Introduction
Liver cancer is the second most common cause of
cancer-associated fatality worldwide, and is one of the few types
of neoplasm with a steadily increasing incidence and mortality
(1–3). Liver cancer subtypes include
hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma,
mixed hepatocellular cholangiocarcinoma (HCC-CCA) and rarer types,
such as hepatoblastoma (HB) (4). HB
is the most common pediatric malignant liver cancer (5). Although the survival rate for HB has
increased from ~30 to >80% with the application of surgical
resection, chemotherapy and liver transplantation (6), the molecular development of this
aggressive embryonal tumor remains largely uncharacterized
(7). Further research regarding the
underlying molecular mechanisms will aid to improve the diagnosis
and treatment of patients with HB.
Human LAG1 longevity assurance homolog 2 (LASS2),
also known as ceramide synthase 2 or tumor metastasis suppressor
gene 1, is a 45-kDa membrane protein (8). The expression of LAG1 longevity
assurance homolog 2 (LASS2) protein is primarily confined to the
nuclear and endoplasmic reticular membranes (9). A previous study demonstrated that LASS
2 knockout induced autophagy and the unfolded protein response,
through its participation in the regulation of endoplasmic
reticulum stress (10). As a
recently identified tumor suppressor gene, LASS2 may also serve an
important role in inhibiting the invasion and metastasis of various
types of tumor (11–16). The silencing of LASS2 enhances the
growth, invasion and migration of HCC (11,12),
bladder cancer (13,14), breast cancer (8,15) and
prostate cancer (16) cells by
regulating ATPase activity. In addition, the reduced tumor
expression of LASS2 protein was identified to be associated with a
worse prognosis in patients with meningioma (17); LASS2 tumor expression was also
observed to be negatively correlated with tumor size, tumor
differentiation and TNM stage in patients with HCC (18). Although its effects on HCC
metastasis and invasion have been reported, to the best of our
knowledge, the precise role of LASS2 in tumorigenesis and its
potential effects on HB cell proliferation, apoptosis and cell
cycle in vitro have yet to be investigated. Therefore, the
proliferation, apoptosis and cell cycle-associated nuclear factor
(NF)-κB p65/p27/cyclin D1/cyclin-dependent kinase 4 (CDK4)
signaling pathway were considered to explore the potential role of
LASS2 in the tumorigenesis of human HB.
Materials and methods
Adv-LASS2-green fluorescent protein
(GFP) recombinant adenovirus vector construction
To construct the recombinant adenovirus vector
Adv-LASS2-GFP, the LASS2-GFP (human LASS2 coding sequence,
NM_022075; 627 ng/µl) and the pShuttle-CMV recombinant shuttle
vector (BAC Biological Technology Co., Ltd., Beijing, China) were
digested with HindIII and NotI (New England Biolabs,
Inc., Ipswich, MA, USA). The digested vector and insert segments
were ligated with T4 DNA ligase. Transformation, plasmid
mini-extraction and sequencing were performed to obtain a verified
pShuttle-LASS2-GFP recombinant shuttle plasmid. Following this, the
pAdxsi vector (BAC Biological Technology Co., Ltd.) and
pShuttle-LASS2-GFP were separately digested with I-CeuI and I-SceI,
followed by ligation and transformation, to obtain a
pAdxsi-LASS2-GFP viral plasmid. Extraction of the viral plasmids
was performed, linearized pAdxsi-LASS2-GFP viral plasmid was
transfected into 293 cells using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), which was
followed by collection and amplification to finally obtain a
recombinant Adv-LASS2-GFP with a titer of 1.2×1010
plaque-forming units/milliliter.
Cell culture and transfection
Human HB HepG2 cells (19) were kindly provided and authenticated
using short tandem repeat markers by Stem Cell Bank, Chinese
Academy of Sciences (Shanghai, China). It is notable that the HepG2
cells are typically misidentified as a HCC cell line, which is
frequently erroneously used in HCC research (20,21).
Cells were cultured in the recommended medium,
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.), supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 U/ml
streptomycin. Cells were incubated at 37°C in a humidified
atmosphere containing 5% CO2. Cells in the exponential
phase of growth were counted, seeded in 6-well plates and
transfected with the recombinant adenoviruses (Adv-LASS2-GFP or
Adv-GFP control) at 50–70% confluence following 24-h incubation.
All assays were performed in triplicate.
Determination of the subcellular
localization of LASS2 by confocal laser scanning microscopy
Adv-GFP or Adv-LASS2-GFP vectors were transfected
into HepG2 cells that were subsequently incubated for 48 h. Cells
were placed on slides, washed three times with phosphate-buffered
saline (PBS) and fixed by incubation in 4% paraformaldehyde for 30
min at room temperature. The nuclei were stained with
4′,6-diamidino-2-phenylindole (DAPI) in the dark for 5 min at room
temperature and washed three times. A confocal microscope (LSM710;
Carl Zeiss AG, Oberkochen, Germany) was used to analyze the
expression of the GFP-LASS2 protein, which was detected with the
GFP filter at 525 nm, following excitation at 488 nm. The cell
nuclei were identified with laser excitation at 340 nm and emission
at 488 nm. Images were acquired at ×200 magnification.
Cell counting kit-8 (CCK-8) cell
viability assay
Cell viability and cell number were assessed using a
CCK-8 assay (Beyotime Institute of Biotechnology, Shanghai, China).
HepG2 cells were seeded into 96-well plates at 104
cells/well. pAdv-GFP or pAdv-LASS2-GFP vectors were transfected
into the cells, which were subsequently incubated for 0, 24, 48, 72
or 96 h. The cells were incubated with 10 µl CCK-8 solution for 1
h. The optical density (OD) was measured at 450 nm. The cell
proliferation rate (%) = (OD value of the test well - OD value of
the background control well) / (OD value of the control well - OD
value of the background control) × 100%.
Colony formation assay
The transfected cells were collected and seeded in
6-cm sterile petri dishes at a density of 103
cells/well. At 14 days from the initial appearance of colonies, the
cells were washed twice with PBS, fixed with 4% paraformaldehyde
for 20 min at room temperature and stained with crystal violet for
20 min at room temperature. Subsequently, the number of colonies
(≥50 cells) was determined.
Cell cycle analysis
HepG2 cells were transfected with Adv-GFP or
Adv-LASS2-GFP for 48 h. Subsequently, the cells were harvested,
washed with PBS and prepared into single-cell suspension
(106 cells/ml, 1 ml/group). The precipitate was removed
by centrifugation at 800 × g for 5 min at room temperature and the
cells fixed in 500 µl 70% ice-cold ethanol for 2 h, or overnight.
Following this, cells were incubated with 100 µl RNAase at 37°C for
30 min and stained with 400 µl propidium iodide (Nanjing KeyGen
Biotech, Nanjing, China) for 30 min at 4°C while protected from
light. The stained cells were subjected to flow cytometry using a
flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA, USA) and
the proportion of cells phases in G0/G1, S
and G2/M were analyzed using ModFit software (version
3.3; Verity Software House, Inc., Topsham, ME, USA).
