Introduction
Individuals with malignancies suffer from the stress
of the cancerous disease whereas cells are affected by
intracellular stress. In eukaryotic cells, the endoplasmic
reticulum (ER) is important for the biosynthesis of organelles and
assembly of proteins (1). This
state, when the accumulation of misfolded or unfolded proteins
exceeds the ability of the body to clear them, ER homeostasis
imbalance [named ER stress (ERS)] occurs in order to deal with the
intracellular microenvironment changes (2). The published data has revealed that
ERS is closely associated with the occurrence and development of
multiple diseases including rheumatic disease (3), diabetes (4,5) and
prostate cancer (6). Endoplasmic
reticulum protein 29 (ERp29) is a reserved protein in ER (7), and it has been suggested that ERp29
may be involved in the aggressive behaviors of cancer cells
(8). One previous study
demonstrated that a triple-protein signature, including ERp29,
chloride intracellular channel 4 and second mitochondria-derived
activator of caspases/direct IAP-binding protein with low PI, may
predict the prognostic risk of colorectal cancer (9). However, the function of ERp29 in
colorectal cancer (CRC) remains unclear; it is potentially
associated with ERS and should be further clarified.
ERS has been receiving increasing attention in the
study of cancer cells as it is considered to activate
caspase-12-induced apoptosis (10,11).
Cancer development studies have revealed an increasing risk of
hepatic tumorigenesis and the promotion of virus-associated
nasopharyngeal carcinogenesis in cells with ERS (12,13).
ERp29, as a molecular chaperone protein, is distributed
ubiquitously and abundantly in mammalian tissues and its
predominant subcellular localization is in the rough ER (14,15).
Certain published data implies that ERp29 participates in
ERS-associated cell reactivity. The exact function of ERp29 appears
to be diverse, depending on the tissue and cell type. ERp29
deficiency may result in lower apoptosis sensitivity in the adult
thyrocytes of ERp29−/− mice during stress response
(16). ERp29 presents a favorable
function in breast cancer cell survival against doxorubicin-induced
genotoxic stress and against ionizing radiation, and it also
demonstrates resistance to radiation treatment in nasopharyngeal
carcinoma and mouse intestinal epithelial cells (17–19).
Molecular and phenotypic remodeling of epithelial-mesenchymal
transition (EMT) revealed advanced malignancy with the loss of
cell-cell junctions, acquired cellular motility and invasiveness
(20). It is unclear whether ERp29
serves a function in the ERS-associated EMT of cancer. Therefore,
it is necessary to denote the function of ERp29 in CRC, in
particular in its malignancy and aggressiveness during ERS.
The present study investigated whether ERp29 during
ERS was involved in interfering with the malignant behaviors of
CRC, as well as its possible mechanisms. This was achieved by
constructing an ERS model in CRC cell lines using conditioned media
supplemented with tunicamycin (TM) and 4-phenylbutyric acid
(4-PBA), as previously described by Reiling et al (21) and Kim et al (22), thus demonstrating a novel function
of ERp29 during ERS in regulating malignant behaviors of CRC
cells.
Materials and methods
Cell culture and treatment
All cell lines (SW620, HCT116, HT29, LS174T, SW480,
LOVO and DLD1) were purchased from American Type Culture Collection
(Manassas, VA, USA). Cells were cultured in RPMI-1640 (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and
incubated at 37°C in a 5% CO2 incubator. Cancer cells
were inoculated into a 6-well plate overnight, then the medium was
replaced with a new basal medium containing TM (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) or/and 4-PBA
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 24 or 48 h. TM
and 4-PBA were dissolved in dimethyl sulfoxide as a stock
solution.
Western immunoblotting
Proteins were extracted from the CRC cells (SW620,
HCT116, HT29, LS174T, SW480, LOVO and DLD1) in each group. The cell
lysates were removed to detect protein expression using
radioimmunoprecipitation assay lysis buffer containing protease
inhibitor (Beyotime Institute of Biotechnology, Shanghai, China) at
4°C for 30 min. Each group of protein samples was quantified using
a Bincinchoninic Acid Protein assay kit (Beyotime Institute of
Biotechnology). Cell lysates (25 µg) were separated on 10% SDS-PAGE
and then transferred to polyvinylidene fluoride (PVDF) membranes.
The membranes were blocked with 5% skimmed milk in PBS containing
0.1% Tween-20 (PBS-Tween-20) for 1 h at room temperature and then
incubated with specific primary antibodies at 4°C overnight. The
rabbit monoclonal antibodies used were heat shock protein family A
(Hsp70) member 5 (GRP78; 1:500; cat. no. 11578-1-AP; ProteinTech
Group, Inc., Chicago, IL, USA), heat shock protein 90 β family
member 1 (GRP94; 1:500; cat. no. 0811; Novus Biologicals, LLC,
Littleton, CO, USA), ERp29 (1:500; cat. no. GTX102225; GeneTex,
Inc., Irvine, CA, USA), CUL5 (1:500; cat. no. BS755; Biogot
Technology Co., Ltd., Nanjing, China), snail family transcriptional
repressor 1 (SNAIL; 1:500; cat. no. 3879S; Cell Signaling
Technology, Inc., Danvers, MA, USA), vimentin (1:500; cat. no.
3932S; Cell Signaling Technology, Inc.), E-cadherin (1:500; cat.
no. 8834S; Cell Signaling Technology, Inc.), β-catenin (1:500; cat.
no. 9562S; Cell Signaling Technology, Inc.), replication protein A2
(RPA2; 1:500; cat. no. 52448S; Cell Signaling Technology, Inc.),
signal sequence receptor subunit 4 (SSR4; 1:500; cat. no. ab180262;
Abcam, Cambridge, MA, USA) and tubulin folding cofactor A (TBCA;
1:500; cat. no. 12304-1-AP; ProteinTech Group, Inc.). Next, the
membranes were washed with PBS-Tween-20 five times and incubated
with anti-mouse or -rabbit secondary antibodies (1:5,000; cat. nos.
ZB-2305 or ZB-2306; Zhongshan Golden Bridge Biotechnology, Beijing,
China) at room temperature for 1 h. Protein bands were visualized
with an enhanced chemiluminescence western blotting detection
system (Thermo Fisher Scientific, Inc.) and analyzed by the Tanon
5200 image acquisition system (Tanon Science and Technology Co.,
Ltd., Shanghai, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
First, total RNA was extracted from CRC cells (SW620
and HCT116) using a TRIzol Reagent kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol, and
then cDNA was synthesized using the Reverse Transcription kit
(Takara Biotechnology, Co., Ltd., Dalian, China) according to the
manufacturer's protocol. PCR was performed using Ex Taq™ DNA
Polymerase (Takara Bio, Inc., Otsu, Japan) and an ABI PRISM 7500
Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reactions were performed as follows:
Predegeneration at 95°C for 30 sec, and PCR for 40 cycles at 95°C
for 5 sec and 60°C for 34 sec. The relative level of gene
expression was normalized to GAPDH and calculated using
2−∆∆Cq [∆Cq=Cq (ERp29 or other genes)-Cq (GAPDH)]
(23). Primers used for the RT-qPCR
analysis of ERp29, GRP78, GRP94 and GAPDH were as follows: ERp29
forward, 5′-AAAGCAAGTTCGTCTTGGTGA-3′ and reverse,
5′-CGCCATAGTCTGAGATCCCCA-3′; GRP78 forward,
5′-CATCACGCCGTCCTATGTCG-3′ and reverse, 5′-CGTCAAAGACCGTGTTCTCG-3′;
GRP94 forward, 5′-GCTGACGATGAAGTTGATGTGG-3′ and reverse,
5′-CATCCGTCCTTGATCCTTCTCTA-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. Each sample was tested in
triplicate.
Immunofluorescence assays
At room temperature, the cells (SW620 and HCT116)
were fixed with 4% paraformaldehyde for 30 min, then 1% Triton
X-100 (cat. no. 0694; Amresco, LLC, Solon, OH, USA) was added for
10 min to puncture the cell membrane. Bovine serum albumin (1%) was
used for non-specific binding. Next, the cells were incubated with
the antibodies for ERp29 (1:100; sc-49658; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), GRP78 (1:50; cat. no.
11578-1-AP; ProteinTech Group, Inc.) and CUL5 (1:50; cat. no.
BS755; Biogot Technology Co., Ltd.) at 4°C overnight, and then at
room temperature, stained with an anti-rabbit or mouse IgG (H+L)
secondary antibody for 1 h (1:100, cat. no. ZF-0511; and 1:100,
cat. no. ZF-0513; Zhongshan Golden Bridge Biotechnology), followed
by 4′,6-diamidino-2-phenylindole staining (5 µg/ml; Beyotime
Biotechnology) for 10 min at room temperature. Finally, the cells
were observed via a confocal fluorescence microscope (FV1000;
Olympus Corporation, Tokyo, Japan) at ×200 magnification.
Cell Counting Kit 8 (CCK8) assays
A total of 103 cells (SW620 and HCT116)
were seeded into each well of a 96-well plate, and then CCK8
reagent (cat. no. CK04; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) at a ratio of CCK8:serum-free RPMI-1640 medium of
1:9, was added to measure cell proliferation induction at 0, 24,
48, 72, 96 and 120 h. Subsequent to adding the CCK8 reagent, the
cells were incubated at 37°C for 2 h. Optical density was obtained
from cell growth curve analysis.
