Introduction
Lung cancer is one of the main causes of
cancer-associated mortality worldwide. The two major
histopathological groups of lung cancer are non-small cell lung
cancer (NSCLC), which accounts for 80–85% of cases, and small-cell
lung cancer (SCLC), which accounts for 15–20% of cases (1,2). SCLC
is an aggressive lung cancer subtype characterized by rapid
expansion and metastasis of cells with neuroendocrine features.
Presently, platinum-based chemotherapy is the first-line treatment
for patients with SCLC; however, patients have a low median
survival time (9–12 months) (3,4), and
experience high toxicity and intolerance. In addition, recurrence
occurs rapidly in the majority of patients with SCLC, and patients
with recurrence have a median survival of only 4–5 months when
treated with further systemic therapy (5). No targeted drugs are currently used as
SCLC treatments. Therefore, novel therapeutic strategies for SCLC
are urgently needed.
Gambogenic acid (GNA) is an active compound isolated
from gamboge, which is a resin exuded from the Garcinia
hanburyi tree (6,7). GNA has been reported to have more
potent anticancer effects and less systemic toxicity than gambogic
acid (GA), another active compound present in gamboge (8–10).
Induction of apoptosis has been characterized as the main molecular
and biochemical effect of GNA in various cancer cell lines and
animal models of carcinogenesis (11–15).
GNA inhibits the proliferation of A549 cells by inducing cell
apoptosis and cell cycle arrest (13). GNA can also cause glycogen synthase
kinase 3β-dependent G1 arrest in lung cancer cells (16). Although several studies have
reported the anticancer activity of GNA in NSCLC (11–17),
whether GNA can exert antitumor effects in SCLC remains
unknown.
In the present study, we aimed to investigate the
effects of GNA on SCLC in vitro and in vivo. Notably,
we found that GNA significantly suppressed the proliferation of
SCLC cells and cell cycle progression. GNA also promoted cell
apoptosis and modulated the expression of apoptosis-related
proteins. The findings indicated that GNA may be a useful
therapeutic reagent for SCLC treatment.
Materials and methods
Reagents
RPMI-1640, fetal bovine serum (FBS) and
penicillin/streptomycin were purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Dimethyl sulfoxide (DMSO) was
obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany), The
Annexin V-FITC Apoptosis Detection kit and the TUNEL Apoptosis
Assay kit were purchased from BD Biosciences (San Jose, CA, USA).
Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). All antibodies were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Cell culture and drug treatment
The human SCLC cell lines, NCI-H446 and NCI-H1688,
were purchased from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). The cells were cultured in RPMI-1640
medium supplemented with 10% FBS, 1×105 µg/ml penicillin
and 1×105 µg/ml streptomycin at 37°C in a humidified
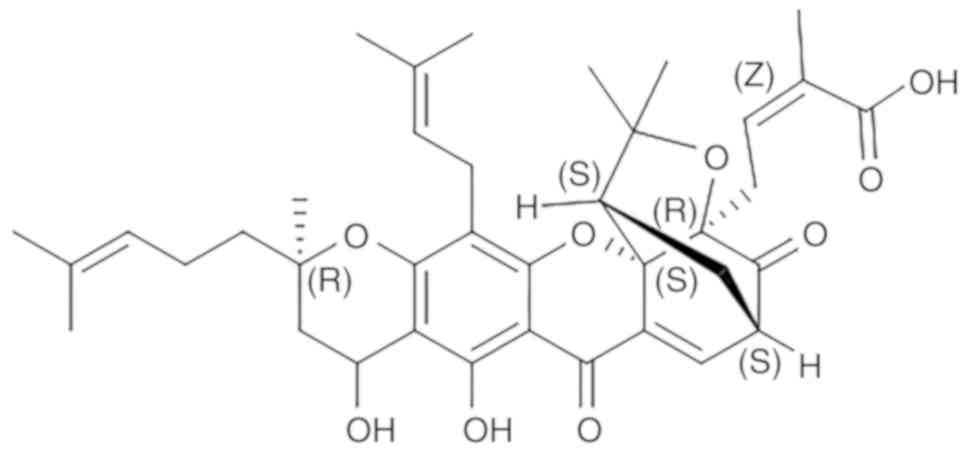
atmosphere containing 5% carbon dioxide. GNA (Fig. 1; purity>98%; Ronghe Medical
Technology, Shanghai, China) was dissolved in DMSO to 100 mM as
stock solution, and the final concentration of DMSO was <0.1
percent and stored at −20°C.
CCK-8 assay
NCI-H446 and NCI-H1688 (4×103) cells were
seeded into 96-well plates, respectively. Then various
concentrations of GNA (NCI-H446: 0, 0.6, 1.0, 1.4, 1.8, 2.2 and 2.4
µM; NCI-H1688: 0, 1.2, 1.6, 2.0, 2.4, 2.8 and 3.2 µM) was added for
24, 48 and 72 h. Cell Counting Kit-8 (CCK-8) reagent was added to
each tested well of the plate after incubation with several
concentrations of GNA for 24, 48 and 72 h, and then incubated for 4
h at 37°C. The optical density at 450 nm was detected using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
A total of three independent experiments were performed.
Cell cycle analysis
SCLC cells were treated with various concentrations
of GNA (NCI-H446: 0, 0.6 and 1.2 µM; NCI-H1688: 0, 1.5 and 2.2 µM)
for 24 h. After 24 h, the cells were harvested and fixed in cold
70% ethanol. The cells were then incubated with propidium iodide
(PI; Cell Cycle kit; BD Biosciences) and then analyzed by flow
cytometry (FACSCalibur; BD Biosciences). Three independent
experiments were performed.
Cell apoptosis assay
SCLC cells were treated with various concentrations
of GNA (NCI-H446: 0, 1.4 and 2.0 µM; NCI-H1688: 0, 2.4 and 3.0 µM)
for 24 h. After 24 h, the cells were trypsinized and washed with
phosphate-buffered saline (PBS). Then, the cells were incubated
with binding buffer containing Annexin V-FITC and PI according to
the manufacturer's protocol (Annexin V-FITC Apoptosis Detection
kit; BD Biosciences) and then analyzed by flow cytometry
(FACSCalibur; BD Biosciences). Three independent experiments were
performed.
