Introduction
Intraocular lymphoma (IOL) represents a malignant,
extranodal form of non-Hodgkin lymphoma (NHL) mainly located in the
vitreous, retina and optic nerve head. It is a subtype of primary
central nervous system lymphoma (PCNSL) that occurs in isolation
[termed primary IOL (PIOL)] or with a simultaneous intracerebral
mass (1). IOL can develop either as
an isolated manifestation or as a recurrent disease. Approximately
15–25% of patients with PCNSL present with intraocular involvement
at diagnosis or during the follow-up period (2), accounting for 1% of intracranial
tumors. Vitreoretinal lymphoma is the main type of IOL.
IOL is a typical masquerade syndrome and shares some
symptoms, such as floaters and blurred vision, with posterior
uveitis, vitritis and retinitis (3). These shared symptoms can result in
difficult or delayed diagnoses. Approximately 80% of patients with
IOL have bilateral eye involvement (4). Although examinations, including
optical coherence tomography (OCT) and B-type ultrasonography can
provide valuable insight for diagnosis, cytological analysis is an
indispensable method for diagnostic confirmation. The main
pathological type is diffuse large B cell lymphoma.
It has to be determined whether IOL should be
considered a distinct lymphoma entity. For the rarity of the
disease, little is known about the differences between IOL and
PCNSL without intraocular involvement (non-IOL). An increasing
number of studies have suggested that IOL and PCNSL are distinct in
terms of genetic features and recommended clinical actions
(5,6). Additionally, Wang et al
(7) used a single nucleotide
polymorphism microarray to demonstrate that patients with IOL with
central nervous system (CNS) lesions at diagnosis had distinct
genetic characteristics compared with patients with isolated PIOL
and secondary IOL following systemic lymphoma, which may reveal the
high heterogeneity and distinctive biological behavior of the
disease. Recent studies have concentrated on isolated PIOL
(8,9), concluding that it is a less aggressive
form of the disease (10,11). However, to the best of our
knowledge, only a few studies to date have focused on IOL with CNS
involvement or following intracerebral mass due to the difficulty
of diagnosis and rarity of the disease (12,13).
As a result, questions concerning the biological behavior of IOL
remain unresolved.
In this study, we hypothesized that patients with
PCNSL with intraocular involvement would have a higher rate of
relapse for the following reasons: i) It is difficult to detect
intraocular infiltration due to atypical symptoms; ii) the unique
genetic makeup of invading tumor cells may result in treatment
insensitivity; and iii) intravenous chemotherapy with high-dose
methotrexate (HD-MTX) is insufficient for the treatment of IOL due
to delivery impediments caused by the blood-ocular barrier. In this
study, we focus on the systemic impact of intraocular involvement
on the prognosis of patients with PCNSL.
Patients and methods
Patient eligibility
From October, 2009 to October, 2016, 103 patients
with PCNSL were consecutively enrolled at our Huashan Hospital and
Huashan Hospital North, Shanghai, China. Their clinical data were
prospectively analyzed. All PCNSL diagnoses were confirmed by
pathological examinations following stereotactic puncture or
surgical tumor reduction. Brain magnetic resonance imaging (MRI),
systemic computed tomography (CT) scans, positron emission
tomography-CT (PET/CT), bone marrow biopsy and Karnofsky
performance scores (KPS) were obtained to aid the diagnosis and
evaluation of the disease state. Cerebrospinal fluid (CSF) samples
were obtained when it could be done safely and without the
possibility of herniation from increased intracranial pressure. The
following patients were excluded in the research: i) Patients with
systemic lymphoma; ii) patients with severe lesions affecting
cardiac, liver or renal function; iii) HIV-positive and
immunosuppressed patients; and iv) patients with PCNSL suspected to
have intraocular involvement, but who rejected vitreous biopsy.
All patients with PCNSL were screened by
ophthalmological assessment (including slit-lamp examination, OCT
and ophthalmologic B-ultrasound) when they were first admitted to
our hospital. Routine ophthalmical testing was also performed after
every 3 chemotherapeutic cycles. Vitreous biopsies were performed
when the above-mentioned inspections suggested IOL. The diagnosis
of IOL was made when lymphoma cells were found in the vitreous
fluid. The identified patients were divided into the IOL and
non-IOL groups according to their intraocular involvement status
during disease progression.
