Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common cancer worldwide (1,2).
Cancer in the head and neck region can arise from several
functional disorders associated chewing, speech, swallowing and
respiration. Therefore, therapy for HNSCC is required to not only
cure the disease, but to also preserve the function of the affected
area in order to maintain the quality of life of patients with
HNSCC. Despite the recent advancements in surgery, chemotherapy and
radiotherapy, limited improvements in treating metastatic HNSCC
have been achieved (3,4). Two-thirds of all patients present
advanced stage III or IV tumors with low locoregional control rates
and the long-term survival of patients with HNSCC has remained
insufficient (5,6). Therefore, novel agents for potential
alternative HNSCC treatments with greater efficacy are urgently
required. The present study focused on the human regenerating gene
(REG) as a reliable biomarker for predicting HNSCC
progression. REG was first identified in regenerating
pancreatic islets in studies on diabetology in 1988 (7). REG family proteins are classified into
4 subfamilies: Types I, II, III and IV. The human REG family
consists of 5 members: REG Iα, REGIβ, REG III,
hepatocarcinoma-intestine-pancreas/pancreatitis-associated-protein
and REG IV (8–13). The REG family of proteins has
been revealed to serve roles in normal tissue regeneration
(14,15) and also the progression of various
types of cancers, including esophageal, gastric, lung, liver,
colorectal and prostate cancer (16–25).
Recently, we reported that REG III expression was associated
with improved survival rates for HNSCC, and that REG III
enhanced chemo- and radiosensitivity in vitro (26). In addition, as REG III
contributes to the improvement of the prognosis of HNSCC, in our
previous study we searched for the substance that induced the
expression of REG III and finally revealed that resveratrol
(3,4′,5-trihydroxy-trans-stilbene) significantly increased
REG III expression in HNSCC cells, and also significantly
inhibited cell growth, enhanced chemo- and radiosensitivity, and
blocked HNSCC cell invasion in vitro (27). The aim of the present study was to
investigate the effect of resveratrol on cancer progression in
HNSCC in vivo for clinical application.
Materials and methods
Cell culture and reagent
Human hypopharyngeal cell carcinoma FaDu cells were
obtained from American Type Culture Collection (ATCC; Manassas, VA,
USA) and grown and maintained in Dulbecco's modified Eagle's medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.) and supplemented with 100 U/ml penicillin G and 100 µg/ml
streptomycin and 250 ng/ml amphotericin B (Antibiotic-Antimycotic;
Gibco; Thermo Fisher Scientific, Inc.). The cells were incubated in
5% CO2/95% air with a humidified atmosphere at 37°C.
3,4′,5-Trihydroxy-trans-stilbene (resveratrol) was obtained
from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Resveratrol was dissolved in dimethyl sulfoxide (DMSO;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and then diluted
with normal saline to achieve the correct dose in 300 µl of 2%
DMSO.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using a
RNAprotect® Cell Mini kit (Qiagen GmbH, Hilden, Germany)
from FaDu cells. cDNA was reverse-transcribed from 0.5–2 µg samples
of total RNA using a High Capacity cDNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) as previously
described (26–33). cDNA was subjected to RT-PCR with the
following primers, which were synthesized and prepared by NGRL
(Sendai, Japan): β-actin (NM_001101) sense,
5′-GCGAGAAGATGACCCAGA-3′ and antisense, 5′-CAGAGGCGTACAGGGATA-3′;
and REG III (AB161037) sense 5′-GAATATTCTCCCCAAACTG-3′ and
antisense, 5′-GAGAAAAGCCTGAAATGAAG-3′.
RT-qPCR was performed using the KAPA SYBR FAST qPCR
Master Mix (Kapa Biosystems; Roche Diagnostics, Indianapolis, IN,
USA) and Thermal Cycler Dice Real-Time System (Takara Bio, Inc.,
Otsu, Japan) as previously described (25–33).
qPCR was performed with the following thermocycling conditions: An
initial step of 3 min at 95°C followed by 40 cycles of 3 sec at
95°C and 20 sec at 60°C. The level of the target mRNA was
normalized to the mRNA level of β-actin as an internal
standard.
Animals
BALB/c nude male mice were purchased from CLEA
Japan, Inc. (Tokyo, Japan). All protocols were approved by the
Animal Care and Use Committee of Nara Medical University (Nara,
Japan). Each cage housed 3 mice with food and water available ad
libitum in a pathogen-free environment with a 12-h light/dark
cycle.
In vivo model for resveratrol-induced
REG III expression in HNSCC cells
A total of 6 BALB-c nude male mice were used in each
experiment. FaDu cells (1×106 cells/100 µl saline) were
implanted subcutaneously into the right flanks of BALB/c nude male
mice (4–5 weeks old). When the tumors reached ~100 mm3
volume, the mice were randomly assigned into 2 groups (day 0):
Vehicle control (normal saline and 2% DMSO) and resveratrol groups.
