Introduction
According to the World Health Organization, gastric
cancer (GC) was the fifth most common malignancy and the third
leading cause of cancer-associated mortality worldwide in 2012. In
addition, it was estimated to cause 723,000 cases of mortality in
2012; notably, half of the reported cases occur in East Asia,
particularly in China (1). The risk
factors for GC include Helicobacter pylori infection,
dietary factors, cigarette smoking, occupation, physical activity
and obesity (2); however, the
processes involved in the development of GC remain to be completely
elucidated. It is therefore essential to explore mechanisms
underlying gastric carcinogenesis, in order to identify molecular
biomarkers for earlier detection and novel molecular targets for
more effective therapy.
MicroRNAs (miRNAs/miRs) were initially discovered in
Caenorhabditis elegans; lin-4 was revealed to inhibit lin-14
translation via the binding of lin-4 transcripts to the 3′
untranslated region (3′UTR) of lin-14 mRNA (3). Since then, thousands of miRNAs, which
are non-coding RNA genes ~22 nt long, have been discovered in
worms, flies, vertebrates and plants. By targeting the 3′UTR of
target mRNAs, miRNAs either inhibit translation or induce mRNA
degradation (4). Accumulating
evidence has revealed that miRNAs are involved in the regulation of
various cellular processes, including development, proliferation,
differentiation, migration, apoptosis and cell cycle progression
(5–7). In our previous study, it was reported
that miR-194 is aberrantly overexpressed in GC tissues compared
with in adjacent tissues. Furthermore, miR-194 has exhibited a
strong ability to promote cell proliferation, migration and
tumourigenicity (8).
The Wnt/β-catenin signalling pathway has been
reported to serve a crucial role in embryonic development, tissue
regeneration and numerous other biological processes. Mutations or
dysregulated expression of components of the Wnt pathway are
associated with various types of cancer (9). Numerous miRNAs have been demonstrated
to directly regulate the Wnt signalling pathway in human
malignancies, including pancreatic (10), colon (11) and breast cancer (12). Our previous study revealed that, in
GC, by targeting a negative regulator of the Ηedgehog signalling
pathway, SUFU negative regulator of Ηedgehog signaling (SUFU),
overexpression of miR-194 resulted in significant nuclear
accumulation of β-catenin, which subsequently activated the
Wnt/β-catenin signalling pathway and promoted GC progression
(8).
In this follow-up study, it was demonstrated that
miR-194 was highly expressed in GC tissues, whereas SUFU was
downregulated in GC tissues and cell lines, thus suggesting that
miR-194 may be a carcinogenic miRNA, whereas SUFU may act as a
tumour suppressor. The nuclear accumulation of β-catenin was
significantly reduced following suppression of miR-194.
Furthermore, the Wnt/β-catenin signalling transduction pathway was
blocked, at least in part, when miR-194 was inhibited. Inhibition
of miR-194 also increased cell apoptosis of GC cells. Taken
together, these findings provided information on the mechanism
underlying GC pathogenesis and may contribute to the development of
novel targeted therapies.
Materials and methods
Tissue samples
The present study was approved by the Ethics
Committee of The Shenzhen University School of Medicine (Shenzhen,
China). All patients (n=62; age, 34–84 years; female to male ratio,
1:2.1, recruited between January 2016 and December 2017) provided
written informed consent, according to the institutionally approved
protocol. All GC tissues and adjacent normal tissues were obtained
from Shenzhen Second People's Hospital (Shenzhen, China). The
samples were pathologically and histologically confirmed to be GC
tissues. Specimens were submerged in RNAlater solution and were
stored in liquid nitrogen until use. Total RNA was subsequently
isolated using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol.
Cell culture and cell
transfection
Immortalised human normal gastric epithelial cells
(HFE145) were obtained from Dr Hassan Ashktorab at Department of
Medicine and Cancer Center, Howard University and Dr Duane Smoot at
Meharry Medical Center (Nashville, TN, USA). BGC-823 and SGC7901
cells were obtained from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). AGS cells were obtained from the
American Type Culture Collection (Manassas, VA, USA). MKN28 cells,
which are known to be MKN74 derivatives (13), were obtained from China
Infrastructure of Cell Line Resources (Beijing, China). All cells
were cultured in Dulbecco's modified Eagle's medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
foetal bovine serum (HyClone; GE Healthcare Life Sciences), 100
U/ml penicillin and 100 mg/ml streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C in a humidified incubator
containing 5% CO2. The miR-194 hairpin inhibitor (cat.
no. IH-300642-05-0002) and its corresponding non-specific control
(cat. no. IN-001005-01-05) were purchased from GE Healthcare
Dharmacon, Inc. (Lafayette, CO, USA). The non-specific control was
designed based on cel-miR-67, mature sequence:
5′-UCACAACCUCCUAGAAAGAGUAGA-3′ (accession no. MIMAT0000039).
Cel-miR-67 has been confirmed to have minimal sequence identity
with miRNAs in human, mouse and rat (14). The miR-194 inhibitor and
non-specific control were transfected using
Lipofectamine® RNAimax (Invitrogen; Thermo Fisher
Scientific, Inc.) at a final concentration of 60 nM, once BGC-823
cells had reached 30–50% confluence. Negative Control (NC) small
interfering (si)RNA (cat. no. siN05815122147-1-5), SUFU siRNAs
(5′-CGGCCTGAGTGATCTCTAT-3′, 5′-GATCCACACCTGCAAGAGA-3′ and
5′-GCAGCTTGAGAGCGTACAT-3′) and β-catenin siRNAs
(5′-GCCACAAGATTACAAGAAA-3′, 5′-GACTACCAGTTGTGGTTAA-3′ and
5′-GATGGACAGTATGCAATGA-3′) were purchased from Guangzhou RiboBio
Co., Ltd. (Guangzhou, China). The NC siRNA, or a mixture of the
three SUFU or β-catenin siRNAs was transfected using
Lipofectamine® RNAimax (Invitrogen; Thermo Fisher
Scientific, Inc.) at a final concentration of 60 nM, once BGC-823
cells had reached 30–50% confluence. Cells were harvested 48 h
post-transfection.
Cell immunofluorescence analysis
BGC-823 cells were transfected with a miR-194
inhibitor and cultured at 37°C for 48 h, or were incubated with 1
µM XAV-939 (cat. no. S1180; Selleck Chemicals, Houston, TX, USA) or
dimethyl sulfoxide (cat. no. D2650; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 16 h at 37°C. The cells were then fixed in
4% formaldehyde (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) for 15 min and washed with PBS three times
prior to permeabilisation with 0.2% Triton X-100 (Beijing Solarbio
Science & Technology Co., Ltd.) (diluted with PBS) for 10 min
at room temperature. Subsequently, the cells were stained with
anti-β-catenin (L54E2) mouse monoclonal antibody (1:100; Cell
Signaling Technology, Inc., Danvers, MA, USA) and anti-SUFU rabbit
polyclonal antibody (1:100, cat. no. ab28083; Abcam, Cambridge, MA,
USA) diluted in 1% bovine serum albumin (cat. no. SW3015; Beijing
Solarbio Science & Technology Co., Ltd.) for 2 h at room
temperature. After washing with PBS, the cells were incubated with
anti-mouse immunoglobulin (Ig)G (H+L), F(ab')2 Fragment (Alexa
Fluor® 594 Conjugate) (1:1,000, cat. no. 8890; Cell
Signaling Technology, Inc.) and anti-rabbit IgG (H+L), F(ab')2
Fragment (Alexa Fluor® 488 Conjugate) (1:1,000, cat. no.
4412; Cell Signaling Technology, Inc.) for 1 h at room temperature
in a dark box Nuclear staining was performed by mounting the cells
in DAPI II (Abbott Laboratories, Abbott Park, IL, USA). The cells
were imaged on a Leica SP5 AOBS confocal microscope (Leica
Microsystems, Inc., Buffalo Grove, IL, USA) and were analysed using
Leica Application Suite X software (Leica Microsystems, Inc.).
Western blotting
BGC-823 cells (30–50% confluent) were transfected
with SUFU siRNA, β-catenin siRNA, non-specific inhibitor and NC
siRNA, miR-194 inhibitor and NC siRNA, or miR-194 inhibitor and
SUFU siRNA; after 48 h, the cells were harvested. For cytoplasmic
and nuclear isolation, proteins were isolated using NE-PER™ nuclear
and cytoplasmic extraction reagents (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Protein
concentrations were estimated using a Bicinchoninic Acid Protein
Assay kit (Pierce; Thermo Fisher Scientific, Inc.). Cell lysates
(30 µg) were electrophoresed on 10% polyacrylamide gels (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and were transferred to
Immobilon-PSQ polyvinylidene difluoride membranes (EMD Millipore,
Bedford, MA, USA). The membranes were blocked with Tris-buffered
saline containing 5% skim milk and 0.1% Tween-20 and were then
incubated with the primary antibodies overnight at 4°C. The primary
antibodies used for western blotting were as follows: Anti-SUFU
(1:1,000, cat. no. 2522; Cell Signaling Technology, Inc.),
anti-β-catenin (1:1,000, cat. no. 8480; Cell Signaling Technology,
Inc.), anti-histone deacetylase 1 antibodies (1:1,000, cat. no.
