Introduction
Bladder cancer is a malignancy with the highest
incidence in the urinary system; 79,030 Americans were diagnosed
with bladder cancer in 2017, and 16,870 of them will succumb to the
disease (1). Urothelial carcinoma
is the most common pathologic type of bladder cancer, and
muscle-invasive bladder cancer (MIBC) is a commonly occurring
disease with a high mortality rate despite comprehensive treatment
(2–7). Patients with MIBC are at risk for
local invasion and distant metastasis, and are usually diagnosed at
advanced stages and have a poor prognosis (2–7). With
the increasing number of patients and the growing complexity of
MIBC, biomarkers are required urgently to predict tumor
progression, yet the development of practical and useful biomarkers
for prognostication has been stagnated even further (4–7).
B7-H3 (B7 homologue 3), which has been recently
discovered as a novel member of the B7 family molecules, plays an
immuno-regulatory role between tumor and immune cells (8,9). B7-H3
is widely expressed in urologic tumors as well as in other human
malignancies, and it is closely related to tumor progression,
metastasis, recurrence and other adverse clinical features
(10–19). Currently, the impact of B7-H3 on
cancer progression has received increasing attention, although its
co-stimulatory or co-inhibitory effect remains contentious
(16–28). However, the role of B7-H3 on tumor
progression in MIBC is not clear.
In the present study, we focused on the association
between the expression level of B7-H3 and the malignant progression
and poor survival in MIBC patients with 10 years follow up, and we
analyzed their associations with various clinicopathological
characteristics. Survival analysis was performed to determine the
prognostic significance of B7-H3 expression on postoperative
survival of patients and to evaluate potential effects on the
progression of MIBC. Moreover, our study showed that suppression of
B7-H3 inhibited proliferation, migration and invasion in
vitro and tumor growth in vivo, and provided some
significant findings for targeted therapy.
Materials and methods
Specimen collection
Following Institutional Review Board's approval, the
retrospective study enrolled 115 MIBC patients undergoing standard
radical cystectomy in the Department of Urology, Fourth Hospital of
Hebei Medical University (Hebei, China), between 2005 and 2006.
Patients were classified according to the 2009 UICC TNM staging as
well as in compliance with 2004 WHO/ISUP classification (2–5). All
patients had no history of preoperative radiotherapy and/or
chemotherapy, nor neoadjuvant chemotherapy. The participants
provided their written informed consents. All patients did not
manifest signs of tumor metastasis, as evidenced by cross-sectional
imaging.
Ethics statement
Human samples and animals in this study were
approved by the Ethics Committee of the Fourth Hospital of Hebei
Medical University. The research involving human participants was
approved by the Fourth Hospital of Hebei Medical University and the
equivalent committee. The participants provided written informed
consent before enrollment in this study. The consent forms were not
be provided in this article due to the large number and they were
all written in the Chinese language. The animal study was conducted
with the approval of the Animal Care and Use Committee of the
Fourth Hospital of Hebei Medical University.
Immunohistochemistry and scoring
Specimens were fixed using formalin, followed by
paraffin embedding and were sliced into 5-µm sections. The latter
were dewaxed by xylene and dehydrated with gradient ethanol, heated
in 1 mmol/l EDTA (pH 8.0) to 121°C, cooled to 90°C, and incubated
for 5 min. Then, the sections were immerged into 3%
H2O2 deionized water for 10 min to eliminate
endogenous peroxidase activity and sealed with goat serum (cat. no.
ZLI-9021; ZSGB-BIO, Beijing, China) for 30 min. After that, the
immunohistochemistry for B7-H3 was carried out on consecutive
sections according to standard pathologic procedures, with the
primary mouse anti-human B7-H3 antibody (1:500 dilution; cat. no.
ab105922; Abcam, Cambridge, UK) and goat anti-mouse IgG (cat. no.
ZB-2305; ZSGB-BIO), then it was dripped with diaminobenzidine (DAB)
for rendering for 3–5 min. Next, sections were then counterstained
with hematoxylin, dehydrated in ethanol, cleared in xylene and
coverslipped. PBS was used to replace the primary antibody as the
negative control.
The scoring method of B7-H3 expression was based on
the stained area and intensity of staining (20). Quantification was made as follows:
Negative/weak, moderate, and strong intensity of B7-H3 expression.
Sections with negative/weak intensity were classified as showing
low B7-H3 expression, whereas sections with moderate and strong
intensity were categorized as high B7-H3 expression (20).
Cell lines and cell culture
The human bladder cancer T24 and 5637 cell lines
were obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China) and cultured according to the instructions from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
These cells were maintained in RPMI-1640 medium supplemented with
10% fetal calf serum (Gibco; 4 weeksc, Inc., Waltham, MA, USA) and
incubated at 37°C in 5% CO2.
Generation of stable cell line
The human B7-H3 (gene ID: 80381) targeting small
hairpin (sh) RNA sequence 5′-TCGTGTGCTGGAGAAAGATCAAACAGAGC-3′
(LV-B7-H3-RNAi) and an OnTargeted and control sequence
5′-GCACTACCAGAGCTAACTCAGATAGTACT-3′ (control shRNA) were used to
generate recombinant lentivirus (10) (purchased from GeneChem Co., Ltd.,
Shanghai, China). As the carrier, recombinant lentivirus GV248 was
titered to 1×108 TU/ml, and the multiplicity of
infection (MOI) was 20. The lentivirus vector was able to express
enhanced green fluorescent protein (EGFP). Reverse
transcription-polymerase chain reaction (RT-PCR), western blotting,
viability and invasion assays were performed to confirm the effect
of B7-H3 silencing.
Gene expression of B7-H3, RT-PCR, and
western blot analysis
Total RNA was extracted and reverse transcribed to
produce cDNA. Forward and reverse primers were as follows:
5′-CCCACAGGTTGCTTTGCTTAA-3′ and 5′-GCAGACCCCTGGAGAACCA-3′ (B7-H3);
5′-CAGCTCACCATGGATGATGATATC-3′ and 5′-AGCCGGCCTTGCACAT-3′
(β-actin). The PCR conditions were 94°C for 2 min, then 36 cycles
or 28 cycles (B7-H3: 36 cycles; β-actin: 28 cycles) at 94°C for 30
sec, 56°C or 59°C (B7-H3: 56°C; β-actin: 59°C) for 30 sec, 72°C for
30 sec, and finally 72°C for 2 min. The nuclear and cytoplasmic
protein was extracted and concentrated and then determined using
the bicinchoninic acid (BCA) method. A total of 50 µg of protein
was transferred onto nitrocellulose filter membranes after 10%
SDS-PAGE. The membranes were blocked and incubated with primary
antibodies, including rabbit anti-B7-H3 (1:200 dilution; cat. no.
ab134161; Abcam, Cambridge, UK), mouse anti-β-actin (1:1,000
dilution; cat. no. ab8226; Abcam), rabbit anti-Ki67 (1:1,000
dilution; cat. no. ab16667; Abcam), mouse anti-proliferating cell
nuclear antigen (PCNA) (1:500 dilution; cat. no. ab29; Abcam),
mouse anti-MMP2 (1:1,000 dilution; cat. no. ab37150; Abcam), and
mouse anti-MMP9 (1:1,000 dilution; cat. no. ab38898; Abcam)
monoclonal antibodies overnight at 4°C, and then incubated with the
secondary antibody (polyclonal goat anti-rabbit cat. no.
605-457-013/mouse cat. no. 605-301-002; 1:10,000 dilution)
(Rockland Immunochemicals Inc., Limerick, PA, USA) for 1 h.
