Introduction
Vulvar cancer is a relatively rare disease,
accounting for 4% of gynecological malignancies globally. The
majority of vulvar cancer cases are of squamous cell carcinoma
(SCC) histology. The incidence of vulvar cancer has increased in
several countries during the last decades, particularly among
younger women (1). In Sweden,
however, the incidence remains at ~3/100,000 women since the 1990s
(2). Vulvar SCC (VSCC) can be
classified as human papilloma virus (HPV)-independent and
HPV-dependent. HPV-independent tumors usually arise later in life
and are often preceded by long-standing vulvar dermatosis (3). The frequency of high-risk HPV-positive
VSCC is reported to be between 28.6 and 44.7%. The most common
HPV-subtype in VSCC is HPV16 (4,5). The
proposed pathologic mechanism in HPV-dependent tumors is
inactivation of tumor suppressor proteins p53 and Rb by the
HPV-derived oncogenic proteins E6 and E7, causing alteration of
signal transduction pathways to promote transformation (6). In HPV-transformed cells, the
p16INK4a/CDK4/pRB pathway is blocked resulting in
accumulation of the cyclin-dependent kinase-4 inhibitor
p16INK4a (3).
Overexpression of p16INK4a has been viewed as a
pseudomarker for high-risk HPV-infection (7). However, according to the study by de
Sanjosé et al only 87.9% of HPV-positive invasive VSCC are
positive for p16INK4a (4). This study as well as another (5), suggest that only tumors with the
combined presence of HPV-DNA and p16INK4a overexpression
should be viewed as truly HPV-driven. HPV- and/or
p16INK4a-positive tumors of female genitalia, as well as
head and neck tumors, have been associated with significant
survival benefits compared to HPV- and p16-negative tumors
(5,8,9).
This study focused on the expression of the
leucine-rich repeats and immunoglobulin-like domains (LRIG) family
of transmembrane proteins and the LIM domain 7 protein (LMO7) in
VSCC. The LRIG protein family includes three members in humans,
LRIG1, LRIG2 and LRIG3 (10–12).
LRIG1 is the most studied family member and has been revealed to
negatively regulate several oncogenic receptor tyrosine kinases
including EGFR, ERBBs 2–4, MET, RET and PDGFR-A (13). Substantial evidence suggests that
LRIG1 functions as a tumor suppressor in various contexts (13,14).
LRIG1 was revealed to be a positive prognostic factor in cervical
SCC and cervical adenocarcinoma (13). Less is known about the functions and
prognostic values of LRIG2 and LRIG3. LMO7 has been revealed to
interact with LRIG1 and LRIG3 (15). LMO7 is a proposed stabilizer of
adherence junctions and transcription factor for muscle related
genes. It has also been associated with different human cancers
(16–18). Loss of LMO7 in a mouse model led to
spontaneous lung adenocarcinomas (19) and low expression of LMO7 in human
lung adenocarcinomas has been reported to be associated with poor
prognosis (16). Conversely, in
another study, high expression of LMO7 was a negative prognostic
factor in LRIG1 expressing non-small cell lung cancers (NSCLC)
(20).
There are a few prognostic factors in VSCC, of which
FIGO-stage and lymph node status are the most important (21). In a recent systematic review
investigating known prognostic factors in VSCC, results were
contradictory. Hence, there is a need for additional prognostic
factors for clinical decision-making in VSCC (22). The aim of the present study was to
investigate possible prognostic values of LRIG1-2 and LMO7 in VSCC
and their possible association to HPV- and
p16INK4a-status in tumors of patients from northern
Sweden.
Materials and methods
Patients and specimens
Patients diagnosed with VSCC in the northern region
of Sweden between 1990 and 2013 were identified through the Swedish
Cancer Registry. Out of 258 correctly classified patients treated
at the University Hospital in Umeå, 34 declined to participate, 81
were excluded due to missing clinical data and in 31 cases material
could not be obtained. Finally, this study was based on 112
patients with an age range of 37–94 years. Patients' records were
retrieved from the Department of Oncology at the University
Hospital in Umeå and clinical data were collected. Formalin-fixed,
paraffin-embedded (FFPE) specimens from diagnostic biopsies or
resection material from the time of diagnosis were retrieved from
the biobank at Västerbotten County Council (Umeå, Sweden).
FFPE-material was handled and stored in room temperature. All
patients alive at the start of the study signed informed consent
for the use of their tissues. This research was approved by the
Regional Ethical Review Board at Umeå University.
HPV DNA analysis
DNA was extracted from 25–30 µm FFPE sections using
the QIAamp DNA Tissue FFPE Tissue kit (Qiagen, Inc., Valencia, CA,
USA) according to the manufacturer's instructions. To check for
possible contamination, a negative control consisting of an empty
test tube was included between every fifth sample and treated
likewise. DNA-samples were stored at 20°C.
HPV-PCR analysis was carried out using 125 ng of
extracted DNA with the general primer pair GP5+/6+, amplifying a
fragment of the conserved HPV L1 gene (23). Primer sequences were as follows:
Forward, 5′-TTTGTTACTGTGGTAGATACTAC-3′ and reverse,
5′-GAAAAATAAACTGTAAATCATATTC-3′. The 50 µl PCR mixture consisted of
5 µl GeneAmp 10X PCR Gold buffer, 3.5 mM MgCl2 (both
from Applied Biosystems Life Technologies; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 200 µM of each dNTP (GeneAmp
dNTP mix) (Thermo Fisher Scientific, Inc.), 25 pmol of each primer
and 1 unit of AmpliTaq Gold DNA Polymerase (Applied Biosystems Life
Technologies; Thermo Fisher Scientific, Inc.). Amplification was
performed in a Biometra professional thermocycler (Thermo Fisher
Scientific, Inc.) or a T100 thermal cycler (Bio-Rad Laboratories,
Hercules, CA, USA). The reaction was initiated with denaturation
for 4 min at 94°C, followed by 40 amplification cycles of
denaturation at 94°C for 1 min, annealing at 44°C for 1 min and
elongation at 72°C for 2 min. The final cycle ended with a
prolonged elongation step at 72°C for 10 min. PCR products were run
on a 2% agarose gel in 50 mM Tris/37 mM Borate/1.3 mM EDTA and
stained with 0.5X GelRed (Biotium, Inc., Hayward, CA, USA). Gels
were visualized under UV-light. Fragments of 130–150 bp were
considered HPV-positive.
