Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
seventh most common cancer type worldwide, with >500,000 new
cases of HNSCC diagnosed worldwide every year. Approximately 60% of
HNSCC are diagnosed at an advanced stage, and the prognosis is
poor, despite the numerous forms of treatment available (1,2). In
recent decades, the 5-year survival rate has improved (3). Although the development of new
therapies and advanced examination techniques has prolonged the
life of the patients, HNSCC is considered difficult to treat, with
the exception of early stage tumors (3). In order to increase patient quality of
life, a strategy for the early detection of HNSCC is urgently
required. Several biomarkers have been associated with the
diagnosis and prognosis of HNSCC, but few have demonstrated
adequate clinical efficacy (4).
Programmed death-ligand 1 (PD-L1) is an immune
co-stimulatory molecule that belongs to the B7-H gene family
(5). It is expressed on many tumor
cell types and the surface of immune cells, including B cells, T
cells, myeloid dendritic cells and macrophages (6). PD-L1 serves an important role in
regulating cellular immunity. PD-L1 combined with programmed cell
death protein 1 (PD-1) inhibits the migration and proliferation of
T cells, as well as the secretion of cytotoxic mediators, thus
limiting its antitumor effects on tumor cells (7,8).
Therefore, anti-PD-L1 monoclonal antibodies (αPD-L1) are effective
in oncotherapy, and antitumor immunity may be enhanced by
inhibiting the expression of PD-L1 (9). Anti-PD-L1 monoclonal antibodies have
shown considerable promise in the treatment of melanoma, renal cell
carcinoma and non-small cell lung cancer (10). The PD-L1/PD-1 axis has gained
increasing attention in cancer immunotherapy and immunopathogenesis
(11). The majority of research has
focused on antitumor immunity, particularly in T cells. However,
tumor-intrinsic PD-L1 signals have not been extensively
investigated (12). Recently, the
regulation of tumor cell proliferation by PD-L1 family members has
gained increasing attention; it has been revealed that PD-L1
participates in epithelial-mesenchymal transition (EMT) regulation
(13,14), and is closely associate with cell
cycle progression (15,16) and the expression of proliferation
marker Ki-67 in human breast cancer (16). Based on these previous findings, it
was speculated that tumor-intrinsic PD-L1 signaling may have broad
biological effects, which require further investigation.
In the present study, the function of PD-L1 in HNSCC
was investigated. Immunohistochemical analysis was used to detect
PD-L1 expression in HNSCC tissues. Next, it was demonstrated that
PD-L1 promoted HNSCC cell proliferation in vitro and in
vivo. In addition, the potential mechanisms underlying the
PD-L1-mediated increase in HNSCC cell proliferation were explored.
The current research aimed to provide experimental evidence for the
use of PD-L1 as a therapeutic target in HNSCC.
Materials and methods
Patient selection and tissue
microarray (TMA)
The independent tissue microarrays were purchased
(LP803 and LP804; US Biomax, Inc., Rockville, MD, USA). The tissue
microarrays consisted of 110 laryngocarcinoma tissues, five normal
laryngeal tissues and five samples of normal adjacent laryngeal
tissue (NAT), which included 106 men and 14 women. Detailed tissue
microarray information is presented in Tables I and II.
 | Table I.LP803 tissue microarray patient
characteristics. |
Table I.
LP803 tissue microarray patient
characteristics.
| Characteristic | Number of patients
(%) |
|---|
| Number of
patients | 80 (100) |
| Mean age,
years | 57.35±8.47 |
| Sex |
|
|
Female | 9 (11.25) |
|
Male | 71 (88.75) |
| Localization |
|
|
Larynx | 80 (100) |
|
Differentiation |
|
| 1 | 24 (33.8) |
| 2 | 42 (59.2) |
| 3 | 5 (7.0) |
 | Table II.LP804 tissue microarray patient
characteristics. |
Table II.
LP804 tissue microarray patient
characteristics.
| Characteristic | Number of patients
(%) |
|---|
|
|---|
| A, Patients with
laryngocarcinoma |
|---|
| Number of
patients | 40 (100) |
| Mean age,
years | 55.25±13.39 |
| Sex |
|
|
Female | 5 (12.5) |
|
Male | 35 (87.5) |
| Localization |
|
|
Larynx | 40 (100) |
| T
classification |
|
| T1 | 3 (10.3) |
| T2 | 11 (38) |
| T3 | 3 (10.3) |
| T4 | 12 (41.4) |
| N
classification |
|
| N0 | 22 (73.3) |
| N+ | 8 (26.7) |
|
Differentiation |
|
| 1 | 12 (41.4) |
| 2 | 15 (51.7) |
| 3 | 2 (6.9) |
|
| B, Healthy
controls |
|
| Number of
patients | 10 (100) |
|
NAT | 5 (50) |
| Normal
laryngeal tissue | 5 (50) |
Immunohistochemical staining and
evaluation
PD-L1 antibody (cat. no. 13684; Cell Signaling
Technology, Inc., Danvers, MA, USA) was used for
immunohistochemical staining, and the method of immunohistochemical
staining was performed as previously described (17). To further analyze the
immunohistochemical staining results, all TMAs were scored for
frequency (0–4) and intensity (0–3) under an Olympus BX51
microscope (Olympus Corp., Tokyo, Japan). The scores of frequency
were assigned when 0–25, 26–50, 51–75 and 76–100% of the tumor
cells stained positive. The scores of intensity (0–3) respectively
represented: 0, negative; 1, weak; 2, moderate; and 4, strong. The
composite expression scores (CES) utilized the following formula:
CES = intensity × frequency.
Cell culture and transfection
Two HNSCC cell lines (Cal-27 and FaDu) were
purchased from Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). All cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.), 20 mg/ml ampicillin and 20 mg/ml
kanamycin at 37°C in an incubator with 5% CO2.
To generate the stable PD-L1-overexpressing
(PD-L1over) and PD-L1 knockdown (PD-L1RNAi;
lentivirus transduction particles containing PD-L1 shRNA) cell
lines, as well as negative control (NC) groups (PD-L1over
NC and PD-L1RNAi NC), lentivirus transduction
particles containing GFP label (cat. nos. GOCL3581014115 and
GICL2481014115; Shanghai GeneChem Co., Ltd., Shanghai, China) were
transfected (multiplicity of infection=20) into Cal-27 and FaDu
cells (2×105 cells/well), and the stable transfected
Cal-27 and FaDu cell lines were selected by culturing for 1 week in
complete medium (DMEM with 10% FBS, ampicillin and kanamycin),
which also contained puromycin (2 µg/ml).
Cell proliferation and colony
formation assay
Cell proliferation was determined using Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) and EdU incorporation (cat. no. C10310-1;
Guangzhou RiboBio Co., Ltd., Shanghai, China) assays, according to
the manufacturer's instructions. For the CCK-8 assay, cells were
seeded in 96-well plates at 1×103 cells/well in DMEM
with 10% FBS, and treated with rapamycin (10 nM) for the total
culture period of 72 h. The absorbance was measured at 450 nm by a
microplate reader (Perkin Elmer) at 0, 24, 48 and 72 h. For the EdU
proliferation assay, tumor cells (5×106 cells/well) were
plated into 6-well plates in DMEM with 10% FBS, and directly
labeled using the Cell-Light™ EdU Apollo® 567 in
vitro Imaging kit, according to the manufacturer's
instructions.
