell apoptosis, cells were suspended in 500 µl
binding buffer at a density of 2×106 cells/ml, and
incubated with Annexin V-fluorescien isothiocyanate and propidium
iodide (PI; BD Biosciences, San Jose, CA, USA) for 15 min in the
dark at room temperature. For cell cycle analysis, 2×106
cells/ml A2058 and A375 cells were fixed using 75% ethanol at 4°C
for 12 h. PI was added into A2058 and A375 cells transfected with
miR-590-5p NC, inhibitors or mimics and incubated for 20 min in the
dark at room temperature. Cell apoptosis and cell cycle was then
analyzed using a flow cytometer and Kaluza Analysis Software
version 2.0 (Beckman Coulter, Inc., Brea, CA, USA).
Western blot analysis
A375 and A2058 cells were washed in PBS three times
prior to proteins being extracted. Then the cells were lysed using
RIPA buffer for 30 min on ice (Xi'an Jing Cai Biological Technology
Co., Ltd., Xi'an, China), each protein sample (30 µg) was denatured
in SDS sample buffer and separated via 10% SDS/PAGE gel. Separated
proteins were transferred onto polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA) blocked with 5% bovine serum
albumin (Beyotime Institute of Biotechnology, Haimen, China) for 2
h at room temperature, and incubated overnight with primary
antibodies at 4°C. Blotting was performed with primary antibodies
against YAP1 (1:300; cat no. 14074; Cell Signaling Technology,
Inc., Danvers, MA, USA). Goat anti-rabbit immunoglobulin
horseradish peroxidase-conjugated F(ab)2 fragments (1:5,000; cat
no. TA130071; OriGene Technologies, Inc., Rockville, MD, USA) were
used as secondary antibodies and incubated for 2 h at room
temperature. β-actin (1:4,000; cat no. ab8226; Abcam, Cambridge,
UK) was used as a loading control and incubated overnight at 4°C.
Blots were then washed three times (10 min/wash) in tris buffered
saline with 0.1% Tween-20 and developed using an enhanced
chemiluminescence system (Xi'an Jing Cai Biological Technology Co.,
Ltd.). ImageJ 1.8.0 (National Institutes of Health, Bethesda, MD,
USA) was used to analyze the gray values of each blot.
Tumour xenograft assays
For tumorigenesis assays, A2058 cells were
engineered to stably overexpress miR-590-5p and luciferase, using a
lentiviral-based system (cat no. 73153; pLenti6.3; Shanghai
GeneChem, Inc., Shanghai, China) In brief, pri-miR-590-5p sequence
was cloned into pLenti6.3 vector (Shanghai GeneChem, Inc.). Then,
pLenti-miR-590-5p-Luci (50 nmol) was co-transfected into 293 cells
with psPAX2 and PMD2G by using Lipofectamine 2000®
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Once the 293 cells were incubated with oligonucleotides
for 8 h at 37°C, the culture medium was changed to DMEM
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (HyClone; GE Healthcare Life Sciences).
Following transfection, viral particles were collected at 48 h, and
centrifuged together at 1,000 × g for 5 min at 4°C, then filtered
through 0.45 nm filter. Xenograft tumors were generated by the
subcutaneous injection of A2058 cells (5×106), including
A2058 pLenti-Luciferase and A2058 pLenti-miR-590-5p-Luciferase,
into the hind limbs of 4–6 weeks old Balb/C female athymic nude
mice (nu/nu; Animal Center of Xi'an Jiaotong University, Xi'an,
China; n=5 for each group). All mice were housed and maintained
under specific pathogen-free conditions at 18–22°C, with 20%
humidity, a 12-h light and 12-h dark cycle and ad libitum
access to food. The tumor size was measured using an Xenogen IVIS
Kinetic imaging system and vernier caliper. Tumor volume was
determined by the formula: 0.5×AxB2, where A represents
the diameter of the base of the tumor and B represents the
corresponding perpendicular value. When the mean diameter reach 1.2
cm or progressive tumor growth was evident, the mice were
euthanasized by placing mice in sealed chambers where 5% isoflurane
was introduced. Then, the tumors were collected and weighed. All
experiments were ethically approved by the Animal Care and Use
Committee of Xi'an Jiaotong University and performed in accordance
with institutional guidelines (23).
Luciferase assays
A total of 5,000 293 cells were seeded in a 96-well
plate at 70% confluence. The YAP1 wild type (WT) 3′-UTR firefly
luciferase construct (pMir-YAP1 WT- 3′-UTR) was generated by
inserting YAP1 WT 3′-UTR into pMir-Report vector (Ambion; Thermo
Fisher Scientific, Inc.). Mutations were introduced in potential
miR-590-5p binding sites using the QuikChange site-directed
mutagenesis kit (Stratagene; Agilent Technologies, Inc., Santa
Clara, CA, USA) according to the manufacturer's protocol. Then, a
final concentration of 100 nM miR-590-5p NC or mimics were
transfected into 293 cells along with 30 ng pMir-YAP1 WT or mutant
3′-UTR luciferase reporter and 10 ng Renilla luciferase
reporter using Lipofectamine® 2000, as previously
stated. Cells were collected 48 h post-transfection, and luciferase
assays were performed using a Photinus pyralis-Renilla
reniformis dual luciferase reporter assay system (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol. The ratio of Photinus pyramid to Renilla of each
lysate luciferase activity was determined by an Orion II Microplate
Illuminometer (Titertek-Berthold, South San Francisco, CA, USA).
Relative activities were expressed as the fold change in luciferase
activity.
Statistical analysis
Statistical analysis was performed using IBM SPSS
statistical software (version 21.0; IBM Corp., Armonk, NY, USA). A
volcano plot of established oncogenic and tumor suppressor miRNA
profiles in A375 cells compared with HM controls was produced, and
an adjusted P-value for each miRNA was analyzed by Bonferroni's
correction. Potential targets of miR-590-5p were determined by
integrating the results of multiple prediction algorithms of
TargetScan [TargetScan human 7.2 (24), www.targetscan.org/], PicTar [PicTar (25), https://pictar.mdc-berlin.de/] and miRNAda [miRNAda
(26), http://www.microrna.org/; search term used, miR-590-5p
mammal) accessed on the 12th December 2016. The
differences in characteristics between 2 groups were examined using
a paired Student's t-test. The differences in characteristics
between 3 groups was examined using one-way analysis of variance
followed by a least significant difference-t-test to detect the
differences between every 2 groups. The differences of miRNA
expressions in A375 and HM cells was examined by hierarchical
cluster analysis. All P-values were determined from 2-sided tests.
P-value <0.05 was considered to indicate a statistically
significant difference. The data were presented as the mean ±
standard deviation from three independent experiments.