A0238; ABclonal Biotech Co., Ltd., Wuhan, China) and anti-GAPDH
monoclonal antibody (1:5,000, cat. no. 5174; Cell Signaling
Technology, Inc.). Following secondary antibody incubation (cat.
no. 7074, 1:3,000; Cell Signaling Technology, Inc.) and washing,
the membranes were visualised using Pierce™ Enhanced
Chemiluminescence Western Blotting Substrate (cat. no. 32106;
Thermo Fisher Scientific, Inc.). Densitometric analyses of western
blots from three independent subcellular fractionation experiments,
with normalization to the control, were performed using ImageJ
1.50i software (National Institutes of Health, Bethesda, MD, USA).
The relative expression levels of the control condition were
designated as 1.
RNA extraction, TaqMan®
Array 96-well Human WNT Pathway Plate assay and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Cells were transfected as aforementioned. Total
cellular RNA was extracted from cultured cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Briefly, 1 ml
TRIzol® reagent per 10 cm2 culture dish
surface area was added directly to the cells in the culture dish,
and the cells were lysed for 2 min on ice. The lysate was
transferred to a tube and 0.2 ml chloroform per 1 ml
TRIzol® reagent was added for homogenisation. After
centrifugation at 12,000 × g for 15 min at 4°C, the aqueous phase
was placed into a new tube. An equal volume of ethanol was added to
the aqueous phase and mixed thoroughly. After incubation for 10 min
at room temperature, the mixture was centrifuged at 12,000 × g for
10 min at 4°C. Finally, the RNA pellet was collected and washed
twice with 75% ethanol, after which, the RNA was resuspended in
RNase-free water. cDNA was synthesised using the Advantage
RT-for-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China) with
random primers, according to the manufacturer's protocol.
TaqMan® Array 96-well Human WNT Pathway Plates (Thermo
Fisher Scientific, Inc.) were used to quantify the expression
levels of Wnt signalling-associated genes, according to the
manufacturer's protocols. 18S, GAPDH, hypoxanthine
phosphoribosyltransferase 1 and glucuronidase β were used as
internal controls. Primer sequences are listed in the instructions
for the TaqMan® Array Human WNT Pathway kit. miR-194
TaqMan® RT-PCR amplicons (Thermo Fisher Scientific,
Inc.) were used to confirm the expression of miR-194, according to
the manufacturer's protocol. RNU6B TaqMan® RT-PCR
amplicon (Thermo Fisher Scientific, Inc.) served as an internal
control. The mRNA expression levels for other genes were determined
by RT-qPCR performed on a CFX96 real-time PCR detection system
(Bio-Rad Laboratories, Inc.) using the SYBR-Green Master Mix
(Takara Biotechnology, Co., Ltd.). The thermocycling conditions
were as follows: Initial denaturation at 95°C for 30 sec, followed
by 40 cycles at 95°C for 5 sec, annealing at 60°C for 20 sec and
extension at 72°C for 30 sec. The 2−ΔΔCq method was used
for quantitative analysis (15).
The specific primer sequences (Sangon Biotech Co., Ltd., Shanghai,
China) used for PCR amplification were as follows: GAPDH, forward
(F) 5′-AGCCTCAAGATCATCAGCAA-3′ and reverse (R)
5′-CCATCACGCCACAGTTTCC-3′; SUFU, F 5′-GACCCTGGTTACAAATTCTGTTGA and
R 5′-AGGCAGGATGGAGACCTTCAG-3′; casein kinase 2 α2 (CSNK2A2), F
5′-AATGTTCGTGTAGCCTCAAGGTACTTC-3′ and R
5′-CAAGCTGGTCATAGTTGTCCTGTCC-3′; frizzled (FZD)-related protein
(FRZB), F 5′-CGATCTGCTCTTCTTCCTCTGTGC-3′ and R
5′-CACGAGTGGCGGTACTTGATGAG-3′; FZD class receptor 7 (FZD7), F
5′-CGGCATCACCACTGGCTTCTG-3′ and R 5′-CCTTGCTGCTGTGGCTAAGTCTG-3′;
F-box and WD repeat domain containing 11 (FBXW11), F
5′-TGAACGAATGGTACGCACTGATCC-3′ and R 5′-CGTCCACACCGCCAGTTAGATTC-3′;
Wnt family member (WNT)7A, F 5′-CTGGAACTGCTCTGCACTGGGA-3′ and R
5′-GTACAGGCAGCTGTGATGGCGT-3′; and WNT7B, F
5′-CCCCCTCCCTGGATCATGCACA-3′ and R 5′-GCCACCACGGATGACAGTGCT-3′.
GAPDH was used as the internal control.
Apoptosis analysis
Cells were transfected as aforementioned. After 48 h
transfection, the cells were dissociated with Accutase®
solution, centrifuged at ~200 × g for 5 min at room temperature and
resuspended at 1×106 cells/ml in Annexin-binding buffer
(cat. no. V13242; Invitrogen; Thermo Fisher Scientific, Inc.).
Subsequently, 5 µl fluorescein isothiocyanate-conjugated Annexin V
and 1 µl propidium iodide stock solution (diluted to a
concentration of 100 µg/ml with Annexin-binding buffer) were added
to a 100-µl cell suspension and incubated for 15 min at room
temperature. Finally, 400 µl Annexin-binding buffer was added to
each tube prior to flow cytometric analysis. Flow cytometry data
were analysed by FlowJo 7.6.1 software (FlowJo, LLC, Ashland, OR,
USA) and the experiments were repeated independently three
times.
Statistical analysis
All statistical analyses were performed using SPSS
16.0 (SPSS, Inc., Chicago, IL, USA). Data comparisons between two
groups were made using Student's t-test. For comparisons in
datasets containing multiple groups, one-way analysis of variances
followed by Dunnett's test was used. Spearman's correlation
analysis was used to determine the correlation between miR-194 and
SUFU expression in paired GC samples. Data are presented as the
means ± standard error of the mean derived from three independent
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
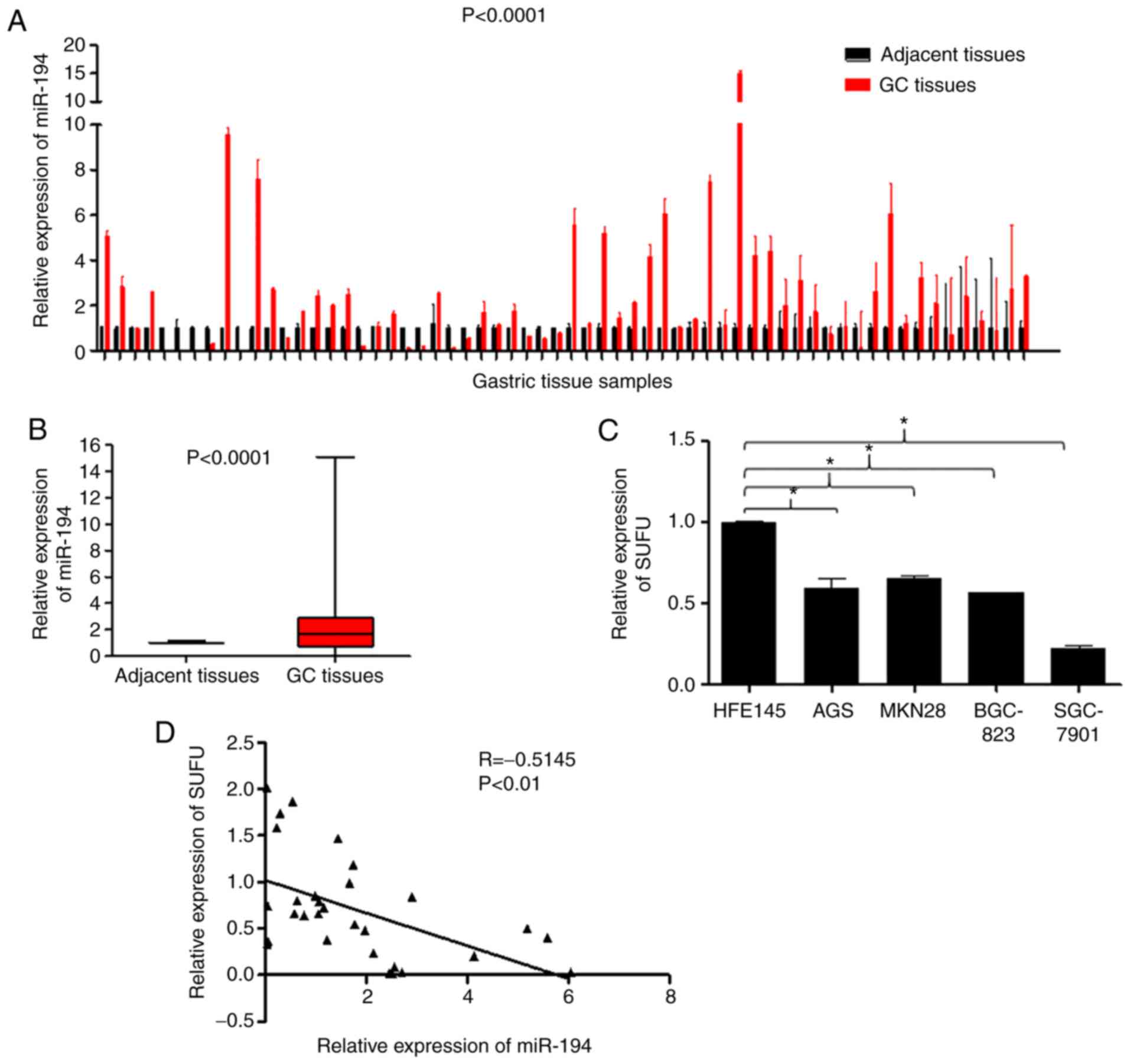
miR-194 expression is elevated and
SUFU expression is downregulated in GC
To explore the role of miR-194 in the carcinogenesis
and development of GC, the present study detected the expression
levels of miR-194 in 62 tumour tissues and paired adjacent
non-cancerous tissues. RT-qPCR analysis indicated that miR-194 was
markedly upregulated in the tumour tissues compared with in the
adjacent non-cancerous tissues (Fig. 1A
and B; P<0.0001). In addition, SUFU mRNA expression was
detected in the HFE145 human normal gastric epithelial cell, and in
the AGS, MKN28, BGC-823 and SGC-7901 human GC cell lines. As shown
in Fig. 1C, SUFU expression was
reduced in GC cell lines compared with in the HFE145 normal gastric
epithelial cell line (P<0.05). Furthermore, Spearman's
correlation analysis indicated a negative correlation between
miR-194 and SUFU expression in 30 paired GC specimens (Fig. 1C; r=−0.5148, P<0.01), which was
in line with our previous findings (8). These results suggested that miR-194
may be carcinogenic, whereas SUFU may act as a tumour suppressor.
