Introduction
Recently, a highly glycosylated
glycosylphosphatidylinositol-anchored protein, known as Ly6/Plaur
domain-containing 8 (LYPD8), was identified as a novel molecule
contributing to the segregation of intestinal bacteria and
intestinal epithelia in the large intestine (1). Lypd8, which is anchored to intestinal
epithelial cells in the uppermost epithelial layer, is
constitutively shed into the intestinal lumen and preferentially
binds to flagellated bacteria from various genera (2). Mice lacking LYPD8 lack the
bacteria-free space immediately above the epithelial layer of the
colon, indicating that LYPD8 is critical for the segregation of
intestinal bacteria and colonic epithelia (3). Furthermore, LYPD8 is also expressed in
the colonic epithelia, and the expression of LYPD8 is reduced in
the colon of some patients with ulcerative colitis (UC) (4). However, the biological action and
functions of LYPD8 in colorectal cancer (CRC) remain to be fully
elucidated.
Previous studies have confirmed that several
cytokines are involved in the transition from inflammation to tumor
(5). Interleukin-6 (IL-6) is an
inflammatory factor with a wide range of biological effects,
including tumor-promoting effects (6). The levels of IL-6 have been observed
to be significantly increased in the serum of patients with UC, and
the degree of the increase was associated with the disease
(7). The expression of IL-6 is
regulated by nuclear factor-κB (NF-κB), which is expressed in
multiple cells and is involved in regulating the transcription of
tumor proliferation-related genes (8). NF-κB is typically present as a dimer
or heterodimer, such as the P65/P50 heterodimer, in cells (9). Tumor necrosis factor-α (TNF-α) is
another important inflammatory factor, and increased TNF-α
expression is present in serum and colon mucosa of patients with
CRC (10). TNF-α promotes the
progression of CRC, and its mechanism may be associated with
activation of the NF-κB signaling pathway (11).
The signal transducer and activator of transcription
3 (STAT3) signaling pathway acts as a bridge between inflammation
and tumors (12). This signaling
pathway is a positive feedback regulator in various types of
cancer, including CRC, prostate, lung, breast, pancreatic and
ovarian cancer (13). The
activation of STAT3 can induce the abnormal transcription of
oncogenes associated with cell proliferation, apoptosis and
differentiation (14). Numerous
cytokines, including IL-6 and TNF-α, exert biological effects in
cancer cells through the STAT3 signaling pathway (15). Previous studies have demonstrated
that the overexpression of IL-6 can increase the anti-apoptotic
effect in cancer cells through the IL-6-STAT3 signaling pathway
(16).
In the present study, to evaluate the effects of
LYPD8 in the biological function of cancer, the expression of LYPD8
was examined, and the corresponding levels of STAT3/P65
phosphorylation in and secretion of IL-6/TNF-α from CRC tissues at
different tumor stages were compared with corresponding
precancerous tissue and normal tissue. Following this, IL-6/TNF-α
secretion, STAT3/P65 dephosphorylation and cell proliferation and
migration were detected when LYPD8 was overexpressed in CRC
cells.
Materials and methods
Histological analysis
A total of 40 patients with CRC (range, 45–70 years
old) who underwent surgery between 2014 and 2015 at Sir Run Run
Shaw Hospital (Hangzhou, China) were recruited. All experiments
using human tissues were approved by the local institutional Ethics
Committee (institutional review board reference no. 20140213-19).
Written informed consent was obtained from patients prior to their
inclusion in the study. The histological types, disease stages and
cancer cell contents in each formalin-fixed paraffin-embedded
section were examined by experienced pathologists. Normal tissue
samples (>3 cm from the edge of cancer tissues) and precancerous
tissue samples (within 3 cm from the edge of cancer tissues) were
used as controls. None of the patients included in the present
study had received preoperative radiotherapy, chemotherapy or
immunotherapy prior to surgery. The tissues were fixed overnight in
4% paraformaldehyde solution, dehydrated and embedded in paraffin.
The tissue sections were then deparaffinized in xylene and
rehydrated in graded ethanol, followed by antigen microwave
retrieval. The sections were blocked with 3%
H2O2 and 10% goat serum (cat. no. ab7481;
Abcam, Cambridge, UK), and then incubated overnight at 4°C with
STAT3 antibody (dilution 1:100; cat. no. ab68153; Abcam) and P65
antibody (dilution 1:100; cat. no. ab32536; Abcam). The sections
were then incubated at 37°C for 1 h with FITC/PE-conjugated
antibody (dilution 1:200; cat. no. A0562/A0423; Beyotime Institute
of Biotechnology, Haimen, China). The nuclei were stained DAPI
(Beyotime Institute of Biotechnology) for 5 min. All sections were
observed under a confocal laser microscope (LSM780; Carl Zeiss AG,
Oberkochen, Germany).
Western blotting
The preparation of protein extracts from the
tissues/cells and electrophoretic separation were performed as
previously described (17).
Following electrophoresis, the proteins were transferred onto a
polyvinylidene difluoride (PVDF) membrane (Merck KGaA, Darmstadt,
Germany), which was blocked in 5% non-fat milk (for 1 h) and
further incubated with a STAT3 antibody (dilution 1:1,000; cat. no.
ab68153; Abcam), phosphorylated (p)-STAT3 antibody (dilution
1:1,000; cat. no. AF3294; Affinity Biosciences, Zhenjiang, China),
P65 antibody (dilution 1:1,000; cat. no. ab32536; Abcam), p-P65
antibody (dilution 1:1,000; cat. no. AF2006; Affinity Biosciences)
and GAPDH antibody (dilution 1:2,000; cat. no. AP0063; Bioworld
Technology, Inc., St. Louis Park, MN, USA) overnight at 4°C.