Results
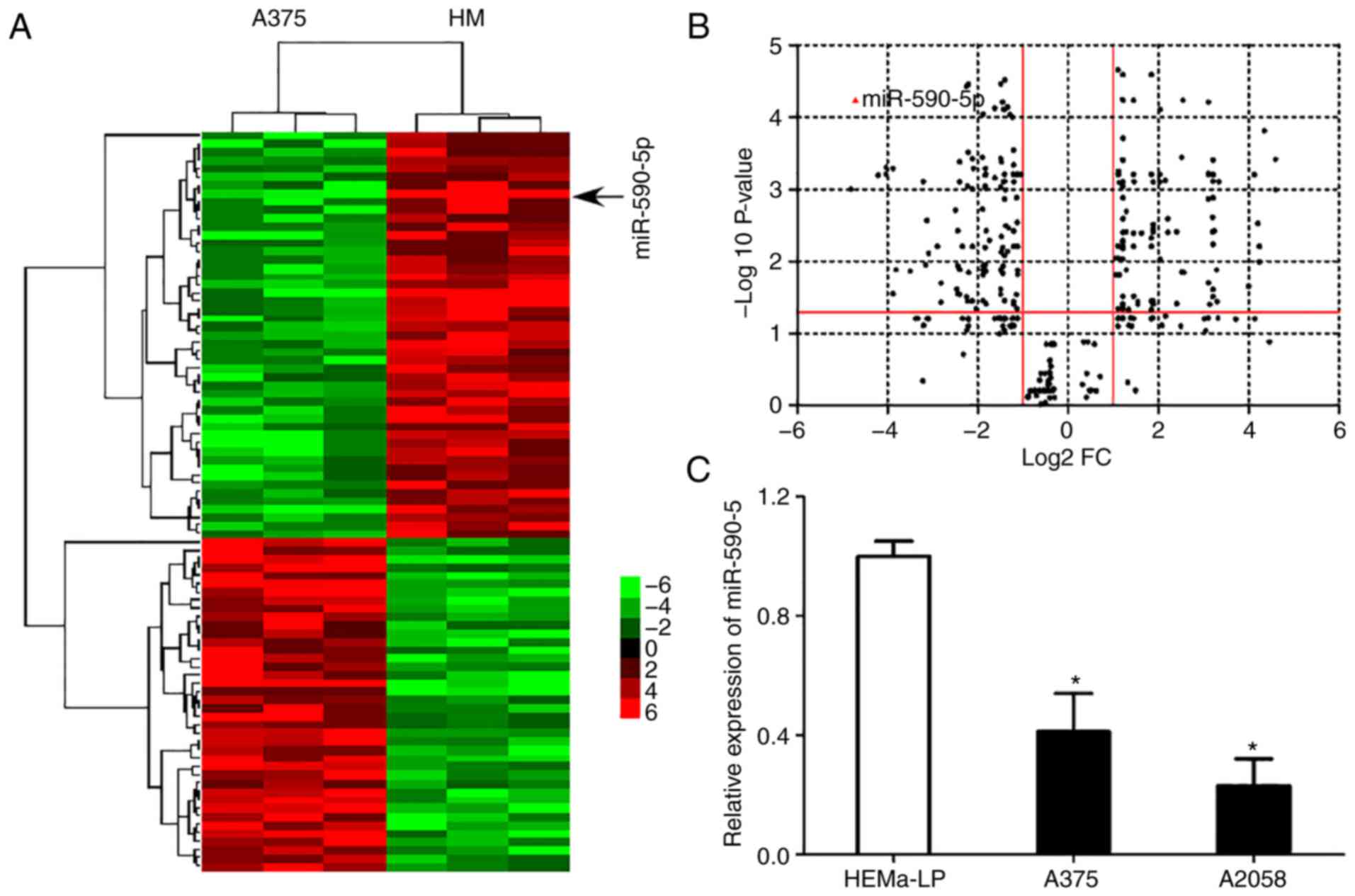
miR-590-5p is downregulated in MM
cells
To identify the miRNAs involved in the
carcinogenesis and progression of MM, established oncogenic and
tumor suppressor miRNA screening was detected in normal HMs and the
MM cell line A375 by RT-qPCR (Table
II). Hierarchical cluster analysis identified that miR-590-5p
was commonly downregulated in A375 cells compared with the
representative controls (Fig. 1A).
A volcano plot revealed a significant difference in the expression
of miR-590-5p in A375 cells compared with the controls (>4-fold
change; P<0.0001; Fig. 1B). The
significant downregulation of miR-590-5p was further confirmed
using RT-qPCR in MM cell lines compared with HEMa-LP (P<0.05;
Fig. 1C).
 | Table II.Established miRNAs detected in the
present study. |
Table II.
Established miRNAs detected in the
present study.
| miRNA name | Function | PMID |
|---|
| miR-590-5p | Tumor
suppressor | 28433598 |
| miR-663 | Oncogene | 28765921 |
| miR-33a | Tumor
suppressor | 28763799 |
| miR-137 | Tumor
suppressor | 28757416 |
| miR-30a | Tumor
suppressor | 28757413 |
| miR-200c | Tumor
suppressor | 28727734 |
| miR-378 | Tumor
suppressor | 28725241 |
| miR-7 | Tumor
suppressor | 28693382 |
| miR-215 | Tumor
suppressor | 28693279 |
| miR-195 | Tumor
suppressor | 28693232 |
| miR-30a-5p | Tumor
suppressor | 28672911 |
| miR-874 | Tumor
suppressor | 28670493 |
| miR-1180 | Tumor
suppressor | 28670370 |
| miR-136 | Tumor
suppressor | 28656883 |
| miR-497 | Tumor
suppressor | 28656286 |
| miR-31 | Tumor
suppressor | 28656284 |
| miR-503 | Tumor
suppressor | 28656281 |
| miR-202 | Tumor
suppressor | 28656198 |
| miR-105-5p | Tumor
suppressor | 28654905 |
| miR-139-5p | Tumor
suppressor | 28653604 |
| miR-539 | Tumor
suppressor | 28653599 |
| miR-30d | Tumor
suppressor | 28651493 |
| miR-493 | Tumor
suppressor | 28651234 |
| miR-491 | Tumor
suppressor | 28648665 |
| miR-337 | Tumor
suppressor | 28641487 |
| miR-26a | Tumor
suppressor | 28640257 |
| miR-199a-3p | Tumor
suppressor | 28639901 |
| miR-557 | Tumor
suppressor | 28639890 |
| miR-195 | Tumor
suppressor | 28639885 |
| miR-455-3p | Tumor
suppressor | 28633632 |
| miR-187 | Tumor
suppressor | 28627639 |
| miR-320 | Tumor
suppressor | 28627594 |
| miR-101 | Tumor
suppressor | 28609840 |
| miR-193a-3p | Tumor
suppressor | 28600480 |
| miR-4728-3p | Tumor
suppressor | 28594651 |
| miR-564 | Tumor
suppressor | 28588702 |
| miR-18a | Tumor
suppressor | 28588697 |
| miR-17-5p | Tumor
suppressor | 28588663 |
| miR-186 | Tumor
suppressor | 28587405 |
| miR-148a | Tumor