Transwell assays
The lower chambers (cat. no. 353097; BD Biosciences,
San Diego, CA, USA) were supplemented with 500 µl 10% fetal bovine
serum in RPMI-1640 medium as a chemotactic factor. Next, a total of
5×104 cells (SW620 or HCT116) were mixed with serum-free
RPMI-1640 medium in the upper chambers for 24 or 48 h at 37°C and
5% CO2. Cells in the upper chamber were removed with a
cotton swab. Next, at room temperature, the chambers were fixed
with 4% paraformaldehyde for 30 min and Giemsa staining (A:B=1:2)
was applied for 20 min. Finally, the cells in the lower chamber
were observed and counted under a light microscope at ×200
magnification.
Animal models
The study was approved by the Southern Medical
University Animal Care and Use Committee (Southern Medical
University, Guangzhou, China). For the animal studies, 4-week-old
nude mice (15–20 g; male), which were obtained from the
Experimental Animal Center of Southern Medical University and fed
in specific pathogen-free conditions (housed at 20–25°C in 55–60%
relative humidity, using a 12 h light/12 h dark cycle and provided
with commercial food and water ad libitum throughout) were
randomly distributed into 4 groups, with 5 mice in each group. A
total of 5×106 SW620 cells were subcutaneously injected
for the assessment of cancer cell growth. In addition,
5×106 cells were injected into the spleens of nude mice
to simulate the experimental metastasis of the liver. After 3
weeks, the subcutaneous masses of tumors or liver metastases were
detected using in vivo imaging (FX Pro; Bruker Scientific
Technology Co., Ltd., Beijing, China). Next, the nude mice were
sacrificed by cervical dislocation and the subcutaneous masses of
tumors or liver metastases were removed from the animals for
histopathological analysis.
Hematoxylin and eosin staining
(H&E)
Animal tumor tissues were fixed in 10% neutral
buffered formalin for 24 h and embedded in paraffin; tumor tissues
were cut into 3-µm sections. At room temperature, the tissues
sections were deparaffinized twice in 100% dimethylbenzene for 25
min and rehydrated in a descending series of ethanol concentrations
(100, 95, 80, 70 and 50%). Thereafter, all tissues sections were
stained with hematoxylin (cat. no. G1120; Solarbio) at room
temperature for 5 min and washed under running tap water for 5 min,
1% hydrochloric acid alcohol for 10–15 sec and running tap water
for 20 min. Next, all tissues slices were stained with eosin for 2
min at room temperature (cat. no. G1120; Solarbio). Dehydration in
a descending series of ethanol concentrations (70, 80, 95 and 100%)
and vitrification by 100% dimethylbenzene for 5 min was then
performed. Finally, the tissues were observed under a light
microscope (magnification, ×200 or 400).
Co-immunoprecipitation
Cell (SW620 and HCT116) extracts were collected as
described in the western immunoblotting section. Next, the cell
extracts were incubated with protein A+G agarose (Santa Cruz
blotechnology, Inc.) for 1 h at 4°C. The protein A+G agarose was
removed and the antibodies for ERp29 (1:20; cat. no. sc-49658;
Santa Cruz Biotechnology, Inc.) or CUL5 (1:25; cat. no. BS755;
Biogot Technology Co., Ltd.) or control IgG (1-2/100–500 µg of
total protein; Santa Cruz Biotechnology, Inc.) were added for
incubation overnight at 4°C. Once protein A+G agarose was added for
8 h at 4°C, the beads were washed with cold PBS and then
centrifuged at 670.8 × g for 10 min at 4°C. The supernatant was
collected. The subsequent processing was performed in accordance
with western blotting as described above.
Gene transfection carriers
Overexpression plasmid and lentivirus vectors of
ERp29 were constructed by and purchased from Shanghai GenePharma
Co., Ltd according to the manufacturer's protocols (Shanghai,
China). The lentivirus vectors silencing ERp29 were purchased from
Shanghai GeneChem Co., Ltd., according to the manufacturer's
protocols (Shanghai, China). The effective silencing fragment for
ERp29 was CCCTGGATACGGTCACTTT. SW620 and HCT116 cells were seeded
in 6-well plates at a density of 5×106 cells/well. After
24 h, ERp29-overexpressing or control cell lines were constructed
by infecting cells with lentivirus ERp29, shERp29 and control
vector at 37°C for 48 h. The cells were then subjected to
immunoblot analysis. The siRNA specific for CUL5
(UUCCGGUAUCAAGUCCUCCTT) or control siRNA (UUCUCCGAACGUGUCACGUTT)
were purchased from Guangzhou Dahong Biosciences (Guangzhou,
China), according to the manufacturer's protocols. SW620 and HCT116
cells were seeded in 6-well plates and control siRNA or CUL5 siRNA
was transfected using Lipofectamine® 2000 according to
the manufacturer's protocols (Thermo Fisher Scientific, Inc.).
After 48 h, the cells were collected and analyzed by
immunoblotting.
Tumor samples and
immunohistochemistry
The 457 tumor samples of CRC were supplied by the
Nanfang Hospital of Southern Medical University (Guangzhou, China).
The patients included 244 men and 213 women (median age, 60 years;
range, 19–86 years). The patients and/or their relatives were
written informed consent to the use of their clinical materials for
research purposes and ethical approval was obtained from the Ethics
Committee of Southern Medical University. At room temperature,
tumor and paracarcinoma tissues were fixed in 10% neutral buffered
formalin for 24 h and cut into 3-µm thick sections. Tissues were
deparaffinized and rehydrated following conventional processes. The
tissues were then blocked with goat serum for 1 h at room
temperature. Next, the expression of ERp29 and CUL5 was detected
and immunostained using primary antibodies (ERp29, 1:300, cat. no.
ab11420, Abcam; CUL5, 1:250, cat. no. BS755, Biogot Technology Co.,
Ltd.) at 4°C overnight. At room temperature, the tissues were
incubated for 1 h with anti-mouse or anti-rabbit HRP-labeled IgG
polymer and then DAB (1:50) for 1 min (EnVision Detection System;
cat. no. K500711; Dako; Agilent Technologies, Inc., Santa Clara,
CA, USA). The immunochemistry results for ERp29 and American Joint
Committee on Cancer (AJCC) stages were evaluated as previously
described (9,24). The staining intensity of CUL5 was
scored as weak (0–25%) or strong (26–100%) based on the percentage
of the positive staining areas of the carcinoma-involved area.
Statistical analysis
The data are presented as the mean ± standard
deviation. Independent samples t-test or one-way analysis of
variance with Tukey's post hoc test was adopted for the comparison
of independent experimental groups. The association between ERp29
expression and the clinicopathological features was obtained using
a χ2 test, and the expression of CUL5 using a Fisher's
exact test. The Kaplan-Meier method was used to obtain survival
curves. All statistics were analyzed by SPSS software (version
13.0; SPSS, Inc., Chicago, IL, USA) and GraphPad Prism (version
6.02; GraphPad Software Inc., La Jolla, CA, USA). P≤0.05 was
considered to indicate a statistically significant difference.
Results
ERp29 is involved in ERS of CRC
The present study initially detected the endogenous
expression of ERp29 in seven CRC cell lines. The cell lines that
produced a lower expression, SW620 and HCT116, were selected for
subsequent experiments (Fig. 1A).
Next, an ERS model of TM incubation was constructed to demonstrate
time-concentration effects. Once the SW620 and HCT116 cells were
cultured in media supplemented with 20 µg/ml TM for 24 and 48 h,
respectively, the post-treated cells produced significantly
increased expression of molecules associated with ERS response,
including GRP94 and GRP78, which are considered to be
representative proteins of ERS (25–27)
and localized in the ER, when compared with untreated cells
(P<0.05; data not shown). To investigate whether ERp29
expression reacted to TM-induced-ERS, it was revealed that ERp29
expression was significantly upregulated under TM stimulation when
compared with untreated cells (P<0.05; data not shown).
Therefore, 20 µg/ml TM was incubated with SW620 cells for 24 h and
HCT116 cells for 48 h, respectively, in order to induce the state
of ERS. 4-PBA is able to neutralize the effect of ERS as described
by Choi et al (28).
Different concentrations (0, 2.5, 5 and 10 mmol/l) of 4-PBA were
selected to neutralize the TM induction effects. Concentration
assessment revealed that 10 mmol/l of 4-PBA was cytotoxic to the
cultured cells, whereas 5 mmol/l of 4-PBA was optimal to treat the
cells (data not shown). The results revealed that GRP78 and GRP94
expression was substantially decreased in the SW620 cells at 24 h
and HCT116 cells at 48 h, respectively when compare with TM-treated
cells (Fig. 1B). Simultaneously,
ERp29 expression was also substantially downregulated when compared
with TM-treated cells (Fig. 1B).
Therefore, 5 mmol/l 4-PBA was determined to counteract the state of
TM-induced ERS. The quantitative PCR of ERp29, GRP78 and GRP94
produced mRNA expression levels consistent with the protein levels,
with significantly increased expression levels in the TM-treated
groups compared with the control and significantly decreased
expression levels in the TM+4-PBA group compared with the
TM-treated group (P<0.01; Fig.
1C). The immunofluorescence assay revealed that ERp29 and GRP78
co-localized in SW620 and HCT116 cells; this indicated that ERp29
was located in the ER and may be associated with ERS (Fig. 1D). These data revealed that cancer
cells during ERS produce an enhanced expression of ERp29.