Blood samples were collected from patients under
aseptic precautions and were subjected to centrifugation for 4 min
at 600 × g after the blood coagulated. The serum was then separated
and stored at 4°C in refrigerators. The serum lactate dehydrogenase
(LDH) concentration was measured using the Lactate Dehydrogenase
acc. to IFCC ver.2 (LDHI2) kit (Roche Diagnostics GmbH, Mannheim,
Germany). The serum β2-microglobulin (β2-MG) concentration was
measured using a latex enhanced immunoturbidimetric assay and tests
were directed by the manufacturer of the kit (Zybio, Chongqing,
China). All the measurements were performed on an Roche cobas 8000
modular analyzer series at Huashan Hospital. Tumor tissues were
fixed in 4% formalin, embedded in paraffin and sectioned at 4
microns. The slices were deparaffinized in xylol and rehydrated in
graded alcohol. Antigen retrieval with 10 mM citrate buffer (pH
6.2) at 95°C was used. After the blocking of non-specific antibody
binding by incubating with 5% BSA, the sections were incubated with
anti-Ki-67 monoclonal antibody at a pre-diluted concentration (cat.
no. MAB-0672; Maixin Biotechnologies, Fuzhou, China) at room
temperature for 45 min and HRP-conjugated secondary antibody for 1
h at 37°C. To reveal endogenous peroxidase activity, the slides
were incubated with DAB substrate and stained with hematoxylin. All
the Ki-67 stained slides were scanned by microscopy.
Treatment
The patients were treated with HD-MTX-based
chemotherapy with or without whole-brain radiotherapy (WBRT).
Rituximab, idarubicin (IDA) and teniposide (VM-26) were also
administered to the patients with PCNSL in combination with HD-MTX
and/or radiotherapy. In total, 16 patients received intravitreal
injections of MTX following the identification of intraocular
involvement. They were initially treated with intravitreal
injections of MTX (400 µg/0.1 ml) in the affected eyes twice a week
for 4 weeks, then once a week for 8 weeks, and finally once a month
for 9 months. An intrathecal injection of MTX was administered to
patients with meningeal involvement. Patients received salvage
drugs (such as high-dose Ara-C and pemetrexed) or radiotherapy at
first relapse.
This study received approval from the Ethics
Committee of the Institutional Review Board of Huashan Hospital,
Fudan University. All the patients voluntarily participated in this
study and provided informed consent.
Statistical analysis
Progression-free survival (PFS) was defined as the
time from entry into the study to the first sign of disease
progression, and overall survival (OS) was defined as the time from
study entry to death. The Mantel-Haenszel Pearson Chi-square test
or Fisher's exact test were used to evaluate the differences in
categorical data between the 2 groups. PFS, OS and 95% confidence
interval (CI) values for PFS and OS were estimated using the
Kaplan-Meier method. The log-rank test was used to perform
univariate analyses. Variables with a univariate analysis P-value
<0.2 were examined using the Cox proportional hazards regression
