Introduction
Breast cancer has the highest cancer incidence rate
among women in the USA and it is also the second leading cause of
cancer-associated mortality in women following lung cancer
(1). The American Cancer Society
(ACS) conducted a statistical analysis of the incidence and
mortality rates of breast cancer in the USA, which revealed that
although the incidence rate is increasing, the mortality rate is
declining (2). The study identified
that the reduction in breast cancer-associated mortality may be
attributed to both improvements in treatment, including the
development of adjuvant chemotherapy and hormonal therapy in the
1980s, and targeted therapies in the 1990s, as well as the
popularization of breast cancer screening (2). The ACS statistics have also demonstrated
that in the USA, 74% of breast cancer cases are hormone receptor
(HR)+/human epidermal receptor (HER2)−
(luminal A) and 12% are HR−/HER2−
[triple-negative breast cancer (TNBC)], which indicates that TNBC
accounts for a relatively high proportion of cases among the
different molecular classifications of breast cancer (2). In China, breast cancer has the highest
incidence rate among all cancer types among females; however, the
mortality rate is second following lung cancer (3).
According to different expression levels of estrogen
receptor (ER), progesterone receptor (PR), HER2 and Ki-67, breast
cancer can be divided into four different types: Luminal A, luminal
B, HER2-positive and TNBC (4).
Luminal A and B type breast cancers are positive for HRs;
therefore, adjuvant endocrine therapy can be used for treatment
(4,5).
HER2-overexpressing breast cancers can be treated with targeted
therapy, including trastuzumab (4,5). However,
for TNBC, as there is no targeted therapy index, adjuvant
chemotherapy is commonly used for treatment (4,5).
Therefore, the study of the targeted therapy index of TNBC is of
utmost clinical significance.
Obesity and being overweight are risk factors for
breast cancer (6). Metabolic syndrome
has been confirmed as a risk factor for breast cancer among
post-menopausal women and is defined as having at least three of
the following: Abdominal obesity, hyperlipidemia, high levels of
low-density lipoprotein, high blood glucose and high blood pressure
(7). According to the National
Enhanced Cancer Surveillance System in Canada, a high-cholesterol
diet can increase breast cancer incidence by 48% in post-menopausal
women (8). As for pre-menopausal
women, low levels of high-density lipoprotein (HDL) can also
increase the incidence of breast cancer (9,10). In
animal experiments, a high level of cholesterol has been shown to
promote the growth and metastasis of mammary tumors (11). Nelson et al (12) demonstrated that a metabolite termed
27-hydroxycholesterol (27HC) was produced following cholesterol
metabolism. Their study also identified that sterol 27-hydroxylase,
which converts cholesterol into 27HC, was highly expressed in
breast cancer; In addition, 27HC can activate two receptors [ER and
liver X receptor (LXR)] in ER+ breast cancer cells to
promote their growth and dissemination. Through animal experiments,
Nelson et al (12) also
revealed that the growth of ER+ breast cancer relies on
ER activation and the activation of LXR promotes cancer metastasis.
This previous study demonstrated that the metabolism of cholesterol
plays an important role in HR-positive breast cancer. However, to
the best of our knowledge, the effects of cholesterol metabolism in
HR-negative TNBC remain to be investigated.
Cholesterol is a major component of the cell
membrane. As important carriers of cholesterol metabolism,
ATP-binding cassette transporter number 1 (ABCA1) and ATP-binding
cassette sub-family G number 1 (ABCG1) can increase the levels of
HDL and decrease intermediate-density lipoprotein to promote
cholesterol metabolism and transport from cells (13). ABCA1 and ABCG1 are regulated by the
lipid metabolism homeostasis regulator, LXR. An LXR agonist can
increase the expression levels of ABCA1 and ABCG1. By contrast, an
LXR inhibitor can downregulate the expression levels of ABCA1 and
ABCG1 (14,15). LXR mediates cholesterol out-flow
through the ABCA1/apolipoprotein A1/HDL or ABCG1/HDL pathway
(16,17).
LXR has two subtypes: LXR-α and LXR-β. LXR-α is
highly expressed in adipose tissue, the liver, adrenal glands,
lungs and the gastrointestinal tract, while LXR-β is widely
expressed (18). ABCA1 is highly
expressed in normal mammary epithelial cells and neoplastic breast
tissues. ABCA1 is also associated with lymph node metastasis in
breast cancer (19). The present
study detected the expression levels of LXR-α, LXR-β, ABCA1 and
ABCG1 in TNBC tissues and in non-cancerous mammary tissues in
preliminary experiments (data not shown). LXR-α did not localize to
the cell nucleus; therefore, the current study investigated the
expression of LXR-β, ABCA1 and ABCG1, which were associated with
cholesterol metabolism in the TNBC tissues and in the non-cancerous
mammary tissues.
Materials and methods
Clinical samples
A total of 96 TNBC tissue chips embedded in paraffin
were obtained from Guilin Fanpu Biotech, Inc. In addition, the
present study obtained 20 paraffin-embedded non-cancerous mammary
tissue samples, 10 clinical samples of TNBC tissue and 5 clinical
samples of non-cancerous mammary tissue, which were stored at
−80°C. Both types of tissues were provided by the Department of
Pathology, Traditional Chinese Medicine Hospital of Wenling. All
patients were female, aged 29 to 79 years and of Han ethnicity. The
specimens were collected from January, 2005 to December, 2015.