Mice were treated intraperitoneally with resveratrol (100
mg/kg/day) or vehicle until the completion of the experiment. At 30
days following treatment, mice were sacrificed with intraperitoneal
administration of pentobarbital (100 mg/kg) and the tumor tissues
were harvested. The mRNA levels of intrinsic REG III were
then examined in tumor tissues via RT-qPCR.
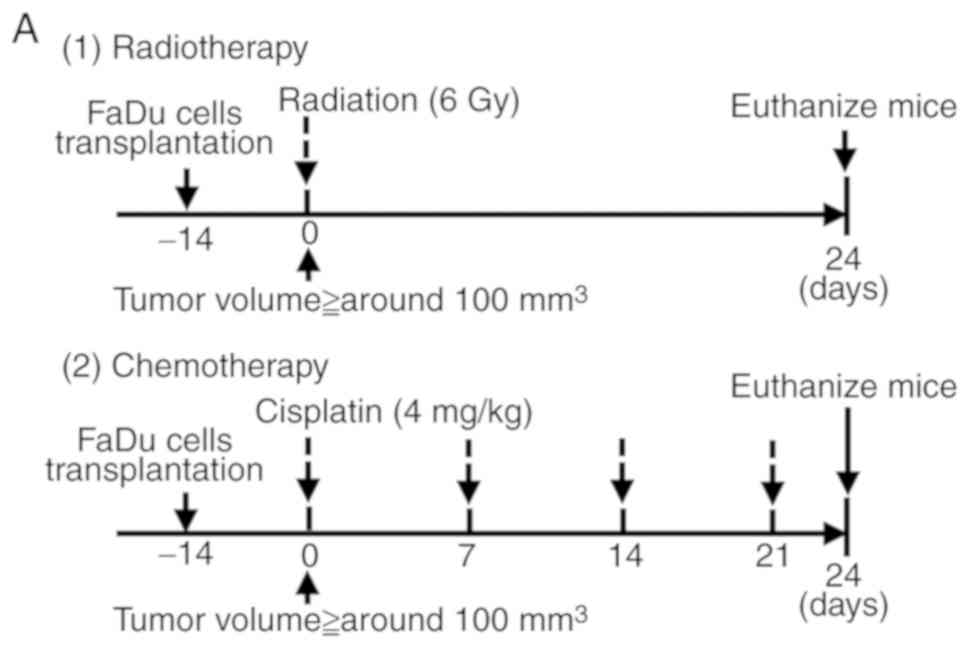
In vivo model of REG III-induced
chemo- and radiosensitivity
A total of 12 BALB-c nude male mice (4–5 weeks old)
were used to establish a xenograft model in each experiment. FaDu
cells transfected with the REG III expression plasmid
(FaDu-REG III cells) or the neomycin-resistance gene alone
(FaDu-mock cells) were used as previously described (26). FaDu-REG III or FaDu-mock cells
(1×106 cells in 100 µl volume) were implanted
subcutaneously in the right flanks of BALB/c nude male mice (4–5
weeks old). When the tumor sizes reached ~100 mm3, the
mice were randomly assigned to the treatment groups (n=3/group; day
0). For chemosensitivity experiments, the treatment groups
consisted of the vehicle control (normal saline) and cisplatin. On
day 0, cisplatin (Nihon Kayaku Co., Tokyo, Japan; 4 mg/kg/week) or
normal saline was administered intraperitoneally; a total of 4
injections of cisplatin were administered. For the radiosensitivity
experiments, the treatment groups consisted of vehicle control
(normal saline) and 6 Gy irradiation. Mice were exposed to
radiation using a MBR-1520R system (Hitachi, Ltd., Tokyo, Japan)
operated at 150 kV and 20 mA as previously described (27), which delivered the dose at 0.8
Gy/min. For both the chemo- and radiosensitivity experiments, the
tumor size was assessed every 3 days. The tumor volume was
calculated using the following formula:
Volume=[Lx(W)2]/2, where L is the length and W is the
width. At 24 days post-treatment, the mice were sacrificed with
intraperitoneal administration of pentobarbital (100 mg/kg) and the
tumor tissues were harvested.