model for multivariate analysis. Results were considered
statistically significant at P≤0.05. The statistical tests were
conducted using STATA 12.0 software.
Results
Patient characteristics
Of the 103 patients with PCNSL eligible for
enrollment into this study, 21 patients (20.4%, including 12 males
and 9 females) presented with intraocular involvement as confirmed
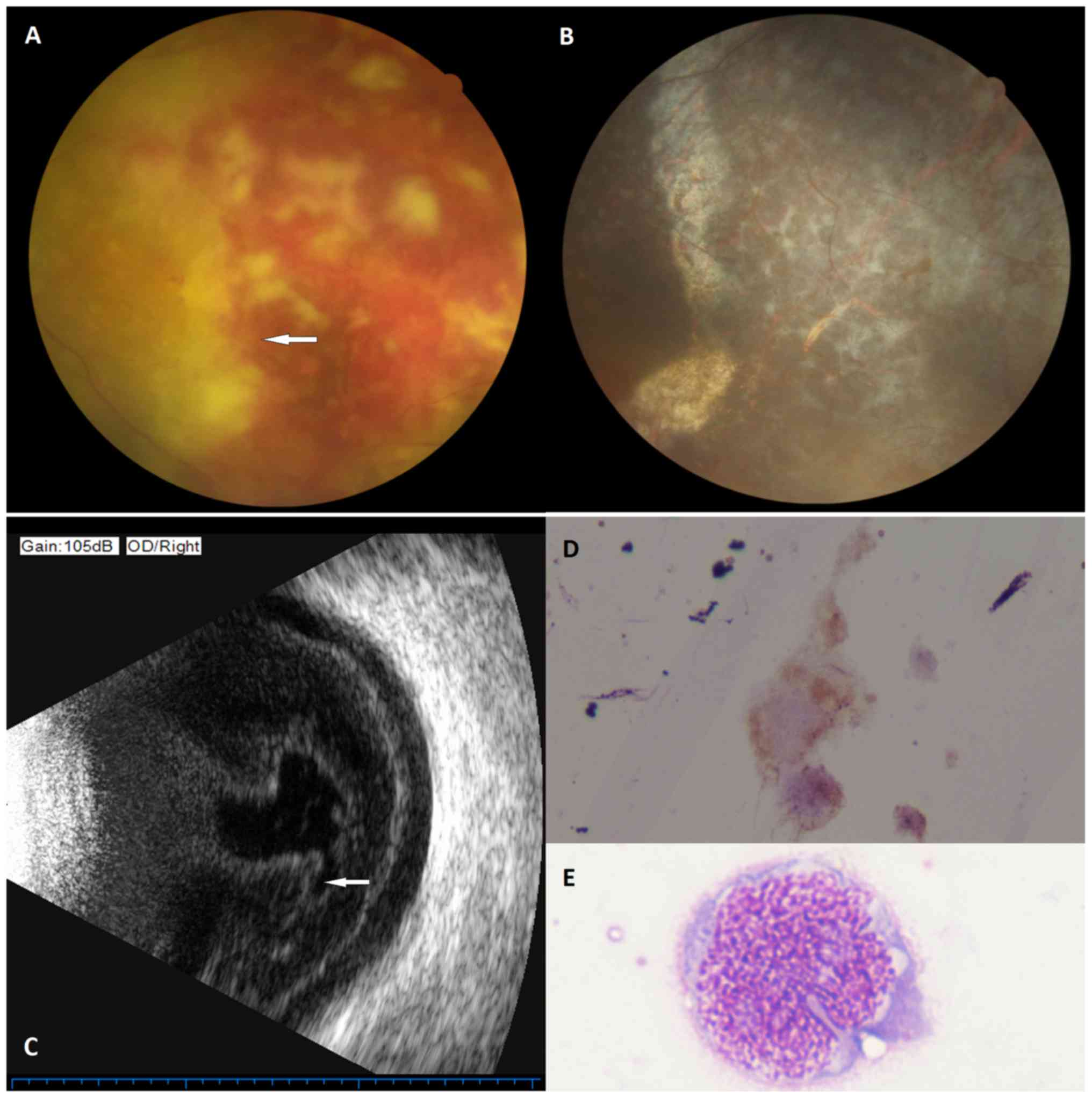
by the cytomorphological examination of vitreous aspirate (Fig. 1). The median follow-up period was 21
months (ranging from 3 to 89 months). The mean age at the onset of
IOL was 55 years (ranging from 37 to 71 years). The main clinical
characteristics of the eligible patients are summarized in Table I. No significant differences were
observed between the patients with and without intraocular
involvement as regards age, sex, clinical presentations and
laboratory data (including Ki-67, serum LDH and
β2-MG).
 | Table I.Characteristics of patients with PCNSL
with and without intraocular involvement. |
Table I.
Characteristics of patients with PCNSL
with and without intraocular involvement.
| Characteristic | Non-IOL (n=82) | IOL (n=21) | P-value |
|---|
| Follow-up time,
median (range) | 20 (3–89) | 27 (4–83) | 0.842 |
| Age, median
(range) | 55.5 (23–75) | 55 (37–71) | 0.947 |
| ≥60 years | 27 | 4 | 0.216 |
| Sex |
| Male | 56 | 12 | 0.336 |
|
Female | 26 | 9 |
|
| KPS, median
(range) | 70 (30–90) | 60 (30–80) |
|
|
30–40 | 28 | 8 | 0.735 |
|
>40 | 54 | 13 |
|
| Diagnosis |
|
Surgery | 40 | 11 | 0.768 |
|
Biopsy | 42 | 10 |
|
| Lesions |
|
Single | 35 | 13 | 0.115 |
|
Multiple | 47 | 8 |
|
| Initial
therapy |
|
Chemotherapy | 47 | 14 | 0.437 |
|
Chemotherapy+radiotherapy | 35 | 7 |
|
| Ki-67 |
| Median
(range) | 60 (0–95) | 75 (20–99) |
|
|
| 48/82 | 10/21 | 0.350 |
|
≥80 | 16/48 | 5/10 | 0.318 |
| LDH |
| Median
(range) | 203.8
(109–638) | 182 (139–385) | 0.763 |
| Elevated β
2-MG | 24/54 | 3/14 | 0.117 |
| Meningeal
involvement | 16/40 | 5/12 | 0.918 |
| Disease
relapse | 38 | 15 | 0.04 |
|
Brain | 38 | 3 |
|
|
Eye | 0 | 6 |
|
| Brain
and eye | 0 | 6 |
|
| Time to diagnosis,
median (range) | 1.0 (0.2–27.0) | 1.0 (0.5–16.0) | 0.775 |
Characteristics of IOL
Among the 21 patients with IOL, 11 patients had
bilateral eye involvement and 10 patients had unilateral eye
involvement over the course of the disease. In total, 16 patients
had clinical symptoms including the following: Blurred vision,
photophobia, fundus hemorrhage, floaters, ocular pain and ocular
movement limits. However, 5 patients were asymptomatic when
diagnosed. A total of 7 patients presented with IOL at the onset of
diagnosis, and 14 patients presented with intraocular involvement
along the disease course. In total, 20 patients had a contaminant
CNS mass during the course of the disease, and 6 had both
parenchymal and ocular involvement at diagnosis. Only 1 patient was
diagnosed with PIOL. The clinical signs of the patients with IOL
are summarized in Table II.
 | Table II.Clinical signs of patients with
intraocular lymphoma. |
Table II.
