Introduction
Pancreatic carcinoma is a common type of cancer
associated with high mortality rates and is the fourth leading
cause of cancer-associated mortality globally (1). Pancreatic ductal adenocarcinoma (PDAC)
is the most common type of pancreatic cancer. The 5-year overall
survival rate for patients with PDAC is ~5% and median survival
time is <6 months (2). Surgical
removal of the tumor is the most effective and preferred therapy
(3). However, PDAC is characterized
by invasive growth and early metastasis, often manifesting in
advanced clinical stage at presentation and rapid postoperative
recurrence, and only one fifth of patients with PDAC are diagnosed
early enough to be candidates for surgical resection (4). Furthermore, due to the frequent
recurrence of PDAC, the median survival time even among surgically
resected cases is <2 years (1).
Therefore, an understanding of the mechanisms of PDAC metastasis
and the identification of the factors involved in early metastasis
are required to improve treatment and disease outcomes in PDAC.
PDAC is associated with significant intra- and
peritumorial inflammation (5).
Chronic pancreatitis is a risk factor for PDAC, and new-onset
pancreatitis is a common symptom of PDAC. Upregulation of
inflammation-associated signaling modulates PDAC progression and
therapeutic resistance by inducing proliferation and metastasis,
and by suppressing apoptosis (6,7).
Macrophage migration inhibitory factor (MIF) is a pleiotropic
inflammatory cytokine that is associated with carcinogenesis
(8). Through autocrine or paracrine
signaling, MIF interacts with cluster of differentiation (CD)74,
its primary receptor, and C-X-C chemokine receptor type 4, its
co-receptor, to activate the AKT serine/threonine kinase (AKT) and
extracellular-signal-regulated kinase (ERK) pathways (9,10).
Previous studies from different groups have identified an increased
expression level and tumor-promoting functions of MIF in PDAC
(11–14). MIF knockdown inhibits ERK1/2 and AKT
phosphorylation, and upregulates p53 expression, in turn leading to
cell cycle arrest and apoptosis in pancreatic cancer cells
(11,12). Recently, Yang et al (13) reported a novel signaling pathway
whereby MIF upregulates miR-301b, which subsequently targets
nuclear receptor subfamily 3 group C member 2. Inhibition of this
signaling axis may reduce metastasis and prolong survival in a
mouse model of PDAC (13).
Furthermore, MIF induces the epithelial to mesenchymal transition
and invasion of PDAC cells (13,14).
Nevertheless, the role of MIF in pancreatic cancer is not clearly
defined.
In the present study, MIF were demonstrated to
enhance cyclin D1 and matrix metalloproteinase (MMP)-2 expression
by activating AKT and ERK signaling; subsequently promoting
metastasis of PDAC cells. We also show that upregulation of MIF is
a frequent event in PDAC, and correlates with unfavorable prognosis
in pancreatic cancer.
Materials and methods
PDAC tissue samples
The present study was approved by the Human Research
Ethics Committees of the First Affiliated Hospital of Sun Yat-sen
University (approval no. 201515; Guangzhou, China), according to
the Declaration of Helsinki. All of patients enrolled in the
present study provided written informed consent.
Human PDAC tissues were collected from 85 patients
who underwent resection at the First Affiliated Hospital of Sun
Yat-sen University between 2003 and 2007. Patients did not receive
any local or systemic chemotherapeutic treatments prior to the
surgery. Tumor histopathology was independently classified
according to the World Health Organization Classification of Tumors
by two pathologists (15). All
patients were followed postoperatively to assess survival outcomes.
The relevant characteristics of patients are listed in Table I.
 | Table I.Association between MIF Expression
and clinical features. |
Table I.
Association between MIF Expression
and clinical features.
|
|
| MIF |
|
|---|
|
|
|
|
|
|---|
| Variables | Case no. | Low | High |
P-valuea |
|---|
| Sex |
|
|
| 0.156 |
|
Male | 51 | 22 | 29 |
|
|
Female | 34 | 20 | 14 |
|
| Age, years |
|
|
| 0.023b |
|
>50 | 69 | 30 | 39 |
|
|
≤50 | 16 | 12 | 4 |
|
| Tumor size, cm |
|
|
| 0.614 |
|
>3 | 48 | 22 | 26 |
|
| ≤3 | 37 | 18 | 17 |
|
|
Differentiation |
|
|
| 0.892 |
| Well
and moderate | 52 | 26 | 26 |
|
|
Poor | 33 | 16 | 17 |
|
| TNM |
|
|
| 0.332 |
|
III/IV | 36 | 20 | 16 |
|
|
I/II | 49 | 22 | 27 |
|
| Lymphatic
spread |
|
|
| 0.036b |
| No | 49 | 29 | 20 |
|
|
Yes | 36 | 13 | 23 |
|
| Hepatic
metastasis |
|
|
| 0.750 |
| No | 66 | 32 | 34 |
|
|
Yes | 19 | 10 | 9 |
|
| Serum CEA,
ng/ml |
|
|
| 0.022b |
|
≥10 | 49 | 19 | 30 |
|
|
<10 | 36 | 23 | 13 |
|
Immunohistochemistry (IHC)
Formalin-fixed paraffin-embedded PDAC tissues were
cut into 5 µm sections, processed for antigen retrieval by pressure
cooking in 10 mM citrate buffer (pH 6.0) and blocked with
UltraCruz® Blocking reagent (cat. no. sc-516214; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) at room temperature for
1 h, followed by incubation at 4°C overnight with rabbit polyclonal
antibody against human MIF (cat. no. sc-20121; Santa Cruz
Biotechnology, Inc.). Immunostaining was performed with the
ChemMate DAKO EnVision Detection kit for
Peroxidase/DAB/Rabbit/Mouse (cat. no. K 5007; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) according to the
manufacturers protocol, which resulted in a brown-colored
precipitate at the antigen site.
MIF in PDAC tissues was evaluated under a light
microscope at ×400 magnification. For each specimen, five images of
representative areas were acquired, and a total of 1,000 tumor
cells were counted. IHC scoring was performed according to a
modified Histo-score (H-score) (16), which includes an assessment of the
fraction of positive cells and the intensity of staining. The
intensity was assigned a score of 0–3, representing no staining for
0, weak staining for 1, moderate staining for 2, and strong
staining for 3. The fraction score was based on the proportion of
positively stained cells (0–100%). The intensity and fraction
scores were multiplied to obtain an H-score that ranged between 0
and 3, and represented the expression level of MIF protein.
