Introduction
Mantle cell lymphoma (MCL) is a type of
non-Hodgkin's lymphoma (NHL) and is molecularly identified by
chromosomal translocation t(11;14) (q13;q32). This disease is
characterized by quick relapses plus poor outcomes over the
long-term, predominantly affecting older adults (1). The clinical course of MCL is mostly
invasive, and most cases have extensive extranodal infiltration and
bone marrow invasion. In younger patients, randomized trials have
established the advantage of dose-intensified,
cytarabine-containing indu-ction with or without transplantation of
stem cells. In elderly patients, treatment with R-CHOP (rituximab,
doxorubicin hydrochloride, cyclophosphamide, vincristine and
prednisone) has greatly prolonged overall survival (2). Yet, the great majority of patients
unfortunately will suffer relapse. Therefore, the pathogenesis of
MCL and effective therapeutic drugs for this disease are still
under active exploration.
Microarray technology is widely utilized for the
study of general genetic aberrations in MCL (3). Nevertheless, there are studies
integrating these microarray datasets to identify key genes in MCL.
Herein, we analyzed two sizeable and representative gene expression
profiles to identify differentially expressed genes (DEGs) between
MCL and normal lymph gland. In addition, functional enrichment
analysis and protein-protein interaction (PPI) analysis were
performed for the DEGs, to uncover key genes in MCL. We found that
matrix metalloproteinase 9 (MMP9) plays a vital role in these key
genes.
Invasion and metastasis are important biological
chara-cteristics of malignant tumors including MCL. Tumor growth,
invasion and metastasis involve a complex multistep process,
including the division and proliferation of tumor cells, the
degradation of the extracellular matrix, cell escape through the
basement membrane, migration into the blood circulation system,
tumor cell growth at secondary sites and a series of activities, at
the same time accompanied by the formation of new blood vessels
(4). Studies have shown that MMP9,
a member of the matrix metalloproteinase (MMP) family is highly
expressed in various tumors and plays a major role in the growth,
metastasis and angiogenesis of primary and secondary tumors
(5–8).
MMPs inhibitors (MMPIs) have been a major focus of
research in recent years. Since the 1990s, the world's
major pharmaceutical companies have invested heavily in the
research and development of MMPIs. At present, more than a dozen
MMPIs have entered the clinical stage and there are also a large
number of compounds that are undergoing pre-clinical research.
These chemically synthesized MMPIs can be broadly classified into
peptide MMPIs, non-peptide MMPIs, tetracycline MMPIs and
bisphosphonate MMPIs (9). As a
result, MMPIs are of great significance as a new antitumor agent.
Moreover, peptide drugs have low adverse reactions, low
immunogenicity, and are easy to synthesize and transform, which
makes these agents worthy of further research.
The affinity of the molecules against their target
proteins were generated with computational techniques. Molecular
docking is a method of binding orientation prediction of molecules
with protein targets (10).
Therefore, molecular docking is regarded as a vital technique in
drug design. The aim of the present study was to screen new cyclic
peptides as activated MMP9 inhibitors with which to target
hydrophobic pockets of MMP9 by using computational docking
techniques.
Materials and methods
Microarray data
Two sizeable gene expression profiles including
lymph nodes of MCL and normal lymph nodes (GSE32018 and GSE9327)
(11,12) were acquired from the GEO (Gene
Expression Omnibus) (http://www.ncbi.nlm.nih.gov/geo). The GEO database is
a functional genomics data repository. It stores microarray and
sequencing data. The two gene expression profiles included 62 MCL
samples and 14 normal lymph node samples.
Data processing
GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r/) is a program
that allows the investigator to compare two or more groups of
samples within a GEO dataset. This is performed to identify DEGs
across experimental circumstances. GEO2R analyzes original
submitter-supplied processed microarray data using the GEO query
and limma R packages (http://www.bioconductor.org/packages/release/bioc/html/limma.html)
from the Bioconductor project. In the present study, GEO2R was
applied to obtain DEGs in MCL compared with normal lymph nodes. The
false-positive results for the microarray were then corrected by
adjusted P-value (adj. P-value) using Benjamini-Hochberg method.
The cut-off criterion was set as P<0.05 and ‘|logFC| >1’.
Gene Ontology (GO) and Pathway
Analysis
The Database for Annotation, Visualization and
Integrated Discovery (DAVID; http://david.abcc.ncifcrf.gov/) is a website that
offers the interpretation of genes. For our study, the DAVID
database was used to perform pathway analysis. Gene Ontology holds
three hierarchies: Cellular component (CC), Biological process (BP)
and Molecular function (MF) (13).
Pathway Analysis is a functional analysis that can
map genes to KEGG pathways (13).
This study performed GO analysis and KEGG pathway analysis using
only overlapped DEGs in the two independent datasets (GSE32018 and
GSE9327). The significance of the GO and pathway term enrichment in
the DEGs are denoted by P-value. The cut-off point was set as
P<0.05.
Establishment of PPI network
STRING (http://string-db.org) was utilized to identify
interaction networks of protein products of these DEGs. The cut-off
criterion was set as ‘Confidence score ≥0.7’. The Cytoscape 3.5.1
software (Institute of Systems Biology, Seattle, WA, USA) was used
to build protein-protein interaction (PPI) networks among the DEGs
in MCL. The hub genes were identified by Cytohubba plugin (14), and the top 10 nodes were selected as
ranked by degree. Molecular Complex Detection (MCODE) (15) was applied in order to find clusters
of genes in the PPI network.
Receptor refinement
Cyclic peptides were docked against activated MMP9
by the Molecular Operating Environment (MOE) software 2016.08
(Chemical Computing Group ULC, Montreal, Quebec, Canada)
(http://www.chemcomp.com/). The 3D structure of
MMP9 was obtained from the database of the Protein Data Bank (PDB)
(https://www.rcsb.org/) using PDB ID:1L6J.
Optimization of the structure occurred by adding hydrogen using
software. To optimize the structure, H2O molecules were
detached from the structure and 3D protonation was conducted to
change the state into the ionization level. Moreover, energy
minimization was performed using certain parameters. Docking
studies used this minimized structure.