In addition, SUFU may be targeted by miR-194 in GC.
Inhibition of miR-194 in BGC-823 cells
induces cytoplasmic translocation of β-catenin and SUFU
Extensive evidence has suggested that sustained
activation of the Wnt/β-catenin signalling pathway is involved in
the carcinogenesis of GC (16–18).
It is well known that miR-194 is highly expressed and SUFU is
expressed at low levels in BGC-823 cells (8). In addition, our previous study
revealed that SUFU is the functional target of miR-194 and
suppression of miR-194 in BGC-823 cells allows β-catenin to
translocate to the cell membrane in GC (8). To further ascertain the effects of
miR-194 on SUFU and β-catenin, immunofluorescence staining assays
were conducted in BGC-823 cells (Fig.
2A). miR-194 was downregulated using a hairpin inhibitor
(Fig. 2B). As shown in Fig. 2A, inhibition of miR-194 resulted in
marked translocation of β-catenin to the cell membrane. In
addition, the SUFU distribution pattern changed from predominantly
nuclear to nuclear-cytoplasmic. This result was consistent with
that observed in cells treated with XAV-939, a Wnt/β-catenin
signalling inhibitor, thus suggesting that miR-194 may be involved
in the localisation of not only β-catenin, but also SUFU.
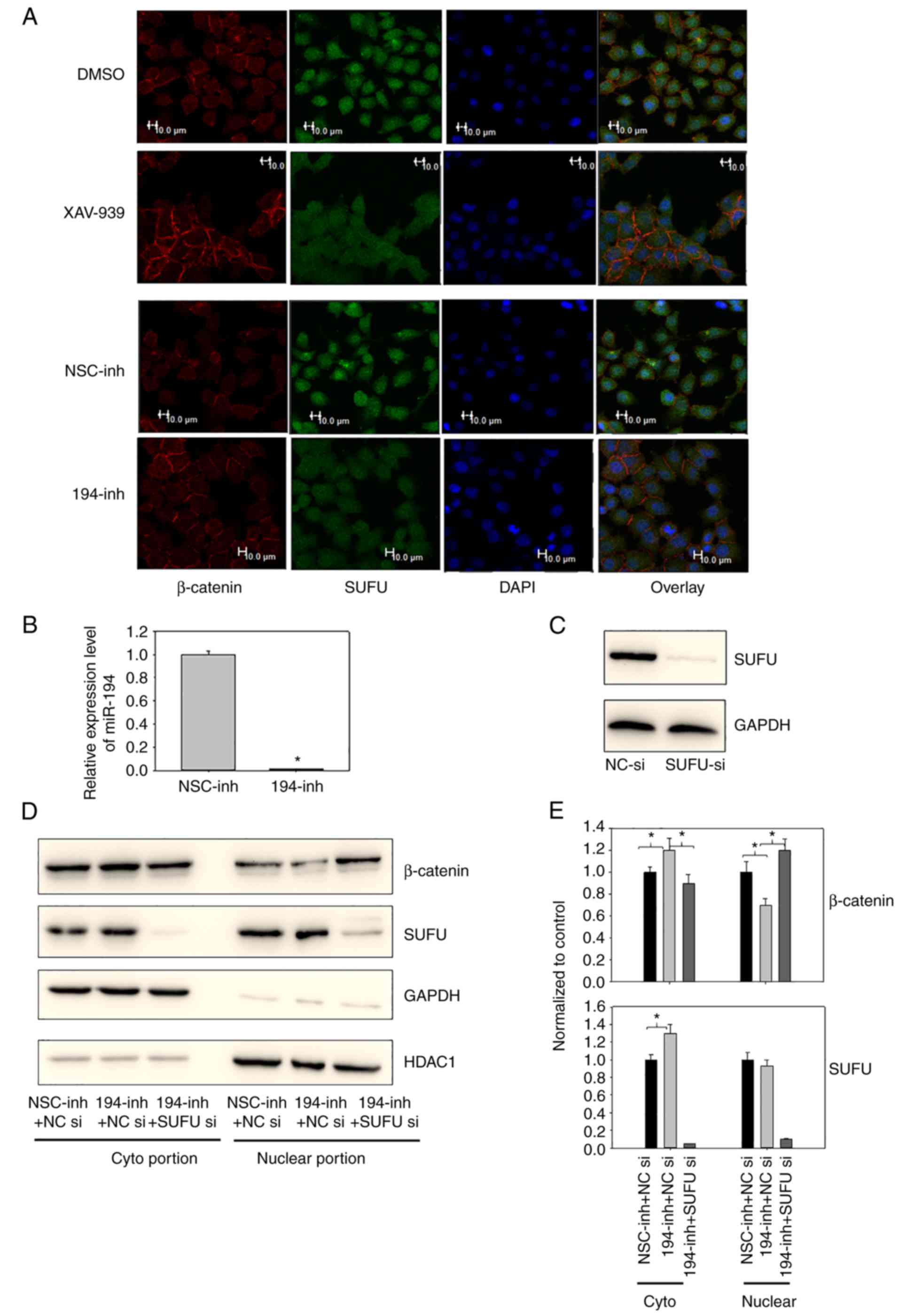 | Figure 2.(A) Inhibition of miR-194 caused
β-catenin to translocate to the cell membrane and caused SUFU to
translocate to the cytoplasm. BGC-823 cells were transfected with a
miR-194 inhibitor for 48 h or were incubated with 1 µM XAV-939 for
16 h. Subsequently, subcellular β-catenin and SUFU localisation was
assessed by immunofluorescence staining. Scale bar, 10 µm. (B)
Reverse transcription-quantitative polymerase chain reaction
analysis of miR-194 was performed on BGC-823 cells 48 h following
miR-194 inhibitor transfection to confirm successful transfection.
Student's t-test was performed to analyse results. (C) SUFU
expression was successfully knocked down using SUFU siRNA; BGC-823
cells were transfected with NC or SUFU siRNAs, and 48 h
post-transfection, the cell lysates were immunoblotted with a SUFU
antibody. (D) Suppression of SUFU alleviated the cytoplasmic
translocation of β-catenin induced by miR-194 inhibition. BGC-823
cells were transfected with a miR-194 inhibitor or a miR-194
inhibitor and SUFU siRNA, and 48 h post-transfection, the cell
lysates were fractionated into cytoplasmic and nuclear portions.
Cell lysates were then immunoblotted with β-catenin and SUFU
antibodies. (E) Densitometric analyses of western blots from three
independent experiments, with normalization to the control, were
performed with ImageJ software. The relative expression levels of
the control condition were designated as 1. One-way analysis of
variance followed by Dunnett's test was used for statistical
analysis, *P<0.05. DMSO, dimethyl sulfoxide; GC, gastric cancer;
HDAC1, histone deacetylase; miR-194, microRNA-194; NC, negative
control; NSC, non-specific; si/siRNA, small interfering RNA; SUFU,
SUFU negative regulator of Ηedgehog signaling. |
To confirm the immunofluorescence results, and to
determine if inhibition of miR-194-induced β-catenin translocation
was mediated by SUFU, the subcellular localisation of β-catenin and
SUFU were examined when miR-194 was inhibited alone or in
combination with SUFU siRNAs. Western blotting was initially
conducted to confirm that SUFU expression was successfully knocked
down using SUFU siRNAs (Fig. 2C).
