Introduction
In terms of incidence and mortality rates, lung
cancer is the most commonly diagnosed cancer and is the most
frequent cause of death worldwide (1). Males are affected by lung cancer twice
more frequently than females. However, a more dynamic increase in
the incidence is observed in females. The peak incidence of lung
cancer is observed after 50 years of age with more than 50% of
cases >65 years of age (2). Lung
cancers are classified as small cell (~17%) and non-small cell lung
cancers (NSCLC), which account for ~80% of all lung cancer cases.
There are three basic histological subtypes of NSCLC, i.e. squamous
cell carcinoma, adenocarcinoma and large cell carcinoma which
account for 90% of all diagnosed cases of NSCLC (3). Factors which are directly linked to
the course and prognosis of lung cancer are the following:
Histological type of cancer, pTNM staging system, and presence of
the EGFR gene mutation and EML4-ALK fusion gene (4).
All proteins from the metallothionein (MT) family
are characterized by the ability to bind heavy metal ions such as
zinc, copper, mercury, lead or cadmium. These proteins bind free
ions of the above metals into inactive complexes, thus, playing a
key role in protecting cells from the negative effects of heavy
metals (5). Apart from the
detoxification function, MTs are also involved in maintaining
homeostasis of metal ions essential for proper functioning of the
organism. Reversible binding of zinc ions leads to the influence of
MTs on the activity of numerous enzymes and transcription factors
dependent on the presence of the above ions. These enzymes and
transcription factors are responsible for intracellular processes
such as DNA replication, transcription, translation and the
processes of proliferation, differentiation and cell apoptosis
(6,7). Moreover, MTs demonstrate strong
antioxidant properties due to their cysteine-rich peptide
structure. MTs constitute one of the main elements of cell
protection against reactive oxygen species next to glutathione
(GSH) and antioxidant enzymes [superoxide dismutase (SOD), catalase
(CAT) and glutathione peroxidase (GPx)] (8,9). It
has been postulated that the role of MTs in carcinogenesis and
cancer progression may be related to the regulation of
proliferation processes and cell differentiation (10–13).
MTs serve as a direct reservoir of zinc ions indispensable for the
processes of the biosyntheses of nucleic acids and proteins which
occur intensively in cancer cells undergoing mitosis. This is
confirmed not only by an increased MT level in the area of
hyperplasia but also by translocation of protein from the cytoplasm
to the cell nucleus in the DNA synthesis phase (S-phase) observed
in cancer cells in vitro (12–14).
Moreover, it has also been demonstrated that MTs can increase the
proliferative potential of cancer cells through the interaction
with p53 protein and inhibition of apoptosis (15,16).
Involvement of MTs in the development of cancer cell resistance to
chemotherapy and radiotherapy is also a significant issue related
to cancer. This phenomenon is most probably related to antioxidant
properties of these proteins (8,17).
Testis-specific metallothionein-like protein,
tesmin, is also known as metallothionein-like 5 protein,
testis-specific MTL-5, or CXC domain containing protein 2. It is a
60-kDa protein which has cysteine-rich motifs (CXC domain),
characteristic of MTs. These domains are located between amino
acids 161–175 and 173–188 of the peptide sequence of tesmin
(18). In humans, the locus for the
tesmin gene is on 11q13.3 (19).
The specific characteristics of this protein are shown in Table I.
 | Table I.Differences in the characteristics
between tesmin protein and metallothioneins (18,19,25). |
Table I.
Differences in the characteristics
between tesmin protein and metallothioneins (18,19,25).
|
Characteristics | Tesmin |
Metallothioneins |
|---|
| Number of known
isoforms | I–III | I–IV |
| Molecular
weight | 27; 32 or 60
kDa | 6–7 kDa |
| Gene locus | 11q13.3 | 16q13 |
| Number of coding
exons | 9 | 3 |
| Presence of
aromatic acids | Present | Absent |
| Promoter
regions-dependent on the presence of metals and the TATA box | Absent | Present |
The presence of tesmin has been confirmed in many
species of plants and animal cells. Tesmin was firstly described in
mouse testicular tissue on day 8 of postnatal development which
coincided with the entry of germ cells into meiosis (20). This was confirmed by other authors
who observed the occurrence of tesmin expression at the stage of
meiotic prophase I, during the division of male and female germ
cells of rats (21). Therefore, a
hypothesis on the probable use of tesmin as a specific marker for
germ cell differentiation was proposed. Moreover, this protein is
mainly found in the cytoplasm of spermatocytes and is translocated
to the nucleus during the G2/M phase of meiotic division (17,22),
which may be indicative of the role of tesmin in the regulation of
other genes responsible for spermatogenesis. However, lack of
tesmin expression in organized chromosomes during meiosis can
suggest that this protein is not permanently bound to chromatin
(18).
Nuclear expression of tesmin can be, as in the case
of MTs, a response to stress related to the presence of high
concentrations of heavy metal ions. In in vitro studies, the
occurrence of the above localization of tesmin expression was
observed after experimental stimulation of a COS-1 cell culture
(fibroblast cell line from the kidney of African green monkey)
using cadmium chloride and zinc chloride (22). This observation was confirmed in an
in vivo experiment using immunohistochemical and
immunofluorescence methods to study the localization of tesmin
expression in the testicular tissue of mice which had previously
been given an intravenous cadmium solution (22). The change in tesmin localization due
to the cadmium solution was found to be related to the occurrence
of features of apoptosis in mouse testicular cells (22). A similar phenomenon was observed in
rats in which tesmin expression was also increased both in the
cytoplasm and nucleus of the spermatogonium as the result of zinc
supplementation (23).
Expression of tesmin mRNA was noted in mouse
embryonic ovaries and testes, embryonic renal tissue, brain, liver,
heart and mature myocardial tissue. This may indicate that this
protein is not specific to germ cells only (21,24).
It has also been postulated that tesmin can be a
coactivator for nuclear mineralocorticoid (aldosterone and
deoxycorticosterone) receptor, which is a ligand-dependent
transcription factor (24). This
observation was not confirmed in the case of cortisol which also
displays an insignificant mineralocorticoid activity (24).
To date, the expression of tesmin in adult humans
has been observed only in gastric carcinoma and prostate cancer
(25,26). Decreased expression of tesmin mRNA
was associated with the single nucleotide polymorphism phenomenon
and the presence of risk alleles of genes promoting the development
of prostate cancer (27). In
addition, online databases contain information about the high
expression of tesmin mRNA in various cancer cell lines (Figs. S1–S3).
The aim of the present study was to examine the
expression of tesmin in NSCLC cases and its association with the
clinicopathological data.