RNA oligoribonucleotides, tumor cell,
lines and transfection
Small interfering RNA (siRNA) duplexes that target
the human MIF mRNA (si-MIF) were designed using BLOCK-iT™ RNAi
Designer software (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou,
China). Negative control RNA duplexes (NC) for siRNA were not
homologous to any known human sequence. The nucleotide sequences of
si-MIF and NC are as follows: si-MIF sense,
5′-GGGUCUACAUCAACUAUUAdTdT-3′ and antisense,
5′-UAAUAGUAGUUGUAGACCCdTdT-3′; NC-sense,
5′-UUCUCCGAACGUGUCACGUdTdT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAGAAdTdT-3′.
Human panc-1 and Bxpc-3 pancreatic cancer cell lines
were obtained from the Cell Bank of Type Culture Collection of
Chinese Academy of Sciences (Shanghai, China) and maintained in a
humidified 5% CO2 incubator at 37°C. Human panc-1
pancreatic cancer cell lines were cultured in Dulbeccos modified
Eagles medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.)
and Bxpc-3 pancreatic cancer cells were cultured in RPMI-1640
(RPMI; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences,
Logan, UT, USA). RNA oligonucleotides were transfected using
Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.).
A total of 5×104 cells/well were transfected with 50 nM
RNA in a 24-well plate.
RNA isolation, reverse
transcription-semi-quantitative polymerase chain reaction
(RT-sqPCR) and RT-qPCR
Total RNA was isolated from cells or frozen tumor
tissues using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturers protocol. RNA concentration
and quality were evaluated according to spectrometric determination
at 260 and 280 nm. A total of 2 µg of total RNA was subjected to
DNase I digestion (Fermentas; Thermo Fisher Scientific, Inc.),
followed by RT using Moloney murine leukemia virus reverse
transcriptase (Promega Corporation, Madison, WI, USA) at 42°C for 1
h followed by termination at 75°C for 5 min. Then, mRNA levels of
MIF, cyclin D1, and MMP-2 were analyzed by RT-sqPCR (for cDNA of
PDAC tissues and cell lines) or by SYBR-Green (cat. no. A25742;
Applied Biosystems; Thermo Fisher Scientific, Inc.) RT-qPCR (for
cDNA of cell lines). The specific primers used to amplify the MIF,
cyclin D1, or MMP-2 genes and the housekeeping GAPDH gene were as
follows: MIF forward, 5′-GCAGAACCGCTCCTACAGCA-3′ and reverse,
5′-GGCTCTTAGGCGAAGGTGGA-3′; Cyclin D1 forward,
5′-GCTGCTCCTGGTGAACAAGC-3′ and reverse, 5′-CACAGAGGGCAACGAAGGTC-3′;
MMP-2 forward, 5′-AGAGTGCATGAACCAACCAG-3′ and reverse,
5′-TGTTCAGGTATTGCATGTGCT-3′; GAPDH forward,
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse, 5′-GACAAGCTTCCCGTTCTCAG-3′.
GAPDH expression served as an internal control. For RT-sqPCR, the
cDNA was amplified as follows: 94°C for 30 sec, 58°C for 30 sec and
72°C for 30 sec for 28 cycles; and a final extension at 72°C for 5
min. The products were resolved on a 1.5% agarose gel and
visualized using ethidium bromide staining with GeneSnap software
(version 1.2.0; Syngene, Frederick, MD, USA). RT-qPCR was performed
using a 7800 fast real-time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with the following thermocycling
conditions: 94°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec.
The gene expression levels were normalized to expression of GAPDH
to calculate the 2−ΔΔCq value (17).
Immunoblotting assay
Proteins from cells or frozen tumor tissues were
extracted with RIPA buffer (Invitrogen; Thermo Fisher Scientific,
Inc.) and quantified using a bicinchoninic acid assay. The total
protein (20 µg/lane) was separated on a 12% polyacrylamide gel, and
transferred to a methanol-activated PVDF membrane (EMD Millipore,
Billerica, MA, USA). The membrane was blocked in Tris-buffered
saline-Tween-20 (TBST) containing 5% bovine serum albumin
(Guangzhou Jetway Biotech Co., Ltd., Guangzhou, China) at room
temperature for 1 h, then subsequently immunoblotted with primary
rabbit polyclonal antibodies against MIF (cat. no. sc-20121; Santa
Cruz Biotechnology, Inc.), cyclin D1 (cat. no. 2978; Cell Signaling
Technology, Inc., Beverly, MA, USA), MMP-2 (cat. no. 40994; Cell
Signaling Technology, Inc.) or GAPDH (cat. no. BM1623; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) at 4°C overnight and
then with the secondary antibody (anti-rabbit IgG horseradish
peroxidase-conjugated; cat. no. 7074; Cell Signaling Technology,
Inc.) at room temperature for 1 h. All the primary antibodies were
diluted at a ratio of 1:1,000 and the secondary antibody was
diluted at a ratio of 1:5,000. Protein bands were visualized using
Clarity Western ECL substrate (Bio-Rad Laboratories, CA, USA). The
intensity of each band was densitometrically quantified using
ImageJ software (version 1.0; National Institutes of Health,
Bethesda, MD, USA).
Cell cycle analysis
Cell cycle analyses were performed using a detergent
containing hypotonic solution (Krishans reagent) containing 10
µg/ml propidium iodide (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) according to the manufacturers protocol and
fluorescence-activated cell sorting (FACS) with a Gallios flow
cytometry system (Beckman Coulter, Inc., Brea, CA, USA) assay. Data
were analyzed using Kaluza software (version 1.5a; Beckman Coulter,
Inc.). Nuclear debris and overlapping nuclei were gated out.
