Introduction
Gallbladder cancer (GBC) is the fifth most common
cancer among the various types of gastrointestinal malignancies,
and the most commonly occurring malignant tumor of bile tract
cancer (BTC) worldwide (1).
Currently, the major therapeutic options include surgical
resection, radiation therapy and chemotherapy, and R0 complete
surgical resection is the only effective treatment (2). Although new improvements have been
made in the diagnosis and therapy of GBC, leading to better
prognoses, only 30% of GBC patients are considered to be suitable
candidates for surgery, and the overall 5-year survival rate is
<5% (3). Additionally,
post-operative recurrence and metastasis also serve a crucial role
in the progression of GBC. Therefore, identifying early diagnostic
markers and novel therapeutic targets is urgently required.
Hypoxia exerts a crucial role in the tumor
microenvironment, particularly in solid tumors (4). Accumulating evidence shows that
hypoxia plays a key role in tumorigenesis, including angiogenesis
and migration (5–7). Hypoxia-inducible factor-1α (HIF-1α) is
sensitive to cellular oxygen levels, and is stabilized under
conditions of hypoxia such that it may activate its target genes
(8), particularly vascular
endothelial growth factor (VEGF) (9). The role of VEGF in hypoxia-induced
angiogenesis has been extensively studied in numerous types of
cancer, including GBC (10). The
microenvironment in numerous types of solid tumors is affected
under hypoxic conditions, and results in the overexpression of
HIF-1α (11,12). Therefore, targeting the expression
of HIF-1α may represent a novel strategy for cancer therapy.
Metformin, which is a member of the biguanide
family, is predominantly used for the treatment of type 2 diabetes
by increasing insulin sensitivity (13). Recently, epidemiologists discovered
that diabetic patients who were treated with metformin had a lower
risk, and lower incidence, of multiple types of cancer (14,15).
Furthermore, accumulating evidence has demonstrated the antitumor
effect of metformin on various cancer types, including biliary
tract cancer (BTC) (16). HIF-1α is
a heterodimeric transcription factor, comprising α and β subunits,
that is associated with glucose uptake and angiogenesis (17). Guimaraes et al (18) demonstrated that metformin was able
to inhibit HIF-1α and led to an increase in cell death in squamous
cell carcinoma. Zhou et al (19) showed that metformin could inhibit
the expression of HIF-1α via inhibition of the enhanced cellular
oxygenation capability in hepatocellular carcinoma HepG2 cells.
However, the role of metformin in cellular migration in a hypoxic
microenvironment in GBC-SD cells, and its associated mechanism,
have yet to be fully elucidated.
In the present study, it was demonstrated that
HIF-1α is overexpressed in GBC tissues, and that HIF-1α
overexpression is correlated closely with lymph node metastasis and
tumor-lymph node-metastasis (TNM) stages. These results suggested
that hypoxia promoted cell migration and increased the expression
of HIF-1α and VEGF. Furthermore, it was shown that metformin
reversed hypoxia-induced migration by targeting the HIF-1α/VEGF
pathway. In conclusion, the present study demonstrated that HIF-1α
may contribute towards tumor migration via overexpression of VEGF
in GBC, while metformin is able to inhibit tumor migration by
targeting the HIF-1α/VEGF pathway.
Materials and methods
Patient tissue samples
The present study was approved by the Clinical
Research Ethics Committee of the First Affiliated Hospital of
Zhengzhou University. Tumor and adjacent non-tumorous tissues were
collected from 34 patients with GBC who had undergone
cholecystectomy at the First Affiliated Hospital of Zhengzhou
University between June 2016 and May 2017. Normal gallbladder
tissues from patients with chronic cholecystitis were included for
comparison. Non-cancerous tissues were obtained from soft tissue
located >1 cm from the edge of the tumor, and non-cancerous
tissues were observed under a microscope by a pathologist. Tissues
were immediately snap-frozen in liquid nitrogen following surgical
resection and stored at −80ºC prior to analysis.
Complete clinicopathological and laboratory data were collected
from each subject. Patients who had received radiotherapy,
chemotherapy or immunotherapy prior to surgery were excluded. As
described by the American Joint Committee on Cancer (AJCC) in 2010
(20), clinical stage was
classified according to the TNM staging system.
Cell culture and transfection
Human GBC-SD cells were purchased from the Shanghai
Cell Bank (Chinese Academy of Sciences) and cultured in RPMI-1640
medium (HyClone™; GE Healthcare Life Sciences, Beijing, China)
supplemented with 10% fetal bovine serum (FBS; GemCell™), 100 U/ml
penicillin and 100 mg/l streptomycin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) under a humidified atmosphere of 5%
CO2 at 37ºC. The small-interfering RNA HIF-1α
(siHIF-1α) and the negative control were purchased from GenePharma
Biotechnology (Shanghai, China). Transfection was performed using
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific
Inc., Waltham, MA, USA) following the manufacturer's protocol. The
siHIF-1α-1 primer sequences were 5′-GCCGAGGAAGAACUAUGAATT-3′
(sense) and 5′-UUCAUAGUUCUUCCUCGGCTT-3′ (antisense); the siHIF-1α-2
primer sequences were 5′-GCUGAUUUGUGAACCCAUUTT-3′ (sense) and
5′-AAUGGGUUCACAAAUCAGCTT-3′ (antisense). The sequences of the
negative control were 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and
5′-ACGUGACACGUUCGGAGAATT-3′ (antisense).
Immunohistochemistry (IHC)
For IHC, the slides were immersed in heated antigen
retrieval solution (10 mmol/l citrate buffer, pH 6.0), and
subsequently treated with 3% hydrogen peroxide
(H2O2) for 10 min. After washing with PBS,
the slides were incubated with diluted primary antibody (HIF-1α;
cat. no. 20960-1-AP, 1:100 dilution; VEGF, cat. no. 19003-1-AP,
1:100 dilution; Proteintech, Wuhan, China) at 4ºC
overnight and then the secondary antibody (cat. no. PV-9000, 1:500
dilution; ZSGB-BIO, Beijing, China) for 20 min at room temperature.
The reaction was developed using a 3,3′-diaminobenzidine (DAB) kit
(cat. no. ZLI-9017; ZSGB-BIO, 1:50 dilution in buffer). Finally,
the slides were counterstained in hematoxylin prior to dehydration
and mounting. Staining reactions were determined on examination
under a light microscope (CX31, Olympus, Japan; original
magnification, ×200).
RNA extraction and real-time
quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissue samples using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. RNA was transcribed to cDNA according
to the manufacturer's protocol by using the Takara PrimeScript
First Strand cDNA Synthesis kit (Takara Biotechnology Co., Ltd.,
Dalian, China). SYBR-Green (Takara Biotechnology Co., Ltd.) was
added to quantify the expression of HIF-1α, according to the
protocol provided. The primers used were as follows: HIF-1α,
5′-TGCAACATGGAAGGTATTGC-3′ (sense), and 5′-TTCACAAATCAGCACCAAGC-3′
(antisense); and β-actin, 5′-CCTGGCACCCAGCACAAT-3′ (sense), and
5′-GGGCCGGACTCGTCATAC-3′ (antisense). PCR reaction conditions were
performed as followed: 95ºC for 60 sec, and 40 cycles of
95ºC for 15 sec, 60ºC for 15 sec, and
72ºC for 45 sec The relative expression levels were
determined using the comparative ΔΔCq method (21).