Patients with recurrent tumors were excluded. The Ethics Board of
the Traditional Chinese Medicine Hospital of Wenling reviewed and
approved the experimental protocol for assaying the surgical
materials and written informed consent was obtained from the
patients.
Immunohistochemistry (IHC)
Paraffin-embedded tissue samples were sectioned
(serial 4-µm-thick sections), baked, dewaxed and hydrated. Antigen
retrieval was performed with Tris-EDTA (pH 9.0) at a high pressure
for 6 min. The sections with nuclear expression were treated for 30
min using 0.3% Triton solution at 37°C to permeabilize the
membranes. Primary antibodies were diluted in PBS and incubated
overnight at 4°C with the tissue sections. The following antibodies
were used: Anti-LXR-β (1:100; rabbit polyclone; cat. no. SC-1001;
Santa Cruz Biotechnology, Inc.), anti-ABCA1 (1:250; mouse
monoclonal; cat. no. ab18180; Abcam) and anti-ABCG1 (1:250; mouse
monoclonal; cat. no. ab52617; Abcam). PBS was used instead of the
primary antibody as a negative control. The sections were then
incubated with horseradish peroxidase (HRP)-conjugated second
antibody (Dako REAL EnVision Detection System, cat. no. K4063,
Agilent) for 30 min at room temperature. The sections were stained
with 3,3′-diaminobenzidine for 2–10 sec at room temperature and
then completely rinsed with water. In addition, hematoxylin
staining for 3 min at room temperature was performed prior to
washing with water. Differentiation for 2 sec was performed using
1% hydrochloric acid in alcohol. Lithium carbonate was added as a
blue stain for 30 sec and then dehydrated. The sections were
treated with clearing agents to ensure transparency before neutral
resins were used for sealing.
Quantum dot-based IHC (QD-IHC)
Until the secondary antibody was added, the protocol
for QD-IHC was the same as for that described above for IHC. The
biotinylated goat anti-rabbit or anti-murine IgG (1:200; cat. no.
111-065-006; Jackson ImmunoResearch) was diluted in 2% bovine serum
albumin (BSA; Gibco; Thermo Fisher Scientific, Inc.) and incubated
for 30 min at room temperature prior to blocking for 20 min in 2%
BSA. Quantum dot-conjugated streptavidin probes (QD-SA) (1:400;
QS605; Wuhan Jianyuan Quantum Dots Co., Ltd.) diluted in 2% BSA
were dripped onto the sections and incubated for 1 h in a 37°C
incubator, as previously described (20,21). The
sections were sealed with 90% glycerol buffer.
The same ranking criteria were used for IHC and
QD-IHC, and a double-blind experiment was performed for both. Two
senior pathological experts observed the sections under ×200
magnification and the positive area (PA) was calculated. The PA
ranking was as follows: 0 (PA, ≤20%), 1 (PA, 21–40%), 2 (PA,
41–60%) and 3 (PA >60%). The intensity staining (IS) was
evaluated under high magnification and ranked as follows: 0
(negative), 1 (weak) and 2 (strong). The final results were
determined by intensity distribution (ID), where ID=PA × IS. An ID
≤2 represented a negative or weak expression, while an ID >2
represented a strong expression (21). The criteria were suitable for LXR-β,
ABCA1 and ABCG1 indices. A high expression was a strong expression,
a low expression was a weak expression and a negative expression
was described as negative.
Extraction of tissue proteins
Breast tissues obtained from surgery that were
stored at −80°C were cut into sections (1–2 mm thickness).
Subsequently, 500 µl pre-cooled RIPA lysis buffer (Wuhan Kerui
Biotech Co., Ltd.) was added to 100 mg tissue and homogenized with
a homogenizer [Tiangen Biotech (Beijing) Co., Ltd.]. The
homogenates were lysed on ice for 30 min prior to centrifugation
with 12,000 × g for 15 min at 4°C for later use. The protein
concentration was detected using the BCA method (Wuhan Kerui
Biotech Co., Ltd.), according to the manufacturer's protocol.
Western blot analysis
SDS-PAGE (8% resolving gel and 5% stacking gel) was
used to resolve proteins prior to transfer onto polyvinylidene
difluoride membranes (EMD Millipore). The membranes were blocked
for 1 h in 5% skim milk at room temperature prior to incubation
with primary antibody overnight at 4°C. The reference antibody used
was β-actin (Sigma-Aldrich; Merck-KGaA). The primary antibodies
used were the same as for IHC. The antibody dilutions were as
follows: Anti-ABCA1 (1:800), anti-ABCG1 (1:800) and anti-LXR-β
(1:300). The membranes were incubated with the secondary antibody
(1:800; cat. no. 111-065-006; Jackson ImmunoResearch) for 1 h at
room temperature. An enhanced chemiluminescent detection system
(Pierce; Thermo Fisher Scientific, Inc.) was used to examine the
signals, according to the manufacturer's instructions. Quantity One
software (version 4.52; Bio-Rad, Inc.) was used to examine the
densitometry, according to the manufacturer's instructions. This
experiment was repeated 3 times.