Design of the peptide
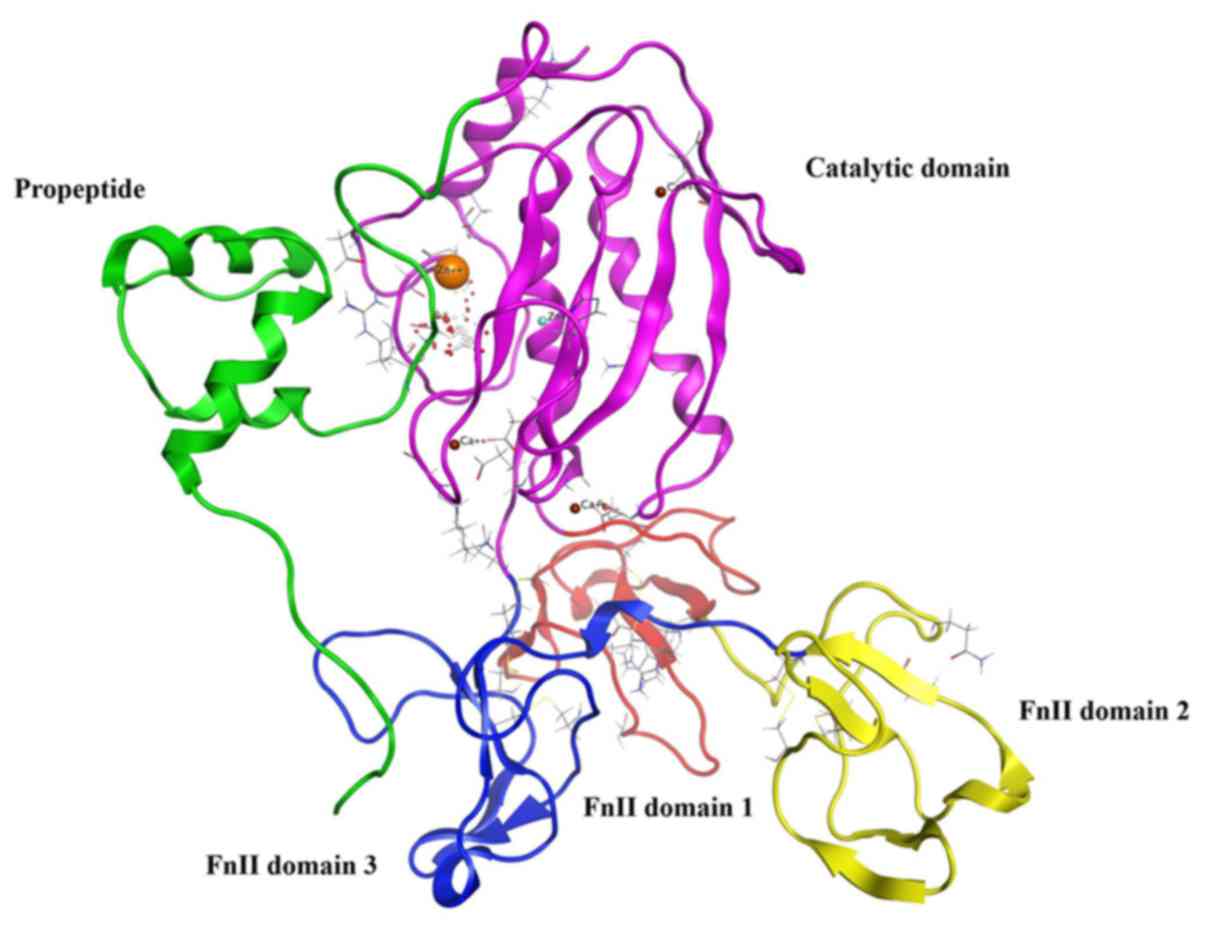
MMP9 usually exists as proMMP9, and the construction
of proMMP9 includes the propeptide, the catalytic domain plus three
fibronectin type II (FnII) domains (Fig. 1) (16). ProMMP9 is triggered by
autoproteolytic cleavage of the propeptide, in vivo or in
vitro, by reacting with an organomercurial compound or other
proteases (17). The stretches of
propeptide residues 97–100 that are inserted into the active-site
cleft, block access to the catalytic zinc. Cleavage of the
propeptide opens up the active site, facilitating interactions with
substrate or inhibitors. According to the study on the propeptide,
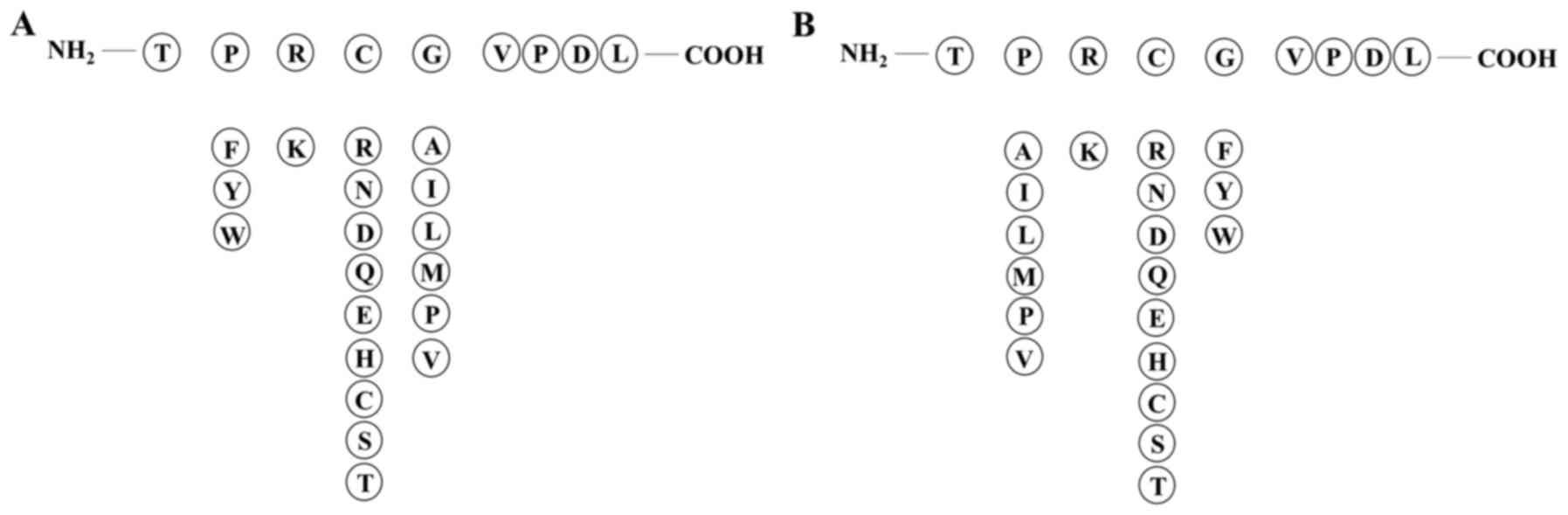
we selected amino acid sequence TPRCGVPDL from the propeptide
including key amino acid residues 97–100 PRCG as the template
peptide. Non-polar aromatic amino acid (F/Y/W) was added in key
amino acids, and there were two alternatives: replacement of the
first position amino acid P or the fourth position G. The second
position of key amino acid residues had a likeness of interaction
with polar positive charged amino acid residue K, whereas the
preference for the third position according to the amino acid
properties was polar amino acids (R/N/D/Q/E/H/C/S/T) (Fig. 2). Six peptides were designed to
investigate their potential against active MMP9. Furthermore,
disulfide bridges were created by adding cysteine residues at both
ends in order to convert these peptides into more stable cyclic
peptides. ACD/ChemSketch software 12.0 (ACD/Labs, Toronto, Ontario,
Canada) (www.acdlabs.com) was used to convert the
designed peptides into their respective 3D structures.
Peptide refinement
All of the peptides were optimized. The MOE software
was used to achieve this task, by adding hydrogen. Energy of the
peptides was minimized using parameters such as gradient: 0.05,
Force Field: MMFF94X, Chiral Constraint and Current Geometry. In
addition, Conformational Search of these peptides was conducted by
the LowModeMD method, and the results were saved in mdb database
for further docking studies.
Molecular docking
Docking of these peptides with the active MMP9 was
conducted with the algorithm of the MOE software. Parameters were
set as Re-scoring function, London dG; placement, Triangle matcher;
Retain, 5; Refinement, Force Field; and Re-scoring 2, London dG.
The docking program of MOE provides the correct conformation (with
the rotation of bonds, structure of the molecule is not rigid) of
the ligand. S score was the basis for the choice of top
conformation for each peptide. The top conformations also received
further evaluation in order to study the hydrogen bonding/π-π
intera-ctions.
Results
Identification of DEGs in MCL
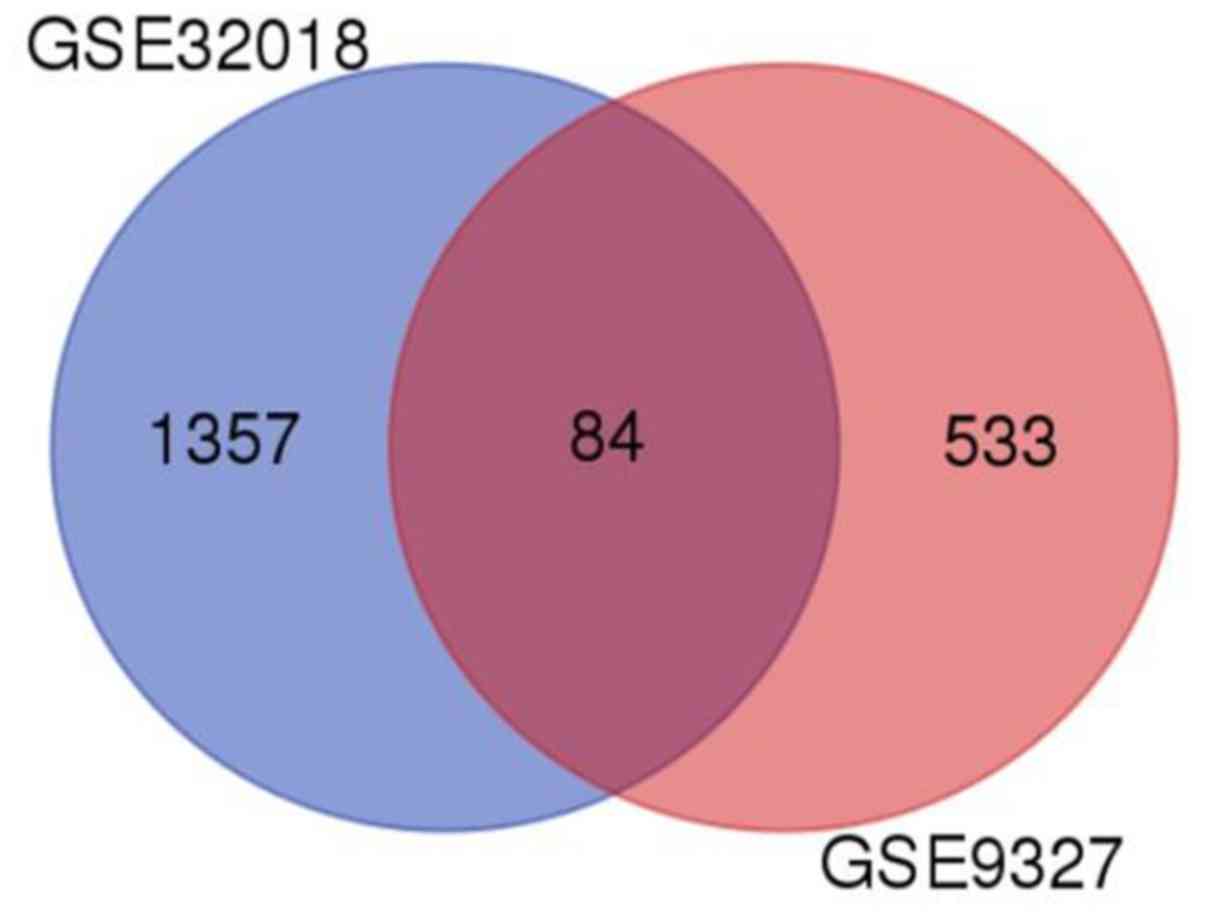
GEO2R analysis demonstrated that a total of 1,441
and 617 DEGs were acquired in the GSE32018 and GSE9327 datasets,
respectively. Moreover, 84 DEGs were also identified in both
datasets (Fig. 3).