Finally, the gray values were analyzed using Odyssey V3.0 software
(http://www.filedudes.com/Odyssey_Browser-download-175211.html).
Cell proliferation
Colony formation assay was used to test the ability
of proliferation. For this assay, 6-well plates were used to seed
cell suspensions (300 cells/well). After incubation, cells were
fixed in methyl hydrate for 10 min. Then the colonies were stained
with Crystal violet and counted using an optical microscope
(ContourGT-K 3D Optical Microscope; Brook Technology Co., Ltd.,
Beijing, China).
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) assay
MTT assay was also used to observe and compare cell
proliferative ability. A total of 2×103 cells were
plated into a well of 96-well plates, and 10 µl of 5 mg/ml MTT was
added into each well and continued to culture for 4 h. Then after
dimethyl sulfoxide addition, the plates were placed on a microplate
autoreader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Optical
density was read at 570 nm wavelength and cell growth curves were
determined according to the optical density value.
Cell cycle analysis
Cells from the two different groups as previously
described were at serum starvation for 24 h and then digested by
trypsin followed by washing with PBS buffer. After that, the cells
were fixed with 75% ethanol at 4°C overnight. After the
centrifugation was performed at 300 × g for 5 min, 1 ml 1X PBS was
used for re-suspension. RNase A (0.5 ml) was then applied to the
re-suspended cells for 20 min and subsequently stained with 1 mM PI
at 37°C for 15 min. Finally, cell cycle distribution of the
different groups was detected by flow cytometry (Becton-Dickinson;
BD Biosciences, San Jose, CA, USA) and cell cycle distribution was
analyzed with Summit 4.3 software (Beckman Coulter, Inc., Brea, CA,
USA).
Cell scratch assays
Cells were seeded to full confluency in 6-well
plates. A scratch was introduced in the middle of each well using a
sterile pipette tip. The medium was discarded and replaced with a
fresh one. The rate of migration towards the center of the wound
was determined at the indicated time points using vernier caliper
(72 h).
Cell invasion assays
The invasion assays were performed with an 8.0-µm
pore inserts in a 24-well Transwell chambers (Corning Costar Inc.,
Corning, NY, USA). For this assay, 2×105 cells were
isolated and added to the upper chamber of a Transwell coated with
Matrigel (BD Bioscience, Mountain View, CA, USA). RPMI-1640 medium
with 10% FBS was added to the lower chamber and incubated for 24 h.
Cells that had migrated to the bottom of the filter were stained
with a three-step stain set (Thermo Fisher Scientific, Inc.,
Manassas, VA, USA). The cells in each chamber were counted under a
ContourGT-K 3D optical microscope (Brook Technology Co., Ltd.).
Xenograft assays in vivo
Sixteen female Athymic BALB/c nude mice (age, 5
weeks; weight, 20 g) were provided by Slac Laboratory Animal Co.,
Ltd. (Shanghai, China), and they were housed in a pathogen-free
animal facility at 20°C under an independent filtration airtight
fume hood with 12-h light/12-h dark cycle with access to distilled
food and water ad libitum, and randomly assigned to groups
(8 mice/group). A total of 2×106 cells were injected
subcutaneously into nude mice and the tumor volume was then
measured. Mice were divided into two groups which were treated
differently using T24/control cells and T24/B7-H3 shRNA cells. The
tumors were measured by Vernier caliper on day 14, 17, 21, 23, 26
and 29. Twenty-nine days after inoculation, the mice were
sacrificed by cervical dislocation, and the final volume of tumor
tissues was determined. The following formula was used for
calculating tumor volume (V): V (mm3) = tumor length
(mm) × tumor width (mm)2/2. To investigate the
correlation between B7-H3 and tumor metastasis, T24/control cells
and T24/B7-H3 shRNA cells were injected into the tail vein of mice,
respectively. After 4 weeks, metastasis in the lung was detected
and the tumors were harvested. The animal study was conducted with
approval of the Animal Care and Use Committee of the Fourth
Hospital of Hebei Medical University. All applicable international,
national, and/or institutional guidelines for the care and use of
animals were followed. Animal surgery was performed with care to
alleviate pain.
Statistical analysis
Data were analyzed with SPSS 22.0 software (IBM
Corp., Armonk, NY). For the immunohistochemistry experiments,
associations between B7-H3 expression level and the
clinicopathological features were evaluated using χ2
tests. Associations of survival and tumor progression with B7-H3
expression were estimated by Kaplan-Meier method and log-rank
tests. For in vitro and in vivo experiments using T24
cells, data are shown as the mean ± standard deviation (SD) of 3
repetitions. Student's t-test was used for statistical comparisons.
A value of P<0.05 was assigned to indicate statistical
significance.
Results
Associations between B7-H3 expression
level and clinicopathological features in MIBC patients
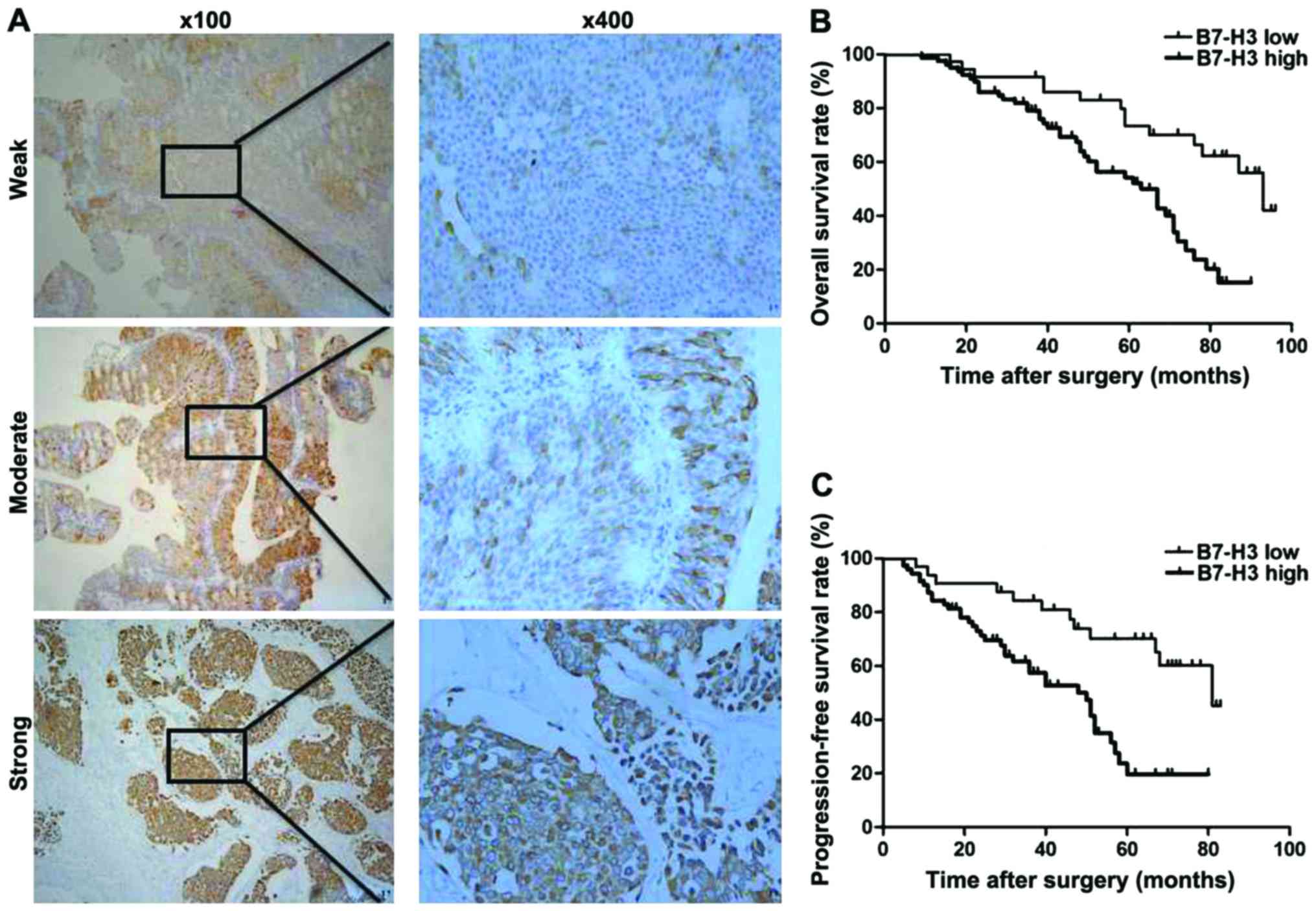
Weak, moderate and strong intensity of B7-H3
expression are shown in Fig. 1. In
addition, high B7-H3-positive expression was found in 79/115
(68.7%) cases, and low B7-H3 expression was observed in 36/115
(31.3%) cases (Table I). High B7-H3
expression was significantly associated with distant metastasis
(P=0.014) and vascular invasion (P=0.031) compared to the
low-expression group. No significant associations were identified
between B7-H3 expression and other pathological factors, including
sex, age, tumor grade, tumor stage, recurrence and lymph node
metastasis (all P>0.05, Table
I).