Materials and methods
Patients
An immunohistochemical (IHC) study was performed on
121 paraffin blocks from patients diagnosed with NSCLC and treated
between February 1998 and November 2010 at the Department and
Clinic of Thoracic Surgery of Wroclaw Medical University (Wroclaw,
Poland). In addition, we used 20 paraffin blocks with tissue
samples from the resection margin of patients with NSCLC as a
control. All patients were treated surgically and tissue specimens
were prepared prior to chemotherapy. To evaluate the
histopathological subtype of NSCLC we used anti-p63 (for lung
squamous cell carcinoma) and TTF-1 (for adenocarcinoma) IHC
reactions. Patients from the IHC group were followed up for
32.76±40.55 months (median, 17.0; range, 0.5–145 months). During
this period 64 patients succumbed to the disease. However, 19
patients were lost to follow-up. The experiment was performed in
accordance with the ethical standards and following approval of the
Ethics Committee of Wroclaw Medical University (decision no. KB
455/2009 and KB 40/2017) and all patients provided a written
statement of informed consent for the use of the material samples
for scientific research.
Additionally, we used 20 tissue samples from NSCLC
cases quick frozen in liquid nitrogen and paired 20 control lung
tissue samples from the surgical margin of these patients. Patients
from the RT-PCR and WB groups were followed up for 47.89±25.59
months (median, 63.98; range, 2–72 months). Nine patients died
during the follow-up. However, none of the patients were lost to
follow-up.
The demographic and clinicopathological
characteristics of the patients are presented in Table II.
 | Table II.NSCLC cases and tumor
characteristics. |
Table II.
NSCLC cases and tumor
characteristics.
| Parameters | IHC (n=121) | WB/RT-PCR
(n=20) |
|---|
| Mean age
(range) | 62.36±8.68
(39–87) | 64.65±15.28
(52–76) |
| Sex, n (%) |
|
|
|
Male | 95
(78.51) | 14 (70) |
|
Female | 26
(21.49) | 6
(30) |
| Tumor size, n
(%) |
|
|
| T1 | 32
(26.45) | 9
(45) |
| T2 | 65
(53.72) | 8
(40) |
| T3 | 11
(9.09) | 3
(15) |
| T4 | 13
(10.74) | 0
(0) |
| Lymph node
involvement, n (%) |
|
|
| N0 | 54
(44.63) | 15 (75) |
| N1, N2,
N3 | 67
(55.37) | 5
(25) |
| Grade, n (%) |
|
|
| G1 | 9
(7.44) | 0
(0) |
| G2 | 78
(64.46) | 13 (65) |
| G3 | 32
(26.45) | 6
(30) |
| No
data, n (%) | 2
(1.65) | 1
(5) |
| pTNM, n (%) |
|
|
| I | 45
(37.19) | 10 (50) |
| II | 23
(19.01) | 7
(35) |
|
III | 49
(40.50) | 3
(15) |
| IV | 4
(3.31) | 0
(0) |
| Stage, n (%) |
|
|
|
Early | 68
(56.20) | 17 (85) |
|
Advanced | 53
(43.80) | 3
(15) |
| Histology, n
(%) |
|
|
|
Adeno | 60
(49.59) | 10 (50) |
|
Plano | 55
(45.45) | 9
(45) |
|
Mixed | 6
(4.96) | 1
(5) |
| Tesmin IHC, n
(%) |
|
|
| 0 | 14
(11.57) | – |
|
1-12 | 107 (88.43) | – |
Immunohistochemistry
The paraffin blocks with NSCLC cases were cut into
4-µm sections. The immunohistochemical reactions were performed
using anti-tesmin rabbit polyclonal antibody (cat. no. NBP2-13624;
Novus Biologicals, LLC, Littleton, Centennial, CO, USA) in a 1:400
dilution and anti-Ki-67 mouse monoclonal antibody, Clone MIB-1
(Dako, Glostrup, Denmark) ready-to-use. Immunohistochemical
reactions were performed using Dako Autostainer Link 48 (Dako).
Visualization of the reactions was carried out using EnVision™ FLEX
High pH (Link) reagents (Dako), according to the manufacturer's
instructions. Positive IHC reaction for tesmin antigen was assessed
using the immunoreactive scale (IRS) by Remmele and Stegner
(28). This scale evaluates the
percentage of positive cancer cells (A) and their intensity of
color reaction (B). The final result is a product of these two
values (AxB; Table III). Before
the IHC experiments, according to the antibody manufacturer's
instructions, we performed reactions to the positive and negative
controls (Fig. S4A and B).
 | Table III.The assessment scale of
immunohistochemical specimens, according to Remmele and Stegner
(28). |
Table III.
The assessment scale of
immunohistochemical specimens, according to Remmele and Stegner
(28).
| Number of | Intensity of | Percentage of |
|---|
| points | color reaction | reacting cells |
| 0 | No reaction | 0% |
| 1 | Weak reaction | <10% |
| 2 | Moderate
reaction | 10-50% |
| 3 | Strong
reaction | 51-80% |
| 4 | – | 81-100% |
Additionally, nuclear expression intensity for Ki-67
antigen was determined using a scale analyzing the percentage of
the number of cancer cells demonstrating nuclear expression of the
studied antigen, according to the following scale: 0% - 0p., 1–10 %
−1p., 11–25 % −2p., 26–50 % −3p., 51–100 % −4p. All specimens were
assessed using an Olympus BX-41 light microscope (Olympus Corp.,
Tokyo, Japan). Moreover, p63 and TTF-1 antigen expression was used
to confirm the histological type of the tumor [TTF-1 (+),
adenocarcinoma; p63 (+), squamous cell carcinoma].
Cell lines
Normal lung fibroblasts (IMR-90) and the lung cancer
cell lines NCI-H1703 (lung squamous cell carcinoma), NCI-H522
(adenocarcinoma of the lung) and A549 (adenocarcinoma of the lung)
were obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The following cell culture media were used:
minimum essential medium (MEM) supplemented with non-essential
amino acids for the IMR90 cell line, RPMI-1640 for the NCI-H1703
and NCI-H522 cell line and high-glucose Dulbecco's modified Eagle's
medium for the A549 cell line. All of the media were additionally
supplemented with L-glutamine to a final concentration of 2 mM, and
with fetal bovine serum (FBS) to a final concentration of 10%. All
of the cell culture media and reagents were provided by
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). In addition, we
performed in vitro knockdown experiments on the NCI-H1703
NSCLC cell line using Tesmin Silencer siRNA s18519 and s18520
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), receiving the
silencing of the tesmin expression at the height of ~55 kDa
(Fig. S4C).