Extraction of total RNA from tissue
samples and reverse transcription (RT)
Frozen breast tissue samples obtained from surgery
were sectioned and TRIzol solution (Invitrogen; Thermo Fisher
Scientific, Inc.) was added at 1 ml/100 mg tissue prior to
homogenization. Total RNA was extracted using phenol-chloroform.
RNA was reverse transcribed to cDNA using the RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions.
Quantitative polymerase chain reaction
(qPCR)
qPCR was performed on a 96-well plate with
SYBR-Green Real-time PCR Master mix (Toyobo Life Science),
according to the manufacturer's instructions. Bio-Rad CFX Manger
3.1 software (Bio-Rad Laboratories, Inc.) was used for data
collection. Each experiment was conducted with at least 3
independent replicates. The qPCR conditions were as follows: Two
initial cycles at 95°C for 180 sec and 95°C for 6 sec and 39 cycles
of 60°C for 9 sec and 72°C for 12 sec with the primers (LXR-β
forward, 5′-CAGCGAGTCTTCCAGAAGGG-3′ and reverse,
5′-TGGGCTTGAGGTGTAAGCTG-3′; ABCA1 forward,
5′-GGGAGAGCACAGGCTTTGAC-3′ and reverse, 5′-CACTCACTCTCGCTCGCAA-3′;
ABCG1 forward, 5′-CCTGTCTGATGGCCGCTTT-3′ and reverse,
5′-CACCTCATCCACCGAGACAC-3′; and β-actin forward,
5′-TCACCATGGATGATGATATCGC-3′ and reverse,
5′-GAATCCTTCTGACCCATGCC-3). RT-qPCR quantification was performed
using the 2−ΔΔCq method (22).
Statistical analysis
SPSS 21.0 software (IBM Corp.) and GraphPad Prism
5.0 software (GraphPad Software, Inc.) were used for the analysis.
The χ2 test was used to analyze the differences in the