Xenograft nude mouse model
Eighteen BALB/c nude male mice at 4–6 weeks-old,
with body weight of 18–22 g, were purchased from the Vital River
Laboratory Animal Technology Co., Ltd. (Beijing, China) and
randomly divided into three groups. Each group consisted of six
mice. The mice were raised in specific pathogen-free environment,
with 12-h light/dark cycles and free access to standard rodent food
and water. NCI-H446 cells (1×106) were suspended in 100
µl PBS and injected subcutaneously in the left armpit of mice. Once
the tumors were established (~1 cm3), the mice were
injected intravenously with different GNA concentrations (4 and 12
mg/kg) or normal saline at 1 p.m. every 2 days. The total injection
time for all mice was 30 min. The weight of the mice and tumor
sizes were recorded 2 days before and after GNA treatment. The
tumor volume was calculated using the formula: length ×
(width2)/2. Careful daily rationing, weighing out how
much food was left and assessing their appetite was performed. When
the mice developed loss of appetite, all of them were intravenously
anesthetized by 1% pentobarbitone sodium (30 mg/kg) and then
sacrificed by decapitation. The maximum tumor diameter was 15 mm
(tumor weight percentage: 9%). Nude mice were sacrificed on the
28th day after the administration, and the tumor body was peeled
and weighed. Concurrently, the heart, liver, spleen, lung and
kidney organs were dissected. After three washes with saline, a
portion of the tumors were immersed in the liquid nitrogen and then
transferred to a −80°C refrigerator for TUNEL detection, and the
remaining portion of the tumor and the organs were placed in 4%
paraformaldehyde solution for subsequent western blotting and
hematoxylin and eosin (H&E) detection. In the experiment, there
were no multiple tumors in nude mice. All of the protocols were
approved by the Animal Ethics Committee of the Medical School,
Southeast University (Nanjing, China).
Western blotting
The expression of apoptosis-related proteins
following treatment with GNA were analyzed by western blotting.
In vitro, NCI-H446 cells were respectively treated with 0,
1.1 and 1.3 µM GNA, and the concentration was 0, 2.0 and 2.3 µM for
NCI-H1688 cells. The dose of the drug was decided at the beginning
of the in vivo xenograft nude mouse model. The cells and
tumor tissue protein were lysed on ice in RIPA buffer (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) containing a protease
inhibitor cocktail (Merck KGaA) and were quantified using a BCA
Assay kit (Thermo Fisher Scientific, Inc.). The proteins lysates
were then separated by 8–12% SDS-PAGE and transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked in 5% non-fat milk and were
incubated overnight at 4°C with diluted (1:1,000) specific primary
antibodies against caspase-3 (cat. no. 9664), −8 (cat. no. 9496)
and caspase-9 (cat. no. 52873), Bax (cat. no. 5023), Bcl-2 (cat.
no. 15071), p53 (cat. no. 2527), poly[ADP-ribose] polymerase 4
(PARP) (cat. no. 5625), β-actin (cat. no. 4970) (Cell Signaling
Technology, Inc.). Subsequently, the membranes were washed with
TBST buffer and were incubated with the appropriate secondary
antibodies (dilution 1:5,000; anti-rabbit IgG: cat. no. 14708;
anti-mouse IgG: cat. no. 58802; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) for 1 h at room temperature. The blots were
detected using ECL reagents (Thermo Fisher Scientific, Inc.).
β-actin and GAPDH were used as loading controls. Three independent
experiments were performed and the ImageJ (version 1.44p analysis
system; NIH; National Institute of Mental Health, Bethesda, MD,
USA) was used to measure the intensity of the bands.
Transferase dUTP nick end-labeling
(TUNEL) analysis
A TUNEL assay was performed by using In Situ
Cell Death Detection kit (BD Biosciences) following the
manufacturer's protocol. Fluorescence emitted from tissue sections
was analyzed, and the images were captured using a fluorescence
microscope (Nikon Corp., Tokyo, Japan).
Histological analysis
The lung, liver, kidney, spleen and heart tissues
from the SCLC xenograft model mice were fixed in 10%
paraformaldehyde and stained with H&E. Histopathological
changes were observed by light microscopy.
Statistical analysis
All the data are presented as the mean ± standard
deviation of three independent experiments. One-way analysis of
variance (ANOVA) followed by Dunnett's test were used to analyze
the data. Differences were considered statistically significant at
P<0.05.
Results
GNA inhibits the proliferation of SCLC
cell lines
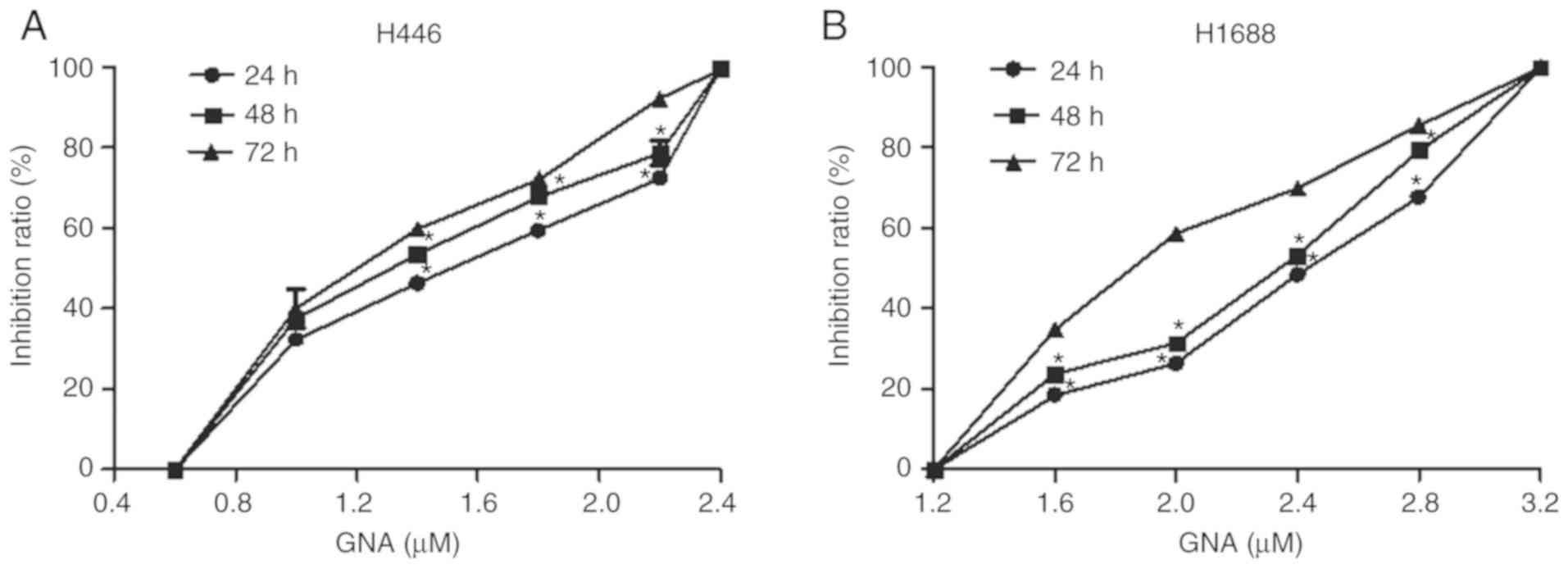
CCK-8 assay results demonstrated that GNA
significantly suppressed the proliferation of NCI-H446 cells at
0.6–2.4 µM in a time- and dose-dependent manner. The
IC50 in NCI-H446 cells was 1.4 µM (Fig. 2A). Additionally, the suppressive
effect of GNA on the proliferation of NCI-H1688 cells was time- and
dose-dependent at 1.2–3.2 µM with an IC50 value of 2.4
µM (Fig. 2B).