 | Table I.Association of B7-H3 expression and
clinicopathological characteristics of the patients with MIBC
(N=115). |
Table I.
Association of B7-H3 expression and
clinicopathological characteristics of the patients with MIBC
(N=115).
|
|
| B7-H3
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Features | No. of
patients | Low n=36 | High n=79 | χ2 | P-value |
|---|
| Age (years) |
|
|
| 3.565 | 0.059 |
|
<65 | 46 | 19 | 27 |
|
|
|
≥65 | 69 | 17 | 52 |
|
|
| Sex |
|
|
| 0.344 | 0.558 |
|
Male | 99 | 32 | 67 |
|
|
|
Female | 16 | 4 | 12 |
|
|
| Tumor stage |
|
|
| 3.789 | 0.052 |
| T2 | 31 | 14 | 17 |
|
|
|
T3/T4 | 84 | 22 | 62 |
|
|
| Tumor grade |
|
|
| 1.981 | 0.159 |
|
Low | 23 | 10 | 13 |
|
|
|
High | 92 | 26 | 66 |
|
|
| Lymph node
metastasis |
|
|
| 1.669 | 0.196 |
|
Yes | 35 | 8 | 27 |
|
|
| No | 80 | 28 | 52 |
|
|
| Recurrence |
|
|
| 0.713 | 0.398 |
|
Yes | 61 | 17 | 44 |
|
|
| No | 54 | 19 | 35 |
|
|
| Distant
metastasis |
|
|
| 6.038 | 0.014 |
|
Yes | 48 | 9 | 39 |
|
|
| No | 67 | 27 | 40 |
|
|
| Vascular
invasion |
|
|
| 4.675 | 0.031 |
|
Yes | 74 | 17 | 54 |
|
|
| No | 41 | 19 | 25 |
|
|
Associations between B7-H3 expression
level and clinical outcomes in MIBC patients
In our 10 year follow-up study, Kaplan-Meier
survival analysis revealed that the estimated cancer overall
survival (OS) rates (standard error) at 1, 5 and 10 years in
patients with high B7-H3 expression were 98.7% (1.3%), 4.5% (4.9%)
and 0% (0%) in contrast to 100% (0%), 58.1% (8.7%) and 48.7% (9.9%)
in patients with low B7-H3 expression, respectively. The estimated
cancer progression-free survival (PFS) rates at 1, 5 and 10 years
following RC in patients with high B7-H3 expression were 71.8%
(5.1%), 16.8% (4.6%), 0% (0%) in contrast to 94.4% (3.8%), 52.0%
(9.2%), 43.4% (11.0%) in patients with low B7-H3 expression,
respectively. Therefore, high B7-H3 expression was significantly
associated with a much shorter OS and PFS in 115 MIBC patients
(P<0.001, P<0.001; Table II
and Fig. 1B and C).
 | Table II.Association of overall and
progression-free survival and different levels of B7-H3 expression
in patients with MIBC (N=115). |
Table II.