protein expression levels of LXR-β, ABCA1 and ABCG1 detected by IHC
and QD-IHC between the TNBC and non-cancerous mammary tissues, as
well as to determine the association between ABCA1 immunostaining
and the clinicopathological parameters of the patients with TNBC.
All values for LXR-β, ABCA1 and ABCG1 expression detected by
western blot analysis and RT-qPCR are expressed as the means ±
standard error of the mean. Statistical analysis was performed
using a Student's t-test for 2 groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
A high expression of ABCA1 is
associated with the breast cancer histological grade
IHC and QD-IHC were used to observe the expression
levels of LXR-β, ABCA1 and ABCG1 in the 96 TNBC tissue samples and
the 20 non-cancerous mammary tissue samples (Figs. 1–3). The
results, which are presented in Tables
I and II, demonstrated that the
expression of ABCA1 was significantly higher in the TNBC tissues,
compared with the non-cancerous mammary tissues (P<0.05), while
the expression levels of LXR-β and ABCG1 in the TNBC tissues and
non-cancerous mammary tissues did not exhibit any significant
difference (P>0.05). Evidently, ABCA1 is highly expressed in the
TNBC tissues, which indicates that ABCA1 is a specific marker for
TNBC.
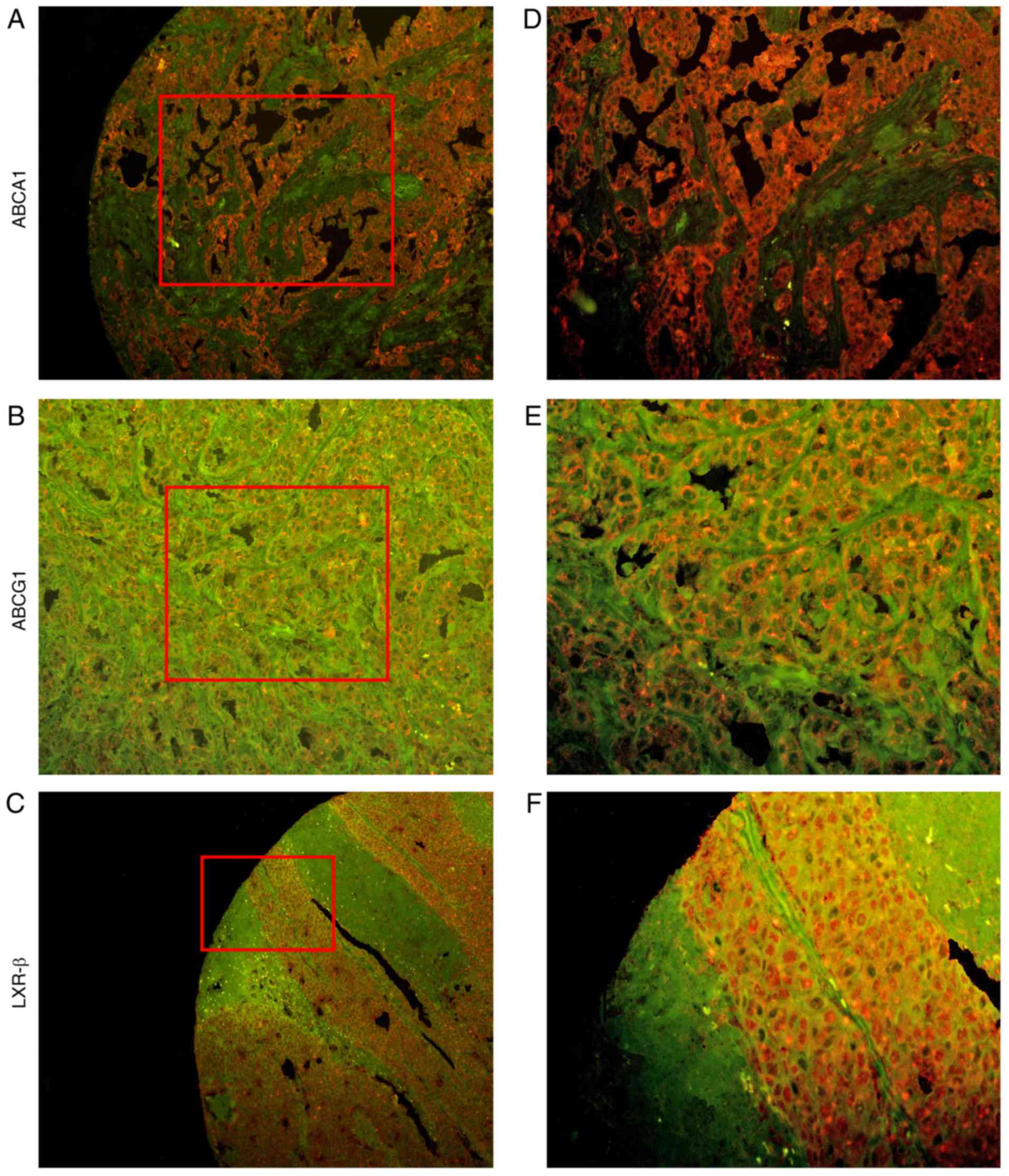
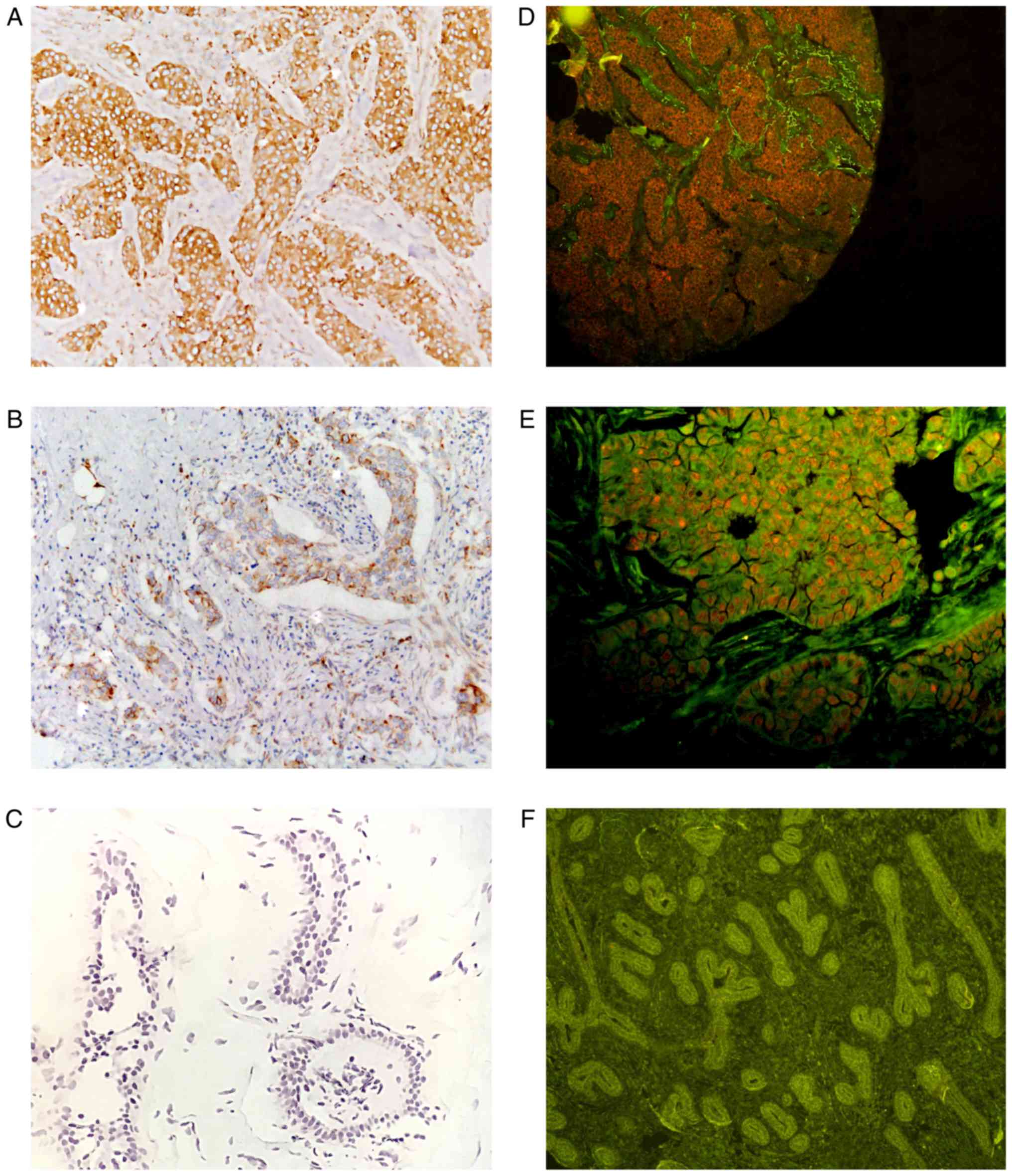 | Figure 1.Staining intensity following IHC and
QD-IHC. (A, B and C) The difference in staining intensity with ×20
magnification following IHC. The brown areas represent positive
expression, while the blue areas represent hematoxylin staining.
