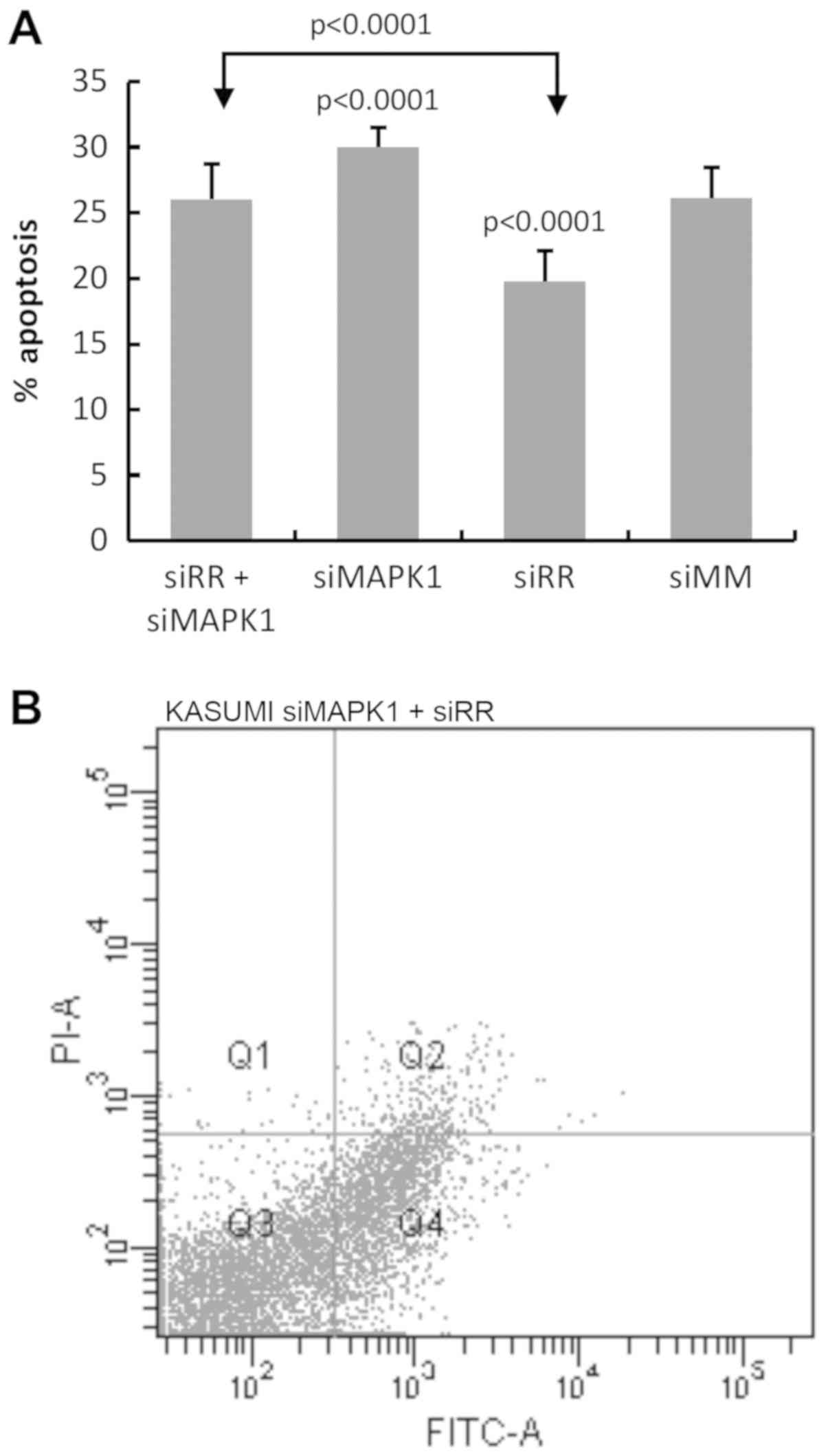
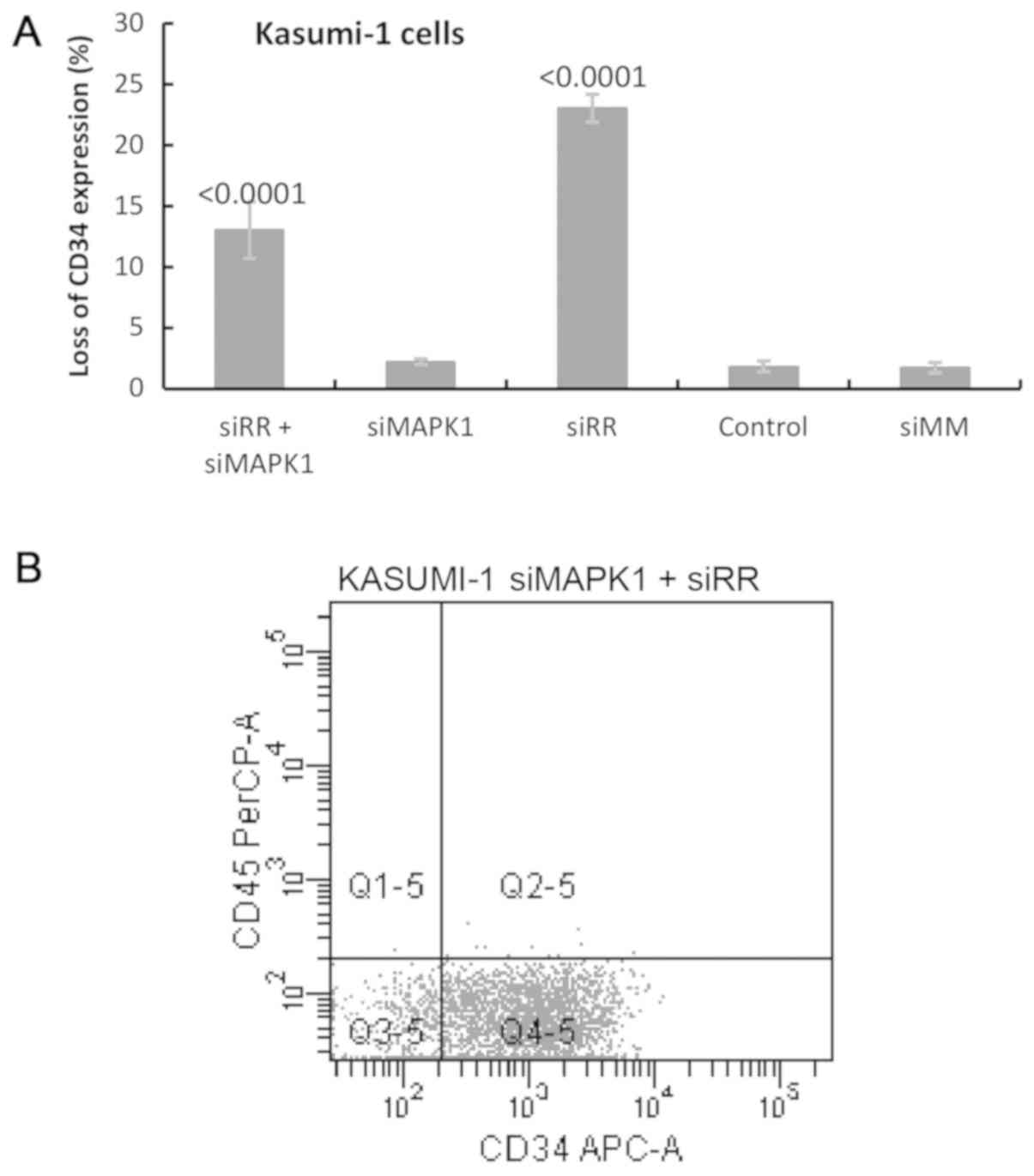
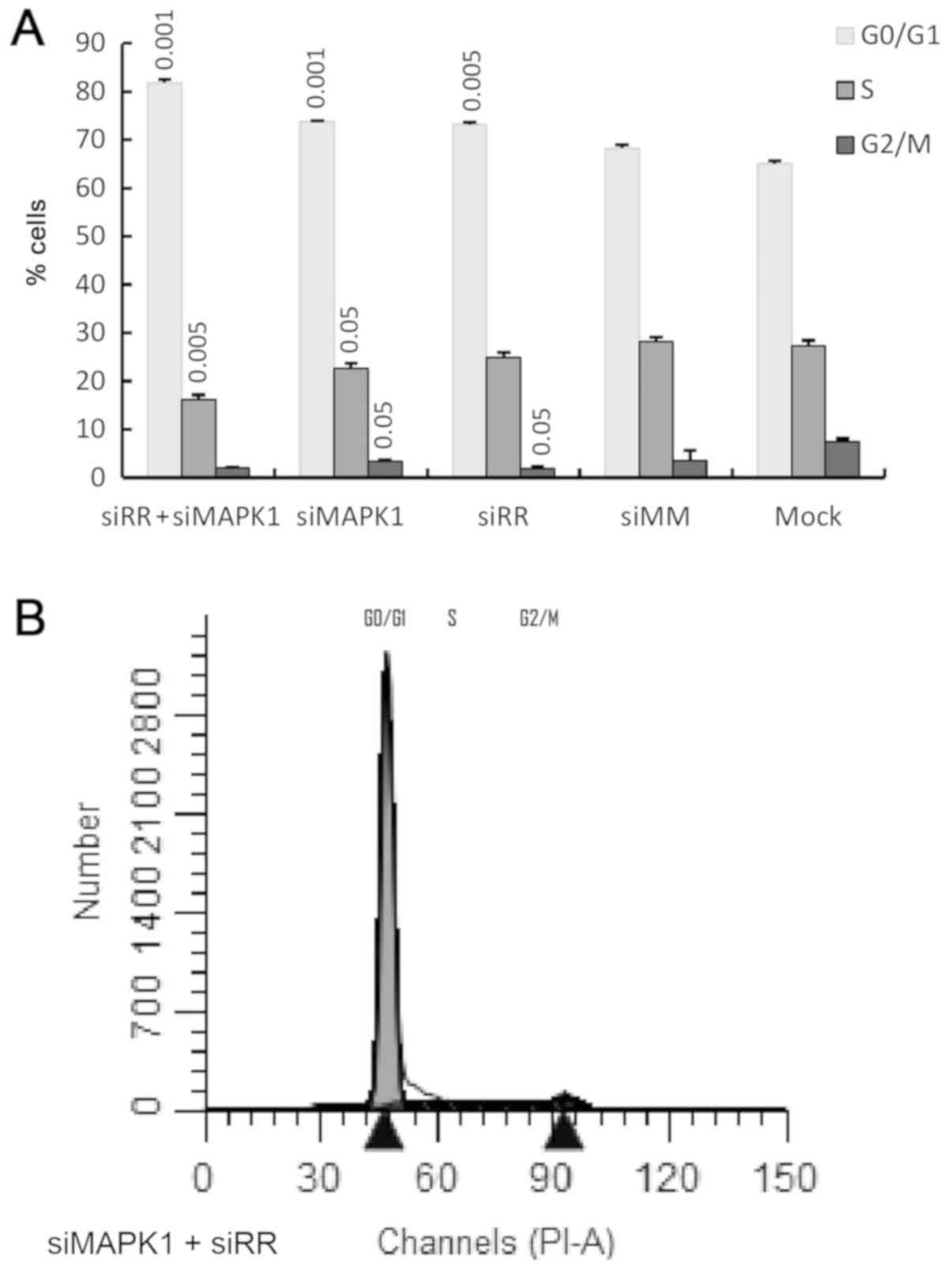
|
1
|
Grimwade D, Hills RK, Moorman AV, Walker
H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ and Burnett
AK; National Cancer Research Institute Adult Leukaemia Working
Group, : Refinement of cytogenetic classification in acute myeloid
leukemia: Determination of prognostic significance of rare
recurring chromosomal abnormalities among 5876 younger adult
patients treated in the United Kingdom Medical Research Council
trials. Blood. 116:354–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nucifora G and Rowley JD: AML1 and the
8;21 and 3;21 translocations in acute and chronic myeloid leukemia.