To identify samples that were incorrectly classified
as HPV-negative when using the GP5+/6+ primer pair due to
disruption of the L1 gene, GP5+/6+ -negative samples were
re-analyzed using the general primer pair CpI/IIG (24). Primer sequences were as follows:
Forward, 5′-TTATCWTATGCCCAYTGTACCAT-3′ and reverse,
5′-ATGTTAATWSAGCCWCCAAAATT-3′. The 50-µl PCR mixture consisted of 5
µl GeneAmp 10X PCR Gold buffer, 200 µM of each dNTP (GeneAmp dNTP
mix), 3 mM MgCl2, 17 pmol CpI, 26 pmol CpIIG, and 1 unit
of AmpliTaq Gold DNA Polymerase. The amplification consisted of
denaturation for 5 min at 94°C, followed by 40 amplification cycles
of denaturation at 95°C for 1 min, annealing at 55°C for 1 min and
elongation at 72°C for 2 min. The final cycle ended with a
prolonged elongation step at 72°C for 4 min. Samples with products
of ~188 bp were considered positive.
To assess the quality of the DNA-preparations,
samples negative for both primer pairs were run on PCR using the
s14 sense/antisense primers (25).
Primer sequences were as follows: Forward,
5′-TCGAAAGGGGAAGGAAAAGA-3′ and reverse, 5′-CAGTGACATGGACAAAAGTG-3′.
The 50-µl PCR mixture consisted of 5 µl GeneAmp 10X PCR Gold
buffer, 200 µM of each dNTP (GeneAmp dNTP mix), 1.5 mM
MgCl2, 15 pmol of each primer and 1 unit of AmpliTaq
Gold DNA Polymerase. The amplification consisted of denaturation
for 1 min at 94°C, followed by 40 amplification cycles of
denaturation at 94°C for 30 sec, annealing at 50°C for 30 sec and
elongation at 72°C for 45 sec. The final cycle ended with a
prolonged elongation step at 72°C for 5 min. Samples with products
of ~127 bp were considered to contain amplifiable DNA.
Genotyping was performed using the
PapilloCheck® HPV screening-kit and the CheckScanner™
laser scanner (Serial no. 700×0177) (Greiner Bio-One North America
Inc., Monroe, NC, USA). In brief, this method detects 24 different
HPV types through PCR amplification of a 350-bp fragment of the E1
gene and hybridization to specific DNA probes on a DNA chip. The
HPV-types detected by the assay included eighteen high-risk types
(16, 18, 45, 31, 33, 52, 58, 35, 59, 56, 51, 39, 68, 73, 82, 53, 66
and 70) and six low-risk types (6, 11, 40, 42, 43 and 44/55).
Immunohistochemistry
FFPE sections (4 µm) were deparaffinized,
rehydrated, and rinsed in water. Immunohistochemistry was performed
using Ventana standard procedure on a Ventana BenchMark ULTRA
instrument (Ventana Medical Systems, Inc., Tucson, AZ, USA).
Antigen retrieval was performed with a CC1 buffer (Ventana Medical
Systems, Inc.). Antibodies used were as follows: Rabbit anti-LRIG1
(product. no. AS184165; AgriSera AB, Vännäs, Sweden), 22 µg/ml;
rabbit anti-LRIG2-151 (12), 3
µg/ml; rabbit anti-LMO7-1250 (15),
24 µg/ml; rabbit anti-LMO7 (cat. no. HPA020923; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), 2 µg/ml; mouse monoclonal
anti-p16INK4a (E6H4) (Ventana Medical Systems, Inc.), 1
µg/ml. For validation of anti-LRIG1 and anti-LMO7 antibodies,
please refer to Figs. S1 and S2,
respectively.
Evaluation and classification of
immunostaining
Evaluation of immunostainings was performed by a
senior pathologist without knowledge of the disease outcome.
Immunoreactivity was scored based on both staining intensity and
percentage of immunoreactive epithelial cells within the tumor.
Intensity was evaluated on a four-grade semi-quantitative scale; no
staining, weak intensity, moderate intensity or strong intensity.
For statistical purposes, intensity scores were grouped into
no/weak and moderate/strong. The percentage of positive cells was
grouped into ‘at or above median’ or ‘below median’ to obtain
relatively even groups. For simplicity, at or above median is
henceforth referred to as ‘high’ and below median as ‘low’ staining
percentage. For p16INK4a immunostaining, only cases with
a strong nuclear and cytoplasmic expression in a continuous segment
of at least 10–20 cells were considered positive.
Statistical analyses
Patient characteristics were analyzed with the
independent sample t-test for comparison of means or the Pearson
Chi-square test for ordinal variables. Two-sided P-values were
reported. Disease-free survival (DFS) was defined from the date of
diagnosis to the date of accession to the patients' records or the
date of recurrence [according to pathological anatomical diagnosis
(PAD) or the date of first recorded clinical progression]. Death
without documented recurrence was censored at the date of death.
Overall survival (OS) was defined as the time from the date of
diagnosis to the date of death irrespective of cause. If a patient
was still alive at the time of accessing the patients' records, the
case was censored at that date. DFS and OS were illustrated in
Kaplan-Meier graphs and a log-rank test was used for comparison of
variables. In the figures presented, the x-axis was truncated at
120 months of follow-up. In the multivariate analysis, tumors were
grouped into low and high percentage of positively-stained cells
with a cut-off value at median. All parameters revealing a
significant difference when comparing survival were included. The
median age in each group was compared with the Mann-Whitney test.
P-values <0.05 were considered to indicate a statistically
significant difference. All statistical analyses were performed
using the SPSS software (IBM SPSS Statistics for Windows, version
23; IBM Corp., Armonk, NY, USA). Due to failure of DNA preparation
or immunohistochemical staining, occasional data were missing in
various statistical analyses.
Results
Study population
The 112 patients diagnosed with VSCC during the
period of 1990 to 2013 from whom clinical data and representative
material could be obtained were included in the study. The cases
that were excluded due to lack of material exhibited no significant
difference compared to those that were included regarding age,
FIGO-stage or histopathological grade.