(D, E and F) The difference in staining intensity with (D and F)
×10 magnification and (E) ×20 magnification following QD-IHC. The
red areas represent the positive expression of the cells, while the
green areas represent a negative expression. (A and D) Strong
staining (score, 2); (B and E) weak staining (score, 1); (C and F)
negative staining (score, 0). (A, B, D and E) are TNBC tissues,
while (C and F) are non-cancerous mammary tissues. IHC,
immunohistochemistry; QD-IHC, quantum dot-based
immunohistochemistry; TNBC, triple-negative breast cancer. |
 | Table I.Positivity rate in the expression of
LXR-β, ABCA1 and ABCG1 in TNBC tissues and non-cancerous mammary
tissues examined by IHC. |
Table I.
Positivity rate in the expression of
LXR-β, ABCA1 and ABCG1 in TNBC tissues and non-cancerous mammary
tissues examined by IHC.
|
| TNBC tissues | Non-cancerous
tissues |
|
|---|
|
|
|
|
|
|---|
|
| n | High, n (%) | Low, n (%) | n | High, n (%) | Low, n (%) | P-value |
|---|
| LXR-β | 96 | 60 (62.5) | 36 (37.5) | 20 | 8 (40) | 12 (60) | 0.063 |
| ABCA1 | 96 | 62 (64.6) | 34 (35.4) | 20 | 6 (30) | 14 (70) | 0.004 |
| ABCG1 | 96 | 53 (55.2) | 43 (44.8) | 20 | 11 (55) | 9 (45) | 0.986 |
 | Table II.Positivity rate in the expression of
LXR-β, ABCA1, and ABCG1 in TNBC tissues and non-cancerous mammary
tissues examined by QD-IHC. |
Table II.
Positivity rate in the expression of
LXR-β, ABCA1, and ABCG1 in TNBC tissues and non-cancerous mammary
tissues examined by QD-IHC.
|
| Breast cancer
tissues | Non-cancerous
tissues |
|
|---|
|
|
|
|
|
|---|
|
| n | High, n (%) | Low, n (%) | n | High, n (%) | Low, n (%) | P-value |
|---|
| LXR-β | 96 | 63 (65.6) | 33 (34.4) | 20 | 9 (45) | 11 (55) | 0.084 |
| ABCA1 | 96 | 64 (66.7) | 32 (33.3) | 20 | 6 (30) | 14 (70) | 0.002 |
| ABCG1 | 96 | 55 (57.3) | 41 (42.7) | 20 | 12 (60) | 8 (40) | 0.823 |
Statistical analysis was performed according to the
pathological characteristics of the 96 patients. It was identified
that the expression of ABCA1 was associated with the breast cancer
histological grade (Table III; IHC,
P<0.001; QD-IHC, P<0.001). However, age and
tumor-node-metastasis (TNM) stage were not significantly associated
with the expression of ABCA1 (P>0.05).
 | Table III.The association between ABCA1 protein
expression and the clinicopathological parameters of the 96
patients with TNBC. |
Table III.
The association between ABCA1 protein
expression and the clinicopathological parameters of the 96
patients with TNBC.