Clinical signs of patients with
intraocular lymphoma.
|
Characteristics | No. of
patients |
|---|
| Single eye
involvement | 10 |
| Bilateral eye
involvement | 11 |
| Symptomatic | 16 |
| Asymptomatic | 5 |
| With parenchyma
involvement at diagnosis | 20 |
| Without parenchyma
involvement at diagnosis | 1 |
| Intraocular
involvement initially | 7 |
| Intraocular
involvement during disease progression | 14 |
Treatment
All the patients were treated with HD-MTX with or
without WBRT; in some cases, this treatment was combined with
rituximab, IDA and VM-26. A total of 15 patients in the IOL group
and 66 patients in the non-IOL group responded to the HD-MTX-based
therapy. The overall response (OR) rates were 71.4 and 80.5%,
respectively (P=0.545). In total, 15 patients in the IOL group and
57 patients in the non-IOL group achieved complete remission
(P=0.864). No significant differences were observed between the
responses of the 2 groups (Table
III).
 | Table III.Outcomes of the HD-MTX-based therapy
in the IOL and non-IOL patients. |
Table III.
Outcomes of the HD-MTX-based therapy
in the IOL and non-IOL patients.
|
| Non-IOL (%) | IOL (%) | P-value |
|---|
| OR rate, % | 80.5 | 71.4 | 0.545 |
| CR rates, % | 69.5 | 71.4 | 0.864 |
A total of 16 patients received intravitreal
injections of MTX at a dose of 400 µg/0.1 ml after presenting with
intraocular involvement. From this group, 15 experienced a
clearance of intraocular tumor cells following ≤15 cycles of
intravitreal MTX injections. One patient experienced disease
progression in both the eyes and in the calvarium after 7 injection
cycles. The main complication of this treatment was reversible
corneal injury, which usually occurred after the first 8 weeks of
injections. Other complications included complicated cataracts,
increased tension, vitreous hemorrhage and optic atrophy.
Disease relapse
Of the 21 patients with IOL, 15 (71.4%) experienced
disease relapse. In total, 3 patients had isolated brain
parenchymal relapse, 6 patients had isolated eye relapse, and 6
patients had relapses concurrently involving the brain parenchyma
and the eyes. In the non-IOL group, 38 patients (46.3%) had brain
relapse (P=0.04) (Table I). The
2-year PFS rates of the IOL and non-IOL groups were 23.8 and 23.2%,
respectively (P=0.951). The 2-year OS rates of the IOL group and
non-IOL group were 61.9 and 41.4%, respectively (P=0.093).
Survival data
The median follow-up periods of the IOL and non-IOL
groups were 27 months (ranging from 4 to 83 months) and 20 months
(ranging from 3 to 89 months), respectively (P=0.842). The median
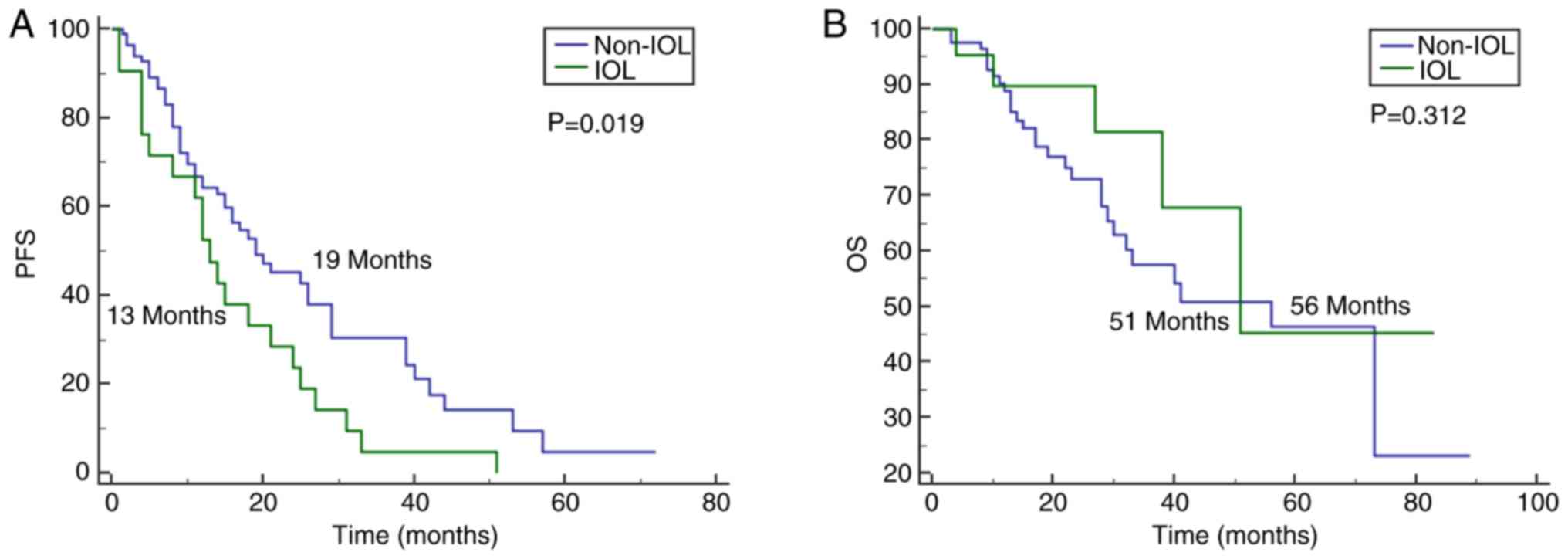
PFS in the IOL group was 13 months (95% CI 9.6–16.3), whereas that
in the non-IOL group was 19 months (95% CI 11.2–26.8; P=0.019)
(Fig. 2A). The median PFS in the
IOL group was significantly shorter compared with the median PFS of
patients without intraocular involvement. The median OS of the
patients with IOL was 51 months vs. 56 months for the patients
without IOL (non-IOL patients; 95% CI 37.2–74.8; P=0.312). No
significant difference was found between the 2 groups (Fig. 2B). A univariate analysis identified
intraocular involvement as the only factor predictive of PFS
(Table IV) and age (≥60) as the
only factor predictive of OS. Furthermore, a multivariate analysis
using backward stepwise selection and including the factors with a
P-value <0.20 from the univariate analyses, demonstrated that
intraocular involvement was a significant risk factor for PFS
(Table V) and age (≥60 years) was a
significant risk factor for OS (Table
VI). The risk of death was not affected by intraocular
involvement.