Blood. 86:1–14. 1995.PubMed/NCBI
|
|
3
|
Miyoshi H, Kozu T, Shimizu K, Enomoto K,
Maseki N, Kaneko Y, Kamada N and Ohki M: The t(8;21) translocation
in acute myeloid leukemia results in production of an AML1-MTG8
fusion transcript. EMBO J. 12:2715–2721. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang KS, Fan YH, Stass SA, Estey EH, Wang
G, Trujillo JM, Erickson P and Drabkin H: Expression of AML1-ETO
fusion transcripts and detection of minimal residual disease in
t(8;21)-positive acute myeloid leukemia. Oncogene. 8:983–988.
1993.PubMed/NCBI
|
|
5
|
Heidenreich O, Krauter J, Riehle H,
Hadwiger P, John M, Heil G, Vornlocher HP and Nordheim A: AML1/MTG8
oncogene suppression by small interfering RNAs supports myeloid
differentiation of t(8;21)-positive leukemic cells. Blood.
101:3157–3163. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trombly DJ, Whitfield TW, Padmanabhan S,
Gordon JA, Lian JB, van Wijnen AJ, Zaidi SK, Stein JL and Stein GS:
Genome-wide co-occupancy of AML1-ETO and N-CoR defines the t(8;21)
AML signature in leukemic cells. BMC Genomics. 16:3092015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hildebrand D, Tiefenbach J, Heinzel T,
Grez M and Maurer AB: Multiple regions of ETO cooperate in
transcriptional repression. J Biol Chem. 276:9889–9895. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan Y, Zhou L, Miyamoto T, Iwasaki H,
Harakawa N, Hetherington CJ, Burel SA, Lagasse E, Weissman IL,
Akashi K and Zhang DE: AML1-ETO expression is directly involved in
the development of acute myeloid leukemia in the presence of
additional mutations. Proc Natl Acad Sci USA. 98:10398–10403. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maiques-Diaz A, Chou FS, Wunderlich M,
Gómez-López G, Jacinto FV, Rodriguez-Perales S, Larrayoz MJ,
Calasanz MJ, Mulloy JC, Cigudosa JC and Alvarez S: Chromatin
modifications induced by the AML1-ETO fusion protein reversibly
silence its genomic targets through AML1 and Sp1 binding motifs.
Leukemia. 26:1329–1337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klampfer L, Zhang J, Zelenetz AO, Uchida H
and Nimer SD: The AML1/ETO fusion protein activates transcription
of BCL-2. Proc Natl Acad Sci USA. 93:14059–14064. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Linggi B, Müller-Tidow C, van de Locht L,
Hu M, Nip J, Serve H, Berdel WE, van der Reijden B, Quelle DE,
Rowley JD, et al: The t(8;21) fusion protein, AML1 ETO,
specifically represses the transcription of the p14(ARF) tumor
suppressor in acute myeloid leukemia. Nat Med. 8:743–750. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vangala RK, Heiss-Neumann MS, Rangatia JS,
Singh SM, Schoch C, Tenen DG, Hiddemann W and Behre G: The myeloid
master regulator transcription factor PU.1 is inactivated by
AML1-ETO in t(8;21) myeloid leukemia. Blood. 101:270–277. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pabst T, Mueller BU, Harakawa N, Schoch C,
Haferlach T, Behre G, Hiddemann W, Zhang DE and Tenen DG: AML1-ETO
downregulates the granulocytic differentiation factor C/EBPalpha in
t(8;21) myeloid leukemia. Nat Med. 7:444–451. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rio-Machín A, Menezes J, Maiques-Diaz A,
Agirre X, Ferreira BI, Acquadro F, Rodriguez-Perales S, Juaristi
KA, Alvarez S and Cigudosa JC: Abrogation of RUNX1 gene expression
in de novo myelodysplastic syndrome with t(4;21)(q21;q22).
Haematologica. 97:534–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta
1773. 1263–1284. 2007.
|
|
16
|
Morgan MA, Dolp O and Reuter CW:
Cell-cycle-dependent activation of mitogen-activated protein kinase
kinase (MEK-1/2) in myeloid leukemia cell lines and induction of
growth inhibition and apoptosis by inhibitors of RAS signaling.