Wound healing assays
GBC-SD cells were seeded at a density of
1×106/ml into 6-well plates and cultured overnight at
37ºC. After the GBC-SD cells had been spread over the
plates, a vertical long wound was scratched into the cells, and the
cells were washed with PBS twice. Images of the cells were captured
at that time-point; GBC-SD cells were pre-cultured under hypoxia
for 2 h and treated with 20 mM metformin, maintaining the cells in
culture under hypoxic conditions with 2% FBS for 48 h, after which
time further images were captured. The wound closure ratio was
calculated as follows: (wound width at 0 h-wound width at 48
h)/wound width at 0 h.
Migration assay
A total of 1×105 cells were suspended in
100 µl serum-free medium and added to the upper chamber of a
Transwell plate, and 600 µl 10% FBS medium was added to the bottom
chamber of the Transwell plate. GBC-SD cells were pre-cultured
under hypoxia for 2 h, and treated with 20 mM metformin, following
incubation for 24 h; those cells that had stuck to the lower
surface of the membrane were washed with PBS and fixed in 100%
methanol for 20 min, and then stained with 0.1% crystal violet for
10 min at room temperature. The cellular migration through the
membrane was visualized using an inverted microscope (IX71,
Olympus, Japan; original magnification, ×100).
Western blot analysis
Whole cell lysates were extracted with RIPA buffer
on ice for 20 min. Protein concentration was determined using a BCA
kit assay (Beyotime Institute of Biotechnology). Each extract
containing ~30 µg protein was subjected to 10% SDS-polyacrylamide
gel electrophoresis (PAGE), and then transferred onto
polyvinylidene difluoride (PVDF) membranes (Merck Millipore,
Bedford, MA, USA). The membranes were blocked in PBS containing 5%
non-fat dry milk for 1 h at room temperature, and were then
incubated with the primary antibodies against HIF-1α (cat. no.
20960-1-AP; 1:300 dilution), VEGF (cat. no. 19003-1-AP; 1:800
dilution) and β-actin (cat. no. 60008-1-Ig, 1:5,000 dilution; all
from Proteintech, Wuhan, China) overnight at 4°C, followed by six
washes for 5 min with PBS. The membranes were subsequently
incubated with peroxidase (HRP)-conjugated goat anti-rabbit IgG
(cat. no. IH-0031, 1:5,000 dilution; DingGuo BioTech Co., Ltd.,
Beijing, China) or peroxidase (HRP)-conjugated goat anti-mouse IgG
(cat. no. IH-0011, 1:5,000, dilution; DingGuo BioTech Co., Ltd.)
for 1 h at room temperature, and washed six times with PBS. Blots
were detected using an enhanced chemiluminescence western blotting
detection kit (Thermo Scientific™ Pierce™ BCA™ Protein assay;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol.
Experimental xenograft model
The animal protocol was approved by the
Institutional Animal Care and Use Committee of the First Affiliated
Hospital of Zhengzhou University. Twelve female immunodeficient
BALB/c nude mice at 4 weeks of age, with an initial body weight of
16±2 g, were purchased from Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). The animals were raised
under pathogen-free conditions at the Institute of Medicine,
Zhengzhou University at a temperature of 25±2°C and a relative
humidity of 70±5% under controlled light conditions (12/12 h
light/day cycle) and fed with standard laboratory food and water.
GBC-SD cells in the exponential phase of growth were resuspended in
200 µl serum-free culture medium at a density of 5×106
cells, and subsequently tumor xenografts were established by
subcutaneous inoculation of the GBC-SD cells into the right flank
of nude mice. The mice were randomly divided into two groups (with
6 mice/group): i) The negative group, which was administered PBS;
and ii) the metformin group, which was administered 350 mg/kg
metformin (22,23) (intra-gastric infusion, daily). The
volume of the tumors was measured every 4 days. Maximum allowable
tumor volume based on ethical guidelines was <2,500
mm3. Tumor volume was calculated using calipers and
estimated according to the following formula: Tumor volume
(mm3) = (length × width2)/2. One month
afterwards, the animals were sacrificed by cervical dislocation
after anesthetized by 1% pentobarbital, and the tumor tissue was
removed and measured. Xenograft tumors were harvested and cut into
4-µm sections for IHC analysis.
Statistical analysis
All data are presented as the mean ± standard
deviation from at least three independent experiments. Differences
between groups were assessed using the Student's t-test followed by
Shapiro-Wilk W test, or one-way (ANOVA) followed by Bonferroni
test, or a χ2 test. P<0.05 was considered to indicate
a statistically significant difference, and analyses were performed
using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
Results
HIF-1α is upregulated in GBC
tissue
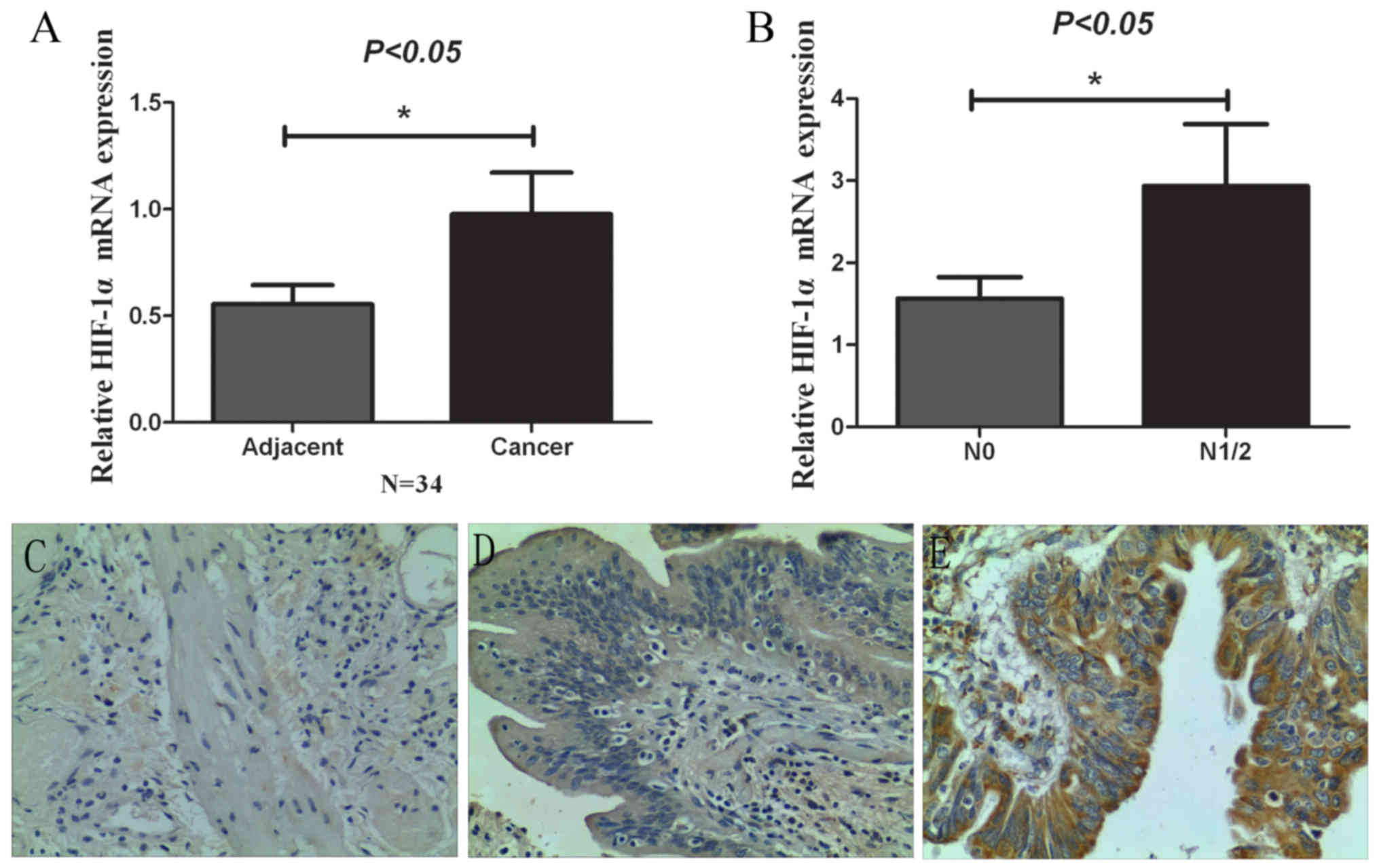
The expression of HIF-1α in 34 paired GBC and
adjacent normal gallbladder tissues was investigated via qRT-PCR
and IHC experiments. As showed in Fig.