Annexin V-allophycocyanin
(APC)/7-amino-actinomycin D (7-AAD) double staining apoptosis
assay
Transfected HepG2 cells were harvested with 0.25%
trypsin without EDTA, washed twice with cold PBS and resuspended in
500 µl binding buffer. Following this, 5 µl Annexin V-APC and 5 µl
7-AAD (Nanjing KeyGen Biotech) were added in sequence according to
the manufacturer's protocol. Cells were incubated for 15 min at
room temperature while protected from light, then immediately
analyzed using flow cytometry as described above and CellQuest
software 6.0 (BD Biosciences).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
Apoptosis-associated nuclear DNA fragmentation was
detected using a commercial biotin dUTP-labeling TUNEL kit (cat.
no. KGA702; KeyGen Biotech) according to the manufacturer's
instructions. Briefly, HepG2 cells were seeded onto slides in
6-well plates. Following 48 h of incubation, the slides were dried
and washed with PBS three times. Cells were fixed with 4%
paraformaldehyde for 30 min at room temperature and permeabilized
with 1% Triton X-100. Subsequently, cells were incubated in 3%
H2O2 in methanol for 10 min at room
temperature to block endogenous peroxidase activity, washed in PBS
and incubated with a mixture of TdT and fluorescein isothiocyanate
dUTP solutions in a humidified chamber at 37°C for 60 min. This was
followed by washing in PBS and incubated with
streptavidin-conjugated horseradish peroxidase (dilution, 1:100;
from the TUNEL kit) in a humidified chamber at 37°C for 30 min.
When cells were washed with PBS, DAPI solution was applied,
followed by counterstaining with hematoxylin. The dehydrated
sections were cleared in xylene, mounted with neutral balsam and
enclosed with coverslips. Over 200 cells with brown granules in the
nuclei were considered to be TUNEL-positive. Cells were assessed in
at least three high-powered fields of view (magnification, ×200)
under an optical microscope (Olympus Corporation, Tokyo, Japan) to
determine the mean percentage of positive cells.
Intracellular reactive oxygen species
(ROS) measurement
Intracellular ROS were monitored using
dihydroethidium (DHE; Nanjing KeyGen Biotech) as the probe. HepG2
cells were trypsinized, washed with PBS and resuspended in 1 ml
serum-free medium supplemented with 10 µM DHE at density of
1×106/ml following transfection. Cells were incubated at
37°C for 20 min in the dark and inverted every 3–5 min to maximize
the contact between the probe and cells. Following this, cells were
washed three times with serum-free medium to remove excess DHE.
Cellular ROS were subsequently measured using flow cytometry, and
fluorescence was read using a fluorescence spectrometer with a 488
nm excitation and 605 nm emission wavelength.
Mitochondrial membrane potential (ΔΨm)
assays
ΔΨm was measured using the JC-1 apoptosis detection
kit (Nanjing KeyGen Biotech). Briefly, HepG2 cells transfected with
the Adv-GFP or Adv-LASS2-GFP vectors were incubated for 48 h. Cells
were collected, washed with PBS, resuspended in 500 µl JC-1 working
solution and incubated at 37°C with 5% CO2 for 20 min.
Cells were collected by centrifugation (800 × g for 5 min at room
temperature), washed twice with 1X incubation buffer and
resuspended in 500 µl 1X incubation buffer. The concentration of
retained JC-1 dye was determined by flow cytometry with an
excitation wavelength of 488 nm and an emission wavelength of 530
nm.
Ca2+-ATPase assays
ATPases decompose ATP to produce ADP and inorganic
phosphate (22). Therefore,
Ca2+-ATPase activity was determined using a colorimetric
method to detect the inorganic phosphate content. Transfected cells
were digested and centrifuged as described above. A total of 500 µl
cell suspension was added to each tube to a density of
106 cells/ml. The cells were disrupted by ultrasonic
homogenization. The detection of inorganic phosphate was performed
with the Ultra Trace Ca2+-ATPase assay kit (Nanjing
KeyGen Biotech) according to the manufacturer's instructions.
Intracellular Ca2+
concentration [Ca2+]i determination
Transfected cells were washed twice with PBS and a
single-cell suspension was prepared by centrifugation of
106cells/ml at 800 g for 5 min. Calbryte 630 AM (AAT
Bioquest, Inc., Sunnyvale, CA, USA) was dissolved in dimethyl
sulfoxide to prepare a 2 mM stock solution; a working solution of
20 µM Calbryte 630 AM in Hank's balanced salt solution was also
prepared. A total of 500 µl Calbryte 630 AM working solution was
added into each well, and the dye-loaded plate was incubated for 60
min, followed by incubation at room temperature in the dark for a
further 15 min. Cells were collected by centrifugation at 800 × g
for 5 min at room temperature, and washed twice with 1X Hank's
buffer with HEPES (HHBS) to remove excess dye. Cells were
resuspended in 500 µl 1X HHBS and analyzed using flow cytometry for
Calbryte 630 AM content, with an excitation wavelength of 610 nm
and an emission wavelength of 640 nm, to determine the
[Ca2+]i.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from HepG2 cells using the
RNAiso Plus reagent (Takara Bio Inc., Otsu, Japan). cDNA was
synthesized using the PrimeScript RT Master Mix kit (Takara Bio
Inc.). qPCR was performed with the SYBR Premix Ex Taq II kit
(Takara Bio Inc.) and the ABI 7500 Real-Time PCR instrument
(Applied Biosystems; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. The thermocycling conditions used
with the ABI7500 detection system were as follows: 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec.
Relative quantification was performed using the comparative Cq
(2−ΔΔCq) method (23).
All data were normalized to the internal control, GAPDH. The
specific primers for human LASS2 and GAPDH used in the present
study were as follows: GAPDH, sense 5′-GGAGCGAGATCCCTCCAAAAT-3′ and
antisense 5′-GGCTGTTGTCATACTTCTCATGG-3′ (197 bp product); and
LASS2, sense 5′-ATCGTCTTCGCCATTGTT-3′ and antisense
5′-CGGTCACTGCGTTCATCT-3′ (233 bp product).
Immunofluorescence
Cells were cultured on glass coverslips and
transfected for 48 h, washed with PBS for 5 min, fixed with 4%
pre-cooled paraformaldehyde for 20 min, washed in PBS three times
for 5 min each time and incubated with PBS containing 0.1% Triton
X-100 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) on ice for 10
min. Two drops of 3% H2O2-methanol solution
were added onto each coverslip for 10 min at room temperature to
inactivate endogenous peroxidase, followed by washing three times
with PBS again. Cells were blocked with ready-to-use normal goat
serum kit (cat. no. AR0009; Boster Biological Technology,
Pleasanton, CA, USA) for 20 min at 37°C and incubated with 50 µl
rabbit anti-human NF-κB p-p65 (Ser536; dilution, 1:100; cat. no.
3033; Cell Signaling Technology, Inc., Danvers, MA, USA) on each
coverslip overnight at 4°C. Following this, cells were incubated
with tetramethylrhodamine-conjugated goat anti-rabbit IgG secondary
antibodies (dilution, 1:100; cat. no. KGAA99; Nanjing KeyGen
Biotech) at 37°C in the dark for 1 h. Cells were counterstained
with DAPI for 5 min at room temperature and the coverslips were
mounted in fluoroshield with DAPI histology mounting medium (cat.
no. F657; Sigma-Aldrich; Merck KGaA) and the slides were scanned
under a confocal microscope (LSM710; Carl Zeiss AG).
Western blot analysis
The total protein was extracted from HepG2 cells
using a protein extraction kit (Nanjing KeyGen Biotech); protein
concentrations were measured using a bicinchoninic acid assay kit
(Nanjing KeyGen Biotech) according to the manufacturer's protocols.