Effect of GNA on the cell cycle in
SCLC cells
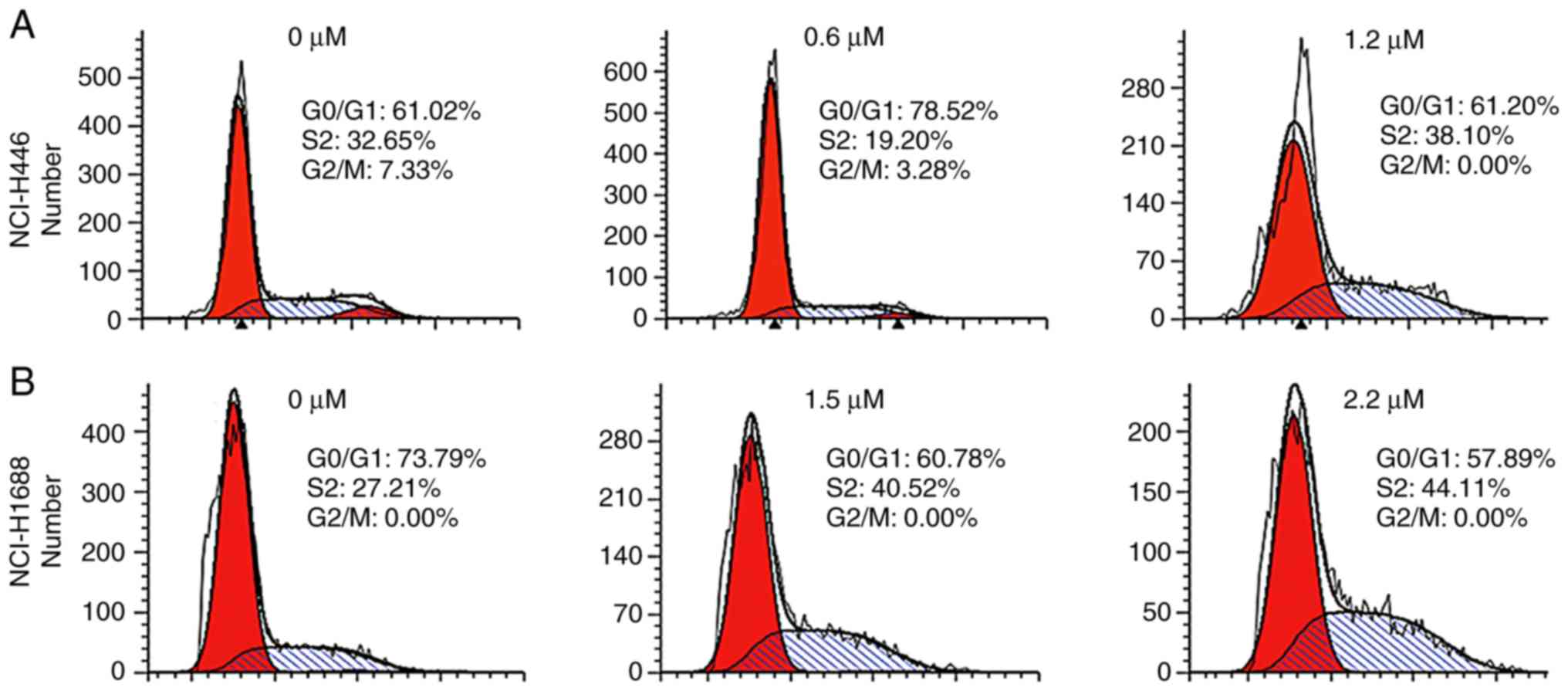
To determine whether the inhibition of SCLC cell
proliferation by GNA was mediated by cell cycle arrest, the cell
cycle phases were examined in NCI-H446 and NCI-H1688 cell lines
that were treated with different concentrations of GNA. For
NCI-H446, the data revealed that low doses of GNA arrested cell
cycle progression in the G0/G1 phase, while higher concentrations
blocked the cycle in the S phase (Fig.
3A). However, the same effect was not observed in NCI-H1688
cells, which was blocked at the S phase in a dose-dependent manner
(Fig. 3B). Therefore, higher
concentrations of GNA inhibited cell cycle progression in SCLC cell
lines by inducing arrest at the S phase. According to ANOVA and
Dunnett's test, significant differences were observed between the
experimental groups and the control group.