1A, the results obtained demonstrated that the mRNA expression
of HIF-1α was higher in GBC tissues compared with that observed in
the adjacent normal gallbladder tissues through RT-PCR experiments.
As showed in Fig. 1B, the mRNA
expression of HIF-1α in lymph node metastasis cancer tissues was
higher (N1/2) than that in non-lymph node metastasis cancer tissues
(N0), which suggested that HIF-1α was upregulated in GBC tissues at
the mRNA level, particularly in lymph node metastasis cancer
tissues. Moreover, we also analyzed the HIF-1α protein expression
levels through using IHC. As showed in Fig. 1C-E, expression of HIF-1α was higher
in GBC tissues compared with that in the adjacent normal
gallbladder tissues and chronic cholecystitis tissues, which was
consistent with the PCR results. Furthermore, the association
between HIF-1α and clinicopathological features was also analyzed.
As shown in Table I, aberrant
expression of HIF-1α was associated with lymph node metastasis and
TNM stage. These results indicate that HIF-1α may serve an
important role in progression of GBC.
 | Table I.Association between HIF-1α expression
and the clinicopathological features of the GBC cases. |
Table I.
Association between HIF-1α expression
and the clinicopathological features of the GBC cases.
|
|
| HIF-1α
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | No. of cases | Low | High | P-value |
|---|
| Age (years) |
|
|
| 0.692 |
|
<60 | 10 | 4 | 6 |
|
|
≥60 | 24 | 7 | 17 |
|
| Sex |
|
|
| 0.714 |
|
Female | 17 | 6 | 11 |
|
|
Male | 17 | 5 | 12 |
|
| Histological
grade |
|
|
| 1.000 |
| Well
and moderate | 24 | 8 | 16 |
|
|
Poor | 10 | 3 | 7 |
|
| N status |
|
|
| 0.024 |
| N0 | 21 | 10 | 11 |
|
|
N1/2 | 13 | 1 | 12 |
|
| Stone |
|
|
| 0.580 |
| No | 30 | 9 | 21 |
|
|
Yes | 4 | 2 | 2 |
|
| Tumor size
(cm) |
|
|
| 0.271 |
|
<5 | 23 | 9 | 14 |
|
| ≥5 | 11 | 2 | 9 |
|
| Clinical stage |
|
|
| 0.002 |
|
I–II | 9 | 7 | 2 |
|
|
III–IV | 25 | 4 | 21 |
|
Hypoxia induces cell migration and
increases the expression of HIF-1α and VEGF
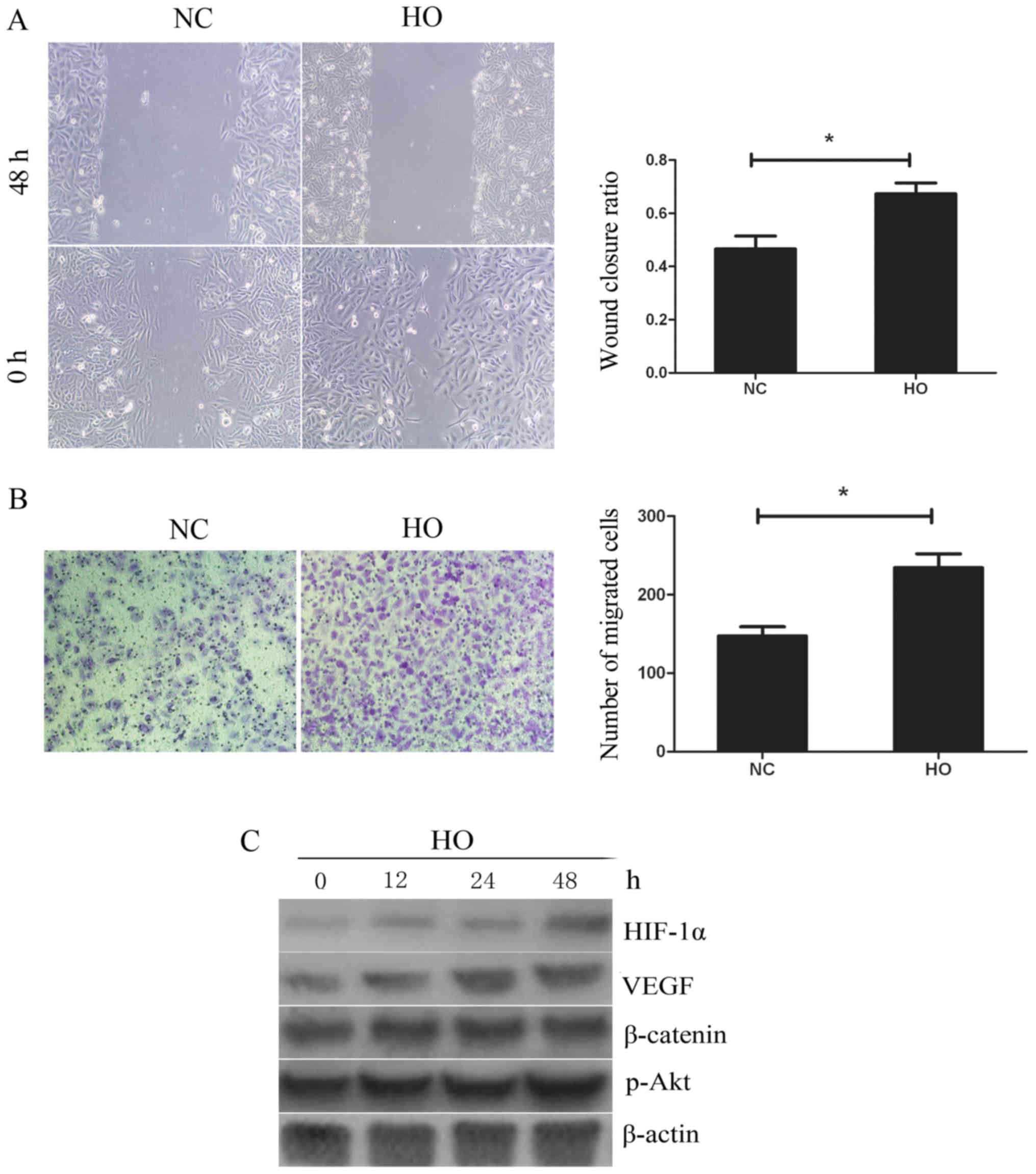
GBC-SD cells were cultured under conditions of
hypoxia (94% N2, 5% CO2, 1% O2) or
normoxia. Wound healing and Transwell assays were performed to
detect the ability of the cells to metastasize. As shown in
Fig. 2A, compared with the normoxia
treatment group, treatment with hypoxia significantly promoted the
ability of cells to migrate. Fig.