Association of overall and
progression-free survival and different levels of B7-H3 expression
in patients with MIBC (N=115).
|
|
| Overall survival %
(±SE) |
| Progression-free
survival % (±SE) |
|
|---|
|
|
|
|
|
|
|
|---|
| B7-H3
expression | No. of
patients | 1-year | 5-year | 10-year | P-value | 1-year | 5-year | 10-year | P-value |
|---|
|
|
|
|
|
| <0.001 |
|
|
| <0.001 |
| High | 79 | 98.7 (1.3) | 14.9 (4.5) | 0 (0.0) |
| 71.8 (5.1) | 16.8 (4.6) | 0 (0.0) |
|
| Low | 36 | 100 (0.0) | 58.1 (8.7) | 47.7 (9.9) |
| 94.4 (3.8) | 52.0 (9.2) | 43.4 (11.0) |
|
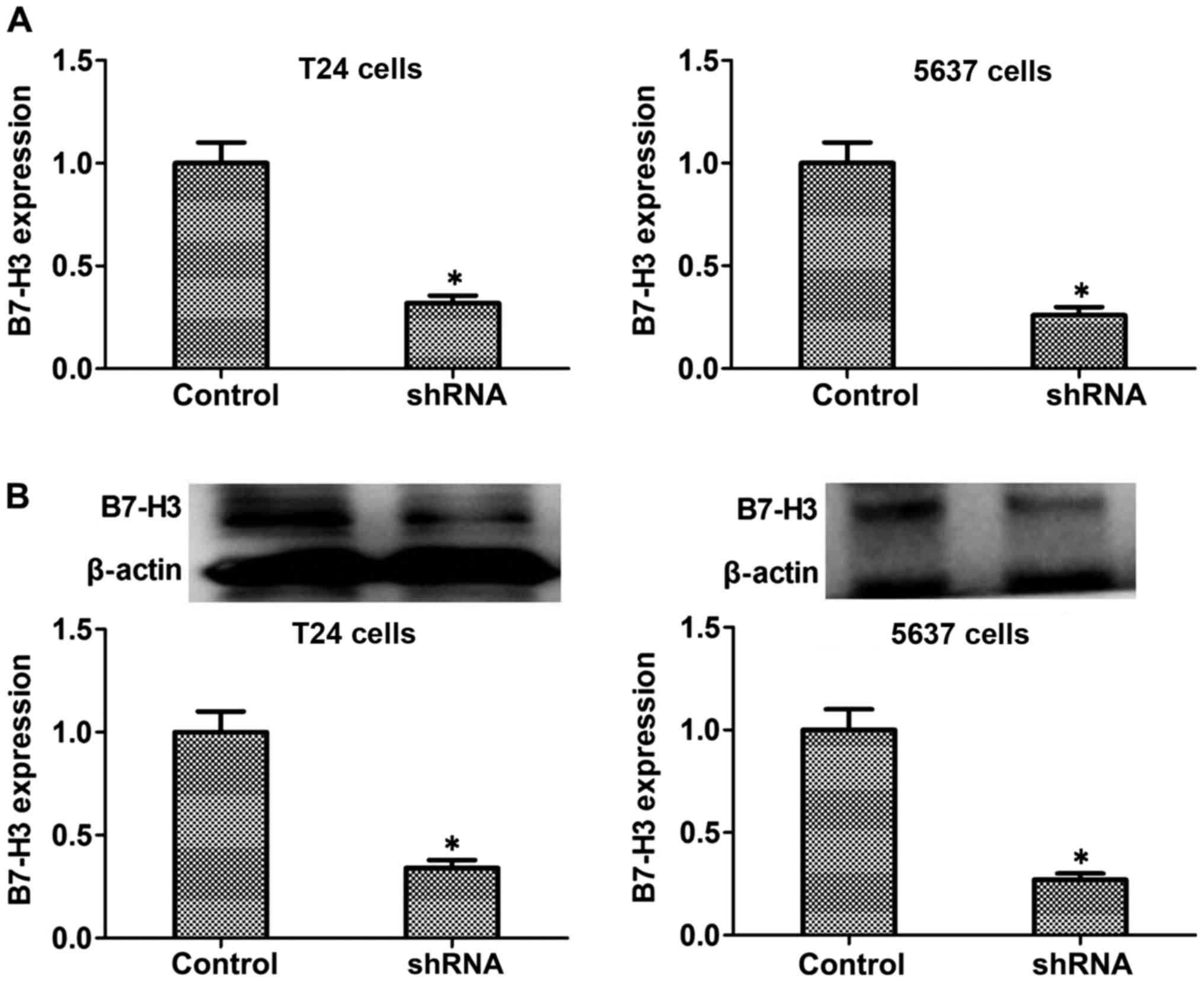
Lentivirus-based RNA interference
transfection markedly downregulates B7-H3 mRNA and protein
expression of T24 or 5637 cells
RT-PCR and western blot experiments were used to
detect the expression level of B7-H3 mRNA and protein in
vitro. Silencing of B7-H3 in T24 and 5637 cells was performed
by recombinant lentivirus vector LV-B7-H3 shRNA interference
transfection. The B7-H3 mRNA and protein expression in B7-H3 shRNA
cells was significantly decreased compared to the control cells
(Fig. 2). Through this way, we
successfully established cancer cells with the silencing of B7-H3
by shRNA.
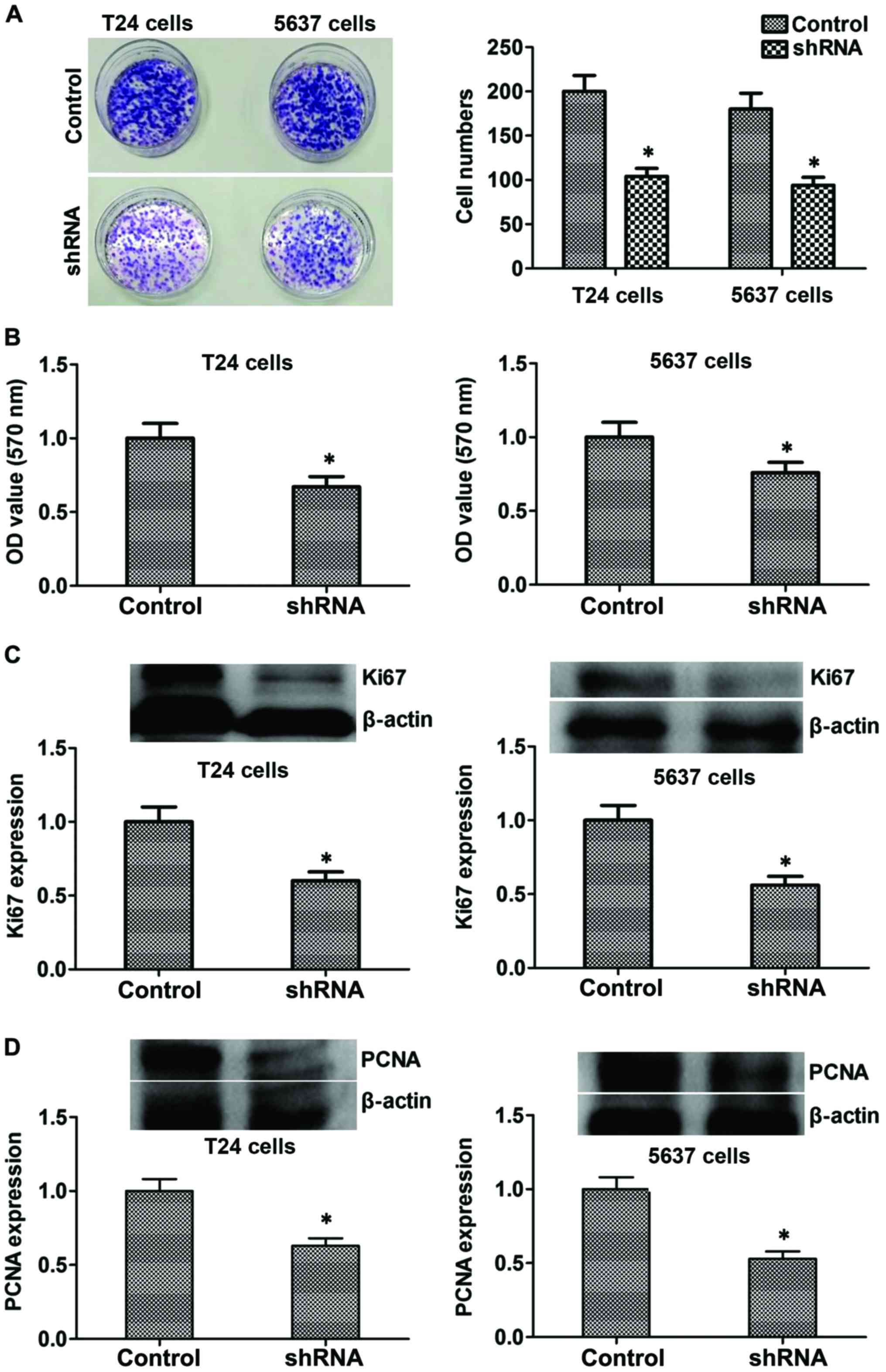
Suppression of B7-H3 inhibits cancer
cell proliferation by regulating Ki67 and PCNA proteins in
vitro
Colony formation and MTT assay were used to evaluate
the effects of B7-H3 silencing on bladder cancer cells. Compared to
control cells, stable silencing of B7-H3 in B7-H3 shRNA cells
markedly reduced its ability to proliferate in vitro
(P<0.05; Fig. 3A and B). The
above mentioned results suggested that B7-H3 silencing
significantly reduced the proliferative potential of cancer cells.
Furthermore, to explore the mechanism, we tested the expression
level of proliferation-related proteins such as Ki67 and PCNA, and
the results showed that the expression of protein Ki67 and PCNA
decreased in the B7-H3 shRNA group compared with the control
(P<0.05, respectively, Fig. 3C and
D). Considered together, our results indicated that B7-H3
silencing could reduce the proliferation by regulating related
proteins such as Ki67 and PCNA in vitro.
