Introduction
Renal cell carcinomas (RCCs) are a heterogeneous
group of different kidney tumors, with clear cell RCC (ccRCC) being
the most prevalent histological subtype (1,2). The
majority of sporadic ccRCC cases are characterized by loss or
inactivation of von Hippel-Lindau (VHL) tumor-suppressor
gene, resulting in accumulation of hypoxia inducible factors (HIFs)
and overexpression of HIF-driven genes that are partially
responsible for cell proliferation, angiogenesis and tumor growth
(1,2). If diagnosed at early stages, clear
cell tumors are usually curable by surgical treatment; whereas
advanced ccRCC are characterized by worse prognosis compared to
non-clear cell subtypes of RCC (3).
The majority patients usually report no symptoms and approximately
a third of patients with ccRCC are diagnosed at the metastatic
stage of the disease. Despite surgical treatment of primary
disease, approximately half of the patients with primarily
localized tumors will eventually develop metastases or recurrent
disease (1,3). The molecular background of ccRCC has
been extensively studied resulting in better understanding of the
ccRCC molecular background and development of novel therapeutic
strategies (2,4). However, the benefits of adjuvant
treatments for advanced or metastatic ccRCC still require
improvement (1). In addition, there
is a lack of reliable molecular prognostic and predictive markers
that could be routinely used for improved patient monitoring and
targeted as effective treatment strategies (1,5).
Nuclear factor-κB (NF-κB) is a protein complex that
controls the expression of genes involved in immune response and
cell survival and is often upregulated in human cancer (6,7)
including RCC (8). The classical
(canonical) NF-κB pathway comprises heterodimer of the
transcription factors RelA/p50. In the resting state, heterodimers
are sequestered in the cytoplasm by NF-κB inhibitors (Iκ-B).
Canonical NF-κB signaling can be induced by pro-inflammatory
mediators including lipopolysaccharides, cytokines or CD40 ligand
(6,7). Inhibitor of nuclear factor κB kinase
subunit B (IKBKB or IKKβ) is part of the Iκ-B kinase (IKK) complex
that activates the transcription factor NF-κB (9). Upon activation, IKBKB phosphorylates
Iκ-B leading to its ubiquitination and degradation, releasing
RelA/p50 from inhibition. Following translocation to the nucleus,
RelA/p50 binds to κB sites within promoters and regulates the
transcription of target genes to increase the expression of
pro-survival and pro-inflammatory factors (6,7,9).
Generation of IKBKB−/− mice and further
experimentation performed using IKBKB-deficient cells demonstrated
that this protein is essential for activation of NF-κB and
functions as a dominant kinase in the canonical NF-κB cascade
(6,10,11).
Sustained activation, defective regulation and
overexpression of proteins of the NF-κB pathway is observed in
certain tumors and tumor-derived cell lines, and is associated with
the malignant phenotype in the majority of cases (12). NF-κB transcription factors have been
extensively researched because of their involvement in stromal
communication with cancer cells and role in establishing the tumor
microenvironment. Cancer cells, a variety of non-cancerous
tumor-associated immune cells and fibroblasts exhibit altered NF-κB
signaling, which is often associated with intratumoral
immunosuppression and development of multidrug resistance (7,12). In
addition to its major role in the activation of NF-κB pathway,
IKBKB was demonstrated to phosphorylate NF-κB-unrelated factors,
including tumor suppressor p53 or forkhead box O3 transcription
factors, targeting them for degradation by the ubiquitin-proteasome
pathway (13,14). It was also demonstrated that IKBKB
is a target for prolyl hydroxylase-mediated hydroxylation and,
therefore, hypoxia increases the expression and activity of IKBKB
in cultured cancer cells (15);
whereas the protein product of VHL tumor suppressor gene
(pVHL) negatively regulates IKBKB (16).
In the present study, the expression of IKBKB in
tumors and matched non-cancerous renal tissues of patients with
ccRCC was investigated. The association of IKBKB protein expression
with clinicopathological parameters and survival of ccRCC patients
was assessed.
Patients and methods
Patients and the collection of
samples
Specimens were obtained from postoperative material
of 66 patients with histologically confirmed ccRCC (33 men and 33
women; mean age ± standard deviation, 63.2±10.6; range 27–83 years)
operated on at the Department of Oncological Surgery, Warmia and
Mazury Oncological Center in Olsztyn (Olsztyn, Poland) between
March 2010 and July 2014. None of patients had a second neoplastic
disease or had previously undergone chemo- or radiotherapy. The
specimens of the tumor and matched, macroscopically unchanged renal
tissue were obtained from surgically resected kidney. Specimens for
RNA or protein extraction were immediately frozen in liquid
nitrogen and stored at −80°C until further analysis. Tumor and
kidney fragments for histological and immunohistochemical studies
were fixed in 4% buffered formaldehyde for 48–72 h at room
temperature, dehydrated using a series of alcohol solutions in
ascending concentrations (50, 60, 70, 85 and 99.8%; at room
temperature), cleared with xylene (1:1 ×ylene and 99.8% alcohol
solution followed by three xylene immersions; at room temperature)
and processed into paraffin blocks. Clinical staging was based on
the American Joint Committee on Cancer criteria (17). The tumor nuclear grading was
characterized by a pathologist according to the Fuhrman system
(18). Clinicopathological and
demographic data of the patients as well as their overall survival
(OS) records were collected during the study. The median follow-up
time was 40.6 months.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted and reverse transcribed
using the method described previously (19). The IKBKB transcripts in
tissue homogenates were determined by qPCR and normalized to
peptidylprolylisomerase A (PPIA) and TATA box
binding protein (TBP) mRNAs using TaqMan Fast Advanced
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and the respective TaqMan Gene Expression Assay
(IKBKB, #Hs00233287_m1; PPIA, #Hs99999904_m1; and
TBP, #Hs00427620_m1; Applied Biosystems; Thermo Fisher
Scientific, Inc.). qPCR reactions were performed using ABI
7500/7500 Fast Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) according to the protocol described by
Kowalczyk et al (20). The
following thermocycling conditions were used: polymerase activation
for 20 sec at 95°C, then 40 cycles of denaturation for 3 sec at
95°C and annealing/extension for 30 sec at 60°C. The ΔΔCq method
(21) was used to determine the
fold differences [relative quantification (RQ)] in expression
between the paired samples of ccRCC and unchanged renal tissue. On
the basis of IKBKB RQ (ccRCC vs. renal tissue), specimens
were divided into two groups, regarded as IKBKB ‘upregulated’
(RQ≥1.5) and ‘no change and downregulated’ (RQ<1.5).