2B also shows the presence of 234.4±17.7 migrated cells per
high-power field in the hypoxia group, which was significantly
higher when compared with that in the normoxia treatment group
(147.4±11.7). To further elucidate the molecular mechanism involved
in cell migration, the expression of HIF-1α, VEGF, β-catenin and
p-Akt was investigated using western blotting. As shown in Fig. 2C, following the culture of GBC-SD
cells under conditions of hypoxia for 12, 24 and 48 h, the
expression level of HIF-1α and VEGF in GBC-SD cells was increased,
while the expression level of β-catenin and p-Akt exhibited no
obvious changes. These data indicate that hypoxia may increase cell
migration by activating HIF-1α and VEGF.
Downregulation of HIF-1α by siRNA and
2-methoxyestradiol (2-ME), an HIF-1α inhibitor, suppresses
hypoxia-induced migration and downregulates the expression of
VEGF
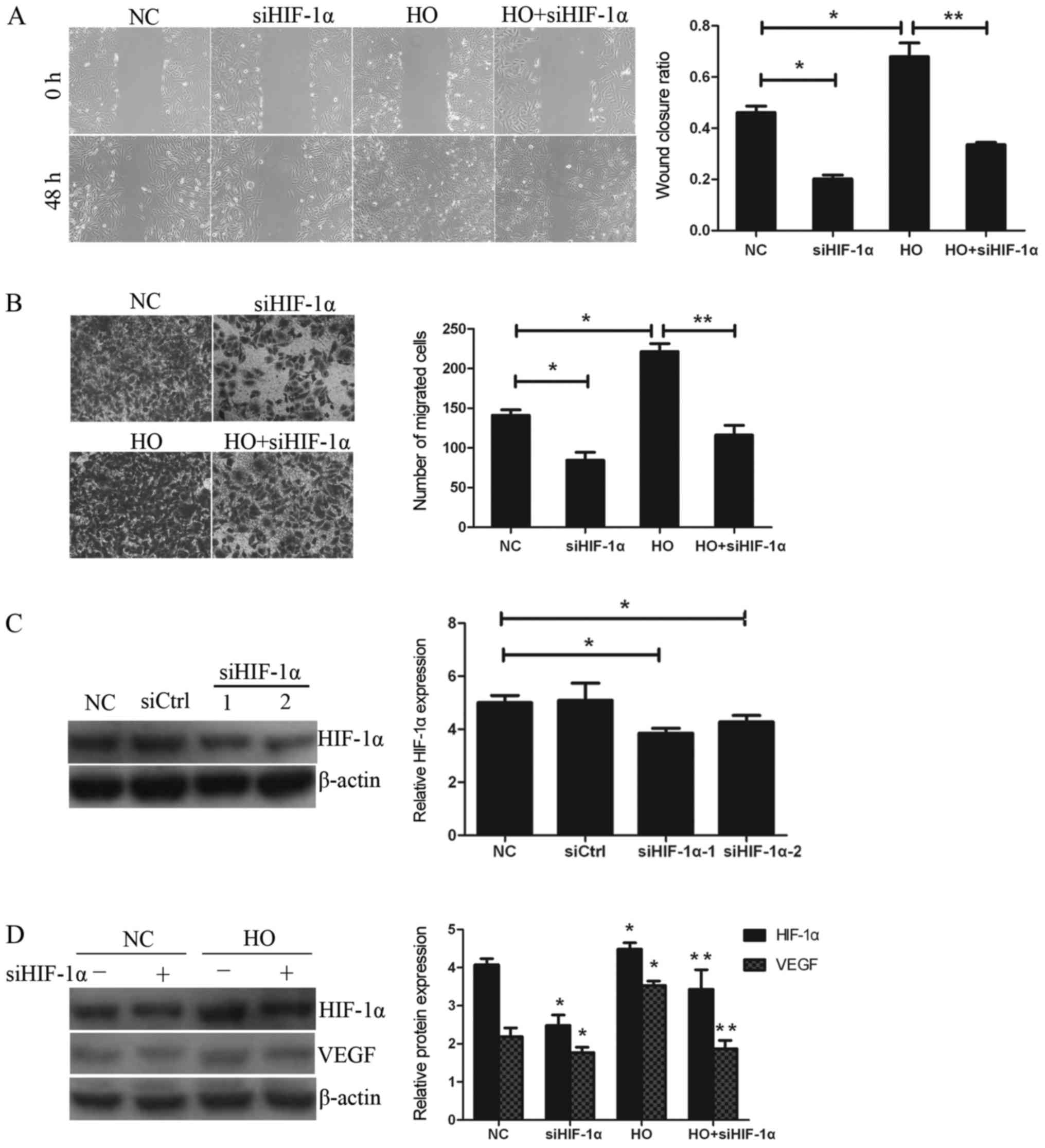
The ability of the GBC-SD cells to migrate was
investigated using wound healing and Transwell assays. According to
the data in Fig. 3C, HIF-1α was
effectively inhibited by siRNA HIF-1α (siHIF-1α). As shown in
Fig. 3A and B, following
downregulation of the expression of HIF-1α by specific inhibitor
siHIF-1α, the wound closure ratios in the hypoxia treatment (HO)
group and the HO+siHIF-1α treatment group were 0.679±0.053 and
0.335±0.009, respectively. Furthermore, the numbers of migrating
cells in the aforementioned treatment groups were 221.6±10.1 and
116.2±12.2, respectively. Therefore, it was observed that the wound
closure ratio and the number of migrating cells in the HO+siHIF-1α
treatment group were significantly decreased compared with the HO
treatment group. Subsequently, to examine whether HIF-1α is
involved in hypoxia-induced migration in GBC-SD cells, the ability
of the cells to migrate following downregulation of the expression
of HIF-1α by siHIF-1α was investigated. As shown in Fig. 3D, siHIF-1α effectively reversed the
increased expression of HIF-1α and VEGF induced by hypoxia.