Real-time PCR (qPCR)
The mRNA expression of MTL5 was analyzed
using 20 cases of NSCLC [10 cases of adenocarcinoma (AC) and 10
cases of squamous cell carcinoma (SCC)], 20 cases of control as
well as cell cultures (NCI-H1703, A549, NCI-H522 and IMR90). Total
RNA was extracted from the studied tissues and cell lines with
RNeasy Mini kit (Qiagen, Hilden, Germany) according to the
manufacturer's protocol. To eliminate genomic DNA contamination,
on-column DNase digestion was performed using RNase-Free DNase Set
(Qiagen). The concentration and quality of RNA samples were
assessed by spectrophotometry using NanoDrop 1000 (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). First-strand cDNA
was synthesized with the High-Capacity cDNA Reverse Transcription
kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
real-time PCR was performed using 7900HT Fast Real-Time PCR System
and TaqMan Gene Expression Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.). β-actin (ACTB) was used as an
endogenous control. The following sets of primers and TaqMan probes
were used in the studies: Hs01127481_m1 for MTL5 and
Hs99999903_m1 for ACTB (Applied Biosystems; Thermo Fisher
Scientific, Inc). The reactions were conducted in triplicates under
the following conditions: Polymerase activation at 50°C for 2 min,
denaturation at 94°C for 10 min followed by 40 cycles of
denaturation at 94°C for 15 sec and annealing with synthesis at
60°C for 1 min. The relative expression of MTL5 mRNA was
calculated using the ΔΔCq method (29).
Protein isolation, SDS-PAGE and
western blot analysis
Western blot technique was used to determine tesmin
expression levels in fresh frozen tissues from 20 NSCLC cases (10
AC and 10 SCC), 20 control cases and cell cultures (NCI-H1703,
A549, NCI-H522 and IMR90). Whole protein lysates from the tissue
samples were obtained using T-PER Tissue Protein Extraction reagent
(Thermo Fisher Scientific, Inc.) with the addition of Halt™
Protease Inhibitor Cocktail (Thermo Fisher Scientific, Inc.) and
0.2 mM PMSF (Sigma-Aldrich; Merck KGaA). Protein concentrations
were quantified using the Pierce BCA Protein Assay kit (Thermo
Fisher Scientific, Inc.) and NanoDrop™ 1000 (NanoDrop Technologies;
Thermo Fisher Scientific, Inc.) spectrophotometer. Equal amounts of
total protein (30 µg) were mixed with Laemmli sample buffer and
resolved on 10% acrylamide gel by SDS-PAGE (30). After the electrophoresis, the
samples were transferred to Immobilon-P polyvinylidene difluoride
(PVDF) membranes (Merck KGaA) in the XCell SureLock™ Mini-Cell
Electrophoresis System (Thermo Fisher Scientific, Inc.). Next, the
membranes were blocked in 4% bovine serum albumin (BSA; Merck KGaA)
solution in TBST buffer (0.2 M Tris, 1.5 M NaCl and 0.1% Tween-20).
After blocking, the membranes were incubated overnight at 4°C with
the primary rabbit anti-human tesmin polyclonal antibody (cat. no.
NBP2-13624; Novus Biologicals), diluted at 1:200. Furthermore, the
membranes were incubated with the secondary HRP-conjugated donkey
anti-rabbit antibody (cat. no. 715-035-152; Jackson ImmunoResearch
Laboratories, West Grove, PA, USA), diluted at 1:3,000 for 1 h at
room temperature. Finally, the membranes were rinsed and treated
with the Luminata Classico (Merck KGaA) chemiluminescent substrate.
The reactions were visualized using the ChemiDoc Imaging system
(Bio-Rad Laboratories, Hercules, CA, USA). β-actin detected with
primary rabbit anti-human β-actin antibody (cat. no. 4970; Cell
Signaling Technology, Leiden, The Netherlands) diluted at 1:1,000
and secondary HRP-conjugated donkey anti-rabbit antibody (cat. no.
711-035-152; Jackson ImmunoResearch Laboratories) diluted at
1:3,000 were used as an internal control to normalize the amount of
tesmin. Densitometric analysis of obtained results was performed
with the use of Image Lab 6.0.1 software (Bio-Rad
Laboratories).
Immunofluorescence (IF)
Cells were fixed with 4% paraformaldehyde for 12 min
at room temperature (RT) and permeabilized using 0.2% Triton X-100
for 10 min. The cells were incubated overnight at 4°C with primary
anti-tesmin antibodies (cat. no. NBP2-13624; dilution 1:200; Novus
Biologicals). Subsequently, secondary fluorescein isothiocyanate
(FITC)-conjugated anti-rabbit antibodies were applied (dilution
1:1,000; for 1 h at RT; Jacksons Immunoresearch Laboratories, Ely,
Cambridgeshire, UK). The sections were mounted in a DAPI-containing
medium (ProLong Gold Antifade reagent with DAPI) (Thermo Fisher
Scientific, Inc.) and analyzed using a confocal laser scanning
microscope FV3000 Fluoview (Olympus Corp.). Omitting the addition
of primary antibody was performed as the respective negative
controls.
Statistical analysis of the obtained
results
The Shapiro-Wilk test was used to evaluate the
normality assumption of the examined groups. The Mann-Whitney or
Wilcoxon tests were used to compare the differences in the
expression of examined markers in all groups of patients and the
clinicopathological data. Additionally, the Spearman's correlation
test was used to analyze the existing correlations. The
Kaplan-Meier method was used to construct survival curves. The
Mantel-Cox method was performed to evaluate the analysis of
survival. Additionally, the Kaplan-Meier plotter web-based tool was
used to draw Kaplan-Meier curves and to analyze patient survival
with high and low expression of MTL5 mRNA (31). All statistical analyses were
performed using GraphPad Prism 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA). The results were considered statistically
significant at P<0.05.