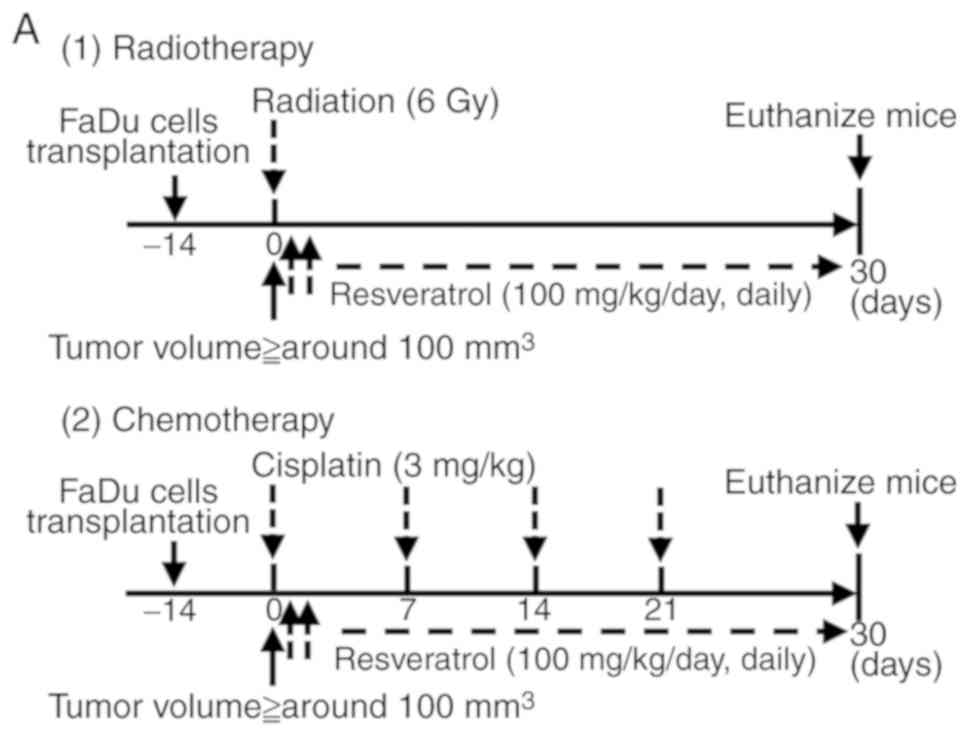
In vivo model for resveratrol-induced
chemo- and radiosensitivity
A total of 12 BALB-c nude male mice were used in
each study. FaDu cells (1×106 cells/100 µl saline) were
implanted subcutaneously in the right flanks of BALB/c nude male
mice (4–5 weeks old). When the tumors reached ~100 mm3
volume, the mice were randomly assigned into the following
treatment groups (n=3/group; day 0). For the chemosensitivity
experiments, the treatment groups consisted of the vehicle control
(normal saline and 2% DMSO), vehicle and cisplatin, resveratrol
alone and cisplatin + resveratrol. On day 0, the mice were treated
intraperitoneally with resveratrol or vehicle until the completion
of the experiment; resveratrol was administered at doses of 100
mg/kg for 30 consecutive days. Cisplatin (3 mg/kg/week) or normal
saline was administered intraperitoneally on day 0; a total of 4
cycles were administered. For the radiosensitivity experiments, the
treatment groups consisted of the vehicle control (normal saline
and 2% DMSO), vehicle and 6 Gy irradiation, resveratrol (100
mg/kg/day) alone and 6 Gy irradiation + resveratrol (100
mg/kg/day). For both the chemo- and radiosensitivity experiments,
the tumor size was measured every 3 days. Following 30 days of
treatment, the mice were sacrificed and the tumor tissues were
harvested.
Statistical analysis
Data were expressed as the mean ± standard error.