For the colony formation assay, cells were seeded
into 6-well plates (200 cells/well) and incubated in complete
medium for 12 days at 37°C. The 6-well plates were washed with PBS
and stained with 0.1% crystal violet at room temperature for 15
min. Colonies which consisted of >50 cells were counted under an
Olympus IX51 microscope (Olympus Corp.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated with TRIzol™ reagent (Thermo
Fisher Scientific, Inc.). RNA (1 µg) was reverse transcribed using
the Super RT Reverse Transcriptase reagent kit (Beijing CoWin
Biotech Co., Ltd., Beijing, China) according to the manufacturer's
instructions. qPCR was conducted in a 25 µl reaction system, using
the 7500 Fast Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and amplified with transcript-specific
primers and SYBR®-Green Master Mix (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Relative gene expression was calculated using the 2−ΔΔCq
method (18), with GAPDH as the
internal control. PD-L1 (cat. no. HQP008443) and GAPDH (cat. no.
HQP006940) primers were purchased from GeneCopoeia, Inc.
(Rockville, MD, USA). The primer sequences were as follows: PD-L1
forward, 5′-TAGAATTCATGAGGATATTTGCTGTCTT-3′ and reverse,
5′-TAGGATCCTTACGTCTCCTCCAAATGTG-3′; GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3.
Xenograft study
Female BALB/c nude mice (n=20; 4 weeks old; 16–18 g)
were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China) and underwent adaptive
feeding 1 week before the experiment. Mice were housed at constant
temperature (20–25°C) and humidity (40–70%) in a 12 h light/dark
cycle, with free access to sterile water and standard chow. The
nude mice were randomly divided into four groups (PD-L1over
NC, PD-L1over, PD-L1RNAi NC and
PD-L1RNAi; n=5 each). Cal-27 cells were selected to
establish subcutaneous xenotransplanted tumor model since Cal-27
cells are more superior than FaDu cells in establishing a
subcutaneous xenotransplanted tumor model. Cells (2×106)
were suspended in PBS (200 µl cell suspension) and injected into
the right side of the mice's backs. Xenograft tumor diameters were
measured every week, and tumor volumes were calculated using the
following equation: Volume = 1/2 × length × width2. The
maximum tumor size was 20 mm. Nude mice were sacrificed and tumors
surgically removed 12 weeks after inoculation.
Western blotting
Cal-27 and FaDu cells were harvested and lysed in
radioimmunoprecipitation assay lysis buffer (Thermo Fisher
Scientific, Inc.) supplemented with protease and phosphatase
inhibitors (Roche Applied Science, Penzberg, Germany). Protein
concentration was determined by the bicinchoninic acid protein
assay. Lysates (20 µg of protein loaded per lane) were resolved by
10% SDS-PAGE, transferred to polyvinylidene difluoride membranes
and immunoblotted with specific primary antibodies (all 1:800)
overnight at 4°C against PD-L1 (cat. no. 9234T; Cell Signaling
Technology, Inc.), protein kinase B (Akt; cat. no. 4691T; Cell
Signaling Technology, Inc.), phosphorylated (p)-AktS473
(cat. no. 4060T; Cell Signaling Technology, Inc.), 70 kDa ribosomal
protein S6 kinase 1 (P70S6K; cat. no. 2708S; Cell Signaling
Technology, Inc.), p-P70S6KT389 (cat. no. 9234T; Cell
Signaling Technology, Inc.) and GAPDH (cat. no. 5174S; Cell
Signaling Technology, Inc.). Following immunoblotting with
IRDye® goat-anti rabbit IgG flourescence secondary
antibodies (dilution 1:20,000; cat. no. 926-32211; LI-COR
Biosciences, Lincoln, NE, USA) at room temperature for 1 h, the
membranes were scanned by an Odyssey infrared imaging system
(LI-COR Biosciences).
Statistical analysis
All values are expressed as the mean ± standard
deviation of three independent experimental repeats. Statistical
analyses were performed in SPSS 19.0 (SPSS, Inc., Chicago, IL,
USA), using one-way analysis of variance with Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
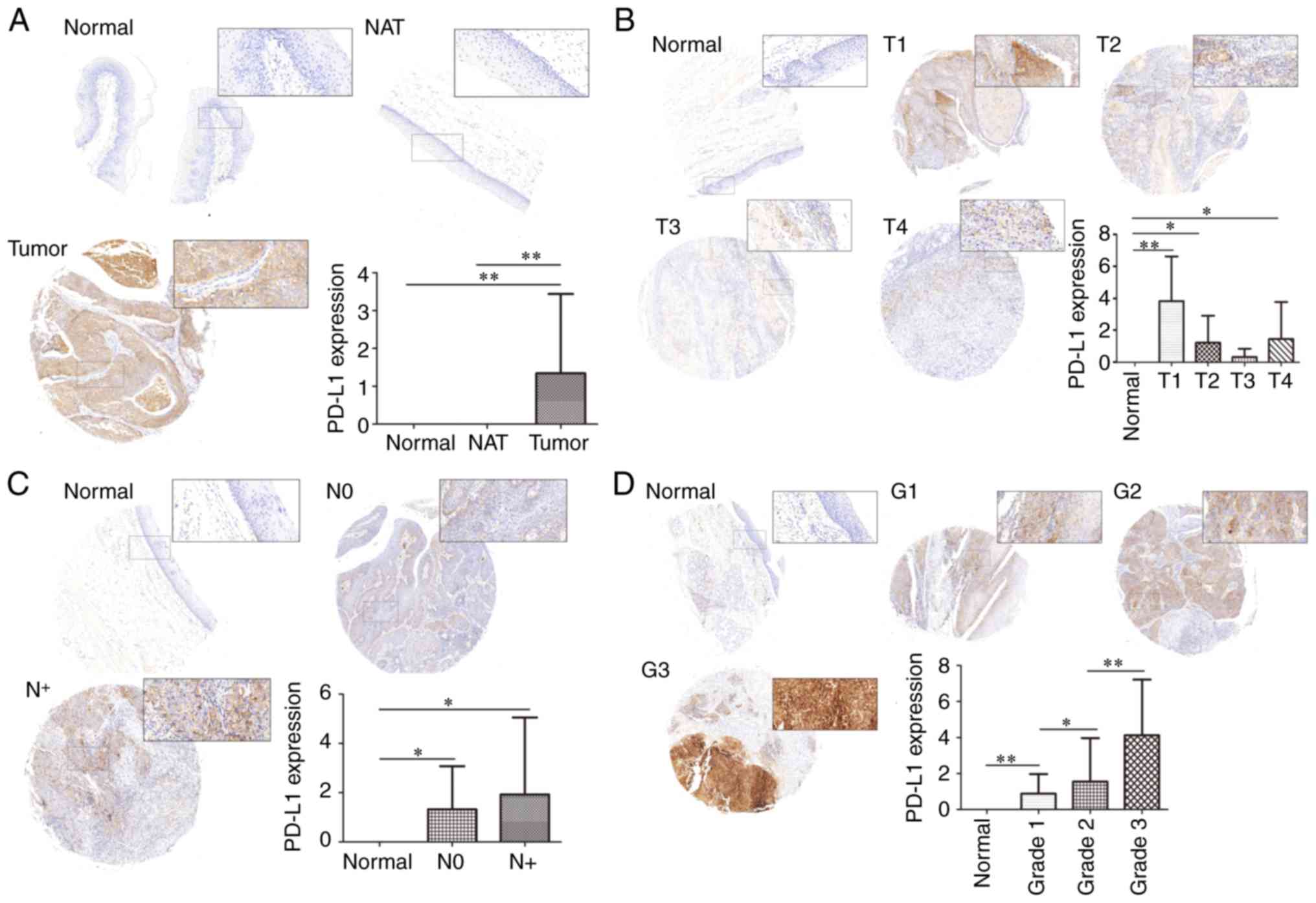
PD-L1 expression in HNSCC
In order to examine the expression of PD-L1 in
HNSCC, NAT and normal tissues, TMAs of HNSCCs stained for PD-L1
were analyzed, and the CES of every clinical sample was measured.