Protein extraction, SDS-PAGE and
western blot analysis
Procedures were performed according to the method
described previously (22) with
some modifications. Briefly, the samples were homogenized in
radioimmunoprecipitation lysis buffer supplemented with 1:100
protease inhibitor cocktail, 1:100 phosphatase inhibitor cocktail
and 5 mM EDTA (Sigma-Aldrich; Merck, KGaA, Darmstadt, Germany).
Homogenates were centrifuged twice at 9,000 × g for 10 min at 4°C.
The protein content in the supernatant was determined by the
Bradford method. Protein lysates were denatured for 5 min at 95°C
and loaded on a 10% polyacrylamide gel (30 µg/lane), separated (10
mA/gel during migration in stacking gel, then 15 mA/gel),
transferred onto polyvinylidene difluoride membrane (Roche
Diagnostics GmbH, Mannheim, Germany) and blocked in 5% nonfat dry
milk for 2 h at room temperature. The level of protein in
homogenates of paired tumor and renal tissue specimens was
determined using rabbit anti-human antibodies against IKBKB
(1:1,000; cat. no. sc-7329; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) and actin (ACTB; 1:100; cat. no. A2066; Sigma-Aldrich;
Merck, KGaA) as the internal loading control. Following overnight
incubation at 4°C with primary antibodies, the membranes were
treated with polyclonal horseradish peroxidase-conjugated goat
anti-rabbit IgG secondary antibodies (diluted 1:40,000; cat. no.
A0545; Sigma-Aldrich; Merck, KGaA) for 60 min at room temperature,
developed with SuperSignal West Pico Chemiluminescent Substrate
(Thermo Fisher Scientific, Inc.) and visualized with G:BOX iChemi
XR imaging system (Syngene Europe, Cambridge, UK). Band intensity
was quantified using ImageJ software (version 1.50i; National
Institutes of Health, Bethesda, MD, USA). IKBKB/ACTB optical
density (OD) ratios were used to determine fold differences in
expression between the paired samples of ccRCC and unchanged renal
tissue. On the basis of their relative IKBKB OD ratios (ccRCC vs.
renal tissue) specimens were divided into two groups, regarded as
IKBKB ‘upregulated’ (OD ratios ≥1.5) and ‘no change and
downregulated’ (OD ratios <1.5).
Immunohistochemistry (IHC) and
evaluation of immunoreactivity
IKBKB immunostaining of the tumor and non-cancerous
kidney 4-µm-thick paraffin sections was performed using the
Autostainer Link 48 supplied with EnVision FLEX reagents (Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) according to the
previously described method (23).
Rabbit antibody directed against human IKBKB (1:1,000; cat. no.
sc-7329; Santa Cruz Biotechnology, Inc.) was applied for 20 min at
room temperature whereas the negative controls were performed by
omitting the primary antibody. The sections were counterstained
with EnVision FLEX Hematoxylin (ready-to-use solution; Dako;
Agilent Technologies, Inc.) for 5 min at room temperature. The
IKBKB immunoreactivity was evaluated using an Olympus BX53 light
microscope (Olympus Corporation, Tokyo, Japan) by two independent
pathologists in a blinded manner regarding the clinicopathological
data of the patients. In doubtful cases, re-evaluation was
performed until a consensus was achieved. Immunoexpression of IKBKB
was assessed in the cytoplasm of cancer cells and non-transformed,
normal epithelial cells of the proximal convoluted tubules (PCTs).
IKBKB immunoreactivity was evaluated according to the imunoreactive
score (IRS) of Remmele and Stegner (24). The IRS scale is based on the
percentage of cells exhibiting positive reaction (0 points, absence
of cells with positive reaction; 1 point, 1–10%; 2 points, 11–50%;
3 points, 51–80%; 4 points, >80% cells with positive reaction)
and reaction intensity (0, no reaction; 1, low intensity reaction;
2, moderate intensity reaction; 3, intense reaction). The final
score is the result of multiplication of both parameters and ranges
from 0 to 12 points). On the basis of their relative IRS ratios
specimens were divided into two groups regarded as ‘upregulated’
(IRS ratio ≥1.5 and tumor-only positive specimens) and ‘no change
or downregulated’ IKBKB immunoreactivity (IRS ratio <1.5 or
renal-only positive specimens).
Cancer genomics data from The Cancer
Genome Atlas (TCGA)
A dataset containing results of RNA-Seq analysis of
469 primary nephrectomy specimens from patients with histologically
confirmed ccRCC (2) was
investigated for mutations, putative copy-number alterations and
expression levels of IKBKB using a cBio Cancer Genomics
Portal (25). The IKBKB
expression data were queried using 1.5-fold change as a z-score
threshold value.
Statistical analysis
Statistical analysis was performed using Prism 6.04
(GraphPad Software, Inc., La Jolla, CA, USA) and Statistica 13.1
(Statsoft, Tulsa, OK, USA). The IKBKB expression levels are
expressed as mean ± standard error. The differences in IKBKB
expression levels between the paired tumor and unchanged renal
tissue specimens were examined by the Wilcoxon matched-pairs test.
Fisher's exact, Mann-Whitney U test and Spearman's rank correlation
were used to assess associations between the patient data and
IKBKB expression levels. Survival curves were plotted
according to the Kaplan-Meier method and the significance of
differences in OS between groups of patients was evaluated by
log-rank test. The uni- and multivariate survival associations were
analyzed using the Cox proportional hazards regression model.
P<0.05 was considered to indicate statistically significant
difference.
