Introduction
Colorectal cancer (CRC), one of the most common
cancer types worldwide, remains a serious threat to human health
with high incidence and mortality globally (1). According to the 2018 statistics by the
American Cancer Society, CRC ranks third in the morbidity and
mortality among all malignancy types in the United States (2). CRC is difficult to detect at an early
stage due to a lack of typical symptoms and signs; therefore, only
39% of patients with CRC have no metastasis at the time of
diagnosis, with a 5-year survival rate of up to 90%; however, the
majority of patients are diagnosed when the disease has distant
metastasis, and the 5-year survival rate of these patients drops to
14%, according to data released by the National Cancer Institute
(2006–2012) (3). Metastasis and
recurrence are considered the primary causes of mortality in
patients with CRC (4). Therefore,
it is urgent and necessary to elucidate the molecular mechanism
underlying the onset and progression (metastasis and recurrence),
which will provide novel therapeutic strategies for CRC.
The onset and progression of CRC are multi-step
processes, starting with hyper-proliferation of epithelial cells,
then forming carcinoma in situ and eventually progressing to
invasive and metastatic carcinoma (5). Gene mutations, including adenomatous
polyposis coli, c-MYC and V-Ki-ras2 Kirsten rat sarcoma viral
oncogene homolog, and epigenetic changes, such as aberrant DNA
methylation, serve key roles in these processes, and these
alterations result in abnormalities in signaling pathways,
including Wnt, mitogen-activated protein
kinase/phosphatidylinositol 3-kinase, transforming growth factor-β
and tumor protein P53, which will further influence a number of
important biological functions of the cells (6–8).
Notably, the nature of gene mutations and epigenetic changes is
that both alter the expression of oncogenes or tumor suppressor
genes (9,10). Gene expression is regulated at
multiple levels, including transcription and translation levels
(11). Previously, non-coding RNAs
(ncRNAs), such as microRNAs (miRNAs) and long ncRNAs (lncRNAs),
have been identified as crucial regulators of gene expression in
numerous human diseases, particularly tumors (12–15).
ncRNAs are generated from non-coding regions that
were previously considered to be junk DNA, without the potential of
translation into proteins (16).
Previously, a novel class of ncRNAs has been identified, termed
Piwi-interacting RNAs (piRNAs), which are characterized by a
3′-terminal 2′-O-methylation (17,18).
piRNAs are named due to their characteristics of exclusive
association with the Piwi subfamily, but not the Ago subfamily, and
they maintain genome integrity by epigenetically silencing
transposons (19,20). Although piRNAs were initially
considered to be only expressed in germ cells, a growing number of
studies identified piRNAs in various human tissues and cells,
including brain tissues and cardiac progenitor cells (21,22),
and they are also abnormally expressed in tumor cells (23,24),
indicating that piRNAs may be involved in the onset and progression
of tumors.
To date, it has been demonstrated that piRNAs are
associated with gastric cancer, breast cancer, lung cancer,
multiple myeloma and bladder cancer and piRNAs serve various roles
in these tumor types, including tumor promotion and suppression in
different tumor types (25–29). Additionally, the mechanisms
underlying piRNAs in tumors are also diverse and include epigenetic
regulation, post-transcriptional regulation and post-translational
regulation (30). These studies
indicated that piRNAs have diverse functions and complex mechanisms
in the tumor context. Although above studies have preliminarily
elucidated the roles of piRNAs in a number of tumor types, the
majority of studies only focused on a specific piRNA and did not
depict the overall changes of piRNAs in tumors, which is not
sufficient to fully understand the complex function of piRNAs in
tumors.
In the present study, to improve the understanding
of the biological function of piRNAs in CRC, the differences in
piRNA expression profiles between CRC tissues and adjacent
non-tumor tissues were compared using second-generation deep
sequencing for small RNAs. Subsequently, the results were validated
by reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). The potential clinical utility of CRC-associated piRNAs
were also assessed by analyzing the association of piRNAs with
clinicopathological features of patients with CRC. To the best of
our knowledge, the present results provided, for the first time, an
overall change of piRNA expression profiles in CRC and an outlook
into clinical applications of piRNAs as a therapeutic target.
Materials and methods
Patients and samples
A total of 83 fresh CRC tissues and matched adjacent
non-tumor tissues were collected from patients with CRC who
underwent surgery from January 2016 to January 2018 at the Second
Hospital of Hebei Medical University (Shijiazhuang, China). The
diagnosis for CRC and histological evaluation were conducted by an
experienced pathologist. All patients exhibited no primary tumors
in other sites and did not receive chemoradiotherapy or biological
therapy prior to surgery. Tissues were placed in RNAstore reagent
(Tiangen Biotech Co., Ltd., Beijing, China) and then stored at
−80°C after being resected from patients. Of the 83 pairs of
samples, 3 pairs were used for deep sequencing for small RNAs, and
the remaining 80 pairs were used for validation. The
clinicopathological characteristics of patients with CRC for small
RNA sequencing analysis and RT-qPCR validation analysis are listed
in Tables I and II, respectively. Written informed consent
was obtained from the recruited patients, and the present study was
approved by the Ethics Committee of the Second Hospital of Hebei
Medical University.
 | Table I.Clinicopathological characteristics
of patients with colorectal cancer for small RNA sequencing. |
Table I.
Clinicopathological characteristics
of patients with colorectal cancer for small RNA sequencing.
| Patients | Sex | Age | Tumor location |
Differentiation | T
stagea | Lymph node
metastasis | AJCC
stagea |
|---|
| 1 | Male | 52 | Rectum | Moderately | T2 | No | I |
| 2 | Male | 75 | Rectum | Poorly | T4 | Yes | III |
| 3 | Female | 62 | Colon | Poorly | T3 | No | II |
 | Table II.Correlation of piRNA expression with
clinicopathological characteristics of patients with colorectal
cancer. |
Table II.
Correlation of piRNA expression with
clinicopathological characteristics of patients with colorectal
cancer.
