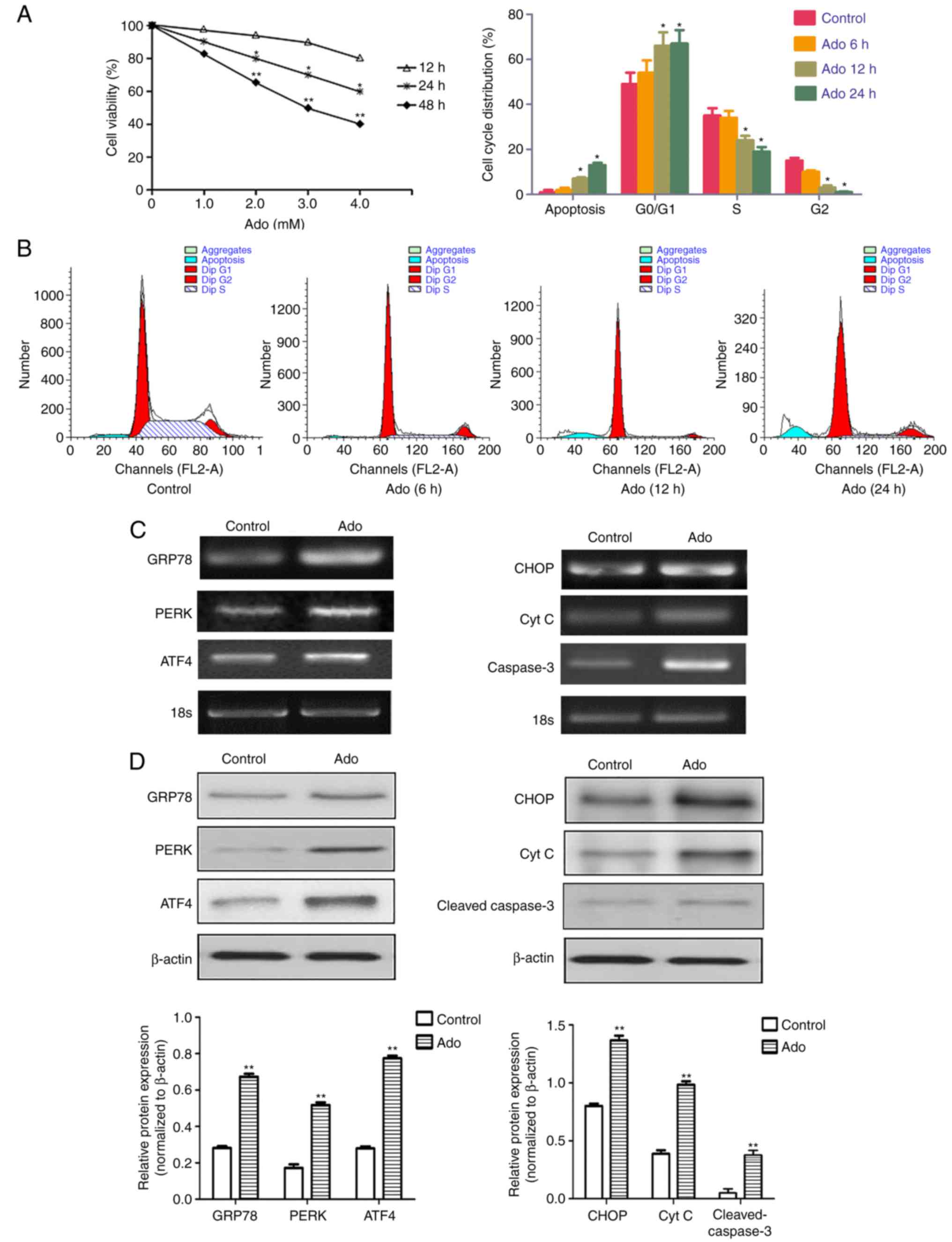
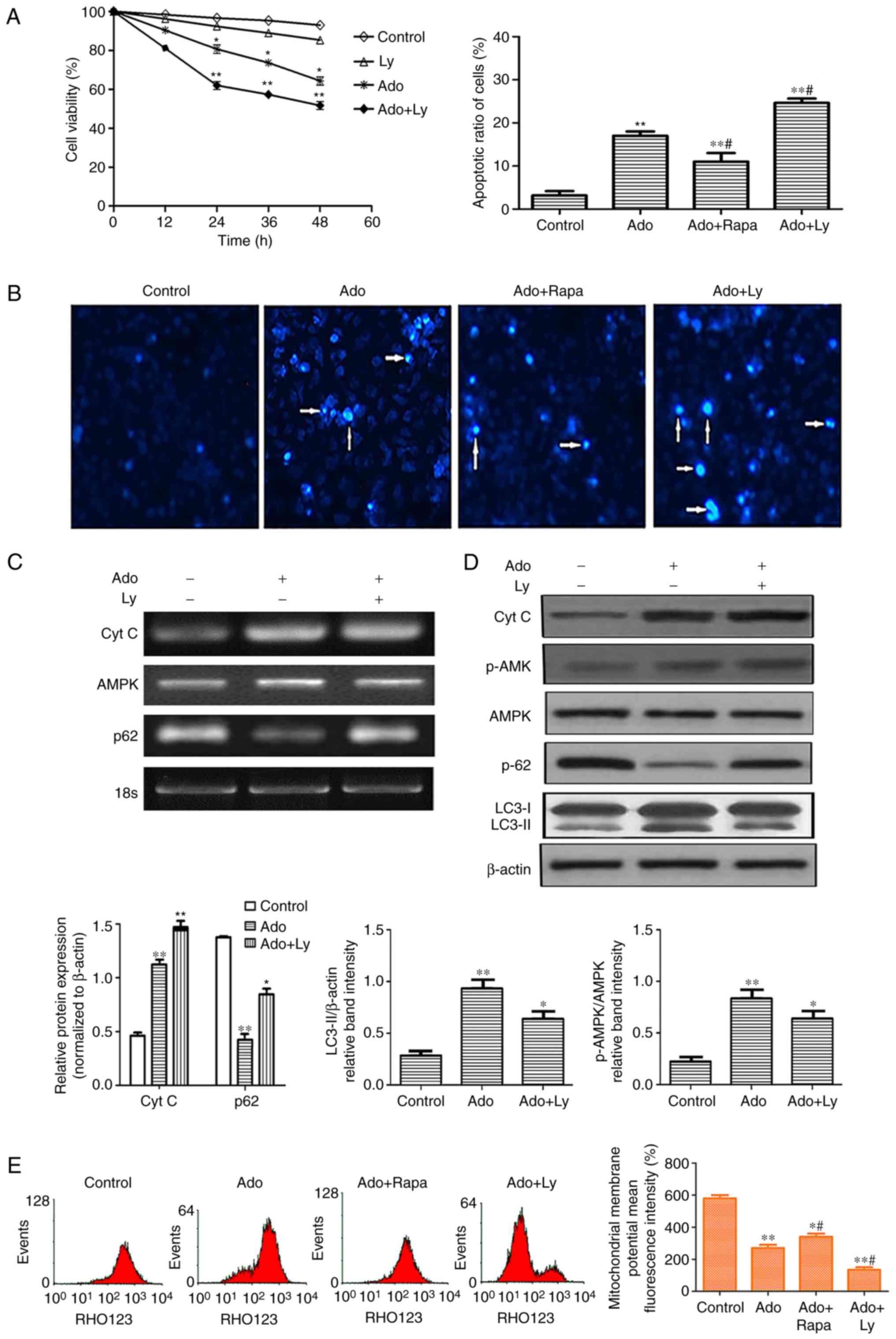
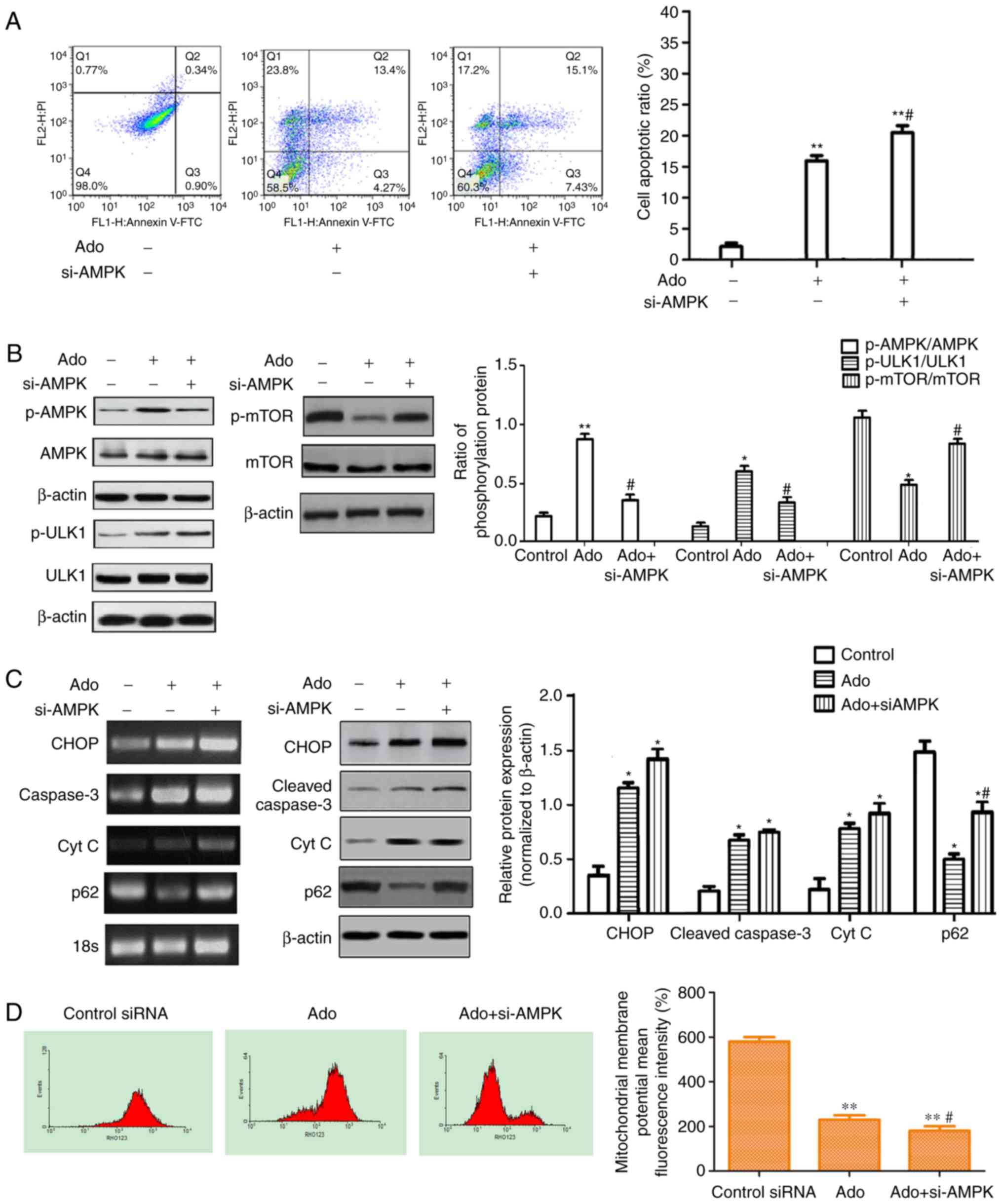
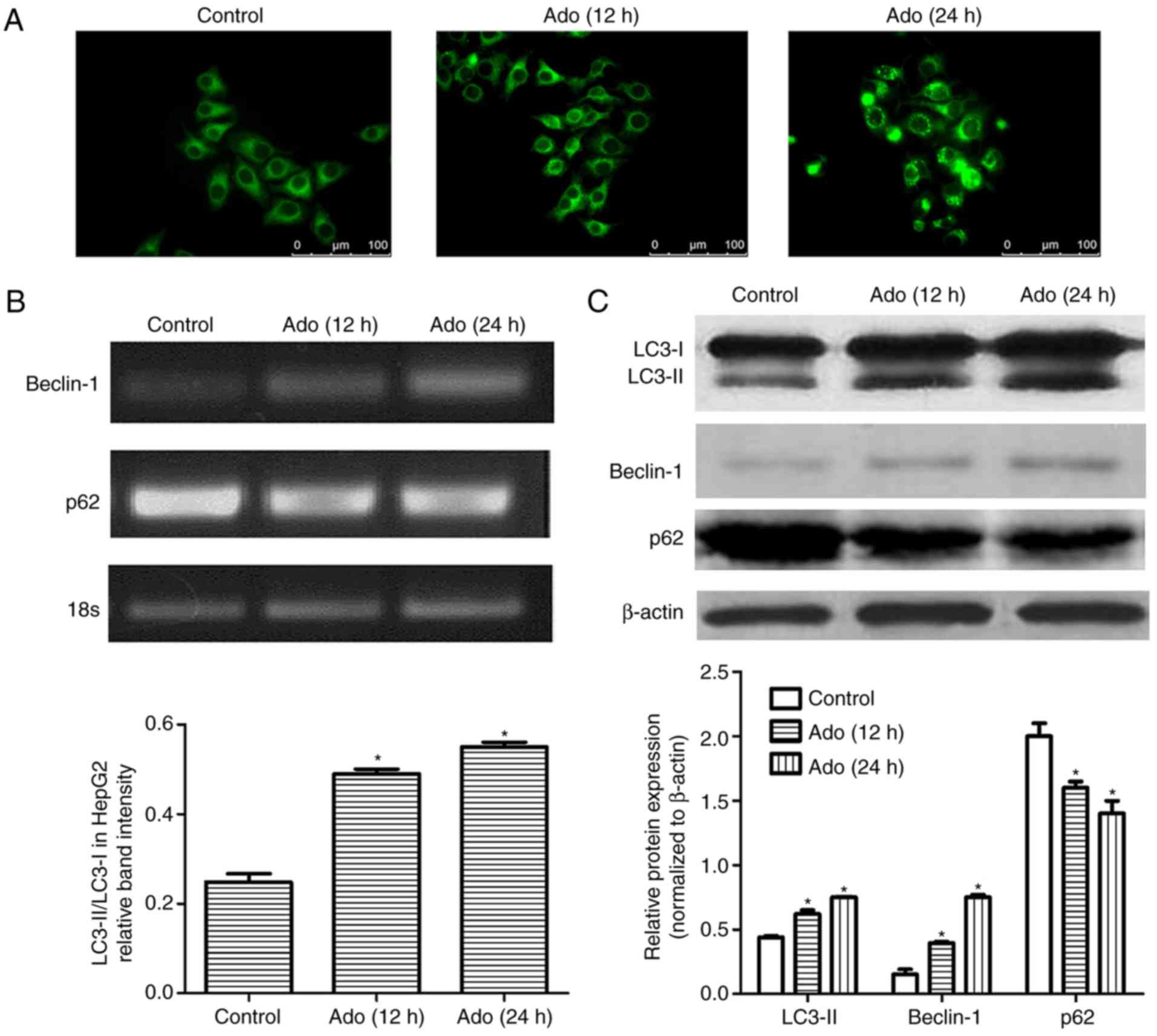
|
1
|
Mokdad AA, Singal AG and Yopp AC: Advances
in local and systemic therapies for hepatocellular cancer. Curr
Oncol Rep. 18:92016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin J, Wu L, Bai X, Xie Y, Wang A, Zhang
H, Yang X, Wan X, Lu X, Sang X, et al: Combination treatment
including targeted therapy for advanced hepatocellular carcinoma.
Oncotarget. 7:71036–71051. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou J, Li G, Zheng Y, Shen HM, Hu X, Ming
QL, Huang C, Li P and Gao N: A novel autophagy/mitophagy inhibitor
liensinine sensitizes breast cancer cells to chemotherapy through
DNM1L-mediated mitochondrial fission. Autophagy. 11:1259–1279.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie WY, Zhou XD, Li Q, Chen LX and Ran DH:
Acid-induced autophagy protects human lung cancer cells from
apoptosis by activating ER stress. Exp Cell Res. 339:270–279. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chi KH, Wang YS, Huang YC, Chiang HC, Chi
MS, Chi CH, Wang HE and Kao SJ: Simultaneous activation and
inhibition of autophagy sensitizes cancer cells to chemotherapy.