suppressor | 28586066 |
| miR-497 | Tumor
suppressor | 28586056 |
| miR-1247-5p | Tumor
suppressor | 28586038 |
| miR-378 | Tumor
suppressor | 28575858 |
| miR-144-3p | Tumor
suppressor | 28574724 |
| miR-211-5p | Tumor
suppressor | 28571042 |
| miR-146a-5p | Tumor
suppressor | 28560455 |
| miR-143-3p | Tumor
suppressor | 28559978 |
| miR-1271 | Tumor
suppressor | 28551819 |
| miR-186 | Tumor
suppressor | 28550686 |
| miR-193b | Tumor
suppressor | 28542597 |
| miR-126 | Tumor
suppressor | 28536606 |
| miR-30b-5p | Tumor
suppressor | 28536082 |
| miR-15a | Oncogene | 28758198 |
| miR-483-5p | Oncogene | 28727371 |
| miR-210-3p | Oncogene | 28693852 |
| miR-193a-3p | Oncogene | 28693273 |
| miR-215 | Oncogene | 28689850 |
| miR-1271 | Oncogene | 28682437 |
| miR-944 | Oncogene | 28680805 |
| miR-138 | Oncogene | 28677784 |
| miR-492 | Oncogene | 28677719 |
| miR-605 | Oncogene | 28673012 |
| miR-661 | Oncogene | 28656235 |
| miR-30e-5p | Oncogene | 28653805 |
| miR-137 | Oncogene | 28610956 |
| miR-96-5p | Oncogene | 28588711 |
| miR-216a | Oncogene | 28579808 |
| miR-142-5p | Oncogene | 28559989 |
| miR-214 | Oncogene | 28559385 |
| miR-141-3p | Oncogene | 28543175 |
| miR-425-5p | Oncogene | 28537672 |
| miR-582 | Oncogene | 28713947 |
| miR-210-3p | Oncogene | 28693582 |
| miR-20a | Oncogene | 28693582 |
| miR-34a | Oncogene | 28599485 |
| miR-146b-5p | Oncogene | 28560062 |
| miR-126 | Oncogene | 28536606 |
| miR-205 | Oncogene | 28476165 |
| miR-18a-5p | Oncogene | 28471447 |
| miR-141 | Oncogene | 28454307 |
| miR-103 | Oncogene | 28445396 |
| miR-556-3p | Oncogene | 28440444 |
| miR-495 | Oncogene | 28401017 |
| miR-221 | Oncogene | 28392366 |
| miR-155 | Oncogene | 28338193 |
| miR-27a | Oncogene | 28327189 |
| miR-181 | Oncogene | 28224609 |
| miR-844 | Oncogene | 28224609 |
| miR-182 | Oncogene | 28122586 |
| miR-125 | Oncogene | 28053194 |
| miR-346 | Oncogene | 27913185 |
| miR-19a | Oncogene | 27830963 |
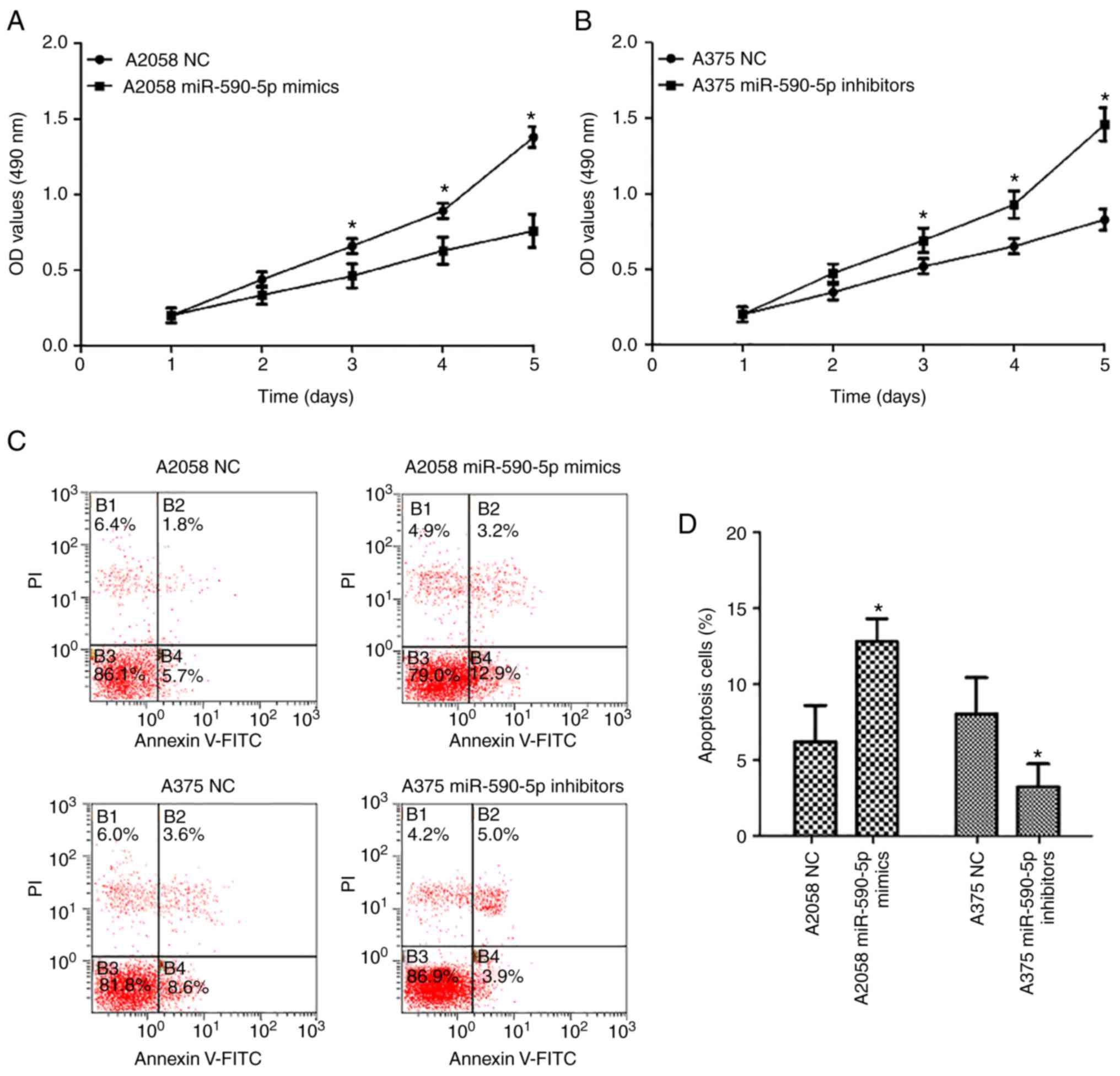
Effect of miR-590-5p on the
proliferation and apoptosis of MM cells
To investigate the functional role of miR-590-5p in
MM cells, gain- and loss-of function experiments were performed by
transfecting miR-590-5p mimics into A2058 cells and miR-590-5p
inhibitors into A375 cells. CCK-8 assays revealed that the
proliferation of A2058 cells transfected with miR-590-5p mimics was
significantly inhibited compared with the normal control
(P<0.05; Fig. 2A). Additionally,
the proliferation of A375 cells transfected with miR-590p-5p
inhibitors was significantly enhanced compared with the normal
control (NC) cells (P<0.05; Fig.