Results
IKBKB expression is downregulated in
ccRCC at the mRNA and protein levels
All tumor and matched unchanged renal tissue samples
of ccRCC patients expressed IKBKB mRNA. IKBKB mRNA
level was reduced or remained unchanged in 63/66 (95.5%), and was
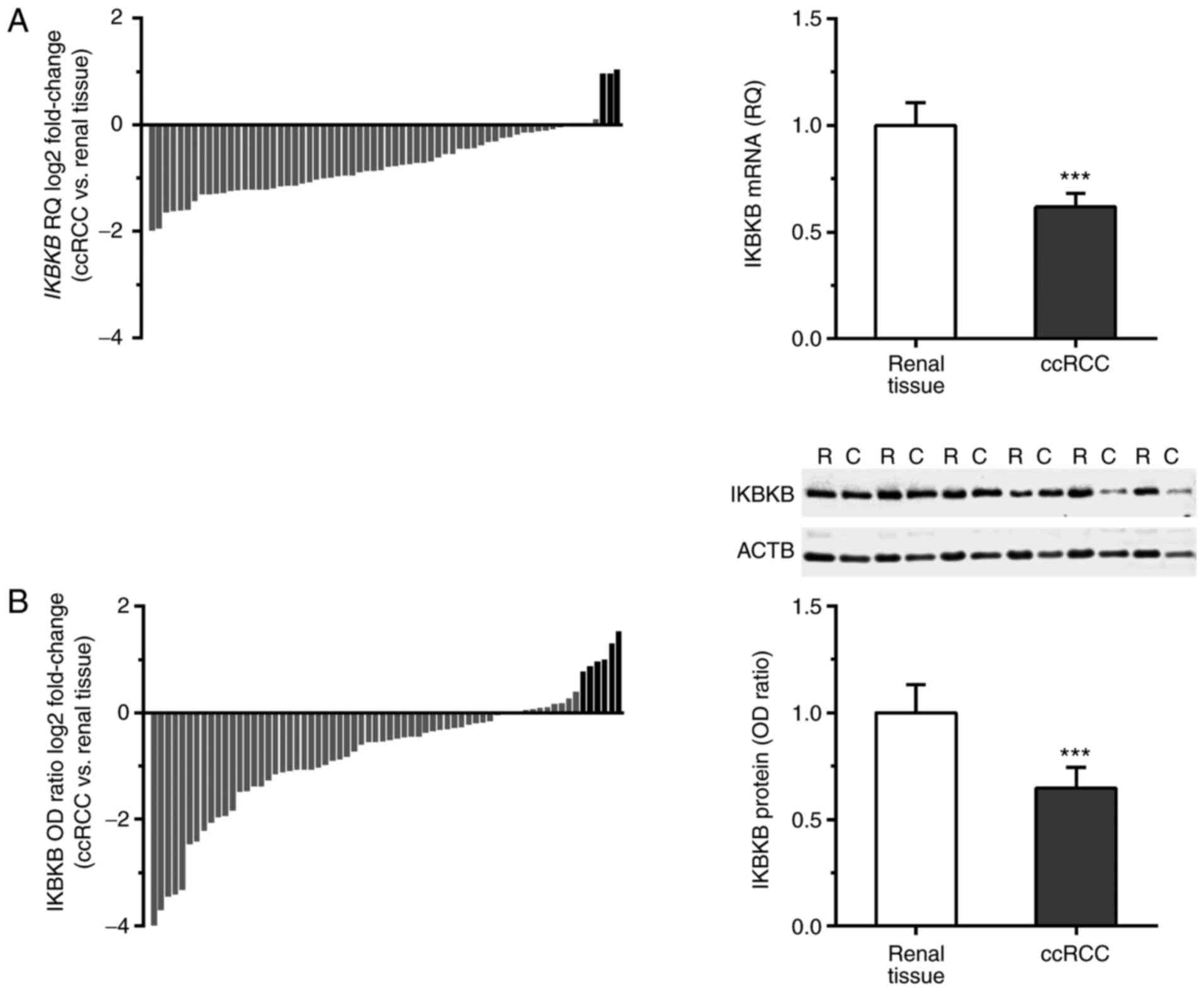
elevated in 3/66 (4.5%) ccRCC cases (Fig. 1A). The average expression level of
IKBKB transcript was significantly decreased in ccRCC
compared with the corresponding renal tissues (RQ 0.62±0.06 and
1.00±0.11, respectively; P<0.001; Fig. 1A).
IKBKB protein was present in all tested homogenates
of tumor and renal tissue as determined by western blotting. The
content of IKBKB protein was reduced or remained unchanged in 60/66
(90.9%) ccRCC cases; whereas, it was elevated in 6/66 (9.1%) of
tumor homogenates (Fig. 1B). The
average IKBKB/ACTB OD was significantly lower in ccRCC samples
compared with the corresponding unchanged kidney tissue (OD ratio
0.65±0.10 and 1.00±0.13, respectively; P<0.001; Fig. 1B).
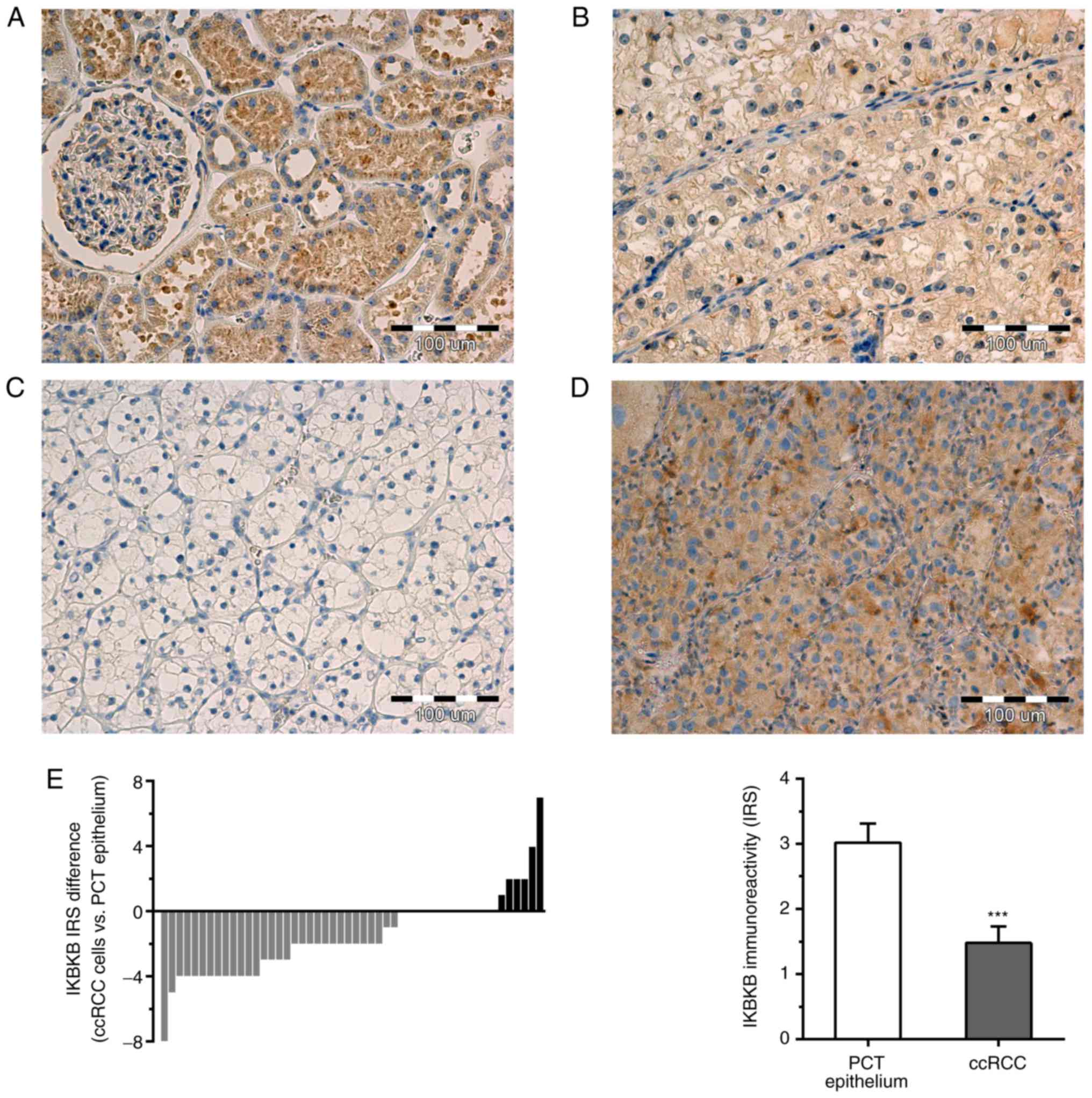
Immunoreactivity of IKBKB was observed in the
cytoplasm of cancer cells and PCT epithelial cells of analyzed
sections (Fig. 2A-D). In addition
to PCT epithelium, the cells of distal convoluted tubules, renal
glomeruli and Bowman's capsule from non-cancerous renal tissue
sections exhibited weak to moderate cytoplasmic IKBKB
immunoreactivity. IKBKB did not exhibit nuclear expression in the
tumor or non-cancerous renal tissue. IKBKB immunoreactivity was
detected in the cancer cells of 26/50 (52%) ccRCC cases. In the PCT
epithelium of matched renal tissue, IKBKB expression was observed
in 41/50 (82%) cases. IKBKB protein expression was downregulated in
31/50 (62%), remained unchanged in 13/50 (26%) and was elevated in
6/50 (13%) of ccRCC cases compared with the matched non-cancerous
tissue (Fig. 2E). The average IKBKB
immunoreactivity was significantly reduced in the tumor cells
compared with the PCT epithelial cells of corresponding
non-cancerous renal tissues (IRS 1.48±0.25 and 3.02±0.29,
respectively; P<0.001; Fig.
2E).
Increased IKBKB immunoreactivity in
ccRCC cells is associated with higher nuclear grade
Elevated immunoexpression of IKBKB protein in cancer
cells compared with normal PCT epithelium was positively associated
with Fuhrman nuclear grade (P=0.0331; Fisher's exact test; Table I). Furthermore, there was ~2-fold
increase in relative immunoreactivity of IKBKB in cancer cells of
G3 tumors compared with G1 and G2 tumors (0.92±0.17 vs. 0.54±0.08;
P=0.0304; Mann-Whitney U test). No other correlations between
IKBKB expression levels and demographic or