Results
IHC
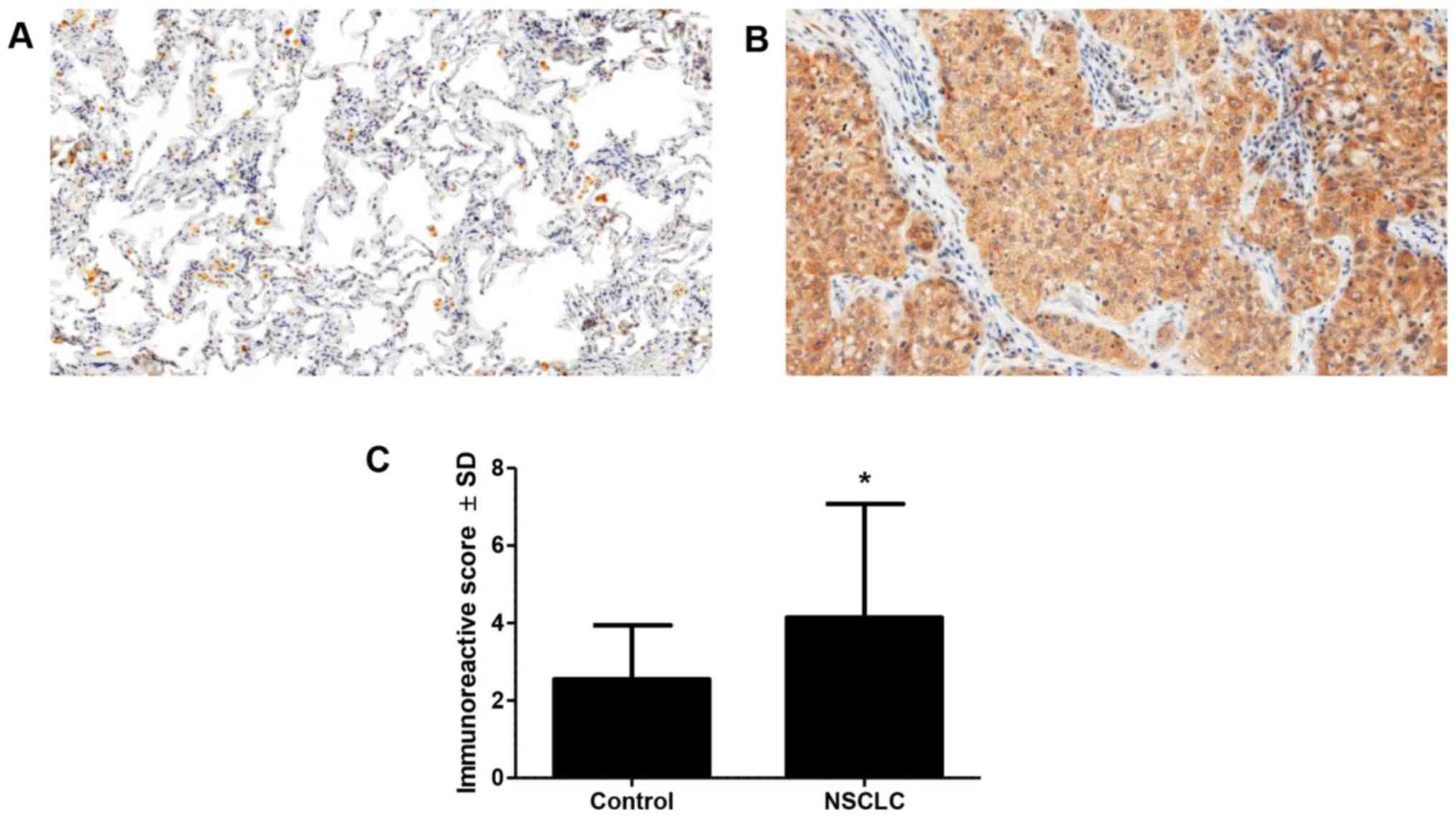
Expression of tesmin was observed in the cytoplasm
and nuclei of cancer cells. In addition, there was also cytoplasmic
expression noted in inflammatory cells and alveolar macrophages
(Fig. 1A and B). Diverse positive
cytoplasmic expression of tesmin was observed in 107 (88.42%) of
cases, positive nuclear expression in 36 (27.75%) cases and
positive low expression in all control cases. Statistical analysis
revealed significantly higher expression of tesmin in cancer cases
compared to that noted in the control (Fig. 1C). Furthermore, we observed
significantly higher expression of tesmin in pT4 compared to pT1-3
cases (3.79±2.95 vs. 6.23±1.96; P<0.01, Mann-Whitney test; data
not shown). We did not reveal any associations between tesmin
expression and G, pN, stage and sex of the patients. Additionally,
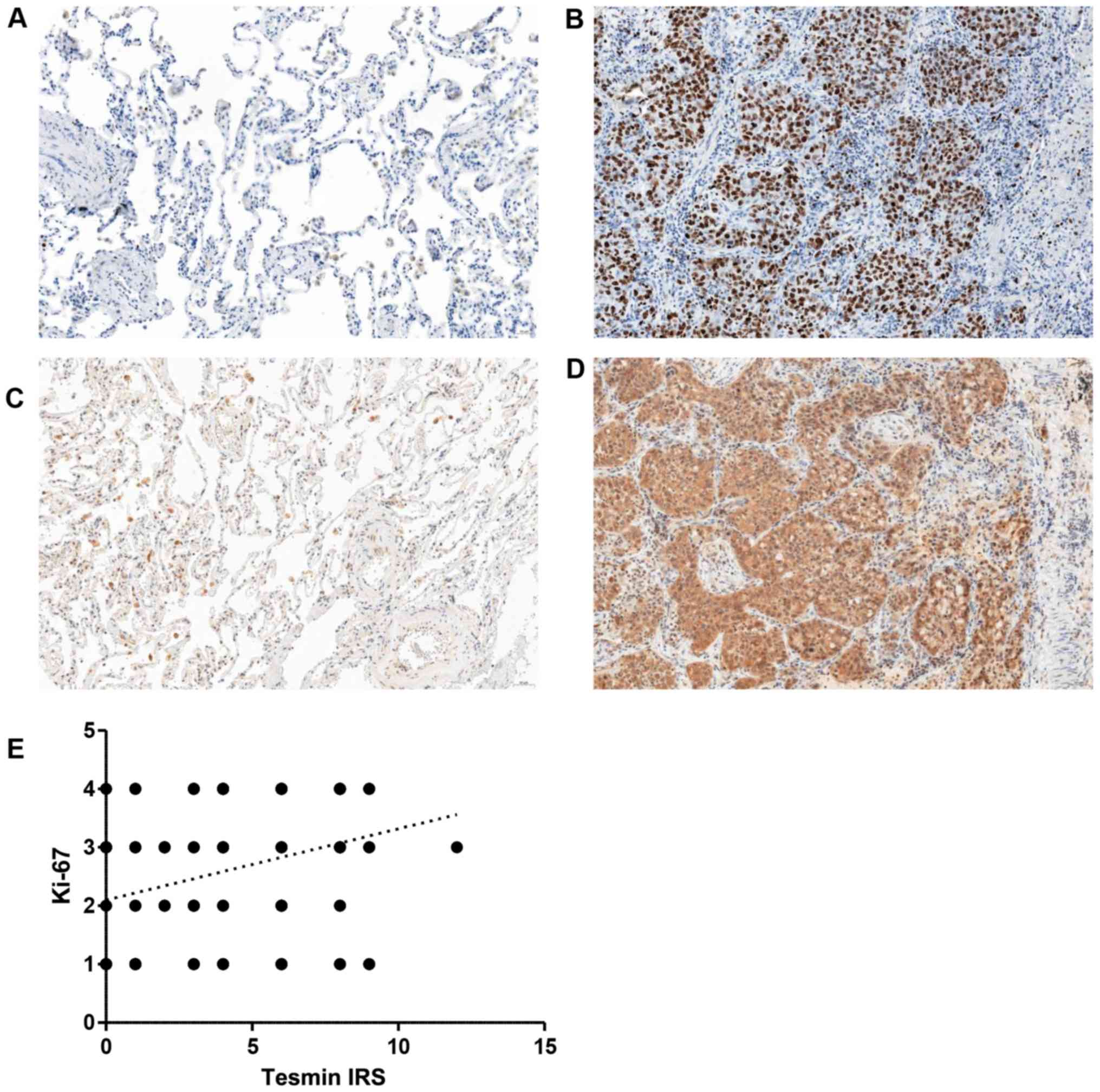
there was a medium positive correlation between expression of
tesmin and Ki-67 (r=0.32, P<0.001, Spearman correlation test;
Fig. 2A-E).
