Introduction
It has been well established that colorectal cancer
(CRC) is one of the most common types of cancer. Among all types of
malignant tumor, the morbidity and mortality rates of CRC are
ranked third overall (1). In China,
the incidence of CRC has been increasing steadily by ≤4.2% per
year, and it is now the second most common cause of
cancer-associated mortality (2).
The majority of patients with CRC undergo surgical resection;
however, recurrence occurs in 20–25% patients following a
‘curative’ operation (3). Multiple
gene alterations, including the inactivation of tumor suppressor
genes and the activation of oncogenes, are associated with the
progression of the normal colonic epithelium into adenoma and
malignant adenocarcinoma (4).
However, there is limited knowledge of the molecular alterations
that occur during the metastasis of CRC (5,6).
Therefore, identification of the relevant genes and their molecular
pathways is required to provide novel targets for the treatment of
metastatic CRC. In recent years, microRNAs (miRNAs) have been
demonstrated to be involved in the development of cancer.
Anoctamin 1 (ANO1) is located on human chromosome
11q13 and contains 26 exons, and encodes a 960-amino acid protein
with 8 transmembrane domains. ANO1 is also referred to as TMEM16A,
ORAOV2, TAOS2, DOG1 or FLJ10261. It is associated with the activity
of calcium-dependent chloride channels expressed on the plasma
membranes of secretory epithelia, smooth muscles and sensory
neurons (7–12). ANO1 is also involved in a variety of
biological functions, including cell proliferation, movement and
attachment (13,14). In a large subset of head and neck
squamous cell carcinoma cases, ANO1 is amplified and highly
expressed (13), and the same is
true for other types of cancer, including breast cancer (15), prostate carcinoma (14), glioblastoma (16), gastrointestinal stromal tumor
(17) and esophageal squamous cell
carcinoma (18). Certain studies
have suggested that ANO1 may be a diagnostic and prognostic
biomarker and a therapeutic target for various types of cancer
(14,19). However, in human malignancies, the
clinical implications of ANO1 remain to be elucidated.
miRNAs are a family of small noncoding RNA
molecules, 21–25 nucleotides long. By targeting mRNAs, miRNAs are
able to control the expression of ~30% of protein-coding genes by
inhibiting their translation (20,21).
miR-144 is enriched in the brain, and exists in normal and
malignant hematopoietic cells (22). Previous reports have demonstrated
that miR-144 serves a role in the regulation of cell proliferation
(23) and apoptosis (24), in addition to the progression of
various types of human cancer, including nasopharyngeal carcinoma
(25), CRC (26), follicular thyroid carcinoma
(19) and pancreatic cancer
(27). In addition, miR-144 has
been demonstrated to target signaling pathways, including the
protein kinase C, Wnt/β-catenin and phosphatase and tensin homolog
(PTEN) pathways (28).
miRanda and TargetScan were first used to identify
miR-144 as an ANO1-targeting miRNA in CRC. Research is limited
regarding the function of miR-144 in CRC by targeting ANO1.
Therefore, the present study aimed to analyze miR-144 expression by
targeting ANO1 in human CRC tissue specimens.
Materials and methods
Materials
A total of 122 tissue samples from patients with CRC
between April 2005 and April 2009 were collected and formalin-fixed
for 48 h at room temperature, and paraffin-embedded. Each patient
underwent surgical resection at The Second Affiliated Hospital of
Nantong University (Nantong, China). Patient characteristics are
presented in Table I. No patients
had received prior radiotherapy or chemotherapy. CRC tumor and
adjacent non-cancerous tissue samples were obtained randomly from
26 patients with CRC. The fresh samples were frozen at −80°C until
required for western blot analysis. Clinicopathological data,
including sex, age, tumor size, tumor-node-metastasis (TNM) stage
and lymph node involvement, were retrospectively analyzed. Staging
and grading of the CRC were performed according to the World Health
Organization classification and the Union for International Cancer
Control (29). Survival time was
defined as the time between surgery and the time of the last
follow-up day, or patient mortality. The present study was
conducted with the approval of the institutional ethics board of
The Second Affiliated Hospital of Nantong University (no.
2006-K013) and the patients with CRC included in the present study
had provided written informed consent.
 | Table I.Association between ANO1 expression
in colorectal cancer tissues and clinical parameters. |
Table I.
Association between ANO1 expression
in colorectal cancer tissues and clinical parameters.
|
|
| ANO1
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinical
parameters | No. of patients
(n=122) | Low level
(n=44) | High level
(n=78) | χ2 | P-value |
|---|
| Sex |
|
|
| 3.303 | 0.069 |
|
Male | 66 | 19 | 47 |
|
|
|
Female | 56 | 25 | 31 |
|
|
| Age, years |
|
|
| 0.458 | 0.499 |
|
≤50 | 35 | 11 | 24 |
|
|
|
>50 | 87 | 33 | 54 |
|
|
| Tumor size, cm |
|
|
| 0.120 | 0.729 |
| ≤5 | 64 | 24 | 40 |
|
|
|
>5 | 58 | 20 | 38 |
|
|
| Tumor location |
|
|
| 0.647 | 0.421 |
|
Colon | 69 | 27 | 42 |
|
|
|
Rectum | 53 | 17 | 36 |
|
|
| Gross type |
|
|
| 1.473 | 0.479 |
|
Massive | 48 | 16 | 32 |
|
|
|
Ulcerative | 53 | 18 | 35 |
|
|
|
Infiltrating type | 21 | 10 | 11 |
|
|
| Histological
differentiation |
|
|
| 26.202 |
<0.001a |
|
Well/moderate | 91 | 21 | 70 |
|
|
|
Poor | 31 | 23 | 8 |
|
|
| Lymph node
metastasis |
|
|
| 1.396 | 0.237 |
|
Negative | 75 | 24 | 51 |
|
|
|
Positive | 47 | 20 | 27 |
|
|
| Invasive depth |
|
|
| 0.054 | 0.817 |
|
T1/T2 | 51 | 19 | 32 |
|
|
|
T3/T4 | 71 | 25 | 46 |
|
|
| TNM stage |
|
|
| 8.816 | 0.003a |
|
I/II | 67 | 32 | 35 |
|
|
|
III/IV | 55 | 12 | 43 |
|
|
Immunohistochemistry
Immunohistochemical staining was performed on 4-µm
thick sections. Tissues were de-waxed in xylene and rehydrated in
alcohol in a descending series, and endogenous peroxidase activity
was suppressed using 3% hydrogen peroxide for 10 min at room
temperature. Antigen retrieval was performed by treatment at 100°C
in 0.01 mol/l sodium citrate buffer (pH 6.0) for 10 min. The
sections were then rinsed with PBS twice for 5 min, and
non-specific binding was blocked using 10% normal goat serum
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 1 h at room
temperature. Sections were incubated at 4°C overnight with a
polyclonal rabbit anti-human ANO1 antibody (1:100; cat. no.
sc-377115; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) in PBS.
Following three 10-min washes in PBS, the sections were incubated
with IRDye® 800CW-conjugated goat anti-rabbit secondary
antibody (1:5,000; cat. no. 925-32211; Rockland Immunochemicals,
Inc., Limerick, PA, USA) for 1 h at 37°C. Following washing in PBS
three times, diaminobenzidine solution was used to develop the
visualization signal. Following hematoxylin counterstaining at room
temperature for 5 min, the sections were dehydrated and mounted.
All slides were examined using a light microscope (Leica
Microsystems GmbH, Wetzlar, Germany) and the results of the
immunohistochemical staining were assessed separately by three
investigators, considering staining frequency as follows: No
staining, 0; 1–25% of cells stained, 1; 25–50% of cells stained, 2;
51–75% of cells stained, 3; and >75% of cells stained, 4.
Staining intensity was rated on a scale of 0–12: 0, negative
staining (−); 1–4, weak staining (+); 5–8, moderate staining (++);
and 9–12, strong staining (+++).