An equal amount of protein (10 µg per lane) for each group was
separated using SDS-PAGE (10% gels) and electrotransferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked in 5% skimmed milk for 2 h at room
temperature and incubated overnight at 4°C with the primary
antibodies, including GAPDH (dilution, 1:5,000; cat. no. EM1101;
Hangzhou HuaAn Biotechnology Co., Ltd., Hangzhou, China),
anti-LASS2 (dilution, 1:300; cat. no. ab85567; Abcam, Cambridge,
UK), cyclin D1 (dilution, 1:5,000; cat. no. ab137875; Abcam), CDK4
(dilution, 1:2,000; cat. no. ab137675; Abcam), NF-κB p65 (dilution,
1:1,000; cat. no. 8242), NF-κB p-p65 (Ser536; dilution, 1:1,000;
cat. no. 3033) and p27kip1 (dilution, 1:1,000; cat. no.
2552; all from Cell Signaling Technology, Inc.). The membranes were
washed and incubated with the secondary antibody (horseradish
peroxidase-conjugated goat anti-rabbit IgG; dilution, 1:4,000; cat.
no. 5220-0283; KPL, Milford, MA, USA) at room temperature for 1–2
h. The membranes were washed, developed using enhanced
chemiluminescent kit (cat. no. SQ101; EpiZyme, Shanghai, China) and
analyzed by densitometry relative to the control gene using Gel-Pro
analyzer 4.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All statistical analyses were performed with SPSS
(version 22.0; IBM Corp., Armonk, NY, USA) and GraphPad Prism
(version 7.0; GraphPad Software, Inc., La Jolla, CA, USA) software.
Data are expressed as the mean ± standard deviation of at least
three independent experiments. One-way analysis of variance with a
least-significant-difference test were used for group comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
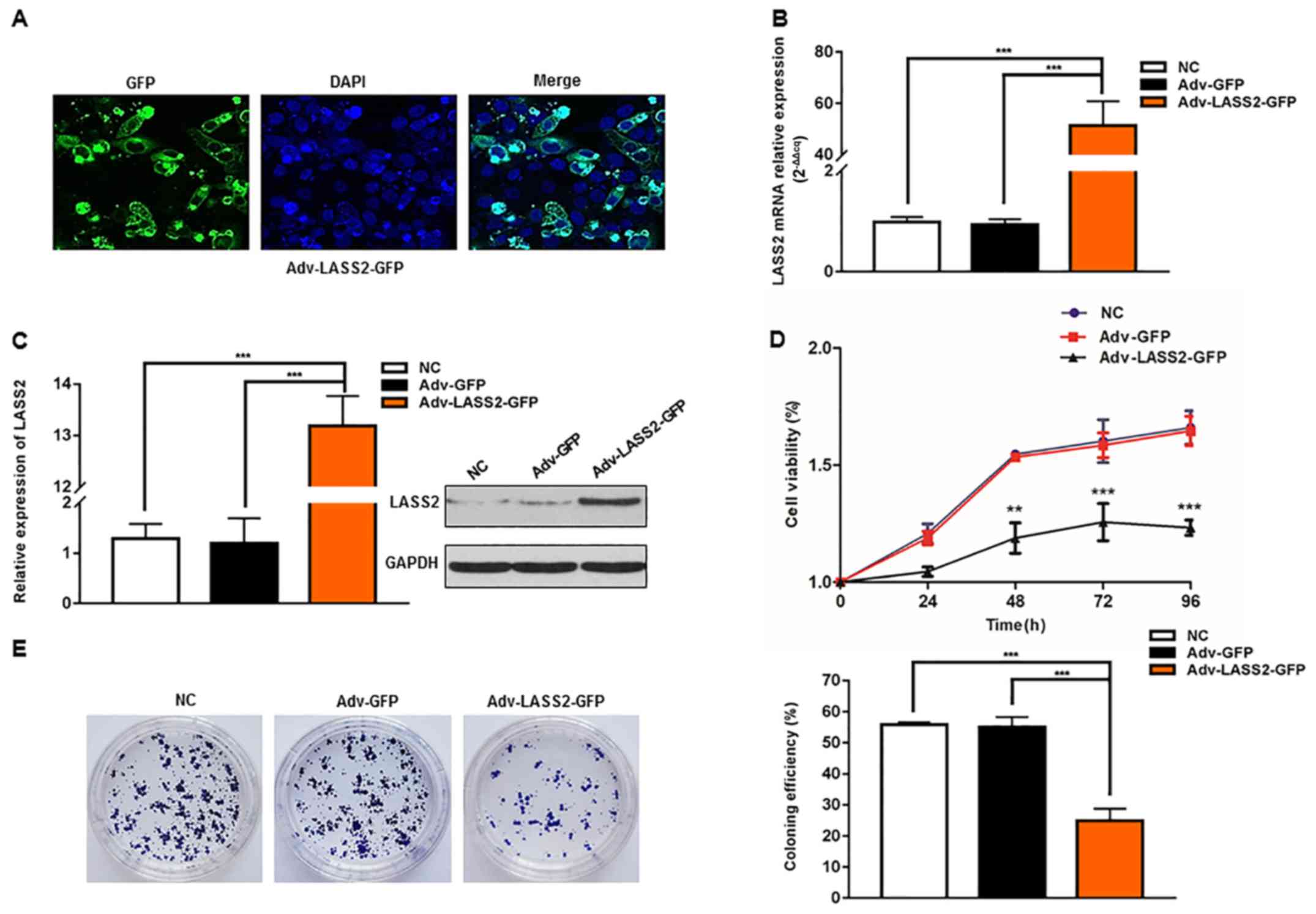
Overexpression of LASS2 inhibits HepG2
cell proliferation
Adenovirus vectors Adv-GFP (vector control) and
Adv-LASS2-GFP were generated and successfully transiently
transfected into HepG2 cells. The subcellular localization of the
LASS2-GFP fusion protein was identified using confocal laser
scanning microscope at 48 h post-transfection. The LASS2-GFP fusion
protein was localized to the nuclear membrane and cytosol (Fig. 1A).
Furthermore, using RT-qPCR, it was identified that
the Adv-LASS2-GFP-transfected cells exhibited a significantly
greater LASS2 mRNA expression level compared with those transfected
with Adv-GFP or negative control (NC) cells (51.62-fold difference;
Fig. 1B). The LASS2 protein
expression levels were also analyzed by western blot analysis. The
increased expression of LASS2 protein was evident in the
Adv-LASS2-GFP transfected cells when compared with the Adv-GFP or
NC cells (10.55-fold; Fig. 1C).
To determine the effect of LASS2 overexpression on
cell proliferation, CCK-8 and colony formation assays were
performed. Cell proliferation was significantly decreased at 48 h
post-transfection with Adv-LASS2-GFP when compared with cells
transfected with Adv-GFP (Fig. 1D).
The overexpression of LASS2 significantly suppressed HepG2 colony
formation (Fig. 1E). These results
indicate that LASS2 overexpression inhibited the proliferation of
HepG2 HB cells.