2B). Cell apoptosis assays revealed that the percentages of
early apoptotic cells significantly increased in A2058 cells
transfected with miR-590-5p mimics compared with NCs (P<0.05),
and decreased in A375 cells transfected with miR-590-5p inhibitors
compared with NCs (P<0.05; Fig. 2C
and D).
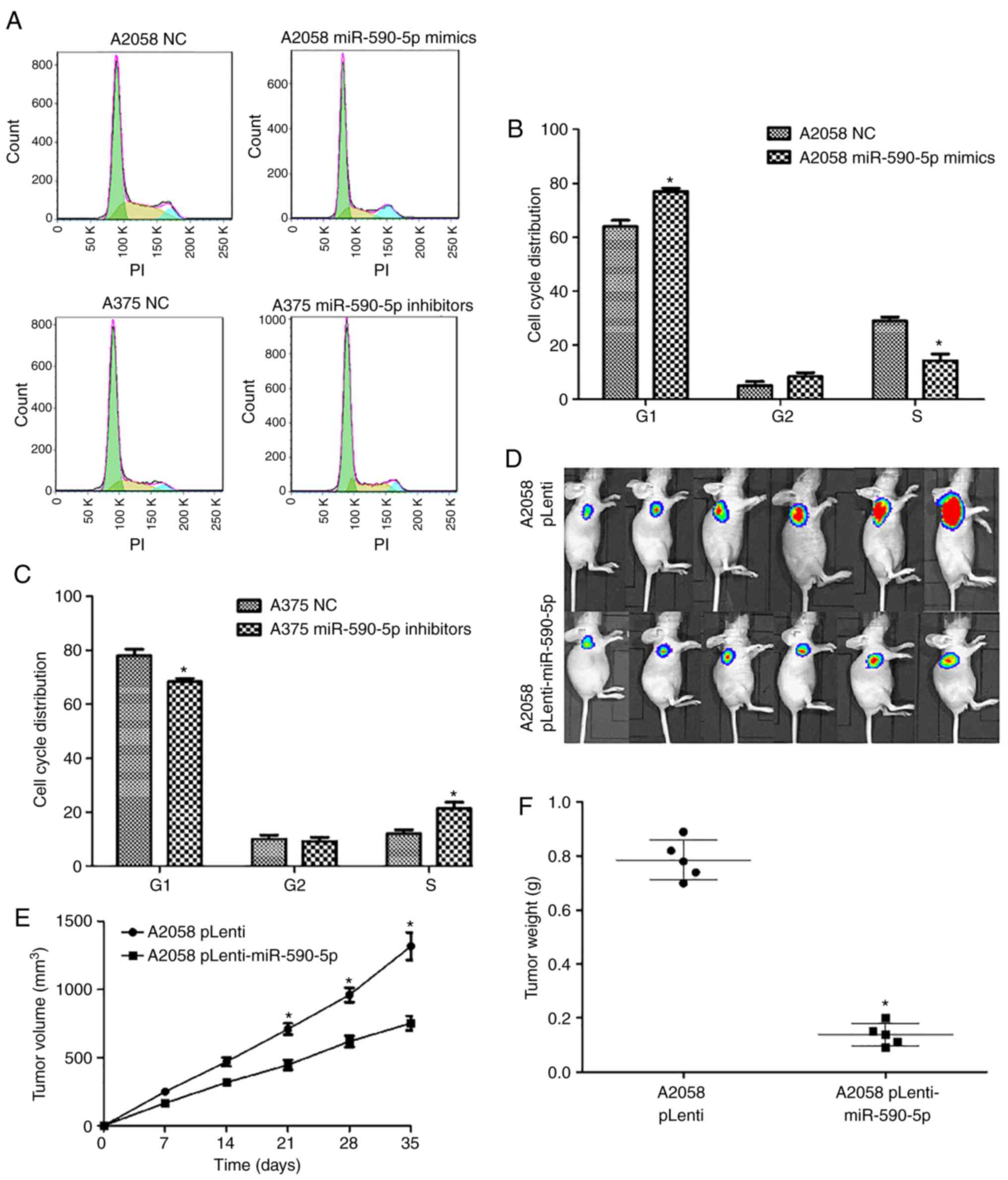
Effects of miR-590-5p on the cell
cycle and tumorigenic ability of MM cells
Flow cytometry assays were performed to determine
the effects of miR-590-5p on the distribution of cells at the
various stages of the cell cycle. Compared to NCs, A2058 cells
transfected with miR-590-5p mimics displayed a significant increase
in the percentage of cells at the G1 stage (P<0.05) and a
significant decrease in the percentage of cells at the S stage
(P<0.05; Fig. 3A and B).
Meanwhile, the proportion of cells at the G1 stage significantly
decreased and the proportion of cells at the S stage significantly
increased in A375 cells transfected with miR-590-5p inhibitors
compared with NCs (P<0.05; Fig.
3C).
Next, the functional roles of miR-590-5p on the
tumorigenic ability of MM cells in vivo were examined. A2058
cells were engineered to stably upregulate miR-590-5p and
luciferase expression and performed tumorigenesis assays in nude
mice. The cells (5×106) were injected into the flanks of
nude mice, and tumor sizes were measured by Xenogen IVIS Kinetic
imaging systems and vernier calipers every 7 days. After 35 days,
the mice were sacrificed and the tumors were collected and weighed.
It was revealed that miR-590-5p exhibited substantial tumor
growth-inhibitory effects as assessed by the Xenogen IVIS200 System
(Fig. 3D). In addition, a
significant reduction of tumor sizes (P<0.05; Fig. 3E) and tumor weight (P<0.05;
Fig. 3F) was observed in the
pLenti-miR-590-5p group compared with the NC group. Altogether
these results indicated miR-590-5p was downregulated in MM cells
and could repress cell proliferation and tumor growth in
vitro and in vivo.