In addition, SUFU expression was knocked down in cells transfected
with SUFU siRNAs and a miR-194 inhibitor, indicating that SUFU
siRNAs were effective under combined transfection conditions
(Fig. 2D and E). In agreement with
the immunofluorescence results, significantly more β-catenin was
translocated to the cyptoplasmic region when cells were transfected
with the miR-194 inhibitor alone. Notably, β-catenin expression was
not significantly altered following miR-194 inhibitor transfection,
as revealed in the western blot analysis presented in our previous
study (8), and in the results of
the TaqMan® Array 96-well Human WNT Pathway Plate assay
(CTNNB1, Table I). These findings
indicated that miR-194 may modulate the Wnt/β-catenin signalling
principally by regulating β-catenin distribution, rather than its
expression. In addition, as shown in Fig. 2D and E, suppression of SUFU
alleviated the β-catenin cytoplasmic translocation induced by
miR-194 inhibition. These findings suggested that miR-194 may
modulate β-catenin subcellular localisation in a SUFU-dependent
manner. Furthermore, consistent with the immunofluorescence
analysis, more SUFU was translocated to the cytoplasmic region when
miR-194 was inhibited. miR-194 directly targets SUFU at its 3′UTR;
in our previous study, the expression levels of total SUFU were
elevated when miR-194 was inhibited (8). Consistently, as shown in Fig. 2D and E, the increased expression of
SUFU was mostly localized to the cytoplasmic region, whereas
nuclear SUFU expression was comparable before and after miR-194
inhibitor transfection. These findings suggested that miR-194 may
regulate the expression and localisation of SUFU.
 | Table I.Relative expression levels of Wnt
signaling pathway-associated genes in BGC-823 cells
post-transfection with β-catenin siRNA or a miR-194 inhibitor. |
Table I.
Relative expression levels of Wnt
signaling pathway-associated genes in BGC-823 cells
post-transfection with β-catenin siRNA or a miR-194 inhibitor.
| Gene | β-catenin
siRNA | miR-194
inhibitor |
|---|
| CTNNB1 | 0.1010 | 0.8991 |
| TCF7 | 0.2024 | 0.2535 |
| KREMEN2 | 0.3353 | 0.6856 |
| FOXN1 | 0.3842 | 0.9433 |
| SFRP4 | 0.4322 | 0.2241 |
| PITX2 | 0.4323 | 0.4980 |
| SFRP1 | 0.4409 | 0.1021 |
| WNT4 | 0.4969 | 0.0816 |
| MYC | 0.5255 | 0.8198 |
| DKK1 | 0.5334 | 0.9630 |
| WNT11 | 0.5505 | 0.9622 |
| AXIN2 | 0.5596 | 0.7282 |
| FZD8 | 0.5795 | 0.7110 |
| NKD1 | 0.5951 | 1.0167 |
| LRP5 | 0.6482 | 0.7654 |
| SLC9A3R1 | 0.6521 | 0.7010 |
| WNT7B | 0.6646 | 0.2173 |
| CSNK1G1 | 0.6665 | 0.8205 |
| SENP2 | 0.6833 | 0.8449 |
| FRZB | 0.6926 | 0.3514 |
| PORCN | 0.6959 | 0.9331 |
| FZD1 | 0.7005 | 0.8605 |
| WNT5A | 0.7087 | 0.9652 |
| CSNK2A2 | 0.7173 | 0.4434 |
| WNT5B | 0.7258 | 0.9242 |
| FZD4 | 0.7523 | 0.7541 |
| PPP2R1A | 0.7633 | 0.7752 |
| LEF1 | 0.7701 | 0.8539 |
| CTNNBIP1 | 0.7743 | 1.8675 |
| TLE3 | 0.7831 | 0.8353 |
| PPP2CA | 0.8020 | 0.8453 |
| CSNK2B | 0.8089 | 0.8229 |
| CSNK1D | 0.8163 | 0.8483 |
| DKK3 | 0.8194 | 1.0270 |
| FZD7 | 0.8226 | 0.0020 |
| FZD10 | 0.8275 | 0.6828 |
| FZD6 | 0.8326 | 0.9536 |
| DVL1 | 0.8365 | 0.7208 |
| WNT6 | 0.8374 | 1.0771 |
| FZD9 | 0.8394 | 0.7183 |
| CSNK2A1 | 0.8427 | 0.7573 |
| EP300 | 0.8494 | 0.8390 |
| DVL3 | 0.8654 | 0.8581 |
| APC | 0.8745 | 1.0586 |
| DVL2 | 0.8824 | 0.8407 |
| AXIN1 | 0.8833 | 0.7570 |
| PYGO1 | 0.8903 | 0.9665 |
| KREMEN1 | 0.8926 | 0.5684 |
| WNT10B | 0.9044 | 0.7967 |
| WNT2B | 0.9231 | 1.2638 |
| GSK3A | 0.9456 | 1.0150 |
| TCF7L2 | 0.9504 | 1.0049 |
| CSNK1G3 | 0.9594 | 0.8100 |
| TLE2 | 0.9770 | 0.7009 |
| TLE4 | 0.9772 | 0.9552 |
| LRP6 | 0.9778 | 0.8770 |
| TCF7L1 | 1.0066 | 0.6388 |
| CBY1 | 1.0073 | 0.7859 |
| FRAT1 | 1.0132 | 0.6921 |
| CSNK1A1 | 1.0142 | 0.7752 |
| FZD2 | 1.0166 | 0.7472 |
| CSNK1G2 | 1.0258 | 0.8320 |
| RHOU | 1.0334 | 0.8648 |
| TLE1 | 1.0401 | 0.9018 |
| FBXW11 | 1.1036 | 0.4307 |
| WNT9A | 1.1052 | 0.6090 |
| WNT10A | 1.1198 | 3.0422 |
| WNT1 | 1.1331 | 1.4755 |
| GSK3B | 1.1437 | 0.8613 |
| FRAT2 | 1.1476 | 0.7411 |
| NLK | 1.1966 | 0.7700 |
| TLE6 | 1.1983 | 0.9940 |
| BTRC | 1.2124 | 0.8591 |
| PYGO2 | 1.2596 | 0.9025 |
| WNT8A | 1.2806 | 0.2067 |
| FZD3 | 1.5928 | 0.8283 |
| WNT7A | Not available | 0.5113 |
miR-194 directly or indirectly
regulates Wnt/β-catenin signalling and other associated genes
involved in signal transduction
It is widely accepted that Wnt signalling is
triggered when Wnt ligands bind to their membrane receptors, FZD
and low-density-lipoprotein-related protein 5/6 (LRP5/6)
co-receptor complex, thus resulting in the inhibition of β-catenin
degradation (19,20). The accumulated β-catenin in the
cytoplasm is translocated to the nucleus, where it interacts with
the lymphoid enhancer factor/T cell factor (LEF/TCF) to
transactivate downstream genes implicated in tumourigenesis.
Our previous study revealed that miR-194 activates
the Wnt/β-catenin signalling pathway by targeting SUFU (8); therefore, it was hypothesised that the
expression of Wnt signalling-associated genes may be altered in
response to inhibition of the Wnt signalling pathway by miR-194
suppression. Therefore, miR-194 was inhibited and β-catenin
expression was knocked down (Fig. 3A
and B), and their gene expression profiles were compared. A
TaqMan® Array 96-well Human WNT Pathway Plate assay was
used to quantify the expression levels of Wnt signalling-associated
genes. The genes were listed from most downregulated to most
upregulated in response to β-catenin siRNA. Notably, the expression
levels of β-catenin were successfully suppressed in the β-catenin
siRNA-transfected cells; however, its expression was relatively
stable following miR-194 inhibition, which agreed with our previous
findings (8). It may be
hypothesized that miR-194 mainly regulates Wnt signalling through
modulating β-catenin localisation, but not its expression.