| Clinicopathological
characteristics | No. (%) | piR-18849 fold
change | P-value | piR-19521 fold
change | P-value | piR-17724 fold
change | P-value |
|---|
| All cases | 80 (100) |
|
|
|
|
|
|
| Sex |
|
| 0.235 |
| 0.849 |
| 0.647 |
|
Male | 46 (57.50) | 2.27
(1.38-6.11) |
| 2.03
(1.20-3.58) |
| 1.25
(0.43-5.64) |
|
|
Female | 34 (42.50) | 3.10
(1.98-6.06) |
| 2.10
(1.47-3.39) |
| 1.83
(0.72-3.17) |
|
| Age |
|
| 0.194 |
| 0.388 |
| 0.614 |
|
≥60 | 49 (61.25) | 3.27
(1.74-6.88) |
| 2.17
(1.42-3.61) |
| 1.52
(0.56-4.15) |
|
|
<60 | 31 (38.75) | 2.33
(1.45-4.45) |
| 2.00
(1.38-3.27) |
| 1.64
(0.42-3.24) |
|
| Tumor location |
|
| 0.725 |
| 0.296 |
| 0.121 |
|
Colon | 39 (48.75) | 3.15
(1.78-6.08) |
| 2.23
(1.47-3.75) |
| 0.97
(0.44-2.74) |
|
|
Rectum | 41 (51.25) | 2.84
(1.37-6.08) |
| 2.06
(1.29-2.96) |
| 1.91
(0.62-6.89) |
|
|
Differentiation |
|
| 0.001 |
| 0.001 |
| 0.329 |
| Well or
Moderately | 56 (70.00) | 2.13
(1.35-4.73) |
| 1.87
(1.12-2.60) |
| 1.36
(0.45-3.22) |
|
|
Poorly | 24 (30.00) | 4.87
(2.64-7.99) |
| 3.22
(1.80-7.89) |
| 1.97
(0.59-8.07) |
|
| T
stagea |
|
| 0.794 |
| 0.618 |
| 0.321 |
| T1 or
T2 | 16 (20.0) | 2.86
(1.75-7.98) |
| 2.03
(1.40-2.97) |
| 2.35
(1.06-3.77) |
|
| T3 | 33 (41.25) | 2.84
(1.46-4.61) |
| 2.07
(1.45-3.39) |
| 1.15
(0.40-3.56) |
|
| T4 | 31 (38.75) | 3.35
(1.59-6.94) |
| 2.09
(1.21-3.68) |
| 1.21
(0.50-3.15) |
|
| Lymph node
metastasis |
|
| 0.043 |
| 0.441 |
| 0.735 |
| No | 50 (62.50) | 2.32
(1.45-4.86) |
| 2.05
(1.18-3.10) |
| 1.65
(0.53-3.55) |
|
|
Yes | 30 (37.50) | 4.58
(1.81-7.28) |
| 2.10
(1.48-4.24) |
| 1.28
(0.43-3.59) |
|
| AJCC
stagea |
|
| 0.249 |
| 0.417 |
| 0.501 |
| I | 14 (17.50) | 2.86
(1.94-9.54) |
| 2.18
(1.38-3.07) |
| 1.98
(0.93-3.90) |
|
| II | 35 (43.75) | 1.96
(1.32-4.45) |
| 1.79
(1.16-3.04) |
| 1.20
(0.42-3.24) |
|
| III or
IV | 31 (38.75) | 4.44
(1.81-7.14) |
| 2.14
(1.48-4.36) |
| 1.36
(0.44-3.31) |
|
Small RNA library construction,
sequencing and data analysis
Total RNA was extracted from 3 pairs of CRC tissues
and matched adjacent non-tumor tissues using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The concentration and quality of RNA were assessed by a
NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc.) and an Agilent 2100 Bioanalyzer (Agilent
Technologies, Inc., Santa Clara, CA, USA). Following passing the
quality control tests, total RNAs from 3 CRC tissues and 3 matched
adjacent non-tumor tissues were pooled separately in equal quantity
(0.4 µg) to generate two sample pools [CRC (T) pool and adjacent
non-tumor (N) pool] and then were sent to Beijing Genomics
Institute (Shenzhen, China) for library preparation and sequencing.
Briefly, small RNAs of 18–40 nt in length were purified from total
RNA by size fractionation using 15% PAGE and sequentially ligated
to 5′ and 3′ adaptors, followed by RT-PCR amplification to produce
sequencing libraries using the TruSeq Small RNA Sample Prep Kit
(Illumina, Inc., San Diego, CA, USA) according to the
manufacturer's protocol. PCR products were gel-purified and
sequenced using Illumina HiSeq 2000 (Illumina, Inc.). Clean reads
were obtained by removing low-quality reads and adaptor sequences
from raw reads. Subsequently, the length distribution of the clean
reads and common and specific sequences between these two samples
were summarized. Small RNA reads were aligned with piRNABank
(http://pirnabank.ibab.ac.in/) to screen
and annotate piRNAs using Bowtie (31). To identify differentially-expressed
piRNAs between CRC samples and adjacent non-tumor samples,
expression levels of each piRNA were normalized using the following
formula: Normalized expression=actual piRNA count/total count of
clean reads × 1,000,000. piRNAs with normalized expression values
<1.0 in both samples were removed. piRNAs with fold change ≥2.0
(log2 ratio ≥1.0 or ≤-1.0) were considered as differentially
expressed.
RT-qPCR of piRNA
Candidate differentially-expressed piRNAs were
further validated in 80 pairs of CRC and adjacent non-tumor tissues
by RT-qPCR. Briefly, total RNAs, including small RNAs, were
isolated from tissues using a miRcute miRNA Isolation kit (Tiangen
Biotech Co., Ltd.) and then reverse transcribed with a miScript
Plant RT kit (Qiagen GmbH, Hilden, Germany), which is a kit
specifically designed for small RNAs with the 2′-O-Me modification
at their 3′ end. RT-qPCR was performed in triplicate with specific
forward primers and universal reverse primers using a miScript
SYBR® Green PCR kit (Qiagen GmbH), according to the
manufacturer's protocols. The reverse transcription process was
performed in two steps of ligation reaction catalyzed by ligase and
reverse transcription reaction catalyzed by reverse transcriptase.
RT-qPCR was performed on an ABI StepOne™ real-time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Amplification
conditions were pre-denaturation at 95°C for 15 min, followed by 40
cycles of denaturation at 94°C for 15 sec, annealing at 55°C for 30
sec and extension at 72°C for 30 sec. The expression of piRNAs was
normalized against U6 small nuclear RNA levels and calculated using
the 2−∆Cq method (32).
Specific primers used in RT-qPCR are detailed in Table III.
 | Table III.Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table III.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
| Name | Sequence
(5′-3′) |
|---|
| hsa-piR-19521 |
GAGTAGAGTGCTTAGTTGAACAG |
| hsa-piR-18849 |
TTTGGCAATGGTAGAACTCACAC |
| hsa-piR-17724 |
TTCCGTAGTGTAGTGGTTATCAC |
| U6 snRNA |
CTCGCTTCGGCAGCACATA |
Statistical analysis
Normally distributed data were expressed as the mean
± standard deviation and non-normally distributed data were
expressed as median with interquartile range. Non-normally
distributed data were analyzed using the Wilcoxon signed rank test
(when paired) or Mann-Whitney U test (when unpaired). The
correlation of piRNA expression with T stage and American Joint
Committee on Cancer stage (7th edition) (33) was analyzed using Spearman's
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using SPSS software version 17.0 (SPSS, Inc., Chicago,
IL, USA).