GNA promotes apoptosis in SCLC cell
lines
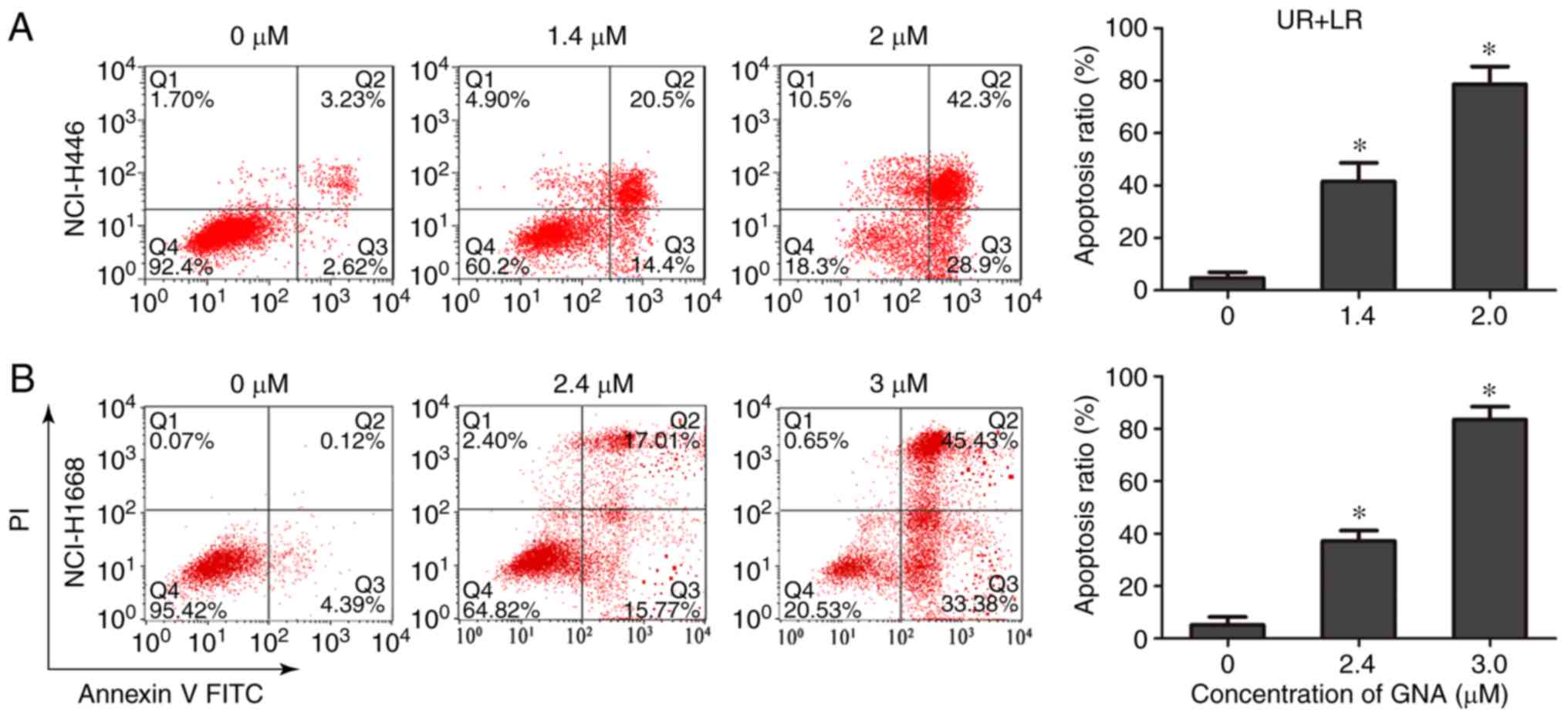
GNA has been previously revealed to inhibit
proliferation and induce apoptosis of A549 cells (11); therefore, experiments were performed
to verify whether GNA had similar effects on NCI-H446 and NCI-H1688
cells. The results demonstrated that GNA increased the rate of cell
apoptosis in a dose-dependent manner (Fig. 4A and B), indicating that GNA induces
apoptosis in SCLC cell lines. According to ANOVA and Dunnett's
test, significant differences were observed between the
experimental groups and the control group.
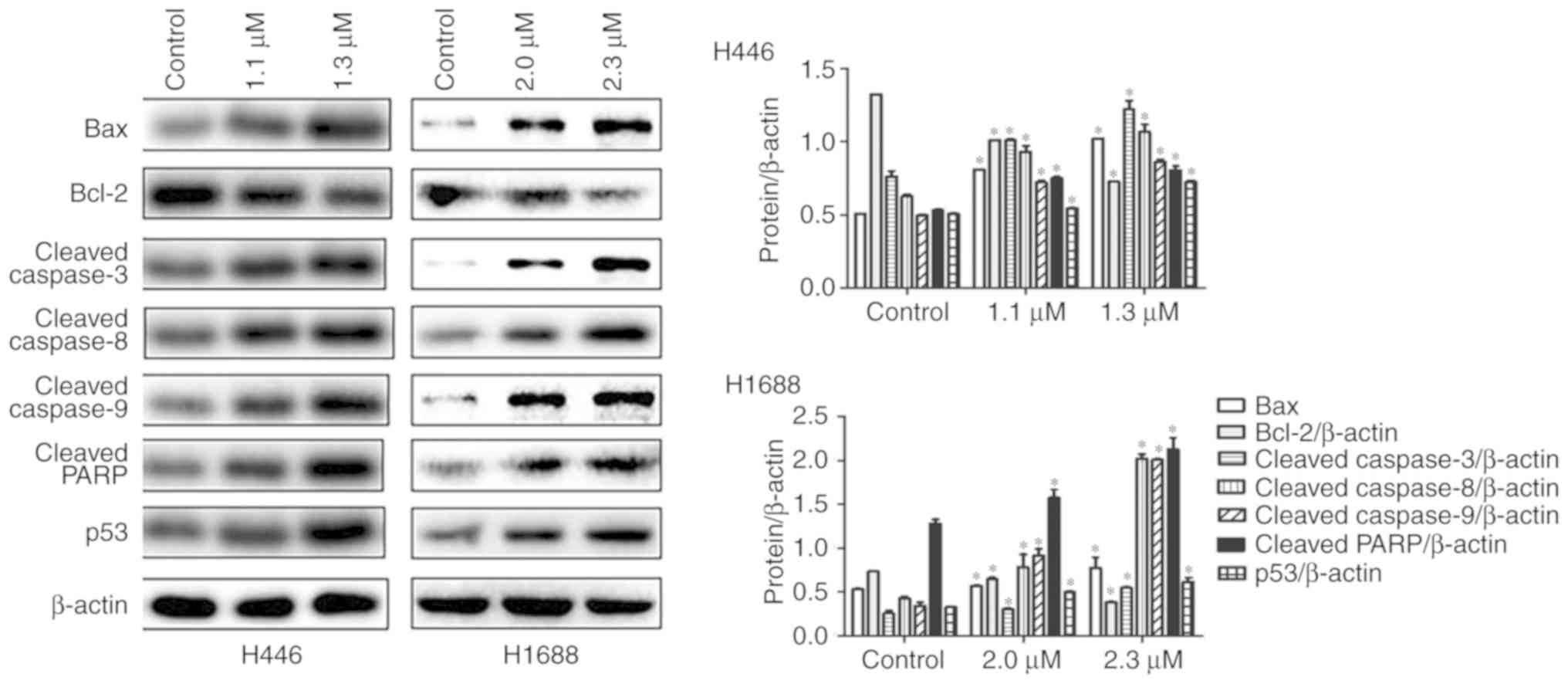
GNA activates apoptotic pathways in
SCLC cells
It was subsequently examined whether GNA-induced
apoptosis of SCLC cells was mediated by activating apoptotic
pathways. After treatment with different concentrations of GNA for
24 h, the expression of apoptosis-related proteins was detected by