Statistically significant differences between groups were
determined by Student's t-test using StatMate IV (Abacus Concepts,
Piscataway, NJ, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
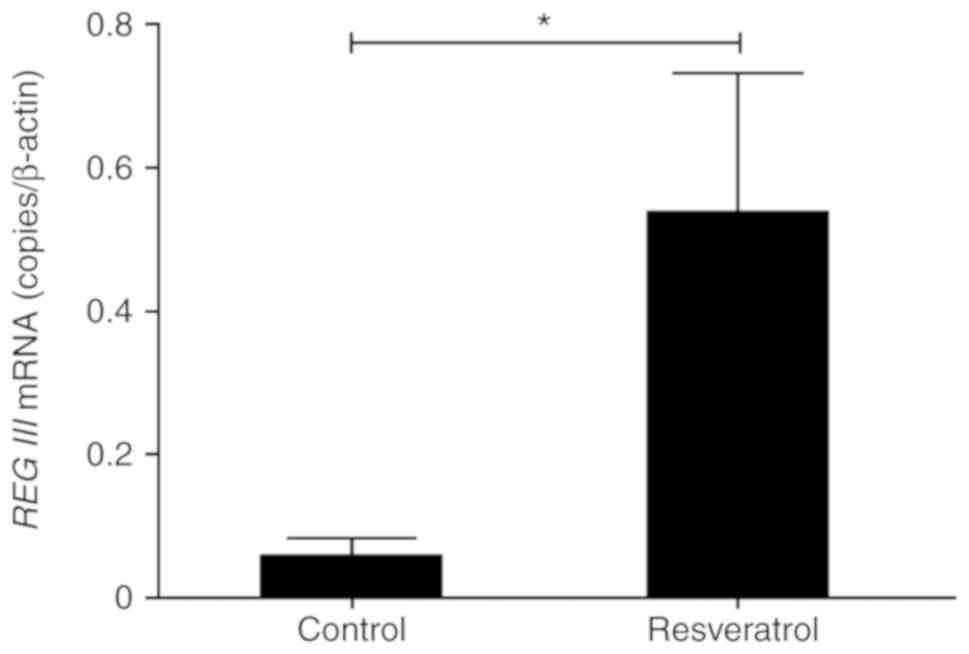
Induction of REG III mRNA by
resveratrol in the tumor tissues of a xenograft mouse model
To elucidate the induction of REG III in
HNSCC cells by resveratrol in vivo, the present study
investigated the levels of REG III mRNA in xenografted FaDu
cells with or without resveratrol. The mRNA levels of REG
III in the resveratrol-treated tumor tissues were significantly
increased by resveratrol when compared with the untreated tumor
tissues, which were used as the control (Fig. 1). This result was in agreement with
the in vitro results we previously reported (27). Therefore, resveratrol induced REG
III mRNA expression in vivo as well as in vitro
in HNSCC.
REG III enhances the efficacy of
radiation or cisplatin therapy in a HNSCC xenograft mouse
model
To evaluate the effect of REG III on chemo-
and radiosensitivity in HNSCC in vivo, the present study
established a HNSCC xenograft model of BALB/c nude mice. An animal
xenograft model was generated via injections in nude mice with FaDu
cells stably transfected with the REG III expression plasmid
(FaDu-REG III) or FaDu cells transfected the neomycin-resistance
gene alone (FaDu-mock) as the control into the right flank. When
tumors reached ~100 mm3 following 2 weeks, the mice were
randomly assigned into 2 groups: Non-treatment, and irradiation or
cisplatin therapy groups (day 0), each specific treatment was then
applied as aforementioned (Fig.
2A). The efficacy of the radiation or cisplatin therapy was
evaluated by monitoring the tumor volume. Regarding the effect of
radiation, there were no significant differences in tumor volume
between the FaDu-REG III and FaDu-mock in the non-treatment groups.
However, in the radiation groups the tumor volume of the FaDu-REG
III-treated mice was significantly inhibited when compared with
FaDu-mock from day 21 (Fig. 2B-D).
Regarding the effect of cisplatin therapy, significant inhibition
of tumor progression was observed in the FaDu-REG III group when
compared with the FaDu-mock-treated groups from day 15, which
corresponded with the results in the radiation groups (Fig. 2B-D). These results indicated that
REG III enhanced chemo- and radiosensitivity in HNSCC in
vivo as well as in vitro, as previously described
(26).
Irradiation or cisplatin therapy with
resveratrol synergistically inhibits HNSCC xenograft tumor growth
in vivo
To assess the in vivo therapeutic potential
of resveratrol, the present study examined tumor progression using
a HNSCC xenograft model of BALB/c nude mice. Tumors in the right
flanks of the mice were established for 2 weeks prior to the
initiation of the treatments, then the mice were randomly assigned
into 4 groups for experiments evaluating cisplatin therapy or
irradiation, respectively (day 0; Fig.
3A). The potential of the treatment was evaluated by assessing
the tumor volume. Regarding the potential of radiation therapy,
mice in the resveratrol alone group had smaller tumor volumes than
mice in the control group; however, the difference was not
statistically significant. Mice in the radiation with resveratrol
group had significantly smaller tumor volumes than mice in the
control group. In addition, the radiation with resveratrol group
exhibited significant antitumor effects when compared with the
resveratrol alone group, or the radiation alone group on day 30
(Fig. 3B-D). Regarding the
potential of cisplatin therapy, mice in the cisplatin alone and
cisplatin with resveratrol groups had significantly smaller tumor
volumes than mice in the control group. In addition, the cisplatin
with resveratrol group exhibited significant antitumor effects when
compared with the resveratrol alone or cisplatin alone groups from
day 24 (Fig. 3B-D). These results
indicated that resveratrol enhanced chemo- and radiosensitivity in
HNSCC in vivo, which was in agreement with the effects
observed with REG III in vivo.
Discussion
Despite the progression in the current cancer
treatments available, such as surgery, radiation and chemotherapy,
these have not been effective in improving the survival rate of
HNSCC, particularly hypopharyngeal carcinoma (1–6).