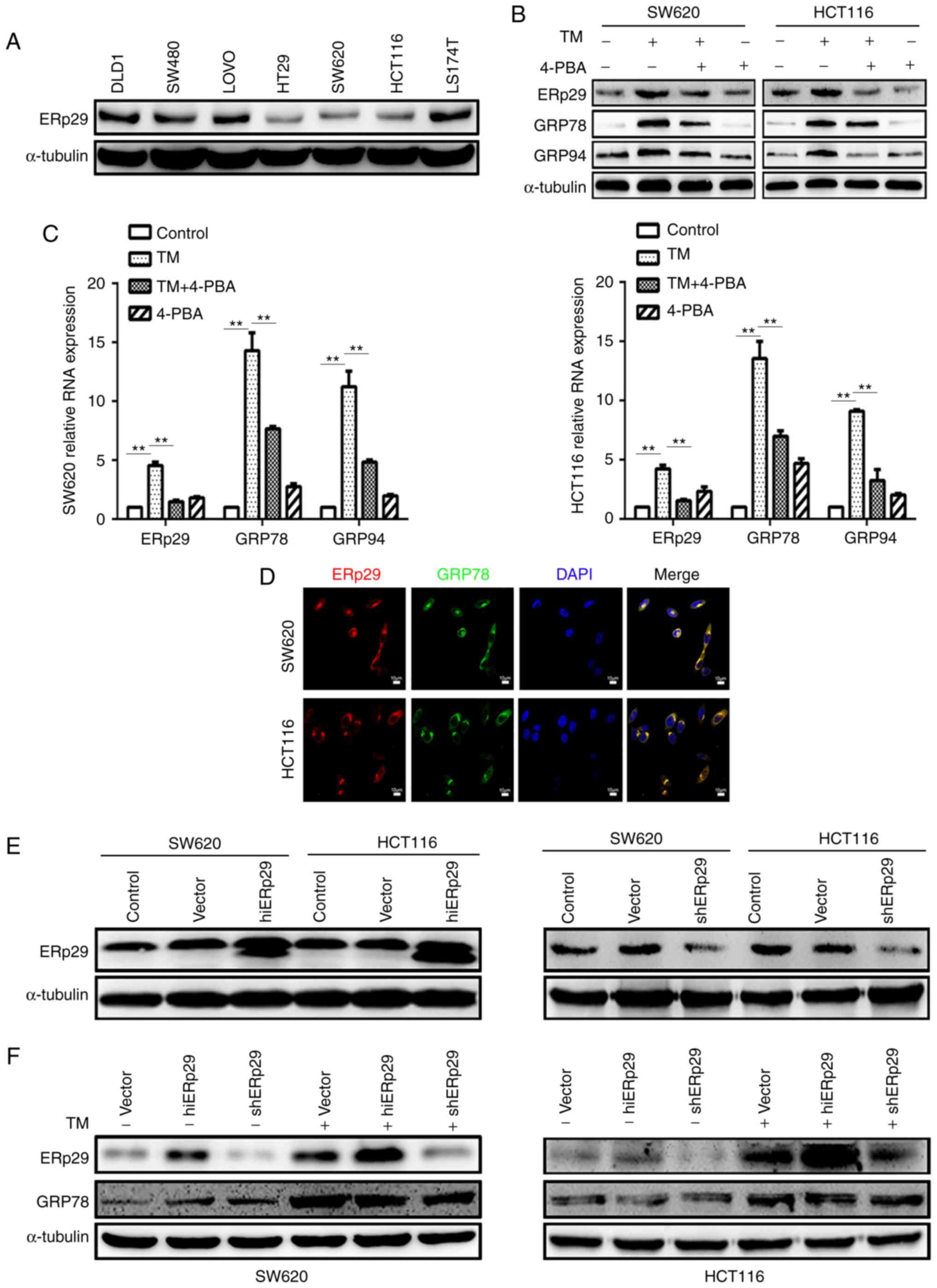 | Figure 1.ERp29 participated in the endoplasmic
reticulum stress of CRC cells. (A) ERp29 was expressed in seven CRC
cell lines. The expression of ERp29, GRP78 and GRP94 in SW620 and
HCT116 cells subsequent to incubation with 20 µg/ml TM and 5 mmol/l
4-PBA for 24 or 48 h, respectively, were measured by (B) western
blotting and (C) reverse transcription-quantitative polymerase
chain reaction. **P<0.01 with comparisons shown by lines. (D)
Immunofluorescent images of ERp29 co-locating with GRP78 in CRC
cells. (E) Constructed overexpressing ERp29 (hiERp29) and silencing
ERp29 (shERp29) stable cell lines. (F) Expression of ERp29 and
GRP78 in hiERp29 and shERp29 cells subsequent to TM stimulation.
ERp29, endoplasmic reticulum protein 29; CRC, colorectal cancer;
GRP78, heat shock protein family A (Hsp70) member 5; GRP94, heat
shock protein 90 β family member 1; TM, tunicamycin; 4-PBA,
4-phenylbutyric acid; hi-, high; sh-, short hairpin RNA. |
Next, ERp29 in association with ERS should be
requisite to investigate. Therefore, the present study established
stably ERp29-overexpressing (hiERp29) and ERp29-silenced [short
hairpin RNA (sh)ERp29] cell lines of SW620 and HCT116, and
confirmed the transfection efficiency (Fig. 1E). It was revealed that
TM-conditioned media was able to induce ERS in hiERp29 and shERp29
cell lines (Fig. 1F). During ERS,
ERp29 was highly upregulated in hiERp29 cells and downregulated in
shERp29 cells. These data demonstrate that ERp29 expression in
cancer cells is positively associated with ERS state.
ERp29 reduces the ERS-induced
inhibition of CRC cell growth
A CCK-8 cell proliferation assay revealed that the
proliferation of SW620 and HCT116 cells was significantly
suppressed by TM, and partly recovered by supplementation with
4-PBA compared with the control cells (P<0.05; Fig. 2A). In contrast, hiERp29 cells during
ERS exhibited a significantly enhanced proliferative rate compared
with the control cells (P<0.05; Fig.
2B), whereas the proliferation of shERp29 cells decreased
significantly (P<0.05; Fig. 2B).
Therefore, ERp29 was a positive factor involved in cancer cell
proliferation during ERS.
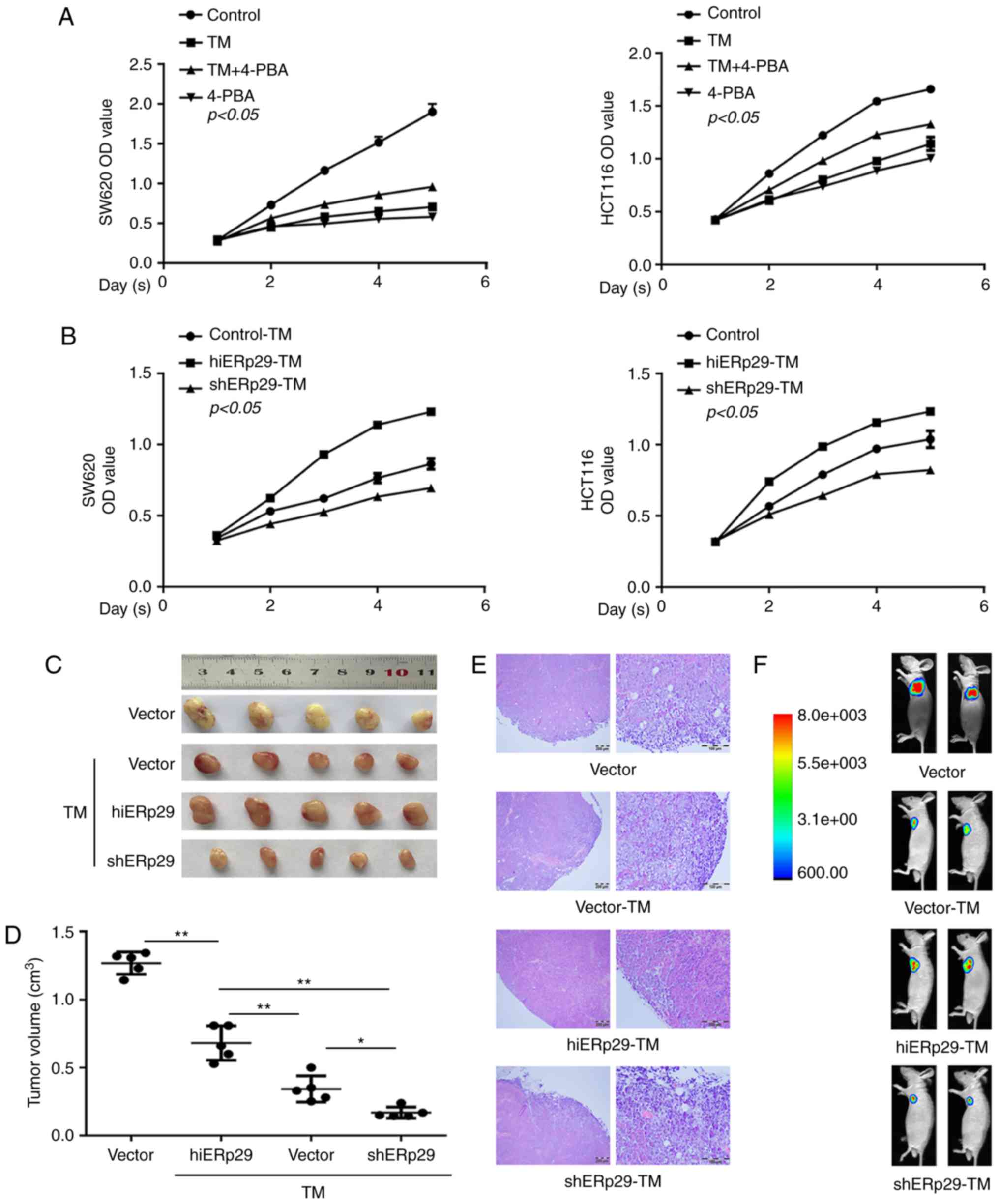 | Figure 2.Growth curve and xenotransplantation
revealed that ERp29 counteracted the endoplasmic reticulum
stress-inducing inhibition of CRC cell proliferation. (A) A Cell
Counting Kit-8 proliferation assay revealed that the proliferation
of CRC cells was significantly slower in SW620-TM and HCT116-TM
cells; correspondingly, the cell growth inhibition receded under
supplementation with 4-PBA. TM, 20 µg/ml; 4-PBA, 5 mmol/l. (B)
TM-treated cells demonstrated a higher proliferation rate in the
hiERp29 group and presented a lower rate in the shERp29 group.