western blotting. The expression of cleaved caspase-3, −8 and −9,
Bax, cleaved PARP and p53 proteins were significantly increased in
the GNA treatment groups compared with the control group. However,
Bcl-2 expression was decreased in GNA treatment groups compared
with the control group (Fig. 5).
Therefore, GNA may induce cell apoptosis by increasing the level of
pro-apoptosis proteins and inhibiting the expression of
anti-apoptosis proteins in SCLC cell lines. According to ANOVA and
Dunnett's test, significant differences were observed between the
experimental groups and the control group.
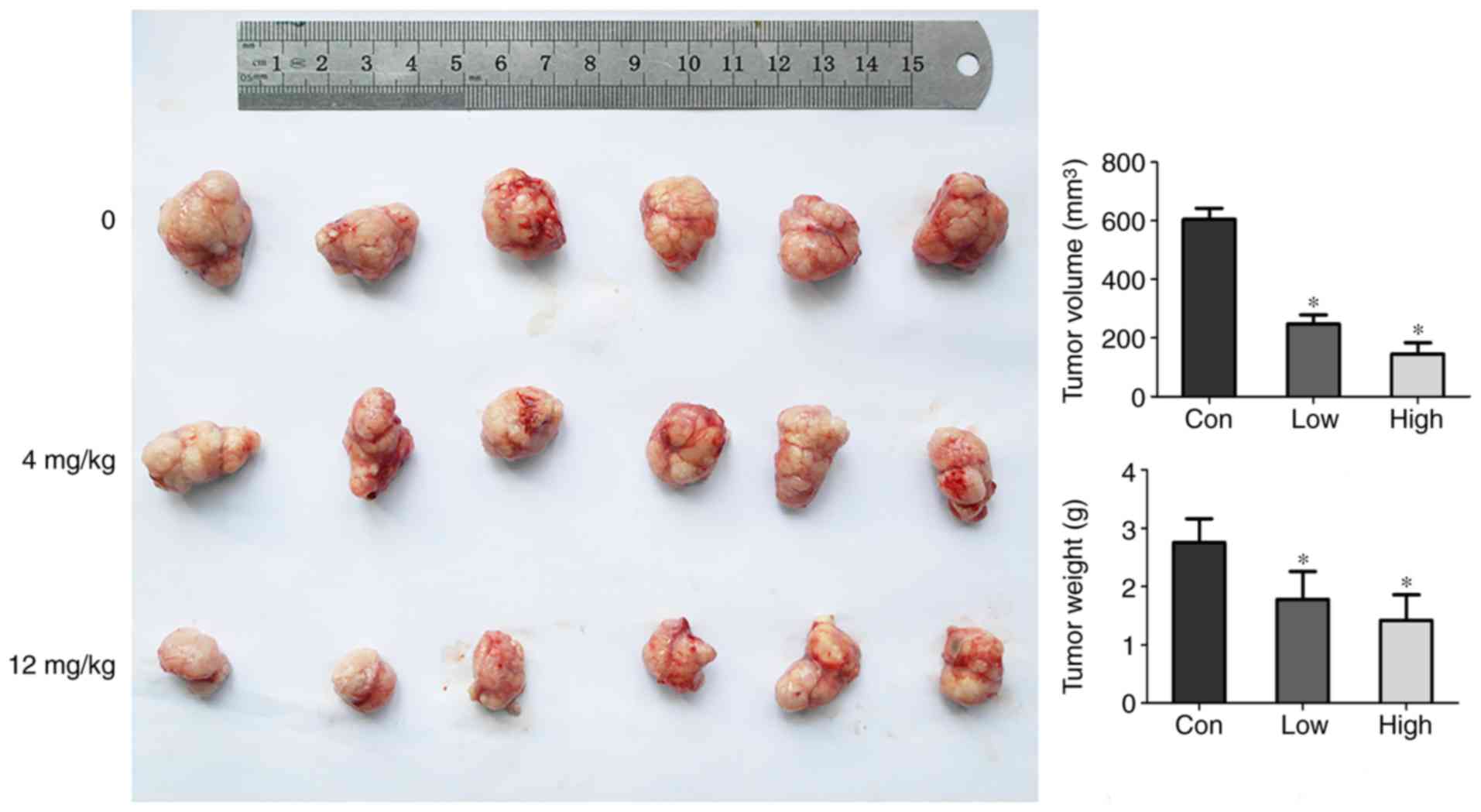
GNA suppresses tumor growth in
vivo
Subsequently, experiments were designed to further
investigate whether GNA inhibited the growth of SCLC tumors in
vivo. GNA-treated mice exhibited tumor tissue loss compared
with the control group (Fig. 6).