|
|
| ABCA1 by IHC |
| ABCA1 by
QD-IHC |
|
|---|
|
|
|
|
|
|
|
|---|
| Parameters | n | High (%) | Low (%) | P-value | High (%) | Low (%) | P-value |
|---|
| Age (years) |
|
|
| 0.151 |
|
| 0.749 |
|
<50 | 68 | 47 (49.0) | 21 (21.9) |
| 48 (50.0) | 20 (20.8) |
|
|
≥50 | 28 | 15 (15.6) | 13 (13.5) |
| 19 (19.8) | 9 (9.4) |
|
| T stage |
|
|
| 0.460 |
|
| 0.898 |
| T2 | 72 | 48 (50.0) | 24 (25.0) |
| 50 (52.1) | 22 (22.9) |
|
| T3 +
4 | 24 | 14 (14.6) | 10 (10.4) |
| 17 (17.7) | 7 (7.3) |
|
| N stage |
|
|
| 0.606 |
|
| 0.581 |
| N0 | 57 | 38 (39.6) | 19 (19.8) |
| 41 (42.7) | 16 (16.7) |
|
| N1 +
2 | 39 | 24 (25.0) | 15 (15.6) |
| 26 (27.1) | 13 (13.5) |
|
| Grade |
|
|
| <0.001 |
|
| <0.001 |
| I +
II | 51 | 42 (43.8) | 9 (9.4) |
| 44 (45.8) | 7 (7.3) |
|
|
III | 45 | 20 (20.8) | 25 (26.0) |
| 23 (24.0) | 22 (22.9) |
|
TNBC tissues exhibit higher ABCA1
protein and mRNA expression levels compared with non-cancerous
mammary tissues
Western blot analysis and RT-qPCR were used to
examine the protein and mRNA expression levels of LXR-β, ABCA1 and
ABCG1 in tissues from 10 patients with TNBC and 5 non-cancerous
mammary tissues that were stored at −80°C following surgery
(Fig. 4). It was identified that the
protein and mRNA expression levels of ABCA1 were significantly
higher in the TNBC tissues, compared with the non-cancerous mammary
tissues (P<0.05). LXR-β mRNA was highly expressed in the TNBC
tissues and expressed at low levels in the non-cancerous mammary
tissues, although the difference was not statistically significant
(P>0.05). The mRNA and protein expression levels of LXR-β and
ABCG1 did not exhibit any significant difference between the TNBC
tissues and non-cancerous mammary tissues (P>0.05). Notably, the
mRNA and protein expression levels of LXR-β were high in the breast
cancer tissues; however, no significant difference was observed
compared with the non-cancerous mammary tissues. Thus, these
results demonstrate that ABCA1 is highly expressed in TNBC tissues,
and suggest that ABCA1 may be closely associated with the
occurrence and development of TNBC.
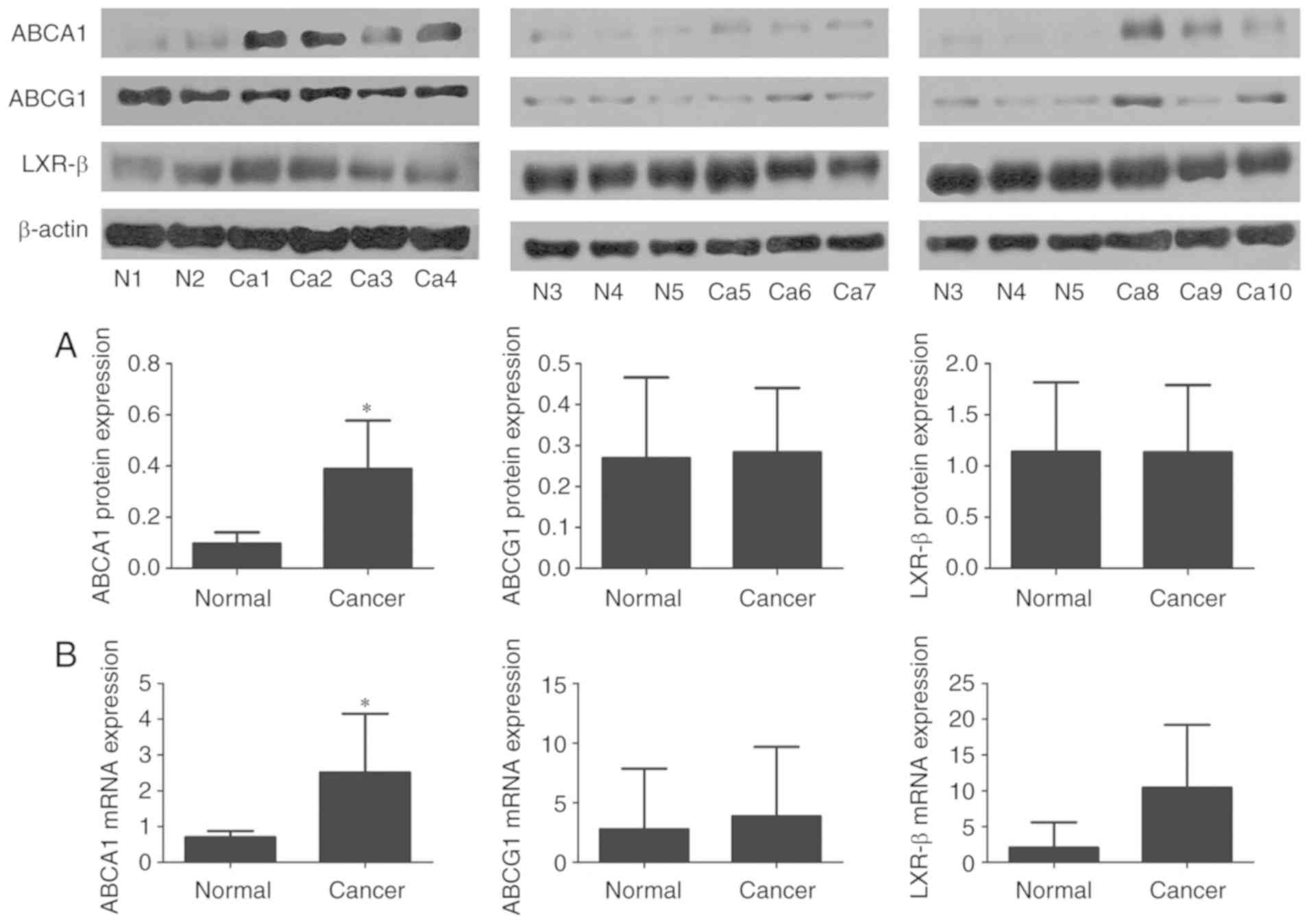 | Figure 4.mRNA and protein expression levels of
ABCA1, ABCG1 and LXR-β in TNBC tissues and non-cancerous mammary
tissues. (A) Protein expression levels. (B) mRNA expression levels.