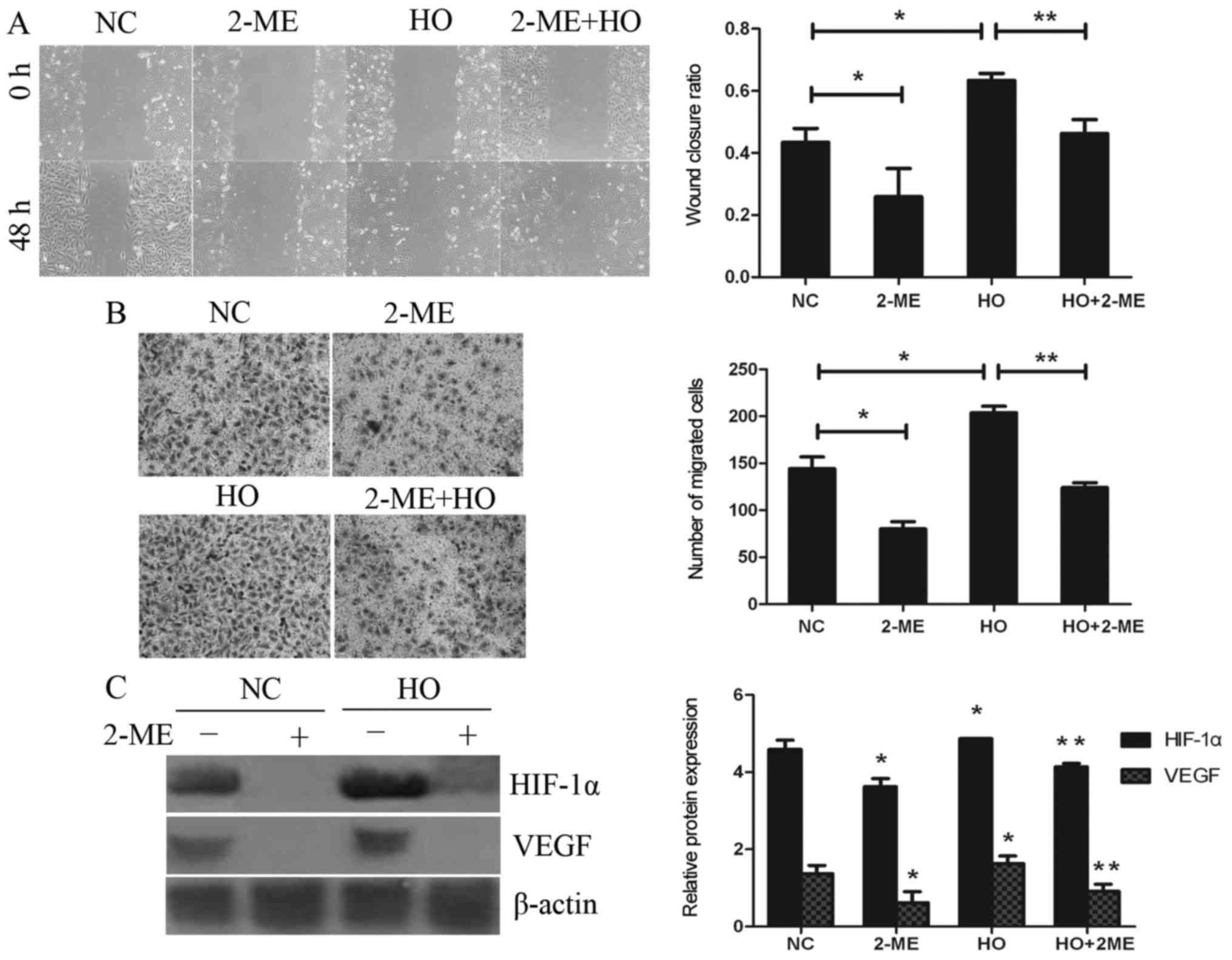
It is noteworthy that similar results were obtained
in subsequent experiments, which explored the effect of the
downregulation of HIF-1α by 2-ME under conditions of normoxia (NC
group) or hypoxia (Fig. 4A and B).
The wound closure ratios in the hypoxia treatment (HO) group and
the HO+2-ME group were 0.633±0.022 and 0.463±0.045, respectively.
In addition, the numbers of migrating cells in the aforementioned
treatment groups were 203.8±7.0 and 124.0±5.2, respectively. The
expression of HIF-1α and VEGF was clearly downregulated by 2-ME
(Fig. 4C), the results of which
were consistent with those of the inhibition of cell migration.
These results further corroborated the finding that hypoxia
promotes cell migration in GBC-SD cells through activation of the
HIF-1α/VEGF pathway.
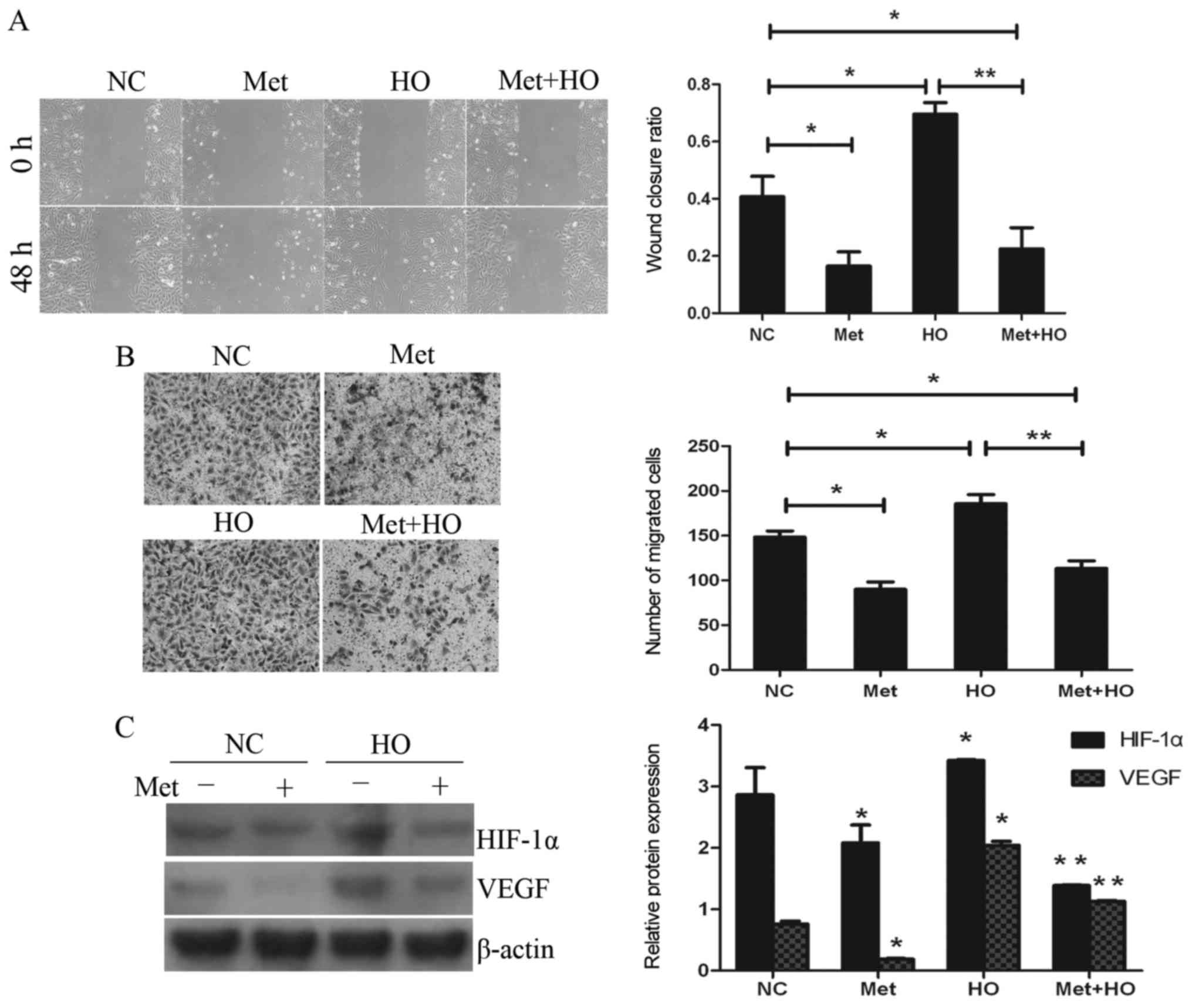
Metformin inhibits hypoxia-induced
migration via downregulation of the expression of HIF-1α and
VEGF
GBC-SD cells were pre-cultured under conditions of
hypoxia for 2 h, and subsequently treated with 20 mmol/l metformin
for 24 or 48 h. As shown in Fig.