Functional enrichment analysis
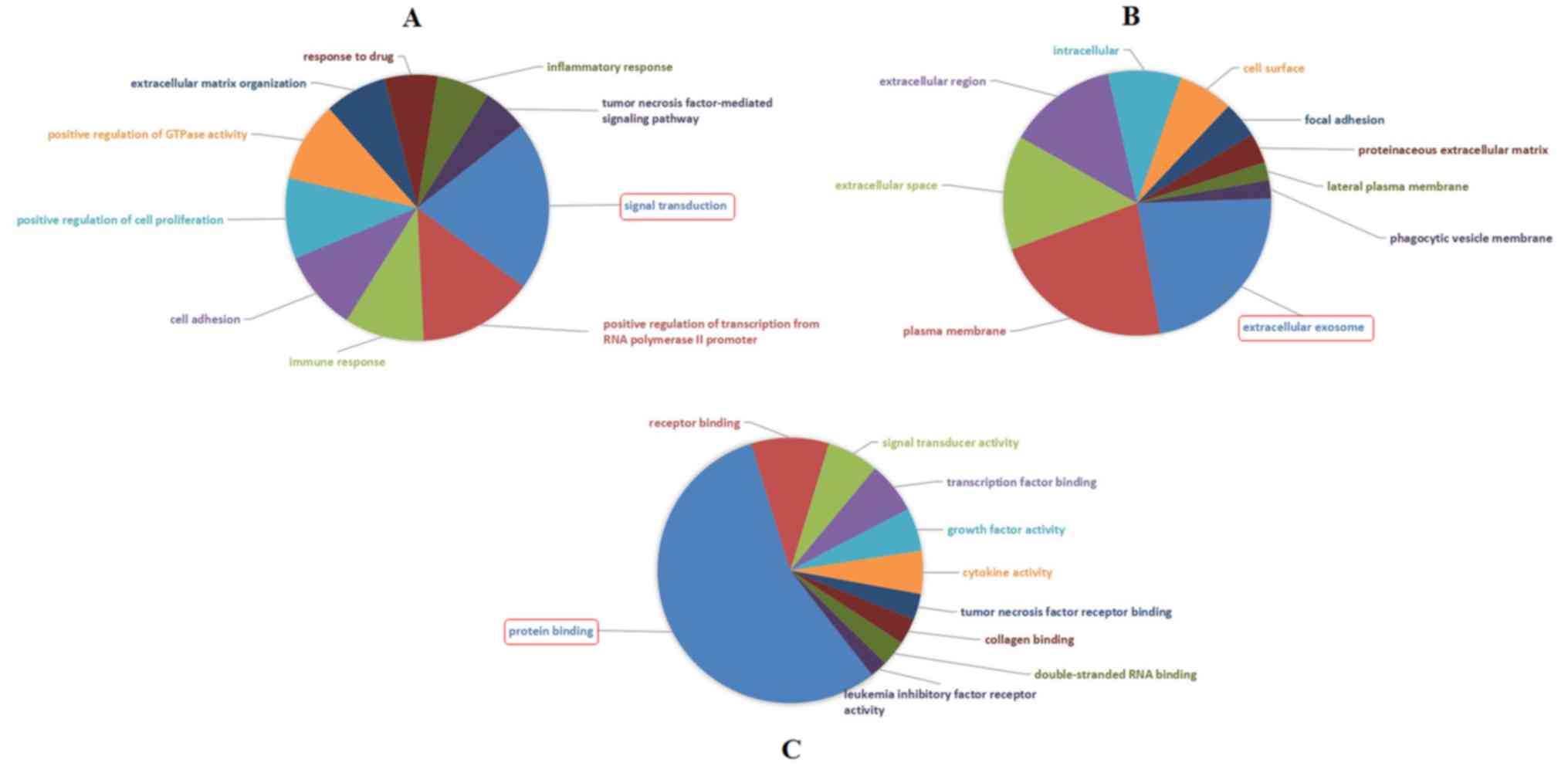
GO analysis illustrated that the most significantly
enriched GO terms corresponded to DEGs were ‘Signal transduction’
(GO: BP) (Fig. 4A), ‘Extracellular
exosome’ (GO: CC) (Fig. 4B) and
‘Protein binding’ (GO: MF) (Fig.
4C).
Additionally, KEGG pathway analysis illustrated that
the DEGs were enriched in 24 pathways, such as ‘Pathway in cancer’,
‘PI3K-Akt signaling pathway’, ‘Cytokine-cytokine receptor
interaction’, ‘Rap1 signaling pathway’, ‘NF-κB signaling pathway’
and ‘Leukocyte trans-endothelial migration’ (Table I).
 | Table I.Enriched pathways corresponding to
differentially expressed genes (DEGs). |
Table I.
Enriched pathways corresponding to
differentially expressed genes (DEGs).