Clinical characteristics
Mean age at diagnosis was 70 years. The mean age at
diagnosis of patients with HPV-negative and HPV-positive tumors was
72 and 66 years, respectively. This difference was significant
(P=0.008). HPV-negative and HPV-positive tumors differed
significantly also regarding lichen sclerosus et atrophicus (LSA)
in disease history (P=0.001) and rate of recurrence or persistence
of disease (P=0.027). Within the HPV-negative group, LSA was more
common, and recurrence was more likely to occur. Cases negative vs.
positive for p16INK4a also differed significantly, and
in the same manner, regarding record of LSA in disease history
(P<0.001) and rate of recurrence (P=0.027). Clinical
characteristics in relation to HPV-status are summarized in
Table I.
 | Table I.HPV status in relation to patient and
tumor characteristics. |
Table I.
HPV status in relation to patient and
tumor characteristics.
| Variables | Category |
HPV−DNA−, n (%) |
HPV−DNA+, n (%) | Total, n | P-value |
|---|
| Mean age at
diagnosis | Years | 72 (range,
37–94) | 66 (range,
44–94) | 70 (mean) | 0.008a |
| Previous
gynecological malignancy | Yes | 3 (4.7) | 1 (2.3) | 4 | 0.644 |
|
| No | 61 (95.3) | 43 (97.7) | 104 |
|
| Hysterectomy before
diagnosis | Yes | 10 (15.4) | 5 (11.4) | 15 | 0.778 |
|
| No | 55 (84.6) | 39 (88.6) | 94 |
|
| Record of LSA prior
to malignancy | Yes | 35 (53.8) | 9 (20.5) | 44 | 0.001a |
|
| No | 30 (46.2) | 35 (79.5) | 65 |
|
| Histopathological
grade | Unknown | 2 (3.1) | 1 (2.4) | 3 | 0.844 |
|
| Poor | 13 (20.0) | 12 (28.6) | 25 |
|
|
| Moderate | 36 (55.4) | 22 (52.4) | 58 |
|
|
| Well | 14 (21.5) | 7 (16.7) | 21 |
|
| FIGO-stage | I | 23 (36.5) | 11 (25) | 34 | 0.558 |
|
| II | 20 (31.7) | 16 (36.4) | 36 |
|
|
| III | 16 (25.4) | 15 (34.1) | 31 |
|
|
| IV | 4 (6.3) | 2 (4.5) | 6 |
|
| Local metastasis at
diagnosis | Yes | 16 (25) | 16 (36.4) | 32 | 0.284 |
|
| No | 48 (75) | 28 (63.6) | 76 |
|
| Distant metastasis
at diagnosis | Yes | 1 (1.6) | 0 (0) | 1 | 0.405 |
|
| No | 63 (98.4) | 44 (100) | 107 |
|
| Recurrence | Yes | 29 (44.6) | 12 (27.3) | 41 | 0.027a |
|
| No | 26 (40.0) | 29 (65.9) | 55 |
|
|
| Persistent
disease | 10 (15.4) | 3 (6.8) | 13 |
|
| Mean time to
recurrence | Months | 48 (range,
0–290) | 67 (range,
0–269) | 56 | 0.124 |
| Vital status | Alive | 23 (35.4) | 22 (50) | 45 | 0.130 |
| Cause of death | Cancer | 16 (24.6) | 9 (20.5) | 25 |
|
|
| Other | 14 (21.5) | 11 (25) | 25 |
|
|
| Unknown | 12 (18.5) | 2 (4.5) | 14 |
|
HPV and p16INK4a
analyses
Forty percent (44/109) of tumors were HPV-positive,
26% (26/99) were p16INK4a-positive and 23% (22/97) were
positive for both HPV and p16INK4a. The latter category
was referred to as HPV-driven in accordance with a recent
hypothesis (4,5). Four tumors were HPV-negative and
p16INK4a-positive whereas 14 tumors were HPV-positive
and p16INK4a-negative. Fifty-seven cases were negative
for both HPV and p16INK4a. In three cases the DNA
preparation was of insufficient quality and in 13 cases
p16INK4a data could not be retrieved. From a total of 44
HPV-positive samples, 41 were subjected to genotyping. Of these, 20
samples were successfully genotyped. Ten samples failed the
internal control, probably due to insufficiency of material or
quality of the sample. In eleven cases, internal control was
satisfactory, however, the genotyping rendered a negative result.
This may be explained by bad quality of the preparation or,
alternatively, disruption of the E1 gene in the specific tumors.
Among successfully typed tumors, HPV16 was the most prevalent type
(13/20) and HPV33 the second most prevalent type (6/20). HPV42 was
found in one of the samples positive for HPV33 and one sample
contained HPV58 only (data not shown).
Associations between HPV- and
p16INK4a-statuses and rates of recurrence and DFS
Both HPV- and p16INK4a-positivity were
associated with a lower rate of recurrence: 34.1% (15/44) of
HPV-positive cases and 60% (39/65) of HPV-negative cases had a
recurrence or persistent disease, 26.9% of
p16INK4a-positive cases and 57.5% of
p16INK4a-negative cases had a recurrence or persistent
disease, and 22.7% of HPV-driven cases and 57.9% of not HPV-driven
cases had a recurrence or persistent disease (data not shown).
HPV-positive and p16INK4a-positive patients also
exhibited a longer DFS than HPV-negative and
p16INK4a-negative patients (P=0.006 and P=0.010,
respectively; Fig. 1B and C).
Similarly, HPV-driven cases revealed a longer DFS than not
HPV-driven cases (P=0.005) (Fig.
1D). However, no significant association between HPV- (P=0.152;
Fig. 1A) or
p16INK4a-status and OS was observed.