clinicopathological parameters of the patients were identified
(Tables I and II).
 | Table I.Associations between demographic and
clinicopathological features of patients with ccRCC and relative
expression of IKBKB at the mRNA and protein levels as
determined by reverse transcription-quantitative polymerase chain
reaction, western blotting and immunohistochemistry. |
Table I.
Associations between demographic and
clinicopathological features of patients with ccRCC and relative
expression of IKBKB at the mRNA and protein levels as
determined by reverse transcription-quantitative polymerase chain
reaction, western blotting and immunohistochemistry.
|
|
| IKBKB mRNA
RQ (tumor vs. renal tissue) | IKBKB protein OD
ratio (tumor vs. renal tissues) |
| IKBKB relative IR
(ccRCC cells vs. PCT epithelium) |
|---|
|
|
|
|
|
|
|
|---|
| Clinicopathological
feature | Number of cases
(%) | <1.5, n (%) | ≥1.5, n (%) | P-value | <1.5 n (%) | ≥1.5 n (%) | P-value | Number of cases
(%) | <1.5 n (%) | ≥1.5 n (%) | P-value |
|---|
| Total | 66 (100) | 63 (95) | 3 (5) |
| 60 (91) | 6 (9) |
| 50 (100) | 44 (88) | 6
(12) |
|
| Sex |
|
|
| 0.2385 |
|
| 0.6724 |
|
|
| 0.1919 |
|
Men | 33 (50) | 33 (100) | 0 (0) |
| 31 (94) | 2 (6) |
| 26 (52) | 21 (81) | 5
(19) |
|
|
Women | 33 (50) | 30 (91) | 3 (9) |
| 29 (88) | 4 (12) |
| 24 (48) | 23 (96) | 1
(4) |
|
| T status |
|
|
| 1.0000 |
|
| 1.0000 |
|
|
| 0.2234 |
|
T1+T2 | 36 (55) | 34 (94) | 2 (6) |
| 33 (92) | 3 (8) |
| 29 (58) | 27 (93) | 2
(7) |
|
| T3 | 30 (45) | 29 (97) | 1 (3) |
| 27 (90) | 3 (10) |
| 21 (42) | 17 (81) | 4
(19) |
|
| Fuhrman grade |
|
|
| 1.0000 |
|
| 1.0000 |
|
|
| 0.0331 |
|
G1+G2 | 49 (74) | 47 (96) | 2 (4) |
| 44 (90) | 5 (10) |
| 37 (74) | 35 (95) | 2
(5) |
|
|
G3+G4 | 17 (26) | 16 (94) | 1 (6) |
| 16 (94) | 1 (6) |
| 13 (26) | 9
(69) | 4
(31) |
|
| Distant
metastases |
|
|
| 1.0000 |
|
| 0.5823 |
|
|
| 0.5756 |
| M0 | 54 (82) | 51 (94) | 3 (6) |
| 48 (89) | 6 (11) |
| 41 (82) | 35 (85) | 6
(15) |
|
| M1 | 12 (18) | 12 (100) | 0 (0) |
| 12 (100) | 0 (0) |
| 9 (18) | 9
(100) | 0
(0) |
|
| Tumor growth |
|
|
| 1.0000 |
|
| 1.0000 |
|
|
| 0.1841 |
|
Kidney-confined | 38 (58) | 36 (95) | 2 (5) |
| 34 (89) | 4 (11) |
| 31 (62) | 29 (94) | 2
(6) |
|
|
Advanced/recurrent | 28 (42) | 27 (96) | 1 (4) |
| 26 (93) | 2 (7) |
| 19 (38) | 15 (79) | 4
(21) |
|
 | Table II.Correlation between demographic and
clinicopathological features of patients with ccRCC and relative
expression of IKBKB at the mRNA and protein levels as
determined by reverse transcription-quantitative polymerase chain
reaction, western blotting and immunohistochemistry. |
Table II.