Western blotting
Radioimmunoprecipitation assay lysis buffer
containing protease inhibitors (Promega Corporation, Madison, WI,
USA) was used to extract the total protein from the tissues. Equal
amounts (30 µg) of protein were examined using the DC protein assay
method (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
separated by SDS-PAGE on a 10% gel, and transferred onto a
polyvinylidene fluoride membrane. The membranes were blocked in 5%
non-fat dry milk in Tween-20 TBS (TBST) buffer (50 mM Tris-HCl, 100
mM NaCl, and 0.1% Tween-20; pH 7.4) at room temperature for 1 h.
The membrane was incubated with a polyclonal rabbit anti-human ANO1
antibody (cat. no. sc-377115; 1:500; Santa Cruz Biotechnology,
Inc.) at 4°C overnight. The other specific primary antibodies used
against each protein in the immunoblotting were as follows:
Anti-EGFR (cat. no. sc-71034), anti-p-EGFR (cat. no. sc-81487),
anti-ERK1/2 (cat. no. sc-81457) and anti-p-ERK1/2 (cat. no.
sc-7976) and anti-β-actin (cat. no. sc-8432) (all 1:1,000; Santa
Cruz Biotechnology, Inc., USA). Following three washes in TBST for
5 min, the membranes were incubated with a horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no.
A6154; 1:1,000; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at
room temperature for 2 h. An Odyssey infrared imaging system
(LI-COR Biosciences, Lincoln, NE, USA) was used to scan the
membrane, and PDQuest software (version, 7.2.0; Bio-Rad
Laboratories, Inc.) was used to analyze the protein bands.
Cell culture and transfection
The normal intestinal epithelial cell line FHC, and
the human colorectal carcinoma cell lines Caco-2, SW620, HCT116,
SW480 and LoVo were purchased from the Cell Bank of the Chinese
Academy of Sciences (Shanghai, China). The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.), except SW480 and SW620
cells, which were cultured in Leibovitz's L-15 Medium (Gibco;
Thermo Fisher Scientific, Inc.) with 10% FBS, at 37°C in a 5%
CO2 atmosphere in a humidified incubator. miR-144 mimics
(miR-144), miR-144 negative control (miR-NC) and the miR-144
inhibitor were designed and synthesized by Shanghai GenePharma Co.,
Ltd. (Shanghai, China). The sequences were as follows: miR-144
mimic sense, 5′-UACAGUAUAGAUGAUGUACU-3′; miR-NC sense, and
5′-UUCUCCGAACGUGUCACGUTT-3′; miR-144 inhibitor,
5′-AGUACAUCAUCUAUACUGUA-3′. The sequences used for ANO1 siRNA were
as follows: ANO1 siRNA forward, 5′-UUUAUUUAGAUGAAUGUCCAG-3′ and
reverse, 5′-GGACAUUCAUCUAAAUAAAUU-3′; control siRNA forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′. SW480 cells (1×106) were
cultured in 6-well plates, and the cell density reached 40–60%
confluence after 24 h. Oligonucleotides (50 nmol) were transfected
into cells using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Following transfection for 48 h, the cells were used for further
cellular function analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissue samples and
cells using the RNeasy RNA Mini kit (Qiagen GmbH, Hilden, Germany).
To detect the expression of mature miR-144, cDNAs were synthesized
using a TaqMan MicroRNA Reverse Transcription kit at a reaction
temperature of 42°C (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and quantified by qPCR using a TaqMan human microRNA assay
kit (Qiagen), according to the manufacturer's protocol. qPCR was
performed for amplification under the following thermocycling
conditions: 95°C for 10 min, followed by 40 cycles at 95°C for 15
sec and 60°C for 60 sec. The relative expression ratio of miR-144
was presented as the fold-change normalized to a U6 endogenous
reference in the normal cell line and was calculated by the
2−ΔΔCt method (30).
Luciferase reporter assay
The ANO1 3′ untranslated regions (UTRs) containing
the wild-type (WT) or mutant (MT) miR-144 binding site were
amplified and cloned into a pGL3-basic vector (Promega
Corporation), and termed ANO1-WT-3′UTR and ANO1-MT-3′UTR,
respectively. The cells were seeded into 24-well plates and
co-transfected with 50 nmol/l pcDNA-miR-144 or the negative control
(NC) using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.). Renilla luciferase activity was used as
an internal control. Following incubation for 24 h, the luciferase
assay was tested using a dual-luciferase reporter assay system
(Promega Corporation), according to the manufacturer's
protocol.
Migration and invasion assays
Transwell chamber plates (24-well) were used to
evaluate cell migration and invasion. For the migration assay,
5×104 cells were suspended in 200 ml serum-free DMEM and
seeded into the upper chamber of each insert. Next, 800 µl DMEM
containing 10% FBS was added to the lower chambers. For the
invasion assay, 40–80 µl Matrigel (BD Biosciences, Franklin Lakes,
NJ, USA) diluted with DMEM was used to coat the chambers and
incubated for 2–4 h at 37°C. A total of 1×105 cells were
suspended in 200 µl DMEM and seeded in the upper chambers, and 800
µl DMEM containing 10% FBS was added to the lower chambers. The
plates were incubated at room temperature for 24 h, and the
migratory and invasive cells were fixed and stained in a 20%
methanol and 0.2% crystal violet solution for 30 min at 37°C. The
numbers of migratory or invasive cells were counted in three fields
in each well under a light microscope at ×200 magnification.
Statistical analysis
The SPSS software package (version 17.0; SPSS, Inc.,
Chicago, IL, USA) was used to perform the statistical analysis. The
miRNA target predicting algorithms TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org/microrna/home.do) were used to
predict potential targets of miR-144. The paired data were analyzed
using a paired Student's t-test, while comparisons between unpaired
groups were performed using an unpaired Student's t-test. The
association between ANO1 expression and clinicopathological
features was analyzed using χ2 test. In addition,
one-way analysis of variance with Tukey's post hoc test was used to
analyze the data differences between groups. The Kaplan-Meier
method was used to calculate overall survival curves, which were
analyzed using the log-rank test. The Cox proportional hazards
regression model was used to perform univariate and multivariate
analysis of several prognostic factors. The results are
representative of at least three independent experiments. Data are
presented as the mean ± standard deviation from three independent
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
Downregulation of miR-144 in CRC cell
lines and tissues
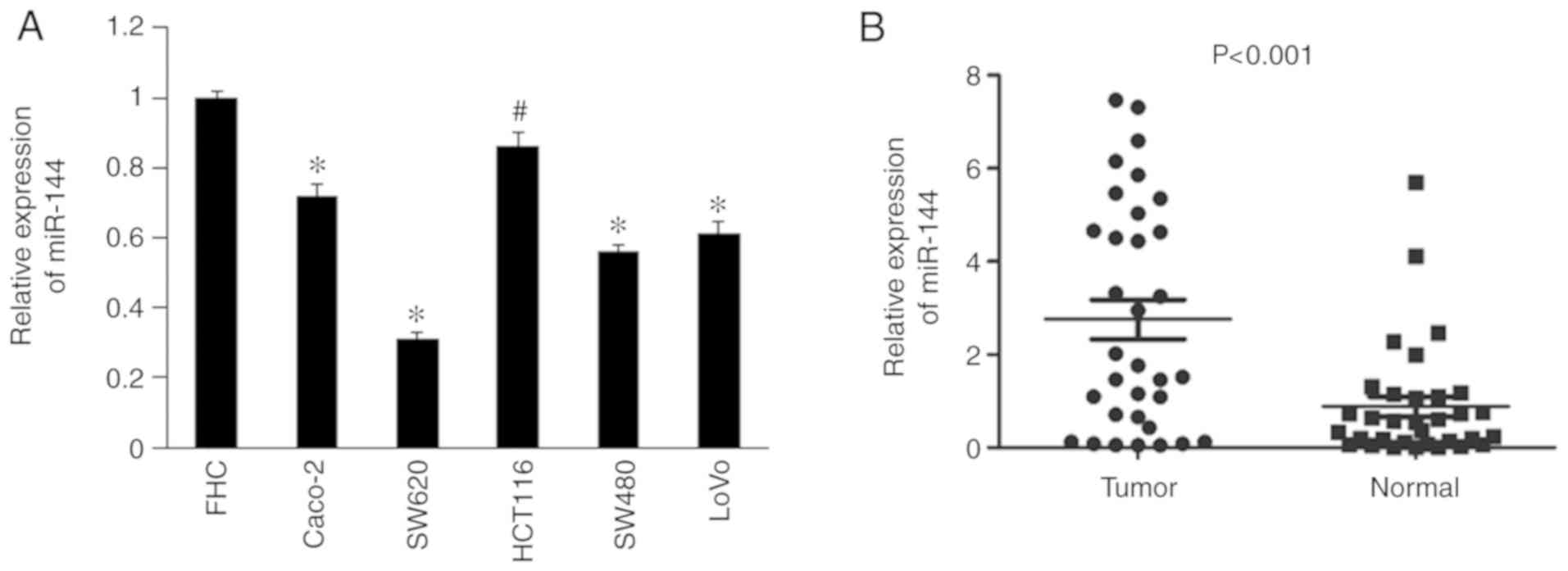
According to the RT-qPCR results, miR-144 levels
were significantly lower in the CRC cell lines, Caco-2, SW620,
HCT116, SW480 and LoVo, compared with normal FHC cells (P<0.05;
Fig. 1A). As presented in Fig. 1B, it was demonstrated that miR-144
expression was significantly decreased in tumor tissues compared
with paracancerous tissues (n=33; P<0.001).