Tumor masses of hiERp29 and shERp29 cells with TM stimulation in
nude mice as presented in a subcutaneous (C) tumor profile, (D)
quantified tumor size, (E) histological images of hematoxylin and
eosin staining (scale bar, 200 and 100 µm) and (F) in vivo
imaging. Error bars represent the mean ± standard deviation of
experiments in triplicate. *P<0.05 and **P<0.01 with
comparisons shown by lines. ERp29, endoplasmic reticulum protein
29; CRC, colorectal cancer; TM, tunicamycin; 4-PBA, 4-phenylbutyric
acid; hi-, high; sh-, short hairpin RNA; OD, optical density. |
Animal models with subcutaneous tumors revealed that
the mean volume of the tumor was 1.2698±0.0732 cm3 (n=5)
in the vector group and 0.3428±0.0862 cm3 (n=5) in the
TM-treated CRC cell group; with a significant difference identified
between these groups (P<0.01; Fig.
2C and D). For TM-treated cells, the mean volume of the tumors
in the hiERp29 group (0.6816±0.1129 cm3, n=5) were
significantly larger compared with that of the shERp29 group
(0.1692±0.0365 cm3, n=5) (P<0.01; Fig. 2C and D). Animal in vivo
images labeled with GFP revealed that the tumor masses of the
hiERp29 group were larger and more intensively fluorescent compared
with those in the shERp29 group (Fig.
2F). Microscopic observation did not reveal any
histopathological differences in the hematoxylin and eosin staining
of these tumor tissues (Fig. 2E).
Thus, animal models revealed that ERp29 promoted CRC cell growth
during ERS.
ERS inhibits the migration and
metastasis of CRC cells, but ERp29 offsets these effects
A Transwell cell migration assay revealed that the
number of invaded cells was significantly decreased following
treatment with TM, and partly retrieved subsequent to being
supplemented with 4-PBA (P<0.01; Fig. 3A and B). Nevertheless, in the
TM-treated groups, the invaded cells were significantly increased
in hiERp29 cells, and were significantly decreased in shERp29 cells
compared with the control (P<0.05; Fig. 3C and D). The results indicated that
during ERS, ERp29 enhanced the migration capacity of CRC cells.
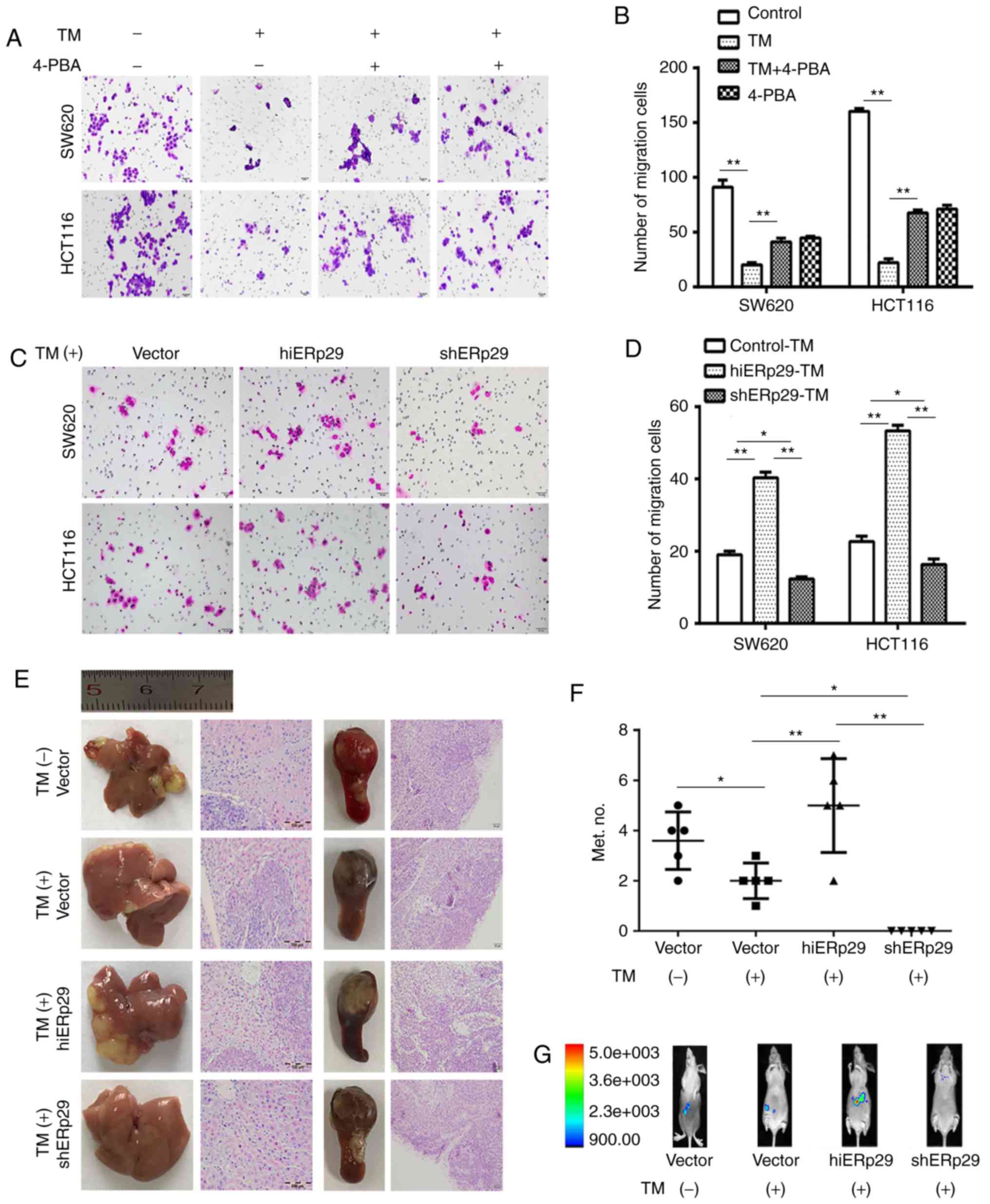 | Figure 3.Endoplasmic reticulum stress inhibits
the migration and metastasis of CRC cells, but ERp29 offsets these
effects. (A) A Transwell invasion assay revealed that the migration
rate of cells was reduced in SW620-TM and HCT116-TM cells;
correspondingly, the cell migration inhibition receded under
supplementation with 4-PBA. TM, 20 µg/ml; 4-PBA, 5 mmol/l. Scale
bar, 50 µm. (B) Quantified Transwell invasion assay results. (C) A
Transwell invasion assay revealed an increase in cell migration in
hiERp29 cells or the decreasing migration of shERp29 cells in
comparison with the control when the cells were stimulated with TM.
Scale bar, 50 µm. (D) Quantified Transwell invasion assay results.
(E) Experimental metastases of nude mice livers and spleen tumors
of inoculated CRC cells with histopathological images. Scale bar,
100 and 50 µm. (F) In vivo imaging of mice peritoneal
cavities and (G) quantified number of liver metastases. Error bars
represent the mean ± standard deviation. *P<0.05 and **P<0.01
with comparisons shown by lines. CRC, colorectal cancer; ERp29,
endoplasmic reticulum protein 29; TM, tunicamycin; 4-PBA,
4-phenylbutyric acid; hi-, high; sh-, short hairpin RNA. |
An experimental metastatic assay revealed that TM
significantly inhibited the liver metastases of mice (n=5,
P<0.05; Fig. 3E and F). In
vivo images revealed that hiERp29 cells produced liver
metastases, but shERp29 cells did not following TM treatment (n=5;
Fig. 3G). Furthermore, during ERS,
macroscopic observations revealed that the metastatic nodules were
more prevalent and larger in the hiERp29 group compared with the
shERp29 group (n=5, P<0.01; Fig. 3E
and F), although the tumor volume generated in the spleen had
little variation amongst all groups (n=5, Fig. 3E). The macroscopic tumor masses were
confirmed by histopathological examination, but they did not
present any histological differences (Fig. 3E). The animal models suggested that
ERp29 may promote metastases during ERS. These results revealed
that ERp29 functions as an impeller in CRC aggressiveness when the
cells are under the ERS state.