 | Table IV.Univariate analysis for risk factors
of PFS and OS. |
Table IV.
Univariate analysis for risk factors
of PFS and OS.
|
| PFS | OS |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Female | 0.992 | 0.610–1.612 | 0.973 | 1.341 | 0.680–2.643 | 0.397 |
| Age, ≥60 years | 1.109 | 0.668–1.839 | 0.689 | 2.004 | 1.009–3.981 | 0.047 |
| KPS <40 | 1.225 | 0.764–1.965 | 0.399 | 1.148 | 0.577–2.811 | 0.695 |
| Radiotherapy | 0.872 | 0.548–1.389 | 0.566 | 1.318 | 0.674–2.576 | 0.419 |
| Multiple lesions in
brain | 1.368 | 0.859–2.177 | 0.187 | 1.758 | 0.882–3.501 | 0.109 |
| Biopsy | 0.968 | 0.622–1.576 | 0.968 | 1.417 | 0.708–2.835 | 0.325 |
| Elevated LDH | 1.139 | 0.612–1.139 | 0.612 | 1.083 | 0.529–2.215 | 0.827 |
| Elevated β2-MG | 0.954 | 0.574–1.585 | 0.857 | 1.481 | 0.749–2.928 | 0.259 |
| Meningeal
involvement | 1.756 | 0.908–3.398 | 0.094 | 1.571 | 0.660–3.738 | 0.307 |
| Ki-67 ≥80 | 0.944 | 0.487–1.831 | 0.866 | 0.568 | 0.204–1.585 | 0.280 |
| Intraocular
involvement | 1.804 | 1.083–3.004 | 0.023 | 0.619 | 0.240–1.599 | 0.322 |
 | Table V.Multivariate analysis for the risk
factors of progression-free survival (PFS). |
Table V.
Multivariate analysis for the risk
factors of progression-free survival (PFS).
|
| PFS |
|---|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value |
|---|
| Intraocular
involvement | 2.600 | 1.255–5.376 | 0.010 |
| Meningeal
involvement | 1.842 | 0.914–3.704 | 0.087 |
| Multiple lesions in
brain | 1.543 | 0.746–3.195 | 0.242 |
 | Table VI.Multivariate analysis for the risk
factors of overall survival (OS). |
Table VI.
Multivariate analysis for the risk
factors of overall survival (OS).
|
| OS |
|---|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value |
|---|
| Age, ≥60 years | 2.183 | 1.091–4.386 | 0.027 |
| Multiple lesions in
brain | 1.923 | 0.958–3.861 | 0.066 |
Discussion
In this study, we prospectively evaluated the
prognostic value of intraocular involvement in patients with PCNSL.
The median follow-up time across both groups was 21 months. The
relapse rate of the patients in the IOL group was significantly
higher than that of the patients in the non-IOL group.
Additionally, a significantly poorer PFS was observed in the IOL
group compared with the non-IOL group. Nonetheless, there was no
significant difference in the OS rate between the 2 groups.
The survival outcomes of patients with PCNSL with
intraocular involvement are controversial. The PFS and OS rates in
the reported studies vary widely and have clear variations. The
main reason for these different survival outcomes is the
heterogeneity of both the inclusion criteria and patient
evaluation. Furthermore, the diagnostic criteria for IOL varies
across different studies due to a lack of standardization. Grimm
et al (14) reported that
patients with PCNSL with and without intraocular involvement had
similar PFS and OS rates; however, their study only included
patients with ocular and parenchymal involvement at diagnosis.