5A, the wound closure ratios in the hypoxia (HO) treatment
group and the hypoxia in combination with metformin (Met+HO) group
were 0.695±0.040 and 0.224±0.074, respectively. These data
indicated that treatment with metformin and hypoxia resulted in a
lower wound closure ratio compared with the hypoxia treatment
group. In addition, the numbers of migrating cells in the
aforementioned treatment groups were 185.8±10.2 and 113.4±8.6,
respectively. The numbers of migrating cells in the Met+HO group
were fewer compared with the HO treatment group (Fig. 5B). These results indicated that
treatment with metformin significantly inhibited hypoxia-induced
migration. To investigate the potential mechanism, the expression
levels of HIF-1α and VEGF were also investigated. As shown in
Fig. 5C, treatment with metformin
decreased the expression of HIF-1α and VEGF. These data suggest
that HIF-1α and VEGF signaling pathway may be involved in
metformin-suppressed cell migration of the GBC-SD cells.
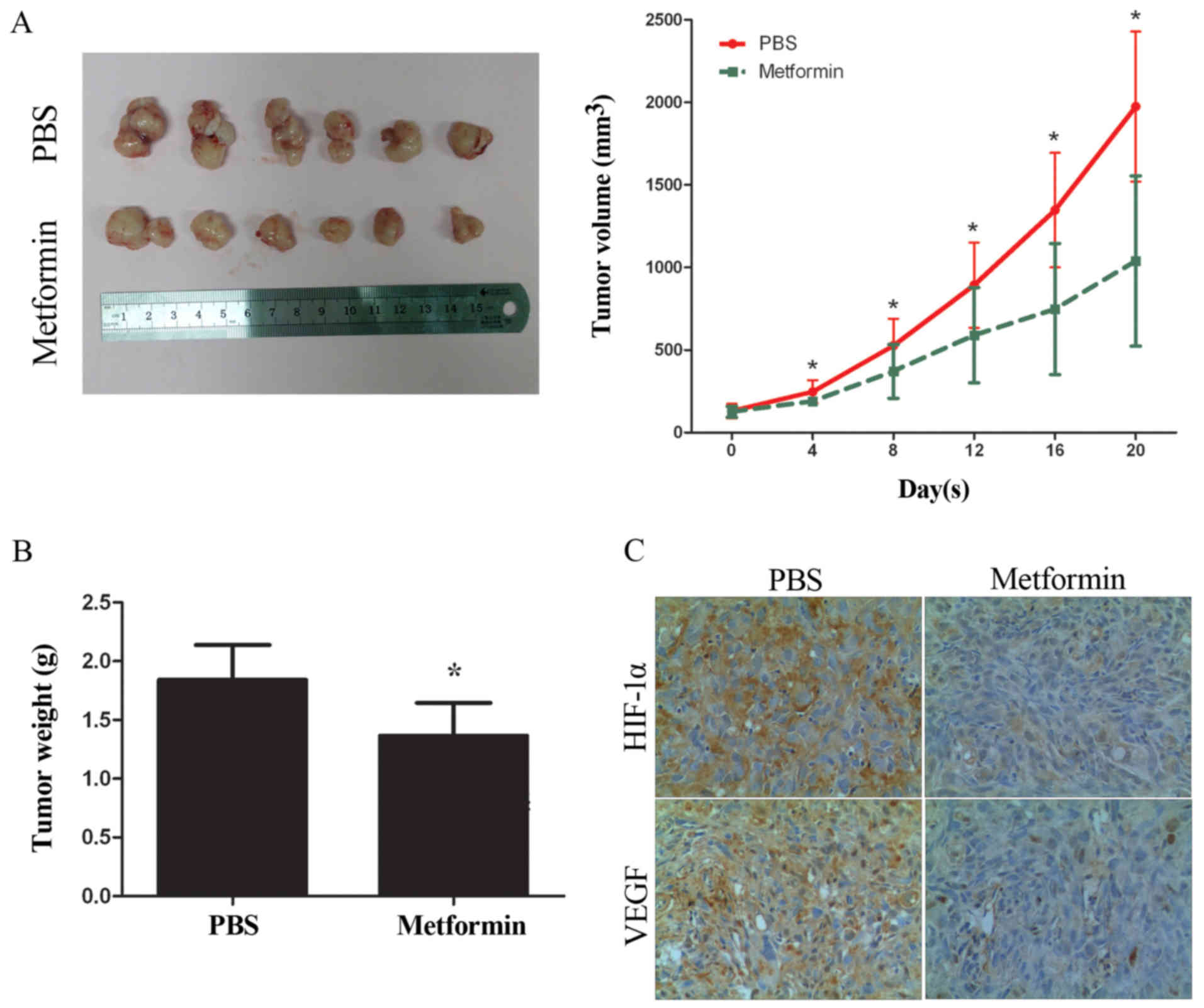
Metformin inhibits GBC cell growth and
downregulates the expression of HIF-1α and VEGF in vivo
Metformin was administered in a xenograft model,
generated by implanting GBC-SD cells into immunodeficient BALB/c
nude mice in order to evaluate their antitumor effect. As shown in
Fig. 6A and B, treatment with
metformin significantly decreased the tumor growth rate and tumor
weight at the endpoint of the animal experiment. Subsequently, the
expression levels of HIF-1α and VEGF in the tumor xenograft tissues
were detected by IHC. As shown in Fig.