Following incubation, the blots were incubated with the appropriate
alkaline phosphatase-labeled secondary antibody (dilution 1:5,000;
cat. no. AF1030; Beyotime Institute of Biotechnology) for 2 h at
room temperature. Following stringent washing with Tris-buffered
saline (TBS) and Tween-20, the membranes were incubated with the
alkaline phosphatase substrate for 5 min at room temperature and
then submitted to fluorescence detection using the VersaDoc 3000
Imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
band intensities were analyzed using ImageJ v1.8.0 software (NIH;
National Institutes of Health, Bethesda, MD, USA).
ELISA
The human IL-6 ELISA kit (cat. no. HM10205;
Bio-Swamp Life Science, Shanghai, China) and human TNF-α ELISA kit
(cat. no. HM10001; Bio-Swamp Life Science) were used for the
quantitation of IL-6 and TNF-α, respectively. The procedure was
performed according to the manufacturer's protocol.
Isolation of RNA and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from the three cell lines
using a Takara MiniBEST Universal RNA Extraction kit (Takara
Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's protocol and quantified with a NanoDrop
spectrophotometer. Total RNA was reverse transcribed with the
PrimeScript® RT reagent kit (Takara Biotechnology Co.,
Ltd.) and random hexamers in a volume of 20 µl according to the
manufacturer's protocol. Then samples (4 samples for each group)
performed out in a PCR gene amplifcation apparatus: 42°C for 60
min, 70°C for 5 min, and then the reaction was terminated, placed
on ice for storage or stored at −20°C. RT-qPCR was performed with z
qPCR kit (SYBR Premix Ex Taq; Takara Biotechnology Co., Ltd.) using
an Applied Bio-Rad CFX96 Sequence Detection system. The following
primers were used for RT-PCR: LYPD8, forward
5′-CGAAAGTTTGAGTGTGCAAATGT-3′ and reverse
5′-CAGAGCAGGGAAAGAGGGTGT-3′; β-actin, forward
5′-TGACGTGGACATCCGCAAAG-3′ and reverse 5′-CTGGAAGGTGGACAGCGAGG-3′.
The qPCR thermocycling conditions were performed as follows:
Pre-deformation 95°C for 10 min, 1 cycle; denaturation 95°C for 15
sec and annealing 60°C for 60 sec, 40 cycles. All reactions were
normalized to the housekeeping gene β-actin to quantify the
relative gene expression and were then analyzed using the
2−ΔΔCq method (18).
Cell culture
Three CRC cell lines (SW480, HT29 and SW620) were
used. SW480 (ATCC® CCL-228™, organism, human; tissue,
colon; disease, colorectal adenocarcinoma), HT29 (ATCC®
HTB-38™, organism, human; tissue, colon; disease, colorectal
adenocarcinoma) and SW620 (ATCC® CCL-227™, organism,
human; tissue, colon; derived from metastatic site, lymph node;
disease, colorectal adenocarcinoma) cells were purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). Three
cell lines within passages 10 were used in all experiments. Three
cell lines were maintained at 37°C in a humidified incubator
containing 5% CO2. The SW480 and SW620 cells were grown
in Dulbecco's modified Eagle's medium (DMEM; Corning, Inc.,
Corning, NY, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientifc Inc., MA, USA). The HT29 cells were
grown in McCoy's 5A medium (Corning, Inc.) supplemented with 10%
FBS.
Cell proliferation and migration
The CCK-8 kit was used to investigate SW480 cell
proliferation. SW480 cells were plated in 96-well plates and grown
for 72 h. The procedure was performed according to the
manufacturer's protocol. Subsequently, the mean percentages of cell
survival and apoptosis were calculated. To inhibit STAT3 and NF-κB
signaling, 0.5, 1 and 2 µM niclosamide (Selleck Chemicals, Houston,
TX, USA) or 5, 15 and 30 µM JSH-23 (Selleck Chemicals) was added to
the cell culture medium. SW480 cell migration was investigated
using Transwell assays. The bottom chambers were filled with medium
with FBS, and the top chambers were seeded with medium without FBS
containing 2×104 SW480 cells. The SW480 cells were
allowed to migrate for 24 h, and non-migrating cells were removed
by scraping. The migratory cells were fixed with 100% methanol and
stained with 0.05% crystal violet. The migratory cells were
observed using a light microscope (Nikon Corp., Tokyo, Japan) and
quantified by manual counting.
Statistical analysis
Statistical significance was assessed with two-way
analysis of variance using GraphPad Prism software (version 5.0;
GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Where significance was revealed, comparisons were made using the
Bonferroni post hoc test. All results are reported as the mean ±
standard deviation.
Results
Correlation of the expression of LYPD8
with STAT3/P65 phosphorylation and IL-6/TNF-α secretion in patients
with CRC
The present study detected the expression and
distribution of STAT3 and P65 in CRC tissues at different stages.
As shown in Fig. 1A, using
immunofluorescence experiments, increased expression levels of
STAT3 and P65 were observed in stage II and IV CRC tissues compared
with those in precancerous tissue. STAT3 and P65 were was expressed
in the cytoplasm and nucleus. The results of the western blotting
showed that the levels of p-P65/P65 and p-STAT3/STAT3 gradually
increased between stage I and III (Fig.
1B and C). These results revealed increased phosphorylation
levels of STAT3 and P65 in CRC tissues.