In vitro tumor cell invasion
assay
Tumor cell invasion was analyzed in 24-well Boyden
chambers with 8-µm pore size polycarbonate membranes (Corning
Incorporated, Corning, NY, USA). The membranes were coated with 60
µg of Matrigel (cat. no. 3432-005-01; R&D Systems, Inc.,
Minneapolis, MN, USA) to form the matrix barrier. Pancreatic cancer
cells transfected with NC or si-MIF were resuspended in 100 µl
serum-free DMEM 36 h post-transfection, and were added to the upper
compartments of the chambers. The lower compartments were filled
with 600 µl DMEM or RPMI-1640 with 10% FBS. Following an incubation
at 37°C for 24 h, the cells remaining on the upper surfaces of the
membrane that had not invaded through the matrix were removed. The
invaded cells on the lower surfaces of the membrane were fixed with
100% methanol at room temperature for 10 min, stained with 0.1%
crystal violet at room temperature for 15 min and counted under a
light microscope at ×400.
Statistical analysis
Associations between MIF expression and
clinicopathological features were examined using the chi-square
test. Overall survival was calculated as the duration between the
date of tumor resection and the time of mortality. Patients who
were lost to follow-up or succumbed to causes unassociated with
PDAC were treated as censored events. Kaplan-Meier estimator plots
were constructed, and the differences between groups were analyzed
using a log rank test. Univariate and multivariate Cox proportional
hazards regression analysis was performed to investigate the
association between the MIF expression or clinical characteristics
of patients and overall survival. Significant prognostic factors
identified using univariate analysis were further evaluated by
multivariate Cox regression analysis. All statistical tests were
two-tailed, and P<0.05 was considered to indicate a
statistically significant difference. Analyses were performed using
SPSS software (version 19.0; IBM Corp., Armonk, NY, USA).
Data are expressed as the mean ± standard error of
the mean from three independent experiments. Differences between
the groups were analyzed by students t-test or one-way analysis of
variance with Bonferronis post hoc test. Analyses were performed
with GraphPad Prism, version 5 (GraphPad Software, Inc., San Diego,
CA, USA).
Results
Overexpression of MIF is a frequent
event in PDAC tissue
It has been reported that MIF is overexpressed in
pancreatic cancer (11,18). To further confirm this, MIF
expression was evaluated in 85 paired PDAC and adjacent
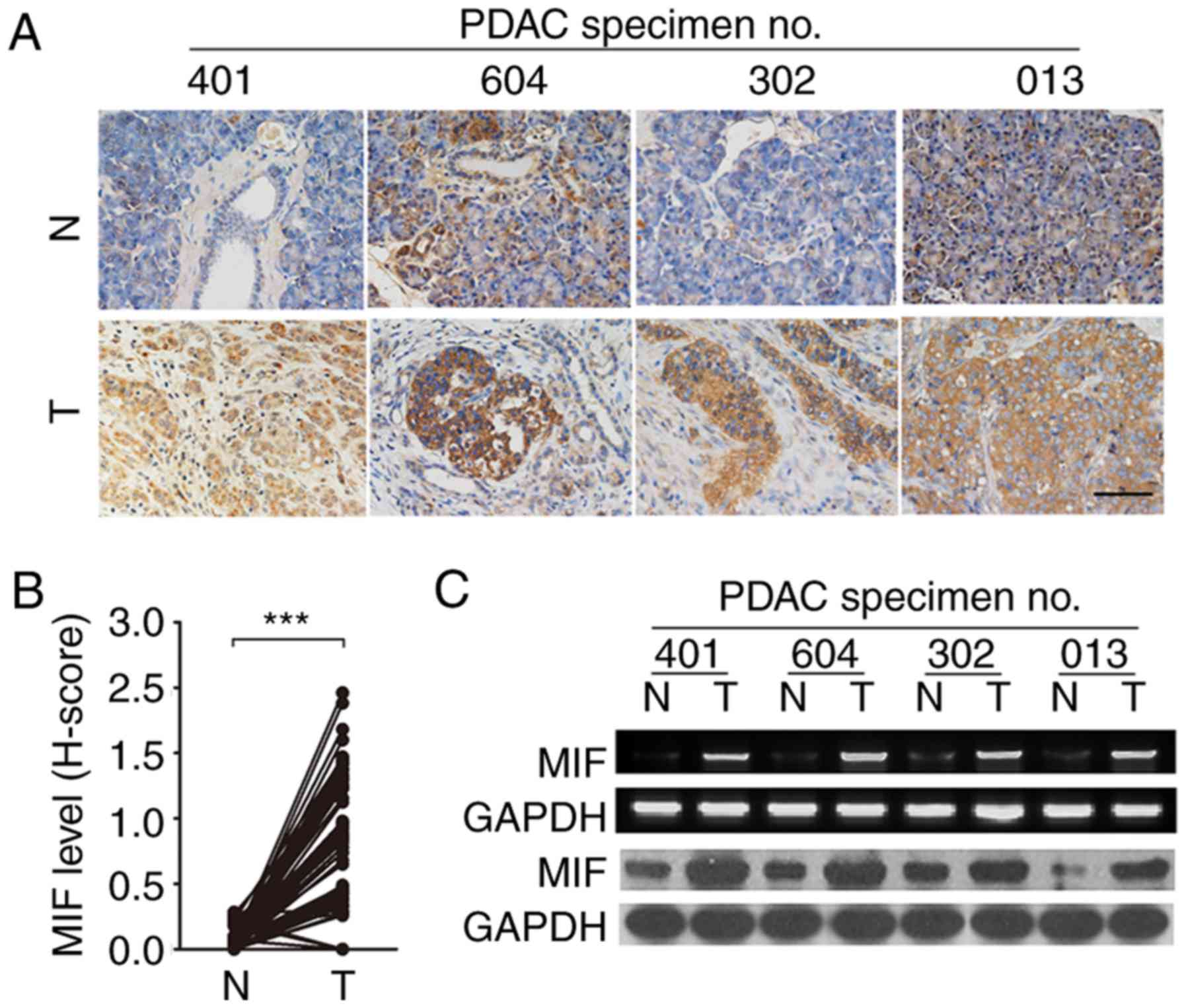
noncancerous tissues by IHC. MIF expression was observed in 75/85
tumor samples (Fig. 1A). Compared
with paired noncancerous tissue, the majority (75/85) of PDAC tumor
tissue exhibited significantly higher MIF expression (Fig. 1B). Consistent with the results from
IHC analysis, RT-sqPCR and immunoblotting assays revealed a similar
trend with increased MIF expression in PDAC tissue at the mRNA and
protein levels (Fig. 1C). These
data indicate that MIF overexpression is prevalent in PDAC.