β-actin served as an internal control. Data are representations of
3 repeated experiments and are presented as the average ± standard
deviation. Western blot analysis (ABCA1, *P<0.05; LXR-β and
ABCG1, P>0.05). RT-qPCR (ABCA1, *P<0.05; LXR-β and ABCG1,
P>0.05). N, non-cancerous mammary tissue; Ca, TNBC tissues;
ABCA1, ATP-binding cassette transporter number 1; LXR-β, liver X
receptor-β; ABCG1, ATP-binding cassette sub-family G number 1;
TNBC, triple-negative breast cancer. |
Discussion
To the best of our knowledge, to date, there is no
similar study available on cholesterol metabolism and ABCA1
expression in TNBC examined using IHC and QD-IHC. From the
histological experiments, the present study identified that ABCA1
was a specific marker for differentiating TNBC tissues from
non-cancerous tissues. Further statistical analysis revealed that
the expression of ABCA1 was associated with the histological grade
of TNBC; however, no significant association with age or TNM stage
was identified. From the above-mentioned results, it is suggested
that ABCA1 may be an oncogene in TNBC; however, further in
vitro studies are required to confirm our findings.
Due to a lack of studies regarding ABCA1 as an
oncogene, the mechanisms of action of ABCA1 as regards the
development and progression of cancer remain unknown. Studies have
demonstrated that ABCA1 is highly expressed in M14 melanoma cell
lines (23), and in LDL1 colon cancer
cell lines and colon cancer tissues (24). The silencing of ABCA1 has been shown
to promote the apoptosis of cancer cells (23). Schimanski et al (19) identified that ABCA1 was highly
expressed in the cell membrane and cytoplasm of breast cancer
cells, and that it was associated with lymph node metastasis.
Another study reported that the overexpression of the ABC
superfamily members, including ABCA1, is an important barrier
against chemotherapy, and sensitivity can be increased by
downregulating or silencing their expression (25).
However, numerous studies have reported that ABCA1
is a tumor suppressor gene that promotes cholesterol metabolism to
inhibit cancer development (26–28).
Studies have demonstrated that ABCA1 exerts antitumor effects and
exhibits a low-to-medium expression in colon cancer cells, as
insufficient levels of ABCA1 can cause an accumulation of
cholesterol in cancer cells, inhibit the release of tumor necrosis
factor in the mitochondria and promote cancer progression (26). Another study demonstrated that LXR
agonists can upregulate ABCA1 expression to inhibit cancer cell
proliferation (27,28). Cells in the S phase require twice the
amount of cholesterol compared with those in the G1 phase for the
completion of DNA synthesis and preparation for the G2 phase and
subsequent mitosis. An LXR agonist can upregulate ABCA1 expression
to inhibit the proliferation of ER-positive breast cancer cells,
promote the efflux of cholesterol from cells and cause
intracellular cholesterol levels in cancer cells to be lower than
that required for the S phase. This interferes with DNA synthesis
in cancer cells, causing them to be unable to complete mitosis,
thereby inhibiting the proliferation of cancer cells (29).
However, the aforementioned mechanism is for
ER-positive breast cancer and other types of cancer cells. To the
best of our knowledge, there is no such study currently available
for TNBC cells. The overexpression of ER can promote reverse
cholesterol transport (29). Unlike
ER-positive breast cancer, TNBC lacks the intermediary effects of
ER. Therefore, further experimental research is warranted to
confirm whether ABCA1 serves as an oncogene in TNBC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HP conducted most of the experiments and wrote the
manuscript; YZ analyzed the experimental data, and was a major
contributor in writing the manuscript; QP collected the clinical
specimens; HC and FC were involved in the conception and design of
the study; JW and DD performed partial of the immunohistochemistry
and quantum dot-immunohistochemistry. All authors have read and
approved the final manuscript and agree to be accountable for all
aspects of the research.
Ethics approval and consent to
participate
For the use of clinical materials, prior approval
was obtained from Traditional Chinese Medical Hospital of Wenling
and written informed consent forms were signed by all the
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Fedewa SA, Goding Sauer A,
Kramer JL, Smith RA and Jemal A: Breast cancer statistics, 2015:
Convergence of incidence rates between black and white women. CA
Cancer J Clin. 66:31–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Curigliano G, Burstein HJ, Winer EP, Gnant
M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn
HJ, et al: De-escalating and escalating treatments for early-stage
breast cancer: The St. Gallen international expert consensus
conference on the primary therapy of early breast cancer 2017. Ann
Oncol. 28:1700–1712. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: Breast cancer, version 4.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
16:310–320. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agnoli C, Grioni S, Sieri S, Sacerdote C,
Ricceri F, Tumino R, Frasca G, Pala V, Mattiello A, Chiodini P, et
al: Metabolic syndrome and breast cancer risk: A case-cohort study
nested in a multicentre italian cohort. PLoS One. 10:e01288912015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu J, La Vecchia C, de Groh M, Negri E,
Morrison H and Mery L; Canadian Cancer Registries Epidemiology
Research Group, : Dietary cholesterol intake and cancer. Ann Oncol.