miR-144 inhibits cell migration and
invasion in vitro
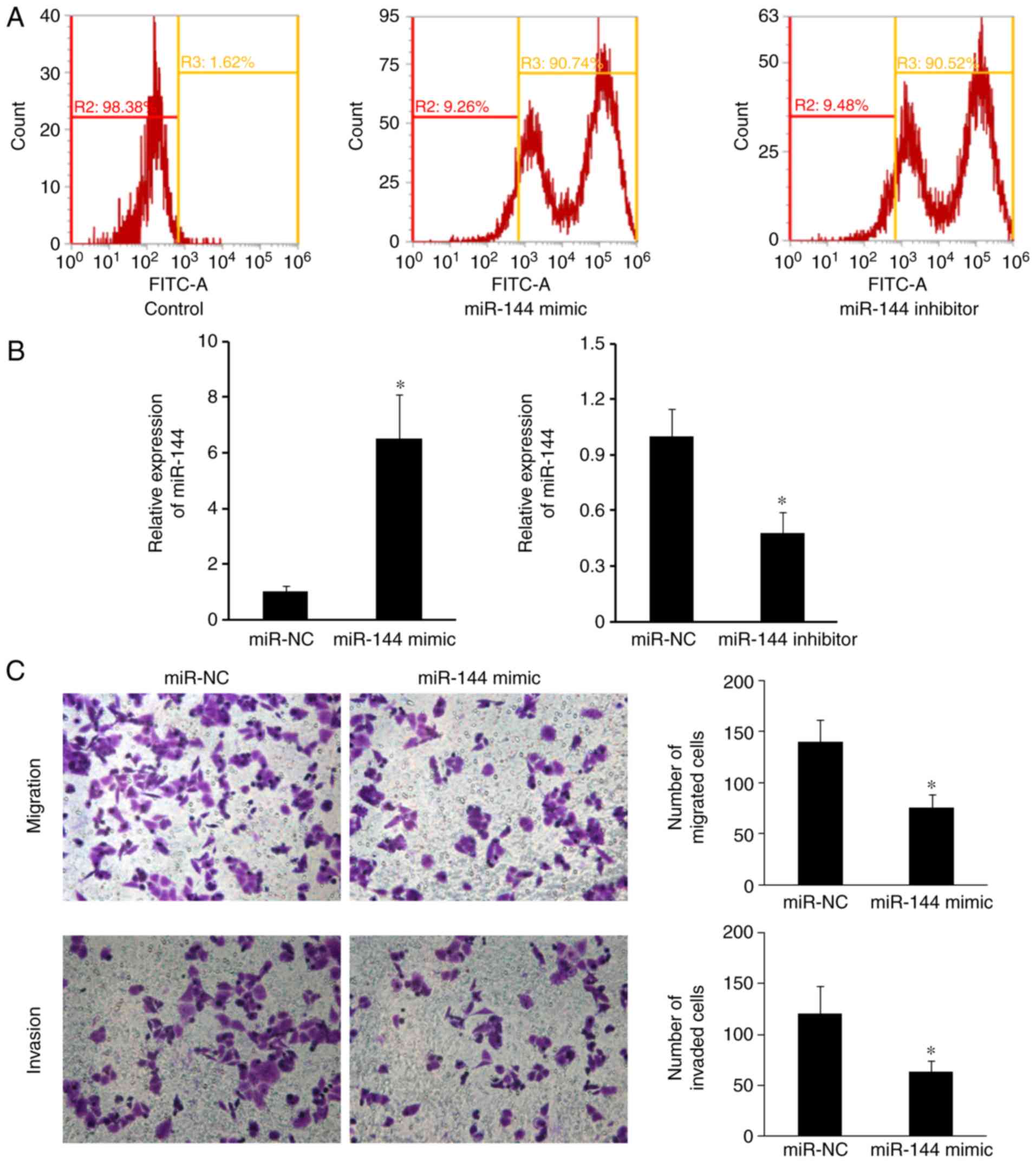
The transfection efficiency was confirmed by flow
cytometry. Flow cytometric analysis confirmed that the transfection
achieved ~90% efficiency (Fig. 2A).