Increased MIF expression is associated
with poor survival of patients with PDAC
Whether MIF overexpression is associated with
clinical features or clinical outcome of patients with PDAC was
investigated. MIF expression was divided into low- and
high-expression groups, based on median expression (cut-off value,
1.08) analyzed by IHC in all PDAC cases The associations between
the MIF expression and clinical features are summarized in Table I. Increased MIF expression was
identified to be associated with advanced age, lymphatic spread and
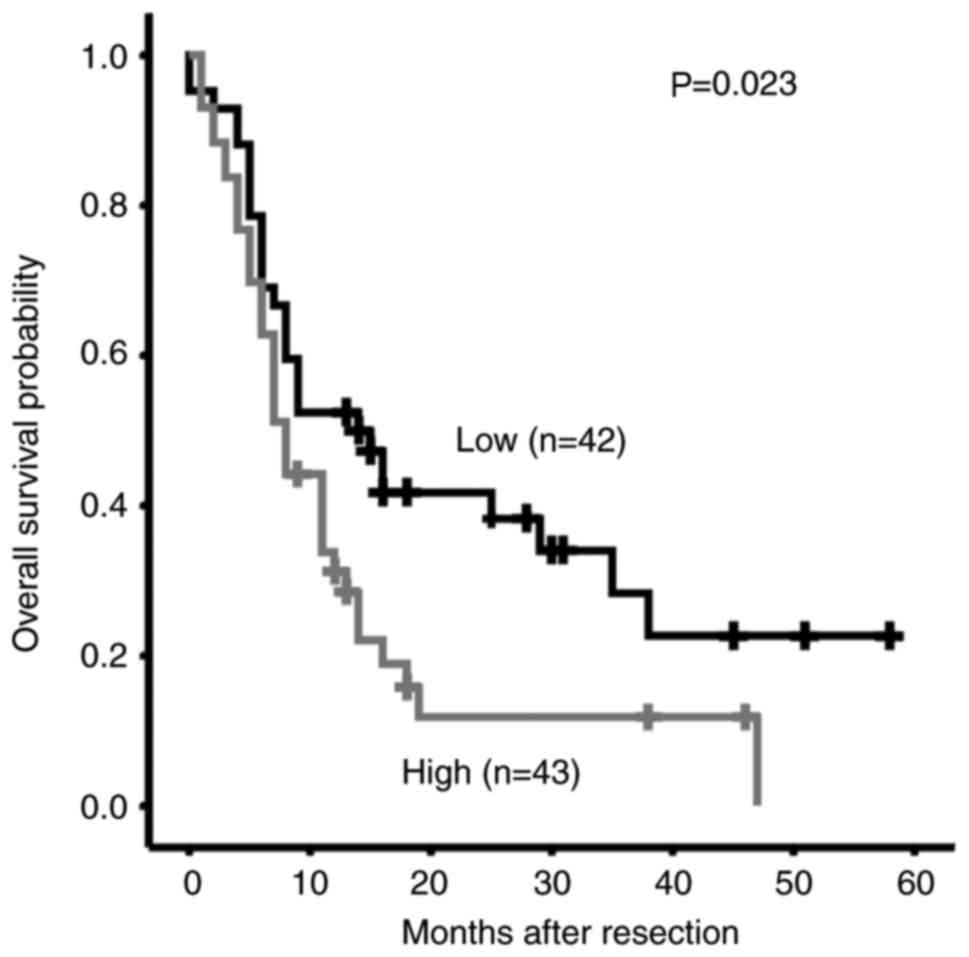
increased serum carcinoembryonic antigen levels (Table I). Furthermore, Kaplan-Meier
analysis revealed that higher MIF levels were significantly
associated with shorter overall survival time of patients with PDAC
(P=0.023; Fig. 2). Cox proportional
hazards regression analysis was performed to exclude confounder
effects. Univariate Cox analysis was first performed to identify
factors that may affect the overall survival of patients with PDAC.
Higher MIF expression, poor differentiation, high tumor node
metastasis (TNM) staging and the presence of metastasis were
associated with inferior survival times (Table II). Multivariate Cox analysis
adjusted for differentiation grade and TNM stage further confirmed
that MIF overexpression was an independent risk factor for poor
overall survival in patients with PDAC (Table II). These data suggest that
overexpression of MIF may predict the poor survival and may
contribute to PDAC metastasis.
 | Table II.Univariate and multivariate analysis
of factors associated with OS. |
Table II.
Univariate and multivariate analysis
of factors associated with OS.
| Clinical
variables | Case no. | HR (95%
CI)a |
P-valuea |
|---|
| Univariate
analysis |
| MIF (High vs.
low) | 43/42 | 1.748
(1.059–2.888) | 0.029b |
| Sex (Male vs.
female) | 51/34 | 1.039
(0.628–1.729) | 0.883 |
| Age (>50 vs. ≤50
years) | 69/16 | 1.372
(0.696–2.706) | 0.361 |
| Tumor size (>3
vs. ≤3 cm) | 48/37 | 1.541
(0.724–2.687) | 0.125 |
| Differentiation
(Poor vs. well/moderate) | 52/33 | 3.858
(2.280–6.526) |
<0.001b |
| TNM stage (III/IV
vs. I/II) | 49/36 | 2.001
(1.401–3.061) |
<0.001b |
| Lymphatic spread
(Yes vs. no) | 36/49 | 2.452
(1.461–4.118) | 0.001b |
| Hepatic metastasis
(Yes vs. no) | 19/66 | 2.200
(1.236–3.916) | 0.007b |
| Serum CEA (≥10 vs.