qPCR
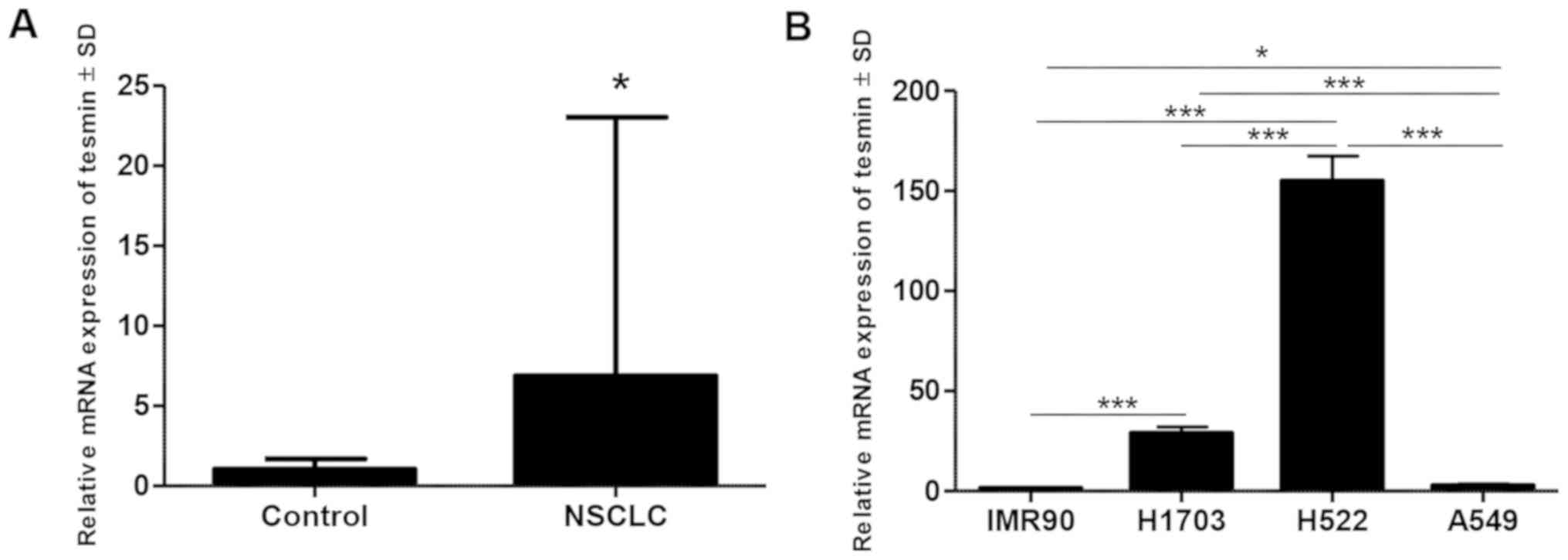
The results of qPCR analysis of MTL5 gene
expression in vivo showed significantly higher expression of
MTL5 in NSCLC compared to that noted in the non-cancerous
tissue from the resection margin (P<0.05, paired t-test)
(Fig. 3A). The MLT5
expression was also higher in non-small cell lung cancer cell lines
(NCI-H1703, A549 and NCI-H522) compared to that noted in the lung
fibroblast culture (IMR90) in the in vitro experiment
(Fig. 3B).
Western blot analysis
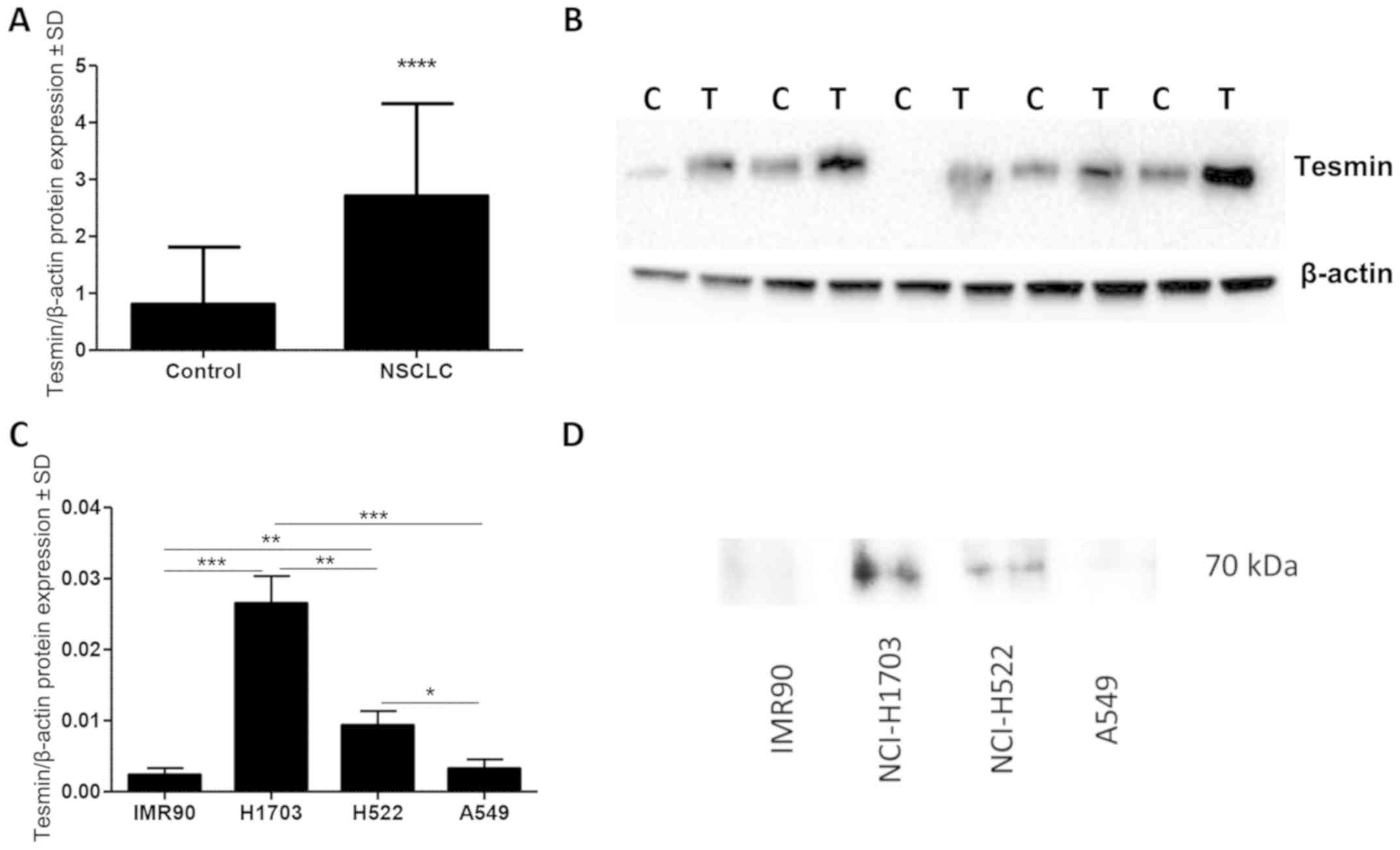
Densitometric analysis of tesmin expression (WB)
showed a significantly higher level of tesmin protein (70 kDa) in
the cancer than that in the adjacent lung tissues (P<0.0001,
paired t-test; Fig. 4A and B).