ERp29 coupling with CUL5 protects CRC
cells from the ERS-mediated depression of malignancy
Two-dimensional electrophoresis revealed different
protein dots between SW620 and hiERp29 SW620 cells, and then they
were confirmed by matrix-assisted laser desorption ionization
time-of-flight mass spectrometry (data not shown) (29,30).
The candidate proteins were CUL5, SSR4, RPA2 and TBCA, and their
expressions were verified using RT-qPCR and western blotting (data
not shown). CUL5, as a scaffolding protein in E3 ligase complexes,
is functionally involved in numerous cellular activities including
cell growth, cell cycle, apoptosis and cancer cell invasion
(31–35). The present study therefore
investigated whether CUL5 was able to confer the synergistic effect
of ERp29 to regulate the biological behavior of tumor cells. To
investigate this, immunofluorescence and co-immunoprecipitation
tests revealed that CUL5 co-localized and interacted with ERp29 in
SW620 and HCT116 cells (Fig. 4A and
B).
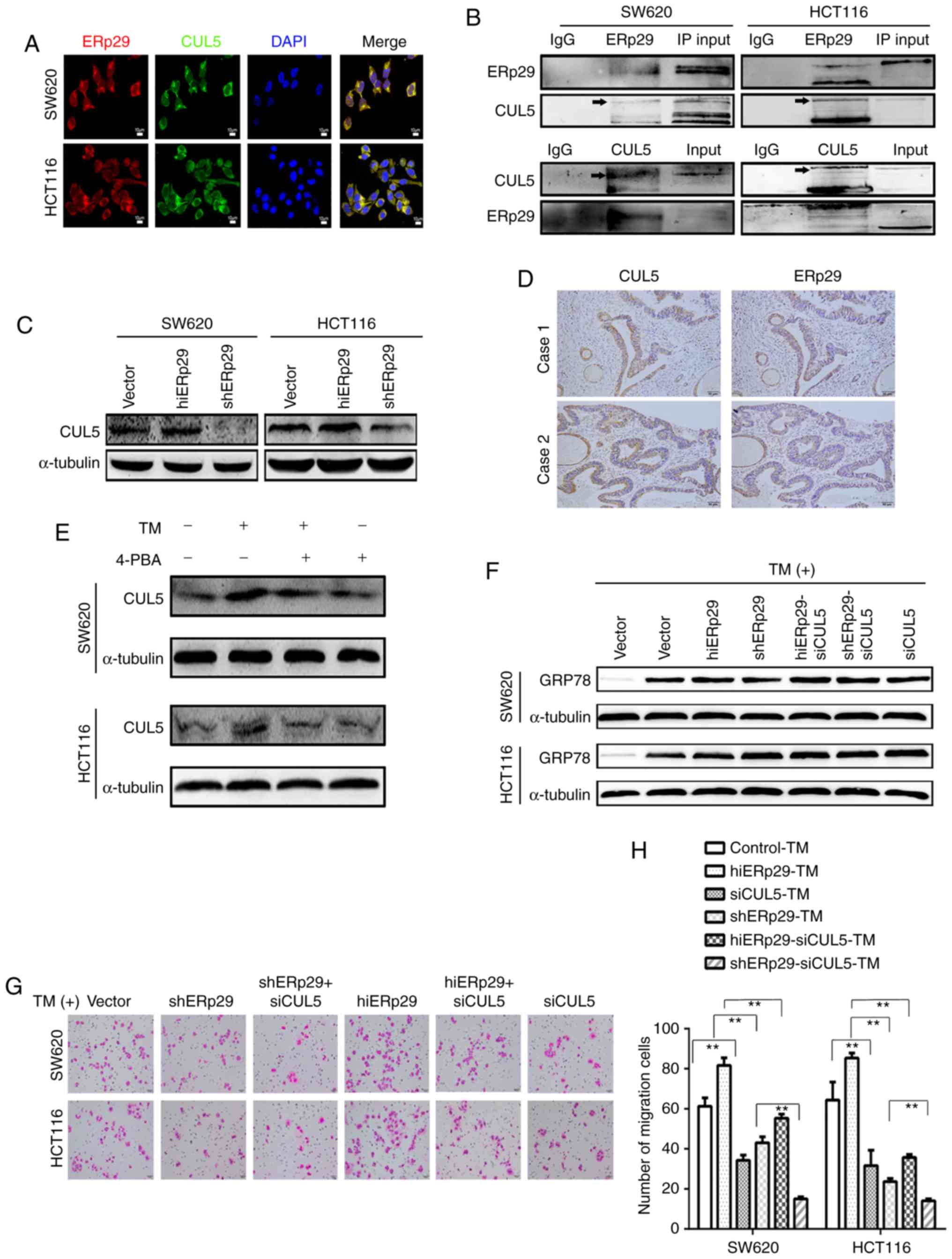 | Figure 4.ERp29 coupling with CUL5 protects CRC
cells from the ERS-mediated suppression of malignancies. (A)
Immunofluorescence images of ERp29 co-locating with CUL5 in CRC
cells. (B) Co-immunoprecipitation revealed that ERp29 interacted
with CUL5 in CRC cells. (C) Expression of CUL5 in hiERp29 and
shERp29 cell lines. (D) Expression of CUL5 and ERp29 in series of
tumor sample sections from patients with CRC; scale bar, 50 µm. (E)
Expression of CUL5 was upregulated with TM stimulation. (F)
hiERp29-siCUL5 and shERp29-siCUL5 cells in ERS. (G) When the
hiERp29 cells or shERp29 cells were affected by the knockdown of
CUL5 and treated with TM, the migration rates were substantially
reduced. Scale bar, 50 µm. (H) Quantified migration rates. Error
bars represent the mean ± standard deviation of experiments in
triplicate. **P<0.01 with comparisons shown by lines. CRC,
colorectal cancer; ERp29, endoplasmic reticulum protein 29; CUL5,
cullin 5; TM, tunicamycin; hi-, high; sh-, short hairpin RNA; si-,
small interfering RNA; ERS, endoplasmic reticulum stress; 4-PBA,
4-phenylbutyric acid; IgG, immunoglobulin G. |
Subsequently, the expression of CUL5 was revealed to
be consistent with that of ERp29 in hiERp29 and shERp29 CRC cells
(Fig. 4C). Immunohistochemistry
examination of serial sections revealed that CUL5 and ERp29 were
co-expressed intensively in 30 cases of human CRC tissues
(χ2=6.239, P<0.05; Fig.
4D). Consistent with the expression of ERp29, CUL5 expression
was substantially increased subsequent to TM treatment and
decreased when supplemented with 4-PBA in cultured cells (Fig. 4E). These data indicated that ERp29
was coupled with CUL5 to create a synergistic effect in the ERS of
CRC cells.
When the knockdown of CUL5 occurred, hiERp29-small
interfering RNA (si)CUL5, shERp29-siCUL5 and siCUL5 cell lines were
obtained (data not shown). Accordingly, GRP78 was highly expressed
in these cells following TM stimulation, meaning that ERS may be
triggered in these cells (Fig. 4F).
The Transwell assay results revealed that the invaded cells were
significantly fewer in the hiERp29-siCUL5 group compared with the
hiERp29 group compared with cells treated with TM (P<0.01).
Similarly, the invaded cells were fewer in the shERp29-siCUL5 group
compared with in the shERp29 group (P<0.01; Fig. 4G and H). These results revealed that
ERp29 should rely on CUL5 to restore invasiveness in CRC cells
during ERS.
ERp29 relies on CUL5 to promote
EMT
EMT provides a novel challenge and opportunity for
the invasive and migratory behaviors of cancer cells. In order to
further understanding of how ERp29 promotes cell migration and
invasion, the expression of EMT-associated proteins were measured.
When SW620 and HCT116 cells were treated with TM, the expression of
E-cadherin, associated with the epithelial phenotype, was
increased, and the expression of mesenchymal phenotype proteins
vimentin, SNAIL and β-catenin, were decreased in comparison with
untreated CRC cells (Fig. 5A). When
the cells were treated with TM and supplemented with 4-PBA, the
epithelial and mesenchymal proteins were restored to the same
expression pattern as that of the blank control (Fig. 5A).