The relative miR-144 expression levels were determined by RT-qPCR
following miR-144 mimic overexpression in SW480 cells. miR-144 mRNA
expression levels were decreased following miR-144 inhibition in
SW480 cells (Fig. 2B). To determine
the effects of miR-144 on the migration and invasion of CRC cells,
SW480 cells transfected with either miR-144 mimic or miR-144
inhibitor were subjected to Transwell assays. The migratory and
invasive abilities of SW480 cells transfected with miR-144 mimic
were reduced compared with the miR-NC group (Fig. 2C; P<0.05). Downregulated
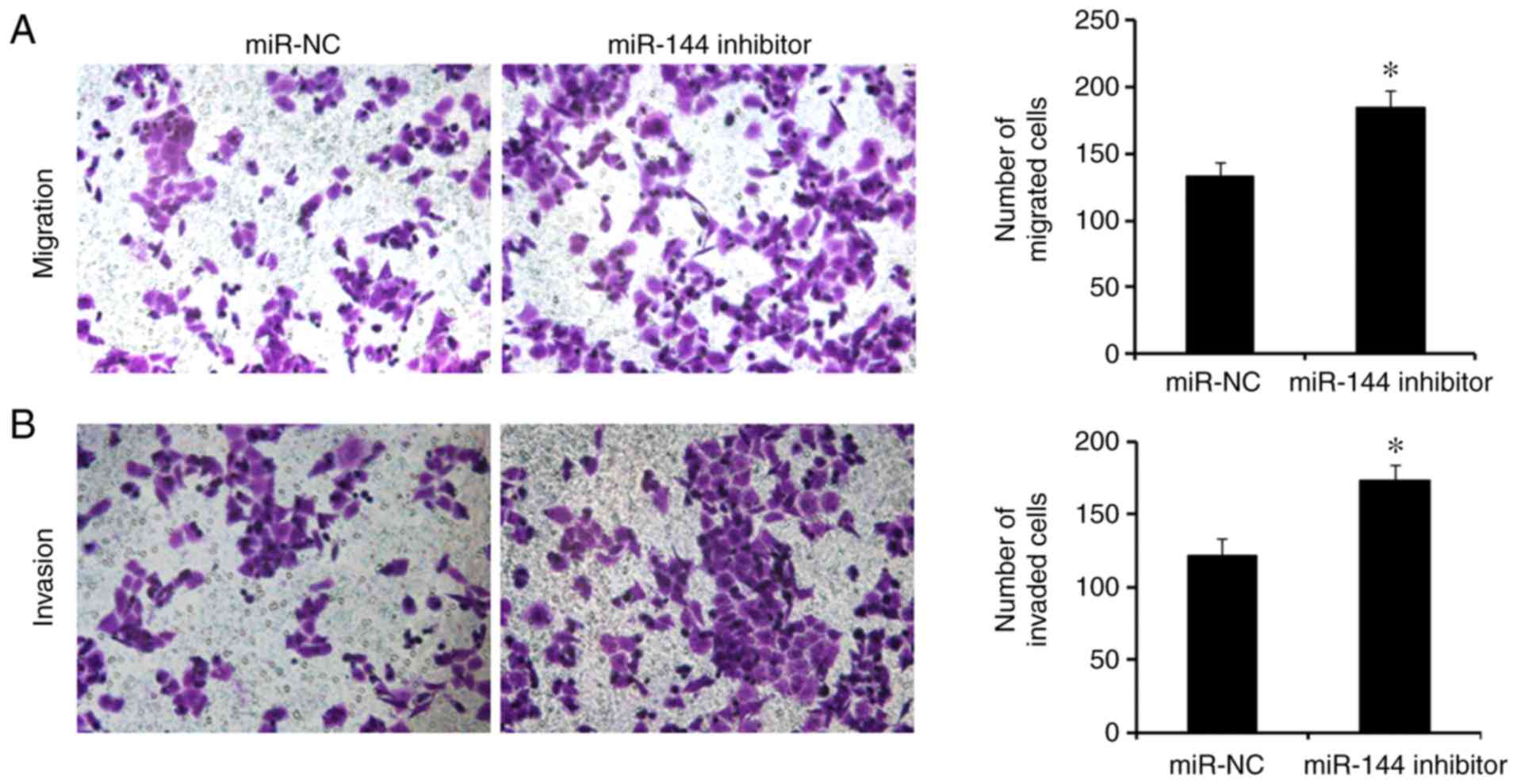
expression of miR-144 via transfection with miR-144 inhibitor
promoted cell migration and invasion in SW480 cells compared with
the miR-NC group (Fig. 3;
P<0.05).
miR-144 targets ANO1 via binding to
its 3′UTR
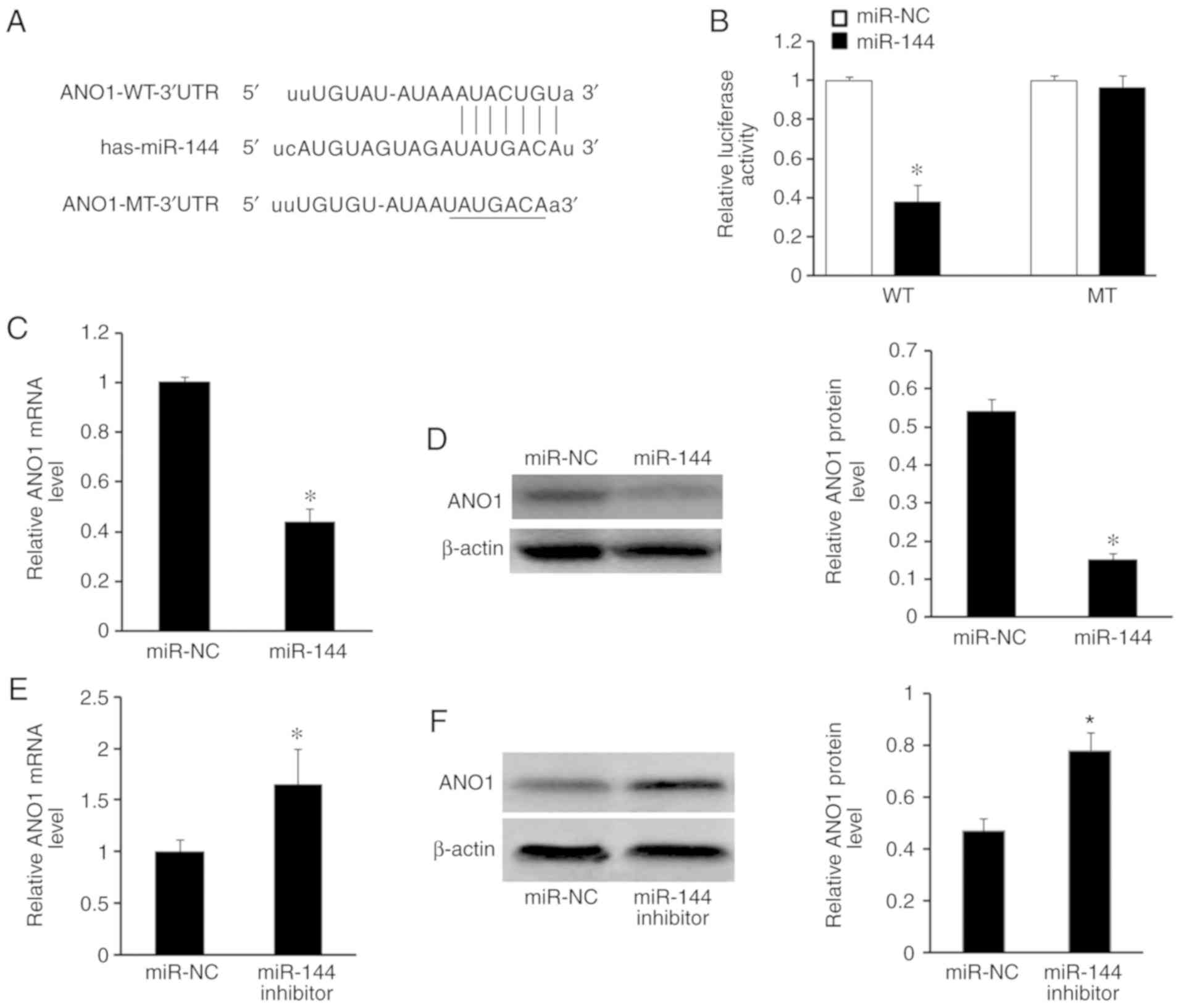
To elucidate the potential molecular mechanism of
miR-144 in CRC progression, putative target genes of miR-144 were
identified using the TargetScan and miRanda databases. It was
revealed that the 3′UTR of ANO1 mRNA contains potential binding
sites for miR-144 (Fig. 4A). A
luciferase activity assay was used to assess whether ANO1 is a
direct target of miR-144. SW480 cells were co-transfected with
ANO1-WT-3′UTR or ANO1-MT-3′UTR, pcDNA-miR-144 or NC. As
demonstrated in Fig. 4B, miR-144
decreased the relative luciferase activity of ANO1-WT-3′UTR in
SW480 cells compared with miR-NC (P<0.05), but not in cells
transfected with ANO1-MT-3′UTR. RT-qPCR and western blot analysis
also demonstrated that the mRNA (P<0.05; Fig. 4C) and protein (P<0.05; Fig. 4D) expression levels of ANO1 were
significantly decreased following miR-144 overexpression in SW480
cells, compared with cells transfected with miR-NC. It was also
observed that the mRNA (P<0.05; Fig.
4E) and protein (P<0.05; Fig.
4F) expression of ANO1 was increased in SW480 cells transfected
with miR-144 inhibitor, compared with cells transfected with
miR-NC, by RT-qPCR and western blot analysis.