In vitro analysis of tesmin expression in cell lines showed
that the presence of tesmin protein in nuclear fraction of cell
lysate was higher in non-small cell lung cancer cell lines
(NCI-H1703, A549 and NCI-H522) compared to the lung fibroblast
culture (IMR90) (Fig. 4C and
D).
IF
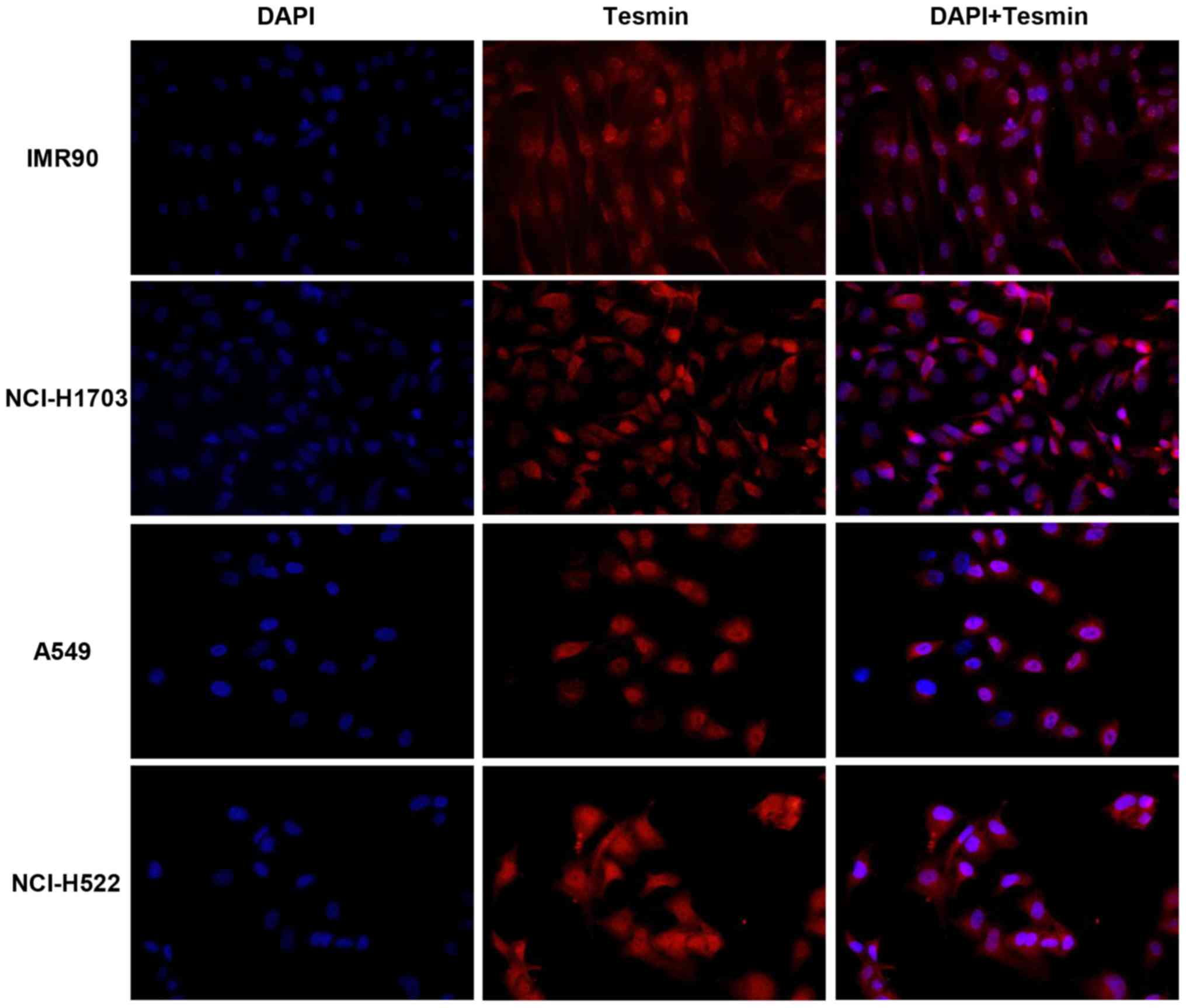
To verify localization of tesmin expression, IF was
performed on lung cancer cell lines. The analysis of the results of
these in vitro studies revealed cytoplasmic and nuclear
positive IF expression of tesmin corresponding to the results of
our IHC studies (Fig. 5).