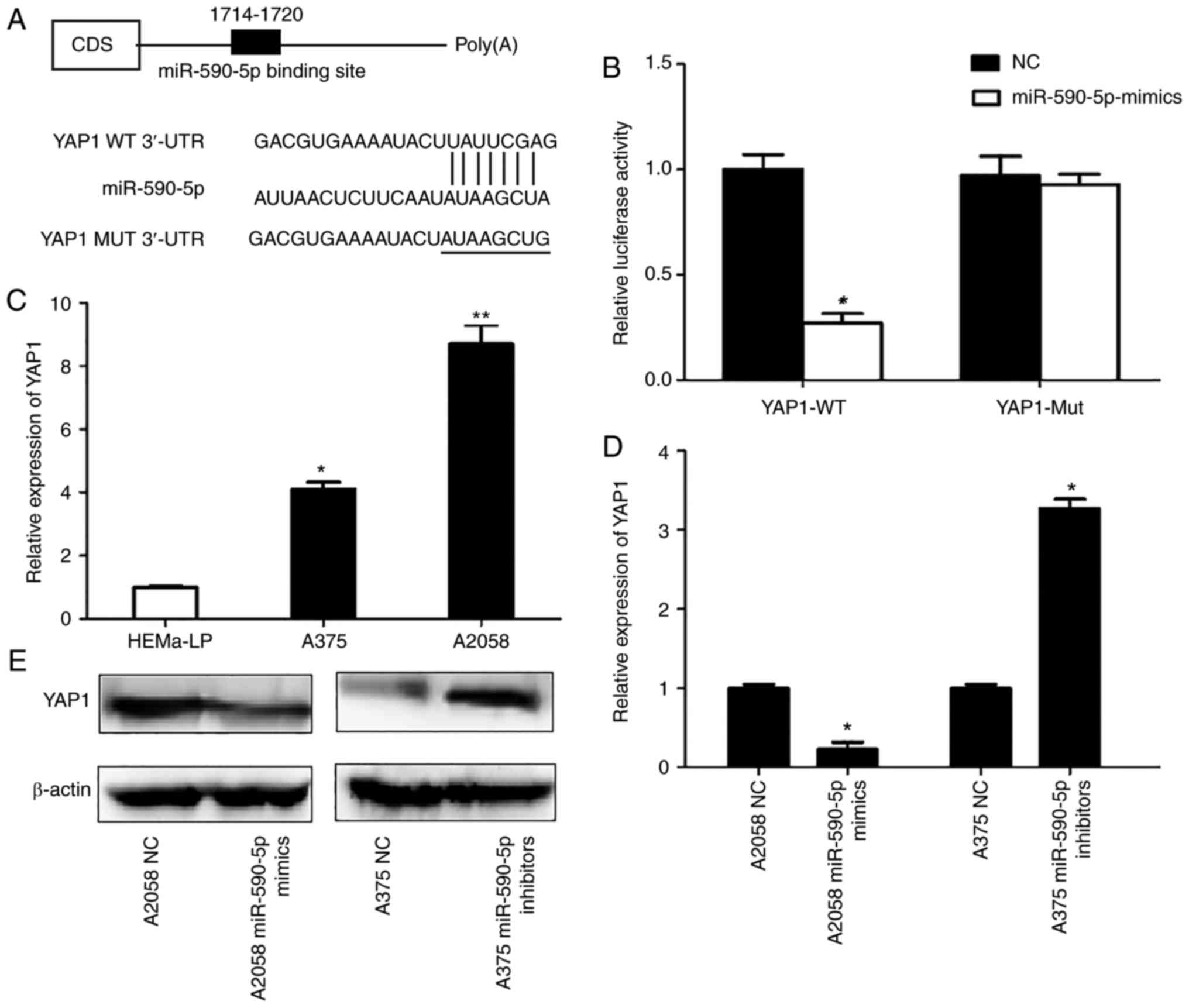
YAP1 is the direct target of
miR-590-5p
To identify the potential targets of miR-590-5p that
may contribute to its tumor growth-inhibitory effects, an unbiased
computational screening was performed by integrating the results of
multiple prediction algorithms (TargetScan, PicTar and miRNAda).
YAP1, which contains a putative miR-590-5p target site, was
selected as a potential target of miR-590-5p in MM cells. YAP1 WT
3′-UTR and Mut 3′-UTR were cloned separately into a luciferase
reporter vector (Fig. 4A).
Luciferase reporter assays demonstrated that 293 cells
co-transfected with YAP1 WT 3′-UTR and miR-590-5p mimics revealed a
>60% significant decrease in the relative luciferase activity
compared with NCs (P<0.05). Conversely, 293 cells co-transfected
with YAP1 Mut 3′-UTR and miR-590-5p resulted in imperceptible
changes in relative luciferase activity compared with the NC
(Fig. 4B).
Next. whether YAP1 expression was inversely
associated with miR-590-5p in MM cells was examined. It was
revealed that YAP1 expression was significantly upregulated in the
two MM cell lines used compared with HEMa-LP cells (P<0.05;
Fig. 4C). Furthermore, RT-qPCR and
western blot results revealed that the mRNA expression levels of
YAP1 were significantly decreased (P<0.05), and the protein
expression of YAP1 decreased in A2058 cells transfected with
miR-590-5p mimics compared with NCs. Conversely, the expression of
YAP1 at the mRNA expression level was significantly upregulated
(P<0.05) and YAP1 protein expression was upregulated in A375
cells transfected with miR-590-5p inhibitors (Fig. 4D and E).
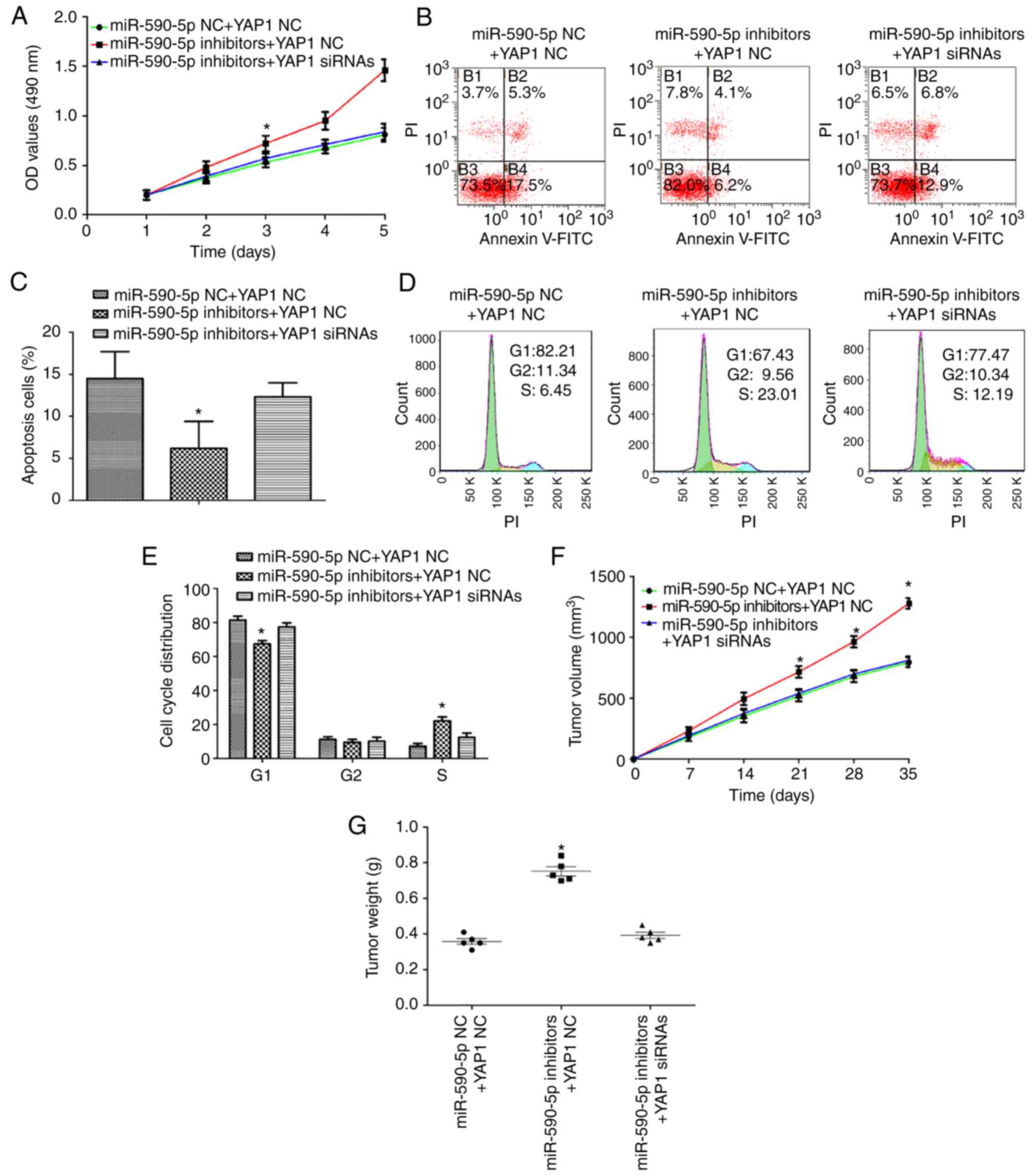
YAP1 is a functional mediator of
miR-590-5p in MM
To determine whether YAP1 is a functional mediator
of miR-590-5p, YAP1 siRNA or NC and miR-590-5p inhibitors were
transfected into A375 cells. As expected, silencing the expression
of YAP1 in A375 cells transfected with miR-590-5p inhibitors
attenuated the effects on cell proliferation (P<0.05; Fig. 5A). In addition, the percentages of
early apoptotic cells were increased in A375 cells transfected with
YAP1 siRNA and miR-590-5p inhibitors compared with A375 cells
transfected with YAP1 NC and miR-590-5p inhibitors (P<0.05;
Fig. 5B and C). It was also
demonstrated that silencing YAP1 may rescue the effects of
miR-590-5p inhibitors on the cell cycle progression of A375 cells
(P<0.05; Fig. 5D and E) in
addition to tumor growth (P<0.05; Fig. 5F and G).
Discussion
In the present study, miR-590-5p was identified to
be downregulated in MM cells by screening established oncogenic and
tumor suppressor miRNAs and RT-qPCR. It was confirmed that
miR-590-5p overexpression is able to significantly inhibit cell
proliferation and induce cell cycle arrest and apoptosis in MM
cells. It was also identified that miR-590-5p may inhibit MM tumor
growth in vivo. Finally, it was demonstrated that YAP1 was
upregulated and inversely associated with miR-590-5p expression in
MM cells and that YAP1 is the direct target and functional mediator
of miR-590-5p in these cells.
Dysregulated miRNAs serve notable roles in the
regulation of carcinogenesis and the progression of multiple types
of cancer (10,27), including MM (11,28,29);
however, the underlying mechanism is poorly understood. Depending
on their different targets, certain miRNAs may function as tumor
suppressors, whilst others function as oncogenes. For example,
miR-21 is upregulated in primary cutaneous melanomas associated
with benign nevi (30,31) and may promote cell invasion by
negatively regulating tissue inhibitor of metalloprotinease-3
(32). Alternatively, miR-125b is
downregulated in the sera of patients with MM and MM cells compared