Correlation between demographic and
clinicopathological features of patients with ccRCC and relative
expression of IKBKB at the mRNA and protein levels as
determined by reverse transcription-quantitative polymerase chain
reaction, western blotting and immunohistochemistry.
|
| IKBKB mRNA
RQ (tumor vs. renal tissue, n=66) | IKBKB protein OD
ratio (tumor vs. renal tissues, n=66) | IKBKB relative IR
(ccRCC cells vs. PCT epithelium, n=50) |
|---|
|
|
|
|
|
|---|
| Variable | Spearman's ρ | P-value | Spearman's ρ | P-value | Spearman's ρ | P-value |
|---|
| Age | −0.2291 | 0.0643 | 0.1107 | 0.3764 | −0.0983 | 0.4971 |
| Tumor size | −0.0366 | 0.7704 | 0.0402 | 0.7486 | −0.0235 | 0.8713 |
Upregulated IKBKB protein expression
is associated with unfavorable prognosis
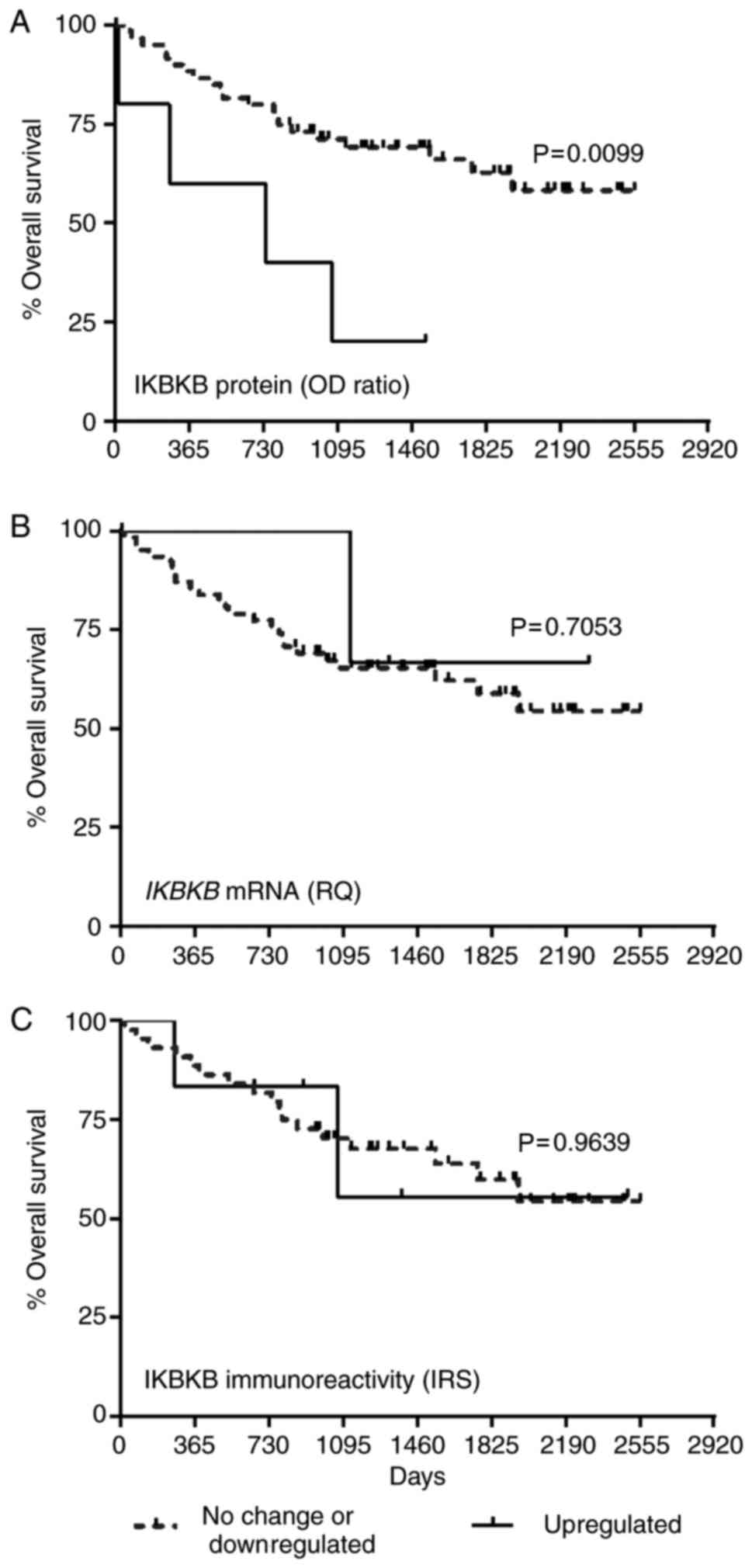
Kaplan-Meier plots presenting the OS of patients
with ccRCC grouped according to the expression levels of IKBKB are
presented in the Fig. 3. Increased
of IKBKB protein expression was significantly associated with
shorter OS of patients with ccRCC (median 25.7 months for
upregulated group vs. ≥85.3 months for not changed or downregulated
IKBKB protein content; P=0.0099; Fig.
3A). The expression of IKBKB transcript and immunoreactivity of
IKBKB in cancer cells did not correlate with OS of patients with
ccRCC (Fig. 3B and C,
respectively). Univariate Cox proportional hazards regression
revealed that IKBKB protein level, T-status of the primary tumor,
nuclear grade, presence of distant metastases and
advanced/recurrent disease were associated with OS of the patients
(Table III). The subsequent
multivariate analysis confirmed that higher IKBKB protein level
[hazard ratio (HR)=5.50; P=0.0020; Table III] and with the presence of
distant metastases (HR=3.99; P=0.0182; Table III) achieved a status of
independent prognostic factors in ccRCC.
 | Table III.Univariate and multivariate Cox
regression analysis of the overall survival rates associated with
different prognostic variables in patients with clear cell renal
cell carcinoma. |
Table III.