6C, the expression levels of HIF-1α and VEGF were decreased in
tumor tissues treated with metformin compared with the PBS group,
the results of which are consistent with those obtained in
vitro.
Discussion
It is well known that GBC greatly threatens human
health due to the high rate of migration and recurrence, and the
5-year survival rate is less than 5% (3,24).
Rapid tumor progression and difficulty in detecting early stage
cancer are major obstacles in offering potentially curative
treatments. Therefore, it is urgent that reliable tumor markers for
early diagnosis are found and that an effective drug for patients
with GBC is developed. In the present study, it has been
demonstrated that HIF-1α was upregulated in 23 out of 34 GBC
tissues compared with that in adjacent non-tumor tissues and
chronic cholecystitis tissues, according to the RT-qPCR and IHC
experiments. Further experiments indicated that HIF-1α
overexpression is significantly associated with both lymph node
metastasis and TNM stage in GBC tissues. These results indicated
that HIF-1α exerts an important role in promoting GBC
progression.
Hypoxia is frequently observed in numerous types of
solid tumors, including GBC (25),
and also plays a crucial role in cancer progression (26). As part of a solid tumor, tumor cells
may take advantage of their biological capacity to adapt to hypoxia
to become even more aggressive (27). Additionally, the hypoxic
microenvironment inside solid tumors limits the effectiveness of
interventional embolization therapy and cytotoxic drugs. HIF-1α,
the key regulatory factor in a hypoxia-influenced microenvironment,
may translocate to the nucleus and induce the transcription of
numerous downstream target genes (17,28).
In the present study, treatment with hypoxia significantly promoted
the ability of cell migration in GBC-SD cells, according to the
results of the wound healing and Transwell assays. The subsequent
experiments also demonstrated that the expression levels of HIF-1α
and VEGF were upregulated under a hypoxic condition, while the
expression levels of HIF-1α and VEGF were present at a low level
under normoxic condition. The HIF-1 complex is composed of two
protein subunits: HIF-1β, which is constitutively expressed, and
HIF-1α, which is not present in normal cells but induced under
hypoxic conditions. The HIF-1α subunit is continuously synthesized
and degraded under normoxic conditions, while it accumulates
rapidly following exposure to low oxygen tensions (29). Herein, GBC-SD cells are gallbladder
cancer cells, not normal cells and previous studies also showed
that HIF-1α was present at a low level in normoxia in GBC-SD cells
(25). To demonstrate the
underlying mechanism of hypoxia-induced migration, siHIF-1α was
applied to the GBC-SD cells. It is noteworthy that siHIF-1α
significantly reversed hypoxia-induced migration, and downregulated
the expression levels of HIF-1α and VEGF. Similar results were also
obtained using 2-ME, a HIF-1α-specific inhibitor. These data
indicated that hypoxia promoted cell migration in GBC-SD cells
through activation of the HIF-1α/VEGF signaling pathway.
Accumulating evidence has confirmed that metformin
may inhibit cell migration or potentiate the effect of
chemotherapeutic agents (30–32).
Numerous studies have also demonstrated that metformin regulates
the AMP-activated protein kinase and extracellular signal-regulated
kinase pathways, and reverses drug resistance (33,34).
In the present study, it was first demonstrated that metformin
inhibited cellular migration, and downregulated the expression of
HIF-1α and VEGF in GBC-SD cells. In addition, it was also shown
that metformin reversed hypoxia-induced migration by targeting the
HIF-1α/VEGF pathway. The present study also investigated further
the role of metformin in GBC-SD cell migration in vivo,
according to an experimental xenograft model. GBC-SD cells were
injected into BALB/c nude mice, and it was shown that metformin
markedly decreased the tumor growth rate and tumor weight at the
endpoint of the xenograft model experiment. Furthermore, the
expression levels of HIF-1α and VEGF in the tumor xenografts
treated with metformin were decreased according to the results of
the IHC experiment, which was consistent with our study in
vitro.
In conclusion, the results of the present study
revealed that HIF-1α is upregulated in GBC tissue, and that the
aberrant expression of HIF-1α is closely associated with lymph node
metastasis and TNM stage. Additional experiments demonstrated that
hypoxia induced the migration of GBC-SD cells by increasing the
expression levels of HIF-1α and VEGF. Furthermore, metformin
reversed the increases in the hypoxia-induced cellular migration by
targeting HIF-1α/VEGF. Thus, the data of the present study provide
evidence that HIF-1α plays a vital role in GBC growth and
migration, and may serve as a therapeutic target for GBC.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural
Science Foundation of China (grant nos. 81401995 and 81702863), the
High School Key Science and Technology Project of Henan Province
(grant no. 19B320039) and the Outstanding Young Talent Research
Fund of Zhengzhou University (grant no. 1421412090).
Availability of data and materials
All data generated or analyzed during the study are
included in this published article.
Authors' contributions
JY, KC, LQ, HT and RL performed the experiments; WZ
and CZ designed the study; JY and RL prepared the study and wrote
the manuscript. All authors read and approved the final manuscript
and agreed to be accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Clinical
Research Ethics Committee of the First Affiliated Hospital of
Zhengzhou University. The animal protocol was approved by the
Institutional Animal Care and Use Committee of the First Affiliated
Hospital of Zhengzhou University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hundal R and Shaffer EA: Gallbladder
cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.PubMed/NCBI
|
|
2
|
Wang J, Narang AK, Sugar EA, Luber B,
Rosati LM, Hsu CC, Fuller CD, Pawlik TM, Miller RC, Czito BG, et
al: Evaluation of adjuvant radiation therapy for resected
gallbladder carcinoma: A multi-institutional experience. Ann Surg
Oncol. 22 Suppl 3:S1100–S1106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu AX, Hong TS, Hezel AF and Kooby DA:
Current management of gallbladder carcinoma. Oncologist.
15:168–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Riffle S, Pandey RN, Albert M and Hegde
RS: Linking hypoxia, DNA damage and proliferation in multicellular
tumor spheroids. BMC Cancer. 17:3382017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ji RC: Hypoxia and lymphangiogenesis in
tumor microenvironment and metastasis. Cancer Lett. 346:6–16. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin YJ, Shyu WC, Chang CW, Wang CC, Wu CP,
Lee HT, Chen LJ and Hsieh CH: Tumor hypoxia regulates forkhead Box
C1 to promote lung cancer progression. Theranostics. 7:1177–1191.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu W, Zhou W, Cheng M, Wang J, Liu Z, He
S, Luo X, Huang W, Chen T, Yan W and Xiao J: Hypoxia activates
Wnt/β-catenin signaling by regulating the expression of BCL9 in
human hepatocellular carcinoma. Sci Rep. 7:404462017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Unwith S, Zhao H, Hennah L and Ma D: The
potential role of HIF on tumour progression and dissemination. Int
J Cancer. 136:2491–2503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen MC, Hsu WL, Hwang PA and Chou TC: Low
molecular weight fucoidan inhibits tumor angiogenesis through
downregulation of HIF-1/VEGF signaling under hypoxia. Mar Drugs.