with healthy volunteers and human epidermal melanocytes,
respectively (33–35). In the present study, it was
demonstrated that miR-590-5p was downregulated in MM cells. As it
was revealed that miR-590-5p inhibited proliferation and induced
apoptosis and cell cycle arrest in MM cells, miR-590-5p may
function as a tumor suppressor gene in MM.
Previously, it was reported that miR-590-5p was
downregulated and functions as a tumor suppressor gene in various
cancer types, including colorectal cancer (36,37)
and breast cancer (20). Meanwhile,
miR-590-5p was demonstrated to be upregulated and function as an
oncogene in cervical cancer (38),
clear cell renal carcinoma (39)
and gastric cancer (40). In
hepatocellular carcinoma, there have been conflicting reports
concerning the expression and function of miR-590-5p. Shan et
al (41) reported that
miR-590-5p was downregulated in six hepatocellular carcinoma cell
lines, inhibited cell growth, induced cell cycle G1 arrest in HepG2
cells by suppressing Wnt family member 5α, c-Myc, and cyclin D1 and
increasing the phosphorylation of β-catenin and the expression of
caspase-3. On the other hand, Jiang et al (42) demonstrated that miR-590-5p levels
were higher in HepG2 cells compared with the normal hepatocellular
cell line L-O2, and functioned as an oncogene to promote the tumor
proliferation and invasion of hepatocellular carcinoma cells by
directly targeting TGF-βRII. The results of the present study
support the tumor suppressor role of miR-590-5p in MM in the
following ways: Firstly, miR-590-5p was downregulated in MM cell
lines; secondly, miR-590-5p exerted anti-tumor effects in
vitro and in vivo; and third, YAP1-which was identified
as an oncogene and upregulated in various types of cancer (43,44)-was confirmed as the direct target and
functional mediator of miR-590-5p in MM cells.
YAP1 is the key downstream effector of Hippo
pathways (45). YAP1 functions as
an oncogene and modulates numerous biological phenotypes of cancer
cells, including proliferation (46), invasion (47), cell cycle progression (48) and cell differentiation (49). YAP1 was identified to be
overexpressed in numerous types of cancer, and is an independent
prognostic predictor of cancer (50,51).
In MM, YAP1-enhanced tumor progression and metastasis through
interacting with the TEA domain transcription factor/TEF, PAR BZIP
transcription factor family of transcription factors (52). Furthermore, the high expression of
YAP1 was significantly associated with the poor outcome of patients
with MM (53). Gene variants of
YAP1 also proved to be independently associated with survival in
patients with cutaneous melanoma (54). Consistent with these previous
studies, the present study revealed that YAP1 was upregulated in MM
cells. Furthermore, YAP1 was identified to be the direct target and
functional mediator of miR-590-5p in MM cells.
It was previously reported miR-590-5p may inhibit
the migration and invasion of cancer cells via the suppression of
YAP1 expression (21). The focus of
this previous study was the effects of miR-590-5p on the migration
and invasion of A375 cells. Though it provided preliminarily
evidence that the functions of miR-590-5p on the migration and
invasion of A375 cells may be mediated by YAP1, this hypothesis was
not confirmed through the use of rescue experiments. In the present
study, the roles of miR-590-5p on the proliferation of A375 and
A2058 cells were investigated in detail. Rescue experiments were
also performed to confirm that the effect of miR-590-5p on the
proliferation of MM cells was mediated by YAP1. It was revealed
that silencing YAP1 is able rescue the effects of miR-590-5p
inhibitors on the cell cycle and tumor growth of A375 cells.