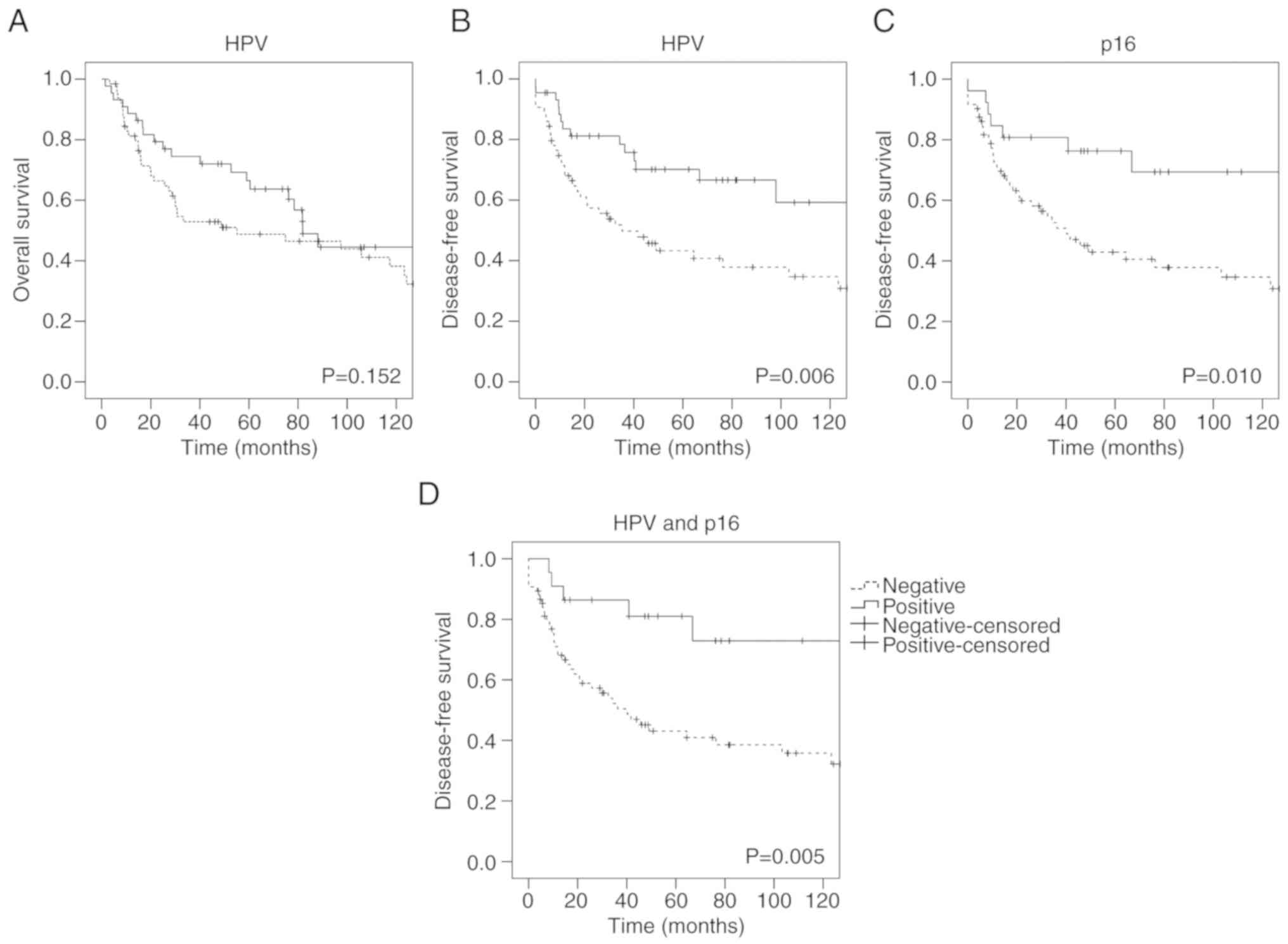 | Figure 1.Kaplan-Meier survival curves
revealing OS and DFS according to HPV- and
p16INK4a-status. (A) HPV-negative vs. HPV-positive
tumors, OS, n=109. (B) HPV-negative vs. HPV-positive tumors, DFS,
n=108. (C) p16INK4a-negative vs.
p16INK4a-positive tumors, DFS, n=98. (D) Not HPV-driven
vs. HPV-driven tumors, DFS, n=97. Of note, the not HPV-driven
tumors were denoted negative and included all cases that were not
concurrently HPV- and p16INK4a-positive. OS, overall
survival; DFS, disease-free survival; HPV, human papilloma
virus. |
LRIG immunohistochemistry
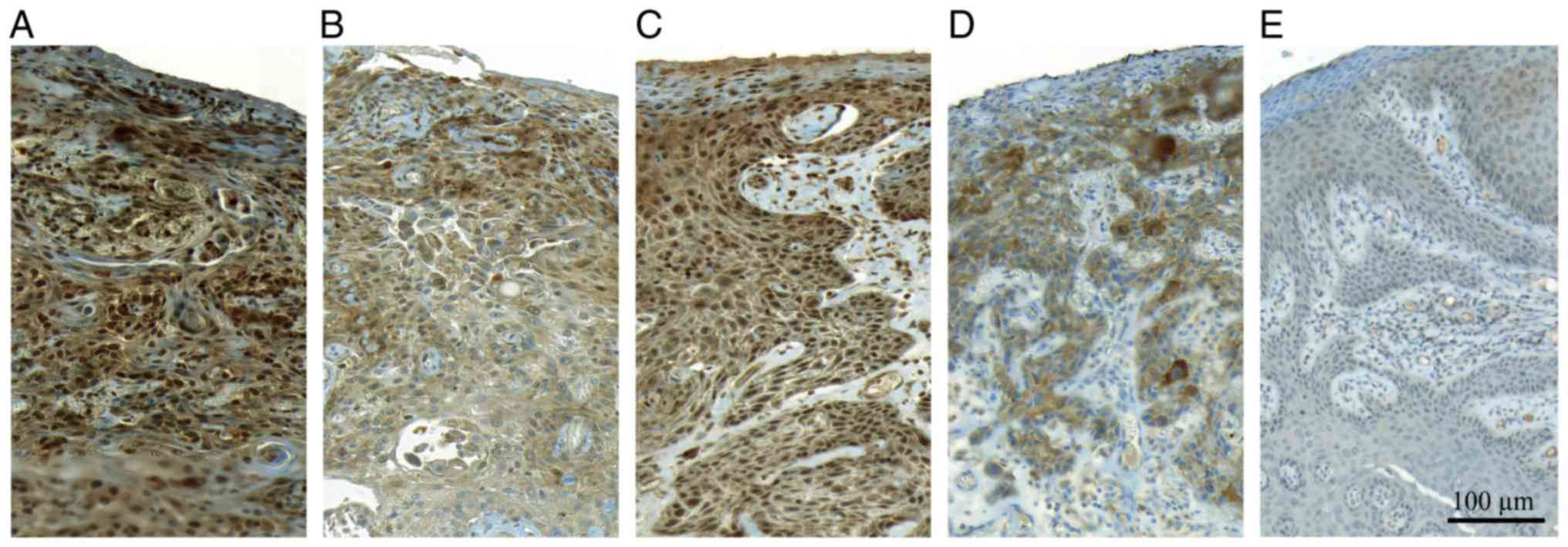
LRIG1 immunoreactivity was observed primarily in
cell nuclei whereas LRIG2 immunoreactivity was primarily
cytoplasmic and membraneous (Fig. 2A
and B). When considering the percentage of immunoreactive tumor
cells, there was a better clinical outcome for patients with a high
proportion of LRIG-positive cells (Fig.