Results
Common and specific reads
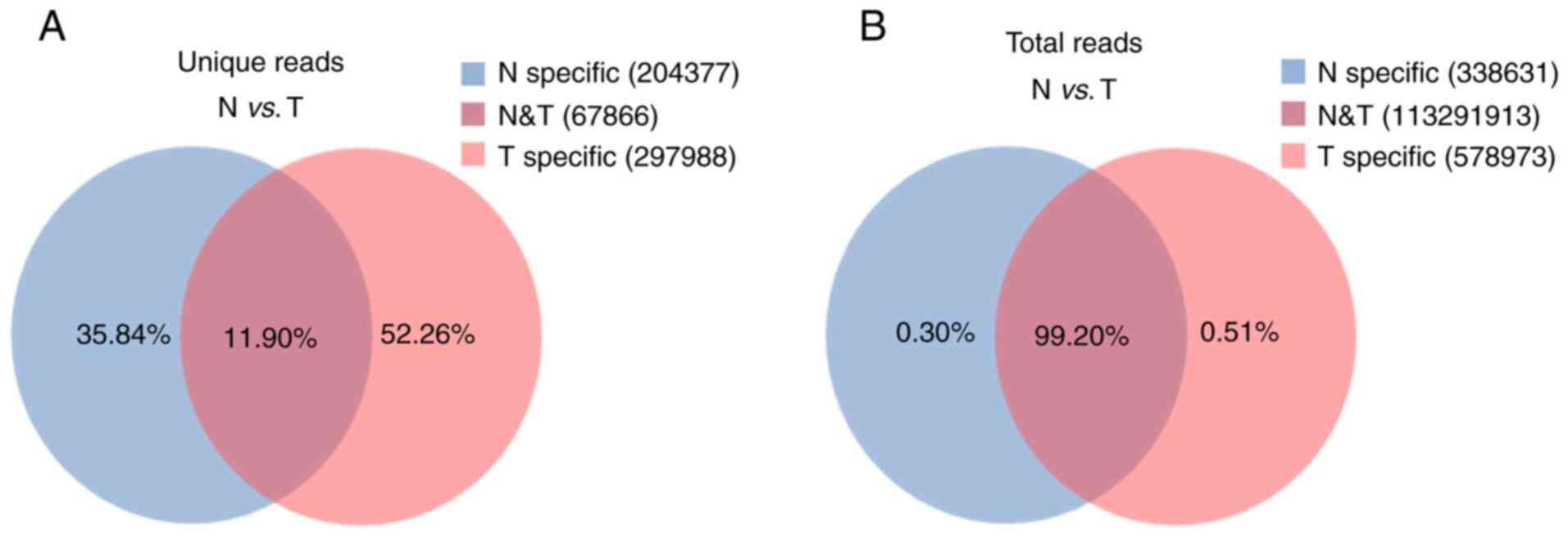
analysis
To evaluate the overall differences of small RNAs in
CRC and adjacent non-tumor tissues, the common and specific reads
were analyzed between the CRC (T) library and adjacent non-tumor
(N) library, including the number of unique reads (types of reads)
and total reads. Small RNA sequencing yielded 570,231 unique reads
with 114,209,517 total reads from these two libraries. Among these
unique reads, only 67,866 (11.90%) unique reads were shared by the
two libraries, whereas 297,988 (52.26%) and 204,377 (35.84%) unique
reads were specific in the T library and N library, respectively
(Fig. 1A). These specific reads
were determined to not be abundant, with only 0.51% of total reads
in the T library and 0.30% of total reads in the N library
(Fig. 1B). These data indicate that
CRC tissues and adjacent non-tumor tissues have diverse small RNA
profiles and that specific small RNAs exhibit low expression
levels.
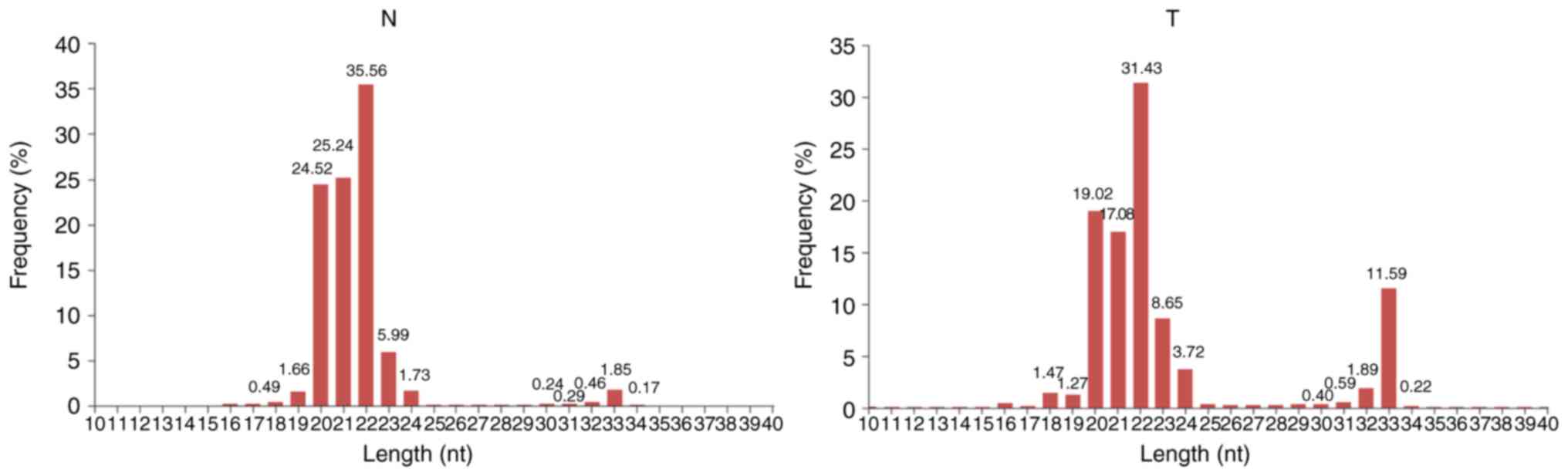
Length distribution of small RNAs
In general, the length of small RNAs ranged from
18–35 nt, and the peak of length distribution was beneficial to
identify the classes of small RNAs. miRNAs were concentrated in 21
or 22 nt, whereas piRNAs were concentrated in 26–32 nt (34). As depicted in Fig. 2, in the N and T libraries, the
lengths of small RNAs were clustered in two ranges: 18–24 and 30–34
nt, and the most abundant cluster was 18–24 nt with a 22 nt peak
point, the canonical length of miRNAs. The percentage of reads
within the 18–24 nt cluster demonstrated no notable difference
between these two libraries (82.64% vs. 95.19%), indicating that
the abundance of miRNAs was not significantly changed in CRC.
However, an increased 30–34 nt peak, primarily comprised of piRNAs,
was determined in the T library compared with the N library (14.69%
vs. 3.01%), indicating that the piRNA pathway is activated in human
CRC.
piRNA annotation
To analyze the differentially expressed piRNAs
between CRC and adjacent non-tumor tissues, piRNAs were first
annotated and screened by mapping clean reads to the piRNA
database. The results demonstrated that 367 unique reads in the N
library and 423 unique reads in the T library, accounting for 0.13%
and 0.12%, respectively, were aligned with piRNA sequences.