PD-L1 expression was compared and associated with clinical
characteristics, including tumor grade and TNM staging.
PD-L1 expression in tumor tissue was significantly
higher than in normal tissue and NAT (P<0.01), with no
significant difference in PD-L1 expression between normal tissue
and NAT (P>0.05; Fig. 1A).
T-stage analysis revealed that although the expression of PD-L1 in
normal tissue was significantly higher in T1 (P<0.01), T2
(P<0.05) and T4 (P<0.05), there was no statistical
significance in PD-L1 expression in T3 (P>0.05; Fig. 1B). It is likely that a larger number
of samples are required to fully investigate the relationship
between T-stage and PD-L1, as the sample sizes of T1 and T3 were
insufficient, and the expected increase in PD-L1 expression with T
stage progression was not observed. For the N-stage, representative
images were presented in Fig. 1C.
The CES of the PD-L1 protein expression revealed that there was no
significant differences between the N-stages (P>0.05). As
N-stage indicates regional lymph node metastasis, these results
suggest that PD-L1 may have no relationship with the regional
metastasis of HNSCC. Finally, the expression of PD-L1 among
different tumor grades was compared respectively, demonstrating
that an increased tumor grade was associated with increased PD-L1
expression (Fig. 1D).
PD-L1 regulates the proliferation and
colony formation in HNSCC cell lines in vitro
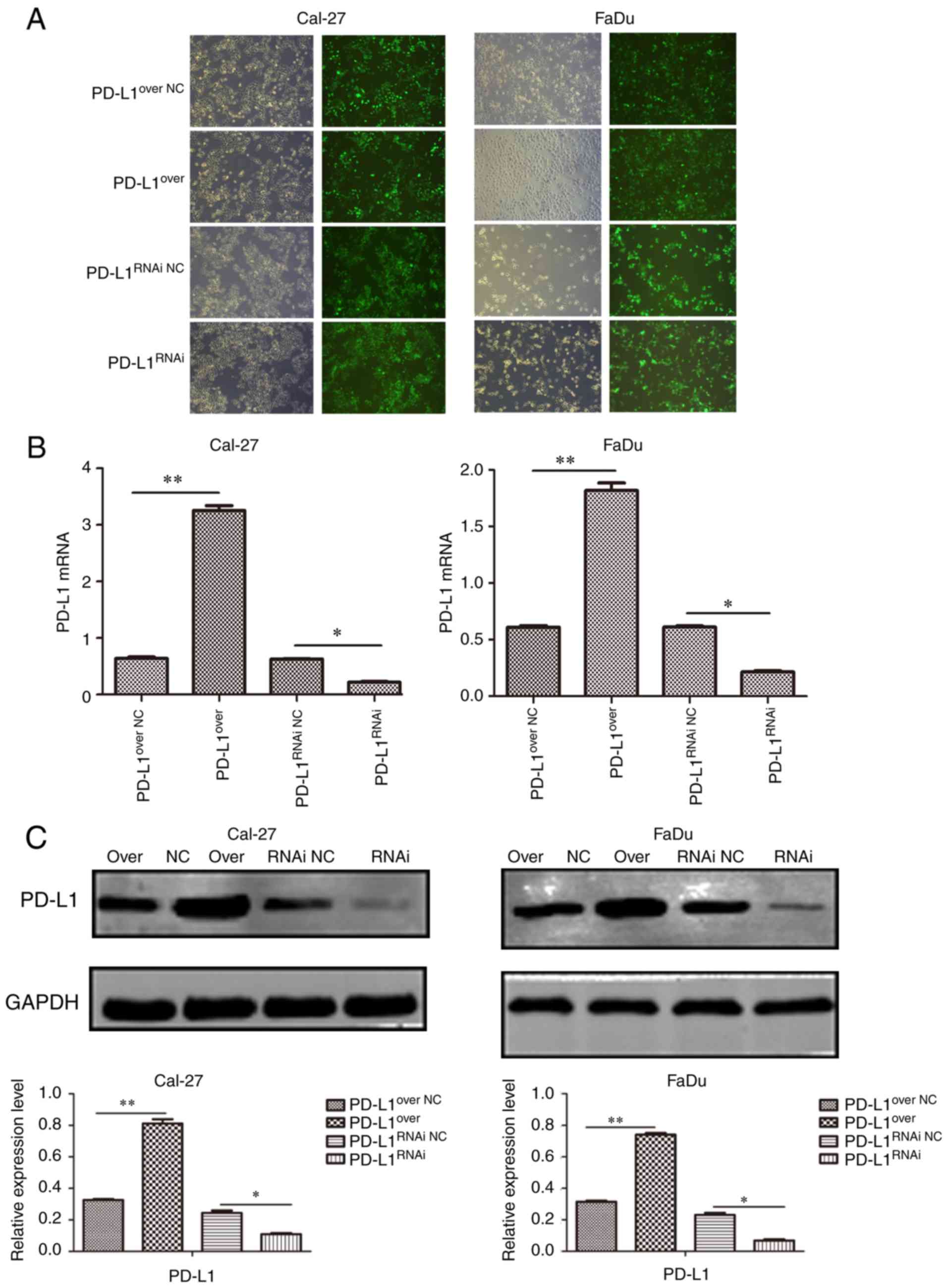
To explore the function of PD-L1, stable
PD-L1-overexpressing (PD-L1over), PD-L1 knockdown
(PD-L1RNAi) and negative control cells (PD-L1over
NC, PD-L1RNAi NC) were generated, and the
expression of PD-L1 was detected by RT-qPCR and western blotting.