Blood. 97:1823–1834. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Towatari M, Iida H, Tanimoto M, Iwata H,
Hamaguchi M and Saito H: Constitutive activation of
mitogen-activated protein kinase pathway in acute leukemia cells.
Leukemia. 11:479–484. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Milella M, Kornblau SM, Estrov Z, Carter
BZ, Lapillonne H, Harris D, Konopleva M, Zhao S, Estey E and
Andreeff M: Therapeutic targeting of the MEK/MAPK signal
transduction module in acute myeloid leukemia. J Clin Invest.
108:851–859. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zaidi SK, Dowdy CR, van Wijnen AJ, Lian
JB, Raza A, Stein JL, Croce CM and Stein GS: Altered Runx1
subnuclear targeting enhances myeloid cell proliferation and blocks
differentiation by activating a miR-24/MKP-7/MAPK network. Cancer
Res. 69:8249–8255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spirin PV, Lebedev TD, Orlova NN,
Gornostaeva AS, Prokofjeva MM, Nikitenko NA, Dmitriev SE, Buzdin
AA, Borisov NM, Aliper AM, et al: Silencing AML1-ETO gene
expression leads to simultaneous activation of both pro-apoptotic
and proliferation signaling. Leukemia. 28:2222–2228. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dennis G Jr, Sherman BT, Hosack DA, Yang
J, Gao W, Lane HC and Lempicki RA: DAVID: Database for annotation,
visualization, and integrated discovery. Genome Biol. 4:P32003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shih LY, Huang CF, Wang PN, Wu JH, Lin TL,
Dunn P and Kuo MC: Acquisition of FLT3 or N-ras mutations is
frequently associated with progression of myelodysplastic syndrome
to acute myeloid leukemia. Leukemia. 18:466–475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bacher U, Haferlach T, Kern W, Haferlach C
and Schnittger S: A comparative study of molecular mutations in 381
patients with myelodysplastic syndrome and in 4130 patients with
acute myeloid leukemia. Haematologica. 92:744–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martinez N, Drescher B, Riehle H, Cullmann
C, Vornlocher HP, Ganser A, Heil G, Nordheim A, Krauter J and
Heidenreich O: The oncogenic fusion protein RUNX1-CBFA2T1 supports
proliferation and inhibits senescence in t(8;21)-positive leukaemic
cells. BMC Cancer. 4:442004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kikushige Y, Yoshimoto G, Miyamoto T, Iino
T, Mori Y, Iwasaki H, Niiro H, Takenaka K, Nagafuji K, Harada M, et
al: Human Flt3 is expressed at the hematopoietic stem cell and the
granulocyte/macrophage progenitor stages to maintain cell survival.
J Immunol. 180:7358–7367. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ikeda H, Kanakura Y, Tamaki T, Kuriu A,
Kitayama H, Ishikawa J, Kanayama Y, Yonezawa T, Tarui S and Griffin
JD: Expression and functional role of the proto-oncogene c-kit in
acute myeloblastic leukemia cells. Blood. 78:2962–2968.
1991.PubMed/NCBI
|
|
30
|
Woźniak J and Kopeć-Szlezak J: c-Kit
receptor (CD117) expression on myeloblasts and white blood cell
counts in acute myeloid leukemia. Cytometry Part B Clin Cytometry.
58:9–16. 2004. View Article : Google Scholar
|
|
31
|
Stone RM, Mandrekar SJ, Sanford BL,
Laumann K, Geyer S, Bloomfield CD, Thiede C, Prior TW, Döhner K,
Marcucci G, et al: Midostaurin plus chemotherapy for acute myeloid
leukemia with a FLT3 mutation. N Engl J Med. 377:454–464.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong Chen, Waters SB, Holt KH and Pessin
JE: SOS phosphorylation and disassociation of the Grb2-SOS complex
by the ERK and JNK signaling pathways. J Biol Chem. 271:6328–6332.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pedram A, Razandi M and Levin ER:
Extracellular signal-regulated protein kinase/Jun kinase cross-talk
underlies vascular endothelial cell growth factor-induced
endothelial cell proliferation. J Biol Chem. 273:26722–26728. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Andreeff M, Jiang S, Zhang X, Konopleva M,
Estrov Z, Snell VE, Xie Z, Okcu MF, Sanchez-Williams G, Dong J, et
al: Expression of Bcl-2-related genes in normal and AML
progenitors: Changes induced by chemotherapy and retinoic acid.