Univariate and multivariate Cox
regression analysis of the overall survival rates associated with
different prognostic variables in patients with clear cell renal
cell carcinoma.
|
| Univariate Cox
regression | Multivariate Cox
regression |
|---|
|
|
|
|
|---|
| Parameter | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| IKBKB mRNA
RQ (upregulated vs. no change/downregulated) | 0.47 | 0.06–3.50 | 0.4645 |
|
|
|
| IKBKB protein OD
ratio (upregulated vs. no change/downregulated) | 4.61 | 1.70–12.5 | 0.0026 | 5.50 | 1.86–16.2 | 0.0020 |
| IKBKB
immunoreactivity (upregulated vs. no change/downregulated) | 1.03 | 0.24–4.49 | 0.9634 |
|
|
|
| Sex (women vs.
men) | 0.70 | 0.32–1.51 | 0.3604 |
|
|
|
| Age (years) | 0.99 | 0.95–1.03 | 0.5269 |
|
|
|
| Tumor size
(cm) | 1.17 | 1.05–1.31 | 0.0052 | 1.07 | 0.88–1.28 | 0.5094 |
| Depth of invasion
(T3 vs. T1+T2) | 4.31 | 1.80–10.3 | 0.0010 | 3.12 | 0.87–11.2 | 0.0815 |
| Fuhrman grade (G3
vs. G1+G2) | 3.33 | 1.49–7.44 | 0.0034 | 1.65 | 0.63–4.33 | 0.3112 |
| Distant metastases
(M1 vs. M0) | 2.78 | 1.19–6.50 | 0.0185 | 3.99 | 1.27–12.6 | 0.0182 |
| Tumor growth
(advanced/recurrent vs. kidney-confined) | 2.37 | 1.08–5.20 | 0.0318 | 0.58 | 0.19–1.77 | 0.3412 |
TCGA dataset analysis
Analysis of cancer genomics data provided by TCGA
revealed that none of 469 ccRCC cases included in the dataset
contained IKBKB sequence mutation. Copy number alterations
were reported only in four ccRCC cases (0.9%). The expression level
of IKBKB mRNA was downregulated in 31/469 (6.6%) and
upregulated in 28 (6%) of queried ccRCC samples while the levels of
IKBKB protein in the tumor did not differ from those in the
matching renal tissue.
Discussion
In the present study, three independent techniques
(RT-qPCR, western blot analysis and IHC) were used to demonstrate
that the expression level of IKBKB is decreased in ccRCC.
The majority of the analyzed tumor specimens exhibited reduced
levels of IKBKB mRNA and protein. However, an upregulated
IKBKB protein level was associated with higher nuclear grade of the
tumor and significantly shorter survival, suggesting an oncogenic
role of this kinase in ccRCC.
Previous studies using patients with RCC, cell lines
and xenograft animal models of RCC identified IKBKB as a
factor associated with the progression of the disease and poor
patient outcomes. A dataset from TCGA containing 469 ccRCC cases
revealed that copy number alterations of IKBKB were rare in
this cancer (~1% of tumors), and no alterations in the IKBKB
sequence were reported in this dataset (2,25).
NF-κB pathway genes rarely contain mutations in cancer (12). Constitutive activation of NF-κB in
certain cancers is usually mediated by alterations of upstream
regulators and/or altered expression levels of NF-κB-associated
genes (12). In the current study,
IKBKB expression was also investigated using the TCGA data
provided by the cBio Cancer Genomics Portal (2,25)
setting a z-score threshold for 1.5-fold change. This evaluation
revealed that the expression level of IKBKB transcript
determined by RNA-Seq was downregulated in 7% and upregulated in 6%
of ccRCC cases, while IKBKB protein levels measured by
reverse-phase protein array remained unaltered in all 469 samples
included in the study (2,25). Although these expression data are
not in line with the results of the current study, this may be
attributed to different sensitivities and specificities of methods
used. However, the prognostic significance of IKBKB reported in
this study is in accordance with analyses based on previously
published study by Peri et al (26). Meta-analysis of ccRCC expression
datasets identified a subset of genes including IKBKB and
NF-κB mediators, matrix metalloproteinase 9, proteasome subunit
β 9 and superoxide dismutase 2, whose elevated
expression levels were associated with worse patient outcomes
(26). Another study established a
model linking pVHL loss to increased activation of NF-κB,
potentially elucidating a previously reported observation that loss
of may be associated with the chemoresistance of ccRCC to tumor
necrosis factor therapy (27).
Recently, it has been reported that IKBKB protein may indirectly
have a role in stabilizing HIF1α and HIF2α in RCC cells via
activation of NF-κB essential modulator (NEMO) protein, a
regulatory subunit of the IKK complex (28). In addition, it was demonstrated that
the degree of NEMO protein expression was downregulated in 62.8%
tumor samples derived from 250 patients with ccRCC, and it was
positively associated with the progression of ccRCC (28). This observation seems to be
analogous to the finding that IKBKB immunoreactivity was decreased
in the majority of analyzed ccRCC specimens, while increased IKBKB
protein expression was associated with higher tumor grade and
poorer patient outcomes. However, additional studies are required
to investigate unknown links between the IKBKB and NEMO proteins
and potential common mechanisms underlying their altered expression
in ccRCC.
Beneficial effects of decreased IKBKB
expression and/or IKBKB protein activity on the effectiveness of
different anticancer approaches were reported previously (29). Treatment of metastatic RCC cell
lines, ACHN and SN12K1, with an anti-oxidant agent, pyrrolidine
dithiocarbamate, decreased expression level of IKBKB and other
components of NF-κB signaling, as assessed by western blotting
(30). The resulting inhibition of
NF-κB pathways exerted anti-proliferative, pro-apoptotic and
anti-angiogenic effects in the tested cell lines (30,31)
and sensitized them to cisplatin treatment (32). Further experiments performed in a
xenograft animal model of RCC demonstrated that the antioxidant
treatment reduced cancer cell proliferation and attenuated tumor
progression, with decreased expression of NF-κB proteins and
upstream kinases, IKBKB and IKKA (33). Notably, accumulation of reactive
oxygen species in the kidneys of superoxide-deficient mice resulted
in decreased expression of IKBKB (34), suggesting that the influence of
oxidative stress on expression of NF-κB-associated genes is tissue-
or disease-specific. Another study performed in RCC cell lines
demonstrated that IKBKB and RelA (p65) were required for the
oncogenic properties of microRNA-21 (miR-21) in ACHN cells
(35). In turn, miR-21 indirectly
increased IKBKB phosphorylation, which upregulated NF-κB and
mechanistic target of rapamycin complex 1 signaling, resulting in
enhanced proliferation, migration and invasiveness of ACHN and
786-O cell lines (35,36). Transfection of RCC cell lines with a
plasmid expressing IKBKB attenuated sunitinib-induced p53 promoter
transcriptional activity (37).