The stably transfected Cal-27 and FaDu cells were established and
representative micrographs showed the immunofluorescence of GFP in
cells (Fig. 2A). It was
demonstrated that the lentiviral transduction particles
successfully altered PD-L1 protein (Fig. 2B) and gene (Fig. 2C) expression. PD-L1RNAi
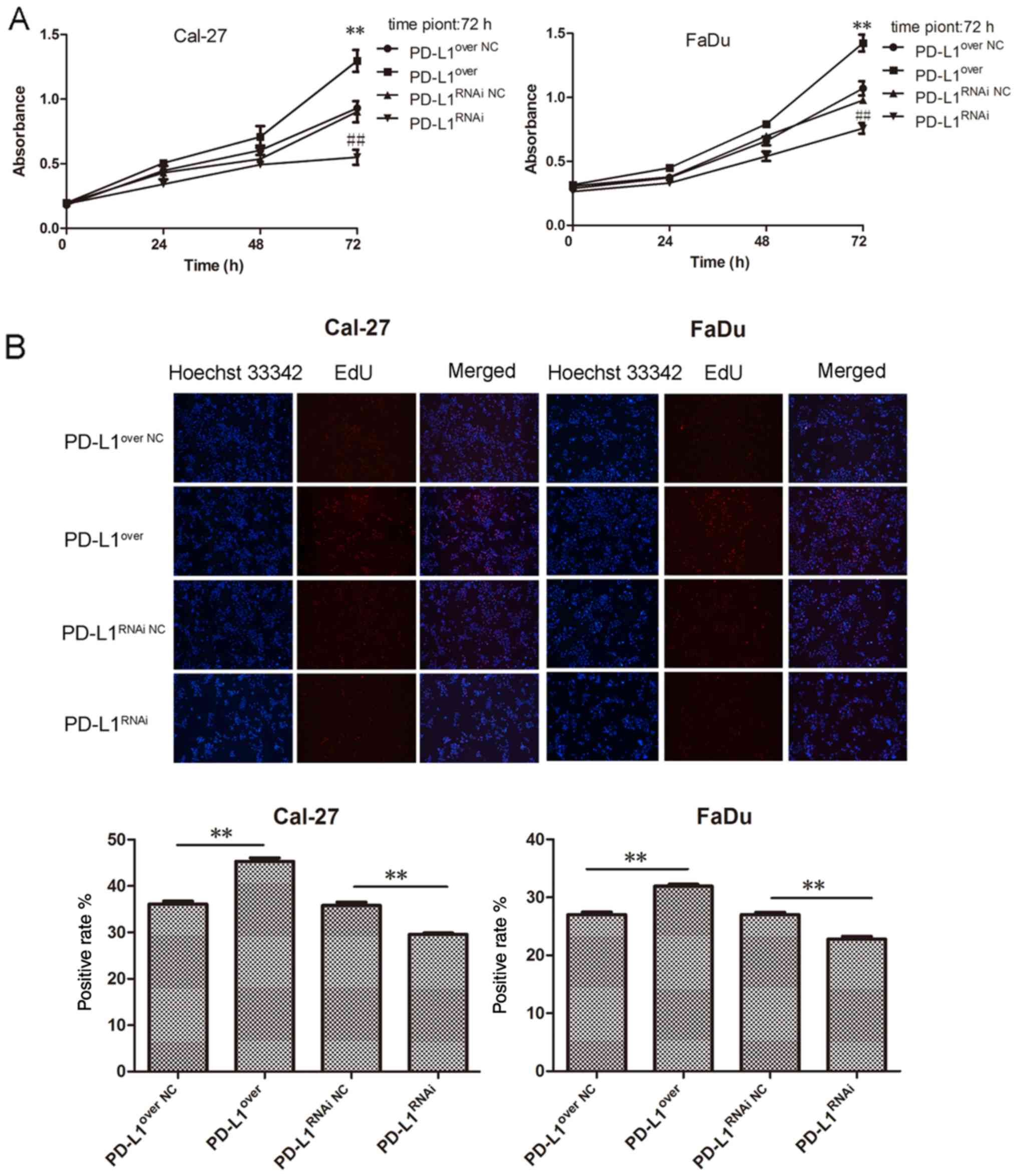
HNSCC cell proliferation was significantly reduced, compared with
its respective control group, with the PD-L1over cells
exhibiting the highest rate of proliferation (Fig. 3A). Furthermore, the EdU
proliferation assay revealed a similar trend, in which the
PD-L1-overexpressing cells exhibited the highest red fluorescence
(Fig. 3B). Furthermore, the colony
forming ability of PD-L1-overexpressing cells was remarkably
increased compared with the PD-L1over NC group, and
colony numbers in the PD-L1 knockdown group were significantly
reduced, compared with the PD-L1RNAi NC group (Fig. 3C).
PD-L1 upregulates mTOR signaling in
HNSCC cell lines
To investigate the potential mechanism by which
PD-L1 promoted cell growth in HNSCC cells, the expression levels of
proteins associated with mTOR signaling and cell proliferation were
detected by western blotting (Fig.
4A). Compared with their corresponding control groups,
p-P70S6KT389 and p-AktS473 expression was
significantly increased on the PD-L1over group, but
significantly decreased in the PD-L1RNAi group (Fig. 4B).
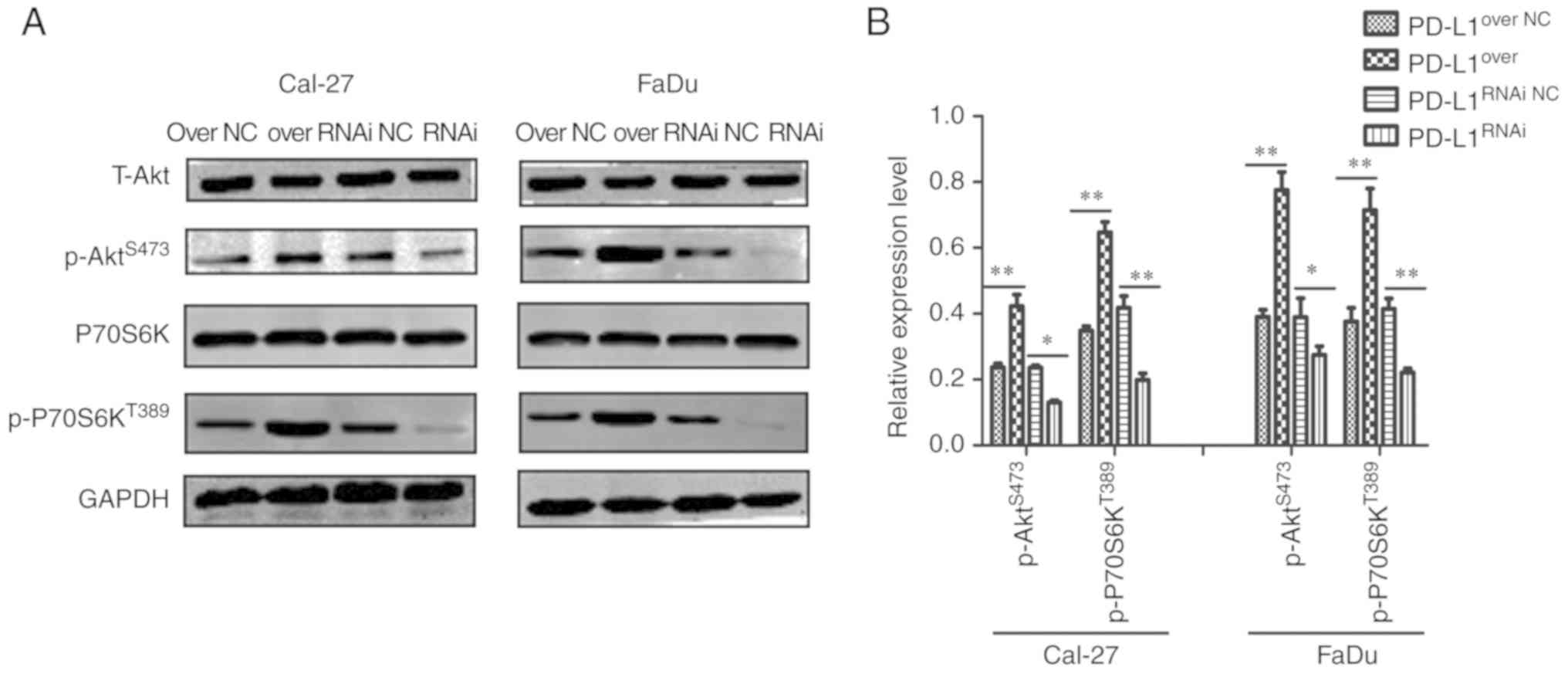 | Figure 4.PD-L1 upregulates mTOR signaling in
HNSCC cell lines. (A) Western blot analysis of T-Akt, P70S6K,
p-P70S6KT389, p-AktS473 and GAPDH expression
in PD-L1over, PD-L1RNAi and respective NC
cells. (B) p-P70S6KT389 and p-AktS473
expression in each group was quantified. *P<0.05 and
**P<0.01. PD-L1, programmed death-ligand 1; T-, total; P70S6K,
70 kDa ribosomal protein S6 kinase 1; p-, phosphorylated; Akt,
protein kinase B; PD-L1over, PD-L1-overexpressing;
PD-L1RNAi, PD-L1 knockdown; NC, negative control. |
Effect of PD-L1 expression on tumor
growth in vivo
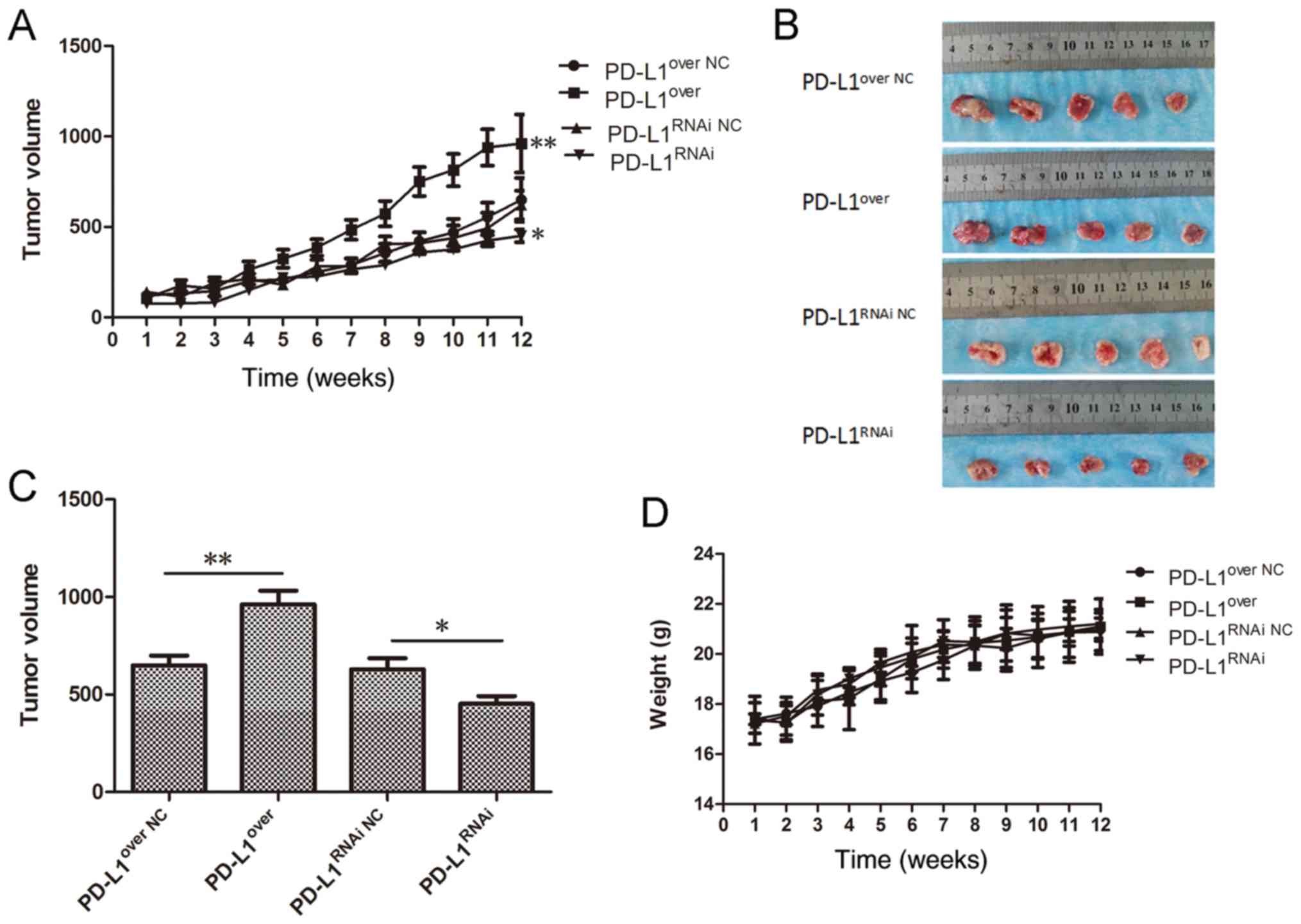
To validate whether the expression of PD-L1 affected
tumor growth in vivo, Cal-27 cells were used to establish a