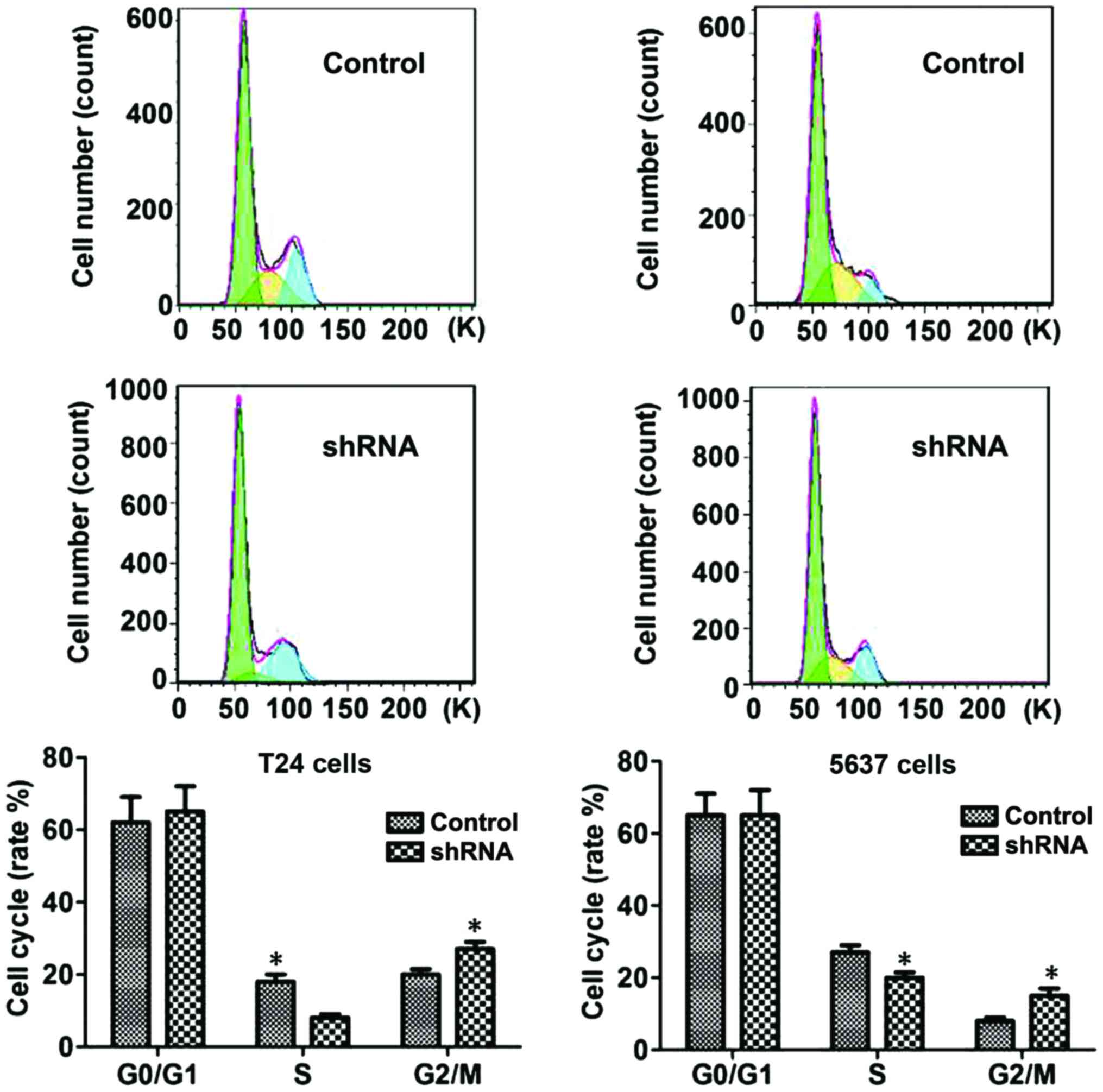
B7-H3 silencing causes G2
phase arrest
The flow cytometric analysis was conducted to
ascertain whether the anti-proliferative effect was due to cell
cycle arrest. Studies showed that significant changes were induced
in S and G2 phases of the cell cycle in B7-H3 shRNA
cells group compared with control cell group: Accumulation of cells
started in sub-G1 phase after 24 h of serum starvation,
and DNA accumulation was observed in the G1 phase with a
significant increase in cell population in the S phase, which
resulted in a significant increase in the cell population in the
G2 phase (P<0.05, respectively; Fig. 4).
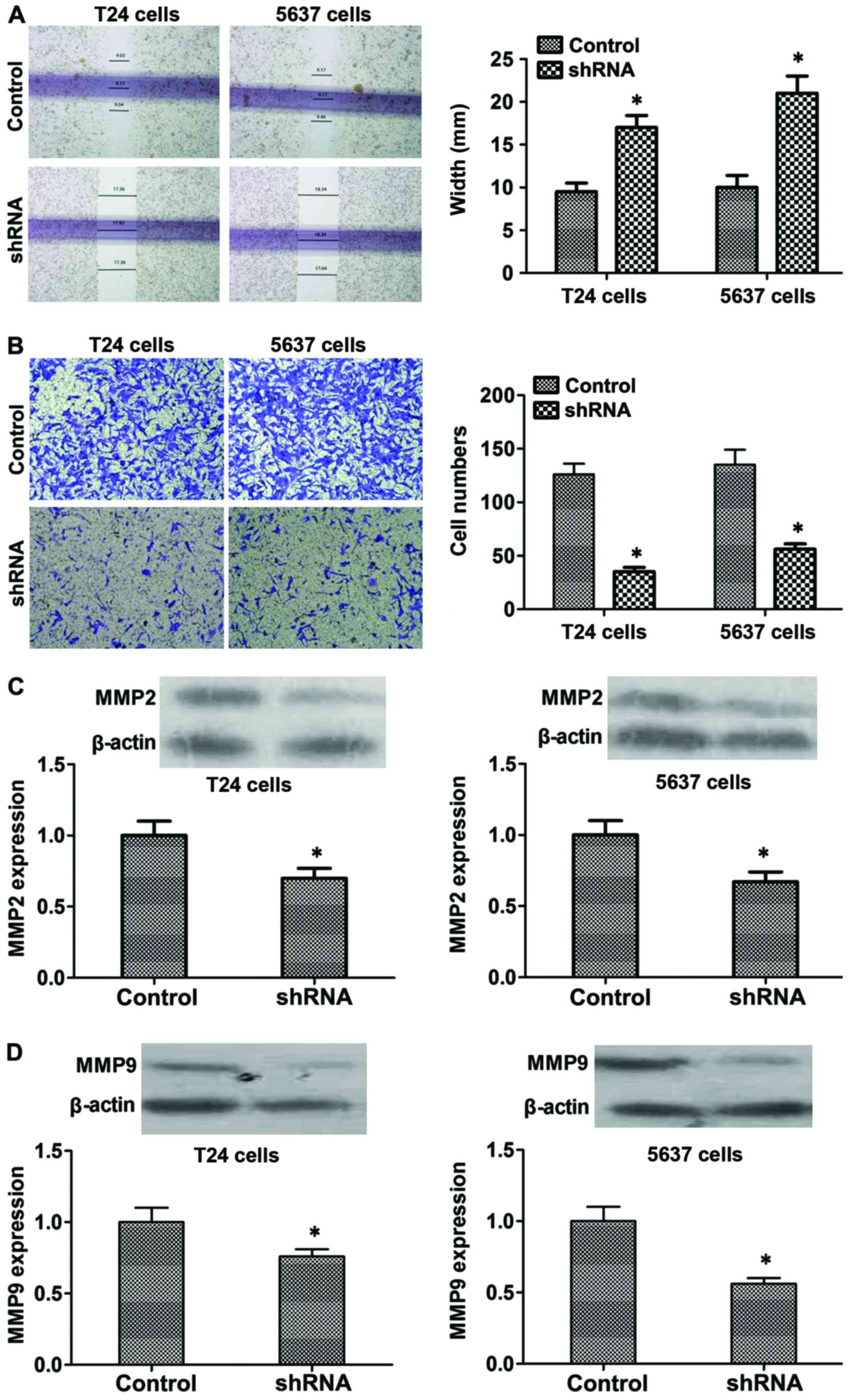
B7-H3 silencing inhibits migration and
invasion by regulating MMP2 and MMP9 proteins in vitro
Moreover, wound healing assay was used to detect
changes in cell migration. As shown in Fig. 5A, compared with the control cells,
B7-H3 shRNA cells showed larger scratch width (P<0.05). In
addition, Transwell assay was used to determine the possible effect
of B7-H3 silencing on regulating cancer cell invasiveness. Data
showed that the invasive capacity of B7-H3 shRNA cells was
decreased compared with the control cells after 48 h (P<0.05;
Fig. 5B). Furthermore, to explore
the mechanism, we tested the expression level of the proteins which
reflect invasion and metastasis such as MMP2 and MMP9, and the
results showed that the expression of MMP2 and MMP9 significantly
decreased in the B7-H3 shRNA group compared with the control
(P<0.05, respectively; Fig. 5C and
D). In summary, our results indicated that B7-H3 silencing
could reduce the invasiveness of cancer cells by regulating the
related proteins such as MMP2 and MMP9.