Chemo- and radioresistance can cause local recurrence and distant
metastasis, which are associated with poor prognosis. Therefore,
identification of reliable biomarkers that enhance sensitivity for
the chemo- and radiotherapy of HNSCC is highly desirable to improve
prognosis. As a biomarker of HNSCC, we have focused on REG,
whose family of proteins have been associated with diseases such as
chronic inflammation and malignant tumors (16–26).
We have previously reported that REG III
expression was associated with an improved survival rate for
patients with HNSCC (26). In
addition, resveratrol significantly increased REG III
expression, could enhance the chemo- and radiosensitivity, and
inhibit cancer progression through the REG III expression pathway
in HNSCC cells in vitro (27). In the present study, the effect of
resveratrol on cancer progression in HNSCC through the REG
III expression pathway in vivo was investigated. The
results of the present study demonstrated that resveratrol
increased the mRNA level of REG III in vivo, which
corresponded with our in vitro results previously reported
(27). This result highlights the
potential of resveratrol in inducing the REG III expression
pathway in vivo.
Resveratrol (3,4′,5-trihydroxystilbene) is a
polyphenolic compound found in grapes and other food products that
provides a number of anti-aging health benefits against metabolism,
cardiovascular disease and carcinogenesis (34–37).
Various previous studies indicated that resveratrol enhanced the
sensitivity of chemo- or radiotherapy (38–42).
Furthermore, some studies have demonstrated that resveratrol
significantly decreased tumor progression (39,40,43).
Such in vitro studies on the associations between
resveratrol and cancer have been reported (34–37,44–50),
while, to the best of our knowledge, there are fewer in vivo
studies.
In the present study, a HNSCC xenograft nude mouse
model was established to evaluate the effect of REG III on
cancer progression in HNSCC in vivo. It was demonstrated
that REG III increased the antitumor effect of radiation or
cisplatin in vivo. In addition, the in vivo
therapeutic potential of resveratrol was evaluated, and the results
revealed that it significantly sensitized HNSCC to irradiation and
cisplatin in vivo, although resveratrol is not likely to be
a primary treatment for HNSCC. These results indicate that
resveratrol has potential for use as an adjuvant anticancer therapy
in HNSCC. In our previous studies, similar results were obtained in
HSC-4 cells as well as FaDu cells (27). In terms of its effect for HNSCC
in vivo, further studies whether HSC-4 cells have similar
results based on the results of this study are required in the
future. Moreover, it is necessary to consider which combination of
chemotherapy, radiotherapy and resveratrol is the most effective
treatment. The present study performed in vivo experiments
using a xenograft mouse model. For clinical applications associated
with the administration of resveratrol, experiments on an
orthotopic transplant mouse model may be required in order to take
into consideration the microenvironment in the future. Concerning
the bioavailability of resveratrol, recent studies have indicated
that resveratrol induces apoptosis or autophagy in several human
cancer cell lines and in an animal model of carcinogenesis
(49,50). It has been reported that resveratrol
induces cell apoptosis and cell cycle arrest via the
caspase/cyclin-cyclin dependent kinase (CDK) signaling pathway
(49). The present study
investigated the anti-carcinogenic potential of resveratrol by
analyzing the REG III expression pathway. Preliminary
experiments were performed to investigate the anti-carcinogenic
mechanism of resveratrol, by analyzing the expression of
apoptosis-related proteins. These preliminary results revealed that
both resveratrol and REG III decreased the expression of
cyclin D1, B-cell lymphoma-xL and activated caspase-3 compared with
the control (data not shown). This indicated that resveratrol may
inhibit cancer progression through the REG III pathway via the
caspase/cyclin-CDK signaling pathway. However, the underlying
mechanism of how resveratrol enhances REG III, and how
REG III enhances the chemo- and radiosensitivity of HNSCC
remains unknown. Therefore, further studies are required in the
future.
In conclusion, the results of the present study
indicate that resveratrol increases the efficacy of cisplatin and
irradiation through the REG III expression pathway,
resulting in the inhibition of tumor growth, in treating HNSCC
in vivo. The present study provides support for clinical
trials using resveratrol as an adjuvant anticancer therapy and may
help improve human HNSCC prognosis. However, additional studies
will be required to fully define the therapeutic potential of
resveratrol for HNSCC.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by
Grants-in-Aids for Scientific Research from the Ministry of
Education, Culture, Sports, Science and Technology of Japan (grant
nos. 15K10822 and 17K11398).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
IO, SM and ST conceived and designed the research.