Association between the expression of
ANO1 and various clinicopathological characteristics
According to the 122 paraffin-embedded colorectal
tissue blocks evaluated by immunohistochemistry, it was
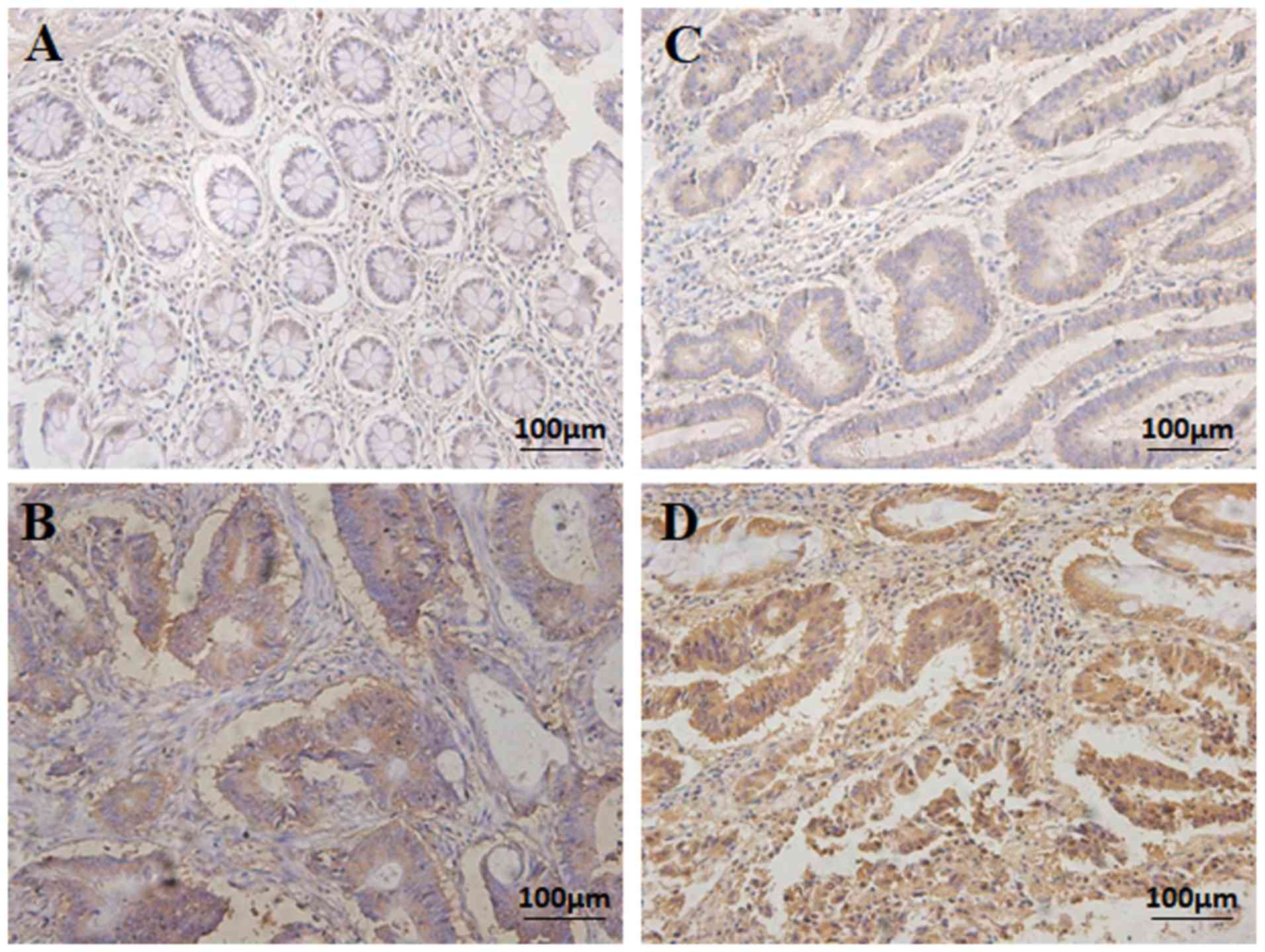
demonstrated that ANO1 was expressed in the CRC tissues samples. A
total of 78/122 (63.93%) cases exhibited high ANO1 expression (ANO1
++ or ANO1 +++), whereas 44/122 (36.07%) exhibited low ANO1
expression (ANO1- or ANO1 +) (Fig.
5; Table I). The adjacent
noncancerous colorectal tissues exhibited no ANO1 staining
(Fig. 5).
According to the χ2 analysis, the
overexpression of ANO1 was associated with clinicopathological
parameters, including histological grade (P<0.001) and TNM stage
(P=0.003). However, it was not associated with sex, age, tumor
location or tumor size (P>0.05; Table I).
Upregulation of ANO1 in CRC tissue
samples
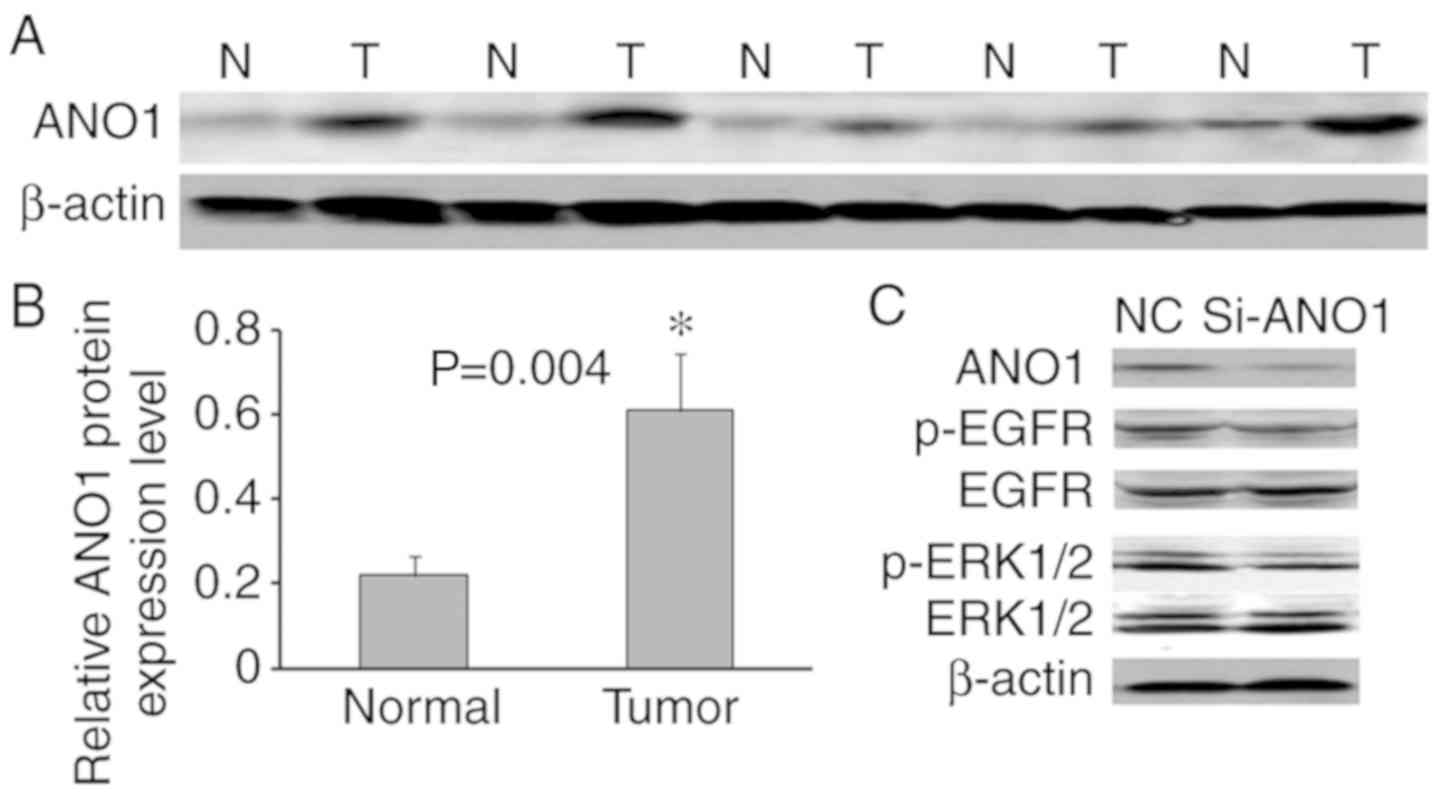
Western blotting was used to analyze the protein
expression of ANO1 in 26 randomly selected CRC and matched
non-tumor tissues. The results of five representative cases are
presented in Fig. 6A. β-actin was
used to normalize ANO1 protein expression. Compared with adjacent
non-tumor tissues in CRC patients, ANO1 protein was upregulated in
69.2% of CRC tissues, and in 26 CRC tissues, the average ANO1
protein expression level was significantly higher compared with
that in the adjacent non-tumor colorectal tissues (P=0.004;
Fig. 6B).