Kreher et al (15)
demonstrated that patients with IOL had significantly inferior PFS
and OS rates compared with non-IOL patients. In this study, not all
patients received an ophthalmological evaluation. In the IOL group
in this study, we included patients who presented with intraocular
involvement either at initial diagnosis or during the course of the
disease. Patients with IOL had an inferior PFS, but similar OS
compared with those in the non-IOL group, and this difference may
have been obtained for the following reasons: i) Many other factors
may influence OS; ii) we changed systemic therapeutic regimens
after disease relapse, which may improve the outcomes to a certain
extent; iii) eye-specific therapy was used in some patients with
IOL, which helped decrease the additional tumor burden; and iv) the
median follow-up time was too short to identify the differences.
Due to the rarity of the disease, further studies are warranted to
confirm our results.
This study demonstrated that 20.4% of patients with
PCNSL presented with intraocular involvement at some point during
the study, which was within the range reported by other studies
(16,17). Although HD-MTX-based therapy
improved the prognosis of patients with PCNSL, up to 60% of
patients PCNSL will eventually relapse (18). Late relapses account for 4% of all
recurrences in the PCNSL population (19). More and more studies are now focused
on the prognostic factors of PCNSL. Kim et al (20) demonstrated that age and the
Memorial-Sloan Kettering Cancer Center prognostic score were
predictive of survival. In the present study, we demonstrated that
patients with intraocular involvement had a higher relapse rate. In
accordance with the findings of this study, Ferreri et al
(21) reported that the prognosis
of PIOL with cerebral involvement was poor and that the 2-year OS
rate was 39%.
The diagnosis of IOL is challenging and misdiagnoses
are not uncommon. Research into this disease requires precise
diagnostic approaches and collaborations between ophthalmologists,
pathologists and oncologists. Common ophthalmological examinations,
such as OCT and flow cytometry, may be sensitive and valuable
methods, but may not result in a definitive diagnosis. Cytological
studies of vitreous biopsies are the mainstay of diagnoses
(22). In the present study, we
selected non-invasive ophthalmological examinations for initial
detection in suspected patients. Diagnosis was then confirmed by
histopathological examinations. This alleviated unnecessary injury
and improved the accuracy of the diagnosis. Furthermore, accurate
diagnoses require the sufficiently rapid transportation of tissue
specimens, good quality specimens, and experienced interpretation
(23). In general, tumor cells in
the specimens are limited in number and are dispersed throughout
the sample. In this study, 5 out of the 21 patients with IOL were
asymptomatic when diagnosed, which indicated that ocular symptoms
are not a necessity in the diagnosis of IOL. Currently, studies
have indicated that a biochemical test of the interleukin
(IL)-10/IL-6 cytokine ratio in the vitreous body helps improve the
diagnostic efficacy (24,25). We also found that this ratio was
higher in the patients IOL than the non-IOL patients (data not
shown), which indicated the promise of this ratio for
diagnosis.
It is noteworthy that intraocular involvement
developed as a recurrent disease during the interval of systemic
MTX therapy in 14 patients with IOL. These observations support the
notion that chemotherapeutic agents, including systemic MTX, may
not adequately penetrate the blood-ocular barrier. The early
recognition of risk factors for intraocular involvement may
facilitate early detection and prompt treatment. Cho and Yu
(26) demonstrated that patients
with PCNSL with a higher Ki-67 level were prone to intraocular
dissemination. Further studies are required to enhance the early
detection of intraocular involvement and to determine which
patients will require eye-dedicated prophylaxis. The findings of
this study indicate that the follow-up ocular examinations during
the course of MTX therapy are also essential.
Due to the blood-ocular barrier, systemic HD-MTX is
not sufficient to achieve targeted therapeutic concentrations in
the eyes. Eye-dedicated therapy, including intravitreal MTX
injection and ocular radiation, is a necessity for patients with
IOL to locally control their disease. Additionally, Akiyama et
al (27) demonstrated that
intravitreal MTX combined with systemic high-dose MTX is effective
at preventing CNS involvement in PIOL. Although repeated
intravitreal MTX injections had many local complications, this
therapy often had a satisfactory effect, and the side-effects were
well tolerated. Only 1 in 16 patients had disease progression from
the left vitreum to the right. Clinical manifestations, such as
anterior uveitis and the development of new lesions were observed
intracalvarium. Similar to many other studies (28 and Refs.
therein), the results of this study demonstrated that repeated
intravitreal injections of MTX are essential and safe for the
treatment of IOL.
Radiotherapy has also been used for IOL treatment
and is considered an effective treatment to control primary
lesions. Isobe et al (29)
demonstrated that 13 of 15 patients with PIOL treated with
radiotherapy reached complete remission. However, there is
significant ocular toxicity associated with this treatment,
including radiation retinopathy and cataract formation. As a
result, radiotherapy is only indicated as a treatment for recurrent
IOL after intravitreal MTX injections or in cases where local
chemotherapy cannot be used (30).
However, Berenbom et al (31) state that intravitreal chemotherapy
does not possess a clear advantage and that radiotherapy may still
be the most appropriate first-line treatment in most cases.