xenograft mouse model. At the 12 week end point, the tumor growth
curve (Fig. 5A) showed that the
tumor volume in the Cal-27-PD-L1over group was
significantly larger than the control group (P<0.01), and the
tumor volume in the PD-L1RNAi group was the smallest
(P<0.05). The tumors were removed and measured on week 12
(Fig. 5B), and the average volume
was calculated (Fig. 5C). These
results demonstrated that PD-L1 accelerated tumor growth,
suggesting an important role for PD-L1 in regulating the tumor cell
growth in vivo. No significant differences in animal weight
were detected (Fig. 5D).
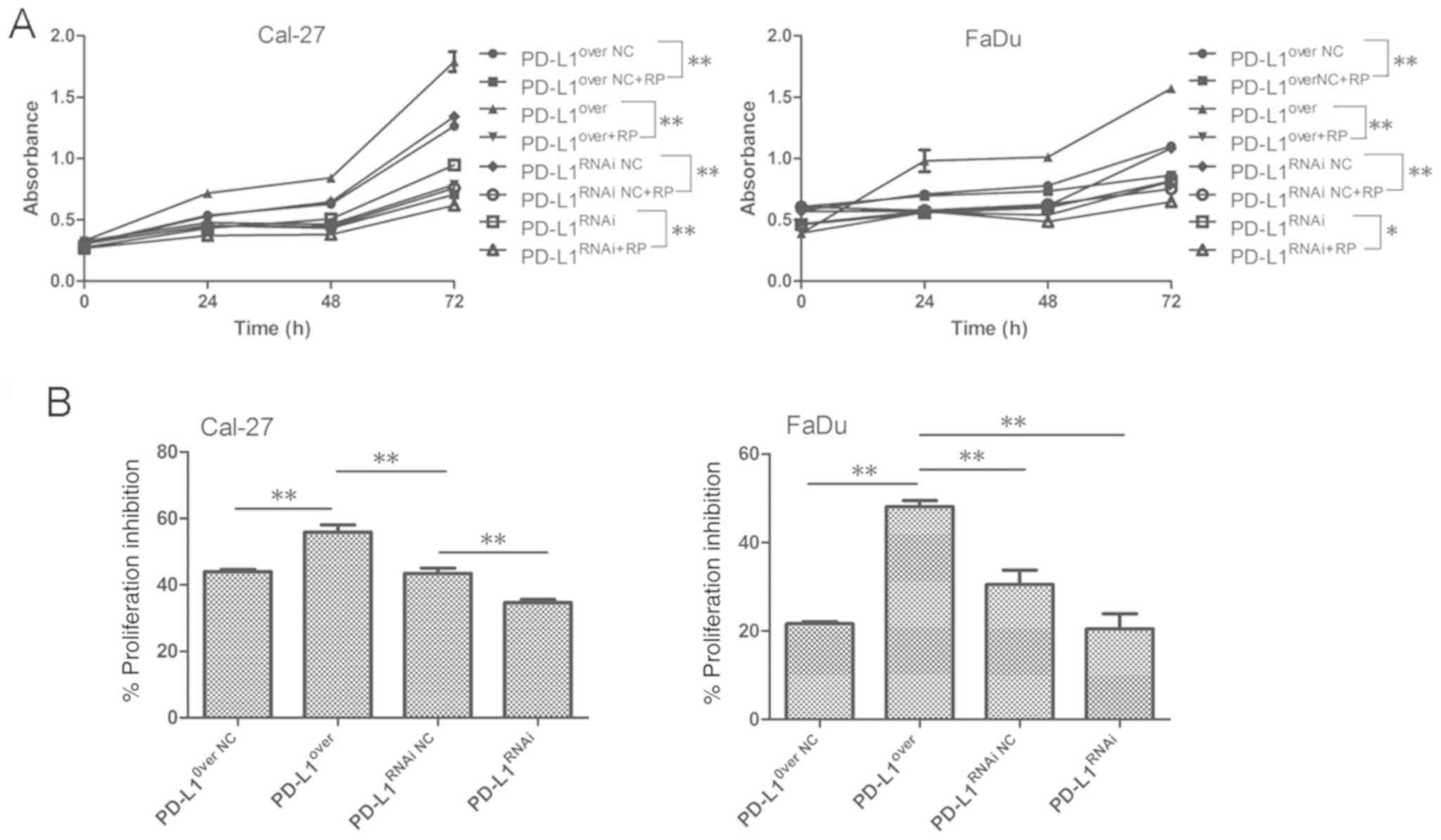
PD-L1 enhances the sensitivity of
HNSCC cells to mTOR inhibitor in vitro
As PD-L1 upregulated the expression of proteins
involved in mTOR signaling, the effects of mTOR inhibitor rapamycin
were investigated. Cellular proliferation was significantly
inhibited in the PD-L1over group following treatment
with mTOR inhibitor. PD-L1over Cal-27 cells were the
most sensitive to rapamycin in the four groups, and the
PD-L1RNAi tumor cells were the most tolerant to
rapamycin-mediated proliferation inhibition (Fig. 6A-C). The result obtained in Cal-27
and FaDu cells were consistent. Although there was no significant
statistical difference between PD-L1RNAi and
PD-L1RNAi NC groups in FaDu cells, it was still
indicated that PD-L1 exerted some regulatory action on tumor cell
proliferation, and mTOR inhibitor was effective in preventing the
PD-L1-driven proliferative effects. As presented in Fig. 4, PD-L1 knockdown reduced the
activation of mTORC1 and mTORC2. In response to rapamycin
treatment, p-P70S6KT389 expression was markedly
suppressed, and p-P70S6KT389 could mediate a negative
feedback loop on phosphoinositide 3-kinase (PI3K)/Akt which was
de-repressed by rapamycin, and elevated the level of
p-AktS473 (Fig. 7A and
B). The proportion of p-P70S6KT389 appeared the most
markedly decreased in PD-L1over+RP cells, compared with
PD-L1over cells; and p-AktS473 of
PD-L1RNAi, which increased, was more than
PD-L1RNAi+RP (Fig. 7B),
further indicating that PD-L1 may have promoted cell growth through
mTOR signaling upregulation.