Therefore, in addition to the results of the previous study,
present understanding of the role of YAP1 in the carcinogenesis and
progression of MM was furthered.
In conclusion, the present study provided evidence
that miR-590-5p directly inhibits YAP1 to reduce the proliferation
and induce apoptosis of MM cells in vitro and is able to
suppress tumor growth in vivo. Enhanced understanding of the
process including cell proliferation and apoptosis that are
regulated by miR-590-5p, and the identification of critical targets
for miR-590-5p including YAP1, provides novel insight into the
mechanism of carcinogenesis and progression in MM.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Foundation of
the First Affiliated Hospital of Xi'an Jiaotong University (grant
no. 2016QN-06), the Fundamental Research Fund for Central
Universities (grant no. xjj2018122) and the Shaanxi Nature Science
Foundation (grant no. 2018JQ8027).
Avaliability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author contributions
LW conceived and designed the experiments. KM, MD
and DH performed the experiments. XM and YZ analyzed the data. WL
contributed reagents, materials and analysis tools. KM and LW wrote
the manuscript.
Ethics approval and consent to
participate
The experimental study was approved by the Ethics
Committee of Xi'an Jiaotong University (Xi'an, China), and informed
consent forms were obtained when the patients who received
circumcision at the First Affiliated Hospital of Xi'an Jiaotong
University were accepted for the study. All procedures performed in
the study involving human foreskin specimens were in accordance
with the ethical standards of the Institutional and/or National
Research Committee and with the 1964 Helsinki declaration and its
later amendments or comparable ethical standards. All the patients
enrolled in this study provided written informed consent for the
use of their excised foreskin.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MM
|
malignant melanoma
|
|
miRNA/miR
|
microRNA
|
|
3′-UTRs
|
3′-untranslated regions
|
|
HM
|
human melanocytes
|
|
YAP1
|
Yes-associated protein 1
|
References
|
1
|
Coricovac D, Dehelean C, Moaca EA, Pinzaru
I, Bratu T, Navolan D and Boruga O: Cutaneous melanoma-A long road
from experimental models to clinical outcome: A review. Int J Mol
Sci. 19:E15662018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roukos DH: PLX4032 and melanoma:
Resistance, expectations and uncertainty. Expert Rev Anticancer
Ther. 11:325–328. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsai J, Lee JT, Wang W, Zhang J, Cho H,
Mamo S, Bremer R, Gillette S, Kong J, Haass NK, et al: Discovery of
a selective inhibitor of oncogenic B-Raf kinase with potent
antimelanoma activity. Proc Natl Acad Sci USA. 105:3041–3046. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eggermont AM, Spatz A and Robert C:
Cutaneous melanoma. Lancet. 383:816–827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adams BD, Parsons C, Walker L, Zhang WC
and Slack FJ: Targeting noncoding RNAs in disease. J Clin Invest.
127:761–771. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moreno-Moya JM, Vilella F and Simón C:
MicroRNA: Key gene expression regulators. Fertil Steril.
101:1516–1523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget.
6:8474–8490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou K, Liu M and Cao Y: New insight into
microRNA functions in cancer: Oncogene-microRNA-tumor suppressor
gene network. Front Mol Biosci. 4:462017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wozniak M, Mielczarek A and Czyz M: miRNAs
in melanoma: Tumor suppressors and oncogenes with prognostic
potential. Curr Med Chem. 23:3136–3153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z, Zheng C, Jiang K, He J, Cao X and
Wu S: MicroRNA-503 suppresses cell proliferation and invasion in
osteosarcoma via targeting insulin-like growth factor 1 receptor.
Exp Ther Med. 14:1547–1553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu L, Xu Q, Li X and Zhang X: MicroRNA-21
regulates the proliferation and apoptosis of cervical cancer cells
via tumor necrosis factor-α. Mol Med Rep. 16:4659–4663. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Markopoulos GS, Roupakia E, Tokamani M,
Vartholomatos G, Tzavaras T, Hatziapostolou M, Fackelmayer FO,
Sandaltzopoulos R, Polytarchou C and Kolettas E:
Senescence-associated microRNAs target cell cycle regulatory genes
in normal human lung fibroblasts. Exp Gerontol. 96:110–122. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin W, Rong X, Dong J, Yu C and Yang J:
miR-142 inhibits the migration and invasion of glioma by targeting
Rac1. Oncol Rep. 38:1543–1550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo W, Wang H, Yang Y, Guo S, Zhang W, Liu
Y, Yi X, Ma J, Zhao T, Liu L, et al: Down-regulated miR-23a
contributes to the metastasis of cutaneous melanoma by promoting
autophagy. Theranostics. 7:2231–2249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bai M, Zhang M, Long F, Yu N, Zeng A and
Zhao R: Circulating microRNA-194 regulates human melanoma cells via
PI3K/AKT/FoxO3a and p53/p21 signaling pathway. Oncol Rep.
37:2702–2710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo S, Guo W, Li S, Dai W, Zhang N, Zhao
T, Wang H, Ma J, Yi X, Ge R, et al: Serum miR-16: A potential
biomarker for predicting melanoma prognosis. J Invest Dermatol.
136:985–993. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang X and Wu X: miRNA expression profile
of vulvar squamous cell carcinoma and identification of the
oncogenic role of miR-590-5p. Oncol Rep. 35:398–408. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou L, Zhao LC, Jiang N, Wang XL, Zhou
XN, Luo XL and Ren J: MicroRNA miR-590-5p inhibits breast cancer
cell stemness and metastasis by targeting SOX2. Eur Rev Med
Pharmacol Sci. 21:87–94. 2017.PubMed/NCBI
|
|
21
|
Wang L, Shi S, Zhou Y, Mu X, Han D, Ge R
and Mu K: miR-590-5p inhibits A375 cell invasion and migration in
malignant melanoma by directly inhibiting YAP1 expression. Xi Bao
Yu Fen Zi Mian Yi Xue Za Zhi. 33:326–330. 2017.(In Chinese).
PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Workman P, Aboagye EO, Balkwill F, Balmain
A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA,
Glennie MJ, et al: Guidelines for the welfare and use of animals in
cancer research. Br J Cancer. 102:1555–1577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 2015. View Article : Google Scholar
|
|
25
|
Chen K and Rajewsky N: Natural selection
on human microRNA binding sites inferred from SNP data. Nat Genet.
38:1452–1456. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Enright AJ, John B, Gaul U, Tuschl T,
Sander C and Marks DS: MicroRNA targets in Drosophila. Genome Biol.
5:R12003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Legras A, Pécuchet N, Imbeaud S, Pallier
K, Didelot A, Roussel H, Gibault L, Fabre E, Le Pimpec-Barthes F,
Laurent-Puig P and Blons H: Epithelial-to-mesenchymal transition
and MicroRNAs in lung cancer. Cancers. 9:E1012017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Varamo C, Occelli M, Vivenza D, Merlano M
and Nigro Lo C: MicroRNAs role as potential biomarkers and key
regulators in melanoma. Genes Chromosomes Cancer. 56:3–10. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng Z, Hao J, Lei D, He Y, Lu L and He L:
Pivotal MicroRNAs in melanoma: A mini-review. Mol Diagn Ther.