The present study has several limitations. The small
sample size is the major limitation of our study due to the low
morbidity rate. As a result, we did not compare the clinical
features of patients with IOL at diagnosis and relapse. Second,
chorioretinal biopsies should be performed if vitreous samples fail
to provide diagnostic tissue. This type of examination increases
reliability in diagnosing and excluding a PIOL that involves the
retina or choroid. However, due to permanent defects in biopsied
nerves and irreversible visual impairment, we did not perform this
examination.
In conclusion, the present study demonstrates IOL
patients have a high risk of disease relapse and a poor PFS, but a
similar OS to the non-IOL group. Our results suggest the importance
of intraocular evaluation for PCNSL patients. Prospective
multicenter studies and collaborative efforts are required to
better understand the pathogenesis and optimal treatment for
IOL.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the China
National Clinical Key Subject Construction Foundation (Oncology
Department, 2013), Shanghai Hospital Development Center (grant no.
16CR2043B), Natural Science Foundation of China (grant no.
81700123), Beijing Medical and Health foundation
(YWJKJJHKYJJ-B17513) and Major Program of Science Foundation of
Shanghai Municipal Commission of Health (no. 201540384).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available upon reasonable
request from the corresponding authors.
Authors' contributions
LZ and JL analyzed the patient data and wrote the
manuscript. KC made contributions to the conception and design of
the study. TD, YY, YM, HK, ZL, NF, JM and QZ participated in the
implementation of the treatment. XX, QW and BC substantially
contributed to the study conception and design, reviewed the
manuscript and gave final approval of the version to be published.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study received approval from the Ethics
Committee of the Institutional Review Board of Huashan Hospital,
Fudan University. All the patients voluntarily participated in this
study and provided informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Abrey LE, Batchelor TT, Ferreri AJ,
Gospodarowicz M, Pulczynski EJ, Zucca E, Smith JR, Korfel A,
Soussain C, DeAngelis LM, et al: Report of an international
workshop to standardize baseline evaluation and response criteria
for primary CNS lymphoma. J Clin Oncol. 23:pp. 5034–5043. 2005,
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan CC, Rubenstein JL, Coupland SE, Davis
JL, Harbour JW, Johnston PB, Cassoux N, Touitou V, Smith JR,
Batchelor TT, et al: Primary vitreoretinal lymphoma: A report from
an international primary central nervous system lymphoma
collaborative group symposium. Oncologist. 16:1589–1599. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peterson K, Gordon KB, Heinemann MH and
DeAngelis LM: The clinical spectrum of ocular lymphoma. Cancer-Am
Cancer Soc. 72:843–849. 1993.
|
|
4
|
Nussenblatt RB, Chan CC, Wilson WH,
Hochman J and Gottesman M: International Central Nervous System and
Ocular Lymphoma Workshop: Recommendations for the future. Ocul
Immunol Inflamm. 14:139–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sung CO, Kim SC, Karnan S, Karube K, Shin
HJ, Nam DH, Suh YL, Kim SH, Kim JY, Kim SJ, et al: Genomic
profiling combined with gene expression profiling in primary
central nervous system lymphoma. Blood. 117:1291–1300. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim MM, Dabaja BS, Medeiros J, Kim S,
Allen P, Chevez-Barrios P, Gombos DS and Fowler N: Survival
outcomes of primary intraocular lymphoma: A single-institution
experience. Am J Clin Oncol. 39:109–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Sato-Otsubo A, Sugita S, Takase H,
Mochizuki M, Usui Y, Goto H, Koyama T, Akiyama H, Miura O, et al:
High-resolution genomic copy number profiling of primary
intraocular lymphoma by single nucleotide polymorphism microarrays.
Cancer Sci. 105:592–599. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mulay K, Narula R and Honavar SG: Primary
vitreoretinal lymphoma. Indian J Ophthalmol. 63:180–186. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Witmer MT: Primary vitreoretinal lymphoma:
Management of isolated ocular disease. Cancer Control. 23:110–116.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stubiger N, Kakkassery V, Gundlach E,
Winterhalter S and Pleyer U: Diagnostics and treatment of primary
vitreoretinal lymphoma. Ophthalmologe. 112:223–230. 2015.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rajagopal R and Harbour JW: Diagnostic
testing and treatment choices in primary vitreoretinal lymphoma.
Retina. 31:435–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho BJ, Kim DY, Park UC, Lee JY, Yoon YH
and Yu HG: Clinical features and treatment outcomes of
vitreoretinal lymphoma according to its association with CNS
lymphoma. Ocul Immunol Inflamm. 26:365–371. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahle G, Touitou V, Cassoux N, Bouyon M,
Humbrecht C, Oesterlé H, Baraniskin A, Soussain C, Nguyen-Them L,
Gaultier C, et al: Optic nerve infiltration in primary central
nervous system lymphoma. Jama Neurol. 74:1368–1373. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grimm SA, McCannel CA, Omuro AM, Ferreri
AJ, Blay JY, Neuwelt EA, Siegal T, Batchelor T, Jahnke K, Shenkier
TN, et al: Primary CNS lymphoma with intraocular involvement:
International PCNSL Collaborative Group Report. Neurology. 71:pp.