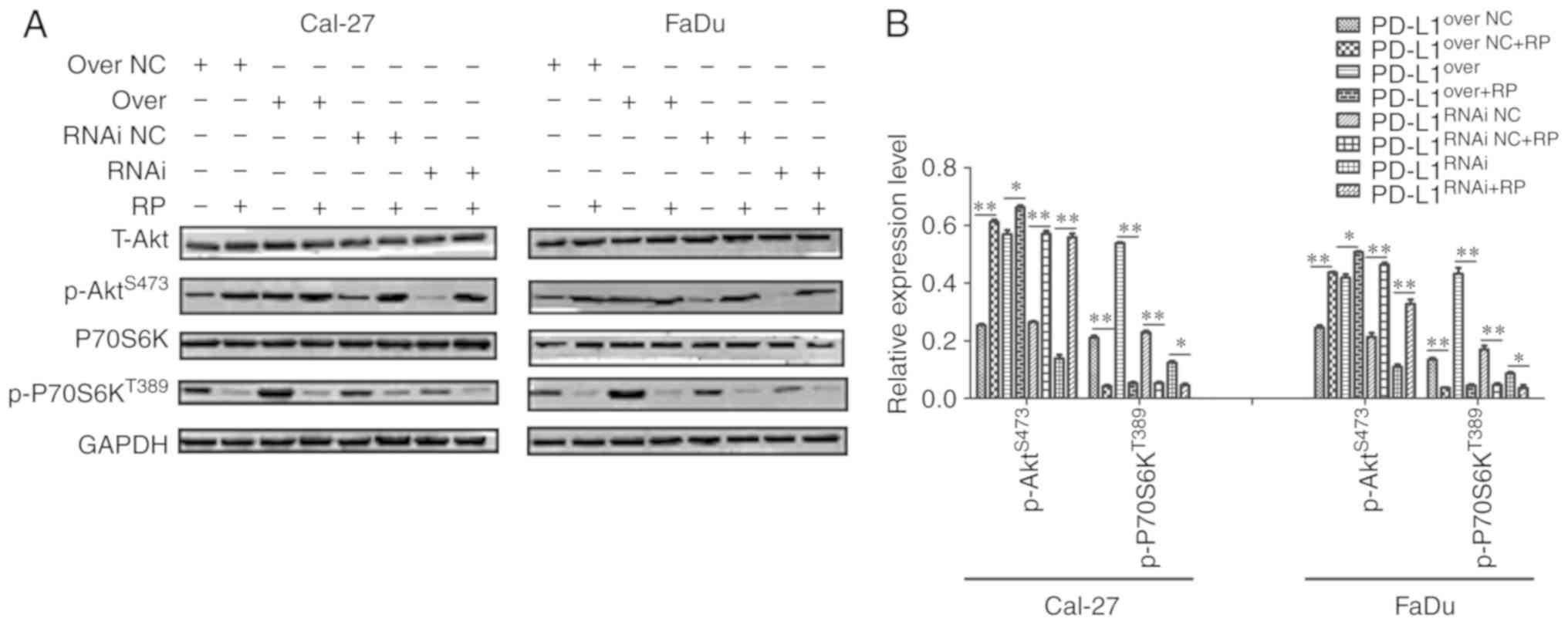 | Figure 7.Rapamycin prevents the PD-L1-mediated
effects on mTOR expression. (A) Western blot analysis of T-Akt,
P70S6K, p-P70S6KT389, p-AktS473 and GAPDH
expression in PD-L1over, PD-L1RNAi and
respective NC cells with or without rapamycin. (B)
p-P70S6KT389 and p-AktS473 expression in each
group was quantified. *P<0.05 and **P<0.01. PD-L1, programmed
death-ligand 1; T-, total; P70S6K, 70 kDa ribosomal protein S6
kinase 1; p-, phosphorylated; Akt, protein kinase B;
PD-L1over, PD-L1-overexpressing; PD-L1RNAi,
PD-L1 knockdown; NC, negative control; mTOR, mammalian target of
rapamycin; RP, rapamycin. |
Discussion
PD-1 is predominantly expressed in activated T/B
cells, where its function is to suppress immune cell activation via
a physiological self-stabilization mechanism (19). Overactivation of T/B cells lead to
autoimmune diseases, and thus PD-1 has a protective role (19,20).
In the tumor microenvironment, infiltrating T cells overexpress
PD-1, and PD-L1 and PD-L2 are highly expressed by tumor cells; the
ligation of PD-1/PD-L1 inhibits T cell activation (21). T cell migration and proliferation is
inhibited, as well as the secretion of perforin and granzymes by
cytotoxic T cells, which greatly limits their killing effects on
tumor cells (22). Blocking the
binding of PD-1 to PD-L1 would likely prevent immune escape,
enhance antitumor immunity and inhibit tumor progression. At
present, the efficacy of PD-1-targeted therapy is very low as a
monotherapy, with an overall response rate of 20–50% in cases with
multiple tumors (23,24). However, it has been suggested that
combining PD-1-targeted therapy with other therapeutic methods may
significantly augment the curative effect and the overall response
rate (25). Therefore, the
combination of PD-1/PD-L1 inhibition with other therapies requires
further research.
As a novel target of immunotherapy, tumor-expressed
PD-L1 mediates cancer immunopathogenesis by negatively regulating T
cells (26). However, increasing
evidence has suggested that PD-L1 has critical functions in
promoting tumor formation and development, without relying on the
immune checkpoint (15,27). Previous studies have confirmed PD-L1
mRNA is upregulated in a wide variety of tumors, such as pulmonary
adenocarcinoma, breast cancer and squamous lung cancer (13,28,29). A
previous study reported that PD-L1 protein expression was increased
in squamous cell carcinoma (SCC) of the head and neck, esophagus
and lung (30). In addition, PD-1
expression is upregulated in response to proinflammatory cytokines
IFN-γ, TNF-α and IL-1β, and PD-L1 blockade by a monoclonal antibody
efficiently augments the effects of adaptive T cell immunotherapy
in a murine model of PD-L1-transfected SCC, and inhibits the growth
of de novo induced PD-L1+ SCC (21,30).