SM, TM and TU conducted the experiments. SM, HO, TKim and TU were
involved in the analysis and interpretation of data. SM wrote the
manuscript. IO and SM revised the manuscript for important
intellectual content. TKit and IO were involved in editing the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All protocols were approved by the Animal Care and
Use Committee of Nara Medical University (Nara, Japan). All
procedures performed in the studies were in accordance with the
1964 Declaration of Helsinki and its later amendments.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cooper JS, Zhang Q, Pajak TF, Forastiere
AA, Jacobs J, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, et
al: Long-term follow-up of the RTOG 9501/intergroup phase III
trial: Postoperative concurrent radiation therapy and chemotherapy
in high-risk squamous cell carcinoma of the head and neck. Int J
Radiat Oncol Biol Phys. 84:1198–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adelstein DJ, Saxton JP, Rybicki LA,
Esclamado RM, Wood BG, Strome M, Lavertu P, Lorenz RR and Carroll
MA: Multiagent concurrent chemoradiotherapy for locoregionally
advanced squamous cell head and neck cancer: Mature results from a
single institution. J Clin Oncol. 24:1064–1071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fung C and Grandis JR: Emerging drugs to
treat squamous cell carcinomas of the head and neck. Expert Opin
Emerg Drugs. 15:355–373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen YJ, Chang JT, Liao CT, Wang HM, Yen
TC, Chiu CC, Lu YC, Li HF and Cheng AJ: Head and neck cancer in the
betel quid chewing area: Recent advances in molecular
carcinogenesis. Cancer Sci. 99:1507–1514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Terazono K, Yamamoto H, Takasawa S, Shiga
K, Yonemura Y, Tochino Y and Okamoto H: A novel gene activated in
regenerating islets. J Biol Chem. 263:2111–2114. 1988.PubMed/NCBI
|
|
8
|
Takasawa S: Regenerating gene (REG)
product and its potential clinical usage. Expert Opin Ther Targets.
20:541–550. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao J, Wang J, Wang H and Lai M: Reg
proteins and their roles in inflammation and cancer of the human
digestive system. Adv Clin Chem. 61:153–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moriizumi S, Watanabe T, Unno M,
Nakagawara K, Suzuki Y, Miyashita H, Yonekura H and Okamoto H:
Isolation, structural determination and expression of a novel reg
gene, human regI beta. Biochim Biophys Acta. 1217:199–202. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lasserre C, Simon MT, Ishikawa H, Diriong
S, Nguyen VC, Christa L, Vernier P and Brechot C: Structural
organization and chromosomal localization of a human gene (HIP/PAP)
encoding a C-type lectin overexpressed in primary liver cancer. Eur
J Biochem. 224:29–38. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nata K, Liu Y, Xu L, Ikeda T, Akiyama T,
Noguchi N, Kawaguchi S, Yamauchi A, Takahashi I, Shervani NJ, et
al: Molecular cloning, expression and chromosomal localization of a
novel human REG family gene. REG III Gene. 340:161–170.
2004.PubMed/NCBI
|
|
13
|
Hartupee JC, Zhang H, Bonaldo MF, Soares
MB and Dieckgraefe BK: Isolation and characterization of a cDNA
encoding a novel member of the human regenerating protein family:
Reg IV. Biochim Biophys Acta. 1518:287–293. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Watanabe T, Yonemura Y, Yonekura H, Suzuki
Y, Miyashita H, Sugiyama K, Moriizumi S, Unno M, Tanaka O, Kondo H,
et al: Pancreatic beta-cell replication and amelioration of
surgical diabetes by Reg protein. Proc Natl Acad Sci USA.
91:3589–3592. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asahara M, Mushiake S, Shimada S, Fukui H,
Kinoshita Y, Kawanami C, Watanabe T, Tanaka S, Ichikawa A, Uchiyama
Y, et al: Reg gene expression is increased in rat gastric
enterochromaffin-like cells following water immersion stress.
Gastroenterology. 111:45–55. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Macadam RC, Sarela AI, Farmery SM,
Robinson PA, Markham AF and Guillou PJ: Death from early colorectal
cancer is predicted by the presence of transcripts of the REG gene
family. Br J Cancer. 83:188–195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harada K, Zen Y, Kanemori Y, Chen TC, Chen
MF, Yeh TS, Jan YY, Masuda S, Nimura Y, Takasawa S, et al: Human
REG I gene is up-regulated in intrahepatic cholangiocarcinoma and
its precursor lesions. Hepatology. 33:1036–1042. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Violette S, Festor E, Pandrea-Vasile I,
Mitchell V, Adida C, Dussaulx E, Lacorte JM, Chambaz J, Lacasa M
and Lesuffleur T: REG IV, a new member of the regenerating gene
family, is overexpressed in colorectal carcinomas. Int J Cancer.