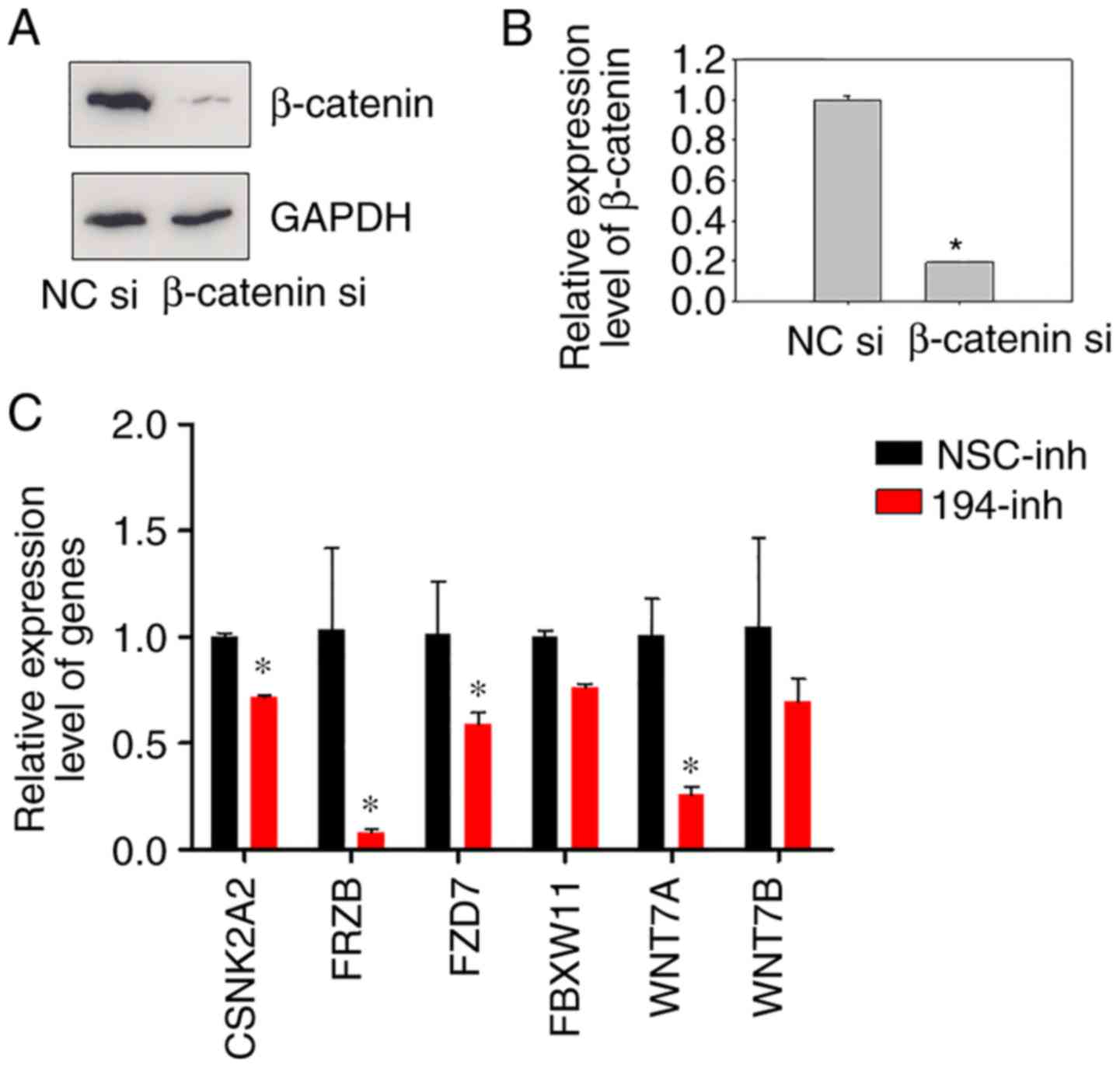 | Figure 3.Inhibition of miR-194 results in
downregulation of Wnt/β-catenin signalling-associated genes. (A)
Transfection efficiency of β-catenin siRNA was evaluated prior to
the TaqMan® Wnt plate assay. BGC-823 cells were
transfected with NC siRNA or β-catenin siRNA, and 48 h
post-transfection, the cell lysates were immunoblotted with a
β-catenin antibody. (B) RT-qPCR of β-catenin was performed on
BGC-823 cells 48 h post-transfection with β-catenin siRNA.
Student's t-test was performed for statistical analysis.
*P<0.05. (C) Relative expression levels of CSNK2A2, FRZB, FZD7,
FBXW11, WNT7A and WNT7B. BGC-823 cells were transfected with NSC
inhibitor or miR-194 inhibitor, and 48 h post-transfection, cells
were harvested, and relative gene expression levels were determined
using RT-qPCR. Data were normalised to GAPDH mRNA. Student's t-test
was performed for statistical analysis. *P<0.05. CSNK2A2, casein
kinase 2 α2; FBXW11, F-box and WD repeat domain containing 11;
FRZB, frizzled-related protein; FZD7, frizzled class receptor 7;
miR-194, microRNA-194; NC, negative control; NSC, non-specific;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; siRNA/si, small interfering RNA; WNT7, Wnt family member
7. |
As shown in Table I,
following inhibition of miR-194, various components of the
Wnt/β-catenin signalling pathway, including members of the Wnt, FZD
G-protein coupled receptor and Disheveled families, LRP5/6,
glycogen synthase kinase 3β, Axin, casein kinase 1/2, β-transducin
repeat containing E3 ubiquitin protein ligase, LEF and TCF, and the
target genes MYC proto-oncogene, bHLH transcription factor and
forkhead box N1 (21) were
downregulated to different extents. Notably, among the seven genes
in which the relative expression levels were <0.5 following
transfection with β-catenin siRNAs, five genes, including TCF7,
secreted FZD-related protein (SFRP)4, paired like homeodomain 2
(PITX2), SFRP1 and WNT4 were also downregulated by >2-fold in
response to miR-194 inhibition. These findings suggested that
similar to β-catenin suppression-mediated effects, inhibition of
miR-194 suppressed the Wnt/β-catenin signalling pathway (Table I).
TCF7, which was the most downregulated gene in
response to β-catenin siRNA transfection, was also downregulated to
a similar degree by miR-194 inhibition. TCF7 (also known as TCF-1)
serves a vital role in Wnt signalling; in the absence of β-catenin,
TCF7 can interact with Groucho corepressors and act as a
transcriptional repressor of Wnt target genes, whereas TCF7 can
activate target gene expression when it binds to β-catenin
(22,23). TCF7 is upregulated in breast cancer
(24), osteosarcoma (25) and renal cell carcinoma (26). In the present study, inhibition of
β-catenin and miR-194 led to the downregulation of TCF7, which
further downregulated Wnt signalling.
Compared with the effects of β-catenin siRNA on
β-catenin-interacting protein 1 (CTNNBIP1; also known as ICAT),
which is a negative regulator of the Wnt signalling pathway that
acts by blocking the interaction between β-catenin and TCF family
members (27,28), CTNNBIP1 was upregulated in response
to miR-194 inhibition. These findings suggested that miR-194 may
regulate the Wnt pathway, similarly to β-catenin, but via different
underlying mechanisms. Furthermore, the results revealed that
inhibition of miR-194 not only downregulated molecules activating
Wnt but also upregulated proteins blocking Wnt signalling activity.
Overall, the Wnt signalling pathway may be inhibited by miR-194
suppression, potentially via SUFU. Wnt-associated genes SFRP
(29,30), nemo-like kinase (31), pygopus family PHD finger 2 (32,33),
FBXW11 (34), PITX2 (35,36),
FRAT genes (37) and
transducer-like enhancer of split genes (38); signal transducers Dickkopf Wnt
signalling pathway inhibitors (39), chibby family member 1, β-catenin
antagonist (40) and kringle
containing transmembrane protein ½ (41); and embryogenesis-associated genes,
such as SUMO-specific peptidase 2 (42), were also detected to better
understand the association between miR-194 suppression and the
Wnt/β-catenin pathway.
Since there is only one well for each gene in the
TaqMan® Array 96-well Human WNT Pathway Plate, to verify
the reliability of the plate assay, the mRNA expression levels of
some genes were detected using RT-qPCR with specifically designed
primers. Genes that were markedly downregulated in response to
miR-194 inhibition were selected for analysis. As expected, the
expression levels of CSNK2A2, FRZB, FZD7, FBXW11, WNT7A and WNT7B
were markedly reduced in BGC-823 cells transfected with a miR-194
inhibitor compared with in those transfected with a non-specific
control inhibitor (Fig. 3C). These
results indicated that inhibition of the Wnt signalling pathway by
miR-194 suppression may downregulate the expression of molecules
involved in Wnt signalling, in agreement with our previous
findings.
miR-194 inhibits cell apoptosis
As Wnt signalling is involved in regulating cell
apoptosis, to explore the effects of miR-194 on programmed cell
death, an apoptosis assay was performed in BGC-823 cells
transfected with miR-194 and non-specific control inhibitors. As
shown in Fig. 4A and B, flow
cytometric analysis revealed that transfection with a miR-194
inhibitor increased apoptosis threefold compared with in the
control group, thus indicating that miR-194 may serve a crucial
role in cell survival, and the pathological elevation of miR-194
may inhibit cell apoptosis. As shown in Fig. 4C and D, the effects of SUFU on
apoptosis were determined. Notably, miR-194 inhibition combined
with SUFU siRNA did not rescue the increased apoptosis; rather, it
promoted apoptosis. These findings suggested that miR-194 may
regulate apoptosis via another mechanism, which requires further
investigation.