In the present study, TMAs were obtained to detect PD-L1 expression
characteristics in HNSCC. PD-L1 expression was significantly higher
in tumor tissue, compared with NAT and normal tissue. Of note, the
expression of PD-L1 progressively increased along with the increase
of the tumor grades. These results suggested that PD-L1 was
associated with HNSCC tumorigenesis. Consistent with the results of
the present study, Strome et al (31) also reported that PD-L1 protein was
upregulated in SCC and highly expressed in 66% (16 of 24) of
freshly isolated SCC samples of the head and neck (31). However, the relationship between
PD-L1 expression and tumor TNM staging remains unclear in certain
reports, as well as its relationship with regional lymph node
metastasis (32,33). In the present study, the results
revealed that PD-L1 expression was not significantly different
between T/N stages. PD-L1/PD-1 plays an important role in directly
regulating the tumor microenvironment, and considering the
complicated effects of PD-L1/PD-1 in the tumor and tumor
microenvironment (9), it is
difficult to illuminate the relationship between PD-L1 and T/N
stages. Additionally, the small sample size may have had an impact
on the results. Hence, further investigation with a larger number
of samples is required.
In addition, the role of PD-L1 as a non-immune
checkpoint was demonstrated, which may be a potential therapeutic
target in HNSCC. The majority of studies focusing on PD-L1 have
predominantly focused on immunity, particularly in T cells
(11,24,31).
Although it has been reported that blocking PD-L1 affects tumor
cell proliferation (12,27,34),
it was uncertain whether PD-L1 inhibition suppresses tumor growth
in HNSCC. Through the generation of stable PD-L1-overexpressing
(PD-L1over), and PD-L1 knockdown (PD-L1RNAi)
cell lines, the present study demonstrated that PD-L1 promoted
tumor growth in vitro and in vivo. In addition,
compared with the control group, PD-L1RNAi HNSCC cell
proliferation decreased, even in the rapamycin-treated RNAi group.
Francisco et al (35)
reported that PD-L1 mediates the development of regulatory T cells
via downregulation of the mTOR pathway, coupled with the
upregulation of phosphatase and tensin homolog (PTEN). In addition,
it has been reported that blocking PD-L1 suppresses mTOR activity
(27,34). Our preliminary experiments confirmed
that PD-L1 had no significant effects on the expression of key
proteins involved in multiple signaling pathways, such as signal
transducer and activator of transcription, mitogen-activated
protein kinase and nuclear factor-κB. Hence, it was concluded that
PD-L1 may have exerted bi-directional effects in regulating the
mTOR pathway, although the exact mechanism remains unclear in
HNSCC.
The PI3K/Akt/mTOR signaling pathway has critical
functions in both solid tumors and hematological malignancies
(36). mTOR regulates cell growth,
motility, and metabolism; this signaling cascade is frequently
upregulated in cancer due to loss of the tumor suppressor PTEN
(37). Glycogen synthase kinase-3
(GSK-3) also interacts with and affects the function of downstream
components of the PI3K/Akt/mTOR signaling network (38). Bertacchini et al (39) reported that dual inhibition of
PI3K/mTOR signaling resensitizes resistant cancer cells to
chemotherapy (39). mTOR contains
two complexes, mTORC1 and mTORC2. p-P70S6KT389 is the
downstream effector of mTORC1, and p-AktS473 is the
downstream effector of mTORC2. mTORC1 and mTORC2 exert important
influences on the growth and survival of tumor cells (40,41).
In the present study, it was demonstrated that PD-L1 increased
p-P70S6KT389 and p-AktS473 expression in
HNSCC cell lines, and cells overexpressing PD-L1 proliferated
faster than control cells. Thus, PD-L1 may have been capable of
regulating both mTORC1 and mTORC2 expression. In addition, mTORC1
inhibitor suppressed p-P70S6KT389 expression and
prevented the PD-L1-mediated increase in proliferation. Cell
proliferation inhibition rate of the PD-L1over cells was
the highest in the four groups following treatment with mTOR
inhibitor, and PD-L1RNAi cells were the most resistance
to rapamycin-mediated proliferation inhibition. In addition,
p-AktS473 expression was increased in the of
PD-L1over+RP cells, compared with the
PD-L1RNAi+RP cells, this may be the combined effect of
p-P70S6KT389-mediated negative feedback loop on
phosphoinositide 3-kinase (PI3K)/Akt which was de-repressed by
rapamycin and low PD-L1-mediated restraint of p-AktS473.
Therefore, further study is required to understand the mutual
influence of both these factors.
Accumulating evidence has revealed that the curative
effect of anti-PD-L1 treatment is superior to anti-cytotoxic
T-lymphocyte protein 4 therapy (42,43).
It has been speculated that anti-PD-L1 may not only prevent
PD-L1-mediated immune escape, but may also restrain PD-L1-mediated
carcinogenesis. This may also be the reason why anti-PD-L1 therapy
has been reported to be more effective than immunotherapy in tumors
(42). In addition, it suggests
that tumor intrinsic PD-L1 signals have an antitumor effect.
In conclusion, the present study revealed that the
expression of PD-L1 was significantly higher in HNSCC compared with
normal tissues or cell lines, and the expression of PD-L1 increased
as the tumor grade progressed. Further, PD-L1 promoted HNSCC cell
proliferation both in vitro and in vivo. PD-L1
depletion led to a downregulation of mTOR signaling, and mTOR
inhibitor prevented the PD-L1-mediated proliferative effect. These
findings increased the current understanding of PD-L1-mediated
carcinogenesis in HNSCC, and may be conducive in discovering novel
therapeutic targets in HNSCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81372880 and
81670910) and the Guidance Fund of the Renmin Hospital of Wuhan
University (grant no. RMYD2018Z12).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZT and AZ conceived and designed the experiments.
AZ, FL, FC, JZ, LW, YW, SC and BX performed the experiments. AZ and
ZT analyzed the data and wrote the paper. ZT and FL revised the
paper. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Wuhan University (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Giraldi L, Leoncini E, Pastorino R,
Wünsch-Filho V, de Carvalho M, Lopez R, Cadoni G, Arzani D,
Petrelli L, Matsuo K, et al: Alcohol and cigarette consumption
predict mortality in patients with head and neck cancer: A pooled
analysis within the International Head and Neck Cancer Epidemiology
(INHANCE) Consortium. Ann Oncol. 28:2843–2851. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kawakita D, Lee YA, Turati F, Parpinel M,
Decarli A, Serraino D, Matsuo K, Olshan AF, Zevallos JP, Winn DM,
et al: Dietary fiber intake and head and neck cancer risk: A pooled
analysis in the International Head and Neck Cancer Epidemiology
consortium. Int J Cancer. 141:1811–1821. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pulte D and Brenner H: Changes in survival
in head and neck cancers in the late 20th and early 21st century: A
period analysis. Oncologist. 15:994–1001. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen EEW, Licitra LF, Burtness B, Fayette
J, Gauler T, Clement PM, Grau JJ, Del Campo JM, Mailliez A, Haddad
RI, et al: Biomarkers predict enhanced clinical outcomes with
afatinib versus methotrexate in patients with second-line recurrent
and/or metastatic head and neck cancer. Ann Oncol. 28:2526–2532.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirano F, Kaneko K, Tamura H, Dong H, Wang
S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, et al: Blockade of
B7-H1 and PD-1 by monoclonal antibodies potentiates cancer
therapeutic immunity. Cancer Res. 65:1089–1096. 2005.PubMed/NCBI
|
|
7
|
Xia Y, Jeffrey Medeiros L and Young KH:
Signaling pathway and dysregulation of PD1 and its ligands in
lymphoid malignancies. Biochim Biophys Acta. 1865:58–71.