103:185–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yonemura Y, Sakurai S, Yamamoto H, Endou
Y, Kawamura T, Bandou E, Elnemr A, Sugiyama K, Sasaki T, Akiyama T,
et al: REG gene expression is associated with the infiltrating
growth of gastric carcinoma. Cancer. 98:1394–1400. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dhar DK, Udagawa J, Ishihara S, Otani H,
Kinoshita Y, Takasawa S, Okamoto H, Kubota H, Fujii T, Tachibana M
and Nagasue N: Expression of regenerating gene I in gastric
adenocarcinomas: Correlation with tumor differentiation status and
patient survival. Cancer. 100:1130–1136. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bishnupuri KS, Luo Q, Murmu N, Houchen CW,
Anant S and Dieckgraefe BK: REG IV activates the epidermal growth
factor receptor/Akt/AP-1 signaling pathway in colon
adenocarcinomas. Gastroenterology. 130:137–149. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayashi K, Motoyama S, Koyota S, Koizumi
Y, Wang J, Takasawa S, Itaya-Hironaka A, Sakuramoto-Tsuchida S,
Maruyama K, Saito H, et al: REG I enhances chemo- and
radiosensitivity in squamous cell esophageal cancer cells. Cancer
Sci. 99:2491–2495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayashi T, Matsubara A, Ohara S, Mita K,
Hasegawa Y, Usui T, Arihiro K, Norimura S, Sentani K, Oue N and
Yasui W: Immunohistochemical analysis of REG IV in urogenital
organs: Frequent expression of REG IV in prostate cancer and
potential utility as serum tumor marker. Oncol Rep. 21:95–100.
2009.PubMed/NCBI
|
|
24
|
Zhou L, Zhang R, Wang L, Shen S, Okamoto
H, Sugawara A, Xia L, Wang X, Noguchi N, Yoshikawa T, et al:
Upregulation of REG I alpha accelerates tumor progression in
pancreatic cancer with diabetes. Int J Cancer. 127:1795–1803. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kimura M, Naito H, Tojo T, Itaya-Hironaka
A, Dohi Y, Yoshimura M, Nakagawara K, Takasawa S and Taniguchi S:
REG Iα gene expression is linked with the poor prognosis of lung
adenocarcinoma and squamous cell carcinoma patients via discrete
mechanisms. Oncol Rep. 30:2625–2631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masui T, Ota I, Itaya-Hironaka A, Takeda
M, Kasai T, Yamauchi A, Sakuramoto-Tsuchida S, Mikami S, Yane K,
Takasawa S and Hosoi H: Expression of REG III and prognosis in head
and neck cancer. Oncol Rep. 30:573–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mikami S, Ota I, Masui T, Itaya-Hironaka
A, Shobatake R, Okamoto H, Takasawa S and Kitahara T: Effect of
resveratrol on cancer progression through the REG III expression
pathway in head and neck cancer cells. Int J Oncol. 49:1553–1560.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ota H, Tamaki S, Itaya-Hironaka A,
Yamauchi A, Sakuramoto-Tsuchida S, Morioka T, Takasawa S and Kimura
H: Attenuation of glucose-induced insulin secretion by intermittent
hypoxia via down-regulation of CD38. Life Sci. 90:206–211. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakagawa K, Takasawa S, Nata K, Yamauchi
A, Itaya-Hironaka A, Ota H, Yoshimoto K, Sakuramoto-Tsuchida S,
Miyaoka T, Takeda M, et al: Prevention of REG I-induced β-cell
apoptosis by IL-6/dexamethasone through activation of HGF gene
regulation. Biochim Biophys Acta. 1833:2988–2995. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamauchi A, Itaya-Hironaka A,
Sakuramoto-Tsuchida S, Takeda M, Yoshimoto K, Miyaoka T, Fujimura
T, Tsujinaka H, Tsuchida C, Ota H and Takasawa S: Synergistic
activations of REG Iα and REG Iβ promoters by IL-6 and
glucocorticoids through JAK/STAT pathway in human pancreatic β
cells. J Diabetes Res. 2015:1730582015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujimura T, Fujimoto T, Itaya-Hironaka A,
Miyaoka T, Yoshimoto K, Yamauchi A, Sakuramoto-Tsuchida S, Kondo S,
Takeda M, Tsujinaka H, et al: Interleukin-6/STAT pathway is
responsible for the induction of gene expression of REG Iα, a new
auto-antigen in Sjögren's syndrome patients, in salivary duct
epithelial cells. Biochem Biophys Rep. 2:69–74. 2015.PubMed/NCBI
|
|
32
|
Tsujinaka H, Itaya-Hironaka A, Yamauchi A,
Sakuramoto-Tsuchida S, Ota H, Takeda M, Fujimura T, Takasawa S and
Ogata N: Human retinal pigment epithelial cell proliferation by the
combined stimulation of hydroquinone and advanced glycation
end-products via up-regulation of VEGF gene. Biochem Biophys Rep.