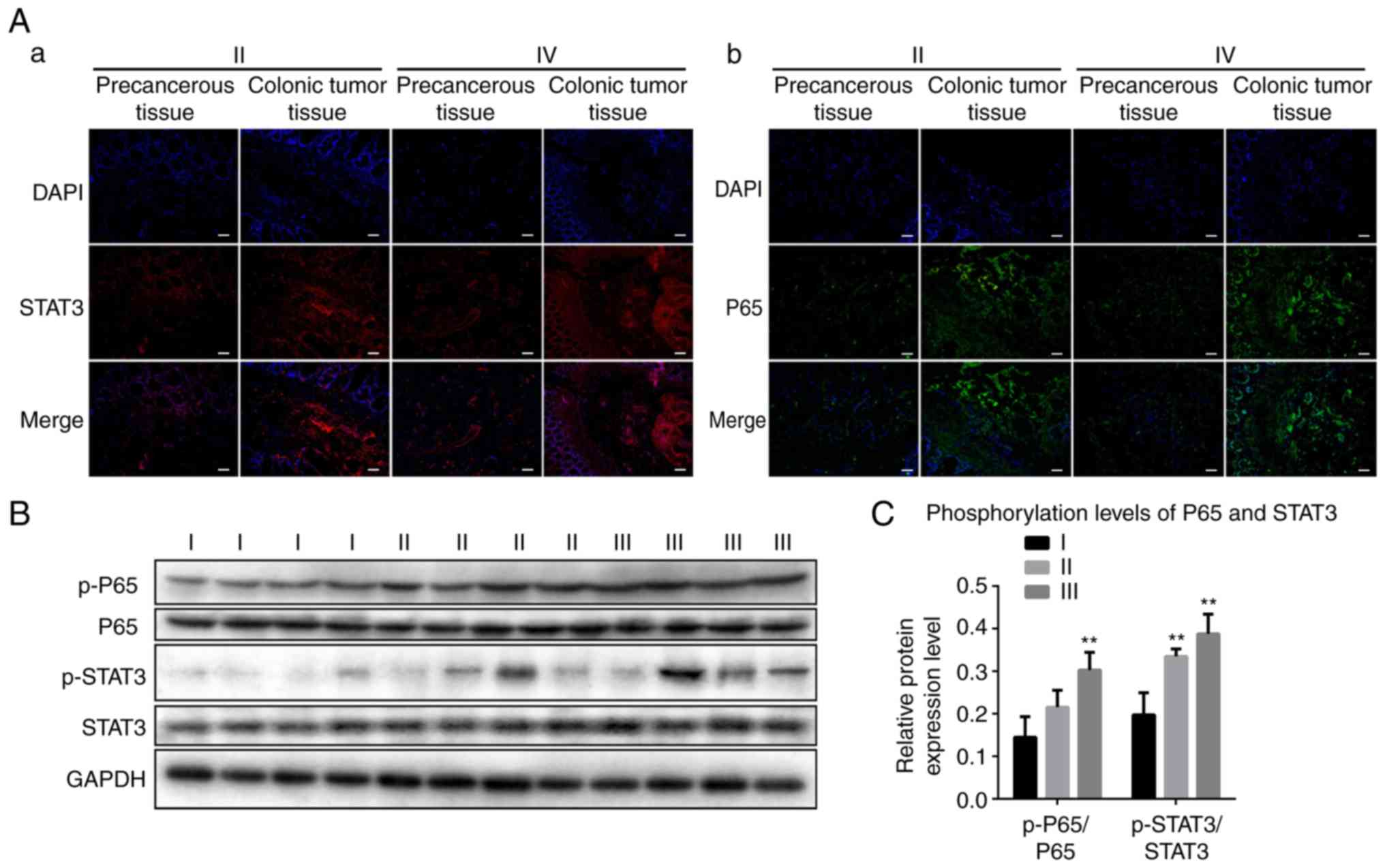 | Figure 1.STAT3 and P65 are activated in colonic
tumor tissues from patients. (A) Representative immunofluorescence
images revealing activated (a) STAT3 and (b) P65 in colonic cancer
tissue and precancerous tissue. Scale bar, 100 µm. (B)
Representative western blotting revealing the expression of p-P65,
P65, p-STAT3 and STAT3 in stages I, II and III colonic tumor
tissues. GAPDH was used as a control. (C) Band intensities of
western blotting for p-P65/P65 and p-STAT3/STAT3 in stage I, II and
III tissues were analyzed. The data are reported as the mean ±
standard deviation of experiments (n=4). **P<0.01,
phosphorylation levels of P65 in stage I vs. in stage III;
**P<0.01, phosphorylation levels of STAT3 in stage I tissues vs.
in stage II, III tissues. STAT3, signal transducer and activator of
transcription 3; p-, phosphorylated. |
Previous studies have demonstrated that STAT3 and
P65 can regulate the biological behavior of tumor cells and the
expression of inflammatory mediators. Therefore, the present study
detected the secretion of IL-6 and TNF-α in CRC tissues at
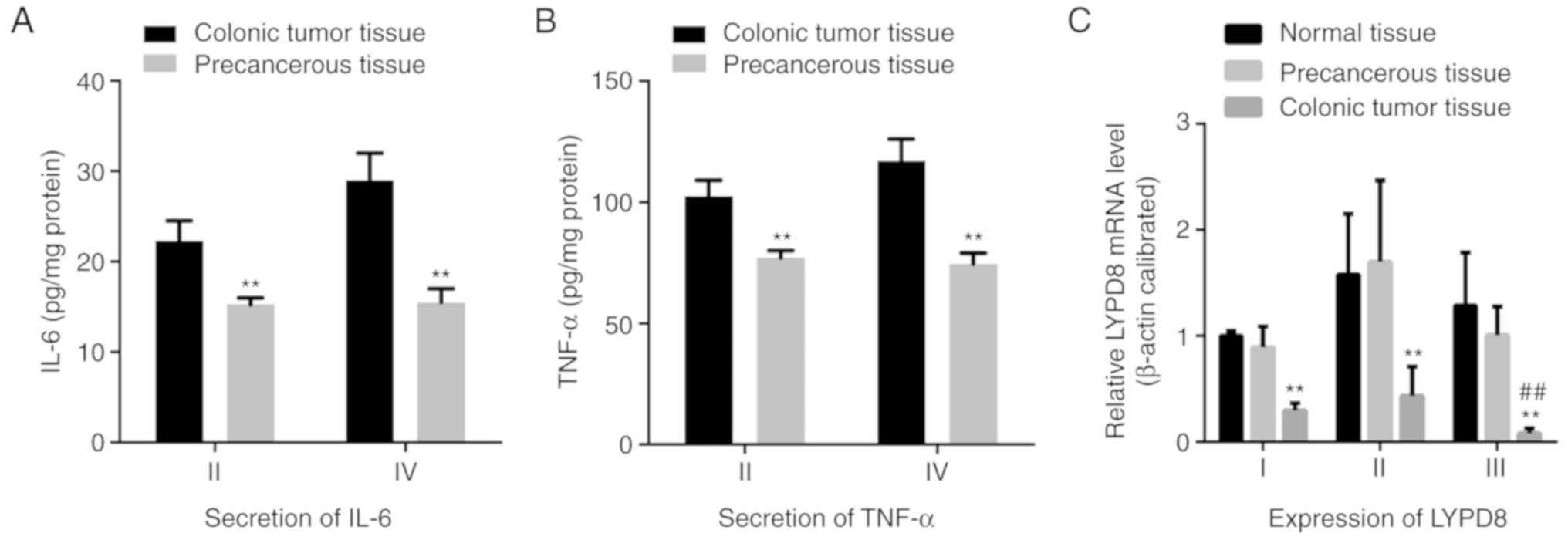
different stages. As shown in Fig. 2A
and B, the secreted levels of IL-6 and TNF-α in stage II and IV
CRC tissues were significantly increased compared with those in
corresponding precancerous tissues. These results revealed
increased expression of IL-6 and TNF-α in CRC tissues. Following
this, the gene expression levels of LYPD8 in stage I, II and III
CRC tissue; precancerous tissue; and normal tissue were assessed
using RT-qPCR analysis (Fig. 2C).