3). For LRIG1, this association was, however, neither
significant when analyzing OS (Fig.
3A-D) nor DFS (data not shown). LRIG2 was significantly
associated with a favorable OS but not DFS in the whole cohort
(Fig. 3E; P=0.001 and P=0.051,
respectively). Among the HPV-negative and not HPV-driven strata,
LRIG2 was significantly associated with a favorable prognosis both
regarding OS (P=0.008 and 0.001, respectively; Fig. 3F and G) and DFS (P=0.031 and 0.009,
respectively). LRIG2 immunoreactivity was also an independent
prognostic marker for OS in multivariate analysis (Table II).
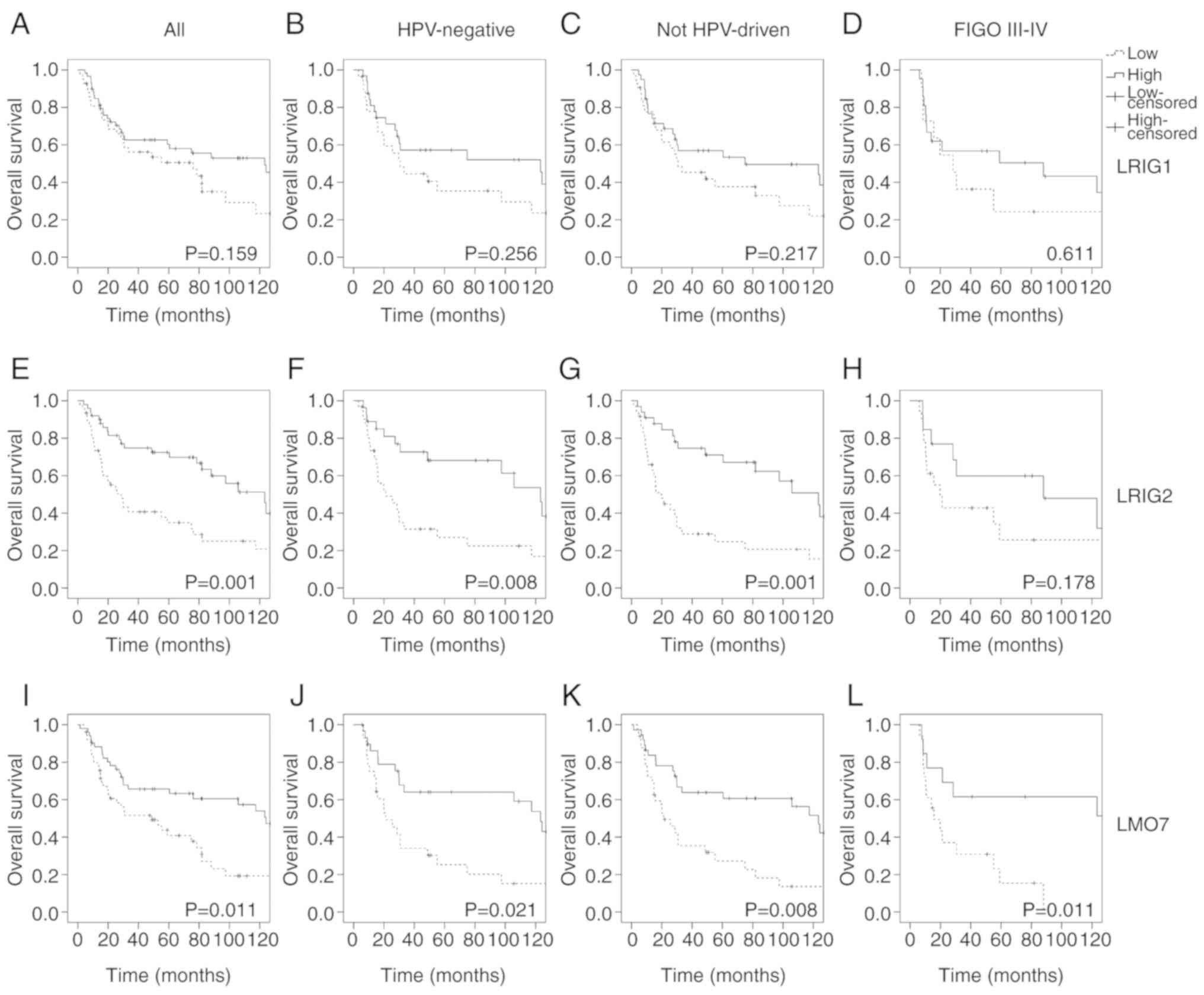 | Figure 3.Kaplan-Meier survival curves
revealing OS according to LRIG and LMO7-1250 immunoreactivities in
the whole cohort and specified subgroups. Patients with tumors of
high staining percentage were compared with patients with low
staining percentage with a cut-off at median. (A) LRIG1, all
tumors, n=101. (B) LRIG1, HPV-negative tumors, n=60. (C) LRIG1, not
HPV-driven tumors, n=71. (D) LRIG1, FIGO stage III–IV, n=32. (E)
LRIG2, all tumors, n=96. (F) LRIG2, HPV-negative tumors, n=58. (G)
LRIG2, not HPV-driven tumors, n=69. (H) LRIG2, FIGO stage III–IV,
n=31. (I) LMO7-1250, all tumors, n=101. (J) LMO7-1250, HPV-negative
tumors, n=58. (K) LMO7-1250, not HPV-driven tumors, n=70. (L)
LMO7-1250, FIGO stage III–IV, n=31. OS, overall survival; LRIG,
leucine-rich repeats and immunoglobulin-like domain; LMO7, LIM
domain 7 protein. |
 | Table II.Cox regression. |
Table II.