13:4436–4451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawamoto M, Onishi H, Ozono K, Yamasaki A,
Imaizumi A, Kamakura S, Nakano K, Oda Y, Suminoto H and Nakamura M:
Tropomyosin-related kinase B mediated signaling contributes to the
induction of malignant phenotype of gallbladder cancer. Oncotarget.
8:36211–36224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valsecchi R, Coltella N, Belloni D,
Ponente M, Ten Hacken E, Scielzo C, Scarfo L, Bertilaccio MT,
Brambilla P, Lenti E, et al: HIF-1α regulates the interaction of
chronic lymphocytic leukemia cells with the tumor microenvironment.
Blood. 127:1987–1997. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao T, Li JZ, Lu Y, Zhang CY, Li Q, Mao J
and Li LH: The mechanism between epithelial mesenchymal transition
in breast cancer and hypoxia microenvironment. Biomed Pharmacother.
80:393–405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Gao Q, Wang D, Wang Z and Hu C:
Metformin inhibits growth of lung adenocarcinoma cells by inducing
apoptosis via the mitochondria-mediated pathway. Oncol Lett.
10:1343–1349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chung HH, Moon JS, Yoon JS, Lee HW and Won
KC: The relationship between metformin and cancer in patients with
type 2 diabetes. Diabetes Metab J. 37:125–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hall C, Stone RL, Gehlot A, Zorn KK and
Burnett AF: Use of metformin in obese women with type i endometrial
cancer is associated with a reduced incidence of cancer recurrence.
Int J Gynecol Cancer. 26:313–317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Wang Z, Li M, Ye Y, Xu Y, Zhang Y,
Yuan R, Jin Y, Hao Y, Jiang L, et al: Chloride intracellular
channel 1 regulates the antineoplastic effects of metformin in
gallbladder cancer cells. Cancer Sci. 108:1240–1252. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He C, Wang L, Zhang J and Xu H:
Hypoxia-inducible microRNA-224 promotes the cell growth, migration
and invasion by directly targeting RASSF8 in gastric cancer. Mol
Cancer. 16:352017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guimaraes TA, Farias LC, Santos ES, de
Carvalho Fraga CA, Orsini LA, de Freitas Teles L, Feltenberger JD,
de Jesus SF, de Souza MG, Santos SH, et al: Metformin increases PDH
and suppresses HIF-1α under hypoxic conditions and induces cell
death in oral squamous cell carcinoma. Oncotarget. 7:55057–55068.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou X, Chen J, Yi G, Deng M, Liu H, Liang
M, Shi B, Fu X, Chen Y, Chen L, et al: Metformin suppresses
hypoxia-induced stabilization of HIF-1α through reprogramming of
oxygen metabolism in hepatocellular carcinoma. Oncotarget.
7:873–884. 2016.PubMed/NCBI
|
|
20
|
Ma F, Wang SH, Cai Q, Zhang MD, Yang Y and
Ding J: Overexpression of LncRNA AFAP1-AS1 predicts poor prognosis
and promotes cells proliferation and invasion in gallbladder
cancer. Biomed Pharmacother. 84:1249–1255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brodowska K, Theodoropoulou S, Hörste
Meyer Zu M, Paschalis EI, Takeuchi K, Scott G, Ramsey DJ, Kiernan
E, Hoang M, Cichy J, et al: Effects of metformin on retinoblastoma
growth in vitro and in vivo. Int J Oncol. 45:2311–2324. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rico M, Baglioni M, Bondarenko M, Laluce
NC, Rozados V, André N, Carré M, Scharovsky OG and Márquez Menacho
M: Metformin and propranolol combination prevents cancer
progression and metastasis in different breast cancer models.
Oncotarget. 8:2874–2889. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang SH, Zhang WJ, Wu XC, Zhang MD, Weng
MZ, Zhou D, Wang JD and Quan ZW: Long non-coding RNA Malat1
promotes gallbladder cancer development by acting as a molecular
sponge to regulate miR-206. Oncotarget. 7:37857–37867.
2016.PubMed/NCBI
|
|
25
|
Sun W, Shen ZY, Zhang H, Fan YZ, Zhang WZ,
Zhang JT, Lu XS and Ye C: Overexpression of HIF-1α in primary
gallbladder carcinoma and its relation to vasculogenic mimicry and
unfavourable prognosis. Oncol Rep. 27:1990–2002. 2012.PubMed/NCBI
|
|
26
|
Batmunkh E, Shimada M, Morine Y, Imura S,
Kanemura H, Arakawa Y, Hanaoka J, Kanamoto M, Sugimoto K and Nishi
M: Expression of hypoxia-inducible factor-1 alpha (HIF-1alpha) in
patients with the gallbladder carcinoma. Int J Clin Oncol.
15:59–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wilson WR and Hay MP: Targeting hypoxia in
cancer therapy. Nat Rev Cancer. 11:393–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song Y, Zheng S, Wang J, Long H, Fang L,
Wang G, Li Z, Que T, Liu Y, Li Y, et al: Hypoxia-induced PLOD2
promotes proliferation, migration and invasion via PI3K/Akt
signaling in glioma. Oncotarget. 8:41947–41962. 2017.PubMed/NCBI
|
|
29
|
Salceda S and Caro J: Hypoxia-inducible
factor 1α (HIF-1α) protein is rapidly degraded by the
ubiquitin-proteasome system under normoxic conditions. Its
stabilization by hypoxia depends on redox-induced changes. J Biol
Chem. 272:22642–22647. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng K and Hao M: Metformin inhibits
TGF-β1-induced epithelial-to-mesenchymal transition via PKM2
relative-mTOR/p70s6k signaling pathway in cervical carcinoma cells.
Int J Mol Sci. 17:E20002016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu T, Wang C, Yang J, Guo Y, Wu Y and Li
X: Metformin inhibits SUV39H1-mediated migration of prostate cancer
cells. Oncogenesis. 6:e3242017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian Y, Tang B, Wang C, Sun D, Zhang R,
Luo N, Han Z, Liang R, Gao Z and Wang L: Metformin mediates
resensitivity to 5-fluorouracil in hepatocellular carcinoma via the
suppression of YAP. Oncotarget. 7:46230–46241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ling S, Xie H, Yang F, Shan Q, Dai H, Zhou
J, Wei X, Song P, Zhou L, Xu X and Zheng S: Metformin potentiates
the effect of arsenic trioxide suppressing intrahepatic
cholangiocarcinoma: Roles of p38 MAPK, ERK3, and mTORC1. J Hematol
Oncol. 10:592017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Harada K, Ferdous T, Harada T and Ueyama
Y: Metformin in combination with 5-fluorouracil suppresses tumor
growth by inhibiting the warburg effect in human oral squamous cell
carcinoma. Int J Oncol. 49:276–284. 2016. View Article : Google Scholar : PubMed/NCBI
|