Although the proportion of unique piRNA reads indicated no notable
changes between these two libraries, the counts of total piRNA
reads increased from 42,999 (0.07%) in the N library to 75,946
(0.15%) in the T library (Table
IV), indicating that the overall expression of piRNAs was
increased in CRC tissues, compared with adjacent non-tumor tissues,
and further implying that piRNAs may be implicated in colorectal
tumorigenesis.
 | Table IV.Number of unique reads and total
reads aligned to piRNA sequences in the N and T libraries. |
Table IV.
Number of unique reads and total
reads aligned to piRNA sequences in the N and T libraries.
|
| N library | T library |
|---|
|
|
|
|
|---|
| Categories | Unique reads
(%) | Total reads
(%) | Unique reads
(%) | Total reads
(%) |
|---|
| piRNA | 367 (0.13) | 42,999 (0.07) | 423 (0.12) | 75,946 (0.15) |
| Other | 271,876
(99.87) | 62,488,846
(99.93) | 365,431
(99.88) | 51,601,726
(99.85) |
| Total | 272,243 (100) | 62,531,845
(100) | 365,854 (100) | 51,677,672
(100) |
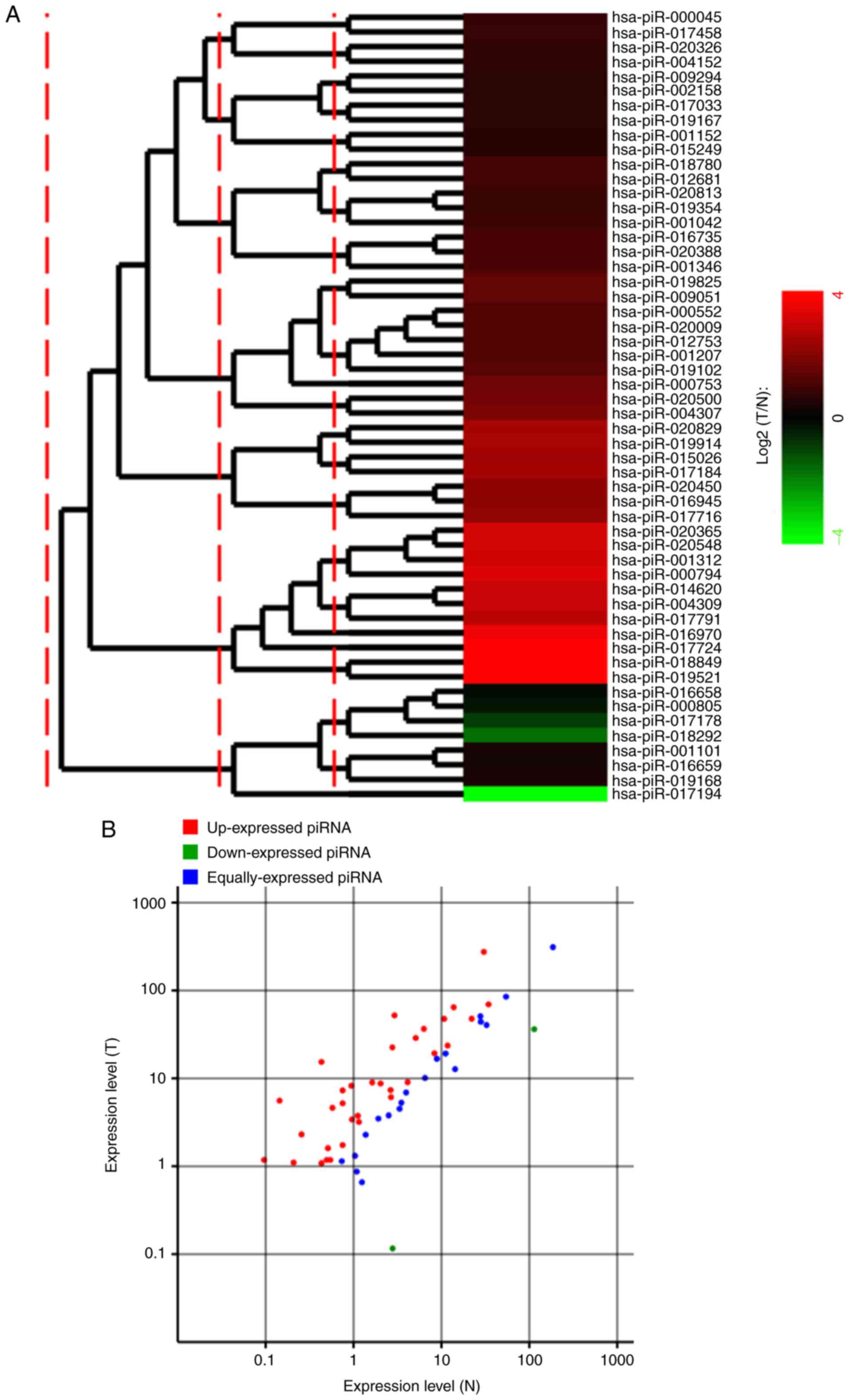
Differentially-expressed piRNAs
between CRC and adjacent non-tumor tissues
Comparison of the expression levels of piRNAs in CRC
and adjacent non-tumor tissues is beneficial for understanding the
roles of piRNAs in the pathogenesis of CRC. In the present study, a
total of 367 unique piRNA reads in the N library and 423 unique
piRNA reads in the T library belonged to 141 registered piRNAs.
Among these piRNAs, 87 piRNAs were excluded due <1.0 normalized
expression values in both libraries. Therefore, 54 piRNAs were
selected for further differential expression analysis. As depicted
in Fig. 3, a total of 35
differentially-expressed piRNAs were identified, of which 33 piRNAs
were upregulated in CRC tissues, whereas only 2 piRNAs were
downregulated in CRC tissues. These differentially-expressed piRNAs
are detailed in Table V.
 | Table V.Differentially expressed piRNAs
between colorectal cancer and adjacent non-tumor tissues by small
RNA sequencing. |
Table V.