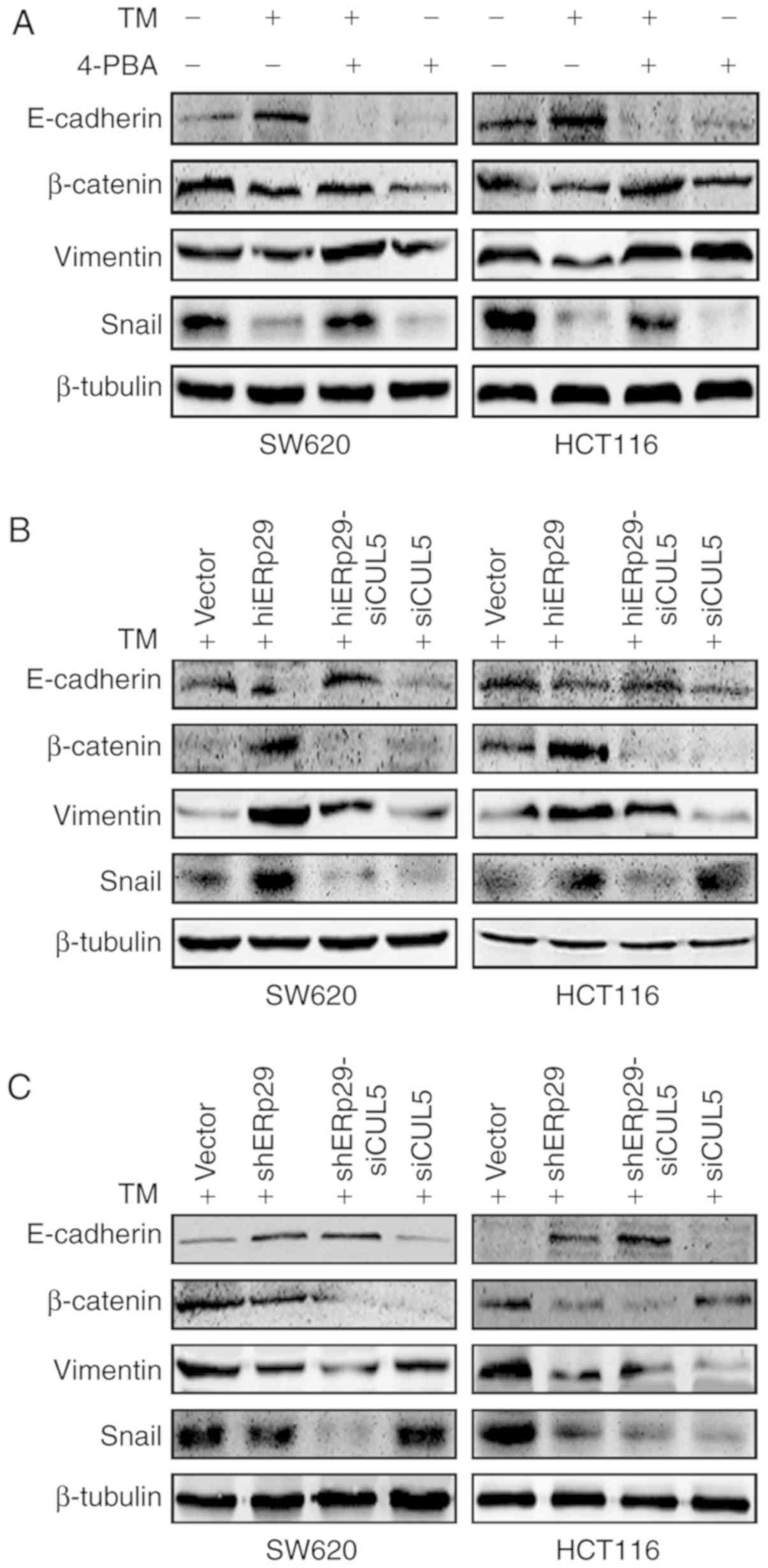 | Figure 5.ERp29 promotes EMT during ERS
depending on the cooperation of CUL5. Western blotting revealed the
expression of EMT-associated proteins in (A) colorectal cancer
cells under TM stimulation and/or supplemented with 4-PBA, (B)
hiERp29 and hiERp29-siCUL5 cells during TM stimulation, and (C)
shERp29 and shERp29-siCUL5 cells during TM stimulation. ERp29,
endoplasmic reticulum protein 29; EMT, epithelial-mesenchymal
transition; CUL5, cullin 5; TM, tunicamycin; hi-, high; sh-, short
hairpin RNA; si-, small interfering RNA; 4-PBA, 4-phenylbutyric
acid; Snail, snail family transcriptional repressor 1. |
In the TM-treated cells, the expression E-cadherin
was decreased and vimentin, SNAIL and β-catenin were increased in
hiERp29 cells in comparison with shERp29 cells or control cells
(Fig. 5B and C). Furthermore, the
expression of E-cadherin was increased and vimentin, SNAIL and
β-catenin were decreased in hiERp29-siCUL5 cells compared with
hiERp29 cells, and the analogous bands of the EMT proteins were
presented in shERp29-siCUL5 cells compared with shERp29 cells
(Fig. 5B and C). Therefore, when
CRC cells were in the state of ERS, the high expression of ERp29
required the cooperation of CUL5 in order to promote EMT.
High expression of ERp29 was
associated with a poor prognosis of patients with CRC
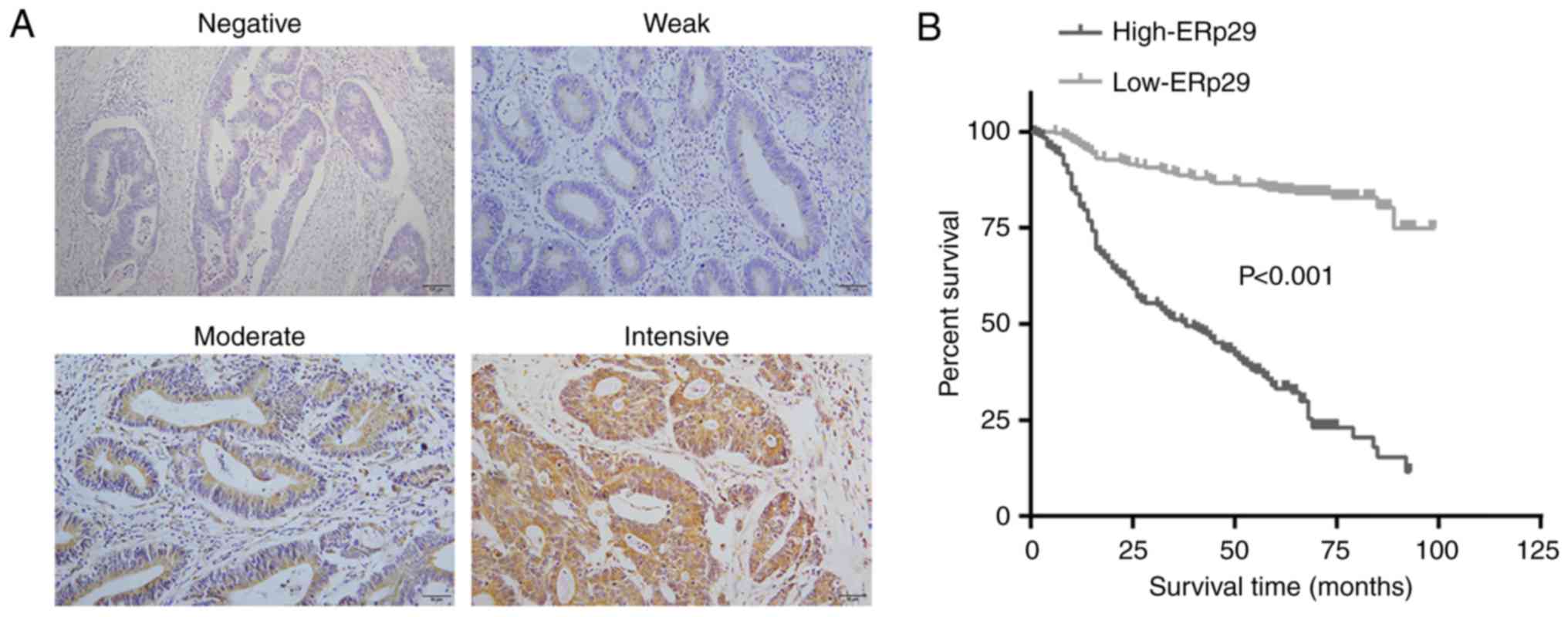
Immunohistochemical examination revealed ERp29
localizing in the cytoplasm of CRC cells of clinical samples, but
demonstrated intensive variation between individuals (Fig. 6A). To further analyze the
association of ERp29 with clinical features, the data revealed that
ERp29 expression levels had no significant association with sex,
age, location and tumor size (Table
I). However, it was revealed that the high expression of ERp29
was associated with lymph node metastasis, AJCC stages, status and
survival time (P<0.001; Table
I). Kaplan-Meier survival analysis revealed that the low
expression of ERp29 was associated with a prolonged survival time
of patients with CRC (P<0.001; Fig.
6B). Clinicopathological analysis demonstrated that the high
expression of ERp29 was associated with aggressive behaviors and an
unfavorable prognosis of CRC.
 | Table I.Expression of ERp29 associated with
clinical characteristics in patients with colorectal cancer
(n=457). |
Table I.
Expression of ERp29 associated with
clinical characteristics in patients with colorectal cancer
(n=457).
|
|
| ERp29
immunoreactivity |
|
|
|---|
|
|
|
|
|
|
|---|
| Feature | No. patients
(%) | Negative (135) | Positive (322) | χ2 | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
≤60 | 221 (48.4) | 72 (53.3) | 149 (46.3) | 1.899 | 0.168 |
|
>60 | 236 (51.6) | 63 (46.7) | 173 (53.7) |
|
|
| Sex |
|
|
|
|
|
|
Male | 244 (53.4) | 63 (46.7) | 181 (56.2) | 3.482 | 0.062 |
|
Female | 213 (46.6) | 72 (53.3) | 141 (43.8) |
|
|
| Location |
|
|
|
|
|
|
Colon | 215 (47.0) | 57 (42.2) | 158 (49.1) | 1.790 | 0.181 |
|
Rectum | 242 (52.0) | 78 (57.8) | 164 (50.9) |
|
|
| AJCC stage |
|
|
|
|
|
| I | 67 (14.7) | 29 (21.4) | 38 (11.8) | 16.947 | <0.001 |
| II | 180 (39.4) | 63 (46.7) | 117 (36.3) |
|
|
|
III/IV | 210 (45.9) | 43 (31.9) | 167 (51.9) |
|
|
| Size (cm) |
|
|
|
|
|
|
<3 | 207 (54.7) | 63 (46.7) | 144 (44.7) | 0.145 | 0.703 |
| ≥3 | 250 (45.3) | 72 (53.3) | 178 (55.3) |
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
Negative | 300 (65.7) | 106 (78.5) | 194 (60.2) | 14.079 | <0.001 |
|
Positive | 157 (34.3) | 29 (21.5) | 128 (39.8) |
|
|
| Status |
|
|
|
|
|
|
Censored | 280 (61.3) | 115 (81.2) | 165 (51.2) | 46.182 | <0.001 |
|
Death | 177 (34.3) | 20 (14.8) | 157 (48.8) |
|
|
| Survival time |
|
|
|
|
|
| ≤60
months | 223 (48.8) | 42 (31.1) | 181 (56.2) | 23.985 | <0.001 |
| >60
months | 234 (51.2) | 93 (68.9) | 141 (43.8) |
|
|
Discussion
Stress, any disturbance of the homeostasis of an
organism, is a ubiquitous phenomenon shaping life throughout eons
of evolution (36). Individuals
with malignancy and their cancer cells have to cope with
extracellular and intracellular stress (37). ERS is one of the known hemostatic
imbalances of bioactivity occurring in an attempt to maintain the
functions of the cells (38,39).