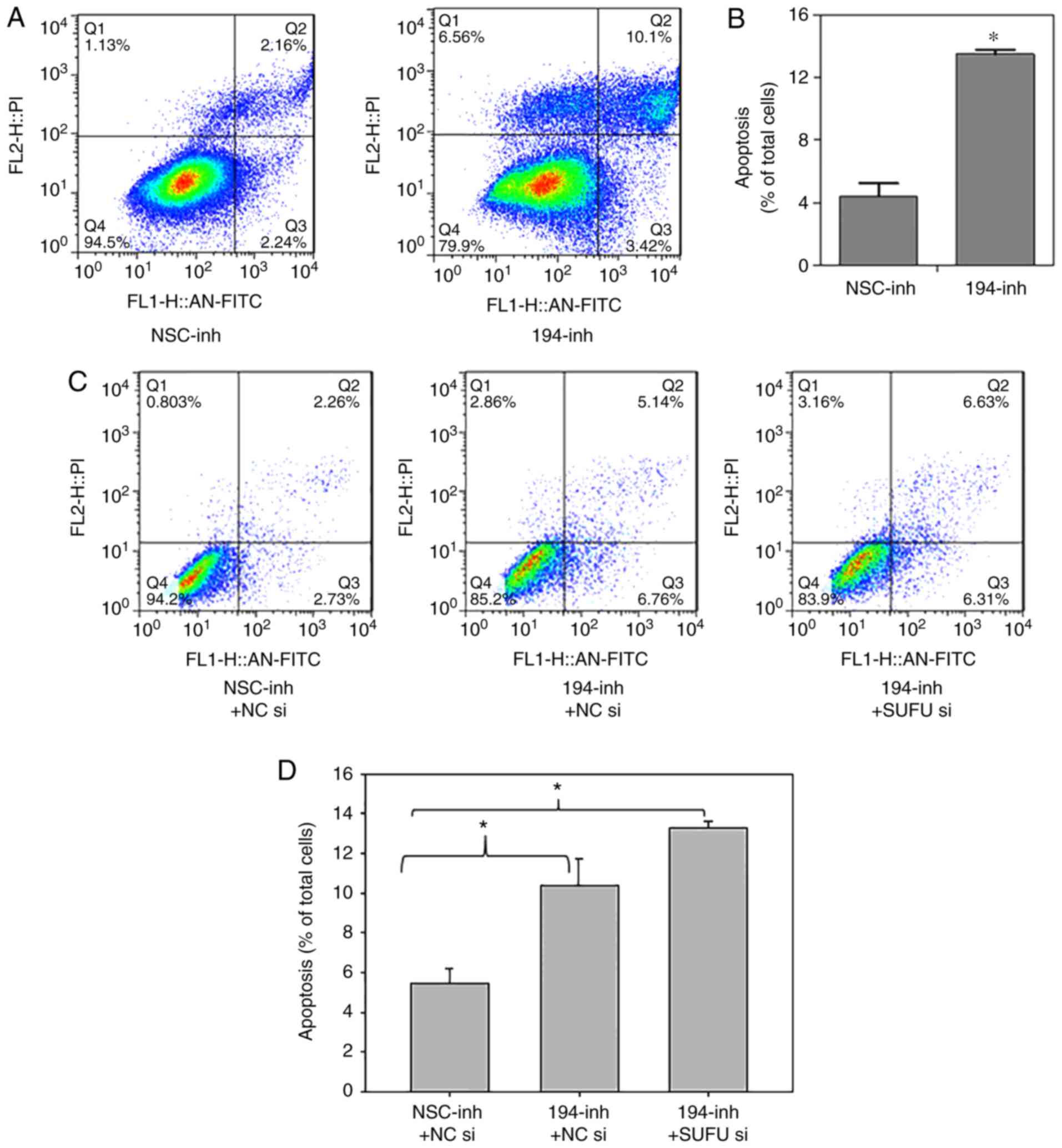 | Figure 4.miR-194 inhibition enhances cell
apoptosis, but not via SUFU. (A and B) BGC-823 cells were
transfected with NSC or miR-194 inhibitors. A total of 48 h
post-transfection, cells were stained with Annexin V-FITC and
apoptosis was analysed. Student's t-test was performed for
statistical analysis. *P<0.05. (C and D) BGC-823 cells were
transfected with miR-194 inhibitor plus NC siRNA or miR-194
inhibitor plus SUFU siRNA, and 48 h post-transfection, cells were
stained with Annexin V-FITC and apoptosis was analysed. One-way
analysis of variance followed by Dunnett's test was used for
statistical analysis. *P<0.05. FITC, fluorescein isothiocyanate;
miR-194, microRNA-194; NC, negative control; NSC, non-specific;
si/siRNA, small interfering RNA; SUFU, SUFU negative regulator of
Ηedgehog signaling. |
Discussion
Our previous study reported that miR-194 activates
the Wnt signalling pathway by targeting SUFU. In the present study,
relatively high miR-194 expression and low SUFU expression was
detected in paired GC tissues and GC cell lines; furthermore,
miR-194 was revealed to exert regulatory effects on SUFU and
β-catenin subcellular localisation, and inhibition of miR-194
resulted in downregulation of Wnt signalling pathway-associated
genes and increased apoptosis.
SUFU has been reported to act as a negative
regulator of the Ηedgehog signal transduction pathway, which
mediates various fundamental processes, including cell growth,
proliferation, survival during embryonic development, and adult
tissue homoeostasis (43,44). SUFU is a major regulator of Gli/Ci
transcription factors in vertebrates and invertebrates. By binding
to the N- and C-terminal SUFU-interacting sites on the Gli/Ci
family of transcription factors, SUFU tethers Gli/Ci in the
cytoplasm and inhibits Gli/Ci activity in the nucleus, thus leading
to inhibition of the transcription of downstream target genes
(45–47). Compared with wild type SUFU, mutant
SUFU is unable to decrease nuclear levels of β-catenin and cannot
inhibit β-catenin/TCF-mediated transcription (48). In humans, children carrying germline
and somatic mutations in the SUFU gene are predisposed to Gorlin's
syndrome tumours and medulloblastomas (49,50).
Downregulation of SUFU has also been reported in glioma (51), lung cancer (52) and other cancers. In GC, low SUFU
expression has been detected in GC samples (53); however, the molecular mechanisms
underlying SUFU dysregulation in gastric carcinogenesis are rarely
reported. In our previous study, it was demonstrated that SUFU is a
target of miR-194, and inhibition of miR-194 causes SUFU
upregulation leading to β-catenin cytoplasmic translocation
(8). The present study further
investigated the regulation of β-catenin and SUFU by miR-194. The
results indicated that more β-catenin was translocated to the
cyptoplasmic region when miR-194 was inhibited alone. Furthermore,
suppression of SUFU alleviated the β-catenin cytoplasmic
translocation induced by miR-194 inhibition, thus suggesting that
SUFU may mediate miR-194 inhibition-induced β-catenin cytoplasmic
localisation. In addition, miR-194 inhibition resulted in elevated
expression of SUFU and translocation of more SUFU to the cytoplasm.
These findings suggested that miR-194 not only regulated
subcellular localisation of β-catenin but also SUFU; however, the
precise mechanisms underlying the distribution of SUFU regulated by
miR-194 in GC require further investigation.
miR-194 expression is lost or downregulated in
glioblastoma, hepatocellular carcinoma, and cervical, ovarian,
colon and lung cancer. Conversely, miR-194 acts as a cancer
promoter in endometrial cancer (54) and oesophageal cancer (55). The role of miR-194 in GC is
controversial. Some independent groups have reported that miR-194
inhibits the proliferation, invasion and migration of GC cells
(56–59), whereas others, including our group,
revealed that miR-194 is overexpressed in GC (60–62).
The present study also indicated that miR-194 may regulate the
distribution of β-catenin to activate Wnt signalling by targeting
SUFU.
In the present study, the expression levels of genes
associated with Wnt signalling and modifiers of Wnt signalling were
detected using a TaqMan® 96-well plate assay and
RT-qPCR. Following miR-194 inhibition, the Wnt pathway was
suppressed. Members of the Wnt family, particularly WNT4/7A/7B/8A,
were markedly downregulated. Furthermore, the expression of an
important negative regulator of β-catenin, CTNNBIP1, exhibited a
twofold increase in miR-194 inhibitor-transfected cells. CTNNBIP1
contains two inhibitory regions, the N-terminal helical domain,
which blocks CBP/p300 binding, and the extended C-terminal region,
which inhibits the interaction of TCF/Lef with β-catenin, leading
to the inhibition of Wnt signalling (28,63).
Another downregulated gene upon miR-194 inhibition, PITX2,
interacts with LEF-1 and β-catenin to increase LEF-1 expression,
resulting in the activation of downstream target genes (35,36).
Notably, the expression of some Wnt negative regulators also
decreased under miR-194 inhibition. For example, FBXW11, which is a
Wnt antagonist that encodes a protein responsible for the
ubiquitylation and subsequent degradation of β-catenin (34). In the present study, FBXW11
expression was reduced following miR-194 inhibitor transfection.
Identified as negative regulators of Wnt signalling, secreted
FZD-related proteins (SFRPs), including SFRP1 and SFRP3 (FRZB),
bind to WNT8 directly and block its activity (29). SFRP1 expression was also decreased
in response to miR-194 inhibition. Therefore, it may be
hypothesized that the overall Wnt signalling activity was inhibited
by miR-194 suppression; however, negative feedback may balance this
inhibition to a certain extent. These data demonstrated that
following inhibition of the Wnt signalling pathway by miR-194
suppression through SUFU, genes relevant to Wnt/β-catenin
signalling were inhibited, indicating a potential therapeutic
effect of miR-194 in GC.