Differentially expressed piRNAs
between colorectal cancer and adjacent non-tumor tissues by small
RNA sequencing.
| piR-name | N-normalized
expression | T-normalized
expression | Log2(T/N) | Regulation |
|---|
| hsa-piR-18849 | 0.1439 | 5.5730 | 5.28 | Up |
| hsa-piR-19521 | 0.4318 | 15.3838 | 5.15 | Up |
| hsa-piR-17724 | 2.9265 | 52.0728 | 4.15 | Up |
| hsa-piR-16970 | 0.0960 | 1.1804 | 3.62 | Up |
| hsa-piR-794 | 0.7516 | 7.2952 | 3.28 | Up |
| hsa-piR-20365 | 30.4165 | 275.2446 | 3.18 | Up |
| has-piR-20548 | 0.2559 | 2.3027 | 3.17 | Up |
| hsa-piR-1312 | 0.9435 | 8.2434 | 3.13 | Up |
| hsa-piR-4309 | 2.7666 | 22.5436 | 3.03 | Up |
| hsa-piR-14620 | 0.5757 | 4.6248 | 3.01 | Up |
| hsa-piR-17791 | 0.7516 | 5.2053 | 2.79 | Up |
| hsa-piR-19914 | 6.3168 | 36.5922 | 2.53 | Up |
| has-piR-20829 | 5.1014 | 28.8132 | 2.50 | Up |
| hsa-piR-17184 | 1.6312 | 8.9787 | 2.46 | Up |
| hsa-piR-15026 | 0.2079 | 1.1030 | 2.41 | Up |
| hsa-piR-17716 | 13.8010 | 64.4379 | 2.22 | Up |
| hsa-piR-20450 | 10.7625 | 47.6028 | 2.15 | Up |
| hsa-piR-16945 | 2.0310 | 8.7465 | 2.11 | Up |
| hsa-piR-4307 | 0.9595 | 3.4057 | 1.83 | Up |
| hsa-piR-20500 | 1.1194 | 3.7540 | 1.75 | Up |
| hsa-piR-753 | 0.5117 | 1.6061 | 1.65 | Up |
| hsa-piR-9051 | 2.6387 | 7.3726 | 1.48 | Up |
| hsa-piR-19825 | 1.1514 | 3.1929 | 1.47 | Up |
| hsa-piR-19102 | 0.4318 | 1.0836 | 1.33 | Up |
| hsa-piR-1207 | 0.4957 | 1.1804 | 1.25 | Up |
| hsa-piR-552 | 0.7516 | 1.7416 | 1.21 | Up |
| hsa-piR-20009 | 8.3318 | 19.2927 | 1.21 | Up |
| hsa-piR-12753 | 2.6706 | 6.1148 | 1.20 | Up |
| hsa-piR-1346 | 4.1259 | 9.0368 | 1.13 | Up |
| hsa-piR-20388 | 0.5437 | 1.1804 | 1.12 | Up |
| hsa-piR-16735 | 22.1007 | 47.7189 | 1.11 | Up |
| hsa-piR-12681 | 34.3825 | 69.5271 | 1.02 | Up |
| hsa-piR-18780 | 11.7700 | 23.6079 | 1.00 | Up |
| hsa-piR-18292 | 113.846 | 36.2632 | −1.65 | Down |
| hsa-piR-17194 | 2.7826 | 0.1161 | −4.58 | Down |
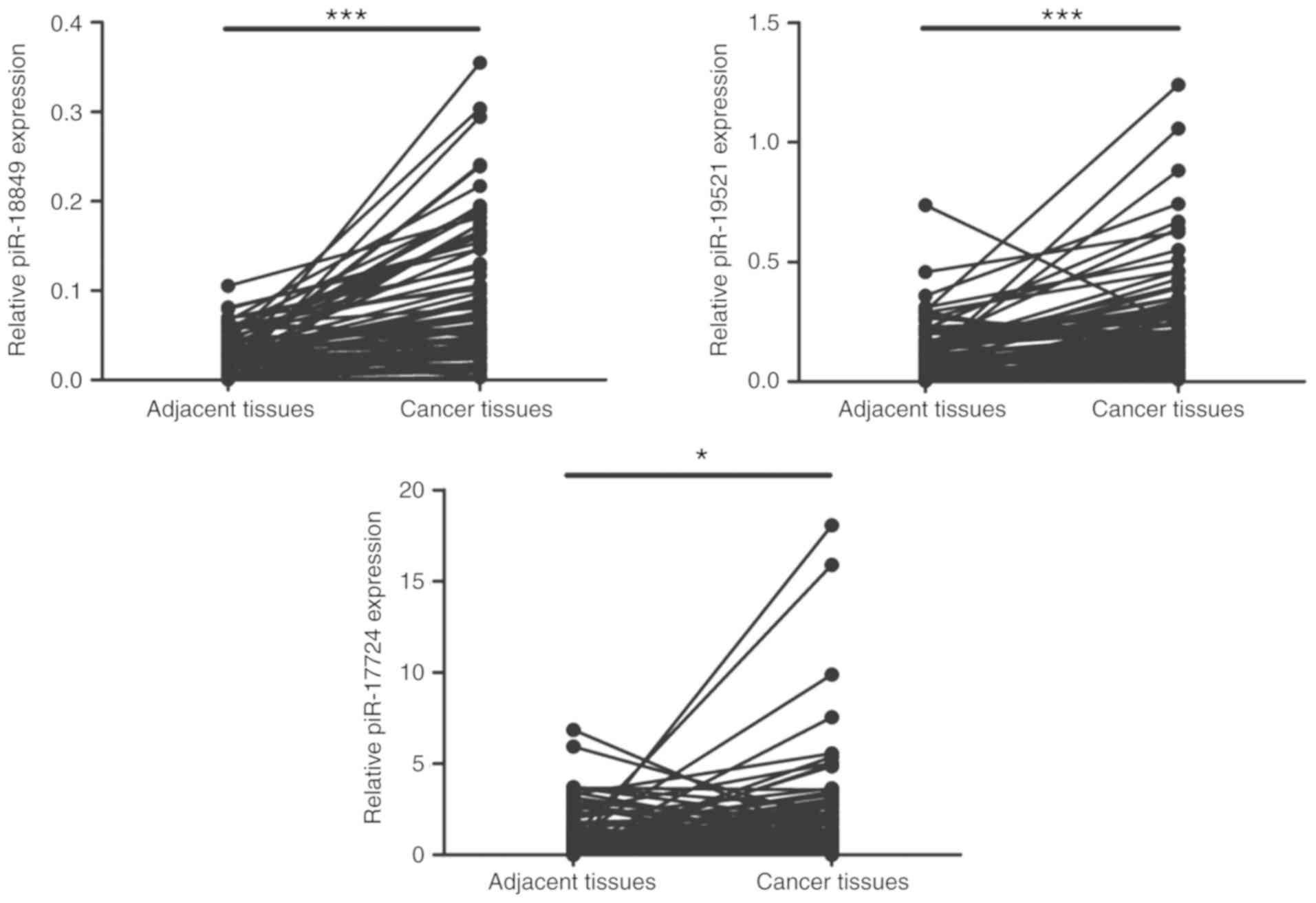
RT-qPCR validation
Since the vast majority of piRNAs were upregulated
in CRC, the focus was on these upregulated piRNAs and the top three
upregulated piRNAs (piR-18849, piR-19521 and piR-17724) were
selected for further validation in 80 matched pairs of CRC and
adjacent non-tumor tissues by RT-qPCR. The relative expression
levels of these three piRNAs, calculated using the 2−∆Cq
method, are depicted in Table VI.