Overexpression of LASS2 induces
G0/G1 cell cycle arrest via modulating cell
cycle regulatory proteins in HepG2 cells
To investigate the role of LASS2 in the regulation
of the cell cycle in HepG2 cells, the effect of its overexpression
on cell cycle progression was assessed. The cell cycle distribution
was analyzed using flow cytometry with propidium iodide. The
results indicated in Fig. 2A
revealed that the overexpression of LASS2 inhibited the cell cycle
progression of HepG2 cells in the G0/G1
phase. In the cells transfected with Adv-LASS2-GFP for 48 h
compared with NC or Adv-GFP groups, the percentage of cells in the
G0/G1 phase was significantly increased to
65.13±3.07%; the proportion of cells in the S and G2/M
phases was reduced, with the cells in S phase significantly
decreasing from 46.11±1.77 to 33.54±2.30% and the cells in the G2/M
phase decreasing from 11.58±1.39 to 2.00±0.84%.
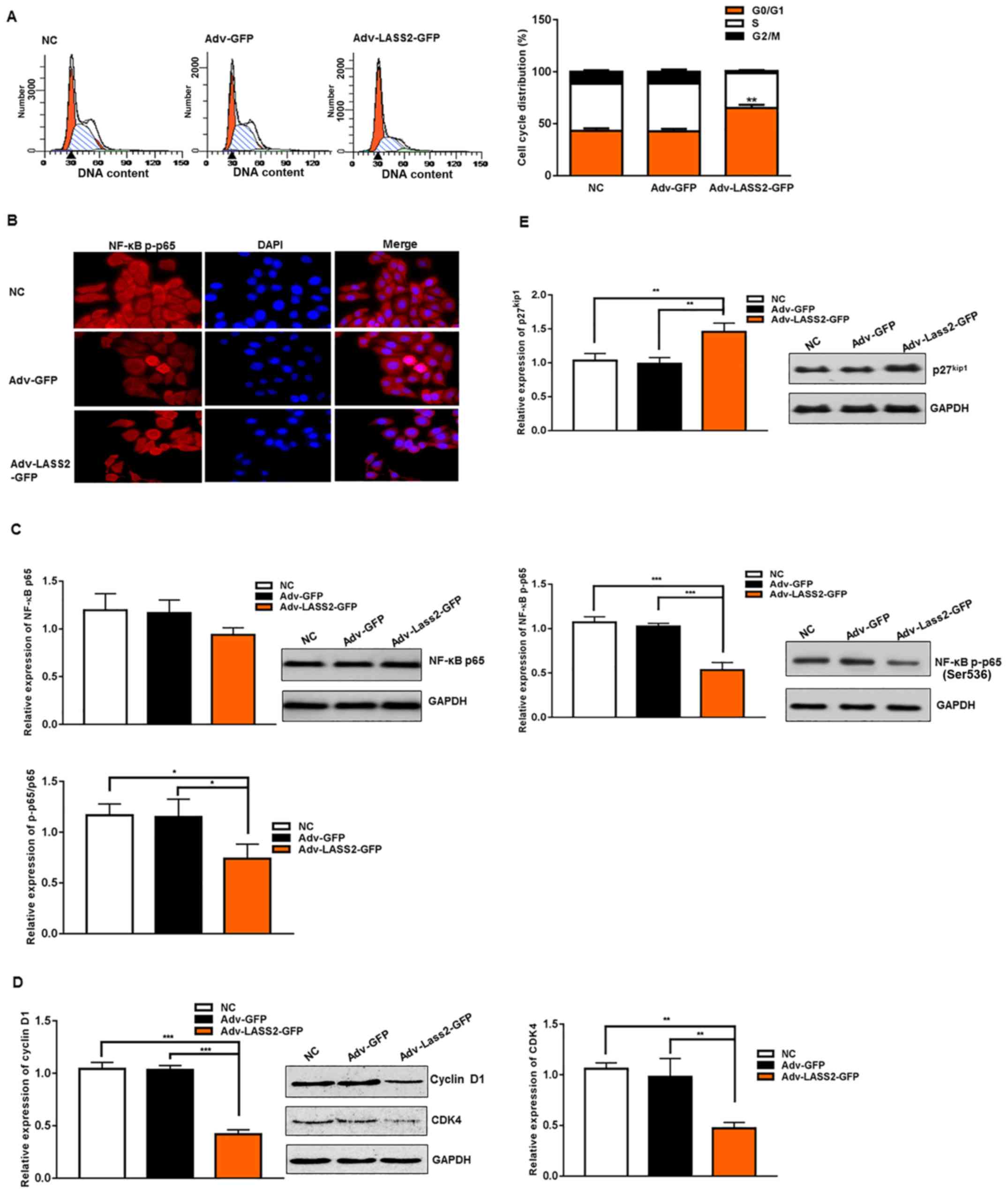 | Figure 2.Overexpression of LASS2 induces
G0/G1 cell cycle arrest via inhibiting NF-κB
p65 activity, downregulates cyclin D1 and CDK4 expression and
upregulates p27 expression. (A) Flow cytometry analysis revealed
arrest of HepG2 cells at the G0/G1 phase in
Adv-LASS2-GFP group following 48 h of transfection. (B)
Immunofluorescence analysis demonstrated the cytoplasm of NF-κB
p-p65 (red) in HepG2 cells (magnification, ×200). The relative
protein expression of (C) NF-κB p65, NF-κB p-p65, (D) cyclin D1,
CDK4 and (E) p27kip were detected using western blot
analysis with specific antibodies. GAPDH was used as a loading
control. *P<0.05, **P<0.01 and ***P<0.001 as indicated or
Adv-LASS2-GFP vs NC or Adv-GFP. DAPI,
4′,6-diamidino-2-phenylindole; CKD4, cyclin-dependent kinase 4;
NF-κB, nuclear factor-κB; LASS2, LAG1 longevity assurance homolog
2; GFP, green fluorescent protein; NC, negative control. |
To explore the potential mechanisms for the
G0/G1 cell cycle arrest induced by LASS2 in
HepG2 cells, the expression of members of the NF-κB
p65/p27kip1/cyclin D1/CDK4 signaling pathway, associated
with the cell cycle and proliferation in cancer cells, were
examined using western blot analysis and immunofluorescence
(Fig. 2B-E).
NF-κB is an inducible transcription factor critical
for the expression of a variety of genes that affect inflammation,
immunity, apoptosis, cell proliferation (24,25)
and the cell cycle (26,27). The present results demonstrated that
NF-κB p65 activity was suppressed following Adv-LASS2-GFP
transfection (Fig. 2B and C).
Immunofluorescence analysis revealed that NF-κB p-p65 was widely
expressed in the cytoplasm, with negative staining in the nuclei
(Fig. 2B). Western blot analysis
indicated that the relative expression of NF-κB p-p65 was
significantly decreased compared with NC or Adv-GFP groups
(Fig. 2C). Although the relative
expression level of inactive NF-κB p65 was also downregulated, the
difference was not statistically significant (Fig. 2C).
CDKs are a family of kinases initially identified
for their role in the regulation of the cell cycle (28). CDK4 is a key partner for cyclin D1
in the regulation of cell cycle progression from the G1
phase (28). The western blot
analysis results indicated that the relative expression of cyclin
D1 and CDK4 were significantly reduced by 40.89±5.91 and
46.95±6.43%, respectively, in the cells transfected with
Adv-LASS2-GFP compared with the control group (Fig. 2D). To investigate the mechanism for
the effect of LASS2 on cyclin D1 expression, western blot analysis
was performed to detect the changes in the expression of the CDK
inhibitor p27. p27 is a negative regulator of cell cycle
progression from G1 to S phase and is considered the
most characteristic of CDK regulators. Furthermore, p27 inhibits
cyclin-CDK complexes such as cyclin D1-CDK4 (29,30).