Since miR-194 has exhibited the ability to activate
Wnt/β-catenin signalling, the present study performed further
experiments to investigate cell apoptosis following miR-194
inhibition. The results indicated that suppression of miR-194
significantly induced apoptosis, strongly implying the carcinogenic
effect of miR-194 in BGC-823 cells. Therefore, inhibition of
miR-194 may reduce miR-194 to its normal levels in patients with GC
and have a potential anticancer therapeutic effect. However,
miR-194 inhibition combined with SUFU siRNA did not rescue the
increased apoptosis; rather, it promoted apoptosis. This finding
suggested that miR-194 may regulate apoptosis via other target
genes, which require further investigation.
In conclusion, the present study revealed that
miR-194 may contribute to carcinogenesis in GC by targeting SUFU.
Notably, miR-194 inhibition resulted in significant translocation
of β-catenin and SUFU from the nucleus to the nucleus-cytoplasm.
Furthermore, this study revealed that miR-194 may regulate, either
directly or indirectly, components of the Wnt/β-catenin signalling
pathway and various related genes involved in signal transduction.
In addition, miR-194 may inhibit cell apoptosis, suggesting it may
be a therapeutic target for GC.
Acknowledgements
The authors would like to thank to Dr Hassan
Ashktorab (Department of Medicine and Cancer Center, Howard
University) and Dr Duane Smoot (Department of Medicine, Meharry
Medical Center) for providing the HFE145 cells.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant no. 81772592) to ZJ; the
National Natural Youth Science Foundation of China (grant no.
31601028) to YP; the Science Technology and Innovation Committee of
Shenzhen (grant no. JCYJ20170818142852491) to ZJ; the Nature
Science Foundation of Guangdong Province (grant no. 2017A030313144)
and Startup Fund of Shenzhen University (grant no. 2018015) to YP;
the Medical Science and Technology Research Foundation of Guangdong
Province (grant no. A2016112), Nature Science Foundation of
Guangdong Province (grant no. 2017A030313479) and The Planned
Science and Technology Project of Shenzhen (grant no.
JCYJ20160422170722474) to XZ; the Guangdong Provincial Department
of Science and Technology (grant no. 2016B090918122), the Science
Technology and Innovation Committee of Shenzhen (grant nos.
JCYJ20160331190123578 and GJHZ20170314154722613) to YW; and NIH
grants DK087454, CA146799, CA173390 and an American Cancer Society
Clinical Research Professorship to SJM. Dr Meltzer is the Harry B.
Myerberg-Thomas R. Hendrix Professor of Gastroenterology.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YP, XZ, ZZ, ZJ, SJM and SL designed the study. YP,
XZ, HL, SD, YH, XFe, RY, YZ, YC, JW and WC performed the
experiments. YQ communicated with patients, asked for written
informed consent and collected GC samples. YW conducted the
statistical analysis. HA and DS provided the HFE145 cell line and
contributed to conception of the study. YP, HL, XFa and ZZ wrote
the manuscript. XFa was also involved in the conception of the
study. YW, SJM and SL reviewed and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Shenzhen University School of Medicine. All
patients provided written informed consent according to the
institutionally-approved protocol.
Patient consent for publication
Written informed consent was obtained from all
patients prior to the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forman D and Burley VJ: Gastric cancer:
Global pattern of the disease and an overview of environmental risk
factors. Best Pract Res Clin Gastroenterol. 20:633–649. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee RC, Feinbaum RL and Ambrost V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carleton M, Cleary MA and Linsley PS:
MicroRNAs and cell cycle regulation. Cell Cycle. 6:2127–2132. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng AM, Byrom MW, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: Rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng Y, Zhang X, Ma Q, Yan R, Qin Y, Zhao
Y, Cheng Y, Yang M, Wang Q, Feng X, et al: MiRNA-194 activates the
Wnt/β-catenin signaling pathway in gastric cancer by targeting the
negative Wnt regulator, SUFU. Cancer Lett. 385:117–127. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou W, Li Y, Gou S, Xiong J, Wu H, Wang
C, Yan H and Liu T: MiR-744 increases tumorigenicity of pancreatic
cancer by activating Wnt/β-catenin pathway. Oncotarget.
6:37557–37569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li T, Lai Q, Wang S, Cai J, Xiao Z, Deng
D, He L, Jiao H, Ye Y, Liang L, et al: MicroRNA-224 sustains
Wnt/β-catenin signaling and promotes aggressive phenotype of
colorectal cancer. J Exp Clin Cancer Res. 35:212016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taipaleenmäki H, Farina NH, van Wijnen AJ,
Stein JL, Hesse E, Stein GS and Lian JB: Antagonizing miR-218-5p
attenuates Wnt signaling and reduces metastatic bone disease of
triple negative breast cancer cells. Oncotarget. 7:79032–79046.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Capes-Davis A, Theodosopoulos G, Atkin I,
Drexler HG, Kohara A, MacLeod RA, Masters JR, Nakamura Y, Reid YA,
Reddel RR, et al: Check your cultures! A list of cross-contaminated
or misidentified cell lines. Int J Cancer. 127:1–8. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin HY, Gonzalez-Martin A, Miletic AV, Lai
M, Knight S, Sabouri-Ghomi M, Head SR, Macauley MS, Rickert RC and
Xiao C: Transfection of microRNA mimics should be used with
caution. Front Genet. 6:3402015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song X, Xin N, Wang W and Zhao C:
Wnt/β-catenin, an oncogenic pathway targeted by H. pylori in
gastric carcinogenesis. Oncotarget. 6:35579–35588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagy TA, Wroblewski LE, Wang D, Piazuelo
MB, Delgado A, Romero-Gallo J, Noto J, Israel DA, Ogden SR, Correa
P, et al: β-Catenin and p120 mediate PPARδ-dependent proliferation
induced by Helicobacter pylori in human and rodent epithelia.
Gastroenterology. 141:553–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Franco AT, Johnston E, Krishna U, Yamaoka
Y, Israel DA, Nagy TA, Wroblewski LE, Piazuelo MB, Correa P and
Peek RM Jr: Regulation of gastric carcinogenesis by Helicobacter
pylori virulence factors. Cancer Res. 68:379–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li VS, Ng SS, Boersema PJ, Low TY,
Karthaus WR, Gerlach JP, Mohammed S, Heck AJ, Maurice MM, Mahmoudi
T, et al: Wnt signaling through inhibition of β-catenin degradation
in an intact Axin1 complex. Cell. 149:1245–1256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He X, Semenov M, Tamai K and Zeng X: LDL
receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling:
Arrows point the way. Development. 131:1663–1677. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Balciunaite G, Keller MP, Balciunaite E,
Piali L, Zuklys S, Mathieu YD, Gill J, Boyd R, Sussman DJ and
Holländer GA: Wnt glycoproteins regulate the expression of FoxN1,
the gene defective in nude mice. Nat Immunol. 3:1102–1108. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cavallo RA, Cox RT, Moline MM, Roose J,
Polevoy GA, Clevers H, Peifer M and Bejsovec A: Drosophila Tcf and
Groucho interact to repress Wingless signalling activity. Nature.
395:604–608. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eastman Q and Grosschedl R: Regulation of
LEF-1/TCF transcription factors by Wnt and other signals. Curr Opin
Cell Biol. 11:233–240. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang SH, Li N, Wei Y, Li QR and Yu ZP:
β-catenin deacetylation is essential for WNT-induced proliferation
of breast cancer cells. Mol Med Rep. 9:973–978. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Zhang S, Xu Y, Zhang Y, Guan H, Li
X, Li Y and Wang Y: Upregulation of miR-192 inhibits cell growth
and invasion and induces cell apoptosis by targeting TCF7 in human
osteosarcoma. Tumour Biol. 37:15211–15220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nikuševa-Martić T, Serman L, Zeljko M,
Vidas Z, Gašparov S, Zeljko HM, Kosović M and Pećina-Šlaus N:
Expression of secreted frizzled-related protein 1 and 3, T-cell
factor 1 and lymphoid enhancer factor 1 in clear cell renal cell
carcinoma. Pathol Oncol Res. 19:545–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang K, Zhu S, Liu Y, Dong X, Shi Z,
Zhang A, Liu C, Chen L, Wei J, Pu P, et al: ICAT inhibits
glioblastoma cell proliferation by suppressing Wnt/β-catenin
activity. Cancer Lett. 357:404–411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tago K, Nakamura T, Nishita M, Hyodo J,
Nagai S, Murata Y, Adachi S, Ohwada S, Morishita Y, Shibuya H, et
al: Inhibition of Wnt signaling by ICAT, a novel
beta-catenin-interacting protein. Genes Dev. 14:1741–1749.