<10 ng/ml) | 49/36 | 1.584
(0.954–2.628) | 0.075 |
| Multivariate
analysis |
| MIF (High vs.
low)a | 43/42 | 1.916
(1.140–3.218) | 0.014b |
| TNM stage (III/IV
vs. I/II) | 49/36 | 2.706
(1.550–4.723) |
<0.001b |
| Differentiation
(Poor vs. well/moderate) | 52/33 | 3.552
(2.051–6.151) |
<0.001b |
Knockdown of MIF suppresses
proliferation and invasion of PDAC cells
The association of MIF expression with clinical
features and poor outcomes for patients with PDAC prompted an
investigation into the potential role of MIF in PDAC growth and
metastasis. Thus, the potential role of MIF in the regulation of
G1/S transition and in vitro invasion of two PDAC cell
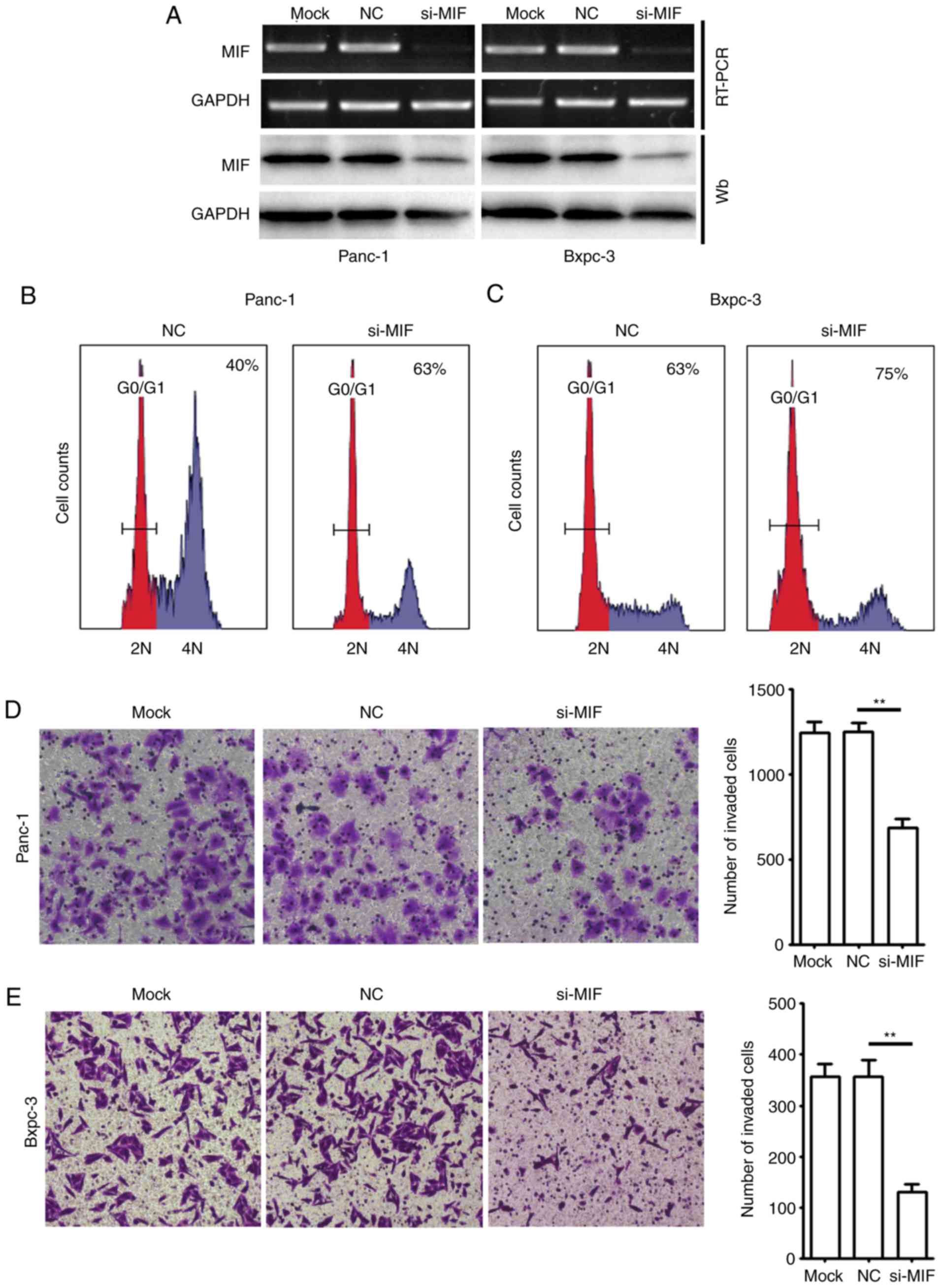
lines, Panc-1 and Bxpc-3, was examined. Cells were transfected with
si-MIF or with si-NC, and were then subjected to a FACS or Boyden
chamber Transwell invasion assay. RT-sqPCR and immunoblotting
assays demonstrated that si-MIF transfection markedly decreased the
mRNA and protein levels of MIF in pancreatic cancer cells (Fig. 3A). Notably, the knockdown of MIF
resulted in a marked accumulation of the G1-population in PDAC
cells (Fig. 3B and C). Furthermore,
si-MIF-transfected Panc-1 and Bxpc-3 cells exhibited a
significantly reduced number of cells invading through the
Transwell chamber (Fig. 3D and
E).
Knockdown of MIF inhibits the
activation of AKT and ERK, and suppresses the expression of cyclin
D1 and MMP-2
The molecular mechanisms underlying the
metastasis-promoting effects of MIF were investigated. The function
of MIF is associated with two major tumor-promoting signaling
pathways, namely the AKT and ERK signaling pathways (9,10)
Consistently, silencing MIF in PDAC cells significantly decreased
the phosphorylation levels of AKT and ERK, but had no effect on the
expression of total AKT and ERK protein (Fig. 4A and B), indicating that MIF
knockdown inhibited the activation of AKT and ERK signaling in PDAC
cells. Cyclin D1 and MMP-2 are important factors upregulated by AKT
and ERK signaling that execute AKT- and ERK-mediated cell invasion
(19–22). The mRNA and protein levels of cyclin
D1 and MMP-2 were significantly reduced in si-MIF-transfected cells
compared with the NC group (Fig. 4C and
D). These data suggest the MIF enhances PDAC metastasis by
activating the AKT and ERK pathways, which in turn upregulates the
expression of cyclin D1 and MMP-2.