The total and phosphorylated levels of epidermal
growth factor receptor (EGFR) and extracellular signal-regulated
kinase (ERK)1/2 were also investigated. Downregulation of ANO1
suppressed the phosphorylation of EGFR and ERK1/2, but had no
effect on the total EGFR and ERK1/2 protein expression levels
(Fig. 6C).
miR-144 suppresses CRC cell migration
and invasion by downregulating ANO1
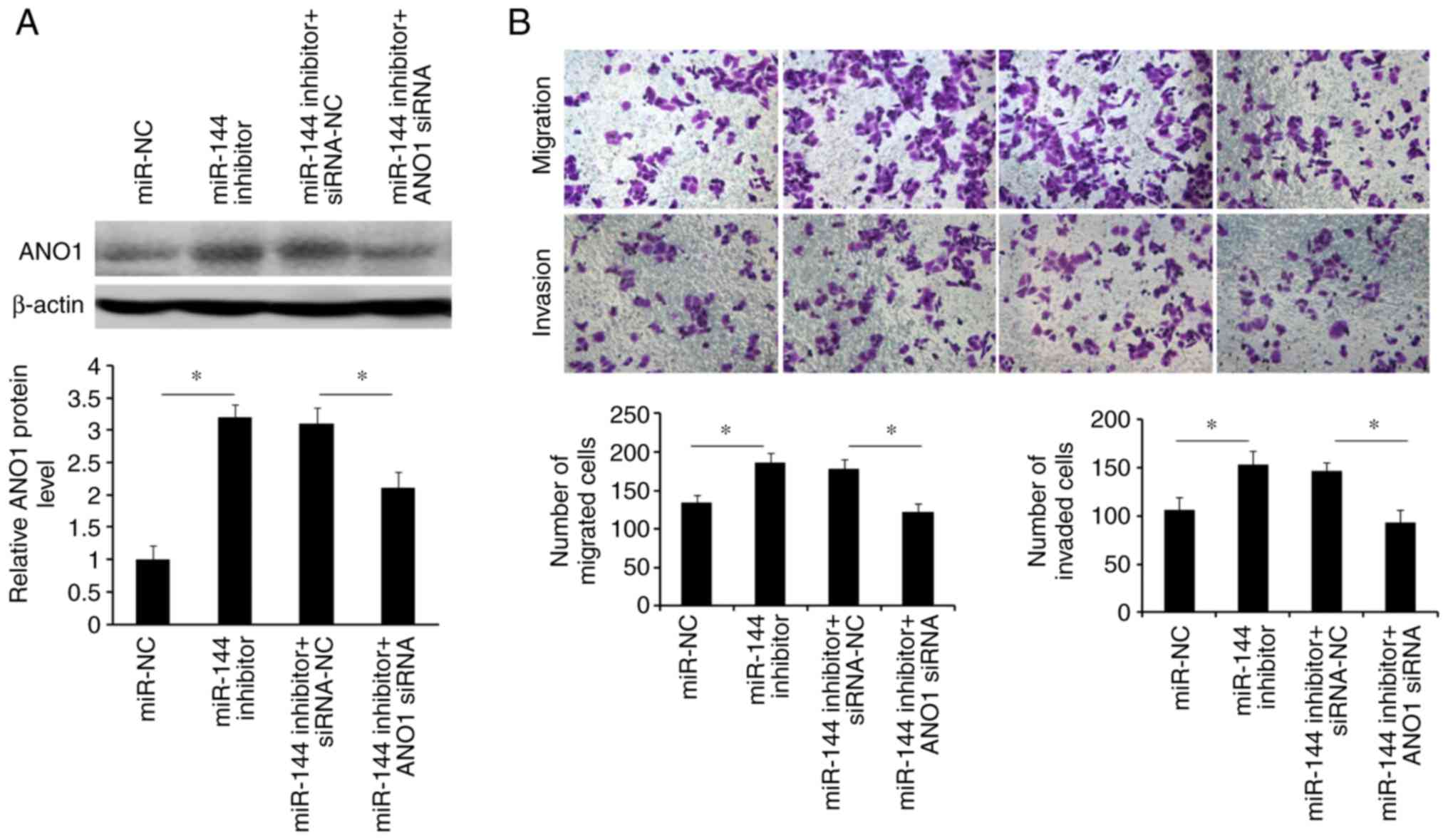
To investigate whether the effects caused by
blocking miR-144 may be abolished by knockdown of ANO1, SW480 cells
were co-transfected with miR-144 inhibitor and si-ANO1. Western
blotting revealed that the induction of ANO1 expression caused by
miR-144 inhibitor may be partly rescued by the suppression of ANO1
expression using si-ANO1 (Fig. 7A).
The Transwell assays indicated that inhibition of ANO1 abrogated
the miR-144 inhibitor-mediated migration and invasion of SW480
cells (Fig. 7B). These results
suggested that miR-144-suppressed CRC cell migration and invasion
was mediated by downregulation of ANO1.
Association between ANO1 expression
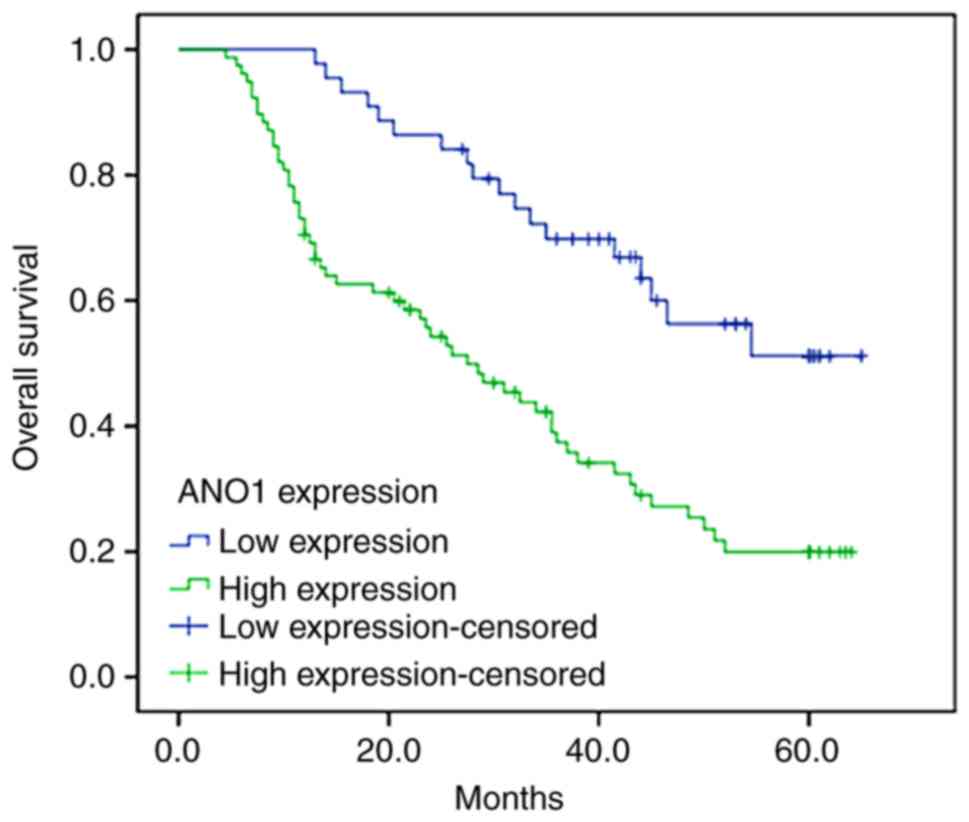
and patient survival
Survival analysis of the high and low ANO1
expression groups was performed to evaluate the prognostic value of
ANO1 in patients with CRC. Compared with the high expression group,
patients exhibiting low ANO1 expression had a longer overall
survival time (P<0.001; Fig. 8).