According to ANOVA and Dunnett's test, significant differences were
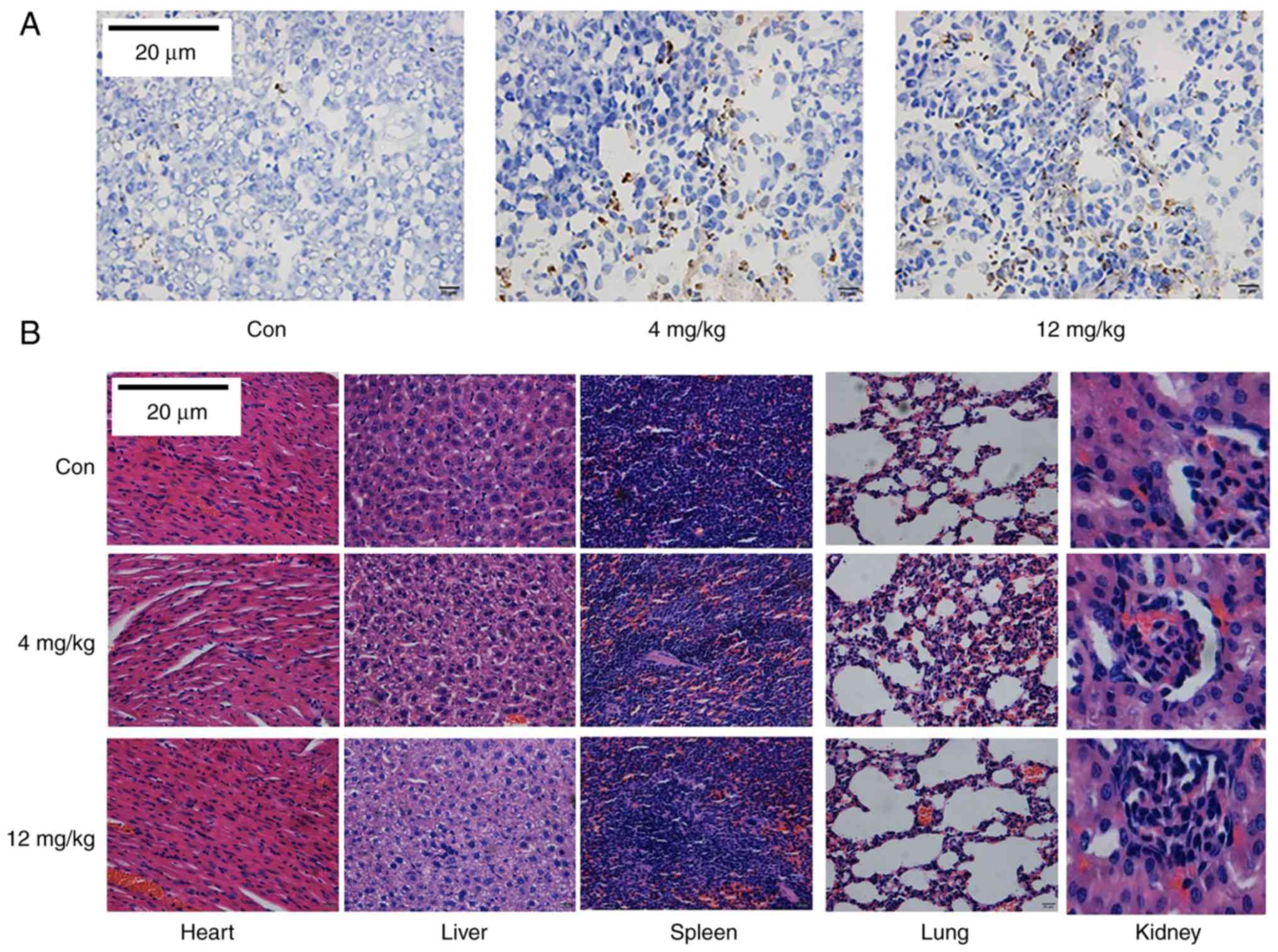
observed between experimental groups and the control group. TUNEL
staining further validated that GNA induced apoptosis in a
dose-dependent manner (Fig. 7A),
which was consistent with the studies performed in vitro.
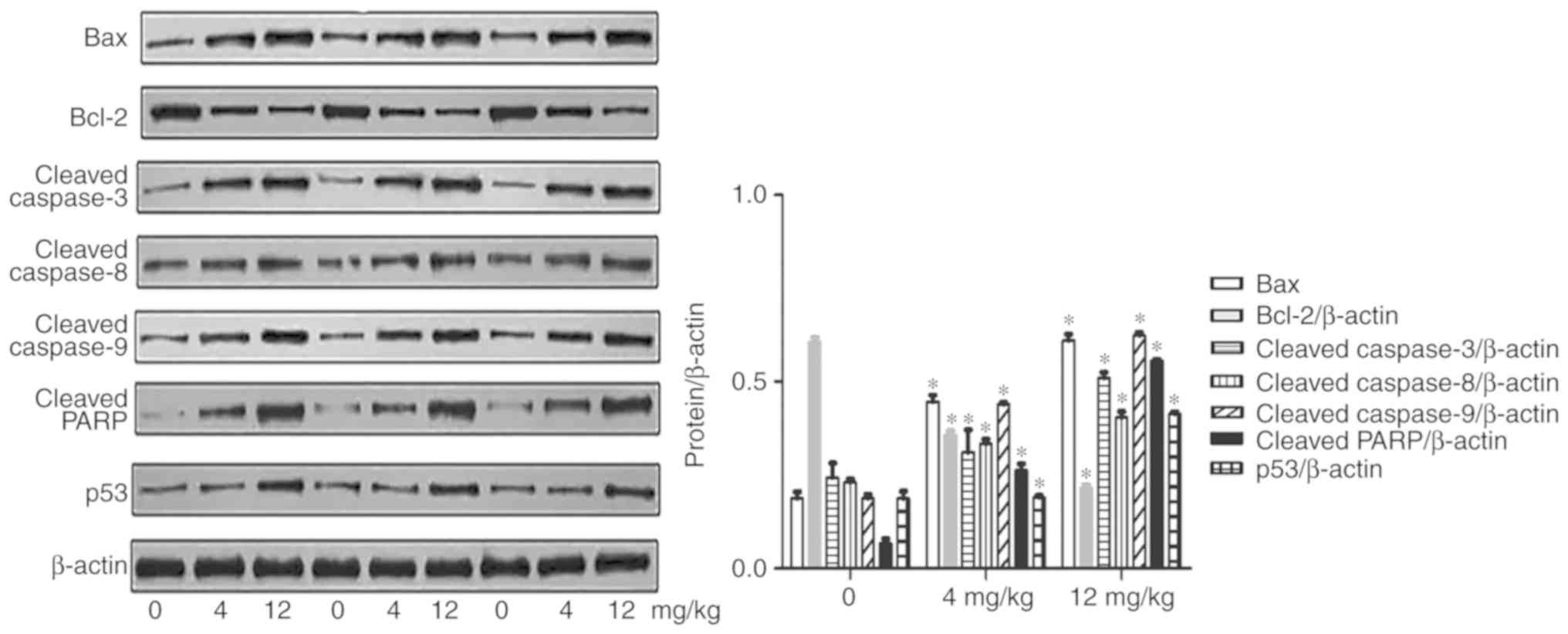
Furthermore, in the in vivo tumor study, the expression of
apoptosis-related proteins exhibited the same trends as observed
in vitro (Fig. 8). The
expression of pro-apoptotic proteins was increased, while the
anti-apoptotic proteins were decreased in the GNA treatment groups
compared with the control group. These findings further
demonstrated that GNA could induce cell apoptosis by activating
apoptosis-related proteins in SCLC cell lines. According to ANOVA
and Dunnett's test, significant differences were observed between
the experimental groups and the control group.