2:123–131. 2015.PubMed/NCBI
|
|
33
|
Takasawa S, Kuroki M, Nata K, Noguchi N,
Ikeda T, Yamauchi A, Ota H, Itaya-Hironaka A, Sakuramoto-Tsuchida
S, Takahashi I, et al: A novel ryanodine receptor expressed in
pancreatic islets by alternative splicing from type 2 ryanodine
receptor gene. Biochem Biophys Res Commun. 397:140–145. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Renaud S and de Lorgeril M: Wine, alcohol,
platelets, and the French paradox for coronary heart disease.
Lancet. 20:1523–1526. 1992. View Article : Google Scholar
|
|
35
|
Frémont L: Biological effects of
resveratrol. Life Sci. 66:663–673. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cal C, Garban H, Jazirehi A, Yeh C,
Mizutani Y and Bonavida B: Resveratrol and cancer: Chemoprevention,
apoptosis, and chemo-immunosensitizing activities. Curr Med Chem
Anticancer Agents. 3:77–93. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kundu JK and Surh YJ: Cancer
chemopreventive and therapeutic potential of resveratrol:
Mechanistic perspectives. Cancer Lett. 269:243–261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG and Pezzuto JM: Cancer chemopreventive activity of resveratrol,
a natural product derived from grapes. Science. 10:218–220. 1997.
View Article : Google Scholar
|
|
39
|
Bai Y, Mao QQ, Qin J, Zheng XY, Wang YB,
Yang K, Shen HF and Xie LP: Resveratrol induces apoptosis and cell
cycle arrest of human T24 bladder cancer cells in vitro and
inhibits tumor growth in vivo. Cancer Sci. 101:488–493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang S, Li W, Sun H, Wu B, Ji F, Sun T,
Chang H, Shen P, Wang Y and Zhou D: Resveratrol elicits
anti-colorectal cancer effect by activating miR-34c-KITLG in vitro
and in vivo. BMC Cancer. 15:9692015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tan L, Wang W, He G, Kuick RD, Gossner G,
Kueck AS, Wahl H, Opipari AW and Liu JR: Resveratrol inhibits
ovarian tumor growth in an in vivo mouse model. Cancer.
122:722–729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tan Y, Wei X, Zhang W, Wang X, Wang K, Du
B and Xiao J: Resveratrol enhances the radiosensitivity of
nasopharyngeal carcinoma cells by downregulating E2F1. Oncol Rep.
37:1833–1841. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yin HT, Tian QZ, Guan L, Zhou Y, Huang XE
and Zhang H: In vitro and in vivo evaluation of the antitumor
efficiency of resveratrol against lung cancer. Asian Pac J Cancer
Prev. 14:1703–1706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Estrov Z, Shishodia S, Faderl S, Harris D,
Van Q, Kantarjian HM, Talpaz M and Aggarwal BB: Resveratrol blocks
interleukin-1beta-induced activation of the nuclear transcription
factor NF-kappaB, inhibits proliferation, causes S-phase arrest,
and induces apoptosis of acute myeloid leukemia cells. Blood.
102:987–995. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liang YC, Tsai SH, Chen L, Lin-Shiau SY
and Lin JK: Resveratrol-induced G2 arrest through the inhibition of
CDK7 and p34CDC2 kinases in colon carcinoma HT29 cells. Biochem
Pharmacol. 65:1053–1060. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
ElAttar TM and Virji AS: Modulating effect
of resveratrol and quercetin on oral cancer cell growth and
proliferation. Anticancer Drugs. 10:187–193. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yeh CB, Hsieh MJ, Lin CW, Chiou HL, Lin
PY, Chen TY and Yang SF: The antimetastatic effects of resveratrol
on hepatocellular carcinoma through the downregulation of a
metastasis-associated protease by SP-1 modulation. PLoS One.
8:e566612013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shankar S, Singh G and Srivastava RK:
Chemoprevention by resveratrol: Molecular mechanisms and
therapeutic potential. Front Biosci. 12:4839–4854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu B, Zhou Z, Zhou W, Liu J, Zhang Q, Xia
J, Liu J, Chen N, Li M and Zhu R: Resveratrol inhibits
proliferation in human colorectal carcinoma cells by inducing G1/S
phase cell cycle arrest and apoptosis through caspase/cyclin CDK
pathways. Mol Med Rep. 10:1697–1702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang G, Guo X, Chen H, Lin T, Xu Y, Chen
Q, Liu J, Zeng J, Zhang XK and Yao X: A resveratrol analog,
phoyunbene B, induces G2/M cell cycle arrest and apoptosis in HepG2
liver cancer cells. Bioorg Med Chem Lett. 22:2114–2118. 2012.
View Article : Google Scholar : PubMed/NCBI
|