| Term | Count | Genes |
|---|
| Pathways in
cancer | 14 | FGF7, MMP9, CDH1,
BIRC3, CXCL12, ITGB1, LAMA4, CCND1, ITGA6, GNAQ, PLCG2, PDGFRA,
GNAS, TRAF5 |
| PI3K-Akt signaling
pathway | 9 | CSH1, LAMA4, CCND1,
FGF7, ITGA6, COL6A3, PDGFRA, ITGB1, SPP1 |
| Cytokine-cytokine
receptor interaction | 8 | TNFSF4, TNFSF11,
IL6ST, PDGFRA, LIFR, TNFRSF8, CXCL12, LTB |
| Focal adhesion | 8 | LAMA4, CCND1,
ITGA6, COL6A3, PDGFRA, BIRC3, ITGB1, SPP1 |
| Rap1 signaling
pathway | 7 | FYB, FGF7, GNAQ,
PDGFRA, CDH1, GNAS, ITGB1 |
| HTLV-I
infection | 7 | CCND1, EGR2, ETS2,
PDGFRA, HLA-A, HLA-DMB, MYBL2 |
| NF-κB signaling
pathway | 6 | TNFSF11, PLCG2,
BIRC3, CXCL12, TRAF5, LTB |
| Small cell lung
cancer | 6 | LAMA4, CCND1,
ITGA6, BIRC3, TRAF5, ITGB1 |
| Leukocyte
transendothelial migration | 5 | CYBB, MMP9, PLCG2,
CXCL12, ITGB1 |
| ECM-receptor
interaction | 5 | LAMA4, ITGA6,
COL6A3, ITGB1, SPP1 |
| Toxoplasmosis | 5 | LAMA4, ITGA6,
HLA-DMB, BIRC3, ITGB1 |
| Platelet
activation | 5 | GNAQ, PLCG2,
GUCY1A3, GNAS, ITGB1 |
| Cell adhesion
molecules (CAMs) | 5 | LAMA4, ITGA6,
COL6A3, ITGB1, SPP1 |
| Jak-STAT signaling
pathway | 5 | LAMA4, ITGA6,
HLA-DMB, BIRC3, ITGB1 |
| Calcium signaling
pathway | 5 | GNAQ, GRIN2D,
PLCG2, PDGFRA, GNAS |
| Epstein-Barr virus
infection | 5 | PLCG2, VIM, HLA-A,
ENTPD1, TRAF5 |
| Inflammatory bowel
disease (IBD) | 4 | MAF, STAT4, RORA,
HLA-DMB |
| Melanoma | 4 | CCND1, FGF7,
PDGFRA, CDH1 |
| Salivary
secretion | 4 | GNAQ, LYZ, GUCY1A3,
GNAS |
| Gap junction | 4 | GNAQ, PDGFRA,
GUCY1A3, GNAS |
| Rheumatoid
arthritis | 4 | TNFSF11, HLA-DMB,
CXCL12, LTB |
| Circadian
entrainment | 4 | GNAQ, GRIN2D,
GUCY1A3, GNAS |
| Inflammatory
mediator regulation of TRP channels | 4 | GNAQ, PLCG2, PRKCH,
GNAS |
| Bladder cancer | 3 | CCND1, MMP9,
CDH1 |
Establishment of the PPI network and
identification of MMP9 as a hub gene
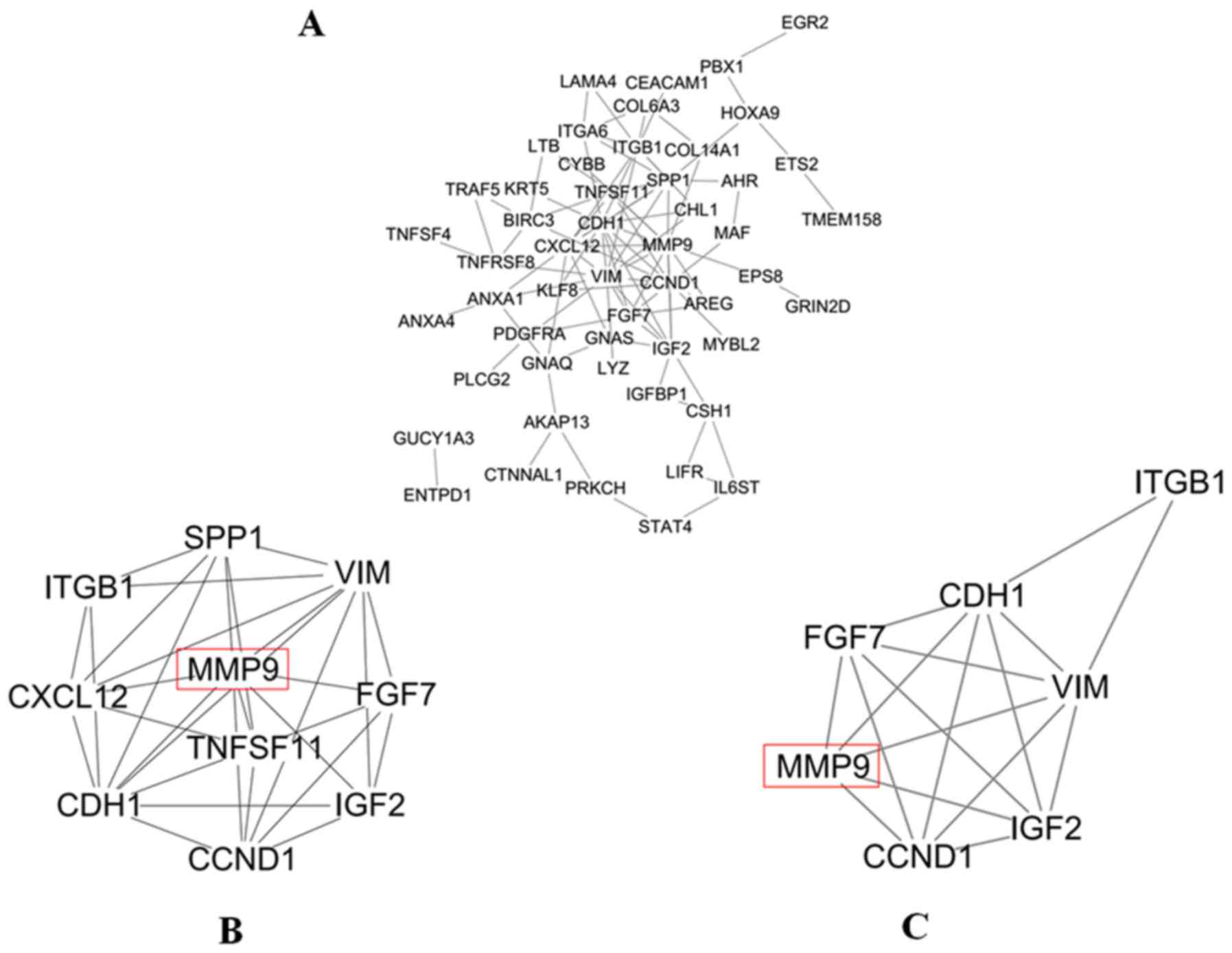
The PPI network of DEGs was constructed by STRING;
it consisted of 52 nodes and 95 edges (Fig. 5A). Moreover, the top 10 degree genes
in the PPI network were selected in MCL using cytohubba, e.g.
VIM, MMP9, CDH1, CCND1, ITGB1, SPP1, CXCL12, IGF2, TNFSF11
and FGF7 genes (Fig. 5B). In
addition, 4 genes in the top 10 degree genes were identified as hub
genes in MCL, e.g. VIM, MMP9, CDH1 and CCND1 genes,
when ‘Degree ≥10’ was set as the cut-off criterion. Then, using
MCODE, clusters were selected from the PPI network. It was shown
that the most significant cluster had 7 nodes and 17 edges. MCODE
analysis demonstrated that the most significant cluster contained
the four hub genes (VIM, MMP9, CDH1 and CCND1)
(Fig. 5C). A member of the
cancer-related genes, MMP9 had the second highest degree in the PPI
network of MCL. Its involvement in invasion and metastasis was
found to be consistent with the malignant biological behavior of
MCL. Meanwhile, the 3D X-ray crystallography structure of MMP9 was
retrieved from the database of PDB using PDB ID:1L6J which had a
resolution of 2.5 Å. Therefore, MMP9 was chosen for follow-up
study.