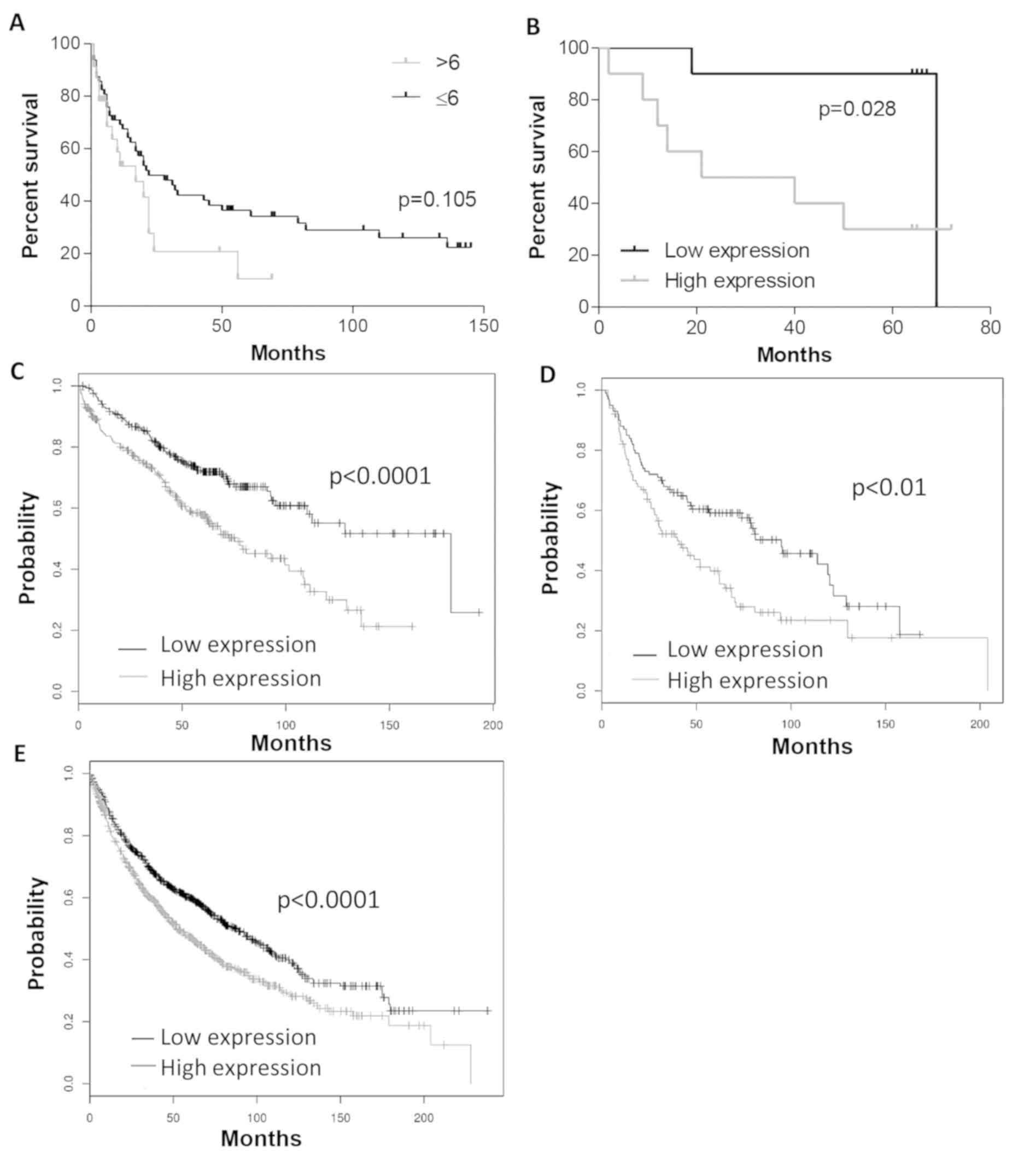
Analysis of survival
Analysis of survival revealed that higher IHC
expression of tesmin was associated with shorter survival (trend
did not reach statistical significance; Fig. 6A). In the WB group higher expression
of tesmin was also associated with shorter survival (P<0.05,
Mantel-Cox test; Fig. 6B). In
addition, using Kaplan-Meier plotter software (31), higher MTL5 mRNA expression
was associated with shorter survival time, especially in groups
with a lower stage of cancer (malignant grade G1, and in stage 1 of
the disease) (Fig. 6C-E).
Discussion
The present study was the first to demonstrate
higher expression of tesmin (MTL5) in non-small cell lung cancer
(NSCLC) cells compared to control tissue. The present study is the
only one that describes in detail the presence of this protein in
NSCLC. Expression of tesmin in NSCLC proves above all that the role
of this protein is much more diverse than the regulation of meiotic
division in germ cells or co-activation of the nuclear
mineralocorticoid receptor (24).
The subcellular location of tesmin was studied in
male and female germ cells and was dependent on the meiotic
division stage. Before meiosis its expression was observed in the
cytoplasm of germ cells whereas during meiosis it was translocated
to the cell nucleus and then it was again located in the cytoplasm
(18). In addition, oxidative
stress was found to induce premature occurrence of nuclear
expression of tesmin. The exact role of this protein in different
cells is still unknown. The findings of this study confirmed the
increased expression of tesmin in the cytoplasm and the nucleus of
lung cancer cells compared to control tissue suggesting that tesmin
may be related to the process of carcinogenesis in NSCLC. In
addition, increased expression of this protein in cases with higher
pT suggests its role in NSCLC progression.
Analysis of the overall survival of NSCLC patients
showed worse prognosis in cases with higher tesmin expression
compared to patients with low expression of the protein
(Kaplan-Meier plotter and our own studies) indicating the influence
of this protein in cancer development.
Similar observations have reported in the case of
proteins from the metallothionein (MT) family in many different
types of cancers. Functional similarity of these proteins with
tesmin (regulation of heavy metal ion concentration, response to
oxidative stress) may suggest a similar mechanism triggering
overexpression of MTs and tesmin and a similar role in the process
of carcinogenesis (12,13). This hypothesis, however, requires
further research.
Additionally, as in the case of MTs, we also found a
positive correlation of tesmin expression with the Ki-67
proliferation antigen, suggesting the influence of tesmin on
stimulation of NSCLC cell proliferation. Considering the previous
reports on tesmin, including its relationship with the meiotic
division of germ cells, this observation seems to be correct.
Nevertheless, further studies are warranted (18).
The obtained immunofluorescence (IF) results based
on tissue material were also confirmed by cell line studies in
which tesmin expression was demonstrated in both the cytoplasm and
the nucleus (IF).
Furthermore, as in the case of tissue sample
studies, in squamous cell carcinoma and lung adenocarcinoma cell
lines, in vitro experiments showed significantly higher
expression of tesmin (by western blotting) and MTL5 mRNA in
relation to the control cell line (IMR90), which may additionally
confirm the involvement of tesmin in the process of neoplastic
transformation.
It seems useful to focus on translocation of tesmin
from the cytoplasm to the nucleus during meiotic divisions and its
relationship with the exposure of germ cells to increased
concentration of heavy metal ions as reported by other authors
(22). In our study, we also
observed tesmin expression in both the cytoplasm and the nucleus.