Cox regression.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| OS | Age (years) |
|
|
|
|
|
|
|
|
<60 | 1 | (ref) |
| 1 | (ref) |
|
|
|
≥60 | 3.8 | 1.85-7.63 |
<0.001a | 4.34 | 1.92-9.81 |
<0.001a |
|
| FIGO |
|
|
|
|
|
|
|
| I +
II | 1 | (ref) |
|
|
|
|
|
| III +
IV | 1.6 | 0.94-2.58 | 0.088 |
|
|
|
|
| HPV-status |
|
|
|
|
|
|
|
|
Negative | 1 | (ref) |
|
|
|
|
|
|
Positive | 0.7 | 0.40-1.12 | 0.125 |
|
|
|
|
| p16 |
|
|
|
|
|
|
|
|
Negative | 1 | (ref) |
|
|
|
|
|
|
Positive | 0.7 | 0.37-1.25 | 0.212 |
|
|
|
|
| LSA |
|
|
|
|
|
|
|
| No | 1 | (ref) |
|
|
|
|
|
|
Yes | 0.71 | 0.43-1.18 | 0.183 |
|
|
|
|
| Local met |
|
|
|
|
|
|
|
| No | 1 | (ref) |
|
|
|
|
|
|
Yes | 1.46 | 0.87-2.46 | 0.155 |
|
|
|
|
| LRIG1 |
|
|
|
|
|
|
|
|
Low | 1 | (ref) |
|
|
|
|
|
|
High | 0.71 | 0.43-1.20 | 0.202 |
|
|
|
|
| LRIG2 |
|
|
|
|
|
|
|
|
Low | 1 | (ref) |
| 1 | (ref) |
|
|
|
High | 0.44 | 0.26-0.75 | 0.003a | 0.41 | 0.24-0.71 | 0.002a |
|
| LMO7-1250 |
|
|
|
|
|
|
|
|
Low | 1 | (ref) |
| 1 | (ref) |
|
|
|
High | 0.54 | 0.32-0.91 | 0.021a | 0.63 | 0.36-1.10 | 0.104 |
|
| LMO7 |
|
|
|
|
|
|
|
|
Low | 1 | (ref) |
|
|
|
|
|
|
High | 0.68 | 0.41-1.15 | 0.149 |
|
|
|
| DFS | Age (years) |
|
|
|
|
|
|
|
|
<60 | 1 | (ref) |
|
|
|
|
|
|
≥60 | 1.77 | 0.92-3.37 | 0.085 |
|
|
|
|
| FIGO |
|
|
|
|
|
|
|
| I +
II | 1 | (ref) |
| 1 | (ref) |
|
|
| III +
IV | 1.92 | 1.11-3.32 | 0.019a | 2.00 | 1.12-3.60 | 0.020a |
|
| HPV-status |
|
|
|
|
|
|
|
|
Negative | 1 | (ref) |
| 1 | (ref) |
|
|
|
Positive | 0.43 | 0.24-0.78 | 0.006a | 0.78 | 0.35-1.74 | 0.538 |
|
| p16 |
|
|
|
|
|
|
|
|
Negative | 1 | (ref) |
| 1 | (ref) |
|
|
|
Positive | 0.35 | 0.16-0.79 | 0.011a | 0.29 | 0.12-0.68 | 0.005a |
|
| LSA |
|
|
|
|
|
|
|
| No | 1 | (ref) |
|
|
|
|
|
|
Yes | 1.16 | 0.68-2.01 | 0.586 |
|
|
|
|
| Local met |
|
|
|
|
|
|
|
| No | 1 | (ref) |
|
|
|
|
|
|
Yes | 1.65 | 0.95-2.87 | 0.076 |
|
|
|
|
| LRIG1 |
|
|
|
|
|
|
|
|
Low | 1 | (ref) |
|
|
|
|
| DFS |
High | 0.49 | 0.46-1.45 | 0.490 |
|
|
|
|
| LRIG2 |
|
|
|
|
|
|
|
|
Low | 1 | (ref) |
|
|
|
|
|
|
High | 0.059 | 0.33-1.02 | 0.059 |
|
|
|
|
| LMO7-1250 |
|
|
|
|
|
|
|
|
Low | 1 | (ref) |
|
|
|
|
|
|
High | 0.67 | 0.38-1.19 | 0.169 |
|
|
|
|
| LMO7 |
|
|
|
|
|
|
|
|
Low | 1 | (ref) |
|
|
|
|
|
|
High | 1.05 | 0.60-1.84 | 0.865 |
|
|
|
When comparing intensity of LRIG1 divided into
no/weak vs. moderate/high, no significant differences were observed
between groups considering OS or DFS (data not shown). However,
when stratified according to HPV-status, no/weak LRIG1 staining was
associated with a better OS in HPV-negative cases (P=0.044) (data
not shown). Stratifications into p16INK4a-status,
HPV-/not HPV-driven tumors or FIGO-stage provided no further
significant findings. Intensity of LRIG2 computed in the same
manner showed no significant differences between groups.
LMO7 immunohistochemistry
LMO7 immunostaining was evaluated using two
different antibodies, one developed by our laboratory (LMO7-1250)
and one commercially available (LMO7; Sigma-Aldrich; Merck KGaA).
The former exhibited staining primarily in the nuclei whereas the
latter stained predominantly around cell membranes and
cytoplasmatically (Fig. 1C-E). High
staining percentage with the LMO7-1250 antibody was significantly
associated with a favorable OS, but not DFS (data not shown), in
the whole cohort (OS: P=0.011; DFS: P=0.139; Fig. 3I). In the HPV-negative and
HPV-driven strata, the association was significant regarding both
OS (P=0.021 and P=0.008, respectively; Fig. 3J and K) and DFS (P=0.038 and
P=0.042, respectively). Additionally, among tumors with FIGO stage
III–IV at diagnosis, high LMO7 staining percentage was associated
with a favorable prognosis (P=0.011; Fig. 3L). The LMO7 antibody also revealed
association with a favorable prognosis but only regarding OS and in
the HPV-negative, p16INK4a-negative and HPV-driven
strata (data not shown). Similar to LRIG immunoreactivity results,
evaluation of staining intensity produced inconclusive results.
Discussion
In the present study, we evaluated the possible
prognostic values of LRIG1-2 and LMO7 expression through
immunohistochemical staining of FFPE material and collection of
clinical data from 112 women diagnosed with VSCC in the northern
region of Sweden. The results revealed that a high percentage of
LRIG2-immunoreactive tumor cells was a significant and independent
positive prognostic marker for OS. LRIG2 was also a significant
positive prognostic factor when considering DFS in the HPV-negative
tumors as well as in the not HPV-driven tumors, suggesting a
disease-specific survival benefit of high LRIG2 expression in
HPV-independent VSCC. This was in contrast to the results in
invasive early stage cervical SCC, where high LRIG2 expression was
correlated to poor survival (26)
and in primary vaginal carcinoma where no significant correlation
was revealed between LRIG2 and patient survival (27). Other studies have revealed LRIG2 to
be a negative prognostic factor in oligodendroglioma (28) although a positive prognostic factor
in glioblastoma/astrocytoma (29).