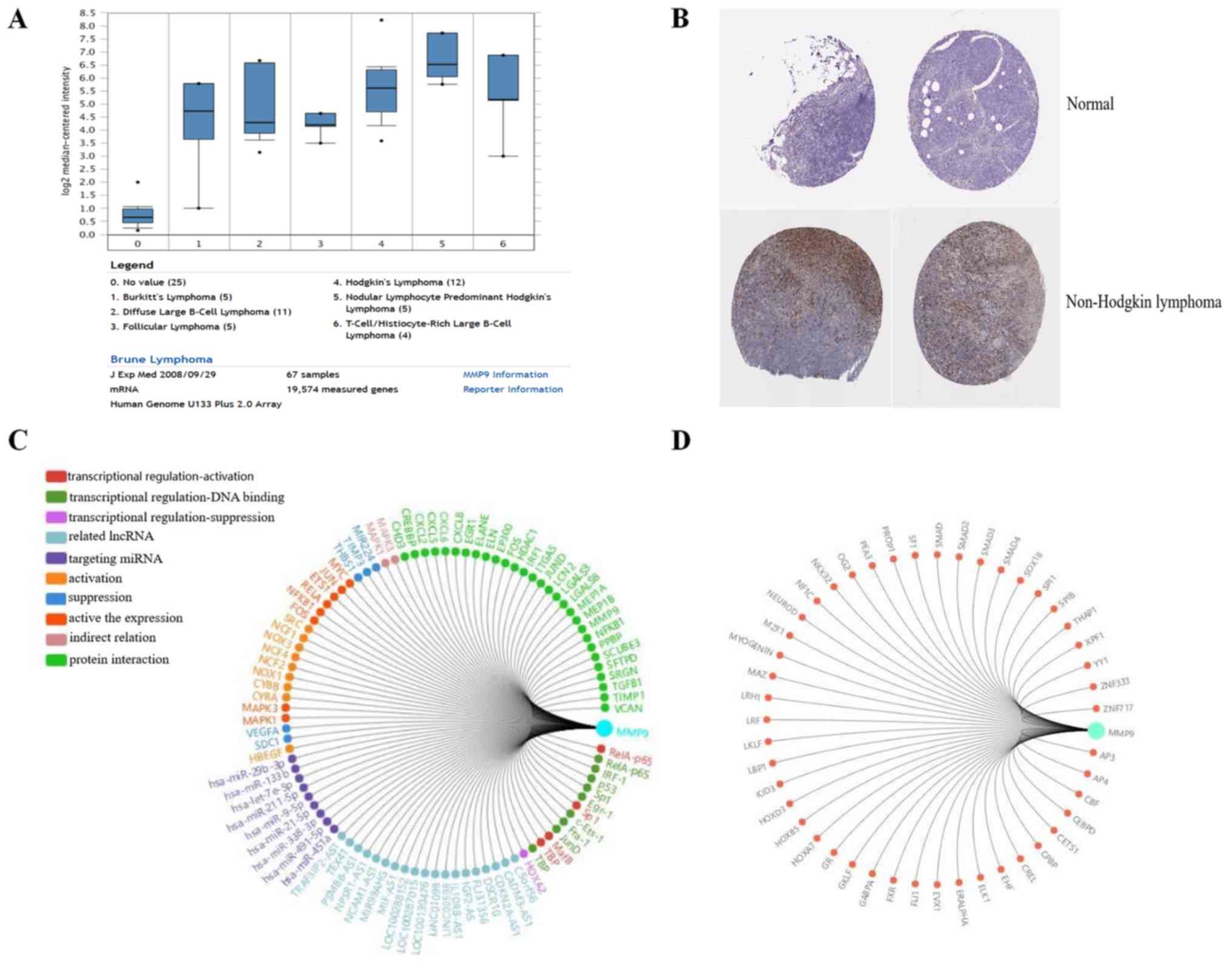
MMP9 serves a key function in lymphoma
progression
The association between MMP9 and lymphoma was
further analyzed. Using Oncomine analysis (18), the expression level of MMP9 was
determined in lymphoma. It was identified that MMP9 was upregulated
in a number of types of lymphoma, including Burkitt's lymphoma,
diffuse large B cell lymphoma, follicular lymphoma and Hodgkin's
lymphoma (19) (Fig. 6A). In addition, the
immunohistochemical staining results of MMP9, obtained from the
Human Protein Atlas database (www.proteinatlas.org), were analyzed, which revealed
that MMP9 was upregulated in non-Hodgkin lymphoma, compared with
normal lymphoid tissue (Fig. 6B)
(http://www.proteinatlas.org/ENSG00000100985-MMP9/pathology/tissue/lymphoma).
Using Gene-RADAR analysis (www.gcbi.com.cn), the regulatory network and related
transcription factors of MMP9 were predicted (Fig. 6C and D). Taken together, MMP9 may be
involved in lymphoma progression.
Molecular docking
MOE docking program generated 10 conformations for
every piece of peptide. Conformations were sorted according to two
parameters: S score plus top ranking conformation with minimum S
score was further analyzed. Peptide 2 was ranked as the top
conformation followed by peptide 3. Other conformations had scores
close to each other (Table II).
The most promising conformation for each peptide was analyzed to
find hydrogen bonding/π-π interactions.
 | Table II.Peptide interaction with MMP9. |
Table II.
Peptide interaction with MMP9.
| Sr. No. | Peptide | S score | RMSD | Interacting
residues | Close contact
residues |
|---|
| Template |
Cys-TPRCGVPDL-Cys | −61.8677 | 6.2413 | Zn500, Leu188,
Glu402, His411 | Leu187, Ala189,
His190, Ala191, Phe192, His401, His405, Asp410, Pro421 |
| 1 |