The present results revealed a significantly upregulated expression
of p27 protein (1.46-fold) in the cells transfected with
Adv-LASS2-GFP compared with the NC group (Fig. 2E).
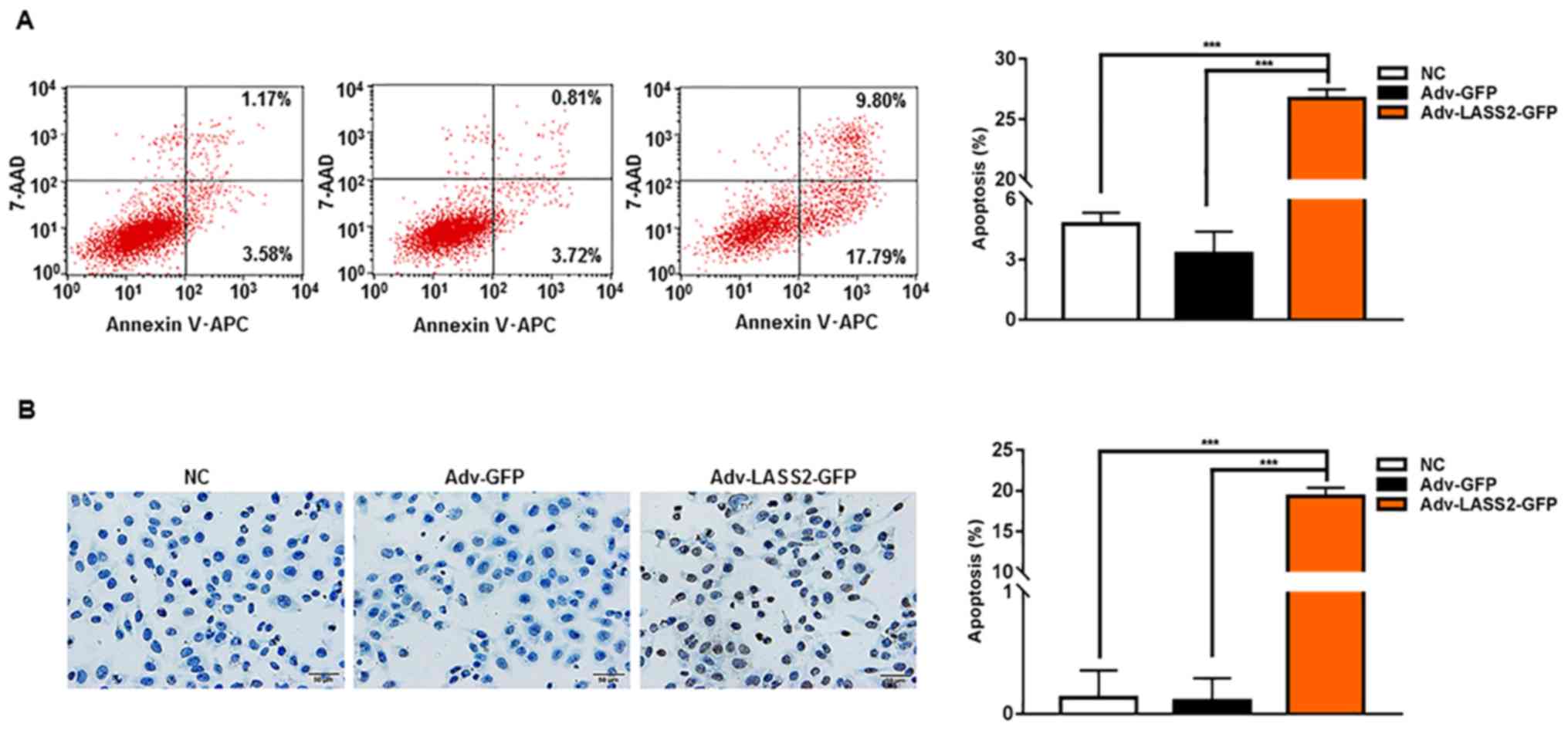
LASS2 induces apoptosis in HepG2 cells
by increasing ROS, reducing ∆Ψm and inducing
[Ca2+]i overload
To confirm whether LASS2 overexpression induced
apoptosis in HepG2 cells, the phosphatidylserine on the surface of
apoptotic cells and nuclear DNA fragmentation during apoptosis was
detected using Annexin V-APC/7-AAD and TUNEL assays, respectively.
The proportion of Annexin V-APC and/or 7AAD-positive cells
(Fig. 3A) and TUNEL-positive cells
(Fig. 3B) was significantly higher
in cells transfected with Adv-LASS2-GFP compared with cells
transfected with Adv-GFP or NC cells.
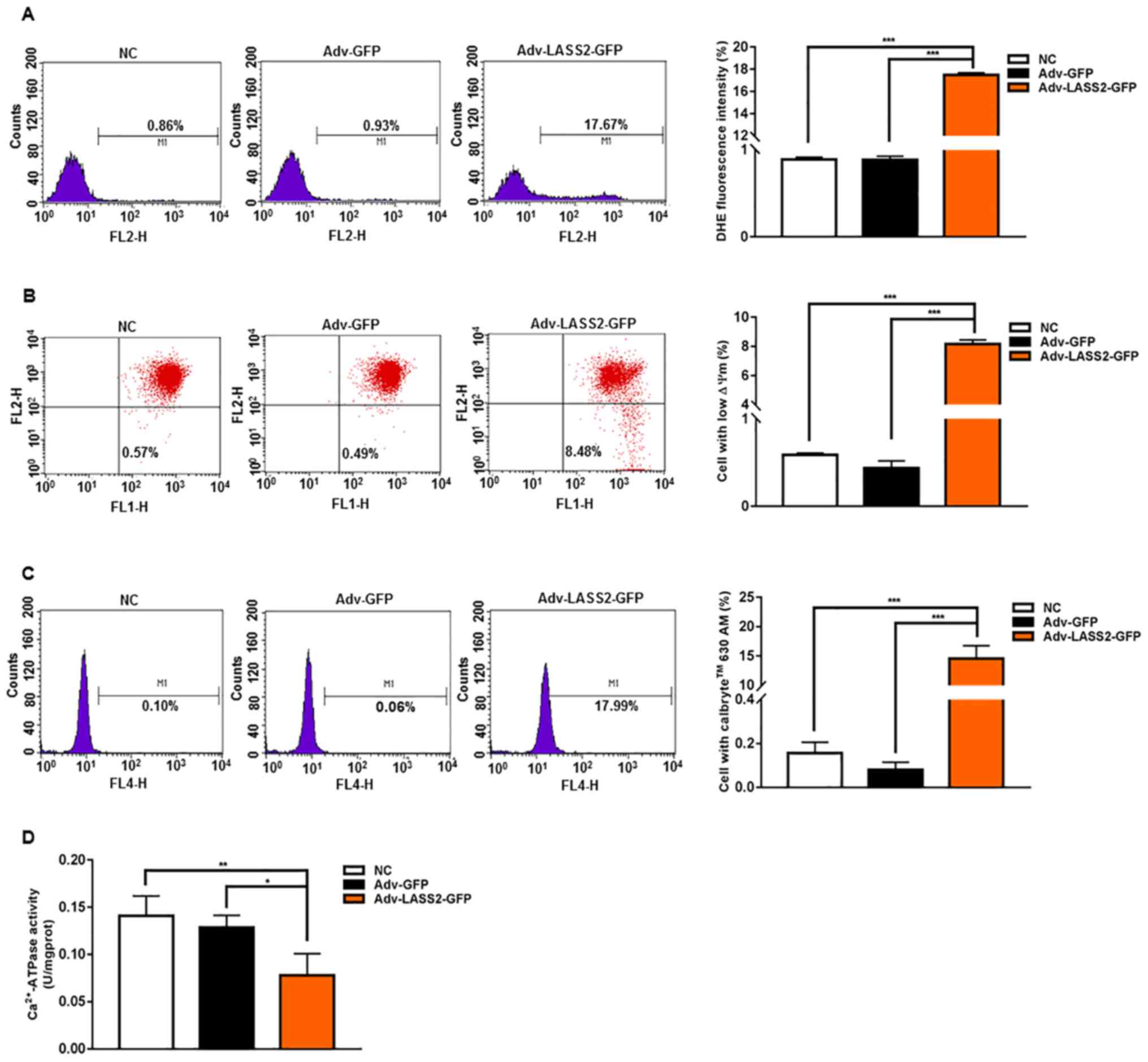
To further investigate the mechanisms of
LASS2-induced apoptosis, changes in intracellular ROS,
∆Ψm and [Ca2+]i were considered.