LRIG1 was not a significant predictor of prognosis in VSCC in our
study, although considerable evidence points to it being a positive
prognostic marker in several other malignancies (13,20).
Thus, results differ regarding the prognostic value of LRIG
proteins in gynecological and other malignancies.
High LMO7 immunoreactivity was associated with
better OS and DFS in our material. Similar to LRIG2, the
association was stronger in HPV-independent tumors. Two antibodies
reactive against LMO7 were used. Their different staining patterns
may be explained by the different epitopes recognized by the
antibodies and may reflect different splice variants of the protein
or masking of epitopes due to post-translational modifications or
protein binding. To the best of our knowledge, it is difficult to
speculate upon how this would affect tumor progression although
there may be a connection to LRIG function since LMO7 has been
revealed to interact with both LRIG1 and LRIG3 in their endogenous
form. LMO7 may also interact with LRIG2 although this has not been
determined. Notably, their protein-protein interaction site is
believed to be the LIM-domain in the C-terminal end of the
LMO7-protein, which is the part recognized by the LMO7-1250
antibody (15). It is, however,
still not possible to draw any clear conclusions about the possible
mechanistic connections that may exist between LRIG proteins, LMO7
and HPV- and p16 status.
Our results revealed that both HPV- and
p16INK4a-positivity conferred a more favorable prognosis
in VSCC. This has been previously revealed (5,30) and
was consistent with findings in other HPV-related cancers (9,31–33).
Tumors with combined HPV- and p16INK4a-positivity
exhibited an even stronger association with a favorable prognosis.
This was consistent with the hypothesis proposed by de Sanjosé
et al according to which the presence of HPV in the absence
of p16INK4a overexpression may be due to a transient HPV
infection, not contributing to carcinogenesis (4,34).
In conclusion, patients with HPV-independent VSCC
had a survival deficit compared to HPV-dependent disease, and our
data suggested a role for LRIG2 and LMO7 as positive prognostic
factors among the HPV-independent cases, and LMO7 among the most
advanced tumors. Thus, these markers could possibly provide means
to facilitate selection of individual treatment strategies among
VSCC patients. However, more research is warranted to further
elucidate the functions and prognostic values of the studied
molecular markers in VSCC.
Supplementary Material
Supporting Data
Acknowledgements
Alexandra Lorenzzi Löfgren, Annika Allard, Birgitta
Ekblom, Charlotte Nordström, Pernilla Andersson, Samuel Kvarnbrink
and Susanne Gidlund are kindly acknowledged for their technical
assistance.
Funding
The present study was supported by grants from the
Swedish Society for Medical Research (SSMF), the Jeanssons
Foundation, the Cancer Research Foundation in Northern Sweden, the
Lions Cancer Research Foundation, Umeå University, and the regional
agreement between Umeå University and Västerbotten County Council
on cooperation in the field of Medicine, Odontology and Health
(ALF).
Availability of data and material
The datasets analyzed during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
KS carried out the DNA-preparations and
PCR-analyses, gathered the approvals and clinical data of patients,
assembled and analyzed the data statistically, wrote the manuscript
and designed the figures. HO classified the tumors according to
histology and differentiation grade, and evaluated the
immunohistochemical stainings. CÖ assisted in statistical analysis
and interpretation of the results. EL and HH provided support and
the materials, helped supervise the study, critically read the
manuscript and suggested changes. DL conceived and designed the
study with the substantial contribution of EL. All authors
critically read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Regional Ethical
Review Board at Umeå University, Umeå, Sweden (Dnr 2013-416-31M).
Written informed consent was obtained from all participants alive
at the start of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kang YJ, Smith M, Barlow E, Coffey K,
Hacker N and Canfell K: Vulvar cancer in high-income countries:
Increasing burden of disease. Int J Cancer. 141:2174–2186. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Register: The National Board of
Health and Welfare, Stockholm. April 23–2018http://www.socialstyrelsen.se/statistics/statisticaldatabase/cancer
|
|
3
|
Del Pino M, Rodriguez-Carunchio L and Ordi
J: Pathways of vulvar intraepithelial neoplasia and squamous cell
carcinoma. Histopathology. 62:161–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Sanjosé S, Alemany L, Ordi J, Tous S,
Alejo M, Bigby SM, Joura EA, Maldonado P, Laco J, Bravo IG, et al:
Worldwide human papillomavirus genotype attribution in over 2000
cases of intraepithelial and invasive lesions of the vulva. Eur J
Cancer. 49:3450–3461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sznurkowski JJ, Żawrocki A and Biernat W:
The overexpression of p16 is not a surrogate marker for high-risk
human papilloma virus genotypes and predicts clinical outcomes for
vulvar cancer. BMC Cancer. 16:4652016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doorbar J: Papillomavirus life cycle
organization and biomarker selection. Dis Markers. 23:297–313.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Santos M, Landolfi S, Olivella A, Lloveras
B, Klaustermeier J, Suárez H, Alòs L, Puig-Tintoré LM, Campo E and
Ordi J: p16 overexpression identifies HPV-positive vulvar squamous
cell carcinomas. Am J Surg Pathol. 30:1347–1356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hellman K, Lindquist D, Ranhem C, Wilander
E and Andersson S: Human papillomavirus, p16INK4A, and
Ki-67 in relation to clinicopathological variables and survival in
primary carcinoma of the vagina. Br J Cancer. 110:1561–1570. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Rorke MA, Ellison MV, Murray LJ, Moran
M, James J and Anderson LA: Human papillomavirus related head and
neck cancer survival: A systematic review and meta-analysis. Oral
Oncol. 48:1191–1201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nilsson J, Vallbo C, Guo D, Golovleva I,
Hallberg B, Henriksson R and Hedman H: Cloning, characterization,
and expression of human LIG1. Biochem Biophys Res Commun.
284:1155–1161. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo D, Holmlund C, Henriksson R and Hedman
H: The LRIG gene family has three vertebrate paralogs widely
expressed in human and mouse tissues and a homolog in Ascidiacea.