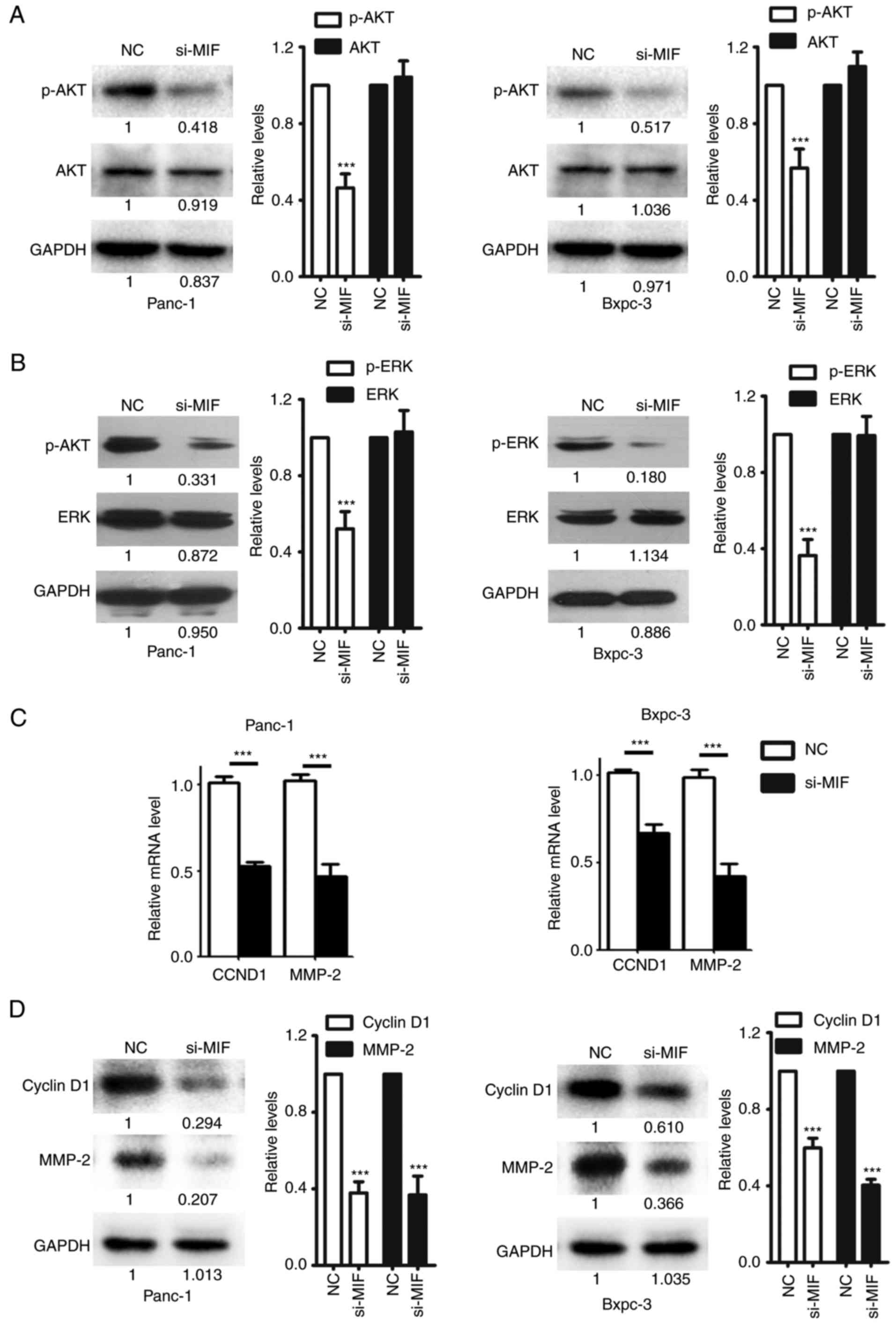 | Figure 4.Silencing of MIF expression
negatively regulates the AKT and ERK signaling pathways. Knockdown
of MIF attenuated AKT and ERK activities in PDAC cells. Panc-1 or
Bxpc-3 cells were transfected with NC or si-MIF and subjected to
immunoblotting for (A) p-AKT and AKT; and (B) p-ERK1/2 and ERK1/2
expression. GAPDH was used as an internal control. Knockdown of MIF
decreased the mRNA and protein levels of CCND1 and MMP-2. Panc-1 or
Bxpc-3 cells transfected with si-MIF or NC were subjected to (C)
reverse transcription- quantitative polymerase chain reaction or
(D) immunoblotting analysis of CCND1 and MMP-2. For (A), (B), and
(D), the intensity of each band was densitometrically quantified
using Image J software and was normalized according to the value of
NC group. Results were reproduced in three independent experiments
and the cumulative data as well as the representative immunoblots
are shown. ***P<0.001. MIF, Macrophage migration inhibitory
factor; PDAC, pancreatic ductal adenocarcinoma; si/siRNA, small
inteferring RNA; NC, negative control siRNA; CCND1, cyclin D1;
MMP-2, matrix metalloproteinase-2; p- phosphorylated; AKT, AKT
serine/threonine kinase; ERK, extracellular-signal-regulated
kinase. |
Discussion
PDAC manifests as a highly aggressive cancer with
poor prognosis. The identification of molecules that are involved
in PDAC tumor progression and aggressiveness may elucidate novel
targets for PDAC treatment. Previous studies have suggested that
MIF, a pro-inflammatory cytokine, facilitates cancer progression,
associating inflammation with pancreatic cancer progression
(12–14,23).
Emerging research has explored the biological effects of MIF in
PDAC (11–14,23).
Similar to other reports that have described a regulatory function
of MIF in cell growth and survival (24–29),
pro-proliferation and anti-apoptosis roles for MIF in PDAC have
been identified (11). Furthermore,
it has been reported that MIF induces epithelial to mesenchymal
transition and enhances tumor aggressiveness in PDAC (13,14).
Additionally, exosome-derived MIF may prime the liver for PDAC
metastasis, and may be a potential biomarker for liver metastasis
(23). Consistently, a
pro-metastasis effect was observed regarding MIF in PDAC in present
study. Taken together, these data highlight the importance of MIF
overexpression in promoting PDAC progression, and suggest that
inhibition of MIF may offer a novel therapeutic option for
treatment of PDAC.