The intracellular ROS level was determined by flow cytometry
analysis with DHE staining. It was revealed that the overexpression
of LASS2 significantly elicited ROS generation at 48 h
post-transfection (Fig. 4A). To
study whether LASS2 induced ∆Ψm collapse in HepG2 cells,
∆Ψm was measured using flow cytometry analysis with JC-1
dye. The LASS2-overexpressing HepG2 cells demonstrated a clear
reduction of ∆Ψm (Fig.
4B). Furthermore, the effect of LASS2 on
[Ca2+]i was determined by flow cytometry
using Calbryte 630 AM fluorescence intensity. LASS2 overexpression
promoted a significant increase of [Ca2+]i in
HepG2 cells (Fig. 4C), leading to
the disruption of intracellular calcium homeostasis.
Ca2+-ATPase is the major active calcium transport
protein in the maintenance of normal [Ca2+]i
in a variety of cell types (31).
Ca2+-ATPase activity was determined using a colorimetric
method by the inorganic phosphate content. A significantly reduced
ATPase activity was detected in cells transfected with
Adv-LASS2-GFP HepG2 cells compared with the Adv-GFP or NC cells
(Fig. 4D).
Discussion
The occurrence and development of tumors is a
complex process involving the gradual dysregulation of multiple
signal networks. The malignant characteristics of tumor cells, such
as unlimited proliferation, apoptosis resistance, invasion and
migration, are associated with mitochondrial dysfunction (32). Until the present day, the majority
of attention has focused on whether LASS2 can inhibit the growth of
cells through repressing the activity of V-ATPase in various types
of cancer cell, including HCC (11,12),
breast cancer (8) and prostate
cancer cells (33). Huang et
al (13) reported that
overexpression of LASS2 induces mitochondrial apoptosis through
downregulating mitochondrial membrane potential in BIU87 and J82
bladder cancer cells. A previous report indicated that LASS2
overexpression induced cell cycle arrest at
G0/G1 phase and induced apoptosis via a
caspase-dependent mitochondrial pathway in HEK293 and 293T cells
(34). Whether LASS2 affects HB
cell proliferation, apoptosis and cell cycle progression via the
mitochondrial and associated NF-кB signaling pathways has not
previously been identified. In the present study, the
antiproliferative effect of LASS2 overexpression was validated, and
it was identified that LASS2 overexpression induced apoptosis and
cell cycle arrest in the G0/G1 phase in HepG2
HB cells. Tang et al (12)
reported that overexpression of LASS2 could increase intracellular
H+ of HCC cells HCCLM3 via V-ATPase and induce cell
apoptosis through the cytochrome c mitochondrial
pathway.
The mitochondria are an important site for the
generation of ROS in eukaryotic cells, and intracellular calcium
stores; at the same time, the mitochondria are the primary switch
for intrinsic apoptosis (35,36).
ROS serve a second messenger role in the activation of
transcription factors (37,38), and not only directly damage
biological macromolecules such as DNA (39), but also activate the apoptotic
signaling pathway (40) and induce
cell cycle arrest (41–43). The present results demonstrated that
LASS2 overexpression increased the ROS concentration, and disrupted
mitochondrial function as evidenced by the loss of ∆Ψm
and the induction of [Ca2+]i overload, which
has not been performed before. An increase in the ROS level could
damage mitochondrial membranes and result in apoptosis through the
oxidation of mitochondrial pores, thereby disrupting the
∆Ψm (44,45). Calcium is a major signaling molecule
in the regulation of various aspects of cell function, including
the regulation of the cell cycle and apoptosis in a wide variety of
cell types (46). Intracellular
calcium homeostasis can be controlled by Ca2+-ATPase,
calcium channels and calcium stores (47). To the best of our knowledge, the
present study is the first to identify that LASS2 overexpression
can inhibit the activity of Ca2+-ATPase; it was
speculated that the inhibition of the calcium pumps promoted
[Ca2+]i overload, leading to the imbalance of
intracellular calcium homeostasis and triggering a chain reaction
leading to the initiation of apoptosis. The production of ROS is
typically induced by mutation, hypoxia, inhibitors and the
production of mitochondrial complexes I–IV (48,49).
Mutation, inhibitors and hypoxia induction were not considered in
the present study. It was hypothesized that LASS2 may bind to
ROS-producing target sites in the mitochondria to generate ROS.
Further study is required to confirm this idea.
Numerous studies have indicated that intracellular
ROS act as potent stimuli of NF-κB activation in various types of
malignant tumor (50–52). However, whether the LASS2-induced
ROS increase activates the NF-κB signaling pathway remains unclear.
Thus, the association between ROS and the NF-κB p65 signaling
pathway was explored. The western blot analysis results indicated
that the phosphorylation of NF-κB p65 was significantly reduced
subsequent to the upregulation of LASS2. The immunofluorescence
staining and confocal microscopy results revealed that
LASS2-elicited ROS generation may have impeded the translocation of
NF-κB p-p65 to the nucleus. NF-κB is important in cell
proliferation, apoptosis and cell cycle regulation to affect normal
and malignant cell growth (53,54).
The activation of NF-κB affects G1/S and G2/M
progression, and the CDK/CDK inhibitor system (55). Thus, the expression of cell cycle
regulatory proteins, such as cyclin D1 and CDK4, and the cell cycle
inhibitor p27 were examined. The data indicated that the
overexpression of LASS2 reduced the relative protein expression of
cyclin D1 and CDK4, and upregulated p27 protein expression. Other
reports have indicated that NF-κB promotes G1-to-S-phase
transition, potentially through cyclin D1 (56,57).