These reports suggest that increased IKBKB content in cancer
cells can compromise the effectiveness of anticancer therapy,
suggesting potential usefulness of IKBKB as both prognostic
and predictive marker in ccRCC.
Altered IKBKB expression and/or IKBKB kinase
activity were previously reported to be associated the with
occurrence and progression of several types of human cancer,
including breast cancer, pancreatic cancer, thyroidal C-cells
carcinoma, acute myeloid leukemia and ovarian cancer (7,8,12,29,38).
However, the available data concerning IKBKB expression are
inconclusive. IKBKB transcript levels were significantly
upregulated in human hepatocellular carcinoma compared with
adjacent normal tissue (39).
Cultured Hs578T breast cancer cells exhibited aberrant expression
and activity of IKBKB compared with untransformed mammary
epithelial cells (40). Reduced
IKBKB expression were demonstrated in glioblastoma tissues
at the mRNA and protein levels (41). However, downregulation of
IKBKB was attributed to microglia/macrophages infiltrating
advanced glioblastoma tumors, indicating the important role of this
kinase for the local microenvironment and antitumor response
(41).
In conclusion, to the best of our knowledge, the
present study is the first comprehensive investigation analyzing
IKBKB expression at the mRNA and protein levels in a cohort
of patients with ccRCC. The results suggest that the three
techniques applied to determine the IKBKB expression,
RT-qPCR, western blotting and IHC, should be used as a
complementary rather than alternative methods. However, additional
methodological studies are required to compare these and other
available assays before conclusions are made. The number of
patients included in the study was enough to disclose associations
of IKBKB protein expression level with clinicopathological
parameters and survival of patients with ccRCC. Therefore, the
findings of the present study demonstrate that the expression of
IKBKB protein may be of clinical relevance in ccRCC. The elevated
content of IKBKB in the ccRCC tumor tissue may be useful as a
potential marker of prognosis, and suggests that the application of
anti-NF-κB treatments may sensitize ccRCC cells to certain adjuvant
therapies. Further studies using a larger number of patients are
required to support this suggestion, and validate prognostic or
predictive value of IKBKB in ccRCC.
Acknowledgements
The authors wish to thank Dr Aleksandra Piotrowska
from the Department of Human Morphology and Embryology, Division of
Histology and Embryology, Wroclaw Medical University (Wroclaw,
Poland) for technical support.
Funding
This study was supported by the National Science
Centre (Poland; grant no. 2012/05/B/NZ4/01832).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BEK, JK and ZK designed the study, analyzed and
interpreted the results; BEK and JK wrote the manuscript draft; ZK
corrected the final version of the manuscript; JGo and PK collected
clinical samples; JGo collected clinicopathological and survival
data; BEK, JK, AEK, ASJ performed qPCR and western blot assays; JGr
and PD performed IHC and evaluated immunoreactivity; BEK performed
the statistical analysis; JK and BEK were managing the project. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Bioethics Committee
for Scientific Research at the University of Warmia and Mazury in
Olsztyn (Olsztyn, Poland; agreements no. 4/2010 and 44/2011).
Written informed consent (as specified in the Declaration of
Helsinki) was obtained from each patients included in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Greef B and Eisen T: Medical treatment of
renal cancer: New horizons. Br J Cancer. 115:505–516. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Genome Atlas Research Network:
Comprehensive molecular characterization of clear cell renal cell
carcinoma. Nature. 499:43–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Znaor A, Lortet-Tieulent J, Laversanne M,
Jemal A and Bray F: International variations and trends in renal
cell carcinoma incidence and mortality. Eur Urol. 67:519–530. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shoji S, Nakano M, Sato H, Tang XY,
Osamura YR, Terachi T, Uchida T and Takeya K: The current status of
tailor-made medicine with molecular biomarkers for patients with
clear cell renal cell carcinoma. Clin Exp Metastasis. 31:111–134.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhatt JR and Finelli A: Landmarks in the
diagnosis and treatment of renal cell carcinoma. Nat Rev Urol.
11:517–525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bradford JW and Baldwin AS: IKK/nuclear
factor-kappaB and oncogenesis: Roles in tumor-initiating cells and
in the tumor microenvironment. Adv Cancer Res. 121:125–145. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morais C, Gobe G, Johnson DW and Healy H:
The emerging role of nuclear factor kappa B in renal cell
carcinoma. Int J Biochem Cell Biol. 43:1537–1549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DiDonato JA, Hayakawa M, Rothwarf DM,
Zandi E and Karin M: A cytokine-responsive IkappaB kinase that
activates the transcription factor NF-kappaB. Nature. 388:548–554.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li ZW, Chu W, Hu Y, Delhase M, Deerinck T,
Ellisman M, Johnson R and Karin M: The IKKbeta subunit of IkappaB
kinase (IKK) is essential for nuclear factor kappaB activation and
prevention of apoptosis. J Exp Med. 189:1839–1845. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka M, Fuentes ME, Yamaguchi K, Durnin
MH, Dalrymple SA, Hardy KL and Goeddel DV: Embryonic lethality,
liver degeneration, and impaired NF-kappa B activation in
IKK-beta-deficient mice. Immunity. 10:421–429. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Erstad DJ and Cusack JC Jr: Targeting the
NF-κB pathway in cancer therapy. Surg Oncol Clin N Am. 22:705–746.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia Y, Padre RC, De Mendoza TH, Bottero V,
Tergaonkar VB and Verma IM: Phosphorylation of p53 by IkappaB
kinase 2 promotes its degradation by beta-TrCP. Proc Natl Acad Sci
USA. 106:2629–26234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang
F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, et al: IkappaB kinase
promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell.
117:225–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cummins EP, Berra E, Comerford KM,
Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE,
Moynagh P, Pouyssegur J and Taylor CT: Prolyl hydroxylase-1
negatively regulates IKappaB kinase-beta, giving insight into
hypoxia-induced NFkappaB activity. Proc Natl Acad Sci USA.