Compared with the precancerous tissue and normal tissue, the gene
expression of LYPD8 was significantly reduced in stage I, II and
III tissues. Furthermore, the expression of LYPD8 was reduced in
stage III tissue compared with that in stage I tissue. Taken
together, when STAT3/P65 is phosphorylated and IL-6/TNF-α is
actively expressed, reduced expression of LYPD8 is noted in colonic
cancer tissue at different stages.
Construction and overexpression of
LYPD8 in CRC cells
The plasmid DNA for overexpressing LYPD8 was
constructed using the eukaryotic expression vector (pIRES2), as
shown in Fig. 3A and B, and the
relative expression levels of LYPD8 in the HT29, SW480 and SW620
cells were examined by RT-qPCR analysis. The lowest expression of
LYPD8 was noted in the SW480 cells, leading to selection of this
cell line for the subsequent experiments. As shown in Fig. 3C, the gene expression of LYPD8 was
significantly increased in the LYPD8 overexpression group (LYPD8
OE) compared with that in the empty pIRES2 group (control). These
results indicated that the LYPD8 overexpression vector had been
successfully used to induce the overexpression of LYPD8 in SW480
cells.
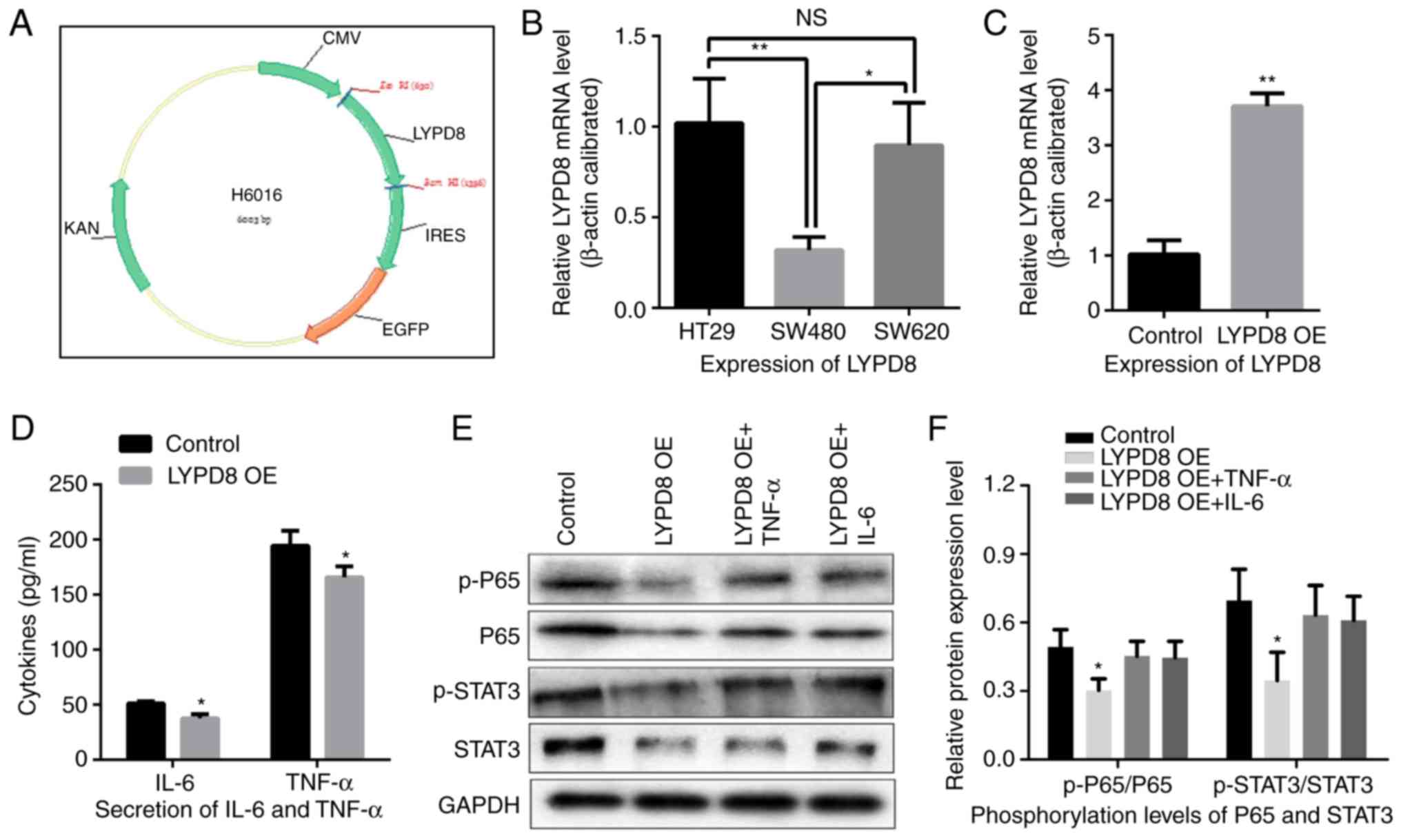 | Figure 3.Effects of the overexpression of LYPD8
on IL-6/TNF-α secretion and STAT3/P65 dephosphorylation in CRC
cells. (A) cDNA coding for the LYPD8 gene was cloned into the
eukaryotic expression vector. (B) Expression levels of LYPD8 in
different CRC cells were determined by RT-qPCR analysis, using
β-actin as a control. (C) Overexpression of LYPD8 via transient
transfection into SW480 cells was confirmed by RT-qPCR analysis.