20:449–455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grignol V, Fairchild ET, Zimmerer JM,
Lesinski GB, Walker MJ, Magro CM, Kacher JE, Karpa VI, Clark J,
Nuovo G, et al: miR-21 and miR-155 are associated with mitotic
activity and lesion depth of borderline melanocytic lesions. Br J
Cancer. 105:1023–1029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Satzger I, Mattern A, Kuettler U,
Weinspach D, Niebuhr M, Kapp A and Gutzmer R: microRNA-21 is
upregulated in malignant melanoma and influences apoptosis of
melanocytic cells. Exp Dermatol. 21:509–514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
del Campo Martin SE, Latchana N, Levine
KM, Grignol VP, Fairchild ET, Jaime-Ramirez AC, Dao TV, Karpa VI,
Carson M, Ganju A, et al: MiR-21 enhances melanoma invasiveness via
inhibition of tissue inhibitor of metalloproteinases 3 expression:
In vivo effects of MiR-21 inhibitor. PloS One. 10:e01159192015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang J, Lu L, Xiong Y, Qin W, Zhang Y,
Qian Y, Jiang H and Liu W: MLK3 promotes melanoma proliferation and
invasion and is a target of microRNA-125b. Clin Exp Dermatol.
39:376–384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kappelmann M, Kuphal S, Meister G,
Vardimon L and Bosserhoff AK: MicroRNA miR-125b controls melanoma
progression by direct regulation of c-Jun protein expression.
Oncogene. 32:2984–2991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alegre E, Sanmamed MF, Rodriguez C,
Carranza O, Martin-Algarra S and Gonzalez A: Study of circulating
microRNA-125b levels in serum exosomes in advanced melanoma. Arch
Pathol Lab Med. 138:828–832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ou C, Sun Z, Li X, Li X, Ren W, Qin Z,
Zhang X, Yuan W, Wang J, Yu W, et al: MiR-590-5p, a
density-sensitive microRNA, inhibits tumorigenesis by targeting
YAP1 in colorectal cancer. Cancer Lett. 399:53–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou Q, Zhu Y, Wei X, Zhou J, Chang L, Sui
H, Han Y, Piao D, Sha R and Bai Y: MiR-590-5p inhibits colorectal
cancer angiogenesis and metastasis by regulating nuclear factor
90/vascular endothelial growth factor A axis. Cell Death Dis.
7:e24132016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chu Y, Ouyang Y, Wang F, Zheng A, Bai L,
Han L, Chen Y and Wang H: MicroRNA-590 promotes cervical cancer
cell growth and invasion by targeting CHL1. J Cell Biochem.
115:847–853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xiao X, Tang C, Xiao S, Fu C and Yu P:
Enhancement of proliferation and invasion by MicroRNA-590-5p via
targeting PBRM1 in clear cell renal carcinoma cells. Oncol Res.
20:537–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shen B, Yu S, Zhang Y, Yuan Y, Li X, Zhong
J and Feng J: miR-590-5p regulates gastric cancer cell growth and
chemosensitivity through RECK and the AKT/ERK pathway. Onco Targets
Ther. 9:6009–6019. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shan X, Miao Y, Fan R, Qian H, Chen P, Liu
H, Yan X, Li J and Zhou F: MiR-590-5P inhibits growth of HepG2
cells via decrease of S100A10 expression and inhibition of the Wnt
pathway. Int J Mol Sci. 14:8556–8569. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang X, Xiang G, Wang Y, Zhang L, Yang X,
Cao L, Peng H, Xue P and Chen D: MicroRNA-590-5p regulates
proliferation and invasion in human hepatocellular carcinoma cells
by targeting TGF-β RII. Mol Cells. 33:545–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zanconato F, Cordenonsi M and Piccolo S:
YAP/TAZ at the Roots of Cancer. Cancer Cell. 29:783–803. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zanconato F, Battilana G, Cordenonsi M and
Piccolo S: YAP/TAZ as therapeutic targets in cancer. Curr Opin
Pharmacol. 29:26–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shibata M, Ham K and Hoque MO: A time for
YAP1: Tumorigenesis, immunosuppression and targeted therapy. Int J
Cancer. Apr 26–2018.(Epub ahead of print). doi: 10.1002/ijc.31561.
View Article : Google Scholar
|
|
46
|
Seo WI, Park S, Gwak J, Ju BG, Chung JI,
Kang PM and Oh S: Wnt signaling promotes androgen-independent
prostate cancer cell proliferation through up-regulation of the
hippo pathway effector YAP. Biochem Biophys Res Commun.
486:1034–1039. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee HJ, Diaz MF, Price KM, Ozuna JA, Zhang
S, Sevick-Muraca EM, Hagan JP and Wenzel PL: Fluid shear stress
activates YAP1 to promote cancer cell motility. Nat Commun.
8:141222017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Strnadel J, Choi S, Fujimura K, Wang H,
Zhang W, Wyse M, Wright T, Gross E, Peinado C, Park HW, et al:
eIF5A-PEAK1 signaling regulates YAP1/TAZ protein expression and
pancreatic cancer cell growth. Cancer Res. 77:1997–2007. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fan Y, Gao Y, Rao J, Wang K, Zhang F and
Zhang C: YAP-1 promotes tregs differentiation in hepatocellular
carcinoma by enhancing TGFBR2 transcription. Cell Physiol Biochem.
41:1189–1198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee K, Lee KB, Jung HY, Yi NJ, Lee KW, Suh
KS and Jang JJ: The correlation between poor prognosis and
increased yes-associated protein 1 expression in keratin 19
expressing hepatocellular carcinomas and cholangiocarcinomas. BMC
Cancer. 17:4412017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hu X, Chen J and Fu Q: Downregulation of
YAP in clear cell renal cell carcinoma contributes to poor
prognosis and progressive features. Ann Clin Lab Sci. 47:36–39.
2017.PubMed/NCBI
|
|
52
|
Lamar JM, Stern P, Liu H, Schindler JW,
Jiang ZG and Hynes RO: The Hippo pathway target, YAP, promotes
metastasis through its TEAD-interaction domain. Proc Natl Acad Sci
USA. 109:E2441–E2450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Menzel M, Meckbach D, Weide B, Toussaint
NC, Schilbach K, Noor S, Eigentler T, Ikenberg K, Busch C,
Quintanilla-Martinez L, et al: In melanoma, Hippo signaling is
affected by copy number alterations and YAP1 overexpression impairs
patient survival. Pigment Cell Melanoma Res. 27:671–673. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yuan H, Liu H, Liu Z, Zhu D, Amos CI, Fang
S, Lee JE and Wei Q: Genetic variants in Hippo pathway genes YAP1,
TEAD1 and TEAD4 are associated with melanoma-specific survival. Int
J Cancer. 137:638–645. 2015. View Article : Google Scholar : PubMed/NCBI
|