NF-κB p65, but not p50, is a downstream mediator of
arsenite-induced p27kip1 protein upregulation in
IKKβ−/− cells (58), and
p27 enhances NF-κB transactivation activity (54). p27 upregulation is particularly
associated with G0 quiescence (59) and the inhibition of cyclin D/CDK4
(60). Taken together, this
suggests that LASS2 overexpression induced
G0/G1 cell cycle arrest via the NF-κB
p65/p27/cyclin D1/CDK4 signaling pathway.
In conclusion, the results of the present study
provide novel evidence to demonstrate that LASS2 is associated with
mitochondrial apoptosis and NF-κB p65/p27/cyclin D1/CDK4 signaling
pathway regulation in HepG2 HB cells. These results may form the
theoretical basis for understanding HB tumorigenesis and provide a
potential therapeutic target for HB, although further study such as
knockdown LASS2 in hepatocytes and other hepatoma cells is required
to verify and explore more potential mechanisms of the LASS2 gene
in vivo and in vitro.
Acknowledgements
Not applicable.
Funding
The present work was supported by grants from
National Natural Science Foundation of China (grant no. 81460317),
Nation High-tech R&D Program of China (grant no. 2014AA022301),
Guizhou Province Medical Science and Technology Project (grant nos.
[2016]1174, LKZ [2011]32 and gzwjkj2015-1-015).
Availability of data and materials
The datasets analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
YY, XY, XM designed the study and drafted the
manuscript. YY, XY, XO, JX performed the experiments. LL, GY
analyzed, interpreted the data and critically revised the
manuscript. YY and TZ performed the statistical analyses. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127 (Suppl 1):S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J, Han KH, Gores G, Llovet JM and
Mazzaferro V: Liver cancer: Approaching a personalized care. J
Hepatol. 62 (Suppl):S144–S156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan X and Qiu Y: Impact of current staging
systems on treatment strategy for HBV-related hepatocellular
carcinoma. Cancer Lett. 379:220–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sia D, Villanueva A, Friedman SL and
Llovet JM: Liver cancer cell of origin, molecular class, and
effects on patient prognosis. Gastroenterology. 152:745–761. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eichenmüller M, Gruner I, Hagl B, Häberle
B, Müller-Höcker J, von Schweinitz D and Kappler R: Blocking the
hedgehog pathway inhibits hepatoblastoma growth. Hepatology.
49:482–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beck A, Trippel F, Wagner A, Joppien S,
Felle M, Vokuhl C, Schwarzmayr T, Strom TM, von Schweinitz D,
Längst G, et al: Overexpression of UHRF1 promotes silencing of
tumor suppressor genes and predicts outcome in hepatoblastoma. Clin
Epigenetics. 10:272018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu JF, Chang HH, Lu MY, Jou ST, Chang KC,
Ni YH and Chang MH: Prognostic roles of pathology markers
immunoexpression and clinical parameters in hepatoblastoma. J
Biomed Sci. 24:622017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mei F, You J, Liu B, Zhang M, Liu J, Zhang
B and Pei F: LASS2/TMSG1 inhibits growth and invasion of breast
cancer cell in vitro through regulation of vacuolar ATPase
activity. Tumour Biol. 36:2831–2844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mizutani Y, Kihara A and Igarashi Y:
Mammalian Lass6 and its related family members regulate synthesis
of specific ceramides. Biochem J. 390:263–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spassieva SD, Mullen TD, Townsend DM and
Obeid LM: Disruption of ceramide synthesis by CerS2 down-regulation
leads to autophagy and the unfolded protein response. Biochem J.
424:273–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu D, Jin H, Jin G, Wang C, Wang N, Hu F,
Luo Q, Chu W, Yao M and Qin W: The asialoglycoprotein receptor
suppresses the metastasis of hepatocellular carcinoma via
LASS2-mediated inhibition of V-ATPase activity. Cancer Lett.
379:107–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang N, Jin J, Deng Y, Ke RH, Shen QJ, Fan
SH and Qin WX: [LASS2 interacts with V-ATPase and inhibits cell
growth of hepatocellular carcinoma]. Sheng Li Xue Bao. 62:196–202.
2010.(In Chinese). PubMed/NCBI
|
|
13
|
Huang L, Luan T, Chen Y, Bao X, Huang Y,
Fu S, Wang H and Wang J: LASS2 regulates invasion and
chemoresistance via ERK/Drp1 modulated mitochondrial dynamics in
bladder cancer cells. J Cancer. 9:1017–1024. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Zuo Y, Ding M, Ke C, Yan R, Zhan
H, Liu J, Wang W, Li N and Wang J: LASS2 inhibits growth and
invasion of bladder cancer by regulating ATPase activity. Oncol
Lett. 13:661–668. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan S, Niu Y, Tan N, Wu Z, Wang Y, You H,
Ke R, Song J, Shen Q, Wang W, et al: LASS2 enhances
chemosensitivity of breast cancer by counteracting acidic tumor
microenvironment through inhibiting activity of V-ATPase proton
pump. Oncogene. 32:1682–1690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu X, You J and Pei F: Silencing of a
novel tumor metastasis suppressor gene LASS2/TMSG1 promotes
invasion of prostate cancer cell in vitro through increase of
vacuolar ATPase activity. J Cell Biochem. 113:2356–2363. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ke RH, Wang Y, Mao Y, Zhang J and Xiong J:
Decreased expression of LASS2 is associated with worse prognosis in
meningiomas. J Neurooncol. 118:369–376. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruan H, Wang T, Yang C, Jin G, Gu D, Deng
X, Wang C, Qin W and Jin H: Co-expression of LASS2 and TGF-β1
predicts poor prognosis in hepatocellular carcinoma. Sci Rep.
6:324212016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
20
|
Zhang ZL, Liu GC, Peng L, Zhang C, Jia YM,
Yang WH and Mao L: Effect of PAK1 gene silencing on proliferation
and apoptosis in hepatocellular carcinoma cell lines MHCC97-H and
HepG2 and cells in xenograft tumor. Gene Ther. 25:284–296. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ceballos MP, Decándido G, Quiroga AD,
Comanzo CG, Livore VI, Lorenzetti F, Lambertucci F,
Chazarreta-Cifre L, Banchio C, Alvarez ML, et al: Inhibition of
sirtuins 1 and 2 impairs cell survival and migration and modulates
the expression of P-glycoprotein and MRP3 in hepatocellular
carcinoma cell lines. Toxicol Lett. 289:63–74. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dunham KR and Selman BR: Interactions of
inorganic phosphate with spinach coupling factor 1. Effects on
ATPase and ADP binding activities. J Biol Chem. 256:10044–10049.
1981.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mankan AK, Lawless MW, Gray SG, Kelleher D
and McManus R: NF-kappaB regulation: The nuclear response. J Cell
Mol Med. 13:631–643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pantano C, Reynaert NL, van der Vliet A
and Janssen-Heininger YM: Redox-sensitive kinases of the nuclear
factor-kappaB signaling pathway. Antioxid Redox Signal.
8:1791–1806. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeng GZ, Wang Z, Zhao LM, Fan JT and Tan
NH: NF-κB and JNK mediated apoptosis and G0/G1 arrest of HeLa cells
induced by rubiarbonol G, an arborinane-type triterpenoid from
Rubia yunnanensis. J Ethnopharmacol. 220:220–227. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li W, Zhi W, Zhao J, Yao Q, Liu F and Niu
X: Cinnamaldehyde protects VSMCs against ox-LDL-induced
proliferation and migration through S arrest and inhibition of p38,
JNK/MAPKs and NF-κB. Vascul Pharmacol. 108:57–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ye D, Luo H, Lai Z, Zou L, Zhu L, Mao J,
Jacob T, Ye W, Wang L and Chen L: ClC-3 chloride channel proteins
regulate the cell cycle by up-regulating cyclin D1-CDK4/6 through
suppressing p21/p27 expression in nasopharyngeal carcinoma cells.