Oncotarget. 7:58075–58088. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rubiolo JA, López-Alonso H, Martínez P,
Millán A, Cagide E, Vieytes MR, Vega FV and Botana LM: Yessotoxin
induces ER-stress followed by autophagic cell death in glioma cells
mediated by mTOR and BNIP3. Cell Signal. 26:419–432. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Artero-Castro A, Perez-Alea M, Feliciano
A, Leal JA, Genestar M, Castellvi J, Peg V, Ramón Y Cajal S and
Lleonart ME: Disruption of the ribosomal P complex leads to
stress-induced autophagy. Autophagy. 11:1499–1519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Netea-Maier RT, Klück V, Plantinga TS and
Smit JW: Autophagy in thyroid cancer: Present knowledge and future
perspectives. Front Endocrinol. 6:222015. View Article : Google Scholar
|
|
9
|
Kovaleva V, Mora R, Park YJ, Plass C,
Chiramel AI, Bartenschlager R, Döhner H, Stilgenbauer S, Pscherer
A, Lichter P, et al: miRNA-130a targets ATG2BDICER1 to
inhibit autophagy and trigger killing of chronic lymphocytic
leukemia cells. Cancer Res. 72:1763–1772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jing Z, Sui X, Yao J, Xie J, Jiang L, Zhou
Y, Pan H and Han W: SKF-96365 activates cytoprotective autophagy to
delay apoptosis in colorectal cancer cells through inhibition of
the calcium/CaMKIIγ/AKT-mediated pathway. Cancer Lett. 372:226–238.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Gong W, Yang ZY, Zhou XS, Gong C,
Zhang TR, Wei X, Ma D, Ye F and Gao QL: Quercetin induces
protective autophagy and apoptosis through ER stress via the
p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis. 22:544–557. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu JL, Hu XL, Guo AY, Wang CJ, Wen YY and
Cang SD: Endoplasmic reticulum stress promotes autophagy and
apoptosis and reverses chemoresistance in human ovarian cancer
cells. Oncotarget. 8:49380–49394. 2017.PubMed/NCBI
|
|
13
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo XL, Hu F, Zhang SS, Zhao QD, Zong C,
Ye F, Guo SW, Zhang JW, Li R, Wu MC, et al: Inhibition of p53
increases chemosensitivity to 5-FU in nutrient-deprived
hepatocarcinoma cells by suppressing autophagy. Cancer Lett.
346:278–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu LX, Deng W, Zhou XT, Chen RP, Xiang
MQ, Guo YT, Pu ZJ, Li R, Wang GF and Wu LF: The mechanism of
adenosine-mediated activation of lncRNA MEG3 and its antitumor
effects in human hepatoma cells. Int J Oncol. 48:421–429. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hajiahmadi S, Panjehpour M, Aghaei M and
Shabani M: Activation of A2b adenosine receptor regulates ovarian
cancer cell growth: Involvement of Bax/Bcl-2 and caspase-3. Biochem
Cell Biol. 93:321–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu S, Hou D, Chen P, Zhang Q, Lv B, Ma Y,
Liu F, Liu H, Song EJ, Yang D, et al: Adenosine induces apoptosis
through TNFR1/RIPK1/P38 axis in colon cancer cells. Biochem Biophys
Res Commun. 460:759–765. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirsch C, Gauss R, Horn SC, Neuber O and
Sommer T: The ubiquitylation machinery of the endoplasmic
reticulum. Nature. 458:453–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu LF, Guo YT, Zhang QH, Xiang MQ, Deng W,
Ye YQ, Pu ZJ, Feng JL and Huang GY: Enhanced antitumor effects of
adenoviral-mediated siRNA against GRP78 gene on adenosine-induced
apoptosis in human hepatoma HepG2 cells. Int J Mol Sci. 15:525–544.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yasuda Y, Saito M, Yamamura T, Yaguchi T
and Nishizaki T: Extracellular adenosine induces apoptosis in
Caco-2 human colonic cancer cells by activating caspase-9/-3 via
A2a adenosine receptors. J Gastroenterol. 44:56–65.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gorman AM, Healy SJ, Jäger R and Samali A:
Stress management at the ER: Regulators of ER stress-induced
apoptosis. Pharmacol Ther. 134:306–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Singh N, Robinson-Taylor KS,
Dorsett-Martin WA, Morris MW Jr, Earl TM and Anderson CD:
Hepatocyte autophagy is linked to C/EBP-homologous protein,
Bcl2-interacting mediator of cell death, and BH3-interacting domain
death agonist gene expression. J Surg Res. 195:588–595. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu LF, Wei BL, Guo YT, Ye YQ, Li GP, Pu ZJ
and Feng JL: Apoptosis induced by adenosine involves endoplasmic
reticulum stress in EC109 cells. Int J Mol Med. 30:797–804. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bromati CR, Lellis-Santos C, Yamanaka TS,
Nogueira TC, Leonelli M, Caperuto LC, Gorjão R, Leite AR, Anhê GF
and Bordin S: UPR induces transient burst of apoptosis in islets of
early lactating rats through reduced AKT phosphorylation via
ATF4/CHOP stimulation of TRB3 expression. Am J Physiol Regul Integr
Comp Physiol. 300:R92–R100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Varshney R, Varshney R, Mishra R, Gupta S,
Sircar D and Roy P: Kaempferol alleviates palmitic acid-induced
lipid stores, endoplasmic reticulum stress and pancreatic β-cell
dysfunction through AMPK/mTOR-mediated lipophagy. J Nutr Biochem.