(D) IL-6/TNF-α secretion was analyzed by ELISA in LYPD8 OE groups
of SW480 cells. (E) Western blotting revealed expression levels of
p-P65, P65, p-STAT3 and STAT3 in the control, LYPD8 OE, LYPD8 OE +
TNF-α and LYPD8 OE + IL-6 groups of SW480 cells. GAPDH was used as
a control. (F) Band intensities of western blotting for p-P65/P65
and p-STAT3/STAT3 in the control, LYPD8 OE, LYPD8 OE + TNF-α and
LYPD8 OE + IL-6 groups were analyzed. The data are reported as the
mean ± standard deviation of experiments (n=4). *P<0.05,
phosphorylation levels of P65 and STAT3 in LYPD8 OE groups vs.
control, LYPD8 OE + TNF-α and LYPD8 OE + IL-6 groups; **P<0.01,
expression of LYPD8 in control group vs. LYPD8 OE group. Control,
empty pIRES2; LYPD8, Ly6/Plaur domain-containing 8; OE,
overexpression; IL-6, interleukin-6; TNF-α, tumor necrosis
factor-α; STAT3, signal transducer and activator of transcription
3; p-, phosphorylated; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; NS, not significant. |
Subsequently, IL-6 and TNF-α secretion were assessed
in the LYPD8-overexpressing SW480 cells using ELISA. As shown in
Fig. 3D, compared with the control
group, IL-6 and TNF-α secretion were decreased in the LYPD8 OE
group. This result revealed that the overexpression of LYPD8
inhibited the secretion of IL-6 and TNF-α in SW480 cells. The
protein expression levels of P65, p-P65, STAT3 and p-STAT3 in SW480
cells were then examined in the control, LYPD8 OE, LYPD8 OE + TNF-α
and LYPD8 OE + IL-6 groups, as demonstrated by western blotting. As
shown in Fig. 3E and F, compared
with the control group, the phosphorylation levels of p-P65/P65 and
p-STAT3/STAT3 were significantly reduced in the LYPD8 OE group.
However, this trend was restored when the SW480 cells were
supplemented with TNF-α (5 ng/ml) and IL-6 (50 ng/ml) in the
culture medium. These results suggest that the overexpression of
LYPD8 in SW480 cells induced the dephosphorylation of P65 and STAT3
and subsequently inhibited the secretion of TNF-α and IL-6.
Overexpression of LYPD8 inhibits CRC
cell proliferation and migration
To investigate cell viability under the
overexpression of LYPD8, CCK-8 assays were performed using SW480
cells. As shown in Fig. 4A, the
overexpression of LYPD8 in SW480 cells inhibited cell viability
compared with the control group. When the SW480 cells were
supplemented with TNF-α (5 ng/ml) and IL-6 (50 ng/ml) in the
culture medium, the cell viability of the LYPD8 OE + TNF-α and
LYPD8 OE + IL-6 groups was similar to that of the control group.
This result indicated that the overexpression of LYPD8 inhibits
SW480 cell viability, whereas TNF-α and IL-6 restore viability.
Furthermore, it was found that niclosamide (STAT3 inhibitor) and
JSH-23 (NF-κB inhibitor) also inhibit cell viability, similar to
the overexpression of LYPD8 in SW480 cells (Fig. 4B). This result indicated that the
overexpression of LYPD8 inhibited SW480 cell viability, and that
this trend was similar to supplementation of STAT3 inhibitor and
NF-κB inhibitor in the culture medium. Transwell assays were used
to examine the effect of the overexpression of LYPD8 on SW480 cell
migration. As shown in Fig. 4C and
D, a more marked inhibitory effect on cell migration was
observed in the LYPD8 OE group compared with that in the untreated
cells group and control group. This result indicated that the
overexpression of LYPD8 inhibits SW480 cell migration.