Leukemia. 13:1881–1892. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
36
|
Salvesen GS and Duckett CS: IAP proteins:
Blocking the road to death's door. Nat Rev Mol Cell Biol.
3:401–410. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Westwick JK, Cox AD, Der CJ, Cobb MH, Hibi
M, Karin M and Brenner DA: Oncogenic Ras activates c-Jun via a
separate pathway from the activation of extracellular
signal-regulated kinases. Proc Natl Acad Sci USA. 91:6030–6034.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang P, Iwasaki-Arai J, Iwasaki H, Fenyus
ML, Dayaram T, Owens BM, Shigematsu H, Levantini E, Huettner CS,
Lekstrom-Himes JA, et al: Enhancement of hematopoietic stem cell
repopulating capacity and self-renewal in the absence of the
transcription factor C/EBP alpha. Immunity. 21:853–863. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamanaka R, Barlow C, Lekstrom-Himes J,
Castilla LH, Liu PP, Eckhaus M, Decker T, Wynshaw-Boris A and
Xanthopoulos KG: Impaired granulopoiesis, myelodysplasia, and early
lethality in CCAAT/enhancer binding protein epsilon-deficient mice.
Proc Natl Acad Sci USA. 94:13187–13192. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang H, Iakova P, Wilde M, Welm A, Goode
T, Roesler WJ and Timchenko NA: C/EBPα arrests cell proliferation
through direct inhibition of Cdk2 and Cdk4. Mol Cell. 8:817–828.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ishiguro A, Spirin KS, Shiohara M, Tobler
A, Gombart AF, Israel MA, Norton JD and Koeffler HP: Id2 expression
increases with differentiation of human myeloid cells. Blood.
87:5225–5231. 1996.PubMed/NCBI
|
|
42
|
Fujimoto T, Anderson K, Jacobsen SE,
Nishikawa Si and Nerlov C: Cdk6 blocks myeloid differentiation by
interfering with Runx1 DNA binding and Runx1-C/EBPalpha
interaction. EMBO J. 26:2361–2370. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rangatia J, Vangala RK, Treiber N, Zhang
P, Radomska H, Tenen DG, Hiddemann W and Behre G: Downregulation of
c-Jun expression by transcription factor C/EBPα is critical for
granulocytic lineage commitment. Mol Cell Biol. 22:8681–8694. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shivakumar L, Minna J, Sakamaki T, Pestell
R and White MA: The RASSF1A tumor suppressor blocks cell cycle
progression and inhibits cyclin D1 accumulation. Mol Cell Biol.
22:4309–4318. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kearsey JM, Coates PJ, Prescott AR,
Warbrick E and Hall PA: Gadd45 is a nuclear cell cycle regulated
protein which interacts with p21Cip1. Oncogene. 11:1675–1683.
1995.PubMed/NCBI
|
|
46
|
Ding Q, Regan SN, Xia Y, Oostrom LA, Cowan
CA and Musunuru K: Enhanced efficiency of human pluripotent stem
cell genome editing through replacing TALENs with CRISPRs. Cell
Stem Cell. 12:393–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shafie NH, Saleem M, Moses EJ, Razak SR
and Yusoff NM: The CRISPR-Cas9 system: A new dawn in gene editing.
J Bioanalys Biomed. 6:45–48. 2014.
|
|
48
|
Kleinstiver BP, Pattanayak V, Prew MS,
Tsai SQ, Nguyen NT, Zheng Z and Joung JK: High-fidelity CRISPR-Cas9
nucleases with no detectable genome-wide off-target effects.
Nature. 529:490–495. 2016. View Article : Google Scholar : PubMed/NCBI
|