Consistent with the results from small RNA sequencing, the RT-qPCR
results demonstrated that the expression levels of piR-18849,
piR-19521 and piR-17724 were consistently increased in CRC tissues,
compared with adjacent tissues (P<0.05; Fig. 4). Among these three piRNAs,
piR-18849 was expressed at the lowest level but had the most
notable difference between these two sets of samples. Notably, the
fold changes from the RT-qPCR results were less than those from
small RNA sequencing, which may be due to the increased sensitivity
of deep sequencing.
 | Table VI.Relative expression levels of the top
three upregulated piRNAs in colorectal cancer. |
Table VI.
Relative expression levels of the top
three upregulated piRNAs in colorectal cancer.
|
| Relative expression
level |
|
|---|
|
|
|
|
|---|
| Name | Adjacent
tissues | Colorectal cancer
tissues | P-value |
|---|
| hsa-piR-18849 | 0.02
(0.01-0.04) | 0.06
(0.03-0.12) | <0.001 |
| hsa-piR-19521 | 0.10
(0.04-0.17) | 0.19
(0.09-0.30) | <0.001 |
| hsa-piR-17724 | 0.63
(0.24-1.39) | 0.92
(0.34-2.33) | <0.05 |
Association between the expression of
piRNAs and clinicopathological features
To clarify the function of the three piRNAs in the
onset and progression of CRC, the correlation of their expression
with clinicopathological characteristics of patients with CRC was
analyzed. As depicted in Table II,
the expression of piR-18849 was positively correlated with lymph
node metastasis potential and negatively correlated with the degree
of tumor differentiation; additionally, CRC with poor
differentiation and high lymph node metastasis had significantly
increased levels of piR-18849 (P<0.05). The expression of
piR-19521 was only negatively correlated with the degree of tumor
differentiation (P=0.001). However, piR-17742 expression levels did
not correlate with any clinicopathological features.
Discussion
It is well known that ncRNAs, as key molecules
regulating gene expression, are widely distributed in various
tissues (35–37). Aberrant expression of ncRNAs is
associated with numerous human disorders (38–40). A
large number of studies demonstrated that lncRNAs and miRNAs are
implicated in a variety of tumor types and may serve as potential
therapeutic targets or diagnostic markers for these tumor types,
including CRC (41–44). The rapid development of the
second-generation deep sequencing technology has provided an
unprecedented platform to comprehensively analyze non-coding
transcriptomes as well as reveal a number of novel non-coding
transcripts in various tissues, organs and disease models.
Furthermore, second-generation sequencing has high sensitivity,
which can avoid some minor differentially-expressed RNAs being
missed. In view of the advantages of second-generation sequencing
and our shortage of funds, studies from Huang et al
(45), Wang et al (46) and Zhang et al (47) were referred to and only 3 pairs of
CRC tissues and adjacent tissues were used for deep sequencing of
small RNAs (≤40 nt). The present data demonstrated that only 11.90%
of small RNAs were shared by CRC and adjacent tissues, indicating
that the expression patterns of small RNAs in CRC and adjacent
tissues were notably different. These observations further implied
that small RNAs may be involved in colorectal carcinogenesis.
To date, the expression patterns of miRNAs and
lncRNAs in tumors have been extensively investigated (48,49).
However, piRNAs, as a class of newly identified small ncRNAs, and
the expression patterns of piRNAs in tumors remain largely unknown,
and the field remains in its infancy. Numerous studies demonstrated
that a number of piRNAs are dysregulated in tumor types, including
gastric cancer, multiple myeloma and bladder cancer, and these
piRNAs are also involved in the onset and progression of these
tumor types (25,28,29).
However, the majority of these studies only focused on a particular
piRNA or profiled piRNA expression patterns in tumor cell lines,
but not in tumor tissues. Based on the current research status, the
differences in piRNA expression patterns in CRC and adjacent
non-tumor tissues were investigated using deep sequencing. The
present results demonstrated that only low proportions of unique
piRNA reads (0.12% vs. 0.13%) and total piRNA reads (0.15% vs.
0.07%) were identified in both the N and T libraries, indicating
that the types of piRNAs were few and that their expression levels
were also low in CRC and adjacent non-tumor tissues. Data from Yang
et al (50) demonstrated
that in normal human testis tissues, 25,845 unique reads
corresponding to 1,051,404 total reads were matched to known piRNA
sequences. Another study by Girard et al (18) identified 52,099 piRNAs in human
testes. The aforementioned data indicate that despite piRNAs being
expressed in somatic cells, including normal somatic cells and
malignant cells, there are fewer types and their expression is
reduced in somatic cells, compared with germ cells. The results
were expected since piRNAs and Piwi are known to be germ
cell-specific and serve key roles in germ cell development,
stemness maintenance, meiosis and spermatogenesis (51).
It is notable that although piRNAs had fewer types
and lower expression compared with miRNAs in CRC and adjacent
tissues, the overall expression levels were notably different
between these two types of tissues, indicating that piRNAs are
dysregulated in CRC and further supporting that piRNAs are
implicated in tumorigenesis (52).
These data also indicate that low levels of piRNAs are sufficient
to generate notable biological effects, similar to lncRNAs
(53).
The signal transduction pathway and epigenetic
status in tumors are similar to those in stem cells, indicating
that tumors are an aberrant stem-like state (54). Therefore, Piwi and piRNAs that are
highly enriched in germline stem cells may also be expressed in
tumor cells. Indeed, studies disclosed that Piwi is overexpressed
in all detected tumor types, as well as associated with tumor
prognosis (55–58). Consistent with the aforementioned
data, the present small RNA sequencing results demonstrated that
the overall expression levels of piRNAs in CRC were increased
compared with adjacent non-tumor tissues. Furthermore, among 35
differentially-expressed piRNAs, 33 piRNAs were upregulated in CRC,
indicating that the majority of piRNAs in CRC may serve
tumor-driving roles, which is consistent with the role of Piwi in
tumors. RT-qPCR further demonstrated that the expression levels of
the top three upregulated piRNAs, piR-18849, piR-19521 and
piR-17724, were increased in CRC, compared with adjacent non-tumor
tissues. Notably, the increased levels of piR-18849 and piR-19521
were significantly correlated with a poorer degree of
differentiation. This may be because piRNAs are highly enriched in
germline stem cells (51), and
tumor cells with a poorer degree of differentiation are more
similar to stem cells (59,60). Therefore, as the degree of tumor
differentiation decreases, the expression of piRNAs may
increase.
In breast cancer, the upregulation of piR-4987 was
associated with lymph node metastasis (45). The present study determined that in
addition to the degree of tumor differentiation, the overexpression
of piR-18849 was also associated with lymph node metastasis in
patients with CRC. piRNAs thus may emerge not only as a potential
therapeutic target but also as an indicator for prognosis in
patients with CRC. However, in the present study, the prognostic
value of identified piRNAs could not be verified due to the short
follow-up period of patients recruited to the study. Additionally,
the specific function of these piRNAs and their roles in the
survival or prognosis of patients will be investigated in
subsequent studies.