Numerous environmental changes including modifying the canonical
protein synthesis machinery may induce cell ERS (40,41).
Molecular chaperone proteins function as a protective measure in
ERS, and ERp29, a pivotal component, has been associated with
cancer of the breast, gallbladder and pancreas (42–44).
However, there is little evidence to demonstrate the biological
functions of ERp29 in CRC, in particular cancer cells in the state
of ERS. A novel observation of the present study was that ERp29 was
able to promote CRC cell growth and metastasis by relying on CUL5
during ERS.
In the present study, ERS was constructed using TM,
which inhibits the synthesis of products used for glycoprotein
chain generation (45,46), and the malignant behaviors, cell
growth, migration and metastatic potential of CRC cells were
weakened compared with the control. 4-PBA facilitates the handling
of mutant proteins and improves protein folding capacity to
suppress ERS (22,47,48).
When the ERS-state CRC cells were supplemented with 4-PBA in the
present study, the malignant behaviors of CRC cells were recovered.
The present study established the fundamental model that ERS
enables to remodify the biological features of CRC cells, and that
neutralizing ERS using 4-PBA may retrieve the decreased malignancy
tendency.
ERp29 belongs to the disulfide isomerase-like
protein family involved in protein secretion (49). The present study confirmed that
ERp29 was involved in CRC cells during ERS, just as ERp29 is
harmonious with the ERS of islet beta cells (50). Overexpression of ERp29 in cultured
cortical neurons improves neuronal survival and outgrowth following
axotomy (51). Downregulation of
ERp29 impairs the cell migration of lung adenocarcinoma cells and
significantly enhances the chemosensitivity to gemcitabine
(52). The present study surveyed
the potential function of ERp29 associated with the aggressive
behaviors of CRC cells during ERS in vivo and in
vitro. Surprisingly, it was revealed that the high expression
of ERp29 enhanced cell growth, invasion and experimental metastases
in CRC cells during ERS. In other words, ERp29 not only maintains
but also enhances sustainable aggressiveness in tumor progression
even though the CRC cells are under a state of ERS.
However, it appears to be known that the underlying
role of ERp29 is to serve CRC cells during ERS, and CUL5 was
identified as a candidate ERp29-modulated protein. Although there
exists no evidence to demonstrate whether ERp29 and CUL5 have
direct interactions with each other at their specific interactive
site, it was revealed that ERp29 needed to be coupled with CUL5 to
promote EMT through the detection ofthe markers of EMT in CRC cells
during ERS. During CUL5 knockdown, ERp29 was unable to maintain the
migration capacity and generate EMT of CRC cells during ERS.
Furthermore, clinical tissue examination revealed that the
co-expression of ERp29 and CUL5 was positive relative to one
another, and the clinicopathological data proved that the high
expression of ERp29 was associated with metastasis and the poor
prognosis of patients with CRC. Therefore, ERp29 should accompany
CUL5 to support malignancy for ERS cancer cells.
In summary, the novel results of the present study
are that cancer cells are also able to maintain malignant
bioactivities when they encounter disadvantageous stresses, and
that ERp29 participates in ERS to counteract the depressed
aggressiveness of CRC. Therefore, determining the regulatory
mechanism of ERp29 during ERS in CRC progression is meaningful for
the diagnosis and treatment of patients with CRC in advanced stages
of the disease.
Acknowledgements
The authors would like to thank to Professor Yanqing
Ding (Southern Medical University, Guangzhou, Guangdong, China) for
providing the experimental laboratory site.
Funding
The present study was supported by the National Key
Research and Development Program of China (grant no.
2016YFC1201801), the National Natural Science Foundation of China
(grant nos. 81672453, 81372584, 81201970 and 81702359), the
Guangdong Provincial Natural Science Foundation of China (grant
nos. 2015A030313243, 2015A030310089 and C1051156), the China
Postdoctoral Science Foundation (grant no. 2017M622736) and the
Guangdong Medical Science and Technology Research Foundation (grant
no. A2017403).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YD, CL and LG conceived and designed the study,
wrote, reviewed and edited the manuscript. LM and YL performed the
methodological development and analysis of the data. NT and GH
acquired the data. YH, DL and QW collected the data. GL, MT and ZJ
performed the animal experiments. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The patients and/or their relatives provided written
informed consent and ethical approval was obtained from the Ethics
Committee of Southern Medical University. All animal experiments
were approved by the Ethics Committee of Southern Medical
University (Guangzhou, China).
Patient consent for publication
The patients and/or their relatives provided written
informed consent for the use of their clinical materials for
research purposes.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ER
|
endoplasmic reticulum
|
|
ERS
|
endoplasmic reticulum stress
|
|
ERp29
|
endoplasmic reticulum protein 29
|
|
CRC
|
colorectal cancer
|
|
EMT
|
epithelial-mesenchymal transition
|
|
TM
|
tunicamycin
|
|
4-PBA
|
4-phenylbutyric acid
|
|
CUL5
|
cullin5
|
|
hiERp29
|
overexpressed ERp29
|
|
SSR4
|
translocon-associated protein subunit
delta
|
|
TBCA
|
tubulin-specific chaperone A
|
|
RPA2
|
replication protein A 32 kDa
subunit
|
References
|
1
|
Araki K and Nagata K: Prtein folding and
quality control in the ER. Cold Spring Harb Perspect Biol.
4:a0154382012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oakes SA and Papa FR: The role of
endoplasmic reticulum stress in human pathology. Annu Rev Pathol.
10:173–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Navid F and Colbert RA: Causes and
consequences of endoplasmic reticulum stress in rheumatic disease.
Nat Rev Rheumatol. 13:25–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hotamisligil GS: Role of endoplasmic
reticulum stress and c-Jun NH2-terminal kinase pathways in
inflammation and origin of obesity and diabetes. Diabetes. 54 Suppl
2:S73–S78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thivolet C, Vial G, Cassel R, Rieusset J
and Madec AM: Reduction of endoplasmic reticulum-mitochondria
interactions in beta cells from patients with type 2 diabetes. PLoS
One. 12:e01820272017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dandekar A, Mendez R and Zhang K: Cross
talk between ER stress, oxidative stress, and inflammation in
health and disease. Methods Mol Biol 1292. 205–214. 2015.
View Article : Google Scholar
|
|
7
|
Demmer J, Zhou C and Hubbard MJ: Molecular
cloning of ERp29, a novel and widely expressed resident of the
endoplasmic reticulum. FEBS Lett. 402:145–150. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao D, Bambang IF, Putti TC, Lee YK,
Richardson DR and Zhang D: ERp29 induces breast cancer cell growth
arrest and survival through modulation of activation of p38 and
upregulation of ER stress protein p58IPK. Lab Invest. 92:200–213.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng YJ, Tang N, Liu C, Zhang JY, An SL,
Peng YL, Ma LL, Li GQ, Jiang Q, Hu CT, et al: CLIC4, ERp29, and
Smac/DIABLO derived from metastatic cancer stem-like cells stratify
prognostic risks of colorectal cancer. Clin Cancer Res.
20:3809–3817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morishima N, Nakanishi K, Takenouchi H,
Shibata T and Yasuhiko Y: An endoplasmic reticulum stress-specific
caspase cascade in apoptosis. Cytochrome c-independent
activation of caspase-9 by caspase-12. J Biol Chem.
277:34287–34294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han J, Back SH, Hur J, Lin YH,
Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M,
et al: ER-stress-induced transcriptional regulation increases
protein synthesis leading to cell death. Nat Cell Biol. 15:481–490.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakagawa H, Umemura A, Taniguchi K,
Font-Burgada J, Dhar D, Ogata H, Zhong Z, Valasek MA, Seki E,
Hidalgo J, et al: ER stress cooperates with hypernutrition to
trigger TNF-dependent spontaneous HCC development. Cancer Cell.
26:331–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsiao JR, Chang KC, Chen CW, Wu SY, Su IJ,
Hsu MC, Jin YT, Tsai ST, Takada K and Chang Y: Endoplasmic
reticulum stress triggers XBP-1-mediated up-regulation of an EBV
oncoprotein in nasopharyngeal carcinoma. Cancer Res. 69:4461–4467.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hubbard MJ, McHugh NJ and Carne DL:
Isolation of ERp29, a novel endoplasmic reticulum protein, from rat
enamel cells. Evidence for a unique role in secretory-protein
synthesis. Eur J Biochem. 267:1945–1957. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shnyder SD and Hubbard MJ: ERp29 is a
ubiquitous resident of the endoplasmic reticulum with a distinct
role in secretory protein production. J Histochem Cytochem.