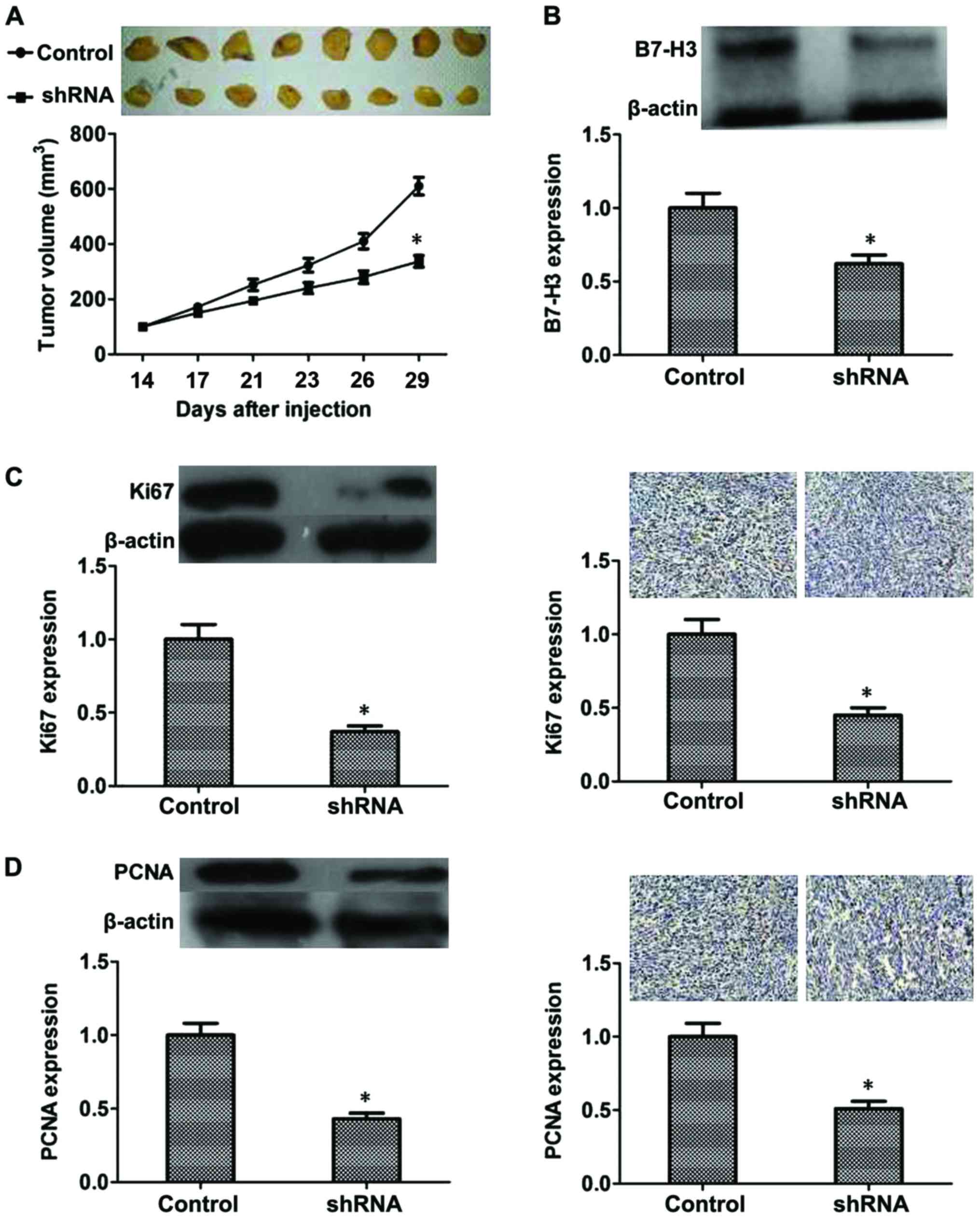
Inhibitory effect of B7-H3 shRNA on
MIBC in vivo
From the results mentioned above, we confirmed that
B7-H3 silencing could inhibit proliferation, cause G2
phase arrest and inhibit cell invasion by regulating related
proteins in vitro. To further demonstrate this, an
experiment was performed in vivo. After 3 weeks, the tumor
volume of the B7-H3 shRNA group was significantly smaller than that
found in the control group (P<0.05) (Fig. 6A). Additionally, we evaluated B7-H3
expression of the mouse tumors by western blot analysis, and the
results revealed that expression of B7-H3 was reduced due to the
knockdown of B7-H3 by shRNA (P<0.05; Fig. 6B). Moreover, we evaluated Ki67 and
PCNA expression in the mouse tumors by western blot analysis and
immunohistochemistry, and the results suggested that expression of
Ki67 and PCNA was also reduced due to the knockdown of B7-H3 by
shRNA (P<0.05, respectively, Fig. 6C
and D). These results confirmed the inhibitory effect of B7-H3
shRNA in vivo.
Discussion
Bladder cancer is a particularly immunogenic
malignancy, and patients with bladder cancer exhibit
tumor-associated immunologic suppression (1–7). B7
homologue 3 (B7-H3) is thought to serve as an accessory
co-regulator of T-cell response after initial antigen priming
(8–15). B7-H3 has been shown to mediate the
proliferation of CD4+ and CD8+ T cells and to
enhance interferon-γ (IFN-γ) production (8). Moreover, B7-H3 may function as a
protective factor in natural killer cell-mediated cytolysis
(8–12). However, other studies have suggested
that B7-H3 is able to inhibit the proliferation of T cells and
reduce the secretion of IFN-γ and interleukin-2 (IL-2) (8–26).
Although there is no consensus between the immunological and
pathophysiologic roles of B7-H3, aberrant B7-H3 expression has been
shown to be closely associated with tumor progression and poor
prognosis in human urologic neoplasms (8). The receptor for B7-H3 has not been
identified and the precise co-stimulatory and co-inhibitory
functions of B7-H3 in immune-regulation remain controversial. A
recent review showed that elevated B7-H3 expression is
significantly associated with poor survival in many types of cancer
(8). Aberrant B7-H3 expression has
been shown to be closely associated with tumor metastasis and worse
prognosis in several different cancers including non-small cell
lung cancer, breast cancer, renal cell cancer, brain cancers
(neuroblastoma and glioma), pancreatic cancer, colon cancer,
melanoma and prostate cancer (8–31).