Cys-TFKEGVPDL-Cys | −62.0522 | 8.4138 | Zn500 | Gly186, Leu187,
His190, His401, His405, His411, Pro421, Met422 |
| 2 |
Cys-TFKEIVPDL-Cys | −85.8988 | 8.1607 | Zn500, His190,
Glu402 | Leu187, Ala189,
His401, His405, His411, Pro421, Met422 |
| 3 |
Cys-TFKEMVPDL-Cys | −82.7207 | 4.0789 | Zn500 | Gly186, Leu187,
Leu188, Ala191, His401, Glu402, His405, His411, Pro421, Met422,
Tyr423 |
| 4 |
Cys-TFKELVPDL-Cys | −68.4973 | 3.3641 | Zn500 | Gly186, Leu187,
Leu188, His401, Glu402, His405, Asp410, His411, Pro421, Met422,
Tyr423 |
| 5 |
Cys-TFKRGVPDL-Cys | −71.9705 | 1.6998 | Zn500, Ala191,
Leu409, Asp410 | Leu187, Phe192,
Pro193, His401, His405, Leu409, Asp410, His411, Pro421 |
| 6 |
Cys-TPKCWVPDL-Cys | −68.3860 | 1.7706 | Zn500, Ala191 | Leu187, Ala189,
His190, Phe192, His401, Glu402, His405, Asp410, His411, Pro421 |
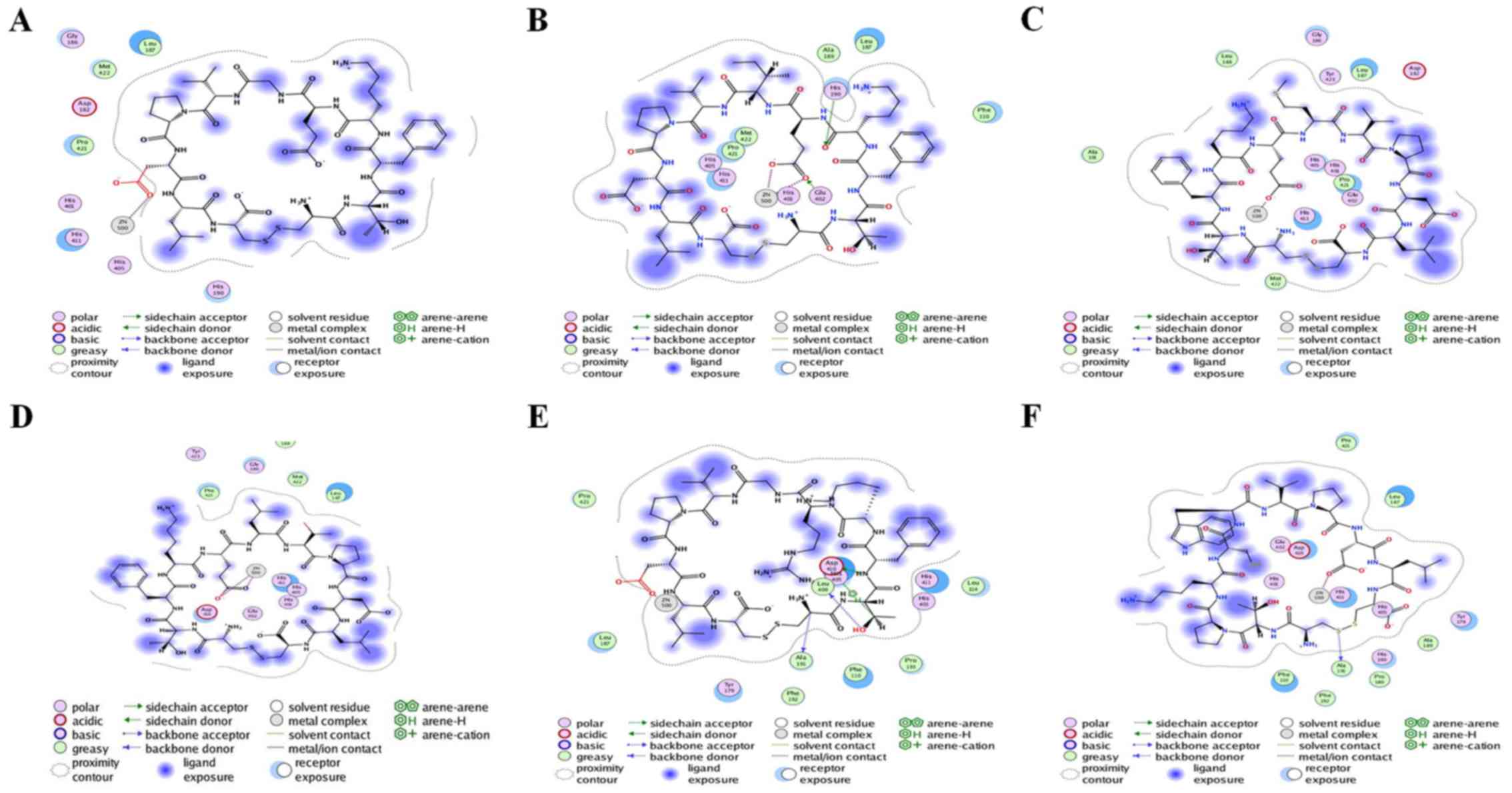
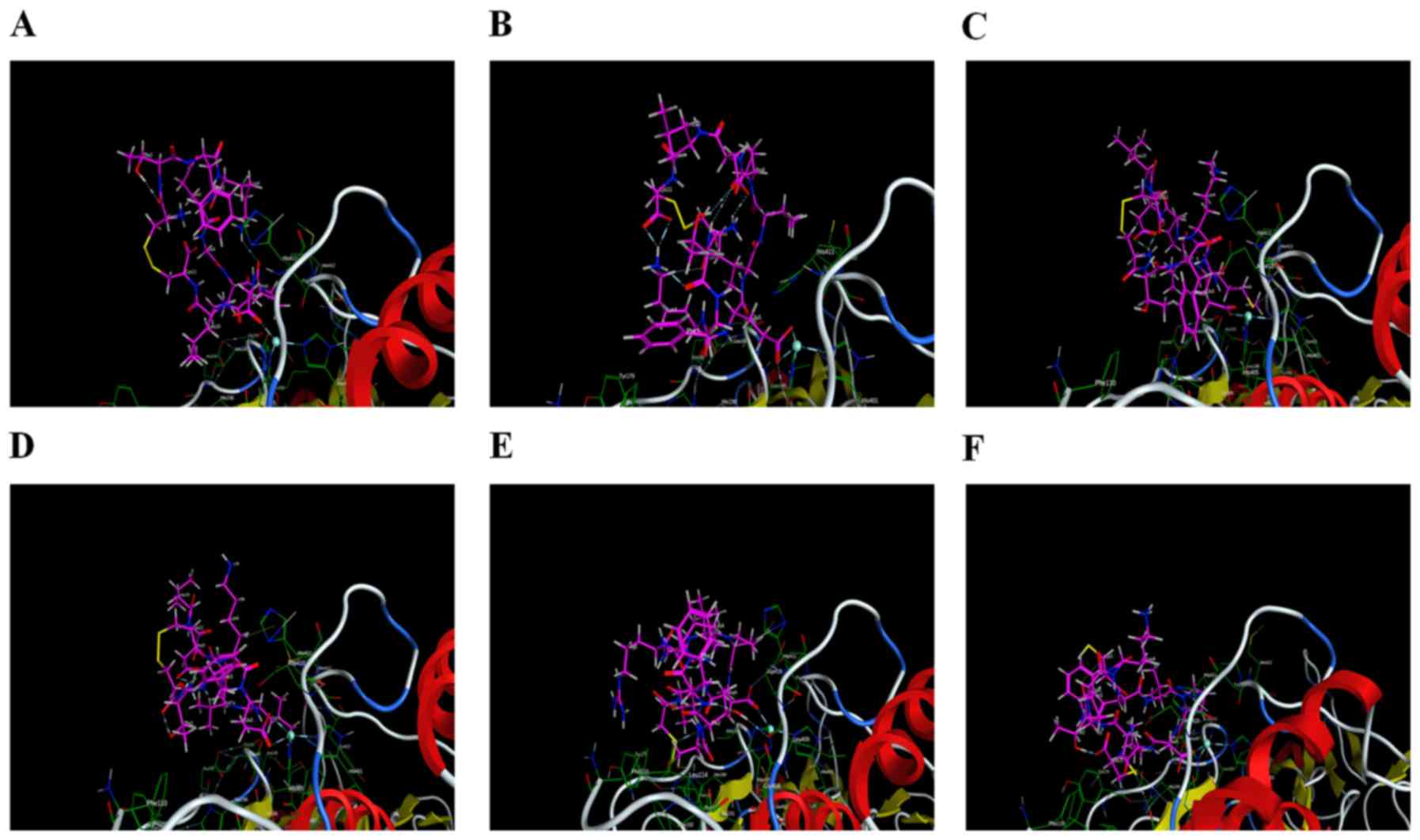
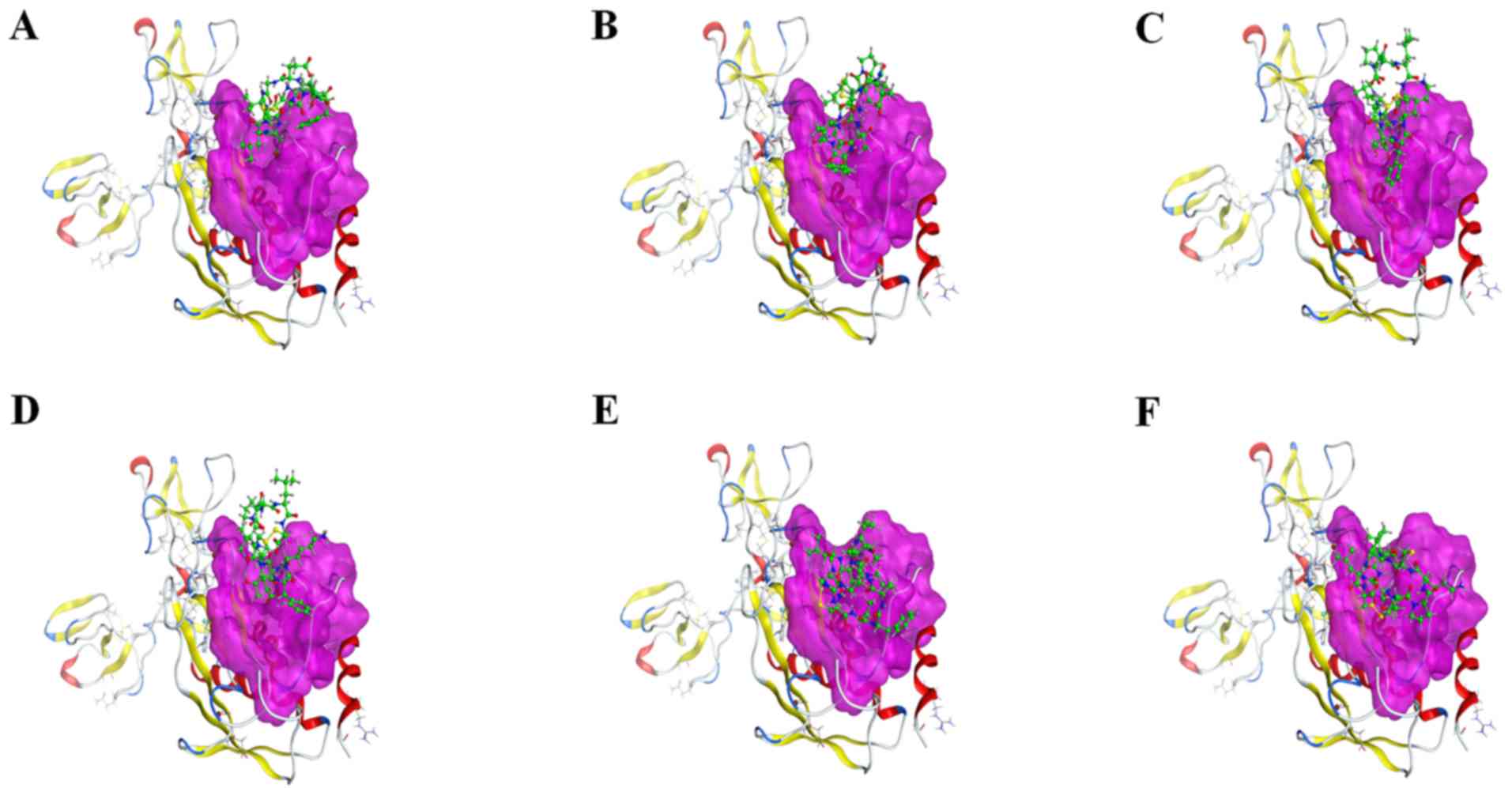
Interaction analysis
Six peptides were found to be able to bind to
Zn2+ in the active site and affect the enzymatic
activity. Peptide 2 also exhibited interactions with the two
residues (His190 and Glu402), in addition to having a minimum S
score. Therefore, peptide 2 could serve as a drug candidate against
active MMP9. Additionally, peptide 5 was ranked third, and it had
potential interactions with Ala191, Leu409 and Asp410 of the active
sites. Peptide 6 had strong interaction with Ala191 of the active
sites. All other peptides (1, 3 and 4) did not have any potential
interaction with the active site but had hydrophobic contact with
active residues of the catalytic domain. Interacting residues of
active sites are shown in Table
II. Interactions between receptor and ligands are shown in
Figs. 7 and 8. The binding mode of ligands with the
receptor protein is shown in Fig.