103:18154–18159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Zhao W, Gao Q, Fan L, Qin Y, Zhou
H, Li M and Fang J: pVHL mediates K63-linked ubiquitination of
IKKβ, leading to IKKβ inactivation. Cancer Lett. 383:1–8. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: Kidney. American Joint Committee
on Cancer: AJCC Cancer Staging Manual. 6th. Springer; New York, NY:
pp. 323–328. 2002
|
|
18
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sliwinska-Jewsiewicka A, Kowalczyk AE,
Krazinski BE, Godlewski J, Kwiatkowski P, Kiewisz J, Grzegrzolka J,
Dziegiel P and Kmiec Z: Decreased expression of SATB2 associates
with tumor growth and predicts worse outcome in patients with clear
cell renal cell carcinoma. Anticancer Res. 38:839–846.
2018.PubMed/NCBI
|
|
20
|
Kowalczyk AE, Krazinski BE, Godlewski J,
Grzegrzolka J, Kiewisz J, Kwiatkowski P, Sliwinska-Jewsiewicka A,
Dziegiel P and Kmiec Z: SATB1 is down-regulated in clear cell renal
cell carcinoma and correlates with MIR-21-5p overexpression and
poor prognosis. Cancer Genomics Proteomics. 13:209–217.
2016.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kowalczyk AE, Krazinski BE, Godlewski J,
Kiewisz J, Kwiatkowski P, Sliwinska-Jewsiewicka A, Kiezun J,
Wierzbicki PM, Bodek G, Sulik M and Kmiec Z: Altered expression of
the PLAGL1 (ZAC1/LOT1) gene in colorectal cancer: Correlations to
the clinicopathological parameters. Int J Oncol. 47:951–962. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kowalczyk AE, Godlewski J, Krazinski BE,
Kiewisz J, Sliwinska-Jewsiewicka A, Kwiatkowski P, Pula B, Dziegiel
P, Janiszewski J, Wierzbicki PM and Kmiec Z: Divergent expression
patterns of SATB1 mRNA and SATB1 protein in colorectal cancer and
normal tissues. Tumor Biol. 36:4441–4452. 2015. View Article : Google Scholar
|
|
24
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
25
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peri S, Devarajan K, Yang DH, Knudson AG
and Balachandran S: Meta-analysis identifies NF-κB as a therapeutic
target in renal cancer. PLoS One. 8:e767462013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qi H and Ohh M: The von Hippel-Lindau
tumor suppressor protein sensitizes renal cell carcinoma cells to
tumor necrosis factor-induced cytotoxicity by suppressing the
nuclear factor-kappaB-dependent antiapoptotic pathway. Cancer Res.
63:7076–7080. 2003.PubMed/NCBI
|
|
28
|
Nowicka AM, Häuselmann I, Borsig L,
Bolduan S, Schindler M, Schraml P, Heikenwalder M and Moch H: A
novel pVHL-independent but NEMO-driven pathway in renal cancer
promotes HIF stabilization. Oncogene. 35:3125–3138. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gamble C, McIntosh K, Scott R, Ho KH,
Plevin R and Paul A: Inhibitory kappa B kinases as targets for
pharmacological regulation. Br J Pharmacol. 165:802–819. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morais C, Pat B, Gobe G, Johnson DW and
Healy H: Pyrrolidine dithiocarbamate exerts anti-proliferative and
pro-apoptotic effects in renal cell carcinoma cell lines. Nephrol
Dial Transplant. 21:3377–3388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morais C, Gobe G, Johnson DW and Healy H:
Anti-angiogenic actions of pyrrolidine dithiocarbamate, a nuclear
factor kappa B inhibitor. Angiogenesis. 12:365–379. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morais C, Gobe G, Johnson DW and Healy H:
Inhibition of nuclear factor kappa B transcription activity drives
a synergistic effect of pyrrolidine dithiocarbamate and cisplatin
for treatment of renal cell carcinoma. Apoptosis. 15:412–425. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morais C, Healy H, Johnson DW and Gobe G:
Inhibition of nuclear factor kappa B attenuates tumour progression
in an animal model of renal cell carcinoma. Nephrol Dial
Transplant. 25:1462–1474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brzóska K, Sochanowicz B, Siomek A,
Olinski R and Kruszewski M: Alterations in the expression of genes
related to NF-κB signaling in liver and kidney of CuZnSOD-deficient
mice. Mol Cell Biochem. 353:151–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bera A, Ghosh-Choudhury N, Dey N, Das F,
Kasinath BS, Abboud HE and Choudhury GG: NFκB-mediated cyclin D1
expression by microRNA-21 influences renal cancer cell
proliferation. Cell Signal. 25:2575–2586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bera A, Das F, Ghosh-Choudhury N, Kasinath
BS, Abboud HE and Choudhury GG: microRNA-21-induced dissociation of
PDCD4 from rictor contributes to Akt-IKKβ-mTORC1 axis to regulate
renal cancer cell invasion. Exp Cell Res. 328:99–117. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu Y, Xu L, Zhang J, Hu X, Liu Y, Yin H,
Lv T, Zhang H, Liu L, An H, et al: Sunitinib induces cellular
senescence via p53/Dec1 activation in renal cell carcinoma cells.
Cancer Sci. 104:1052–1061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim HJ, Hawke N and Baldwin AS: NF-kappaB
and IKK as therapeutic targets in cancer. Cell Death Differ.
13:738–747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang R, Xia Y, Li J, Deng L, Zhao L, Shi
J, Wang X and Sun B: High expression levels of IKKalpha and IKKbeta
are necessary for the malignant properties of liver cancer. Int J
Cancer. 126:1263–1274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Romieu-Mourez R, Landesman-Bollag E,
Seldin DC, Traish AM, Mercurio F and Sonenshein GE: Roles of IKK
kinases and protein kinase CK2 in activation of nuclear
factor-kappaB in breast cancer. Cancer Res. 61:3810–3818.
2001.PubMed/NCBI
|
|
41
|
Mieczkowski J, Kocyk M, Nauman P,
Gabrusiewicz K, Sielska M, Przanowski P, Maleszewska M, Rajan WD,
Pszczolkowska D, Tykocki T, et al: Down-regulation of IKKβ;
expression in glioma-infiltrating microglia/macrophages is
associated with defective inflammatory/immune gene responses in
glioblastoma. Oncotarget. 6:33077–33090. 2015. View Article : Google Scholar : PubMed/NCBI
|