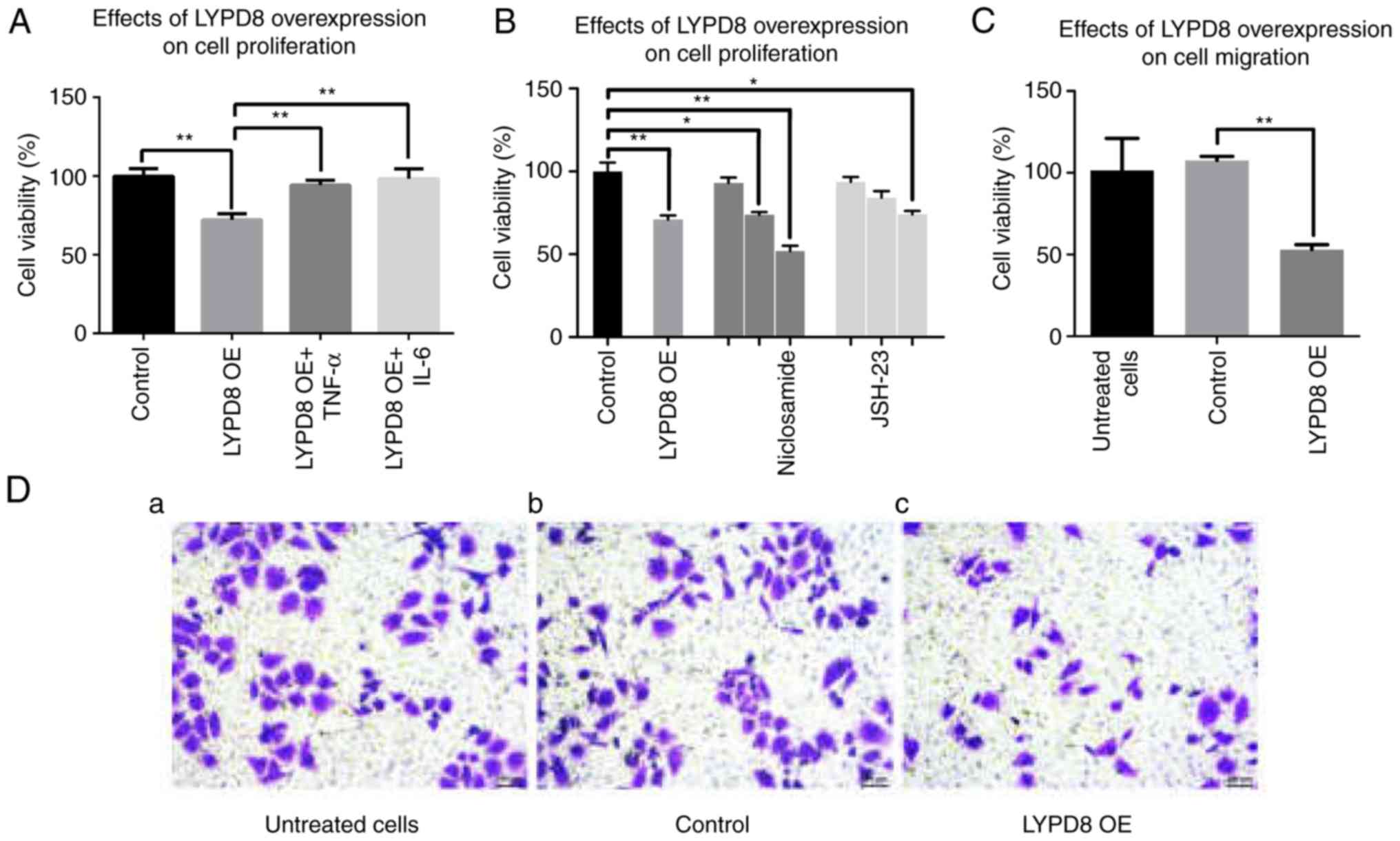 | Figure 4.Effects of the overexpression of LYPD8
on SW480 cell proliferation and migration. (A) Cell viability of
the control, LYPD8 OE, LYPD8 OE + TNF-α and LYPD8 OE + IL-6 groups
of SW480 cells. (B) SW480 cells were treated with different
concentrations (0.5, 1 and 2 µM) of niclosamide and different
concentrations (5, 15 and 30 µM) of JSH-23, respectively. (C)
Numbers of migratory SW480 cells from the untreated, control and
LYPD8 OE groups. (D) Transwell assay of SW480 cells from the (a)
untreated, (b) control and (c) LYPD8 OE groups (magnification,
×200). The data are reported as the mean ± standard deviation of
experiments (n=4). *P<0.05, **P<0.01. Control, empty pIRES2
group; LYPD8, Ly6/Plaur domain-containing 8; OE, overexpression;
IL-6, interleukin-6; TNF-α, tumor necrosis factor-α. |
Discussion
In colonic tumor tissue, a large number of
inflammatory factors promote inflammation and increase incidence
(19). An interactive network
between cancer cells and the surrounding inflammatory
microenvironment is formed, which promotes cancer cell growth,
proliferation and migration (20).
Inflammatory disorder induces the dysfunction of colon
cancer-related oncogenes and tumor suppressor genes, which lead to
tumorigenesis (21). Inflammatory
reactions also lead to the progression and spread of CRC cells
(22). LYPD8 is specifically
expressed in colonic epithelial cells, whereas its expression is
reduced in colonic epithelial cells from patients with UC (23). Mice lacking Lypd8 lack the
bacteria-free space immediately above the epithelial layer of the
colon, indicating that Lypd8 is critical for the segregation of
intestinal bacteria and colonic epithelia (1,3).
Therefore, LYPD8 dysfunction is an important factor in the
generation of colitis and an important regulatory protein in the
development of colon cancer.
IL-6 and TNF-α are important mediators involved in
inflammatory reactions and serve an important regulatory role in
cell behavior (24). IL-6 is a
cytokine that is involved in the physiological and pathological
processes of numerous types of cancer (25). Previous studies have reported that
IL-6 is an important regulator in the progression and metastasis of
certain tumors (26). TNF-α is
secreted by macrophages or activated T cells and can promote cancer
cell growth and metastasis (27). A
high expression of TNF-α leads to disorderly association with other
cytokines and is involved in disease mechanisms (28). The results of the present study
demonstrated that the expression levels of IL-6 and TNF-α in CRC
tumor tissues were significantly increased compared with those in
precancerous tissues, which is consistent with previous studies.