2016.PubMed/NCBI
|
|
8
|
Rollins MR and Gibbons JR: CD80 expressed
by CD8+ T cells contributes to PD-L1-induced apoptosis
of activated CD8+ T cells. J Immunol Res.
2017:76594622017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zandberg DP and Strome SE: The role of the
PD-L1: PD-1 pathway in squamous cell carcinoma of the head and
neck. Oral Oncol. 50:627–632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Topalian SL, Drake CG and Pardoll DM:
Targeting the PD-1/B7-H1(PD-L1) pathway to activate antitumor
immunity. Curr Opin Immunol. 24:207–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clark CA, Gupta HB, Sareddy G, Pandeswara
S, Lao S, Yuan B, Drerup JM, Padron A, Conejo-Garcia J, Murthy K,
et al: Tumor-intrinsic PD-L1 signals regulate cell growth,
pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer
Res. 76:6964–6974. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alsuliman A, Colak D, Al-Harazi O, Fitwi
H, Tulbah A, Al-Tweigeri T, Al-Alwan M and Ghebeh H: Bidirectional
crosstalk between PD-L1 expression and epithelial to mesenchymal
transition: Significance in claudin-low breast cancer cells. Mol
Cancer. 14:1492015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao Y, Zhang L, Kamimura Y, Ritprajak P,
Hashiguchi M, Hirose S and Azuma M: B7-H1 overexpression regulates
epithelial-mesenchymal transition and accelerates carcinogenesis in
skin. Cancer Res. 71:1235–1243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang X, Chen C, Xia F, Yu Z, Zhang Y,
Zhang F, Gu H, Wan J, Zhang X, Weng W, et al: CD274 promotes cell
cycle entry of leukemia-initiating cells through JNK/Cyclin D2
signaling. J Hematol Oncol. 9:1242016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghebeh H, Tulbah A, Mohammed S, Elkum N,
Bin Amer SM, Al-Tweigeri T and Dermime S: Expression of B7-H1 in
breast cancer patients is strongly associated with high
proliferative Ki-67-expressing tumor cells. Int J Cancer.
121:751–758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M,
Meng YL, Yang AG and Wen WH: B7-H1 expression is associated with
poor prognosis in colorectal arcinoma and regulates the
proliferation and invasion of HCT116 colorectal cancer cells. PLoS
One. 8:e760122013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chamoto K, Al-Habsi M and Honjo T: Role of
PD-1 in immunity and diseases. Curr Top Microbiol Immunol.
410:75–97. 2017.PubMed/NCBI
|
|
20
|
Nishimura H, Nose M, Hiai H, Minato N and
Honjo T: Development of lupus-like autoimmune diseases by
disruption of the PD-1 gene encoding an ITIM motif-carrying
immunoreceptor. Immunity. 11:141–151. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boussiotis VA: Molecular and biochemical
aspects of the PD-1 checkpoint pathway. N Engl J Med.
375:1767–1778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baumeister SH, Freeman GJ, Dranoff G and
Sharpe AH: Coinhibitory pathways in immunotherapy for cancer. Annu
Rev Immunol. 34:539–573. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahmad SM, Borch TH, Hansen M and Andersen
MH: PD-L1-specific T cells. Cancer Immunol Immunother. 65:797–804.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kleffel S, Posch C, Barthel SR, Mueller H,
Schlapbach C, Guenova E, Elco CP, Lee N, Juneja VR, Zhan Q, et al:
Melanoma cell-intrinsic PD-1 receptor functions promote tumor
growth. Cell. 162:1242–1256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen L, Gibbons DL, Goswami S, Cortez MA,
Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, et al: Metastasis
is regulated via microRNA-200/ZEB1 axis control of tumour cell
PD-L1 expression and intratumoral immunosuppression. Nat Commun.
5:52412014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mak MP, Pan T, Diao L, Cardnell RJ,
Gibbons DL, William WN, Skoulidis F, Parra ER, Rodriguez-Canales J,
Wistuba II, et al: A patient-derived, pan-cancer EMT signature
identifies global molecular alterations and immune target
enrichment following epithelial to mesenchymal transition. Clin
Cancer Res. 22:609–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ritprajak P and Azuma M: Intrinsic and
extrinsic control of expression of the immunoregulatory molecule
PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol.
51:221–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Strome SE, Dong H, Tamura H, Voss SG,
Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, et al:
B7-H1 blockade augments adoptive T-cell immunotherapy for squamous
cell carcinoma. Cancer Res. 63:6501–6505. 2003.PubMed/NCBI
|
|
32
|
Hsu MC, Hsiao JR, Chang KC, Wu YH, Su IJ,
Jin YT and Chang Y: Increase of programmed death-1-expressing
intratumoral CD8 T cells predicts a poor prognosis for
nasopharyngeal carcinoma. Mod Pathol. 23:1393–1403. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Muenst S, Schaerli AR, Gao F, Däster S,
Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE,
et al: Expression of programmed death ligand 1 (PD-L1) is
associated with poor prognosis in human breast cancer. Breast
Cancer Res Treat. 146:15–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang CH, Qiu J, O'Sullivan D, Buck MD,
Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ,
et al: Metabolic competition in the tumor microenvironment is a
driver of cancer progression. Cell. 162:1229–1241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Francisco LM, Salinas VH, Brown KE,
Vanguri VK, Freeman GJ, Kuchroo VK and Sharpe AH: PD-L1 regulates
the development, maintenance, and function of induced regulatory T
cells. J Exp Med. 206:3015–3029. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Follo MY, Manzoli L, Poli A, McCubrey JA
and Cocco L: PLC and PI3K/Akt/mTOR signalling in disease and
cancer. Adv Biol Regul. 57:10–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jhanwar-Uniyal M, Amin AG, Cooper JB, Das
K, Schmidt MH and Murali R: Discrete signaling mechanisms of mTORC1
and mTORC2: Connected yet apart in cellular and molecular aspects.
Adv Biol Regul. 64:39–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hermida MA, Dinesh Kumar J and Leslie NR:
GSK3 and its interactions with the PI3K/AKT/mTOR signalling
network. Adv Biol Regul. 65:5–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bertacchini J, Frasson C, Chiarini F,
D'Avella D, Accordi B, Anselmi L, Barozzi P, Forghieri F, Luppi M,
Martelli AM, et al: Dual inhibition of PI3K/mTOR signaling in
chemoresistant AML primary cells. Adv Biol Regul. 68:2–9. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thedieck K, Holzwarth B, Prentzell MT,
Boehlke C, Kläsener K, Ruf S, Sonntag AG, Maerz L, Grellscheid SN,
Kremmer E, et al: Inhibition of mTORC1 by astrin and stress
granules prevents apoptosis in cancer cells. Cell. 154:859–874.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu P, Gan W, Inuzuka H, Lazorchak AS, Gao
D, Arojo O, Liu D, Wan L, Zhai B, Yu Y, et al: Sin1 phosphorylation
impairs mTORC2 complex integrity and inhibits downstream Akt
signalling to suppress tumorigenesis. Nat Cell Biol. 15:1340–1350.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stewart R, Morrow M, Hammond SA, Mulgrew
K, Marcus D, Poon E, Watkins A, Mullins S, Chodorge M, Andrews J,
et al: Identification and characterization of MEDI4736, an
antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res.
3:1052–1062. 2015. View Article : Google Scholar : PubMed/NCBI
|