GNA has a low toxicity in vivo
H&E staining of tissues collected from the
xenograft mice was conducted to detect toxicity in vivo. No
apoptotic cell death was observed in the lung, liver, kidney,
spleen or heart tissues from the SCLC xenograft mice (Fig. 7B).
Discussion
The clinical applications of traditional Chinese
medicine in antitumor research has been extensively investigated
(19,20). Among them, GNA has been reported to
exert numerous pharmacological activities, particularly
broad-spectrum anticancer effects (21,18).
In the present study, our results illustrated that GNA inhibited
cell proliferation, induced cell apoptosis and induced cell cycle
arrest in SCLC cells. Furthermore, GNA suppressed tumor growth in a
mouse SCLC xenograft model.
Previous studies have reported that many active
compounds present in traditional Chinese medicines can arrest the
cell cycle in tumor cells, including GNA (14), wogonin (18) and curcumin (22). GNA was previously reported to induce
G1 arrest in non-small cell lung cancer cells (12,13,16)
and breast cancer cells (22). GNA
also induced G2/M phase arrest in multiple myeloma cells (23). Notably, our results revealed that
low doses of GNA arrested cell cycle progression in the G0/G1
phase, whereas a higher concentration caused S-phase arrest of
NCI-H446 SCLC cells. This suggests that the underlying mechanisms
of GNA-induced cell cycle are complex, and further studies are
required to understand this phenomenon.
GNA was revealed to inhibit cell proliferation by
inducing apoptosis in non-small cell lung cancer cells (13,16).
GNA was revealed to induce apoptosis and G0/G1 phase arrest of A549
cells in a dose- and time-dependent manner in vitro.
Apoptosis is a programmed cell death mechanism and is the most
common form of cell death (24,25).
In the present study, we observed that cleaved caspase-3, −8, −9,
Bax, cleaved PARP and p53 protein levels were increased by GNA
treatment, whereas Bcl-2 expression was decreased. Our findings
demonstrated that GNA promoted the apoptosis of SCLC cells by
upregulating pro-apoptotic mediators in vitro and in
vivo. It is important to highlight that no toxicity was
observed following the treatment of nude mice with GNA, which was
confirmed by H&E staining of the mouse lung, liver, tumor,
kidney, spleen and heart tissues (26).
The present study has a number of limitations. The
exact mechanism by which GNA induces cell cycle arrest and
apoptosis remains unknown (16,18,27).
Given the important effects of GNA on inhibiting the proliferation
of SCLC cells, the effective concentrations and potential mechanism
of GNA in SCLC cells remain to be investigated in future
studies.
In conclusion, the findings, based on the
experiments in vivo and in vitro, demonstrated that
GNA could inhibit cell proliferation, induce cell apoptosis and
cell cycle arrest in two SCLC cell lines. In addition, the growth
of SCLC xenograft tumors was also suppressed by GNA. Furthermore,
GNA modulated the levels of apoptosis-related proteins and promoted
the apoptosis of SCLC cells in vitro and in vivo, and
exhibited a relatively low toxicity in mice, which was clearly
different from GA (26). In
conclusion, GNA may be a potential candidate for treatment of
SCLC.
Acknowledgements
The present study was supported by the Innovation
Program for College Graduates of Jiangsu Province, People's
Republic of China (no. SJCX17-0058).
Funding
No funding was received.
Availability of data and materials
The datasets used or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TH and HZ acquired the data and created a draft of
the manuscript; XW, LX and JJ prepared the experimental materials
and performed the in vitro assays; XZ, TH and HZ processed
the interpreted data, performed the statistical analysis and
analyzed the results; XZ, HZ and TH revised and approved the final
version of the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All the animal experiments were conducted under
protocols approved by the Animal Ethics Committee of the Medical
School, Southeast University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Xiaoli Zhu, MD, Chair, Department of Pulmonary
Medicine, Zhongda Hospital, Medical School, Southeast University,
China. She has published >10 SCI-indexed papers in some renowned
journals, such as FEBS Journal and presided over a number of
provincial subjects. In addition, she has published several
influential studies and has held a variety of academic posts.
References
|
1
|
van Meerbeeck JP, Fennell DA and De
Ruysscher DK: Small-cell lung cancer. Lancet. 378:1741–1755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heist RS and Engelman JA: SnapShot:
Non-small cell lung cancer. Cancer Cell. 21:448e442. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noda K, Nishiwaki Y, Kawahara M, Negoro S,
Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, et
al: Irinotecan plus cisplatin compared with etoposide plus
cisplatin for extensive small-cell lung cancer. N Engl J Med.