According to the multivariate Cox regression analysis, it was
demonstrated that the expression of ANO1 and differentiation were
significantly associated with the overall survival time of patients
with CRC (Table II).
 | Table II.Cox proportional hazards model
analysis of prognostic factors. |
Table II.
Cox proportional hazards model
analysis of prognostic factors.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| ANO1 expression
(high vs. low) | 1.06 | 0.85–1.17 | 0.018a | 1.38 | 0.84–1.94 | 0.032a |
| TNM stage (III/IV
vs. I/II) | 0.85 | 0.73–1.05 | 0.776 | 0.92 | 0.56–1.68 | 0.089 |
| Lymph node
metastasis (positive vs. negative) | 0.89 | 0.68–1.16 | 0.042a | 0.54 | 0.28–1.36 | 0.970 |
| Invasive depth
(T3/T4 vs. T1/T2) | 0.68 | 0.43–0.85 | 0.184 | 0.48 | 0.26–1.06 | 0.067 |
| Histology
differentiation (poor vs. well/moderate) | 1.34 | 0.83–1.87 | 0.005a | 1.09 | 0.23–2.58 | 0.003a |
| Tumor location
(rectum vs. colon) | 0.63 | 0.52–1.13 | 0.325 | 0.49 | 0.28–1.17 | 0.092 |
| Tumor size (>5
cm vs. ≤5 cm) | 0.07 | 0.41–1.40 | 0.093 | 0.67 | 0.32–1.48 | 0.296 |
Discussion
Colorectal cancer (CRC) has one of the highest
mortality rates among malignant tumors worldwide. According to the
World Health Organization's International Agency for Research on
Cancer, CRC is the second most common type of malignant tumor in
females and the third most common in males (31). Multiple genetic alterations are
involved in the progression of the normal colonic epithelium into
adenoma and subsequent malignant adenocarcinoma (32). However, understanding of the
molecular alterations in the metastasis of CRC is limited.
ANO1 mediates trans-epithelial ion transport. It
serves an important role in regulating airway fluid secretion, gut
motility, secretory functions of exocrine glands, renal function,
smooth muscle contraction and nociception (10,12,33).
Certain diseases, including cystic fibrosis, asthma, gastroparesis,
hypertension, rotavirus-induced diarrhea and polycystic kidney
disease, are associated with ANO1 dysfunction (34–38).
In a large subset of head and neck squamous cell carcinoma cases,
ANO1 is amplified and highly expressed (13), which is also the case for other
types of cancer including breast cancer (15), prostate carcinoma (15), glioblastoma (16), gastrointestinal stromal tumors
(17), and esophageal squamous cell
carcinoma (18).
Previous studies have demonstrated that miR-144 is
associated with the response to mood stabilizer treatment (28), stress responses (39) and aging diseases (40). In various types of cancer, aberrant
expression of miR-144 has been demonstrated. It has been reported
that downregulation of miR-144 may be associated with poor
prognosis in patients with CRC due to activation of the mTOR
signaling pathway (26). It has
also been revealed that miR-144 may regulate proliferation by
targeting enhancer of zeste homolog 2 in bladder cancer cells
(41). Other observations have
suggested that miR-144 activates RAC-α serine/threonine-protein
kinase signaling via downregulation of PTEN in the tumorigenesis
and tumor progression of breast cancer (42). In the present study, miR-144 was
downregulated in CRC and it significantly inhibited cell migration
and invasion in vitro. Downregulation of miR-144 via
transfection with miR-144 inhibitor significantly promoted CRC cell
migration and invasion. In addition, miR-144 was involved in
ANO1-mediated CRC cell migration and invasion. Furthermore, the
data presented herein suggest that ANO1 serves consistent roles in
the activation of the EGFR/ERK signaling pathway.
Due to the limited number of patients included in
the study, the Cox proportional hazards regression model did not
indicate a significant association between TNM stage and patient
survival. Therefore, a future study may use a larger number of
samples. Furthermore, the present study demonstrated that miR-144
serves a critical role in CRC carcinogenesis. It was identified
that ANO1 is a direct functional target of miR-144. To confirm the
results, the effect of ANO1 overexpression and silencing on the
growth, migration and invasion capabilities of multiple CRC cell
lines may be examined in future studies. It is essential to
understand the molecular mechanism behind the miR-144-mediated
regulation of ANO1 expression.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
Nantong Science and Technology Project (grant no. MS32017005) and
the Hospital Level Subject of The Second Affiliated Hospital of
Nantong University (grant no. YG201603).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
SF, CT and YC had responsibility for the design of
the study. YJ, FL, ZG and YZ performed the experiments. YC, WS and
FL analyzed the data. CT, YJ and WS wrote the manuscript. SF ZG and
YZ revised the manuscript. All authors read and approved the final
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work is appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was conducted with the approval of
the institutional ethics board of The Second Affiliated Hospital of
Nantong University and the patients included in the present study
had provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Ahmedin J: Cancer
statistics, 2015. CA Cancer J Clin. 60:277–300. 2010.PubMed/NCBI
|
|
2
|
Zou L, Zhong R, Lou J, Lu X, Wang Q, Yang
Y, Xia J, Ke J, Zhang T, Sun Y, et al: Replication study in Chinese
population and meta-analysis supports association of the 11q23
locus with colorectal cancer. PLoS One. 7:e454612012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Edge SB: AJCC Cancer Staging Manual. JAMA
J Am Med Association. 304:1726–1727. 2010. View Article : Google Scholar
|
|
4
|
Stoffel EM and Richard CR: Genetics and
genetic testing in hereditary colorectal cancer. Gastroenterology.
149:1191–1203.e2. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cai K, Mulatz K, Ard R, Nguyen T and Gee
SH: Increased diacylglycerol kinase ζ expression in human
metastatic colon cancer cells augments Rho GTPase activity and
contributes to enhanced invasion. BMC Cancer. 14:2082014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karpiński P, Sąsiadek MM and Blin N:
Aberrant epigenetic patterns in the etiology of gastrointestinal
cancers. J Appl Genet. 49:1–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR,
Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG,
Kendrick ML, et al: Ano1 is a selective marker of interstitial
cells of Cajal in the human and mouse gastrointestinal tract. Am J
Physiol Gastrointest Liver Physiol. 296:G1370–G1381. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fen H, Hongkang Z, Meng W, Yang H, Kudo M,
Peters CJ, Woodruff PG, Solberg OD, Donne ML, Huang X, et al:
Calcium-activated chloride channel TMEM16A modulates mucin
secretion and airway smooth muscle contraction. Proc Natl Acad Sci
USA. 109:16354–16359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fen H, Rock JR, Harfe BD, Cheng T, Huang
X, Jan YN and Jan LY: Studies on expression and function of the
TMEM16A calcium-activated chloride channel. Proc Natl Acad Sci USA.
106:21413–21418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hwang SJ, Blair PJ, Britton FC, O'Driscoll
KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM and Ward
SM: Expression of anoctamin 1/TMEM16A by interstitial cells of
Cajal is fundamental for slow wave activity in gastrointestinal
muscles. J Physiol. 587:4887–4904. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Manoury B, Tamuleviciute A and Tammaro P:
TMEM16A/anoctamin 1 protein mediates calcium-activated chloride
currents in pulmonary arterial smooth muscle cells. J Physiol.