Collectively, to the best of our knowledge, the
present study presented, for the first time, global piRNA
expression profiles in CRC and adjacent non-tumor tissues by deep
sequencing for small RNAs. Based on the small RNA sequencing data,
it was determined that the overall expression levels of piRNAs in
CRC tissues were increased, compared with adjacent tissues,
implying that piRNAs may be involved in colorectal tumorigenesis.
These observations will provide a theoretical basis for
piRNA-targeted therapeutic strategies for CRC. However, only 3
pairs of samples were used for deep sequencing in the present
study, which may cause a number of notable piRNAs to be omitted due
to the limited samples. Another notable question was that the focus
was only on those upregulated piRNAs, instead of those
downregulated piRNAs. Therefore, determining what causes
downregulation of a number of piRNAs in CRC, whether the
downregulation of piRNAs is active or passive, and what roles these
downregulated piRNAs serve in CRC will help the comprehensive
understanding of the function of piRNAs in CRC.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos.81702324 and 81602529), the
Natural Science Foundation of Hebei Province, China (grant no.
H2017206141) and the Post-graduate's Innovation Fund Project of
Hebei Province (grant no. CXZZBS2017103).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY, WQ and HQJ conceived and designed the
experiments. JY and CGJ conducted the experiments. DXZ, QD and JH
recruited patients and collected samples as well as patients'
clinicopathological information. JY, WQ, XLX and XYJ analyzed and
interpreted the data. JY drafted the manuscript. HQJ and XYJ
revised the manuscript. HQJ supervised the whole project. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second Hospital of Hebei Medical University
(Shijiazhuang, China). All patients provided written informed
consent prior to participation in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
piRNA
|
Piwi-interacting RNA
|
|
CRC
|
colorectal cancer
|
|
ncRNA
|
non-coding RNA
|
|
miRNA
|
microRNA
|
|
lncRNA
|
long non coding RNA
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Howlader N, Noone A, Krapcho M, Miller D,
Bishop K, Altekruse S, Kosary C, Yu M, Ruhl J and Tatalovich Z:
SEER cancer statistics review. 1975-2013, National Cancer
Institute; Bethesda, MD: https://seer.cancer.gov/archive/csr/1975_2013April.
2016
|
|
4
|
Herszenyi L and Tulassay Z: Epidemiology
of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci.
14:249–258. 2010.PubMed/NCBI
|
|
5
|
Lollini PL, De Giovanni C, Nicoletti G, Di
Carlo E, Musiani P, Nanni P and Forni G: Immunoprevention of
colorectal cancer: A future possibility? Gastroenterol Clin North
Am. 31:1001–1014. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marmol I, Sanchez-de-Diego C, Pradilla
Dieste A, Cerrada E and Rodriguez Yoldi MJ: Colorectal carcinoma: A
general overview and future perspectives in colorectal cancer. Int
J Mol Sci. 18:E1972017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ashktorab H, Daremipouran M, Goel A, Varma
S, Leavitt R, Sun X and Brim H: DNA methylome profiling identifies
novel methylated genes in African American patients with colorectal
neoplasia. Epigenetics. 9:503–512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ashktorab H, Rahi H, Wansley D, Varma S,
Shokrani B, Lee E, Daremipouran M, Laiyemo A, Goel A, Carethers JM
and Brim H: Toward a comprehensive and systematic methylome
signature in colorectal cancers. Epigenetics. 8:807–815. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pulford DJ, Falls JG, Killian JK and
Jirtle RL: Polymorphisms, genomic imprinting and cancer
susceptibility. Mutat Res. 436:59–67. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hatziapostolou M and Iliopoulos D:
Epigenetic aberrations during oncogenesis. Cell Mol Life Sci.
68:1681–1702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schwanhausser B, Busse D, Li N, Dittmar G,
Schuchhardt J, Wolf J, Chen W and Selbach M: Global quantification
of mammalian gene expression control. Nature. 473:337–342. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anastasiadou E, Jacob LS and Slack FJ:
Non-coding RNA networks in cancer. Nat Rev Cancer. 18:5–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taft RJ, Pang KC, Mercer TR, Dinger M and
Mattick JS: Non-coding RNAs: Regulators of disease. J Pathol.
220:126–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Smet EG, Mestdagh P, Vandesompele J,
Brusselle GG and Bracke KR: Non-coding RNAs in the pathogenesis of
COPD. Thorax. 70:782–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goodrich JA and Kugel JF: From bacteria to
humans, chromatin to elongation, and activation to repression: The
expanding roles of noncoding RNAs in regulating transcription. Crit
Rev Biochem Mol Biol. 44:3–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aravin A, Gaidatzis D, Pfeffer S,
Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ,
Kuramochi-Miyagawa S, Nakano T, et al: A novel class of small RNAs
bind to MILI protein in mouse testes. Nature. 442:203–207. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Girard A, Sachidanandam R, Hannon GJ and
Carmell MA: A germline-specific class of small RNAs binds mammalian
Piwi proteins. Nature. 442:199–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aravin AA, Sachidanandam R, Bourc'his D,
Schaefer C, Pezic D, Toth KF, Bestor T and Hannon GJ: A piRNA
pathway primed by individual transposons is linked to de novo DNA
methylation in mice. Mol Cell. 31:785–799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aravin AA and Bourc'his D: Small RNA
guides for de novo DNA methylation in mammalian germ cells. Genes
Dev. 22:970–975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Esposito T, Magliocca S, Formicola D and
Gianfrancesco F: piR_015520 belongs to Piwi-associated RNAs
regulates expression of the human melatonin receptor 1A gene. PLoS
One. 6:e227272011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vella S, Gallo A, Lo Nigro A, Galvagno D,
Raffa GM, Pilato M and Conaldi PG: PIWI-interacting RNA (piRNA)
signatures in human cardiac progenitor cells. Int J Biochem Cell
Biol. 76:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Law PT, Qin H, Ching AK, Lai KP, Co NN, He
M, Lung RW, Chan AW, Chan TF and Wong N: Deep sequencing of small
RNA transcriptome reveals novel non-coding RNAs in hepatocellular
carcinoma. J Hepatol. 58:1165–1173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng J, Guo JM, Xiao BX, Miao Y, Jiang Z,
Zhou H and Li QN: piRNA, the new non-coding RNA, is aberrantly
expressed in human cancer cells. Clin Chim Acta. 412:1621–1625.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng J, Deng H, Xiao B, Zhou H, Zhou F,
Shen Z and Guo J: piR-823, a novel non-coding small RNA,
demonstrates in vitro and in vivo tumor suppressive activity in
human gastric cancer cells. Cancer Lett. 315:12–17. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hashim A, Rizzo F, Marchese G, Ravo M,
Tarallo R, Nassa G, Giurato G, Santamaria G, Cordella A, Cantarella
C and Weisz A: RNA sequencing identifies specific PIWI-interacting
small non-coding RNA expression patterns in breast cancer.