346:85–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lara PN Jr, Natale R, Crowley J, Lenz HJ,
Redman MW, Carleton JE, Jett J, Langer CJ, Kuebler JP, Dakhil SR,
et al: Phase III trial of irinotecan/cisplatin compared with
etoposide/cisplatin in extensive-stage small-cell lung cancer:
Clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol.
27:2530–2535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Network NCC: NCCN Clinical Practice
Guidelines in Oncology (NCCN Guidelines®). 2018.
|
|
6
|
Asano J, Chiba K, Tada M and Yoshii T:
Cytotoxic xanthones from Garcinia hanburyi. Phytochemistry.
41:815–820. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song JZ, Yip YK, Han QB, Qiao CF and Xu
HX: Rapid determination of polyprenylated xanthones in gamboge
resin of Garcinia hanburyi by HPLC. J Sep Sci. 30:304–309.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin T, Fang Q, Peng D, Huang X, Zhu T, Luo
Q, Zhou K and Chen W: PEGylated non-ionic surfactant vesicles as
drug delivery systems for gambogenic acid. Drug Deliv. 20:277–284.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen HB, Zhou LZ, Mei L, Shi XJ, Wang XS,
Li QL and Huang L: Gambogenic acid-induced time- and dose-dependent
growth inhibition and apoptosis involving Akt pathway inactivation
in U251 glioblastoma cells. J Nat Med. 66:62–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen F, Zhang XH, Hu XD, Zhang W, Lou ZC,
Xie LH, Liu PD and Zhang HQ: Enhancement of radiotherapy by ceria
nanoparticles modified with neogambogic acid in breast cancer
cells. Int J Nanomedicine. 10:4957–4969. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang L, Wang M, Cheng H and Li Q:
Gambogenic acid inhibits proliferation of A549 cells through
apoptosis-inducing. Zhongguo Zhong Yao Za Zhi. 36:1217–1221.
2011.(In Chinese). PubMed/NCBI
|
|
12
|
Cheng H, Su JJ, Peng JY, Wang M, Wang XC,
Yan FG, Wang XS and Li QL: Gambogenic acid inhibits proliferation
of A549 cells through apoptosis inducing through up-regulation of
the p38 MAPK cascade. J Asian Nat Prod Res. 13:993–1002. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Q, Cheng H, Zhu G, Yang L, Zhou A, Wang
X, Fang N, Xia L, Su J, Wang M, et al: Gambogenic acid inhibits
proliferation of A549 cells through apoptosis-inducing and cell
cycle arresting. Biol Pharm Bull. 33:415–420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan F, Wang M, Li J, Cheng H, Su J, Wang
X, Wu H, Xia L, Li X, Chang HC, et al: Gambogenic acid induced
mitochondrial-dependent apoptosis and referred to phospho-Erk1/2
and phospho-p38 MAPK in human hepatoma HepG2 cells. Environ Toxicol
Pharmacol. 33:181–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan F, Wang M, Chen H, Su J, Wang X, Wang
F, Xia L and Li Q: Gambogenic acid mediated apoptosis through the
mitochondrial oxidative stress and inactivation of Akt signaling
pathway in human nasopharyngeal carcinoma CNE-1 cells. Eur J
Pharmacol. 652:23–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu XJ, Han QB, Wen ZS, Ma L, Gao J and
Zhou GB: Gambogenic acid induces G1 arrest via GSK3β-dependent
cyclin D1 degradation and triggers autophagy in lung cancer cells.
Cancer Lett. 322:185–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng C, Sun M, Su J and Li Q: Effect of
gambogenic acid on inducing A549 cell apoptosis through regulating
Ras/Raf/Erk signaling pathway. Traditional Chin Drug Res Clin
Plarmacol. 27:189–193. 2016.
|
|
18
|
Su J, Cheng H, Zhang D, Wang M, Xie C, Hu
Y, Chang HC and Li Q: Synergistic effects of 5-fluorouracil and
gambogenic acid on A549 cells: Activation of cell death caused by
apoptotic and necroptotic mechanisms via the ROS-mitochondria
pathway. Biol Pharm Bull. 37:1259–1268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu W, Guo QL, You QD, Zhao L, Gu HY and
Yuan ST: Anticancer effect and apoptosis induction of gambogic acid
in human gastric cancer line BGC-823. World J Gastroenterol.
11:3655–3659. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yi T, Yi Z, Cho SG, Luo J, Pandey MK,
Aggarwal BB and Liu M: Gambogic acid inhibits angiogenesis and
prostate tumor growth by suppressing vascular endothelial growth
factor receptor 2 signaling. Cancer Res. 68:1843–1850. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang T, Wei J, Qian X, Ding Y, Yu L and
Liu B: Gambogic acid, a potent inhibitor of survivin, reverses
docetaxel resistance in gastric cancer cells. Cancer Lett.
262:214–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang K, Tang Y, Sun M, Lu B, Zhu H, Ji O
and Shen Q: The mechanism of neogambogic acid-induced apoptosis in
human MCF-7 cells. Acta Biochim Biophys Sin. 43:698–702. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen R, Zhang H, Liu P, Wu X and Chen B:
Gambogenic acid synergistically potentiates bortezomib-induced
apoptosis in multiple myeloma. J Cancer. 8:839–851. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jing G, Wang JJ and Zhang SX: ER stress
and apoptosis: A new mechanism for retinal cell death. Exp Diabetes
Res 2012. 5895892012.
|
|
26
|
Liu N, Huang H, Liu S, Li X, Yang C, Dou
QP and Liu J: Calcium channel blocker verapamil accelerates
gambogic acid-induced cytotoxicity via enhancing proteasome
inhibition and ROS generation. Toxicol In Vitro. 28:419–425. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li F, Wang Y and Yan Y: Gambogenic acid
induces cell growth inhibition, cell cycle arrest and metastasis
inhibition in choroidal melanoma in a dose-dependent manner. Exp
Ther Med. 13:2456–2462. 2017. View Article : Google Scholar : PubMed/NCBI
|