Although MIF overexpression has been observed in
patients with PDAC, to the best of our knowledge, only one report
has explored the associations between MIF expression, disease
aggressiveness and clinical outcome (14). To date, only one other group has
indicated an association between increased tumor expression of MIF
and decreased survival rates in patients with PDAC following tumor
resection (14). In the present
study, the overexpression of MIF was demonstrated to be a frequent
event in PDAC. Notably, the peritumorial tissues of PDAC expressed
undetectable or low levels of MIF protein in acinar and ductal
cells as well as stromal cells. As known, PDAC is associated with
intra- and peritumorial inflammation, which may significantly
induce MIF expression in peritumorial tissues (30). The variation in MIF expression
between the peritumorial tissues of PDAC samples may be at least
partly due to the differing degree of peritumorial inflammation.
The results of the present study demonstrated that high levels of
MIF were associated with metastasis and inferior survival of
patients with PDAC, and that high MIF expression may serve as an
independent risk factor for poor disease outcome. Using an
siRNA-based strategy, the pro-metastasis effect of endogenous MIF
expression was examined in human PDAC cell lines, further
supporting the role for MIF in PDAC aggressiveness.
PDAC has a high tendency to metastasize. Typically,
PDAC cells first spread to nearby lymph nodes, and later
metastasize to the liver and other organs.2 Thus,
lymphatic spread is a critical early event of PDAC progression.
Consistently, the present study demonstrated that lymphatic spread
and hepatic metastasis are significantly associated with poor
survival of patients with PDAC. The association between high MIF
expression and lymphatic spread indicated that MIF may be a
possible mediator of extensive lymph node metastasis. Furthermore,
the finding that silencing of MIF inhibits the invasion of PDAC
cells in vitro supports the possibility that MIF facilitates
lymph node metastasis of PDAC cells. Nevertheless, this hypothesis
requires further evaluation using additional in vivo
studies.
The present study results revealed that that
silencing of MIF significantly inhibited the expression of MMP-2
and CCND1, suggesting that MMP-2 and CCND1 may be target genes that
mediate the pro-metastasis effects of MIF. MMP-2 is a member of the
matrix metalloproteinase family, is frequently overexpressed in
tumors, and is well known to facilitate invasion and metastasis of
tumor cells by degrading extracellular matrix (31). CCND1, a regulator of cell cycle
(32,33), promotes tumor invasion and
metastasis, according to evidence from clinical studies and in
vivo experiments (34,35). On one hand, nuclear CCND1 and its
binding partner Cdk4 act as a transcriptional regulator of genes
controlling cell adherence and migration (36,37).
On the other hand, CCND1 in the cytoplasm phosphorylates
cytoplasmic and membrane-associated proteins to promote cell
spreading and invasion (38).
Notably, we previously reported that MIF promotes hepatocellular
carcinoma cell growth by positively regulating CCND1 expression
(26). The data from cell cycle
analysis also revealed that silencing of MIF blocked G1/S
transition of PDAC cells (data not shown), indicating that MIF
promoted PDAC cell cycle by inducing CCND1 expression. Taken
together with the results of the present study, these data indicate
that CCND1 may serve a central role in MIF-mediated tumor
progression.
In conclusion, the findings of the current study
identified a MIF/AKT/ERK/CCND1/MMP-2 cascade that promotes PDAC
metastasis. The results suggest that MIF is a candidate prognostic
indicator in patients following resection of PDAC. Additional
pre-clinical studies are necessary to evaluate the effect of
MIF-targeting in pancreatic cancer as a novel therapeutic
approach.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81672417,
81370368, 81172337 and 81502464), and the Science and Technology
Foundation of Guangdong Province grant (grant nos. 2014A020212647
and 2014A020212083).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors contributions
WL and JTX designed the study. DW, RW, AH and ZF
performed the experiments. DW, KW and MH analyzed the experimental
data. AH and MH collected the tissue specimens. WL, JTX, DW and RW
wrote the manuscript. All authors discussed the results and
contributed to the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Human Research
Ethics Committees of the First Affiliated Hospital of Sun Yat-sen
University (approval no. 201515; Guangzhou, China), according to
the Declaration of Helsinki. All of patients enrolled in the
present study provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu SL, Friess H, Kleeff J, Ji ZL and
Buchler MW: Surgical approaches for resection of pancreatic cancer:
An overview. Hepatobiliary Pancreat Dis Int. 1:118–125.
2002.PubMed/NCBI
|
|
4
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:2140–2141. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garcea G, Dennison AR, Steward WP and
Berry DP: Role of inflammation in pancreatic carcinogenesis and the
implications for future therapy. Pancreatology. 5:514–529. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hausmann S, Kong B, Michalski C, Erkan M
and Friess H: The role of inflammation in pancreatic cancer. Adv
Exp Med Biol. 816:129–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gukovsky I, Li N, Todoric J, Gukovskaya A
and Karin M: Inflammation, autophagy, and obesity: Common features
in the pathogenesis of pancreatitis and pancreatic cancer.
Gastroenterology. 144(1199–1209): e11942013.
|
|
8
|
Lippitz BE: Cytokine patterns in patients
with cancer: A systematic review. Lancet Oncol. 14:e218–e228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwartz V, Lue H, Kraemer S, Korbiel J,
Krohn R, Ohl K, Bucala R, Weber C and Bernhagen J: A functional
heteromeric MIF receptor formed by CD74 and CXCR4. FEBS Lett.
583:2749–2757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leng L, Metz CN, Fang Y, Xu J, Donnelly S,
Baugh J, Delohery T, Chen Y, Mitchell RA and Bucala R: MIF signal
transduction initiated by binding to CD74. J Exp Med.
197:1467–1476. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Denz A, Pilarsky C, Muth D, Ruckert F,
Saeger HD and Grutzmann R: Inhibition of MIF leads to cell cycle
arrest and apoptosis in pancreatic cancer cells. J Surg Res.
160:29–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo D, Guo J, Yao J, Jiang K, Hu J, Wang
B, Liu H, Lin L, Sun W and Jiang X: D-dopachrome tautomerase is
over-expressed in pancreatic ductal adenocarcinoma and acts
cooperatively with macrophage migration inhibitory factor to
promote cancer growth. Int J Cancer. 139:2056–2067. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang S, He P, Wang J, Schetter A, Tang W,
Funamizu N, Yanaga K, Uwagawa T, Satoskar AR, Gaedcke J, et al: A
novel MIF signaling pathway drives the malignant character of
pancreatic cancer by targeting NR3C2. Cancer Res. 76:3838–3850.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Funamizu N, Hu C, Lacy C, Schetter A,
Zhang G, He P, Gaedcke J, Ghadimi MB, Ried T, Yfantis HG, et al:
Macrophage migration inhibitory factor induces epithelial to
mesenchymal transition, enhances tumor aggressiveness and predicts
clinical outcome in resected pancreatic ductal adenocarcinoma. Int
J Cancer. 132:785–794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Klöppel G, Hruban RH DS, Longnecker DS,
Adler G, Kern SE and Partanen TJ: Ductal adenocarcinoma of the
pancreasPathology and Genetics of Tumours of the Digestive System.