9.
Discussion
Mantle cell lymphoma (MCL) is a rare and incurable
type of non-Hodgkin's lymphoma. Standard treatment for young
patients includes cytarabine-based induction, which is then
followed up with autologous stem cell transplant. This is well
supported by large randomized trial data. Many patients are not
eligible for this intensive approach due to advanced age,
comorbidities and increased toxicities (20). Molecular targeting strategies have
improved the outcome of MCL patients (21). These targeting strategies include
bortezomib, lenalidomide, temsirolimus, and especially inhibitors
of the B-cell receptor pathway. Nevertheless, the efficacy noted is
not identical for all patients with MCL, partly due to the
complexity of MCL. Therefore, investigation of the molecular
mechanisms involved in MCL and the exploration of specifically
targeted new agents are essential and urgent.
Eighty-four differentially expressed genes (DEGs)
were identified in this study. The DEGs were enriched in 24
pathways, such as ‘Pathway in cancer’, ‘PI3K-Akt signaling
pathway’, ‘Cytokine-cytokine receptor interaction’, ‘Rap1 signaling
pathway’, ‘NF-κB signaling pathway’ and ‘Leukocyte transendothelial
migration’. Among the 84 DEGs, 4 genes were identified as hub genes
in MCL: VIM, MMP9, CDH1 and CCND1 genes.
Additionally, 2 clusters were obtained from the PPI network using
MCODE, and the most significant cluster contained the four hub
genes. Finally, tumor-related gene MMP9 attracted our attention,
and the 3D X-ray crystallography structure of MMP9 in PDB provided
favorable conditions for the study.
Matrix metalloproteinase 9 (MMP9), a member of the
gelatinase family of matrix metalloproteinases (MMPs) that degrades
ECM proteins, plays a major role in microvascular remodeling and
cell migration during morphogenesis and wound healing (22–24).
The expression level of MMP9 can be abnormally elevated in most
tumors, and is subject to complex regulation. This could include a
very complex regulation process at the level of transcription, mRNA
dendritic translocation, and local translation as well as protein
activation (23). Indeed, a high
MMP9 level is associated with a poor prognosis in cancer patients
(25–27).
The invasive nature and migratory capacity of tumor
cells can be reduced by MMP inhibitors (28). The search for MMP inhibitors which
are specifically designed to be safe and effective remains a
‘hotspot’ of cancer research. MMP9, an extracellular acting
Zn2+-dependent endopeptidase, is released
extracellularly in a latent, proform with the enzymatic site
covered by a propeptide which needs to be severed off to expose the
Zn2+ binding region, interact with the substrate and
reveal the activity. From the model of interaction with the
substrate or inhibitor, it can be divided into two parts: i)
Zn2+ binding site of the catalytic activity center; ii)
hydrophobic zone S2, S1′, S1′, S2′ (29). As these active sites are important
in enzymatic activity; therefore, targeting it may block enzymatic
activity.
Computational techniques allow the evaluation of the
binding affinity of compounds before synthesizing them in the
laboratory (30). These
computational techniques provide information concerning
cancer-related genes. They, therefore, aid in the development of
new inhibitory compounds. Molecular docking is such a technique.
Molecular docking is used in binding orientation of small molecules
against their targets. This technique is also vital in the
identification of new inhibitory compounds against certain diseases
(31). This study focused on the
design and molecular docking of cyclic peptides against active
MMP9.
We studied the prospective for six peptides against
active MMP9. The six peptides were stabilized, by adding a
disulfide bridge, which converted them into the much more stable
cyclic forms (32).
In the present study, peptides were docked with
active MMP9 to determine their affinity as MMP9 inhibitors. Only
top conformations were selected. The negative and low score for any
ligand shows favorable interactions between the ligand and the
receptor protein. Our results demonstrated that peptide 2 had a
minimum S score and also had promising interactions with active
site residues. All the peptides had low energy; they therefore
formed a stable structure.
As observed in the study, active MMP9 was blocked by
peptide 2. Thus, it may serve as a drug candidate for active MMP9.
Additionally, peptide 5 and 6 had potential results that could
serve as important drug candidates to block MMP9 activity. However,
the designed cyclic peptides were not tested in mantle cell
lymphoma in vivo nor in vitro. Further studies need
to be conducted on the validity, metabolism, and side effects of
the proposed peptides.
No drug has yet been developed to cure MCL, a rare
and aggressive disease. New and better strategies are required to
develop drug candidates that can treat MCL. This study is based on
bioinformatic analysis of the MCL mechanism, and focuses on drug
candidates that can target MMP9. MMPs are inhibited by compounds
containing zinc-chelating groups, but few inhibitors specific for
MMP9 have been developed; the most promising MMP9 inhibitors also
inhibit other members of the MMP family (33). We used molecular docking to design
selective MMP9 inhibitors based on protein structure. This study
revealed cyclic peptide (CTFKEIVPDLC), which is able to interact
with the sites of active MMP9.
The present study was proven to be valuable prior to
synthesizing drugs. Additionally, cyclic peptides could be used as
future candidates for drugs against MCL, as they possess binding
affinity against MMP9. The results of this study are useful for
drug development. The results can assist in the aided-screening of
drugs against MCL.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets (GSE32018 and GSE9327) (11,12)
analyzed during the current study are available in Gene Expression
Omnibus (GEO) www.ncbi.nlm.nih.gov/pubmed.
Crystallographic data for MMP9 analyzed during the
current study are available in Protein Data Bank (PDB) www.rcsb.org. The expression level of MMP9 analyzed
during the current study is available in Oncomine www.oncomine.com (18). The immunohistochemistry staining
results of MMP9 analyzed during the current study are available in
the Human Protein Atlas database www.proteinatlas.org. The regulatory network and
related transcription factors of MMP9 analyzed during the current
study are available in Gene-RADAR www.gcbi.com.cn.
Authors' contributions
WY designed this study and conducted bioinformatic
analysis and molecular docking. SXL participated in sorting the
data and in drafting of the manuscript. HG participated in drafting
of the manuscript, provided research guidance, and is the
corresponding author. MW participated in drafting of the
manuscript. All authors actively contributed to the paper on an
intellectual level. All authors read and approved the final
manuscript. All authors agreed to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MCL
|
mantle cell lymphoma
|
|
GEO
|
Gene Expression Omnibus
|
|
DEGs
|
differentially expressed genes
|
|
PPI
|
protein-protein interaction
|
|
MCODE
|
Molecular Complex Detection
|
|
PDB
|
Protein Data Bank
|
|
MOE
|
Molecular Operating Environment
|
|
R-CHOP
|
rituximab, cyclophosphamide,
doxorubicin hydrochloride, vincristine, prednisone
|
|
MMP9
|
matrix metalloproteinase 9
|
|
MMPs
|
matrix metalloproteinases
|
|
MMPIs
|
MMP inhibitors
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
3D
|
three-dimensional
|
|
FnII
|
fibronectin type II
|
|
BP
|
biological process
|
|
CC
|
cell component
|
|
MF
|
molecular function
|
|
ECM
|
extracellular matrix
|
References
|
1
|
Li XY, Zhang L, Liu X, Feng L and Wang X:
The antitumor effects of arsenic trioxide in mantle cell lymphoma
via targeting Wnt/β-catenin pathway and DNA methyltransferase-1.
Oncol Rep. 38:3114–3120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen JB, Han X, Jemal A, Ward EM and
Flowers CR: Deferred therapy is associated with improved overall
survival in patients with newly diagnosed mantle cell lymphoma.
Cancer. 122:2356–2363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ratsch BA, Grau M, Döken B, Lenz P and
Lenz G: The use of microarry technologies in mantle cell lymphoma.