The immune response is a regulatory response in the development of
CRC involving inflammatory factors, including IL-6 and TNF-α
(29). The results of the present
study demonstrated that the overexpression of LYPD8 in CRC cells
inhibited the secretion of IL-6 and TNF-α.
IL-6 mediates signaling pathway transduction and the
activation of transcriptional factors through the STAT3 signaling
pathway to regulate tumor cell proliferation and migration
(30). As STAT3 is required for the
survival of intestinal epithelial cells and maintenance of mucosal
integrity, excessive interference with systemic STAT3 activation
can potentially cause gastrointestinal damage (31). When stimulated with extracellular
cytokines, including IL-6, the tyrosine group of STAT3 is
phosphorylated to p-STAT3, and homo or heterodimers form via the
SH2 region. Following this, p-STAT3 dimers are transferred into the
nucleus and induce the abnormal expression of downstream genes
(32). In the present study,
p-STAT3 was detected in SW480 cells of the control, LYPD8 OE, LYPD8
OE + TNF-α and LYPD8 OE + IL-6 groups, and the overexpression of
LYPD8 reduced the phosphorylation of STAT3.
Previous studies have demonstrated that TNF-α
upregulates NF-κB signaling pathway activity (33). NF-κB is a protein that exhibits
transcriptional function. NF-κB initiates gene transcription
involved in pathological processes with or without other cytokines
(34). Due to the stimulating
effect on tumor development by NF-κB, a series of drugs have been
developed that target the activity of NF-κB (35). NF-κB exists in cells is a dimeric
state. P65/p50 heterodimers are the most common state, which are
widely distributed in cells and are involved in the expression and
regulation of multiple genes (36).
The results of the present study demonstrated that the
overexpression of LYPD8 promoted the phosphorylation of P65,
leading to a change of cell behavior. In addition, the results
demonstrated that the secretion of IL-6 and TNF-αin CRC tissues was
significantly increased compared with that in corresponding
precancerous tissues, and the phosphorylation of STAT3/P65 was
induced. By contrast, the expression of LYPD8 was significantly
reduced in stage I, II, and III CRC tissues. These investigations
remain in progress, and the aim of future investigations is to
continue to identify the association between the protein expression
of LYPD8 and the development of CRC.
In conclusion, the present study used clinical
samples and revealed the association between LYPD8 and the
expression of IL-6/TNF-α. The mechanism by which LYPD8 is involved
in regulating the STAT3 signaling pathway in CRC, and the effect of
the overexpression of LYPD8 on CRC behaviors were examined. To the
best of our knowledge, the present study is the first to examine
the associations among LYPD8, inflammatory factors and the
occurrence and development of CRC. To investigate the effect of
LYPD8, the expression of LYPD8 was examined in clinical samples and
then overexpressed in CRC cells. Previous studies had an important
implication for examining the association between the inflammatory
microenvironment and tumors. The present study provides a
theoretical and experimental basis for the design of
anti-inflammatory-targeted treatment for CRC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81771502 and
81701820), the Natural Science Foundation of Zhejiang Province
(grant no. LH19H160001) and the Medical Health Science and
Technology Project of Zhejiang Provincial Health Commission (grant
nos. 2018KY473, 2018PY025 and 2019KY737).
Availability of data and materials
The datasets generated during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
JX and JQ completed all trial procedures, the data
analysis and were major contributors in writing the manuscript.
WZhang was involved in the collection and processing of human
cancer specimens. EC, GZ and GC guided the histopathological study
of immunohistochemistry and all the methods and ideas of the cell
experiments. FW and XS participated in the completion of the PCR
and ELISA test procedures, and participated in the cultivation of
cells. WZhou and ZS designed the overall idea of the experiment and
provided theoretical guidance throughout the process, and
participated in the revision of the manuscript and the processing
of data. All authors read and approved the manuscript and agree to
be accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All experiments using human tissues were approved by
the local institutional Ethics Committee (institutional review
board reference no. 20140213-19). Written informed consent was
obtained from patients prior to their inclusion in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Okumura R, Kurakawa T, Nakano T, Kayama H,
Kinoshita M, Motooka D, Gotoh K, Kimura T, Kamiyama N, Kusu T, et
al: Lypd8 promotes the segregation of flagellated microbiota and
colonic epithelia. Nature. 532:117–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsu C, Okumura R and Takeda K: Human LYPD8
protein inhibits motility of flagellated bacteria. Inflamm Regen.
37:232017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okumura R and Takeda K: Roles of
intestinal epithelial cells in the maintenance of gut homeostasis.
Exp Mol Med. 49:e3382017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nishino K, Nishida A, Inoue R, Kawada Y,
Ohno M, Sakai S, Inatomi O, Bamba S, Sugimoto M, Kawahara M, et al:
Analysis of endoscopic brush samples identified mucosa-associated
dysbiosis in inflammatory bowel disease. J Gastroenterol.
53:95–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Terzić J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology. 138:2101–2114.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y, Han ZP, Zhang SS, Jing YY, Bu XX,
Wang CY, Sun K, Jiang GC, Zhao X, Li R, et al: Effects of
inflammatory factors on mesenchymal stem cells and their role in
the promotion of tumor angiogenesis in colon cancer. J Biol Chem.