Hamilton SR and Aaltonen LA: International Agency for Research on
Cancer Press; Lyon, France: pp. 221–230. 2000
|
|
16
|
Wang R, Zhao N, Li S, Fang JH, Chen MX,
Yang J, Jia WH, Yuan Y and Zhuang SM: MicroRNA-195 suppresses
angiogenesis and metastasis of hepatocellular carcinoma by
inhibiting the expression of VEGF, VAV2, and CDC42. Hepatology.
58:642–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tan L, Ye X, Zhou Y, Yu M, Fu Z, Chen R,
Zhuang B, Zeng B, Ye H, Gao W, et al: Macrophage migration
inhibitory factor is overexpressed in pancreatic cancer tissues and
impairs insulin secretion function of β-cell. J Transl Med.
12:922014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lauring J, Park BH and Wolff AC: The
phosphoinositide-3-kinase-Akt-mTOR pathway as a therapeutic target
in breast cancer. J Natl Compr Canc Netw. 11:670–678. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cohen P and Frame S: The renaissance of
GSK3. Nat Rev Mol Cell Biol. 2:769–776. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Ben QW, Yao WY, Zhang JJ, Chen DF,
He XY, Li L and Yuan YZ: BMP2 induces PANC-1 cell invasion by MMP-2
overexpression through ROS and ERK. Front Biosci. 17:2541–2549.
2012. View Article : Google Scholar
|
|
22
|
Dong QZ, Wang Y, Tang ZP, Fu L, Li QC,
Wang ED and Wang EH: Derlin-1 is overexpressed in non-small cell
lung cancer and promotes cancer cell invasion via EGFR-ERK-mediated
up-regulation of MMP-2 and MMP-9. Am J Pathol. 182:954–964. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Costa-Silva B, Aiello NM, Ocean AJ, Singh
S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et
al: Pancreatic cancer exosomes initiate pre-metastatic niche
formation in the liver. Nat Cell Biol. 17:816–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Richard V, Kindt N, Decaestecker C, Gabius
HJ, Laurent G, Noel JC and Saussez S: Involvement of macrophage
migration inhibitory factor and its receptor (CD74) in human breast
cancer. Oncol Rep. 32:523–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oliveira CS, de Bock CE, Molloy TJ,
Sadeqzadeh E, Geng XY, Hersey P, Zhang XD and Thorne RF: Macrophage
migration inhibitory factor engages PI3K/Akt signalling and is a
prognostic factor in metastatic melanoma. BMC Cancer. 14:6302014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang XH, Jian WH, Wu ZF, Zhao J, Wang H,
Li W and Xia JT: Small interfering RNA (siRNA)-mediated knockdown
of macrophage migration inhibitory factor (MIF) suppressed cyclin
D1 expression and hepatocellular carcinoma cell proliferation.
Oncotarget. 5:5570–5580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C, Zhou X, Li W, Li M, Tu T, Ba X, Wu
Y, Huang Z, Fan G, Zhou G, et al: Macrophage migration inhibitory
factor promotes osteosarcoma growth and lung metastasis through
activating the RAS/MAPK pathway. Cancer Lett. 403:271–279. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bifulco C, McDaniel K, Leng L and Bucala
R: Tumor growth-promoting properties of macrophage migration
inhibitory factor. Curr Pharm Des. 14:3790–3801. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lue H, Thiele M, Franz J, Dahl E,
Speckgens S, Leng L, Fingerle-Rowson G, Bucala R, Lüscher B and
Bernhagen J: Macrophage migration inhibitory factor (MIF) promotes
cell survival by activation of the Akt pathway and role for
CSN5/JAB1 in the control of autocrine MIF activity. Oncogene.
26:5046–5059. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Candido J and Hagemann T: Cancer-related
inflammation. J Clin Immunol. 1 Suppl 33:S79–S84. 2013. View Article : Google Scholar
|
|
31
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sherr CJ and Roberts JM: Living with or
without cyclins and cyclin-dependent kinases. Genes Dev.
18:2699–2711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bienvenu F, Jirawatnotai S, Elias JE,
Meyer CA, Mizeracka K, Marson A, Frampton GM, Cole MF, Odom DT,
Odajima J, et al: Transcriptional role of cyclin D1 in development
revealed by a genetic-proteomic screen. Nature. 463:374–378. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Drobnjak M, Osman I, Scher HI, Fazzari M
and Cordon-Cardo C: Overexpression of cyclin D1 is associated with
metastatic prostate cancer to bone. Clin Cancer Res. 6:1891–1895.
2000.PubMed/NCBI
|
|
35
|
Huang H, Hu YD, Li N and Zhu Y: Inhibition
of tumor growth and metastasis by non-small cell lung cancer cells
transfected with cyclin D1-targeted siRNA. Oligonucleotides.
19:151–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Z, Jiao X, Wang C, Ju X, Lu Y, Yuan L,
Lisanti MP, Katiyar S and Pestell RG: Cyclin D1 induction of
cellular migration requires p27KIP1. Cancer Res.
66:9986–9994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Z, Wang C, Jiao X, Lu Y, Fu M, Quong
AA, Dye C, Yang J, Dai M, Ju X, et al: Cyclin D1 regulates cellular
migration through the inhibition of thrombospondin 1 and ROCK
signaling. Mol Cell Biol. 26:4240–4256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fuste NP, Fernandez-Hernandez R, Cemeli T,
Mirantes C, Pedraza N, Rafel M, Torres-Rosell J, Colomina N,
Ferrezuelo F, Dolcet X, et al: Cytoplasmic cyclin D1 regulates cell
invasion and metastasis through the phosphorylation of paxillin.
Nat Commun. 7:115812016. View Article : Google Scholar : PubMed/NCBI
|