B7-H3 has been confirmed to promote cancer cell migration and
invasion in renal cell cancer and prostate cancer (8,13,14).
Clinical data indicate that B7-H3 may be exploited as a
co-inhibitory molecule in immune evasion process (8–20).
Some scholars speculate that the biological role of B7-H3 may
differ from one tumor type to another and changes during disease
progression. However, little is known about the exact impact of
B7-H3 expression in bladder cancer, and conflicting opinions on the
role of B7-H3 expression in bladder cancer are still being debated
(11,15). As known, bladder cancer is a
heterogeneous disease that spans a broad spectrum from
non-muscle-invasive bladder cancer (NMIBC) to muscle-invasive
bladder cancer (MIBC) (16).
Conclusions from the previous research usually depend on the study
of a mixed cohort including the two types of bladder cancer
patients, for which the lack of separate analysis of subgroups of
MIBC or NMIBC exist. Considering that the risk of tumor invasion
and metastasis with MIBC or NMIBC is quite different, the original
intention of our study is to focus on the independent analysis of
MIBC patients and investigate whether there are different findings,
at least partly, from previous reports. We examined
immunohistochemical expression of B7-H3 in clinical specimens from
115 MIBC patients and evaluated the associations between B7-H3
expression, clinicopathological features and outcomes. Notably, the
present study showed that MIBC patients with high B7-H3 expression
were more likely to manifest advanced tumor stage and a higher
proportion of distant metastasis, whose OS and PFS were
significantly shorter than patients with low B7-H3 expression.
These different results with other studies provide a hypothesis of
the function of B7-H3 at the clinical level, perhaps through
facilitating tumor progression of bladder cancer, especially in
MIBC. Recent studies demonstrated that B7-H3 expression in tumor
cells was correlated with malignant behaviors, such as
proliferation, invasion and metastatic potential which finally
contributes to cancer progression (8,17,18).
In order to verify our clinical findings, we transfected human
bladder cancer T24 and 5637 cells with targeted silencing of B7-H3,
using lentivirus-based RNA stable interference transfection.
There has been previous research to confirm the role
of B7-H3 in migration and invasion. Some studies indicate that
B7-H3 silencing reduces the expression of metastasis-associated
proteins such as matrix metalloproteinase (MMP)-2, MMP-9, signal
transducer and activator of transcription 3 (STAT3) and the level
of secreted interleukin-8 (IL-8) (8,18,19,31).
An additional mechanism suggests that B7-H3 also influences liver
cancer aggressiveness and invasiveness through the JAK3/STAT3/SLUG
signaling pathway (19). In
hepatocellular carcinoma, B7-H3 has been found to promote
epithelial-mesenchymal transition (EMT), and STAT3 has also been
found to induce NF-κB activity, which influences the expression of
IL-8, VEGF and MMPs in the tumor microenvironment (8,30). Xie
et al reported that B7-H3 increases the activity of NF-κB
signaling, which stimulated the in vitro and in vivo
invasion of pancreatic cancer cells (21). It has also been reported that B7-H3
promoted cell migration and invasion through the JAK3/STAT3/MMP9
signaling pathway in colorectal cancer (31). Consistent with these results, our
results demonstrated that B7-H3 could promote cell migration and
invasion in vitro. In addition, we confirmed that B7-H3
promoted cell proliferation, and inhibited G2 phase
arrest in vitro. Furthermore, our results showed that B7-H3
silencing could alter the T24 and 5637 cell behaviors by regulating
their related proteins. However, this finding still needs to be
confirmed in other types of MIBC cells and in different types of
cancer. The central key is investigation of the signaling pathways
and molecular mechanisms, which is the next step underway in future
research. Finally, we confirmed similar results in vivo
before clinical experiments. Recently, a study reported that B7-H3
could promote the migration and invasion of bladder cancer cells
via the PI3K/Akt/STAT3 signaling pathway (32). Although they found that B7-H3 did
not affect cell proliferation in vitro, which was different
from ours, their results proved that B7-H3 had an effect on the
PI3K/Akt/STAT3 signaling pathway which could influence
proliferation. Our results did confirm the function of B7-H3 on the
proliferation by regulating Ki67 and PCNA, and we also proved this
effect in vivo. In addition, we demonstrated that
suppression of B7-H3 significantly caused G2 phase
arrest. Therefore, our results should be reliable and more studies
are needed to explore the complicated mechnism.
Moreover, B7-H3 expression has been reported in
kidney cancer, endothelium of colon, lung, and breast cancers
(27–31). In addition, B7-H3 has been detected
in tumor vasculature, and influenced the cancer microenvironment as
soluble B7-H3 (sB7-H3) in pancreatic cells, which leads to a
significant increase in migration and invasion (21,30).
B7-H3 has also been proven to lead to an upregulation of NF-κB
through a TLR4-dependent mechanism, which leads to a significant
increase in VEGF and IL-8 expression, resulting in tumor invasion
and angiogenesis (21,29).
Considered together, these series of
cellular-function experiments were confirmed with our clinical
findings in the immunohistochemistry assay. The expression of B7-H3
protein in tumor tissues and T24 or 5637 cells indicates its
clinical relevance in MIBC. Our study provided a further
investigation and understanding of the B7-H3 molecular mechanisms
which were involved in the proliferation and metastatic potential
of MIBC. All the results demonstrated that B7-H3 may play a
pro-tumor role in MIBC progression and could be regarded as an
immuno-therapeutic target for MIBC patients.
In conclusion, we first investigated the expression
of co-stimulatory molecule B7-H3 in MIBC tumor specimens, and we
found that patients with high B7-H3 expression manifested high
malignant progression and poor prognosis in MIBC. Then we
demonstrated that B7-H3 could promote the development of MIBC in
vitro and in vivo. Thus, B7-H3 may be a potential novel
biomarker for MIBC.
Acknowledgements
Not applicable.
Funding
The present study was supported by several grants
from the National Natural Science Foundation of China (nos.
81402228 and 81772858).
Availability of data and materials
The dataset supporting the conclusions of this
article is included within the article. Raw data of the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZLX and YZ carried out the experiment of molecular
biology and drafted the manuscript. ZLX and LW participated in the
sequence alignment. ZLX, YZ, LW and HWM participated in the design
of the study and performed the statistical analysis. ZLX, PFL and
BES conceived of the study, participated in its design and
coordination and helped to draft the manuscript. All authors read
and approved the final manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Human samples and animals in this study were
approved by the Ethics Committee of the Fourth Hospital of Hebei
Medical University. The research involving human participants was
approved by the Fourth Hospital of Hebei Medical University and the
equivalent committee. The participants provided written informed
consent before enrollment in this study. The consent forms were not
be provided in this article due to the large number and they were
all written in the Chinese language. The animal study was conducted
with approval of the Animal Care and Use Committee of the Fourth
Hospital of Hebei Medical University. All applicable international,
national, and/or institutional guidelines for the care and use of
animals were followed. Animal surgery was performed with care to
alleviate pain.