23:491–500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ni H, Liu H and Gao R: Serum lipids and
breast cancer risk: A meta-analysis of prospective cohort studies.
PLoS One. 10:e01426692015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Danilo C and Frank PG: Cholesterol and
breast cancer development. Curr Opin Pharmacol. 12:677–682. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alikhani N, Ferguson RD, Novosyadlyy R,
Gallagher EJ, Scheinman EJ, Yakar S and LeRoith D: Mammary tumor
growth and pulmonary metastasis are enhanced in a hyperlipidemic
mouse model. Oncogene. 32:961–967. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nelson ER, Wardell SE, Jasper JS, Park S,
Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V,
et al: 27-Hydroxycholesterol links hypercholesterolemia and breast
cancer pathophysiology. Science. 342:1094–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye D, Lammers B, Zhao Y, Meurs I, Van
Berkel TJ and Van Eck M: ATP-binding cassette transporters A1 and
G1, HDL metabolism, cholesterol efflux, and inflammation: Important
targets for the treatment of atherosclerosis. Curr Drug Targets.
12:647–660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park Y, Pham TX and Lee J:
Lipopolysaccharide represses the expression of ATP-binding cassette
transporter G1 and scavenger receptor class B, type I in murine
macrophages. Inflamm Res. 61:465–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang N, Ranalletta M, Matsuura F, Peng F
and Tall AR: LXR-induced redistribution of ABCG1 to plasma membrane
in macrophages enhances cholesterol mass efflux to HDL.
Arterioscler Thromb Vasc Biol. 26:1310–1316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vedhachalam C, Liu L, Nickel M,
Dhanasekaran P, Anantharamaiah GM, Lund-Katz S, Rothblat GH and
Phillips MC: Influence of ApoA-I structure on the ABCA1-mediated
efflux of cellular lipids. J Biol Chem. 279:49931–49939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang N, Lan D, Chen W, Matsuura F and Tall
AR: ATP-binding cassette transporters G1 and G4 mediate cellular
cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci
USA. 101:9774–9779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Repa JJ and Mangelsdorf DJ: The role of
orphan nuclear receptors in the regulation of cholesterol
homeostasis. Annu Rev Cell Dev Biol. 16:459–481. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schimanski S, Wild PJ, Treeck O, Horn F,
Sigruener A, Rudolph C, Blaszyk H, Klinkhammer-Schalke M, Ortmann
O, Hartmann A and Schmitz G: Expression of the lipid transporters
ABCA3 and ABCA1 is diminished in human breast cancer tissue. Horm
Metab Res. 42:102–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parak WJ, Pellegrino T and Plank C:
Labelling of cells with quantum dots. Nanotechnology. 16:R9–R25.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He Y, Zhao X, Gao J, Fan L, Yang G, Cho WC
and Chen H: Quantum dots-based immunofluorescent imaging of stromal
fibroblasts Caveolin-1 and light chain 3B expression and
identification of their clinical significance in human gastric
cancer. Int J Mol Sci. 13:13764–13780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bachmeier BE, Iancu CM, Killian PH,
Kronski E, Mirisola V, Angelini G, Jochum M, Nerlich AG and Pfeffer
U: Overexpression of the ATP binding cassette gene ABCA1 determines
resistance to Curcumin in M14 melanoma cells. Mol Cancer.
8:1292009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bi DP, Yin CH, Zhang XY, Yang NN and Xu
JY: MiR-183 functions as an oncogene by targeting ABCA1 in colon
cancer. Oncol Rep. 35:2873–2879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kachalaki S, Baradaran B, Majidi J,
Yousefi M, Shanehbandi D, Mohammadinejad S and Mansoori B: Reversal
of chemoresistance with small interference RNA (siRNA) in etoposide
resistant acute myeloid leukemia cells (HL-60). Biomed
Pharmacother. 75:100–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith B and Land H: Anticancer activity of
the cholesterol exporter ABCA1 gene. Cell Rep. 2:580–590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gabitova L, Restifo D, Gorin A, Manocha K,
Handorf E, Yang DH, Cai KQ, Klein-Szanto AJ, Cunningham D, Kratz
LE, et al: Endogenous sterol metabolites regulate growth of
EGFR/KRAS-dependent tumors via LXR. Cell Rep. 12:1927–1938. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scoles DR, Xu X, Wang H, Tran H,
Taylor-Harding B, Li A and Karlan BY: Liver X receptor agonist
inhibits proliferation of ovarian carcinoma cells stimulated by
oxidized low density lipoprotein. Gynecol Oncol. 116:109–116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McDonnell DP, Park S, Goulet MT, Jasper J,
Wardell SE, Chang CY, Norris JD, Guyton JR and Nelson ER: Obesity,
cholesterol metabolism, and breast cancer pathogenesis. Cancer Res.
74:4976–4982. 2014. View Article : Google Scholar : PubMed/NCBI
|