57:212–227. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Imamura K, Ogura T, Kishimoto A, Kaminishi
M and Esumi H: Cell cycle regulation via p53 phosphorylation by a
5′-AMP activated protein kinase activator,
5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, in a human
hepatocellular carcinoma cell line. Biochem Biophys Res Commun.
287:562–567. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oura K, Tadokoro T, Fujihara S, Morishita
A, Chiyo T, Samukawa E, Yamana Y, Fujita K, Sakamoto T, Nomura T,
et al: Telmisartan inhibits hepatocellular carcinoma cell
proliferation control by inducing cell cycle arrest. Oncol Rep.
38:2825–2835. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang S, Kang MS, Ryu E and Myung K:
Eukaryotic DNA replication: Orchestrated action of multi-subunit
protein complexes. Mutat Res. 809:58–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kenific CM and Debnath J: Cellular and
metabolic functions for autophagy in cancer cells. Trends Cell
Biol. 25:37–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park JH, Lee JE, Shin IC and Koh HC:
Autophagy regulates chlorpyrifos-induced apoptosis in SH-SY5Y
cells. Toxicol Appl Pharmacol. 268:55–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Buontempo F, Ersahin T, Missiroli S,
Senturk S, Etro D, Ozturk M, Capitani S, Cetin-Atalay R and Neri
ML: Inhibition of Akt signaling in hepatoma cells induces apoptotic
cell death independent of Akt activation status. Invest New Drugs.
29:1303–1313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xing CG, Zhu BS, Liu HH, Lin F, Yao HH,
Liang ZQ and Qin ZH: LY294002 induces p53-dependent apoptosis of
SGC7901 gastric cancer cells. Acta Pharmacol Sini. 29:489–498.
2008. View Article : Google Scholar
|
|
35
|
Hardie DG, Ross FA and Hawley SA: AMPK: A
nutrient and energy sensor that maintains energy homeostasis. Nat
Rev Mol Cell Biol. 13:251–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ojuka EO, Jones TE, Nolte LA, Chen M,
Wamhoff BR, Sturek M and Holloszy JO: Regulation of GLUT4
biogenesis in muscle: Evidence for involvement of AMPK and
Ca2+. Am J Physiol Endocrinol Metab. 282:E1008–E1013.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth and metabolism. Nat Cell
Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Galardo MN, Riera MF, Pellizzari EH,
Sobarzo C, Scarcelli R, Denduchis B, Lustig L, Cigorraga SB and
Meroni SB: Adenosine regulates Sertoli cell function by activating
AMPK. Mol Cell Endocrinol. 330:49–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aymerich I, Foufelle F, Ferré P, Casado FJ
and Pastor-Anglada M: Extracellular adenosine activates
AMP-dependent protein kinase (AMPK). J Cell Sci. 119:1612–1621.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bolster DR, Crozier SJ, Kimball SR and
Jefferson LS: AMP-activated protein kinase suppresses protein
synthesis in rat skeletal muscle through down-regulated mammalian
target of rapamycin (mTOR) signaling. J Biol Chem. 277:23977–23980.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|