Genomics. 84:157–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holmlund C, Nilsson J, Guo D, Starefeldt
A, Golovleva I, Henriksson R and Hedman H: Characterization and
tissue-specific expression of human LRIG2. Gene. 332:35–43. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindquist D, Kvarnbrink S, Henriksson R
and Hedman H: LRIG and cancer prognosis. Acta Oncol. 53:1135–1142.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Poulin EJ and Coffey RJ: LRIG1 is
a triple threat: ERBB negative regulator, intestinal stem cell
marker and tumour suppressor. Br J Cancer. 108:1765–1770. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karlsson T, Kvarnbrink S, Holmlund C,
Botling J, Micke P, Henriksson R, Johansson M and Hedman H: LMO7
and LIMCH1 interact with LRIG proteins in lung cancer, with
prognostic implications for early-stage disease. Lung Cancer.
125:174–184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamura H, Hori K, Tanaka-Okamoto M,
Higashiyama M, Itoh Y, Inoue M, Morinaka S and Miyoshi J: Decreased
expression of LMO7 and its clinicopathological significance in
human lung adenocarcinoma. Exp Ther Med. 2:1053–1057. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu Q, Guo C, Li Y, Aronow BJ and Zhang J:
LMO7 mediates cell-specific activation of the Rho-myocardin-related
transcription factor-serum response factor pathway and plays an
important role in breast cancer cell migration. Mol Cell Biol.
31:3223–3240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He H, Li W, Yan P, Bundschuh R, Killian
JA, Labanowska J, Brock P, Shen R, Heerema NA and de la Chapelle A:
Identification of a recurrent LMO7-BRAF fusion in papillary thyroid
carcinoma. Thyroid. 28:748–754. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanaka-Okamoto M, Hori K, Ishizaki H,
Hosoi A, Itoh Y, Wei M, Wanibuchi H, Mizoguchi A, Nakamura H and
Miyoshi J: Increased susceptibility to spontaneous lung cancer in
mice lacking LIM-domain only 7. Cancer Sci. 100:608–616. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kvarnbrink S, Karlsson T, Edlund K,
Botling J, Lindquist D, Jirstrom K, Micke P, Henriksson R,
Johansson M and Hedman H: LRIG1 is a prognostic biomarker in
non-small cell lung cancer. Acta Oncol. 54:1113–1119. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prat J and Mutch DG: Pathology of cancers
of the female genital tract including molecular pathology. Int J
Gynaecol Obstet. 143 (Suppl 2):S93–S108. 2018. View Article : Google Scholar
|
|
22
|
Te Grootenhuis NC, Pouwer AW, de Bock GH,
Hollema H, Bulten J, van der Zee AGJ, de Hullu JA and Oonk MH:
Prognostic factors for local recurrence of squamous cell carcinoma
of the vulva: A systematic review. Gynecol Oncol. 148:622–631.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Roda Husman AM, Walboomers JM, van den
Brule AJ, Meijer CJ and Snijders PJ: The use of general primers GP5
and GP6 elongated at their 3′ ends with adjacent highly conserved
sequences improves human papillomavirus detection by PCR. J Gen
Virol. 76:1057–1062. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smits HL, Tieben LM, Tjong-A-Hung SP,
Jebbink MF, Minnaar RP, Jansen CL and Ter Schegget J: Detection and
typing of human papillomaviruses present in fixed and stained
archival cervical smears by a consensus polymerase chain reaction
and direct sequence analysis allow the identification of a broad
spectrum of human papillomavirus types. J Gen Virol. 7:3263–3268.
1992. View Article : Google Scholar
|
|
25
|
Dahlgren L, Mellin H, Wangsa D,
Heselmeyer-Haddad K, Björnestål L, Lindholm J, Munck-Wikland E,
Auer G, Ried T and Dalianis T: Comparative genomic hybridization
analysis of tonsillar cancer reveals a different pattern of genomic
imbalances in human papillomavirus-positive and -negative tumors.
Int J Cancer. 107:244–249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hedman H, Lindström AK, Tot T, Stendahl U,
Henriksson R and Hellberg D: LRIG2 in contrast to LRIG1 predicts
poor survival in early-stage squamous cell carcinoma of the uterine
cervix. Acta Oncol. 49:812–815. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ranhem C, Lillsunde Larsson G, Hedman H,
Lindquist D, Karlsson MG, Hellström AC, Östensson E, Sorbe B,
Hellman K and Andersson S: Expression of LRIG proteins as possible
prognostic factors in primary vaginal carcinoma. PLoS One.
12:e01838162017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holmlund C, Haapasalo H, Yi W, Raheem O,
Brännström T, Bragge H, Henriksson R and Hedman H: Cytoplasmic
LRIG2 expression is associated with poor oligodendroglioma patient
survival. Neuropathology. 29:242–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo D, Nilsson J, Haapasalo H, Raheem O,
Bergenheim T, Hedman H and Henriksson R: Perinuclear leucine-rich
repeats and immunoglobulin-like domain proteins (LRIG1-3) as
prognostic indicators in astrocytic tumors. Acta Neuropathol.
111:238–246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nooij LS, Ter Haar NT, Ruano D, Rakislova
N, van Wezel T, Smit VTHBM, Trimbos BJBMZ, Ordi J, van Poelgeest
MIE and Bosse T: Genomic characterization of vulvar (Pre)cancers
identifies distinct molecular subtypes with prognostic
significance. Clin Cancer Res. 23:6781–6789. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mellin H, Friesland S, Lewensohn R,
Dalianis T and Munck-Wikland E: Human papillomavirus (HPV) DNA in
tonsillar cancer: Clinical correlates, risk of relapse, and
survival. Int J Cancer. 89:300–304. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Attner P, Du J, Näsman A, Hammarstedt L,
Ramqvist T, Lindholm J, Marklund L, Dalianis T and Munck-Wikland E:
Human papillomavirus and survival in patients with base of tongue
cancer. Int J Cancer. 128:2892–2897. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lindquist D, Romanitan M, Hammarstedt L,
Näsman A, Dahlstrand H, Lindholm J, Onelöv L, Ramqvist T, Ye W,
Munck-Wikland E and Dalianis T: Human papillomavirus is a
favourable prognostic factor in tonsillar cancer and its oncogenic
role is supported by the expression of E6 and E7. Mol Oncol.
1:350–355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chaux A, Cubilla AL, Haffner MC, Lecksell
KL, Sharma R, Burnett AL and Netto GJ: Combining routine
morphology, p16INK4a immunohistochemistry, and in situ
hybridization for the detection of human papillomavirus infection
in penile carcinomas: a tissue microarray study using classifier
performance analyses. Urol Oncol. 32:171–177. 2014. View Article : Google Scholar : PubMed/NCBI
|