Oncotarget. 5:9901–9910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng L, Song L, Liu C, Lv X, Li X, Jie J,
Zhao D and Li D: piR-55490 inhibits the growth of lung carcinoma by
suppressing mTOR signaling. Tumour Biol. 37:2749–2756. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan H, Wu QL, Sun CY, Ai LS, Deng J, Zhang
L, Chen L, Chu ZB, Tang B, Wang K, et al: piRNA-823 contributes to
tumorigenesis by regulating de novo DNA methylation and
angiogenesis in multiple myeloma. Leukemia. 29:196–206. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chu H, Hui G, Yuan L, Shi D, Wang Y, Du M,
Zhong D, Ma L, Tong N, Qin C, et al: Identification of novel piRNAs
in bladder cancer. Cancer Lett. 356:561–567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mei Y, Wang Y, Kumari P, Shetty AC, Clark
D, Gable T, MacKerell AD, Ma MZ, Weber DJ, Yang AJ, et al: A
piRNA-like small RNA interacts with and modulates p-ERM proteins in
human somatic cells. Nat Commun. 6:73162015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Langmead B, Trapnell C, Pop M and Salzberg
SL: Ultrafast and memory-efficient alignment of short DNA sequences
to the human genome. Genome Biol. 10:R252009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Williams Z, Morozov P, Mihailovic A, Lin
C, Puvvula PK, Juranek S, Rosenwaks Z and Tuschl T: Discovery and
characterization of piRNAs in the human fetal ovary. Cell Rep.
13:854–863. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaikkonen MU, Lam MT and Glass CK:
Non-coding RNAs as regulators of gene expression and epigenetics.
Cardiovasc Res. 90:430–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun Y, Koo S, White N, Peralta E, Esau C,
Dean NM and Perera RJ: Development of a micro-array to detect human
and mouse microRNAs and characterization of expression in human
organs. Nucleic Acids Res. 32:e1882004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sasaki YT, Sano M, Ideue T, Kin T, Asai K
and Hirose T: Identification and characterization of human
non-coding RNAs with tissue-specific expression. Biochem Biophys
Res Commun. 357:991–996. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Almeida RA, Fraczek MG, Parker S,
Delneri D and O'Keefe RT: Non-coding RNAs and disease: The
classical ncRNAs make a comeback. Biochem Soc Trans. 44:1073–1078.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mestdagh P, Vandesompele J, Brusselle G
and Vermaelen K: Non-coding RNAs and respiratory disease. Thorax.
70:388–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang H, Chen Z, Wang X, Huang Z, He Z and
Chen Y: Long non-coding RNA: A new player in cancer. J Hematol
Oncol. 6:372013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ma Y, Yang Y, Wang F, Moyer MP, Wei Q,
Zhang P, Yang Z, Liu W, Zhang H, Chen N, et al: Long non-coding RNA
CCAL regulates colorectal cancer progression by activating
Wnt/β-catenin signalling pathway via suppression of activator
protein 2α. Gut. 65:1494–1504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yin Y, Zhang B, Wang W, Fei B, Quan C,
Zhang J, Song M, Bian Z, Wang Q, Ni S, et al: miR-204-5p inhibits
proliferation and invasion and enhances chemotherapeutic
sensitivity of colorectal cancer cells by downregulating RAB22A.
Clin Cancer Res. 20:6187–6199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang G, Hu H, Xue X, Shen S, Gao E, Guo
G, Shen X and Zhang X: Altered expression of piRNAs and their
relation with clinicopathologic features of breast cancer. Clin
Transl Oncol. 15:563–568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang H, Zhao Y, Chen M and Cui J:
Identification of novel long non-coding and circular RNAs in human
papillomavirus- mediated cervical cancer. Front Microbiol.
8:17202017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Z, Song N, Wang Y, Zhong J, Gu T,
Yang L, Shen X, Li Y, Yang X, Liu X, et al: Analysis of
differentially expressed circular RNAs for the identification of a
coexpression RNA network and signature in colorectal cancer. J Cell
Biochem. 120:6409–6419. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen Z, Li J, Tian L, Zhou C, Gao Y, Zhou
F, Shi S, Feng X, Sun N, Yao R, et al: MiRNA expression profile
reveals a prognostic signature for esophageal squamous cell
carcinoma. Cancer Lett. 350:34–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Su X, Malouf GG, Chen Y, Zhang J, Yao H,
Valero V, Weinstein JN, Spano JP, Meric-Bernstam F, Khayat D and
Esteva FJ: Comprehensive analysis of long non-coding RNAs in human
breast cancer clinical subtypes. Oncotarget. 5:9864–9876. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang Q, Hua J, Wang L, Xu B, Zhang H, Ye
N, Zhang Z, Yu D, Cooke HJ, Zhang Y and Shi Q: MicroRNA and piRNA
profiles in normal human testis detected by next generation
sequencing. PLoS One. 8:e668092013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Thomson T and Lin H: The biogenesis and
function of PIWI proteins and piRNAs: Progress and prospect. Annu
Rev Cell Dev Biol. 25:355–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ng KW, Anderson C, Marshall EA, Minatel
BC, Enfield KS, Saprunoff HL, Lam WL and Martinez VD:
Piwi-interacting RNAs in cancer: Emerging functions and clinical
utility. Mol Cancer. 15:52016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dick JE: Stem cell concepts renew cancer
research. Blood. 112:4793–4807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu X, Sun Y, Guo J, Ma H, Li J, Dong B,
Jin G, Zhang J, Wu J, Meng L and Shou C: Expression of hiwi gene in
human gastric cancer was associated with proliferation of cancer
cells. Int J Cancer. 118:1922–1929. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
He W, Wang Z, Wang Q, Fan Q, Shou C, Wang
J, Giercksky KE, Nesland JM and Suo Z: Expression of HIWI in human
esophageal squamous cell carcinoma is significantly associated with
poorer prognosis. BMC Cancer. 9:4262009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jiang J, Zhang H, Tang Q, Hao B and Shi R:
Expression of HIWI in human hepatocellular carcinoma. Cell Biochem
Biophys. 61:53–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Qiao D, Zeeman AM, Deng W, Looijenga LH
and Lin H: Molecular characterization of hiwi, a human member of
the piwi gene family whose overexpression is correlated to
seminomas. Oncogene. 21:3988–3999. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hassiotou F and Geddes D: Anatomy of the
human mammary gland: Current status of knowledge. Clin Anat.
26:29–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gisina AM, Lupatov AY, Karalkin PA,
Mainovskaya OA, Petrov LO, Sidorov DV, Yarygin VN and Yarygin KN:
Detection of minor subpopulations of colorectal adenocarcinoma
cells expressing cancer stem cell markers. Bull Exp Biol Med.
151:234–238. 2011. View Article : Google Scholar : PubMed/NCBI
|