2000.PubMed/NCBI
|
|
29
|
Wang S, Krinks M, Lin K, Luyten FP and
Moos M Jr: Frzb, a secreted protein expressed in the Spemann
organizer, binds and inhibits Wnt-8. Cell. 88:757–766. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kiper POS, Saito H, Gori F, Unger S, Hesse
E, Yamana K, Kiviranta R, Solban N, Liu J, Brommage R, et al:
Cortical-bone fragility - insights from sFRP4 deficiency in Pyle's
disease. N Engl J Med. 374:2553–2562. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ota S, Ishitani S, Shimizu N, Matsumoto K,
Itoh M and Ishitani T: NLK positively regulates Wnt/β-catenin
signalling by phosphorylating LEF1 in neural progenitor cells. EMBO
J. 31:1904–1915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Townsley FM, Cliffe A and Bienz M: Pygopus
and Legless target Armadillo/beta-catenin to the nucleus to enable
its transcriptional co-activator function. Nat Cell Biol.
6:626–633. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kramps T, Peter O, Brunner E, Nellen D,
Froesch B, Chatterjee S, Murone M, Züllig S and Basler K:
Wnt/wingless signaling requires BCL9/legless-mediated recruitment
of pygopus to the nuclear beta-catenin-TCF complex. Cell.
109:47–60. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Paluszczak J, Wiśniewska D,
Kostrzewska-Poczekaj M, Kiwerska K, Grénman R, Mielcarek-Kuchta D
and Jarmuż-Szymczak M: Prognostic significance of the methylation
of Wnt pathway antagonists-CXXC4, DACT2, and the inhibitors of
sonic hedgehog signaling-ZIC1, ZIC4, and HHIP in head and neck
squamous cell carcinomas. Clin Oral Investig. 21:1777–1788. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amen M, Liu X, Vadlamudi U, Elizondo G,
Diamond E, Engelhardt JF and Amendt BA: PITX2 and beta-catenin
interactions regulate Lef-1 isoform expression. Mol Cell Biol.
27:7560–7573. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vadlamudi U, Espinoza HM, Ganga M, Martin
DM, Liu X, Engelhardt JF and Amendt BA: PITX2, beta-catenin and
LEF-1 interact to synergistically regulate the LEF-1 promoter. J
Cell Sci. 118:1129–1137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Liu S, Zhu H, Zhang W, Zhang G,
Zhou X, Zhou C, Quan L, Bai J, Xue L, et al: FRAT1 overexpression
leads to aberrant activation of beta-catenin/TCF pathway in
esophageal squamous cell carcinoma. Int J Cancer. 123:561–568.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chodaparambil JV, Pate KT, Hepler MR, Tsai
BP, Muthurajan UM, Luger K, Waterman ML and Weis WI: Molecular
functions of the TLE tetramerization domain in Wnt target gene
repression. EMBO J. 33:719–731. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bafico A, Liu G, Yaniv A, Gazit A and
Aaronson SA: Novel mechanism of Wnt signalling inhibition mediated
by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol.
3:683–686. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li FQ, Mofunanya A, Harris K and Takemaru
K: Chibby cooperates with 14-3-3 to regulate beta-catenin
subcellular distribution and signaling activity. J Cell Biol.
181:1141–1154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mao B, Wu W, Davidson G, Marhold J, Li M,
Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, et al: Kremen
proteins are Dickkopf receptors that regulate Wnt/beta-catenin
signalling. Nature. 417:664–667. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kang X, Qi Y, Zuo Y, Wang Q, Zou Y,
Schwartz RJ, Cheng J and Yeh ET: SUMO-specific protease 2 is
essential for suppression of polycomb group protein-mediated gene
silencing during embryonic development. Mol Cell. 38:191–201. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ingham PW and Placzek M: Orchestrating
ontogenesis: Variations on a theme by sonic Ηedgehog. Nat Rev
Genet. 7:841–850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ingham PW, Nakano Y and Seger C:
Mechanisms and functions of Ηedgehog signalling across the metazoa.
Nat Rev Genet. 12:393–406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Merchant M, Vajdos FF, Ultsch M, Maun HR,
Wendt U, Cannon J, Desmarais W, Lazarus RA, de Vos AM and de
Sauvage FJ: Suppressor of fused regulates Gli activity through a
dual binding mechanism. Mol Cell Biol. 24:8627–8641. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hana Y, Shia Q and Jiang J: Multisite
interaction with Sufu regulates Ci/Gli activity through distinct
mechanisms in Hh signal transduction. Proc Natl Acad Sci USA.
112:6383–6388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Svärd J, Heby-Henricson K, Persson-Lek M,
Rozell B, Lauth M, Bergström A, Ericson J, Toftgård R and Teglund
S: Genetic elimination of suppressor of fused reveals an essential
repressor function in the mammalian Ηedgehog signaling pathway. Dev
Cell. 10:187–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Taylor MD, Zhang X, Liu L, Hui CC,
Mainprize TG, Scherer SW, Wainwright B, Hogg D and Rutka JT:
Failure of a medulloblastoma-derived mutant of SUFU to suppress WNT
signaling. Oncogene. 23:4577–4583. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Smith MJ, Beetz C, Williams SG, Bhaskar
SS, O'Sullivan J, Anderson B, Daly SB, Urquhart JE, Bholah Z, Oudit
D, et al: Germline mutations in SUFU cause Gorlin
syndrome-associated childhood medulloblastoma and redefine the risk
associated with PTCH1 mutations. J Clin Oncol. 32:4155–4161. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Brugières L, Remenieras A, Pierron G,
Varlet P, Forget S, Byrde V, Bombled J, Puget S, Caron O, Dufour C,
et al: High frequency of germline SUFU mutations in children with
desmoplastic/nodular medulloblastoma younger than 3 years of age. J
Clin Oncol. 30:2087–2093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu X, Wang X, Du W, Chen L, Wang G, Cui
Y, Liu Y, Dou Z, Wang H, Zhang P, et al: Suppressor of fused (Sufu)
represses Gli1 transcription and nuclear accumulation, inhibits
glioma cell proliferation, invasion and vasculogenic mimicry,
improving glioma chemo-sensitivity and prognosis. Oncotarget.
5:11681–11694. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee Y, Kawagoe R, Sasai K, Li Y, Russell
HR, Curran T and McKinnon PJ: Loss of suppressor-of-fused function
promotes tumorigenesis. Oncogene. 26:6442–6447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yan R, Peng X, Yuan X, Huang D, Chen J, Lu
Q, Lv N and Luo S: Suppression of growth and migration by blocking
the Ηedgehog signaling pathway in gastric cancer cells. Cell Oncol.
36:421–435. 2013. View Article : Google Scholar
|
|
54
|
Chung TK, Lau TS, Cheung TH, Yim SF, Lo
KW, Siu NS, Chan LK, Yu MY, Kwong J, Doran G, et al: Dysregulation
of microRNA-204 mediates migration and invasion of endometrial
cancer by regulating FOXC1. Int J Cancer. 130:1036–1045. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wijnhoven BP, Hussey DJ, Watson DI, Tsykin
A, Smith CM and Michael MZ: South Australian Oesophageal Research
Group: MicroRNA profiling of Barrett's oesophagus and oesophageal
adenocarcinoma. Br J Surg. 97:853–861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Song Y, Zhao F, Wang Z, Liu Z, Chiang Y,
Xu Y, Gao P and Xu H: Inverse association between miR-194
expression and tumor invasion in gastric cancer. Ann Surg Oncol. 19
Suppl 3:S509–S517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li Z, Ying X, Chen H, Ye P, Shen Y, Pan W
and Zhang L: MicroRNA-194 inhibits the epithelial-mesenchymal
transition in gastric cancer cells by targeting FoxM1. Dig Dis Sci.
59:2145–2152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen X, Wang Y, Zang W, Du Y, Li M and
Zhao G: miR-194 targets RBX1 gene to modulate proliferation and
migration of gastric cancer cells. Tumour Biol. 36:2393–2401. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bao J, Zou JH, Li CY and Zheng GQ: miR-194
inhibits gastric cancer cell proliferation and tumorigenesis by
targeting KDM5B. Eur Rev Med Pharmacol Sci. 20:4487–4493.
2016.PubMed/NCBI
|
|
60
|
Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y,
Yao H, Liu X, Ke Y, Si J, et al: MiR-375 frequently downregulated
in gastric cancer inhibits cell proliferation by targeting JAK2.
Cell Res. 20:784–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tsukamoto Y, Nakada C, Noguchi T, Tanigawa
M, Nguyen LT, Uchida T, Hijiya N, Matsuura K, Fujioka T, Seto M, et
al: MicroRNA-375 is downregulated in gastric carcinomas and
regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer
Res. 70:2339–2349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shiotani A, Uedo N, Iishi H, Murao T,
Kanzaki T, Kimura Y, Kamada T, Kusunoki H, Inoue K and Haruma K: H.
pylori eradication did not improve dysregulation of specific
oncogenic miRNAs in intestinal metaplastic glands. J Gastroenterol.
47:988–998. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Daniels DL and Weis WI: ICAT inhibits
beta-catenin binding to Tcf/Lef-family transcription factors and
the general coactivator p300 using independent structural modules.
Mol Cell. 10:573–584. 2002. View Article : Google Scholar : PubMed/NCBI
|