Semin Hematol. 48:166–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan F, Lu J, Yu W, Zhang Y, Su S, Pang L
and Zhu B: MicroRNA-26b-5p regulates cell proliferation, invasion
and metastasis in human intrahepatic cholangiocarcinoma by
targeting S100A7. Oncol Lett. 15:386–392. 2018.PubMed/NCBI
|
|
5
|
Klassen LMB, Chequin A, Manica GC,
Biembengut IV, Toledo MB, Baura VA, de O Pedrosa F, Ramos EAS,
Costa FF, de Souza EM, et al: MMP9 gene expression regulation by
intragenic epigenetic modifications in breast cancer. Gene.
642:461–466. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang XZ, Cui SZ, Zeng LS, Cheng TT, Li XX,
Chi J, Wang R, Zheng XF and Wang HY: Overexpression of Rab 1B and
MMP9 predicts poor survival and good response to chemotherapy in
patients with colorectal cancer. Aging. 9:914–931. 2017.PubMed/NCBI
|
|
7
|
EI-Sharkawi F, EI Sabah M, Hassan Z and
Khaled H: The biochemical value of urinary metalloproteinases 3 and
9 in diagnosis and prognosis of bladder cancer in Egypt. J Biomed
Sci. 21:722014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pouyanfar N, Monabbati A, Sharifi AA and
Dianatpour M: Expression levels of MMP9 and PIWIL2 in prostate
cancer: a case-control study. Clin Lab. 62:651–657. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong Y, Lu YT, Sun Y, Shi ZH, Li NG, Tang
YP and Duan JA: Recent opportunities in matrix metalloproteinase
inhibitor drug design for cancer. Expert Opin Drug Discov.
13:75–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kist R, Timmers LFSM and Caceres RA:
Searching for potential mTOR inhibitors: Ligand-based drug design,
docking and molecular dynamics studied of rapamycin binding site. J
Mol Graph Model. 80:251–263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gómez-Abad C, Pisonero H, Blanco-Aparicio
C, Roncador G, González-Menchén A, Martinez-Climent JA, Mata E,
Rodríguez ME, Muñoz-González G, Sánchez-Beato M, et al: PIM2
inhibition as a rational therapeutic approach in B-cell lymphoma.
Blood. 118:5517–5527. 2001. View Article : Google Scholar
|
|
12
|
Ruiz-Vela A, Aggarwal M, de la Cueva P,
Treda C, Herreros B, Martín-Pérez D, Dominguez O and Piris MA:
Lentiviral (HIV)-based RNA interference screen in human B-cell
receptor regulatory networks reveals MCL1-induced oncogenic
pathways. Blood. 11:1665–1676. 2008.
|
|
13
|
Liao YX, Zhang ZP, Zhao J and Liu JP:
Effects of fibronectin 1 on cell proliferation, senescence and
apoptosis of human glioma cells through the PI3K/AKT signaling
pathway. Cell Physiol Biochem. 48:1382–1396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan J, Qian X, Song B, An X, Cai T, Zuo Z,
Ding D, Lu Y and Li H: Integrated bioinformatics analysis reveals
that the expression of cathepsin S is associated with lymph node
metastasis and poor prognosis in papillary thyroid cancer. Oncol
Rep. 40:111–122. 2018.PubMed/NCBI
|
|
15
|
Zang Y, Gu L, Zhang Y, Wang Y and Xue F:
Identification of key genes and pathways in uterine leiomyosarcoma
through bioinformatics analysis. Oncol Lett. 15:9361–9368.
2018.PubMed/NCBI
|
|
16
|
Elkins PA, Ho YS, Smith WW, Janson CA,
D'Alessio KJ, McQueney MS, Cummings MD and Romanic AM: Structure of
the C-terminally truncated human Pro MMP9, a gelatin-binding matrix
metalloproteinase. Acta Crystallogr D Biol Crystallogr.
58:1182–1192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Appleby TC, Greenstein AE, Hung M,
Liclican A, Velasquez M, Villaseñor AG, Wang R, Wong MH, Liu X,
Papalia GA, et al: Biochemical characterization and structure
determination of a potent, selective antibody inhibitor of human
MMP9. J Biol Chem. 292:6810–6820. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian Y, Xu L, He Y, Xu X, Li K, Ma Y, Gao
Y, Wei D and Wei L: Knockdown of RAC1 and VASP gene expression
inhibits breast cancer cell migration. Oncol Lett. 16:2151–2160.
2018.PubMed/NCBI
|
|
19
|
Brune V, Tiacci E, Pfeil I, Döring C,
Eckerle S, van Noesel CJ, Klapper W, Falini B, von Heydebreck A,
Metzler D, Bräuninger A, et al: Origin and pathogenesis of nodular
lymphocyte-predominant Hodgkin lymphoma as revealed by global gene
expression analysis. J Exp Med. 205:2251–2268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Steiner RE, Romaguera J and Wang M:
Current trials for frontline therapy of mantle cell lymphoma. J
Hematol Oncol. 11:132018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin P: Optimizing therapy for mantle
cell lymphoma. Hematology Am Soc Hematol Educ Program.
2017:304–309. 2017.PubMed/NCBI
|
|
22
|
Hou C, Miao Y, Ji H, Wang S, Liang G,
Zhang Z and Hong W: 6-Gingerol inhibits hair cycle via induction of
MMP2 and MMP9 expression. An Acad Bras Cienc. 89:2707–2717. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Phillips TM, Fadia M, Lea-Henry TN, Smiles
J, Walters GD and Jiang SH: MMP2 and MMP9 associate with crescentic
glomerulonephritis. Clin Kidney J. 10:215–220. 2017.PubMed/NCBI
|
|
24
|
Sakata K, Satoh M, Someya M, Asanuma H,
Nagakura H, Oouchi A, Nakata K, Kogawa K, Koito K, Hareyama M and
Himi T: Expression of matrix metalloproteinase 9 is a prognostic
factor in patients with non-Hodgkin lymphoma. Cancer. 100:356–365.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xue Q, Cao L, Chen XY, Zhao J, Gao L, Li
SZ and Fei Z: High expression of MMP9 in glioma affects cell
proliferation and is associated with patient survival rates. Oncol
Lett. 13:1325–1330. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klimczak-Bitner AA, Kordek R, Bitner J,
Musial J and Szemraj J: Expression of MMP9, SERPINE1 and miR-134 as
prognostic factors in esophageal cancer. Oncol Lett. 12:4133–4138.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu Y, Ding Z, Jian H, Shen L, Zhu L and Lu
S: Prognostic value of MMP9 activity level in resected stage I B
lung adenocarcinoma. Cancer Med. 5:2323–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Piperigkou Z, Manou D, Karamanou K and
Theocharis AD: Strategies to target matrix metalloproteinases as
therapeutic approach in cancer. Methods Mol Biol. 1731:325–348.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vandooren J, Van den Steen PE and
Opdenakker G: Biochemistry and molecular biology of gelatinase B or
matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev
Biochem Mol Biol. 48:222–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khan MF, Nahar N, Rashid RB, Chowdhury A
and Rashid MA: Computational investigations of physicochemical,
pharmacokinetic, toxicological properties and molecular docking of
betulinic acid, a constituent of Corypha taliera (Roxb.) with
Phospholipase A2 (PLA2). BMC Complement Altern Med. 18:482018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumar A, Srivastava G, Negi AS and Sharma
A: Docking, molecular dynamics, binding energy-MM-PBSA studies of
naphthofuran derivatives to identify potential dual inhibitors
against BACE-1 and GSK-3β. J Biomol Struct Dyn. 19:1–16. 2018.
View Article : Google Scholar
|
|
32
|
Ojo OS, Nardone B, Musolino SF, Neal AR,
Wilson L, Lebl T, Slawin AMZ, Cordes DB, Taylor JE, Naismith JH, et
al: Synthesis of the natural product descurainolide and cyclic
peptides from lignin-derived aromatics. Org Biomol Chem.
16:266–273. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gossage DL, Cieslarová B, Ap S, Zheng H,
Xin Y, Lai P, Chen G, Smith V and Sundy JS: Phase 1b study of the
safety, pharmacokinetics, and disease-related outcomes of the
matrix metalloproteinase-9 inhibitor andecaliximab in patients with
rheumatoid arthritis. Clin Ther. 40:156–165. 2018. View Article : Google Scholar : PubMed/NCBI
|