286:25007–25015. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Waldner M and Neurath M: Master regulator
of intestinal disease: IL-6 in chronic inflammation and cancer
development. Semin Immunol. 26:75–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu X, Huang C, He X, Tian YY, Zhou DX, He
Y, Liu XH and Li J: Feedback regulation of telomerase reverse
transcriptase: New insight into the evolving field of telomerase in
cancer. Cell Signal. 25:2462–2468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oh H and Ghosh S: NF-κB: Roles and
regulation in different CD4+ T-cell subsets. Immunol
Rev. 252:41–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Simone V, Franzè E, Ronchetti G,
Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald
TT, Pallone F, et al: Th17-type cytokines, IL-6 and TNF-α
synergistically activate STAT3 and NF-κB to promote colorectal
cancer cell growth. Oncogene. 34:3493–3503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cortez M, Carmo LS, Rogero MM, Borelli P
and Fock RA: A high-fat diet increases IL-1, IL-6, and TNF-α
production by increasing NF-κB and attenuating PPAR-γ expression in
bone marrow mesenchymal stem cells. Inflammation. 36:379–386. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garbers C, Aparicio-Siegmund S and
Rose-John S: The IL-6/gp130/STAT3 signaling axis: Recent advances
towards specific inhibition. Curr Opin Immunol. 34:75–82. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siveen KS, Sikka S, Surana R, Dai X, Zhang
J, Kumar AP, Tan BK, Sethi G and Bishayee A: Targeting the STAT3
signaling pathway in cancer: Role of synthetic and natural
inhibitors. Biochim Biophys Acta. 1845:136–154. 2014.PubMed/NCBI
|
|
14
|
Chen J, Wang J, Lin L, He L, Wu Y, Zhang
L, Yi Z, Chen Y, Pang X and Liu M: Inhibition of STAT3 signaling
pathway by nitidine chloride suppressed the angiogenesis and growth
of human gastric cancer. Mol Cancer Ther. 11:277–287. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang C, Yang G, Jiang T, Zhu G, Li H and
Qiu Z: The effects and mechanisms of blockage of STAT3 signaling
pathway on IL-6 inducing EMT in human pancreatic cancer cells in
vitro. Neoplasma. 58:396–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu J, Zhu C, Zhang Y, Jiang N, Li S, Su Z,
Akaike T and Yang J: hE-cadherin-Fc fusion protein coated surface
enhances the adhesion and proliferation of human mesenchymal stem
cells. Colloids Surf B Biointerfaces. 109:97–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gleeson M, Bishop NC, Stensel DJ, Lindley
MR, Mastana SS and Nimmo MA: The anti-inflammatory effects of
exercise: Mechanisms and implications for the prevention and
treatment of disease. Nat Rev Immunol. 11:607–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elinav E, Nowarski R, Thaiss C, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Balkwill FR and Mantovani A:
Cancer-related inflammation: Common themes and therapeutic
opportunities. Semin Cancer Biol. 22:33–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Erreni M, Mantovani A and Allavena P:
Tumor-associated macrophages (TAM) and inflammation in colorectal
cancer. Cancer Microenviron. 4:141–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allaire JM, Morampudi V, Crowley SM, Stahl
M, Yu H, Bhullar K, Knodler LA, Bressler B, Jacobson K and Vallance
BA: Frontline defenders: Goblet cell mediators dictate host-microbe
interactions in the intestinal tract during health and disease. Am
J Physiol Gastrointest Liver Physiol. 314:G360–G377. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Popko K, Gorska E, Stelmaszczyk-Emmel A,
Plywaczewski R, Stoklosa A, Gorecka D, Pyrzak B and Demkow U:
Proinflammatory cytokines Il-6 and TNF-α and the development of
inflammation in obese subjects. Eur J Med Res. 15
(Suppl):S120–S122. 2010.
|
|
25
|
Mihara M, Hashizume M, Yoshida H, Suzuki M
and Shiina M: IL-6/IL-6 receptor system and its role in
physiological and pathological conditions. Clin Sci. 122:143–159.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McAllister SS and Weinberg RA: The
tumour-induced systemic environment as a critical regulator of
cancer progression and metastasis. Nat Cell Biol. 16:717–727. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guan YQ, Li Z and Liu JM: Death signal
transduction induced by co-immobilized TNF-α plus IFN-γ and the
development of polymeric anti-cancer drugs. Biomaterials.
31:9074–9085. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Emi Aikawa N, de Carvalho JF, Artur
Almeida Silva C and Bonfá E: Immunogenicity of anti-TNF-alpha
agents in autoimmune diseases. Clin Rev Allergy Immunol. 38:82–89.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zamarron BF and Chen W: Dual roles of
immune cells and their factors in cancer development and
progression. Int J Biol Sci. 7:651–658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo Y, Xu F, Lu T, Duan Z and Zhang Z:
Interleukin-6 signaling pathway in targeted therapy for cancer.
Cancer Treat Rev. 38:904–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Neurath MF and Finotto S: IL-6 signaling
in autoimmunity, chronic inflammation and inflammation-associated
cancer. Cytokine Growth Factor Rev. 22:83–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin L, Hutzen B, Zuo M, Ball S, Deangelis
S, Foust E, Pandit B, Ihnat MA, Shenoy SS, Kulp S, et al: Novel
STAT3 phosphorylation inhibitors exhibit potent growth-suppressive
activity in pancreatic and breast cancer cells. Cancer Res.
70:2445–2454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lü L, Tang D, Wang L, Huang LQ, Jiang GS,
Xiao XY and Zeng FQ: Gambogic acid inhibits TNF-α-induced invasion
of human prostate cancer PC3 cells in vitro through PI3K/Akt and
NF-κB signaling pathways. Acta Pharmacol Sin. 33:531–541. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ben-Neriah Y and Karin M: Inflammation
meets cancer, with NF-κB as the matchmaker. Nat Immunol.
12:715–723. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miller S, Huang R, Sakamuru S, Shukla SJ,
Attene-Ramos MS, Shinn P, Van Leer D, Leister W, Austin CP, Xia M,
et al: Identification of known drugs that act as inhibitors of
NF-kappaB signaling and their mechanism of action. Biochem
Pharmacol. 79:1272–1280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wan F and Lenardo MJ: The nuclear
signaling of NF-kappaB: Current knowledge, new insights, and future
perspectives. Cell Res. 20:24–33. 2010. View Article : Google Scholar : PubMed/NCBI
|