Sci Rep. 6:302762016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
James MK, Ray A, Leznova D and Blain SW:
Differential modification of p27Kip1 controls its cyclin
D-cdk4 inhibitory activity. Mol Cell Biol. 28:498–510. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Philipp-Staheli J, Payne SR and Kemp CJ:
p27(Kip1): Regulation and function of a haploinsufficient tumor
suppressor and its misregulation in cancer. Exp Cell Res.
264:148–168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Caro AA, Evans KL and Cederbaum AI: CYP2E1
overexpression inhibits microsomal Ca2+-ATPase activity
in HepG2 cells. Toxicology. 255:171–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zong WX, Rabinowitz JD and White E:
Mitochondria and Cancer. Mol Cell. 61:667–676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu X, Liu B, Zou P, Zhang Y, You J and Pei
F: Silencing of LASS2/TMSG1 enhances invasion and metastasis
capacity of prostate cancer cell. J Cell Biochem. 115:731–743.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Su J, Yu W, Gong M, You J, Liu J and Zheng
J: Overexpression of a novel tumor metastasis suppressor gene
TMSG1/LASS2 induces apoptosis via a caspase-dependent mitochondrial
pathway. J Cell Biochem. 116:1310–1317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Krammer PH, Kamiński M, Kiessling M and
Gülow K: No life without death. Adv Cancer Res. 97:111–138. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gupta AK, Ghosh K, Palit S, Barua J, Das
PK and Ukil A: Leishmania donovani inhibits inflammasome-dependent
macrophage activation by exploiting the negative regulatory
proteins A20 and UCP2. FASEB J. 31:5087–5101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ko EY, Cho SH, Kwon SH, Eom CY, Jeong MS,
Lee W, Kim SY, Heo SJ, Ahn G, Lee KP, et al: The roles of NF-κB and
ROS in regulation of pro-inflammatory mediators of inflammation
induction in LPS-stimulated zebrafish embryos. Fish Shellfish
Immunol. 68:525–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pannunzio NR and Lieber MR: AID and
reactive oxygen species can induce DNA breaks within human
chromosomal translocation fragile zones. Mol Cell. 68:901–912.e3.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zou Z, Chang H, Li H and Wang S: Induction
of reactive oxygen species: An emerging approach for cancer
therapy. Apoptosis. 22:1321–1335. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hsu YC, Huang TY and Chen MJ: Therapeutic
ROS targeting of GADD45γ in the induction of G2/M arrest in primary
human colorectal cancer cell lines by cucurbitacin E. Cell Death
Dis. 5:e11982014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Park WH: H2O2
inhibits the growth of human pulmonary fibroblast cells by inducing
cell death, GSH depletion and G1 phase arrest. Mol Med Rep.
7:1235–1240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim J, Kim SH, Johnson VJ and Sharma RP:
Extracellular signal-regulated kinase-signaling-dependent G2/M
arrest and cell death in murine macrophages by cadmium. Environ
Toxicol Chem. 24:3069–3077. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kalghatgi S, Spina CS, Costello JC, Liesa
M, Morones-Ramirez JR, Slomovic S, Molina A, Shirihai OS and
Collins JJ: Bactericidal antibiotics induce mitochondrial
dysfunction and oxidative damage in Mammalian cells. Sci Transl
Med. 5:192ra852013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang S, Li T, Zhang L, Wang X, Dong H, Li
L, Fu D, Li Y, Zi X, Liu HM, et al: A novel chalcone derivative S17
induces apoptosis through ROS dependent DR5 up-regulation in
gastric cancer cells. Sci Rep. 7:98732017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tiwari M, Prasad S, Shrivastav TG and
Chaube SK: Calcium signaling during meiotic cell cycle regulation
and apoptosis in mammalian oocytes. J Cell Physiol. 232:976–981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bagur R and Hajnóczky G: Intracellular
Ca2+ sensing: Its role in calcium homeostasis and
signaling. Mol Cell. 66:780–788. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hamanaka RB and Chandel NS: Mitochondrial
reactive oxygen species regulate cellular signaling and dictate
biological outcomes. Trends Biochem Sci. 35:505–513. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Moon DO, Kim MO, Lee JD, Choi YH and Kim
GY: Rosmarinic acid sensitizes cell death through suppression of
TNF-alpha-induced NF-kappaB activation and ROS generation in human
leukemia U937 cells. Cancer Lett. 288:183–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Korn SH, Wouters EF, Vos N and
Janssen-Heininger YM: Cytokine-induced activation of nuclear
factor-kappa B is inhibited by hydrogen peroxide through oxidative
inactivation of IkappaB kinase. J Biol Chem. 276:35693–35700. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jamaluddin M, Wang S, Boldogh I, Tian B
and Brasier AR: TNF-alpha-induced NF-kappaB/RelA Ser(276)
phosphorylation and enhanceosome formation is mediated by an
ROS-dependent PKAc pathway. Cell Signal. 19:1419–1433. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ge QL, Liu SH, Ai ZH, Tao MF, Ma L, Wen
SY, Dai M, Liu F, Liu HS, Jiang RZ, et al: RelB/NF-κB links cell
cycle transition and apoptosis to endometrioid adenocarcinoma
tumorigenesis. Cell Death Dis. 7:e24022016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wolff B and Naumann M: INK4 cell cycle
inhibitors direct transcriptional inactivation of NF-kappaB.
Oncogene. 18:2663–2666. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cicenas J, Kalyan K, Sorokinas A, Jatulyte
A, Valiunas D, Kaupinis A and Valius M: Highlights of the latest
advances in research on CDK inhibitors. Cancers (Basel).
6:2224–2242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hinz M, Krappmann D, Eichten A, Heder A,
Scheidereit C and Strauss M: NF-kappaB function in growth control:
Regulation of cyclin D1 expression and G0/G1-to-S-phase transition.
Mol Cell Biol. 19:2690–2698. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bera A, Ghosh-Choudhury N, Dey N, Das F,
Kasinath BS, Abboud HE and Choudhury GG: NFκB-mediated cyclin D1
expression by microRNA-21 influences renal cancer cell
proliferation. Cell Signal. 25:2575–2586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guo W, Liu J, Jian J, Li J, Wan Y and
Huang C: IKK-β/NF-κB p65 mediates p27(Kip1) protein degradation in
arsenite response. Biochem Biophys Res Commun. 447:563–568. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Poon RY, Toyoshima H and Hunter T:
Redistribution of the CDK inhibitor p27 between different cyclin.
CDK complexes in the mouse fibroblast cell cycle and in cells
arrested with lovastatin or ultraviolet irradiation. Mol Biol Cell.
6:1197–1213. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Orlando S, Gallastegui E, Besson A, Abril
G, Aligué R, Pujol MJ and Bachs O: p27Kip1 and
p21Cip1 collaborate in the regulation of transcription
by recruiting cyclin-Cdk complexes on the promoters of target
genes. Nucleic Acids Res. 43:6860–6873. 2015. View Article : Google Scholar : PubMed/NCBI
|