588:2305–2314. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hawon C, Yang YD, Lee J, Lee B, Kim T,
Jang Y, Back SK, Na HS, Harfe BD, Wang F, et al: The
calcium-activated chloride channel anoctamin 1 acts as a heat
sensor in nociceptive neurons. Nat Neurosci. 15:1015–1021. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ayoub C, Wasylyk C, Li Y, Thomas E, Marisa
L, Robé A, Roux M, Abecassis J, de Reyniès A and Wasylyk B: ANO1
amplification and expression in HNSCC with a high propensity for
future distant metastasis and its functions in HNSCC cell lines. Br
J Cancer. 103:715–726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu W, Lu M, Liu B, Huang Y and Wang KW:
Inhibition of Ca2+-activated Cl− channel
ANO1/TMEM16A expression suppresses tumor growth and invasiveness in
human prostate carcinoma. Cancer Lett. 326:41–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Britschgi A, Bill A, Brinkhaus H, Rothwell
C, Clay I, Duss S, Rebhan M, Raman P, Guy CT, Wetzel K, et al:
Calcium-activated chloride channel ANO1 promotes breast cancer
progression by activating EGFR and CAMK signaling. Proc Natl Acad
Sci USA. 110:E1026–E1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Liu Y, Ren Y, Kang L and Zhang L:
Transmembrane protein with unknown function 16A overexpression
promotes glioma formation through the nuclear factor-κB signaling
pathway. Mol Med Rep. 9:1068–1074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berglund E, Akcakaya P, Berglund D,
Karlsson F, Vukojević V, Lee L, Bogdanović D, Lui WO, Larsson C,
Zedenius J, et al: Functional role of the Ca2+-activated
Cl− channel DOG1/TMEM16A in gastrointestinal stromal
tumor cells. Exp Cell Res. 326:315–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shang L, Hao JJ, Zhao XK, He JZ, Shi ZZ,
Liu HJ, Wu LF, Jiang YY, Shi F, Yang H, et al: ANO1 protein as a
potential biomarker for esophageal cancer prognosis and
precancerous lesion development prediction. Oncotarget.
7:24374–24382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rossing M, Borup R, Henao R, Winther O,
Vikesaa J, Niazi O, Godballe C, Krogdahl A, Glud M, Hjort-Sørensen
C, et al: Down-regulation of microRNAs controlling tumourigenic
factors in follicular thyroid carcinoma. J Mol Endocrinol.
48:11–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha
V, Lindblad-Toh K, Lander ES and Kellis M: Systematic discovery of
regulatory motifs in human promoters and 3′UTRs by comparison of
several mammals. Nature. 434:338–345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Landgraf P, Rusu M, Sheridan R, Sewer A,
Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M,
et al: A mammalian microRNA expression atlas based on small RNA
library sequencing. Cell. 129:1401–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Wang X, Jiang J, Cao Z, Yang B and
Cheng X: Modulation of T cell cytokine production by miR-144* with
elevated expression in patients with pulmonary tuberculosis. Mol
Immunol. 48:1084–1090. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Y, Xie Z, Lin J and Liu P: MiR-144-3p
inhibits cell proliferation and induces apoptosis in multiple
myeloma by targeting c-Met. Am J Transl Res. 9:2437–2446.
2017.PubMed/NCBI
|
|
25
|
Zhang LY, Ho-Fun LV, Wong AM, Kwong DL,
Zhu YH, Dong SS, Kong KL, Chen J, Tsao SW, Guan XY and Fu L:
MicroRNA-144 promotes cell proliferation, migration and invasion in
nasopharyngeal carcinoma through repression of PTEN.
Carcinogenesis. 34:454–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takeshi I, Takehiko Y, Naohiro N, Kogo R,
Sudo T, Tanaka F, Shibata K, Sawada G, Takahashi Y, Ishibashi M, et
al: Downregulation of miR-144 is associated with colorectal cancer
progression via activation of mTOR signaling pathway.
Carcinogenesis. 33:2391–2397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sureban SM, Randal M, Lightfoot SA,
Hoskins AB, Lerner M, Brackett DJ, Postier RG, Ramanujam R,
Mohammed A, Rao CV, et al: DCAMKL-1 regulates
epithelial-mesenchymal transition in human pancreatic cells through
a miR-200a-dependent mechanism. Cancer Res. 71:2328–2338.
2015. View Article : Google Scholar
|
|
28
|
Zhou R, Yuan P, Wang Y, Hunsberger JG,
Elkahloun A, Wei Y, Damschroder-Williams P, Du J, Chen G and Manji
HK: Evidence for selective microRNAs and their effectors as common
long-term targets for the actions of mood stabilizers.
Neuropsychopharmacology. 34:1395–1405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Märkl B, Olbrich G, Schenkirsch G,
Kretsinger H, Kriening B and Anthuber M: Clinical significance of
international union against cancer pN staging and lymph node ratio
in node-positive colorectal cancer after advanced lymph node
dissection. Dis Colon Rectum. 59:386–395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kinzler KW and Vogelstein B: Lessons from
hereditary colorectal cancer. Cell. 87:159–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Faria D, Rock JR, Romao AM, Schweda F,
Bandulik S, Witzgall R, Schlatter E, Heitzmann D, Pavenstädt H,
Herrmann E, et al: The calcium-activated chloride channel Anoctamin
1 contributes to the regulation of renal function. Kidney Int.
85:1369–1381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang CH, Li Y, Zhao W, Lifshitz LM, Li H,
Harfe BD, Zhu MS and ZhuGe R: The transmembrane protein 16A
Ca2+-activated Cl-channel in airway smooth muscle
contributes to airway hyperresponsiveness. Am J Respir Crit Care
Med. 187:374–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Forrest AS, Joyce TC, Huebner ML, Ayon RJ,
Wiwchar M, Joyce J, Freitas N, Davis AJ, Ye L, Duan DD, et al:
Increased TMEM16A-encoded calcium-activated chloride channel
activity is associated with pulmonary hypertension. Am J Physiol
Cell Physiol. 303:C1229–C1243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sondo E, Caci E and Galietta LJ: The
TMEM16A chloride channel as an alternative therapeutic target in
cystic fibrosis. Int J Biochem Cell Biol. 52:73–76. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ousingsawat J, Mirza M, Tian Y, Roussa E,
Schreiber R, Cook DI and Kunzelmann K: Rotavirus toxin NSP4 induces
diarrhea by activation of TMEM16A and inhibition of Na+
absorption. Pflugers Arch. 461:579–589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tanaka T and Nangaku M: ANO1: An
additional key player in cyst growth. Kidney Int. 85:1007–1009.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Katsuura S, Kuwano Y, Yamagishi N,
Kurokawa K, Kajita K, Akaike Y, Nishida K, Masuda K, Tanahashi T
and Rokutan K: MicroRNAs miR-144/144* and miR-16 in peripheral
blood are potential biomarkers for naturalistic stress in healthy
Japanese medical students. Neurosci Lett. 516:79–84. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Persengiev S, Kondova I, Otting N, Koeppen
AH and Bontrop RE: Genome-wide analysis of miRNA expression reveals
a potential role for miR-144 in brain aging and spinocerebellar
ataxia pathogenesis. Neurobiol Aging. 32:2316.e17–e27. 2011.
View Article : Google Scholar
|
|
41
|
Guo Y, Ying L, Tian Y, Yang P, Zhu Y, Wang
Z, Qiu F and Lin J: miR-144 downregulation increases bladder cancer
cell proliferation by targeting EZH2 and regulating Wnt signaling.
FEBS J. 280:4531–4538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu L, Yang Y, Hou J, Zhai C, Song Y, Zhang
Z, Qiu L and Jia X: MicroRNA-144 affects radiotherapy sensitivity
by promoting proliferation, migration and invasion of breast cancer
cells. Oncol Rep. 34:1845–1852. 2015. View Article : Google Scholar : PubMed/NCBI
|