Patient consent for publication
The participants provided written informed consent
before enrollment in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lobo N, Mount C, Omar K, Nair R,
Thurairaja R and Khan MS: Landmarks in the treatment of
muscle-invasive bladder cancer. Nat Rev Urol. 14:565–574. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamat AM, Colombel M, Sundi D, Lamm D,
Boehle A, Brausi M, Buckley R, Persad R, Palou J, Soloway M, et al:
BCG-unresponsive non-muscle-invasive bladder cancer:
Recommendations from the IBCG. Nat Rev Urol. 14:244–255. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kojima T, Kawai K, Miyazaki J and
Nishiyama H: Biomarkers for precision medicine in bladder cancer.
Int J Clin Oncol. 22:207–213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Funt SA and Rosenberg JE: Systemic,
perioperative management of muscle-invasive bladder cancer and
future horizons. Nat Rev Clin Oncol. 14:221–234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamat AM, Hahn NM, Efstathiou JA, Lerner
SP, Malmström PU, Choi W, Guo CC, Lotan Y and Kassouf W: Bladder
cancer. Lancet. 388:2796–2810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trenta P, Calabrò F, Cerbone L and
Sternberg CN: Chemotherapy for Muscle-Invasive Bladder Cancer. Curr
Treat Options Oncol. 17:62016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castellanos JR, Purvis IJ, Labak CM, Guda
MR, Tsung AJ, Velpula KK and Asuthkar S: B7-H3 role in the immune
landscape of cancer. Am J Clin Exp Immunol. 6:66–75.
2017.PubMed/NCBI
|
|
9
|
Hofmeyer KA, Ray A and Zang X: The
contrasting role of B7-H3. Proc Natl Acad Sci USA. 105:10277–10278.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vigdorovich V, Ramagopal UA, Lázár-Molnár
E, Sylvestre E, Lee JS, Hofmeyer KA, Zang X, Nathenson SG and Almo
SC: Structure and T cell inhibition properties of B7 family member,
B7-H3. Structure. 21:707–717. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamato I, Sho M, Nomi T, Akahori T,
Shimada K, Hotta K, Kanehiro H, Konishi N, Yagita H and Nakajima Y:
Clinical importance of B7-H3 expression in human pancreatic cancer.
Br J Cancer. 101:1709–1716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mao Y, Li W, Chen K, Xie Y, Liu Q, Yao M,
Duan W, Zhou X, Liang R and Tao M: B7-H1 and B7-H3 are independent
predictors of poor prognosis in patients with non-small cell lung
cancer. Oncotarget. 6:3452–3461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Crispen PL, Sheinin Y, Roth TJ, Lohse CM,
Kuntz SM, Frigola X, Thompson RH, Boorjian SA, Dong H, Leibovich
BC, et al: Tumor cell and tumor vasculature expression of B7-H3
predict survival in clear cell renal cell carcinoma. Clin Cancer
Res. 14:5150–5157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benzon B, Zhao SG, Haffner MC, Takhar M,
Erho N, Yousefi K, Hurley P, Bishop JL, Tosoian J, Ghabili K, et
al: Correlation of B7-H3 with androgen receptor, immune pathways
and poor outcome in prostate cancer: An expression-based analysis.
Prostate Cancer Prostatic Dis. 20:28–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ingebrigtsen VA, Boye K, Tekle C, Nesland
JM, Flatmark K and Fodstad O: B7-H3 expression in colorectal
cancer: Nuclear localization strongly predicts poor outcome in
colon cancer. Int J Cancer. 131:2528–2536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma J, Ma P, Zhao C, Xue X, Han H, Liu C,
Tao H, Xiu W, Cai J and Zhang M: B7-H3 as a promising target for
cytotoxicity T cell in human cancer therapy. Oncotarget.
7:29480–29491. 2016.PubMed/NCBI
|
|
17
|
Picarda E, Ohaegbulam KC and Zang X:
Molecular pathways: Targeting B7-H3 (CD276) for human cancer
immunotherapy. Clin Cancer Res. 22:3425–3431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tekle C, Nygren MK, Chen YW, Dybsjord I,
Nesland JM, Maelandsmo GM and Fodstad O: B7-H3 contributes to the
metastatic capacity of melanoma cells by modulation of known
metastasis-associated genes. Int J Cancer. 130:2282–2290. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang FB, Wang L, Jia HC, Li D, Li HJ,
Zhang YG and Sun DX: B7-H3 promotes aggression and invasion of
hepatocellular carcinoma by targeting epithelial-to-mesenchymal
transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int.
15:452015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maeda N, Yoshimura K, Yamamoto S, Kuramasu
A, Inoue M, Suzuki N, Watanabe Y, Maeda Y, Kamei R, Tsunedomi R, et
al: Expression of B7-H3, a potential factor of tumor immune evasion
in combination with the number of regulatory T cells, affects
against recurrence-free survival in breast cancer patients. Ann
Surg Oncol. 21 Suppl 4:S546–S554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie C, Liu D, Chen Q, Yang C, Wang B and
Wu H: Soluble B7-H3 promotes the invasion and metastasis of
pancreatic carcinoma cells through the TLR4/NF-κB pathway. Sci Rep.
6:275282016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bachawal SV, Jensen KC, Wilson KE, Tian L,
Lutz AM and Willmann JK: Breast cancer detection by B7-H3-targeted
ultrasound molecular imaging. Cancer Res. 75:2501–2509. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan H, Wei X, Zhang G, Li C, Zhang X and
Hou J: B7-H3 over expression in prostate cancer proB7-H3 role in
the immune landscape of cancer 75. Am J Clin Exp Immunol. 6:66–75.
2017.PubMed/NCBI
|
|
24
|
Li M, Zhang G, Zhang X, Lv G, Wei X, Yuan
H and Hou J: Overexpression of B7-H3 in CD14+ monocytes
is associated with renal cell carcinoma progression. Med Oncol.
31:3492014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baral A, Ye HX, Jiang PC, Yao Y and Mao Y:
B7-H3 and B7-H1 expression in cerebral spinal fluid and tumor
tissue correlates with the malignancy grade of glioma patients.
Oncol Lett. 8:1195–1201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Z, Luther N, Ibrahim GM, Hawkins C,
Vibhakar R, Handler MH and Souweidane MM: B7-H3, a potential
therapeutic target, is expressed in diffuse intrinsic pontine
glioma. J Neurooncol. 111:257–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang R, Chadalavada K, Wilshire J, Kowalik
U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C and
Tabar V: Identifcation and characterization of angiogenesis targets
through proteomic profling of endothelial cells in human cancer
tissues. PLoS One. 8:e788852013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seaman S, Stevens J, Yang MY, Logsdon D,
Graff-Cherry C and St Croix B: Genes that distinguish physiological
and pathological angiogenesis. Cancer Cell. 11:539–554. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ferrara N: VEGF and the quest for tumour
angiogenesis factors. Nat Rev Cancer. 2:795–803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen W, Liu P, Wang Y, Nie W, Li Z, Xu W,
Li F, Zhou Z, Zhao M and Liu H: Characterization of a soluble B7-H3
(sB7-H3) spliced from the intron and analysis of sB7-H3 in the sera
of patients with hepatocellular carcinoma. PLoS One. 8:e769652013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu F, Zhang T, Zou S, Jiang B and Hua D:
B7-H3 promotes cell migration and invasion through the
Jak2/Stat3/MMP9 signaling pathway in colorectal cancer. Mol Med
Rep. 12:5455–5460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Guo G, Song J, Cai Z, Yang J, Chen
Z, Wang Y, Huang Y and Gao Q: B7-H3 Promotes the migration and
invasion of human bladder cancer cells via the PI3K/Akt/STAT3
signaling pathway. J Cancer. 8:816–824. 2017. View Article : Google Scholar : PubMed/NCBI
|