However, nuclear expression of tesmin was not related to the
clinicopathological data of patients. Its potentially different
role in the cell nucleus and the cytoplasm of lung cancer cells
certainly requires further research.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was financed by the National
Science Centre, Poland under the programme ‘Preludium 12’ project
no 2016/23/N/NZ5/02570.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JG designed the study, evaluated the IHC reaction,
prepared the database, performed the statistical analysis, analysed
the results and created a draft of the manuscript; AG performed the
qPCR analysis, prepared the database and analysed the results; MO
and NGP prepared the in vivo WB analysis and all in
vitro experiments, created the database and analysed the
results; AP prepared the IHC analysis, created the database and
analysed the results; KRW prepared the IF analysis and the in
vitro experiments and analysed the results; AR obtained the
in vivo material from patients, collected the clinical data
of patients, prepared the database and analysed the results. PD,
MPO and ZK supervised methodical all experiments, analysed the
results, prepared, reviewed and edited the manuscript. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The experiment was performed in accordance with the
ethical standards and following approval of the Ethics Committee of
Wroclaw Medical University (decision no. KB 455/2009 and KB
40/2017) and all patients provided a written statement of informed
consent for the use of the material samples for scientific
research.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Stewart BW and Wild CP: Word Cancer Report
2014. World Health Organization; Lyon: 2014
|
|
2
|
Didkowska J, Wojciechowska U and Olasek P:
Zachorowania i zgony na nowotwory złośliwe w Polsce. Krajowy
Rejestr Nowotworów, Centrum Onkologii-Instytut im. Marii
Skłodowskiej-Curie. http://onkologia.org.pl/k/epidemiologiaJuly
06–2018
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krzakowski M, Jassem J, Dziadziuszko R,
Kowalski DM, Olszewski W, Orłowski T, Rzyman W and Smorczewska M:
Zalecenia postępowania diagnostyczno-terapeutycznego w nowotworach
złośliwych-2013 r. Nowotwory płuca i opłucnej oraz śródpiersia, Via
Medica. 1:69–101, Gdańsk. 2013.
|
|
5
|
Theocharis SE, Margeli AP and Koutselinis
A: Metallothionein: A multifunctional protein from toxicity to
cancer. Int J Biol Markers. 18:162–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vašák M: Advances in metallothionein
structure and functions. J Trace Elem Med Biol. 19:13–17. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wierzowiecka B, Gomulkiewicz A,
Cwynar-Zajac L, Olbromski M, Grzegrzolka J, Kobierzycki C,
Podhorska-Okolow M and Dziegiel P: Expression of metallothionein
and vascular endothelial growth factor isoforms in breast cancer
cells. In Vivo. 30:271–278. 2016.PubMed/NCBI
|
|
8
|
Theocharis SE, Margeli AP, Klijanienko JT
and Kouraklis GP: Metallothionein expression in human neoplasia.
Histopathology. 45:103–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Romero-Isart N and Vašák M: Advances in
the structure and chemistry of metallothioneins. J In Org Biochem.
88:388–396. 2002.
|
|
10
|
Bay BH, Jin R, Huang J and Tan PH:
Metallothionein as a prognostic biomarker in breast cancer. Exp
Biol Med (Maywood). 231:1516–1521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimoda R, Achanzar WE, Qu W, Nagamine T,
Takagi H, Mori M and Waalkes MP: Metallothionein is a potential
negative regulator of apoptosis. Toxicol Sci. 73:294–300. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cherian MG, Jayasurya A and Bay BH:
Metallothioneins in human tumors and potential roles in
carcinogenesis. Mutat Res. 533:201–209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dziegiel P, Pula B, Kobierzycki C,
Stasiolek M and Podhorska-Okolow M: Metallothioneins in normal and
cancer cells. Adv Anat Embryol Cell Biol. 218:1–117. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Werynska B, Pula B, Kobierzycki C,
Dziegiel P and Podhorska-Okolow M: Metallothioneins in the lung
cancer. Folia Histochem Cytobiol. 53:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ostrakhovitch EA, Olsson PE, Jiang S and
Cherian MG: Interaction of metallothionein with tumor suppressor
p53 protein. FEBS Letters. 580:1235–1238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ostrakhovitch EA, Olsson PE, von Hofsten J
and Cherian MG: P53 mediated regulation of metallothionein
transcription in breast cancer cells. J Cell Biochem.
102:1571–1583. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coyle P, Philcox JC, Carey LC and Rofe AM:
Metallothionein: The multipurpose protein. Cell Mol Life Sci.
59:627–647. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sutou S, Miwa K, Matsuura T, Kawasaki Y,
Ohinata Y and Mitsui Y: Native tesmin is a 60-kilodalton protein
that undergoes dynamic changes in its localization during
spermatogenesis in mice. Biol Reprod. 68:1861–1869. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
HUGO Gene Nomenclature Comitte. http://www.genenames.orgJune 1–2018
|
|
20
|
Sugihara T, Wadhwa R, Kaul SC and Mitsui
Y: A novel testis-specific metallothionein-like protein, tesmin, is
an early marker of male germ cell differentiation. Genomics.
57:130–136. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Olesen C, Møller M and Byskov AG: Tesmin
transcription is regulated differently during male and female
meiosis. Mol Reprod Dev. 67:116–126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuura T, Kawasaki Y, Miwa K, Sutou S,
Ohinata Y, Yoshida F and Mitsui Y: Germ cell-specific
nucleocytoplasmic shuttling protein, tesmin, responsive to heavy
metal stress in mouse testes. J Inorg Biochem. 88:183–191. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maremanda KP, Khan S and Jene G: Zinc
protects cyclophosphamide-induced testicular damage in rat:
Involvement of metallothionein, tesmin and Nrf2. Biochem Biophys
Res Commun. 445:591–596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rogerson FM, Yao YZ, Young MJ and Fuller
PJ: Identification and characterization of a ligand-selective
mineralocorticoid receptor coactivator. FASEB J. 28:4200–4210.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fic M, Pula B, Rogala K and Dziegiel P:
Role of metallothionein expression in gastrointestinal cancers.
Postępy Biol Komórki. 40:5–20. 2013.
|
|
26
|
Oh JH, Yang JO, Hahn Y, Kim MR, Byun SS,
Jeon YJ, Kim JM, Song KS, Noh SM, Kim S, et al: Transcriptome
analysis of human gastric cancer. Mamm Genome. 16:942–954. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Penney KL, Sinnott JA, Tyekucheva S, Gerke
T, Shui IM, Kraft P, Sesso HD, Freedman ML, Loda M, Mucci LA and
Stampfer MJ: Association of prostate cancer risk variants with gene
expression in normal and tumor tissue. Cancer Epidemiol Biomarkers
Prev. 24:255–260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe (German). 8:138–140. 1987.
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar : PubMed/NCBI
|