1355–1360. 2008, View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kreher S, Strehlow F, Martus P, Roth P,
Hertenstein B, Röth A, Birnbaum T, Griesinger F, Rauch M, Kanz L,
et al: Prognostic impact of intraocular involvement in primary CNS
lymphoma: Experience from the G-PCNSL-SG1 trial. Ann Hematol.
94:409–414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jahnke K, Korfel A, Komm J, Bechrakis NE,
Stein H, Thiel E and Coupland SE: Intraocular lymphoma 2000–2005:
Results of a retrospective multicentre trial. Graefes Arch Clin Exp
Ophthalmol. 244:663–669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kaburaki T, Taoka K, Matsuda J, Yamashita
H, Matsuda I, Tsuji H, Tanaka R, Nakazaki K, Nakamura F, Kamiya K,
et al: Combined intravitreal methotrexate and immunochemotherapy
followed by reduced-dose whole-brain radiotherapy for newly
diagnosed B-cell primary intraocular lymphoma. Br J Haematol.
179:246–255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Langner-Lemercier S, Houillier C, Soussain
C, Ghesquières H, Chinot O, Taillandier L, Soubeyran P, Lamy T,
Morschhauser F, Benouaich-Amiel A, et al: Primary CNS lymphoma at
first relapse/progression: Characteristics, management, and outcome
of 256 patients from the French LOC network. Neuro Oncol.
18:1297–1303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nayak L, Hedvat C, Rosenblum MK, Abrey LE
and DeAngelis LM: Late relapse in primary central nervous system
lymphoma: Clonal persistence. Neuro Oncol. 13:525–529. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JE, Yoon DH, Kim S, Lee DH, Kim JH,
Yoon YH, Chi HS, Lee SW, Park CS, Huh J and Suh C: Relapse pattern
and prognostic factors for patients with primary central nervous
system lymphoma. Korean J Hematol. 47:60–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferreri AJ, Blay JY, Reni M, Pasini F,
Gubkin A, Tirelli U, Calderoni A, Zucca E, Cortelazzo S, Chassagne
C, et al: Relevance of intraocular involvement in the management of
primary central nervous system lymphomas. Ann Oncol. 13:531–538.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fend F, Ferreri AJ and Coupland SE: How we
diagnose and treat vitreoretinal lymphoma. Br J Haematol.
173:680–692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coupland SE, Bechrakis NE, Anastassiou G,
Foerster AM, Heiligenhaus A, Pleyer U, Hummel M and Stein H:
Evaluation of vitrectomy specimens and chorioretinal biopsies in
the diagnosis of primary intraocular lymphoma in patients with
Masquerade syndrome. Graefes Arch Clin Exp Ophthalmol. 241:860–870.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sugita S, Takase H, Sugamoto Y, Arai A,
Miura O and Mochizuki M: Diagnosis of intraocular lymphoma by
polymerase chain reaction analysis and cytokine profiling of the
vitreous fluid. JPN J Ophthalmol. 53:209–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan CC, Whitcup SM, Solomon D and
Nussenblatt RB: Interleukin-10 in the vitreous of patients with
primary intraocular lymphoma. Am J Ophthalmol. 120:671–673. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cho BJ and Yu HG: Risk factors for
intraocular involvement in patients with primary central nervous
system lymphoma. J Neurooncol. 120:523–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akiyama H, Takase H, Kubo F, Miki T,
Yamamoto M, Tomita M, Mochizuki M, Miura O and Arai A: High-dose
methotrexate following intravitreal methotrexate administration in
preventing central nervous system involvement of primary
intraocular lymphoma. Cancer Sci. 107:1458–1464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frenkel S, Hendler K, Siegal T, Shalom E
and Pe'Er J: Intravitreal methotrexate for treating vitreoretinal
lymphoma: 10 years of experience. Br J Ophthalmol. 92:383–388.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Isobe K, Ejima Y, Tokumaru S, Shikama N,
Suzuki G, Takemoto M, Tsuchida E, Nomura M, Shibamoto Y and
Hayabuchi N: Treatment of primary intraocular lymphoma with
radiation therapy: A multi-institutional survey in Japan. Leuk
Lymphoma. 47:1800–1805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tropinskaya OF, Vetlova ER, Serova NK,
Golanov AV and Fil'Chenkova NA: Radiotherapy of primary intraocular
lymphoma associated with primary central nervous system lymphoma.
Zh Vopr Neirokhir Im N N Burdenko. 80:74–81. 2016.(In English,
Russian). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Berenbom A, Davila RM, Lin HS and Harbour
JW: Treatment outcomes for primary intraocular lymphoma:
Implications for external beam radiotherapy. Eye. 21:1198–1201.
2007. View Article : Google Scholar : PubMed/NCBI
|