50:557–566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hirsch I, Weiwad M, Prell E and Ferrari
DM: ERp29 deficiency affects sensitivity to apoptosis via
impairment of the ATF6-CHOP pathway of stress response. Apoptosis.
19:801–815. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang D and Putti TC: Over-expression of
ERp29 attenuates doxorubicin-induced cell apoptosis through
up-regulation of Hsp27 in breast cancer cells. Exp Cell Res.
316:3522–3531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi L, Wu P, Zhang X, Qiu Y, Jiang W, Huang
D, Liu Y, Tan P and Tian Y: Inhibiting ERp29 expression enhances
radiosensitivity in human nasopharyngeal carcinoma cell lines. Med
Oncol. 29:721–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang B, Wang M, Yang Y, Wang Y, Pang X,
Su Y, Wang J, Ai G and Zou Z: ERp29 is a radiation-responsive gene
in IEC-6 cell. J Radiat Res. 49:587–596. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee DG, Lee SH, Kim JS, Park J, Cho YL,
Kim KS, Jo DY, Song IC, Kim N, Yun HJ, et al: Loss of NDRG2
promotes epithelial-mesenchymal transition of gallbladder carcinoma
cells through MMP-19-mediated Slug expression. J Hepatol.
63:1429–1439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reiling JH, Clish CB, Carette JE,
Varadarajan M, Brummelkamp TR and Sabatini DM: A haploid genetic
screen identifies the major facilitator domain containing 2A
(MFSD2A) transporter as a key mediator in the response to
tunicamycin. Proc Natl Acad Sci USA. 108:11756–11765. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim HJ, Jeong JS, Kim SR, Park SY, Chae HJ
and Lee YC: Inhibition of endoplasmic reticulum stress alleviates
lipopolysaccharide-induced lung inflammation through modulation of
NF-kappaB/HIF-1α signaling pathway. Sci Rep. 3:11422013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thirunavukarasu P, Talati C, Munjal S,
Attwood K, Edge SB and Francescutti V: Effect of incorporation of
pretreatment serum carcinoembryonic antigen levels into AJCC
staging for colon cancer on 5-year survival. JAMA Surg.
150:747–755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Preissler S, Rato C, Chen R, Antrobus R,
Ding S, Fearnley IM and Ron D: AMPylation matches BiP activity to
client protein load in the endoplasmic reticulum. Elife.
4:e126212015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eletto D, Dersh D and Argon Y: GRP94 in ER
quality control and stress responses. Semin Cell Dev Biol.
21:479–485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Argon Y and Simen BB: GRP94, an ER
chaperone with protein and peptide binding properties. Semin Cell
Dev Biol. 10:495–505. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi SI, Lee E, Jeong JB, Akuzum B, Maeng
YS, Kim TI and Kim EK: 4-Phenylbutyric acid reduces mutant-TGFBIp
levels and ER stress through activation of ERAD pathway in corneal
fibroblasts of granular corneal dystrophy type 2. Biochem Biophys
Res Commun. 477:841–846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krajaejun T, Lohnoo T, Jittorntam P,
Srimongkol A, Kumsang Y, Yingyong W, Rujirawat T, Reamtong O and
Mangmee S: Assessment of matrix-assisted laser desorption
ionization-time of flight mass spectrometry for identification and
biotyping of the pathogenic oomycete Pythium insidiosum. Int
J Infect Dis. 77:61–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao L, Liu L, Wang S, Zhang YF, Yu L and
Ding YQ: Differential proteomic analysis of human colorectal
carcinoma cell lines metastasis-associated proteins. J Cancer Res
Clin Oncol. 133:771–782. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kunkler B, Salamango D, DeBruine ZJ, Ploch
C, Dean S, Grossens D, Hledin MP, Marquez GA, Madden J, Schnell A,
et al: CUL5 is required for thalidomide-dependent inhibition of
cellular proliferation. PLoS One. 13:e01967602018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lubbers J, Lewis S, Harper E, Hledin MP,
Marquez GA, Johnson AE, Graves DR and Burnatowska-Hledin MA:
Resveratrol enhances anti-proliferative effect of VACM-1/cul5 in
T47D cancer cells. Cell Biol Toxicol. 27:95–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kipreos ET, Lander LE, Wing JP, He WW and
Hedgecock EM: cul-1 is required for cell cycle exit in C.
elegans and identifies a novel gene family. Cell. 85:829–839.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao F, Sun X, Wang L, Tang S and Yan C:
Downregulation of MicroRNA-145 caused by hepatitis B virus X
protein promotes expression of CUL5 and contributes to pathogenesis
of hepatitis B virus-associated hepatocellular carcinoma. Cell
Physiol Biochem. 37:1547–1559. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu XM, Wang XB, Chen MM, Liu T, Li YX, Jia
WH, Liu M, Li X and Tang H: MicroRNA-19a and −19b regulate cervical
carcinoma cell proliferation and invasion by targeting CUL5. Cancer
Lett. 322:148–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zelenka J, Koncošová M and Ruml T:
Targeting of stress response pathways in the prevention and
treatment of cancer. Biotechnol Adv. 36:583–602. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Northcott JM, Dean IS, Mouw JK and Weaver
VM: Feeling stress: The mechanics of cancer progression and
aggression. Front Cell Dev Biol. 6:172018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang M, Law ME, Castellano RK and Law BK:
The unfolded protein response as a target for anticancer
therapeutics. Crit Rev Oncol Hematol. 127:66–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hetz C and Papa FR: The unfolded protein
response and cell fate control. Mol Cell. 69:169–181. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schaeffer C, Merella S, Pasqualetto E,
Lazarevic D and Rampoldi L: Mutant uromodulin expression leads to
altered homeostasis of the endoplasmic reticulum and activates the
unfolded protein response. PLoS One. 12:e01759702017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lugea A, Gerloff A, Su HY, Xu Z, Go A, Hu
C, French SW, Wilson JS, Apte MV, Waldron RT and Pandol SJ: The
combination of alcohol and cigarette smoke induces endoplasmic
reticulum stress and cell death in pancreatic acinar cells.
Gastroenterology. 153:1674–1686. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen S, Zhang Y and Zhang D: Endoplasmic
reticulum protein 29 (ERp29) confers radioresistance through the
DNA repair gene, O(6)-methylguanine DNA-methyltransferase, in
breast cancer cells. Sci Rep. 5:147232015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yuan LW, Liu DC and Yang ZL: Correlation
of S1P1 and ERp29 expression to progression, metastasis, and poor
prognosis of gallbladder adenocarcinoma. Hepatobiliary Pancreat Dis
Int. 12:189–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang K, Yao H, Yang Z, Li D, Yang L, Zou
Q, Yuan Y and Miao X: Comparison of ILK and ERP29 expressions in
benign and malignant pancreatic lesions and their
clinicopathological significances in pancreatic ductal
adenocarcinomas. Clin Transl Oncol. 18:352–359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nair S, Xu C, Shen G, Hebbar V,
Gopalakrishnan A, Hu R, Jain MR, Liew C, Chan JY and Kong AN:
Toxicogenomics of endoplasmic reticulum stress inducer tunicamycin
in the small intestine and liver of Nrf2 knockout and C57BL/6J
mice. Toxicol Lett. 168:21–39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gan PP, Zhou YY, Zhong MZ, Peng Y, Li L
and Li JH: Endoplasmic reticulum stress promotes autophagy and
apoptosis and reduces chemotherapy resistance in mutant p53 lung
cancer cells. Cell Physiol Biochem. 44:133–151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yam GH, Gaplovska-Kysela K, Zuber C and
Roth J: Sodium 4-phenylbutyrate acts as a chemical chaperone on
misfolded myocilin to rescue cells from endoplasmic reticulum
stress and apoptosis. Invest Ophthalmol Vis Sci. 48:1683–1690.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zeng M, Sang W, Chen S, Chen R, Zhang H,
Xue F, Li Z, Liu Y, Gong Y, Zhang H and Kong X: 4-PBA inhibits
LPS-induced inflammation through regulating ER stress and autophagy
in acute lung injury models. Toxicol Lett. 271:26–37. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ferrari DM, Nguyen Van P, Kratzin HD and
Söling HD: ERp28, a human endoplasmic-reticulum-lumenal protein, is
a member of the protein disulfide isomerase family but lacks a CXXC
thioredoxin-box motif. Eur J Biochem. 255:570–579. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao J, Zhang Y, Wang L, Xia L, Lu M, Zhang
B, Chen Y and He L: Endoplasmic reticulum protein 29 is involved in
endoplasmic reticulum stress in islet beta cells. Mol Med Rep.
13:398–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang YH, Belegu V, Zou Y, Wang F, Qian
BJ, Liu R, Dai P, Zhao W, Gao FB, Wang L, et al: Endoplasmic
reticulum protein 29 protects axotomized neurons from apoptosis and
promotes neuronal regeneration associated with Erk signal. Mol
Neurobiol. 52:522–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ye W, Zhang R, Hu Y, Xu X and Ying K:
Increased expression of endoplasmic reticulum protein 29 in lung
adenocarcinoma is associated with chemosensitivity to gemcitabine.
Anticancer Drugs. 26:612–619. 2015.PubMed/NCBI
|